Abstract
The world is moving towards decarbonization policies in the energy and industrial sectors to bring down carbon dioxide release and reach net zero emissions. Technologies to capture CO2 and use it as a feedstock to produce CO2-based chemicals and biofuels via chemical or biochemical conversion pathways can potentially reduce the amount of CO2 released. The paper serves the innovative scientific knowledge for CO2 transformation via a biochemical pathway to microalgal biomass with its subsequent treatment to biofuels and bioproducts assuming milder climatic conditions (Central or Eastern Europe, Visegrad countries or climatically related world regions). The recent trends were critically reviewed for microalgal biorefinery to reach the sustainability of microalgal-based chemicals with added value, digestion, hydrothermal liquefaction, pyrolysis, and gasification of microalgal residues. Knowledge-based chemical process engineering analysis, systematic data synthesis, and critical technical evaluation of available life cycle assessment studies evaluated the sustainability of microalgal biorefinery pathways. The research showed that biological CO2 fixation using water, seawater or wastewater to produce third-generation biomass is a promising alternative for bioethanol production via pretreatment, enzymatic hydrolysis, digestion, and distillation, and can be realized on a large scale in an economically viable and environmentally sound manner. Its best economically promising and sustainable pathway is perceived in producing microalgal-based nutraceuticals, bioactive medical products, and food products such as proteins, pigments, and vitamins. Machine learning methods for data mining, process control, process optimization, and geometrical configuration of reactors and bioreactors are the crucial research needs and challenges to implementing microalgal biorefinery in an operational environment.
1. Introduction
The world’s industrialization and rapid economic growth caused a sharp increase in CO2 emissions. The global CO2 emission report [1] demonstrates that total global CO2 emissions permanently increased from 5.0 Gt CO2 in 1940 to 23.2 Gt in 1990, 25.45 Gt in 2000, and 37.12 Gt CO2 in 2021. Due to its negative effect associated with global warming, there is a worldwide effort to decrease its generation significantly. The Forest for Climate Declaration, ratified at the COP24 Congress in Katowice in 2018, calls for equilibrium in the 21st century between greenhouse gases emitted by various sources and those removed by sinks [2]. The Paris Agreement also requires minimizing greenhouse gases from 20 wt % in 2020 by 40 wt % in 2030 and up to 80 wt % in 2050, all compared to 1990.
The V4 countries (also known as V4, Visegrad Group or Visegrad Four) represent the countries in Central Europe formed by Czechia, Hungary, Poland, and Slovakia. The V4 countries sincerely cooperate to keep sustainable regional cooperation at the industrial, economic, and political levels and share intellectual property or culture. Generally known, there is a global effort in sustainable climate and renewable energy development strategies and raw material security, as governmentally policed and regulated by the Paris Agreement, Renewable Directive Strategies, Fit for 55, and Repower E.U. plans.
The V4 countries also plan to implement several decarbonization policies in the energy and industrial sectors to decrease carbon dioxide release and meet net zero emissions by 2050. Annual global shares of CO2 emissions in 2021 [3] from fossil fuels and industry were 0.09 wt % for Slovakia, 0.13 wt % for Hungary, 0.26 wt % for Czechia, and 0.88 wt % for Poland. As for annual EU-27 shares of CO2 emissions in 2021, the values of 1.1 wt % for Slovakia, 1.6 wt % for Hungary, 3.5 wt % for Czechia, and 10.9 wt % for Poland were reported [4]. Regarding the trends of CO2 emissions per country in Figure 1, all the V4 countries tend to reduce annual CO2 emissions. The CO2 release from fossil fuel resources and industry, as shown in Figure 2, was reported as being 4.99 metric tons per person in Hungary, 6.48 metric tons per person in Slovakia, 8.58 metric tons per person in Poland, and 9.24 tons per person in Czechia, all dated in 2021 [5]. Comparing V4 countries, worldwide CO2 released from burning fossil fuels and from industry was 4.69 metric tons per person in 2021. The figure for the EU-27 was 8.35 metric tons per person in 2021 [5]. Deeply analyzing annual CO2 emissions by sector in Figure 3, it is evident that the energy sector (power and heat supply) is also the biggest emitter of CO2 in the V4 countries, EU-27, and in the world, followed by transportation (roads, aviation, maritime, rails), manufacturing and construction industries (steel, cement, chemical, and petrochemical), buildings (heating, cooling), agriculture (farm needs and practices and fishing), and waste treatment technologies (solid waste handling and treatment, incineration, and wastewater treatment).
