Uranium Dissemination with Phosphate Fertilizers Globally: A Systematic Review with Focus on East Africa
Abstract
1. Introduction
1.1. Background Information
1.2. Rationale of the Review
2. Data and Methodology
- Develop research questions;
- Establish search keywords using Boolean operators;
- Choose a reliable search database;
- Limit the article’s publication time interval;
- Import the article abstract to Excel and select the relevant abstract;
- Extract, group, and analyze the data.
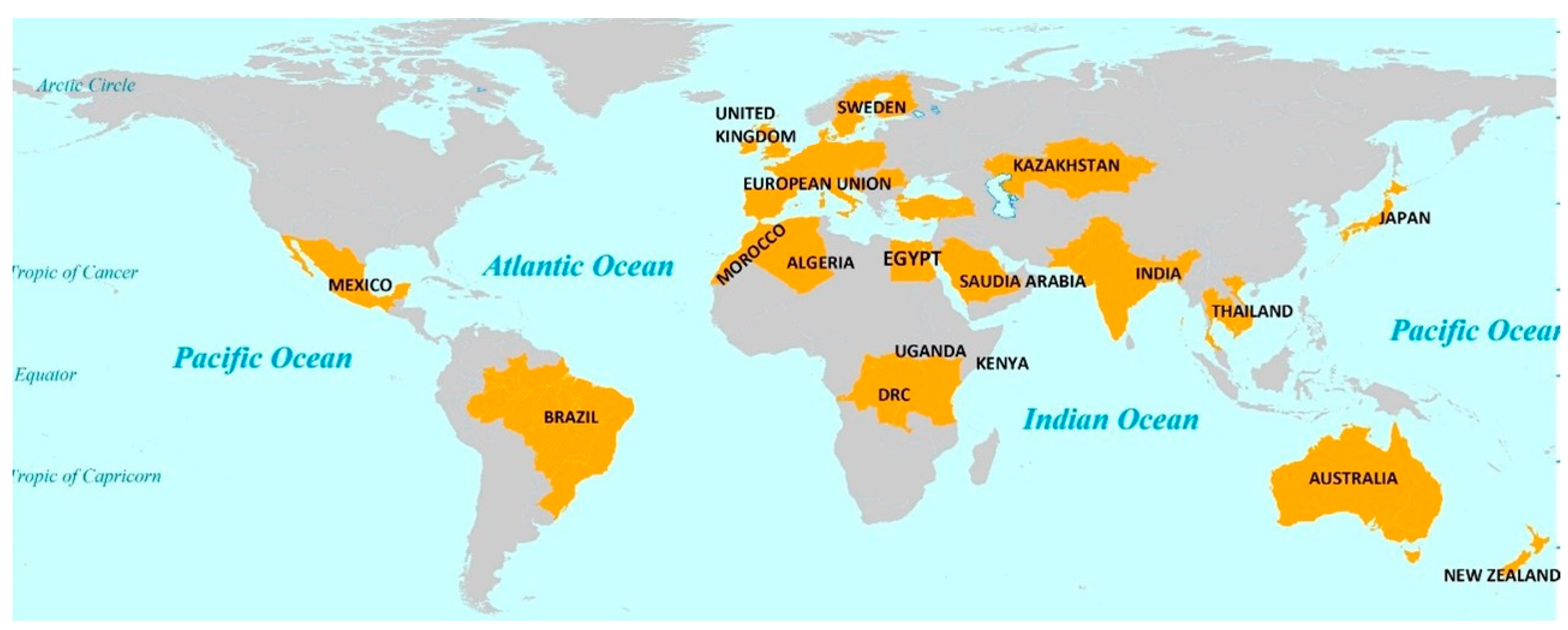
2.1. Countries Included in the Reviewed Study
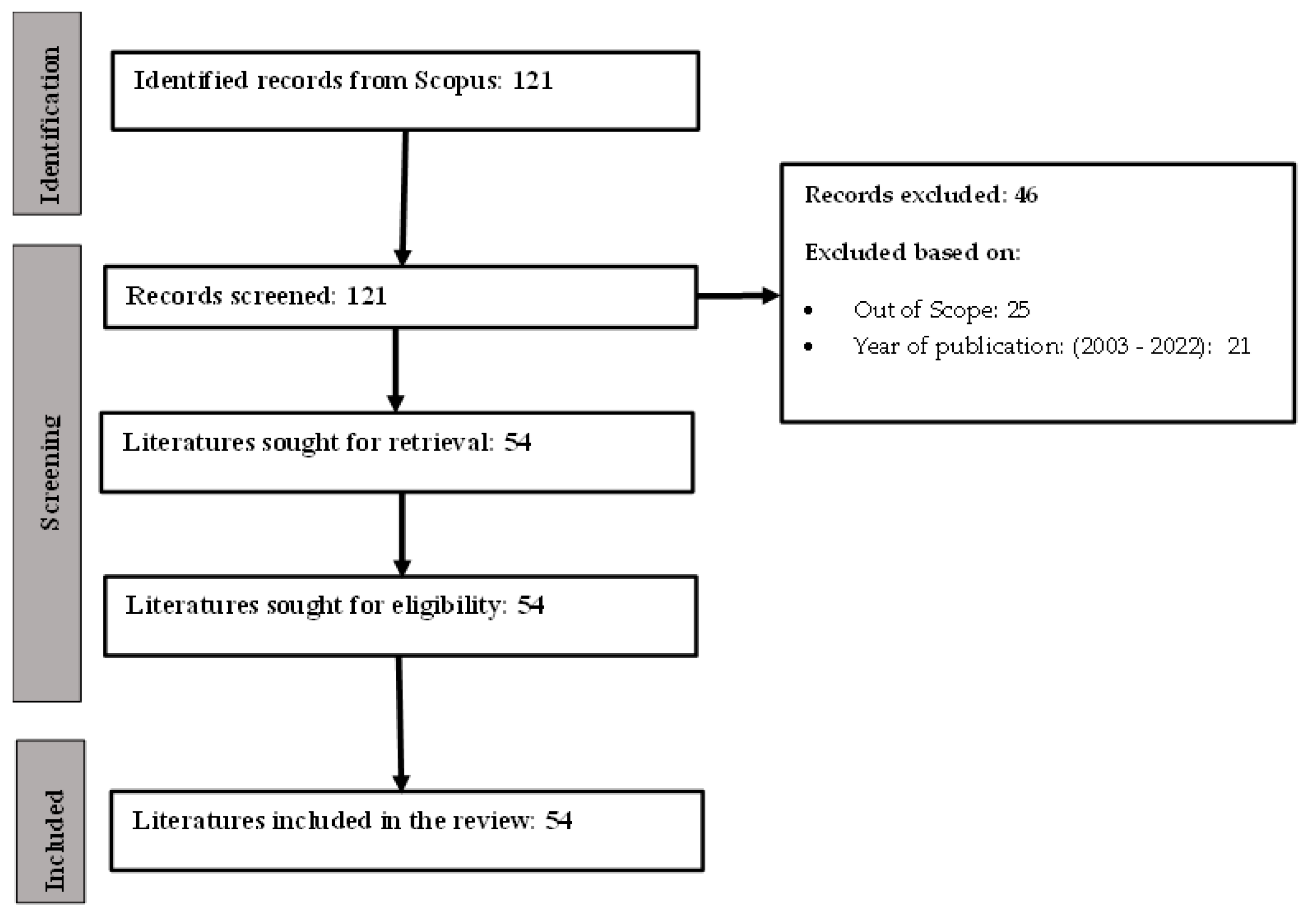
2.2. Literature Sources and Search Strategy
2.3. Inclusion and Exclusion Criteria
- The full text was available for review;
- Scrutinized uranium or radioactivity in phosphate fertilizers in relation to arable or agricultural soils;
- The document type must be a peer referred or reviewed article;
- The year of publication was restricted to twenty years (2003-2022);
- The subject area was not restricted;
- The language was restricted to English.
2.4. Data Collection Process
- The names of the authors and the year of study;
- The country in which the study was carried out;
- Article publication year;
- Sampling environment and study areas;
- Measurement and analytical techniques used;
- Uranium activity concentration (Bq kg−1) or uranium concentration in milligrams per kilogram (mg kg−1) or parts per million (ppm);
- Quantitative reported data (mean, standard deviation, sample size, and uncertainties) from review articles were used. For the sake of uniformity and easy comparisons of various quantitative data, all measurements were converted to ppm (mg kg−1) using the relation: 1 mg kg−1 238U in soil ~12.4 Bq kg−1 238U [61] and the relationship between the activity concentrations of 238U (Bg kg−1) and the concentrations of 238U (mg kg−1): 1 Bq kg−1 = 8.1 × 10−3 mg kg−1 [62].
3. Results and Discussion
3.1. The Focus of the Review
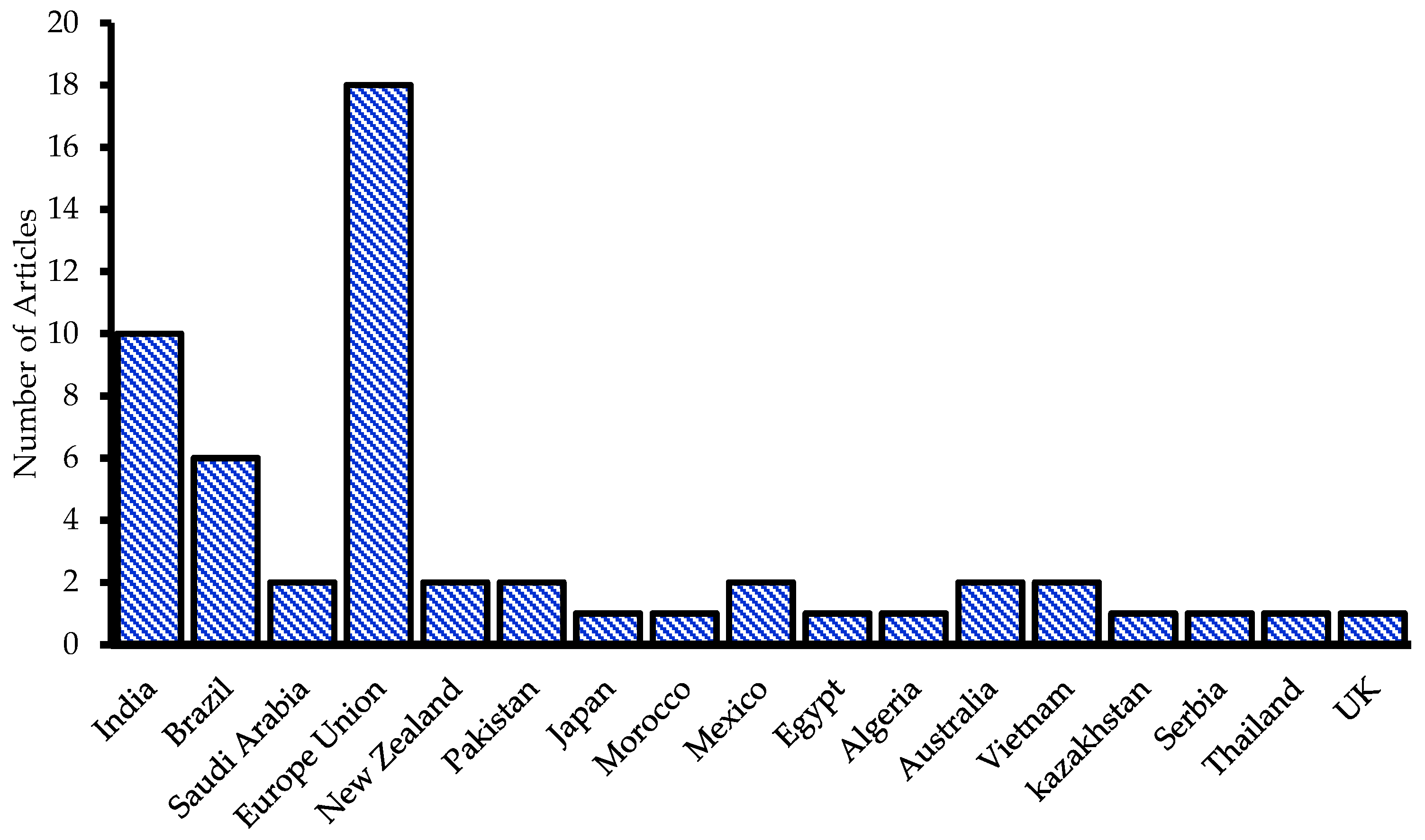
3.2. Geographical Distribution of U in P Fertilizers According to Research Studies
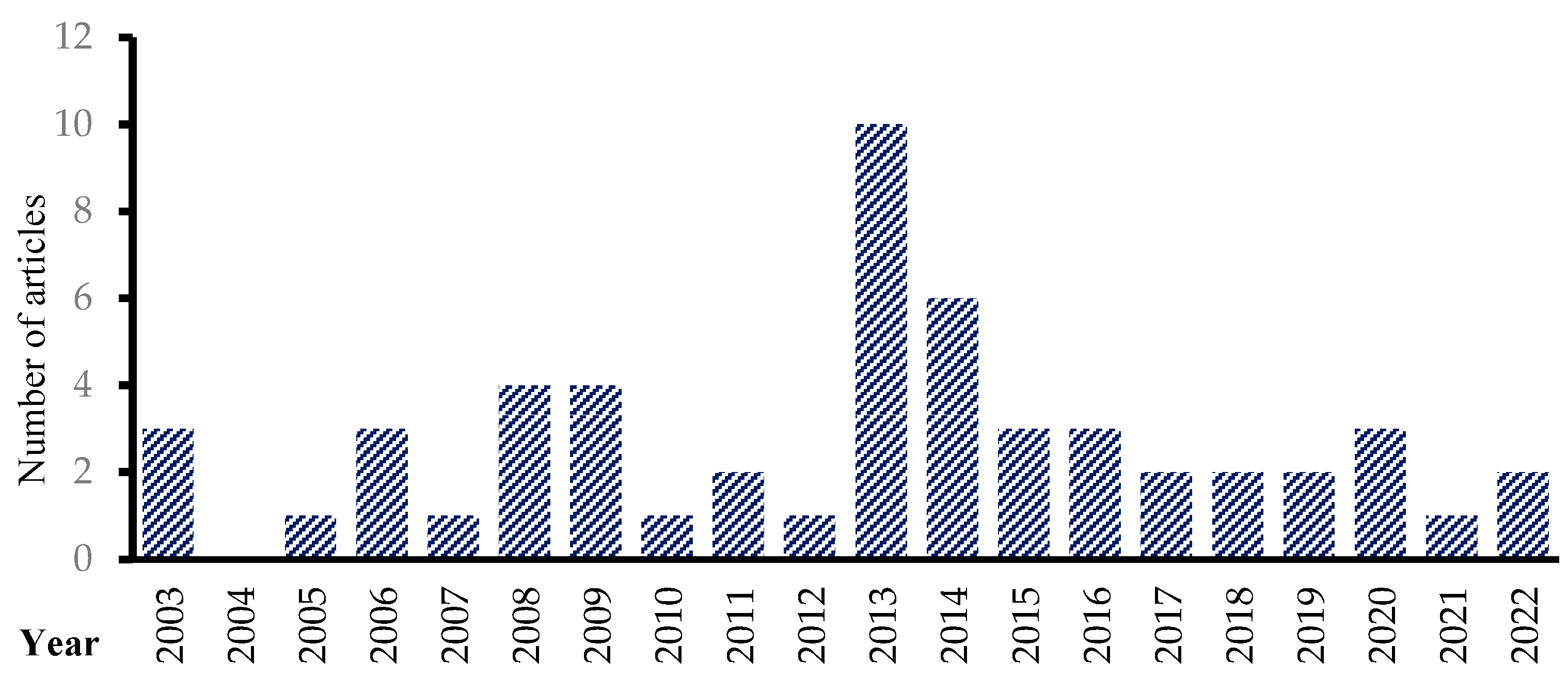
3.3. Descriptive Statistics of the Selected Articles
3.4. U in P Fertilizers and Agricultural Soils
3.4.1. Europe
3.4.2. Australia and New Zealand
3.4.3. Middle East
3.4.4. Africa
3.4.5. Asia
3.4.6. Latin America

3.5. U as a Potential Agricultural Soil Contaminant
3.6. Reported U Uptake by Plants
3.7. The Fate of U Added to Agricultural Soil
3.8. Potential Regulatory Limits of U in P Fertilizers
4. Conclusions and Policy Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Haidar, N.H.S. Uranium Recovery from Phosphates for Self-Sufficient Nuclear Power in the Eastern Mediterranean. Sci. Technol. Nucl. Install. 2022, 2022, 3985408. [Google Scholar] [CrossRef]
- Komar Kawatra, S.; Carlson, J.T. Beneficiation of Phosphate Ore; IntechOpen: London, UK, 2013; p. 168. [Google Scholar]
- Reyes, R.Y.; Ramirez, J.D.; Palattao, B.L.; Tabora, E.U.; Marcelo, E.A.; Vargas, E.P.; Intoy, S.P. Comprehensive Extraction of Uranium, Rare Earth Elements (REE) and Other Valuable Resources from Wet Phosphoric acid. Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:50019773 (accessed on 21 November 2023).
- Beltrami, D.; Cote, G.; Mokhtari, H.; Courtaud, B.; Moyer, B.A.; Chagnes, A. Recovery of uranium from wet phosphoric acid by solvent extraction processes. Chem. Rev. 2014, 114, 12002–12023. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes—A critical review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Hakkar, M.; Arhouni, F.E.; Mahrou, A.; Bilal, E.; Bertau, M.; Roy, A.; Steiner, G.; Haneklaus, N.; Mazouz, H.; Boukhair, A.; et al. Enhancing rare earth element transfer from phosphate rock to phosphoric acid using an inexpensive fly ash additive. Miner. Eng. 2021, 172, 107166. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitras, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Arhouni, F.E.; Hakkar, M.; Mahrou, A.; Belahbib, L.; Mazouz, H.; Haneklaus, N.; Pavón, S.; Bertau, M.; Boukhair, A.; Ouakkas, S.; et al. Better filterability and reduced radioactivity of phosphogypsum during phosphoric acid production in Morocco using a fly ash waste and pure silica additive. J. Radioanal. Nucl. Chem. 2022, 331, 1609–1617. [Google Scholar] [CrossRef]
- Kouzbour, S.; Gourich, B.; Gros, F.; Vial, C.; Allam, F.; Stiriba, Y. Comparative analysis of industrial processes for cadmium removal from phosphoric acid: A review. Hydrometallurgy 2019, 188, 222–247. [Google Scholar] [CrossRef]
- Hoffmann, K.; Huculak-Mączka, M.; Kaniewski, M.; Hoffmann, J. Studies on the use of tributyl phosphate for purification of phosphoric acid. Przem. Chem. 2016, 95, 2276–2280. [Google Scholar]
- Haneklaus, N.H. Unconventional Uranium Resources From Phosphates. Encycl. Nucl. Energy 2021, 286–291. [Google Scholar]
- Ulrich, A.E.; Schnug, E.; Prasser, H.M.; Frossard, E. Uranium endowments in phosphate rock. Sci. Total Environ. 2014, 478, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Al Khaledi, N.; Taha, M.; Hussein, A.; Hussein, E.; El Yahyaoui, A.; Haneklaus, N. Direct leaching of rare earth elements and uranium from phosphate rocks. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479, 012065. [Google Scholar] [CrossRef]
- Diwa, R.R.; Tabora, E.U.; Palattao, B.L.; Haneklaus, N.H.; Vargas, E.P.; Reyes, R.Y.; Ramirez, J.D. Evaluating radiation risks and resource opportunities associated with phosphogypsum in the Philippines. J. Radioanal. Nucl. Chem. 2021, 331, 967–974. [Google Scholar] [CrossRef]
- Campos, D.A.; Blanché, S.; Jungkunst, H.F.; Philippe, A. Distribution, behavior, and erosion of uranium in vineyard soils. Environ. Sci. Pollut. Res. 2021, 28, 53181–53192. [Google Scholar] [CrossRef]