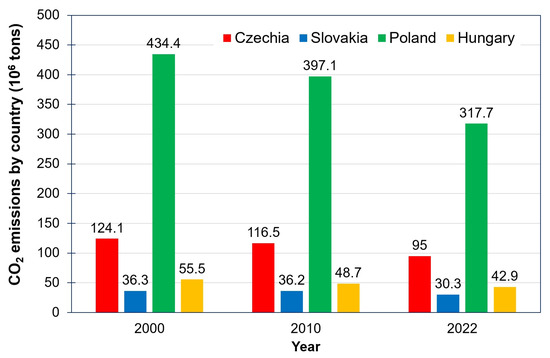
Figure 1.
Annual CO2 emissions by V4 countries. Source: adapted from Our World in Data [3].
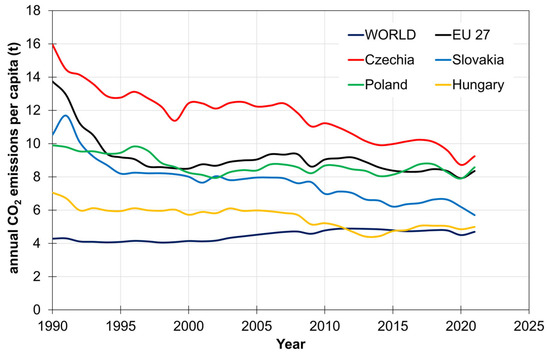
Figure 2.
Annual CO2 emissions per capita. Source: adapted from Our World in Data [5].
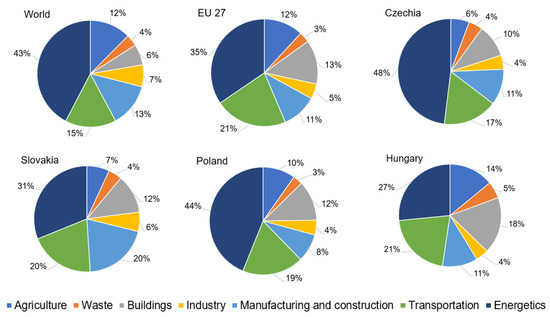
Figure 3.
Annual perceptual CO2 emissions by sectors and country in 2021. Source: grouped data adapted from Our World in Data [6].
There is an opportunity to lower CO2 released through technologies for capturing and using CO2. CO2 can be transformed into advanced biofuels (such as methane, ethanol, and butanol), chemicals (such as urea, methanol, and formic acid) or building materials (including inorganic and organic carbonates) through various chemical, electrochemical, photochemical or biochemical processes [7]. A transformation strategy can aid in achieving decarbonization plans, net zero CO2 emissions, reducing dependence on fossil carbon-based materials [8], and stabilizing power demand through sustainable renewable energy [9]. There are numerous review papers available on CO2 utilization for sustainability. The papers provide an overview of the current state of scientific knowledge in several areas. For example, the National Energy Technology Laboratory [7] gives an interesting overview of technologies that convert CO2 into other substances. Zhao and Itakura [10] address the recent carbon capture, utilization, and storage trends. Alok et al. [11] present the latest developments in transforming CO2 into value-added products using thermal, chemical, electrochemical, photochemical, and biochemical methods. The article compares these methods and provides future perspectives. Rafiee et al. [12] examine the physical and chemical pathways for CO2 conversion from a process integration perspective. Sustainable CO2 utilization pathways are introduced, and their scale-ups, overall applicability, and significant limitations are discussed concerning an industrial environment [13] or from an industrial ecology perspective [14]. Zhi et al. [15] review the chemical pathways for transforming CO2 into X. The review focuses on converting CO2 into synthetic gas or liquid fuels, considering efficiency in power production and risk analysis. Al-Qadri et al. [16] defined the challenges and current state of research on transforming CO2 into dimethyl ether, benzene, toluene, and xylene using zeolite-based catalysts. Alper and Orhan [17] demonstrate recent developments in CO2 conversion processes for polymers, fine chemicals, and inorganics. Szima and Cormos [18] demonstrate the feasibility of producing synthetic natural gas cost effectively. Additionally, Parvez et al. [19] present the potential of CO2 used in thermochemical biomass conversion to adjust syngas H2/CO ratio and improve char surface area, as well as properties of bio-syngas, bio-oil, and biochar. Mohapatra et al. [20] demonstrated the potential, approaches, and limitations of using microalgal strains to fix CO2 from flue gas. Chen et al. [21] provided an overview of strategies, challenges, technological advances, regulations, and microalgae-based CO2 and wastewater treatment policies. A critical review of CO2 capture technologies using solid cycles and viable paths for CO2 conversion has been undertaken by Theofanadis et al. [22]. These technologies are at near commercial readiness. Li et al. [23] found that using CO2 to build construction materials is a promising route for carbon sequestration. They also discussed critical issues for scale-up and industrial perspectives. Ho and Iizuka [24] summarized the research trends on the concept and mechanisms of CO2 carbonation using seawater. They also discussed the challenges and limitations of its application.