- Bergen, B.; Verbeeck, M.; Smolders, E. Trace metal accumulation in agricultural soils from mineral phosphate fertiliser applications in European long-term field trials. Eur. J. Soil Sci. 2022, 73, e13167. [Google Scholar] [CrossRef]
- Bigalke, M.; Imseng, M.; Schneider, S.; Schwab, L.; Wiggenhauser, M.; Keller, A.; Müller, M.; Frossard, E.; Wilcke, W. Uranium Budget and Leaching in Swiss Agricultural Systems. Front. Environ. Sci. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Sun, Y.; Amelung, W.; Wu, B.; Haneklaus, S.; Schnug, E.; Bol, R. Fertilizer P-derived uranium continues to accumulate at Rothamsted long-term experiments. Sci. Total Environ. 2022, 820, 153118. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Amelung, W.; Gudmundsson, T.; Wu, B.; Bol, R. Critical accumulation of fertilizer-derived uranium in Icelandic grassland Andosol. Environ. Sci. Eur. 2020, 32, 1–7. [Google Scholar] [CrossRef]
- Tulsidas, H.; Gabriel, S.; Kiegiel, K.; Haneklaus, N. Uranium resources in EU phosphate rock imports. Resour. Policy 2019, 61, 151–156. [Google Scholar] [CrossRef]
- Abraham, J.; Dowling, K.; Florentine, S. Assessment of potentially toxic metal contamination in the soils of a legacy mine site in Central Victoria, Australia. Chemosphere 2018, 192, 122–132. [Google Scholar] [CrossRef]
- Wu, F.; Wang, J.; Liu, J.; Zeng, G.; Xiang, P.; Hu, P.; Xiang, W. Distribution, geology and development status of phosphate resources. Geol. China 2021, 48, 82–101. [Google Scholar]
- Hore-Lacy, I. Production of byproduct uranium and uranium from unconventional resources. In Uranium for Nuclear Power: Resources, Mining and Transformation to Fuel; Elsevier: Amsterdam, The Netherlands, 2016; pp. 239–251. ISBN 9780081003077. [Google Scholar]
- Zielinski, R.A.; Simmons, K.R.; Orem, W.H. Use of 234U and 238U isotopes to identify fertilizer-derived uranium in the Florida Everglades. Appl. Geochem. 2000, 15, 369–383. [Google Scholar] [CrossRef]
- Steiner, G.; Geissler, B.; Haneklaus, N. Making Uranium Recovery from Phosphates Great Again? Environ. Sci. Technol. 2020, 54, 1287–1289. [Google Scholar] [CrossRef]
- López, L.; Castro, L.N.; Scasso, R.A.; Grancea, L.; Tulsidas, H.; Haneklaus, N. Uranium supply potential from phosphate rocks for Argentina’s nuclear power fleet. Resour. Policy 2019, 62, 397–404. [Google Scholar] [CrossRef]
- Meza, L.H.; Mandour, M.A.; Shalaby, M.H.; Hassan, M.H.; Mohamed, N.A. A thorough investigation of the uranium concentration in phosphate mines: A case study of Minjingu phosphate mine, Arusha, United Republic of Tanzania. Int. J. Low Radiat. 2015, 10, 74–92. [Google Scholar] [CrossRef]
- Boukhenfouf, W.; Boucenna, A. The radioactivity measurements in soils and fertilizers using gamma spectrometry technique. J. Environ. Radioact. 2011, 102, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.K.; El-Arabi, A.G.M. Natural radioactivity in farm soil and phosphate fertilizer and its environmental implications in Qena governorate, Upper Egypt. J. Environ. Radioact. 2005, 84, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Mwalongo, D.A.; Haneklaus, N.H.; Carvalho, F.P.; Lisuma, J.B.; Kivevele, T.T. Influence of phosphate fertilizers on the radioactivity of agricultural soils and tobacco plants in Kenya, Tanzania, and Uganda. Environ. Sci. Pollut. Res. 2023, 30, 83004–83023. [Google Scholar] [CrossRef] [PubMed]
- Qamouche, K.; Chetaine, A.; El Yahyaoui, A.; Moussaif, A.; Fröhlich, P.; Bertau, M.; Haneklaus, N. Uranium and other heavy metal sorption from Moroccan phosphoric acid with argan nutshell sawdust. Miner. Eng. 2021, 171, 107085. [Google Scholar] [CrossRef]
- Nziguheba, G. Overcoming phosphorus deficiency in soils of Eastern Africa: Recent advances and challenges. In Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities; Springer: New York, NY, USA, 2007. [Google Scholar]
- Kifuko, M.N.; Othieno, C.O.; Okalebo, J.R.; Kimenye, L.N.; Ndung’u, K.W.; Kipkoech, A.K. Effect of combining organic residues with Minjingu phosphate rock on sorption and availability of phosphorus and maize production in acid soils of western Kenya. Exp. Agric. 2007, 43, 51–66. [Google Scholar] [CrossRef]
- Ndeleko-Barasa, E.M.; Mucheru-Muna, M.W.; Ngetich, K.F. Agronomic and financial benefits of direct Minjingu phosphate rock use in acidic humic nitisols of Upper Eastern Kenya. Heliyon 2021, 7, e08332. [Google Scholar] [CrossRef]
- Abuli, J.S.; Mugwe, J.N.; Mugendi, D.N. Effects of Phosphorus Sources on Soybean Yield in Central Highlands of Kenya; Kenyatta University Press: Nairobi, Kenya, 2012; pp. 595–601. [Google Scholar]
- Schnug, E.; Lottermoser, B.G. Fertilizer-derived uranium and its threat to human health. Environ. Sci. Technol. 2013, 47, 2433–2434. [Google Scholar] [CrossRef]
- De Souza Braz, A.M.; Da Costa, M.L.; Ramos, S.J.; Dall’agnol, R.; Fernandes, A.R. Long term application of fertilizers in eastern amazon and effect on uranium and thorium levels in soils. Minerals 2021, 11, 994. [Google Scholar] [CrossRef]
- Zlobina, A.; Farkhutdinov, I.; Carvalho, F.P.; Wang, N.; Korotchenko, T.; Baranovskaya, N.; Farkhutdinov, A. Impact of Environmental Radiation on the Incidence of Cancer and Birth Defects in Regions with High Natural Radioactivity. Int. J. Environ. Res. Public Health 2022, 19, 8643. [Google Scholar] [CrossRef] [PubMed]
- Van Dung, N.; Thuan, D.D.; Nhan, D.D.; Carvalho, F.P.; Van Thang, D.; Quang, N.H. Radiation exposure in a region with natural high background radiation originated from rare earth element deposits at Bat Xat district, Vietnam. Radiat. Environ. Biophys. 2022, 61. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Kawasaki, A.; Iiyama, I. Distribution of uranium in soil components of agricultural fields after long-term application of phosphate fertilizers. Sci. Total Environ. 2009, 407, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, I.M.; Geraldo, L.P. Uranium content in phosphate fertilizers commercially produced in Brazil. Appl. Radiat. Isot. 2003, 59, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Kratz, S.; Schnug, E. Rock phosphates and P fertilizers as sources of U contamination in agricultural soils. Uranium Environ. Min. Impact Consequences 2006, 10, 57–67. [Google Scholar]
- Hu, N.; Zhang, H.; Ding, D.; Tan, Y.; Li, G. Influence of Uranium Speciation on Plant Uptake. In Uranium in Plants and the Environment; Springer: Cham, Switzerland, 2020; pp. 181–191. [Google Scholar]
- Kaishwa, S.J.; Marwa, E.M.; Msaky, J.J.; Mwakalasya, W.N. Uranium natural levels in soil, rock and water: Assessment of the quality of drinking water in Singida Urban District, Tanzania. J. Water Health 2018, 16, 542–548. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, X.; Hu, X.; Wei, J.; Tang, C. Mineralogical and geochemical evidence for biogenic uranium mineralization in northern Songliao Basin, NE China. Ore Geol. Rev. 2022, 141, 104556. [Google Scholar] [CrossRef]
- Banzi, F.P.; Kifanga, L.D.; Bundala, F.M. Natural radioactivity and radiation exposure at the Minjingu phosphate mine in Tanzania. J. Radiol. Prot. 2000, 20, 41–51. [Google Scholar] [CrossRef]
- Mwalongo, D.A.; Haneklaus, N.H.; Lisuma, J.B.; Kivevele, T.T.; Mtei, K.M. Uranium in phosphate rocks and mineral fertilizers applied to agricultural soils in East Africa. Environ. Sci. Pollut. Res. 2023, 30, 33898–33906. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; Mansour, N.A.; Fayez-Hassan, M.; Sedqy, E. Assessment of natural radioactivity in fertilizers and phosphate ores in Egypt. J. Taibah Univ. Sci. 2016, 10, 296–306. [Google Scholar] [CrossRef]
- El-Bahi, S.M.; Sroor, A.; Mohamed, G.Y.; El-Gendy, N.S. Radiological impact of natural radioactivity in Egyptian phosphate rocks, phosphogypsum and phosphate fertilizers. Appl. Radiat. Isot. 2017, 123, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; Chang, B.U.; Tokonami, S. Comparison of Natural Radioactivity of Commonly Used Fertilizer Materials in Egypt and Japan. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Schmidtlein, R.K. Phosphate Fertilizers from Morocco and Russia US; International Trade Commission: Washington, DC, USA, 2020; p. 651. [Google Scholar]
- El Bamiki, R.; Raji, O.; Ouabid, M.; Elghali, A.; Yazami, O.K.; Bodinier, J.L. Phosphate rocks: A review of sedimentary and igneous occurrences in Morocco. Minerals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Searchinger, T.; Richard, W.; Craig, H.; Janet, R. Creating a Sustainable Food Future; A Menu of Solutions to Feed Nearly 10 Billion People by 2050; World Resources Institute: Washington, DC, USA, 2018. [Google Scholar]
- Haneklaus, N.; Sun, Y.; Bol, R.; Lottermoser, B.; Schnug, E. To extract, or not to extract uranium from phosphate rock, that is the question. Environ. Sci. Technol. 2017, 51, 753–754. [Google Scholar] [CrossRef]
- Kratz, S.; Schick, J.; Schnug, E. Trace elements in rock phosphates and P containing mineral and organo-mineral fertilizers sold in Germany. Sci. Total Environ. 2016, 542, 1013–1019. [Google Scholar] [CrossRef]