The current literature focuses on reviews of ideas, mechanisms, and process setups for implementing CO2 conversion technologies on an industrial scale. It is essential to note that not all CO2-to-X transformation pathways are universally applicable. For example, certain regions such as Central Europe, Eastern Europe, V4 countries or similar regions may face limitations due to local climatic conditions, power sector and industry structure, and decision-making policies. This study aims to assess the current scientific understanding of the sustainable biochemical conversion of CO2 into microalgae and the subsequent production of biofuel and bio-products. The focus is on identifying the potential benefits, limitations, technical readiness level, and research needs and challenges from a chemical engineering perspective. This review assumes milder climatic conditions in Central or Eastern Europe, Visegrad countries or other climatically related world regions are unfavorable for year-round conventional microalgal cultivation.
2. Methodology
This review has been prepared using a systematic and structured approach to evaluate and synthesize the existing literature on a given subject. As defined above, the review aims to summarize the available information for sustainable CO2 transformation through a biochemical pathway to microalgae, followed by the production of biofuels and bioproducts under milder climatic conditions. This review’s methodology was based on searching for relevant passages in the available literature sources, systematically appraising the resources, encoding the literature, and creating a conceptual scheme using a chemical process engineering perspective.
Firstly, keywords (CO2, microalgae, chemicals, cultivation, biorefinery, fermentation, pyrolysis, gasification, LCA study) were defined to find suitable studies as relevant sources of information for this review. For a comprehensive literature search, relevant databases, journals, and other sources were identified using the Scopus database. As shown in Table 1, many papers and reviews have been published on CO2-to-X conversion pathways since 2015. These papers cover recent trends in microalgal biorefineries, cultivation systems, chemical production, anaerobic co-digestion, and the pyrolysis or gasification of microalgal residues. Furthermore, life cycle assessments of biochemical CO2-to-X pathways have been conducted. Searched studies were then screened and selected. Inclusion (originality, keywords fit, process efficiency, scale-up potential, availability for mild weather conditions) and exclusion (year of publication, relevance to targeted topic, information repeating, technical readiness level, energy requirement, risk assessment) criteria were applied.

Table 1.
The keyword search in the Scopus database has been used since 2015 (dated 2 January 2024). Source: own elaboration.
Chemical process engineering knowledge was used to identify the key data, results, and critical findings from selected studies. This was followed by organizing all the data and presenting the findings coherently, highlighting patterns, trends, and key themes. The aim of the review is to analyze, synthesize, and critically evaluate the potential advantages, limitations, technological maturity, research needs, and challenges of CO2 to X conversion pathways in the biorefinery approach. It focuses on reviewing recent scientific findings on sustainable microalgal biorefinery technological steps, especially those operating under adverse climatic conditions. These include microalgal cultivation systems, algae-based chemicals, anaerobic digestion, and microalgal residue pyrolysis/gasification.
3. Biochemical CO2-to-X Transformation Pathways
Cultivation of microalgae, an intermediate CO2-based product of microalgal biorefineries, is recognized as a promising decentralized alternative to capture and use CO2 emissions using biological transformation. Microalgal strains are generally cultivated in open raceways or closed (flat panel, tubular) cultivation systems. It is generally known that CO2, nutrients (mainly N compounds and phosphates), and sunlight as an energy source are needed for microalgal growth under photosynthetic reaction. There is a chance to directly use cooled flue gas as a nutrient and CO2 source, as presented by Mohapatra et al. [20]. The CO2 amount of 1.83 tons generates one ton of microalgal biomass [25].