- Bigalke, M.; Schwab, L.; Rehmus, A.; Tondo, P.; Flisch, M. Uranium in agricultural soils and drinking water wells on the Swiss Plateau. Environ. Pollut. 2018, 233, 943–951. [Google Scholar] [CrossRef]
- Takeda, A.; Tsukada, H.; Takaku, Y.; Hisamatsu, S.; Nanzyo, M. Accumulation of uranium derived from long-term fertilizer applications in a cultivated Andisol. Sci. Total Environ. 2006, 367, 924–931. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Williams, G.D.Z.; Dwyer, G.S.; Gatiboni, L.; Duckworth, O.W.; Vengosh, A. Evidence for the accumulation of toxic metal(loid)s in agricultural soils impacted from long-term application of phosphate fertilizer. Sci. Total Environ. 2024, 907, 167863. [Google Scholar] [CrossRef]
- IAEA. Energy Neutral Mineral Processing with High Temperature Reactors: Resource Identification, Uranium Recovery and Thermal Processes; International Atomic Energy Agency: Vienna, Austria, 2023; Volume IAEA-TECDO, ISBN 978-92-0-118823-6. [Google Scholar]
- Mengist, W.; Soromessa, T.; Legese, G. Method for conducting systematic literature review and meta-analysis for environmental science research. MethodsX 2020, 7, 100777. [Google Scholar] [CrossRef]
- Harmsen, K.; Haan, F.A.M. de Occurance and behaviour of uranium and thorium in soil and water. Neth. J. Agric. Sci. 1980, 28, 40–62. [Google Scholar]
- UNSCEAR, United Nations Scientific Committee on the Effect of Atomic Radiation. Sources and Effects of Ionizing Radiation; Sources and Effects of Ionizing Radiation: New York, NY, USA, 2000. [Google Scholar]
- Reitsma, F.; Woods, P.; Fairclough, M.; Kim, Y.; Tulsidas, H.; Lopez, L.; Zheng, Y.; Hussein, A.; Brinkmann, G.; Haneklaus, N.; et al. On the sustainability and progress of energy neutral mineral processing. Sustainability 2018, 10, 235. [Google Scholar] [CrossRef]
- Duhan, S.S.; Khyalia, P.; Solanki, P.; Laura, J.S. Uranium Sources, Uptake, Translocation in the soil-plant System and Its Toxicity in Plants and Humans: A Critical Review. Orient. J. Chem. 2023, 39, 303–319. [Google Scholar] [CrossRef]
- Semioshkina, N.; Voigt, G. Soil—Plant transfer of radionuclides in arid environments. J. Environ. Radioact. 2021, 237, 106692. [Google Scholar] [CrossRef]
- Uchida, S.; Tagami, K.; Hirai, I. Soil-to-plant transfer factors of stable elements and naturally occurring radionuclides (1) upland field crops collected in Japan. J. Nucl. Sci. Technol. 2007, 44, 628–640. [Google Scholar] [CrossRef]
- Verbeeck, M.; Salaets, P.; Smolders, E. Trace element concentrations in mineral phosphate fertilizers used in Europe: A balanced survey. Sci. Total Environ. 2020, 712, 136419. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, B.; Amelung, W.; Christensen, B.T.; Pätzold, S.; Bauke, S.L.; Schweitzer, K.; Baumecker, M.; Bol, R. Non-critical uranium accumulation in soils of German and Danish long-term fertilizer experiments. Geoderma 2020, 370, 114336. [Google Scholar] [CrossRef]
- Servitzoglou, N.G.; Stoulos, S.; Katsantonis, D.; Papageorgiou, M.; Siountas, A. Natural radioactivity studies of phosphate fertilizers applied on Greek Farm soils used for wheat cultivation. Radiat. Prot. Dosimetry 2018, 181, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.E. Cadmium governance in Europe’s phosphate fertilizers: Not so fast? Sci. Total Environ. 2019, 650, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Suciu, N.A.; De Vivo, R.; Rizzati, N.; Capri, E. Cd content in phosphate fertilizer: Which potential risk for the environment and human health? Curr. Opin. Environ. Sci. Heal. 2022, 30, 100392. [Google Scholar] [CrossRef]
- Schnug, E.; Haneklaus, N. Uranium, the hidden treasure in phosphates. Procedia Eng. 2014, 83, 265–269. [Google Scholar] [CrossRef]
- Liesch, T.; Hinrichsen, S.; Goldscheider, N. Uranium in groundwater—Fertilizers versus geogenic sources. Sci. Total Environ. 2015, 536, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Rogasik, J.; Kratz, S.; Funder, U.; Panten, K.; Barkusky, D. Uranium in Soils of German Long-Term Fertilizer Experiments; Backhuys Publishers: Leiden, The Netherlands, 2008. [Google Scholar]
- Lottermoser, B.G. Trace metal enrichment in sugarcane soils due to the long-term application of fertilisers, north Queensland, Australia: Geochemical and Pb, Sr, and U isotopic compositions. Aust. J. Soil Res. 2009, 47, 311–320. [Google Scholar] [CrossRef]
- Hilton, J.; Johnston, A.E.; Dawson, C.J. The Phosphate Life-Cycle: Rethinking the Options for a finite Resource. In Proceedings of the Ifs; International Fertiliser Society: Colchester, UK, 2010; p. 23. [Google Scholar]
- Taylor, M.D. Accumulation of uranium in soils from impurities in phosphate fertilisers. Landbauforsch. Volkenrode 2007, 57, 133–139. [Google Scholar]
- Pearson, A.J.; Gaw, S.; Hermanspahn, N.; Glover, C.N.; Anderson, C.W.N. Radium in New Zealand agricultural soils: Phosphate fertiliser inputs, soil activity concentrations and fractionation profiles. J. Environ. Radioact. 2019, 205–206, 119–126. [Google Scholar] [CrossRef]
- Alshahri, F.; Alqahtani, M. Chemical fertilizers as a source of 238U, 40K, 226Ra, 222Rn, and trace metal pollutant of the environment in Saudi Arabia. Environ. Sci. Pollut. Res. 2015, 22, 8339–8348. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.A.; Kinsara, A.A.; Molla, N.I.; Nassef, M.H. Natural radioactivity measurements in agricultural soil, fertilizer and crops in some specific areas of Kingdom of Saudi Arabia. Radiochim. Acta 2014, 102, 513–522. [Google Scholar] [CrossRef]
- Al-Eshaikh, M.A.; Kadachi, A.N.; Mansoor Sarfraz, M. Determination of uranium content in phosphate ores using different measurement techniques. J. King Saud Univ. Eng. Sci. 2016, 28, 41–46. [Google Scholar] [CrossRef]
- Khater, A.E.M.; AL-Sewaidan, H.A. Radiation exposure due to agricultural uses of phosphate fertilizers. Radiat. Meas. 2008, 43, 1402–1407. [Google Scholar] [CrossRef]
- Khater, A.E.M. Uranium and heavy metals in phosphate fertilizers. In Uranium, Mining and Hydrogeology; Springer: New York, NY, USA, 2008; pp. 193–198. [Google Scholar]
- Bianconi, F. Uranium geology of Tanzania: Monograph series on mineral deposits. In Proceedings of the Uranium Mineralization—New Aspects on Geology, Mineralogy, Geochemistry, and Exploration Methods; Friedrich, G., Gatzweiler, R., Vogt, J., Eds.; Bornträger: Aachen, Germany, 1987; pp. 11–25. [Google Scholar]
- Mustonen, R.; Annanmaki, M. Studies on the Radiation Exposure of Workers in Connection with Processing of the Minjingu Phosphate in Tanzania; Supplementary Report to the Finnish Center for Radiation and Nuclear Safety 666/622/87; Finnish Center for Radiation and Nuclear Safety: Helsinki, Finland, 1988. [Google Scholar]
- Makweba, M.M.; Holm, E. The natural radioactivity of the rock phosphates, phosphatic products and their environmental implications. Sci. Total Environ. 1993, 133, 99–110. [Google Scholar] [CrossRef]
- Hameed, P.S.; Pillai, G.S.; Mathiyarasu, R. A study on the impact of phosphate fertilizers on the radioactivity profile of cultivated soils in Srirangam (Tamil Nadu, India). J. Radiat. Res. Appl. Sci. 2014, 7, 463–471. [Google Scholar] [CrossRef]
- Chauhan, P.; Chauhan, R.P.; Gupta, M. Estimation of naturally occurring radionuclides in fertilizers using gamma spectrometry and elemental analysis by XRF and XRD techniques. Microchem. J. 2013, 106, 73–78. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.P.; Kumar, A. Soil to plant transfer of alpha activity in potato plants: Impact of phosphate fertilizers. J. Environ. Health Sci. Eng. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- UNSCEAR. Sources and Effects of Ionizing Radiation; Report to the General Assembly of the United Nations with Scientific Annexes, United Nations Sales; UNSCEAR: Vienna, Austria, 2000. [Google Scholar]
- Punniyakotti, J.; Lakshmi, K.S.; Meenakshisundaram, V.; Manju, N.; Poonguzhali, P. Influence of fertilizers on the natural radioactivity profile of soil samples of agricultural land in Villupuram District, Tamilnadu State, India. J. Radioanal. Nucl. Chem. 2020, 325, 85–92. [Google Scholar] [CrossRef]
- Kant, K.; Upadhyay, S.B.; Sonkawade, R.G.; Chakarvarti, S.K. Radiological risk assessment of use of phosphate fertilizers in soil. Iran. J. Radiat. Res. 2006, 4, 63–70. [Google Scholar]
- Ghosh, D.; Deb, A.; Bera, S.; Sengupta, R.; Patra, K.K. Measurement of natural radioactivity in chemical fertilizer and agricultural soil: Evidence of high alpha activity. Environ. Geochem. Health 2008, 30, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hossain, S.M.; Rahman, M.T.; Halim, M.A.; Ishtiak, M.N.; Kabir, M. Determination of trace metal concentration in compost, DAP, and TSP fertilizers by neutron activation analysis (NAA) and insights from density functional theory calculations. Environ. Monit. Assess. 2017, 189, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nasim-Akhtar; Sabiha-Javied; Tufail, M. Enhancement of natural radioactivity in fertilized soil of Faisalabad, Pakistan. Environ. Sci. Pollut. Res. 2012, 19, 3327–3338. [Google Scholar] [CrossRef]
- Akhtar, N.; Tufail, M.; Hussain, M.Y.; Akram, M. Primordial radionuclides contamination level in fertilized farms soils of Faisalabad-Pakistan. Soil Environ. 2011, 30, 88–94. [Google Scholar]
- Nguyen Van, T.; Vu Ngoc, B.; Huynh Nguyen Phong, T.; Le Cong, H.; Truong Thi Hong, L. Gross alpha, gross beta and activity concentration of 226Ra in some fertilizers commonly used in the south of Vietnam and health risk due to radionuclides transferred from fertilizers to food crops. J. Radioanal. Nucl. Chem. 2018, 317, 463–471. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Huynh, N.P.T.; Vu, N.B.; Le, C.H. Long-term accumulation of 226Ra in some agricultural soils based on model assessment. Agric. Water Manag. 2021, 243, 106453. [Google Scholar] [CrossRef]
- Tagami, K.; Uchida, S. Soil-to-Crop Transfer Factor: Consideration on Excess Uranium from Phosphate Fertilizer. In Uranium in Plants and the Environment; Springer: Cham, Switzerland, 2020; pp. 163–180. [Google Scholar]
- Lauria, D.C.; Ribeiro, F.C.A.; Conti, C.C.; Loureiro, F.A. Radium and uranium levels in vegetables grown using different farming management systems. J. Environ. Radioact. 2009, 100, 176–183. [Google Scholar] [CrossRef]
- Saueia, C.H.R.; Le Bourlegat, F.M.; Mazzilli, B.P.; Fávaro, D.I.T. Availability of metals and radionuclides present in phosphogypsum and phosphate fertilizers used in Brazil. J. Radioanal. Nucl. Chem. 2013, 297, 189–195. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Thu Huynh, N.P.; Le, C.H. Accumulation rates of natural radionuclides (40K, 210Pb, 226Ra, 238U, and 232Th) in topsoils due to long-term cultivations of water spinach (Ipomoea Aquatica Forssk.) and rice (Oryza sativa L.) based on model assessments: A case study in Dong Nai province. J. Environ. Manag. 2020, 271, 111001. [Google Scholar] [CrossRef]
- IAEA. Radiation Protection and Management of NORM Residues in the Phosphate Industry; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Hoyer, M. Uranium contamination of soil and groundwater by phosphate fertilizer application. FOG Freib. Online Geosci. 2013, 35, 707–716. [Google Scholar]
- Birke, M.; Rauch, U.; Lorenz, H. Uranium in stream and mineral water of the Federal Republic of Germany. Environ. Geochem. Health 2009, 31, 693–706. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Chemical aspects of uranium behavior in soils: A review. Eurasian Soil Sci. 2011, 44, 862–873. [Google Scholar] [CrossRef]
- IAEA/NEA. Uranium Resources, Production and Demand; IAEA: Vienna, Austria, 2020; Volume 15. [Google Scholar]
- Considine, T.J. The market impacts of US uranium import quotas. Resour. Policy 2019, 63, 101445. [Google Scholar] [CrossRef]
- Gabriel, S.; Baschwitz, A.; Mathonnière, G.; Fizaine, F.; Eleouet, T. Building future nuclear power fleets: The available uranium resources constraint. Resour. Policy 2013, 38, 458–469. [Google Scholar] [CrossRef]
- Sun, Y.; Haneklaus, N.; Bol, R.; Lottermoser, B.; Schnug, E. Phosphate rock—The chance and need for zero waste activity. In Proceedings of the 8th International Phosphorus Workshop (IPW8), Rostock, Germany, 12–13 September 2016; pp. 12–16. [Google Scholar]
- Vandenhove, H.; Van Hees, M.; Wouters, K.; Wannijn, J. Can we predict uranium bioavailability based on soil parameters? Part 1: Effect of soil parameters on soil solution uranium concentration. Environ. Pollut. 2007, 145, 587–595. [Google Scholar] [CrossRef]
- Chen, B.; Roos, P.; Borggaard, O.K.; Zhu, Y.G.; Jakobsen, I. Mycorrhiza and root hairs in barley enhance acquisition of phosphorus and uranium from phosphate rock but mycorrhiza decreases root to shoot uranium transfer. New Phytol. 2005, 165, 591–598. [Google Scholar] [CrossRef]
- Reimann, C.; Filzmoser, P.; Hron, K.; Kynčlová, P.; Garrett, R.G. A new method for correlation analysis of compositional (environmental) data—A worked example. Sci. Total Environ. 2017, 607–608, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, M.; Zurn, J.D.; Molero, G.; Singh, P.; He, X.; Aoun, M.; Juliana, P.; Bockleman, H.; Bonman, M.; El-Sohl, M.; et al. The role of wheat in global food security. In Agricultural Development and Sustainable Intensification; Routledge: London, UK, 2018; pp. 81–110. [Google Scholar]
- Stanojković, A.; Dukić, D.A.; Mandić, L.; Pivić, R.; Stanojković, A.; Jošić, D. Evaluation of the chemical composition and yield of crops as influenced by bacterial and mineral fertilization. Rom. Biotechnol. Lett. 2012, 17, 7136–7144. [Google Scholar]
- Stojanović, M.; Mihajlović, M.; Lopičić, Z.; Milojković, J.; Šoštarić, T.; Petrović, M. The influence of soil type on maize and wheat uranium uptake. Qual. Assur. Saf. Crop. Foods 2013, 5, 237–242. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Mitra, A.; Voronina, A.; Walther, C. Uranium and Plants: Elemental Translocation and Phytoremediation Approaches; EPA: New York, NY, USA, 2020. [Google Scholar]
- Charro, E.; Moyano, A. Soil and vegetation influence in plants natural radionuclides uptake at a uranium mining site. Radiat. Phys. Chem. 2017, 141, 200–206. [Google Scholar] [CrossRef]
- Porntepkasemsan, B.; Kulsawat, W.; Nochit, P. Impact of phosphate fertilizers on the uranium and thorium of cultivated soils profiles, Kamphaeng Phet, Thailand. J. Phys. Conf. Ser. 2018, 1144, 012072. [Google Scholar] [CrossRef]
- Bigalke, M.; Ulrich, A.; Rehmus, A.; Keller, A. Accumulation of cadmium and uranium in arable soils in Switzerland. Environ. Pollut. 2017, 221, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Walther, C. Uranium in Plants and the Environment; Springer: Cham, Switzerland, 2019; ISBN 3030149617. [Google Scholar]
- Sheppard, S.C.; Sheppard, M.I.; Gallerand, M.O.; Sanipelli, B. Derivation of ecotoxicity thresholds for uranium. J. Environ. Radioact. 2005, 79, 55–83. [Google Scholar] [CrossRef] [PubMed]
- Guillén, J.; Gómez-Polo, F.M. Factors influencing the soil to plant transfer of uranium. In Uranium in Plants and the Environment; Springer: Cham, Switzerland, 2020; pp. 137–147. [Google Scholar]
- Ratnikov, A.N.; Sviridenko, D.G.; Popova, G.I.; Sanzharova, N.I.; Mikailova, R.A. The Behaviour of Uranium in Soils and the Mechanisms of Its Accumulation by Agricultural Plants. In Uranium in Plants and the Environment; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Nkuba, L.L.; Mohammed, N.K. Determination of radioactivity in maize and mung beans grown in the neighborhood of Minjingu phosphate mine, Tanzania. Tanzania J. Sci. 2014, 40, 51–59. [Google Scholar]
- Dos Santos Amaral, R.; Eustaquio de Vasconcelos, W.; Borges, E.; Vita Silveira, S.; Paci Mazzilli, B. Intake of uranium and radium-226 due to food crops consumption in the phosphate region of Pernambuco–Brazil. J. Environ. Radioact. 2005, 82, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, K.; Khandaker, M.U.; Amin, Y.M.; Mahat, R. Uptake and distribution of natural radioactivity in rice from soil in north and west part of peninsular malaysia for the estimation of ingestion dose to man. Ann. Nucl. Energy 2015, 76, 85–93. [Google Scholar] [CrossRef]
- Laurette, J.; Larue, C.; Mariet, C.; Brisset, F.; Khodja, H.; Bourguignon, J.; Carrière, M. Influence of uranium speciation on its accumulation and translocation in three plant species: Oilseed rape, sunflower and wheat. Environ. Exp. Bot. 2012, 77, 96–107. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, W.; Zhou, J.; Luo, D.; Li, Z. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Pavel, L.V.; Cretescu, I. Characterization and remediation of soils contaminated with uranium. J. Hazard. Mater. 2009, 163, 475–510. [Google Scholar] [CrossRef]
- Echevarria, G.; Sheppard, M.I.; Morel, J.L. Effect of pH on the sorption of uranium in soils. J. Environ. Radioact. 2001, 53, 257–264. [Google Scholar] [CrossRef]
- Birke, M.; Rauch, U.; Lorenz, H.; Kringel, R. Distribution of uranium in German bottled and tap water. J. Geochem. Explor. 2010, 107, 272–282. [Google Scholar] [CrossRef]
- Haneklaus, N.; Bayok, A.; Fedchenko, V. Phosphate rocks and nuclear proliferation. Sci. Glob. Secur. 2017, 25, 143–158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwalongo, D.A.; Haneklaus, N.H.; Lisuma, J.B.; Mpumi, N.; Amasi, A.I.; Mwimanzi, J.M.; Chuma, F.M.; Kivevele, T.T.; Mtei, K.M. Uranium Dissemination with Phosphate Fertilizers Globally: A Systematic Review with Focus on East Africa. Sustainability 2024, 16, 1496. https://doi.org/10.3390/su16041496
Mwalongo DA, Haneklaus NH, Lisuma JB, Mpumi N, Amasi AI, Mwimanzi JM, Chuma FM, Kivevele TT, Mtei KM. Uranium Dissemination with Phosphate Fertilizers Globally: A Systematic Review with Focus on East Africa. Sustainability. 2024; 16(4):1496. https://doi.org/10.3390/su16041496
Chicago/Turabian StyleMwalongo, Dennis A., Nils H. Haneklaus, Jacob B. Lisuma, Nelson Mpumi, Aloyce I. Amasi, Jerome M. Mwimanzi, Furaha M. Chuma, Thomas T. Kivevele, and Kelvin M. Mtei. 2024. "Uranium Dissemination with Phosphate Fertilizers Globally: A Systematic Review with Focus on East Africa" Sustainability 16, no. 4: 1496. https://doi.org/10.3390/su16041496
APA StyleMwalongo, D. A., Haneklaus, N. H., Lisuma, J. B., Mpumi, N., Amasi, A. I., Mwimanzi, J. M., Chuma, F. M., Kivevele, T. T., & Mtei, K. M. (2024). Uranium Dissemination with Phosphate Fertilizers Globally: A Systematic Review with Focus on East Africa. Sustainability, 16(4), 1496. https://doi.org/10.3390/su16041496