Microalgal sustainability [26] is nowadays viewed in microalgal-based products, as shown in Figure 4, like bio-oils, bioethanol, proteins, biohydrogen, biogas, biomethane or as carried out by Naims [27], in green chemicals with high added value (amino acids, steroids, essential oils), pigment (chlorophylls, carotenoids) ingredients, food and feed, fertilizers, and advanced fuels, all produced in dependence on microalgal species and CO2 purity. Nowadays, the sustainability and economic feasibility of waste processing technologies are viewed in the context of applying the biorefinery concept. Designing technology in the biorefinery concept means its process and technical setup must be adequately chosen to ensure its economic feasibility, environmental friendliness, and sustainability [28]. The biorefinery’s design must consider the cost of production in conjunction with the cost of sales and the market demand for the targeted commodities. Only a portfolio of high-value-added main products and low-value-added by-products can meet economic attractivity and sustainability needs. Standard selling rates for micro-algae products [27] are, e.g., 0.25 Eur kg−1 to fertilize them; 5–11 Eur kg−1 for micro-algae based powders; as a nutritional supplement 20–50 Eur kg−1; 500 Eur kg−1 for polyunsaturated Omega-3 fatty acids; for microalgae-based oil 625 Eur kg−1; or beta-carotene pigment of 2750 Eur kg−1 that help to reach industrial attractivity and economic feasibility of algal biorefineries [25].
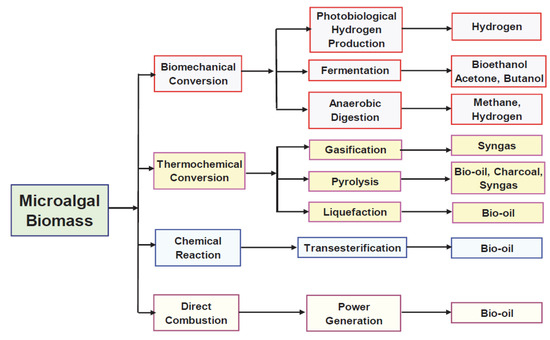
Figure 4.
Technological pathways of microalgal use. Source: adapted from Alam et al. [29].
3.1. Microalgal Cultivation Systems
Conventional cultivation systems may be classified according to their design into three broad categories: open, closed, and hybrid, which combine the best features of open and closed photobioreactor cultivation systems. Open ponds, raceways or inclined thin-layer systems are the most used open cultivation systems. In closed photobioreactors, the culture medium is isolated from the surrounding environment by transparent surfaces, which, compared to open systems, allows for easier control of operating conditions and elimination of contamination of the processed culture medium. The most used closed systems are tubular and flat panel photobioreactors. An illustration of conventional open and closed culture system designs is shown in Figure 5.
Cultivation of microalgae involves several factors that influence the growth and production of such microorganisms. Light (solar, artificial), carbon dioxide, nutrients (especially nitrogen, phosphorus, potassium, and magnesium), and the appropriate temperature of the culture medium are critical parameters for the proper growth of photosynthetic microalgae. Different microalgae species have other requirements for the intensity and spectrum of light radiation [30]. The growth rate of microalgae in photobioreactors can be significantly influenced by the concentration of carbon dioxide and nutrients in the culture batch. Common microalgae species can withstand a relatively wide range of culture medium temperatures. For the most widely used conventional microalgae, such as Chlorella, a range of culture temperatures from 15 to 40 °C is most reported as optimal for proper growth. Some microalgae species (Phaeodactylum) can only grow in a narrow temperature range of 20 to 25 °C [31]. The optimum temperature for cultivating most freshwater microalgae species significantly limits the operating time of cultivation systems. Lower or higher temperatures may cause growth inhibition and subsequent degradation of the cultured microalgae culture due to rotting [32]. Considering the average daily temperatures in Central or Eastern European countries (Czechia, Poland, Slovakia, and Hungary), which exceed 15 °C only in the summer months from approximately June to August [33], microalgae cultivation in outdoor environments is significantly limited. At temperatures below 15 °C, the cultivation process works inefficiently, and the overall yields of microalgae are meager [34]. Cultivation is often limited to the short summer period when temperatures and solar radiation intensities are sufficient for efficient microalgae growth. Failure to do so may result in the need for heat exchanging to maintain the desired temperature of the media. In some cases, artificial lighting may be used [35]. The requirement to heat the culture medium or provide artificial lighting during escalating energy costs leads to substantial operational expenses, ultimately preventing the further commercialization of cultivation systems [34].

Figure 5.
Conventional microalgal cultivation systems. Source: grouped figure adapted from Bitog et al. [36]: (a) raceway system, (b) flat panel, (c) column, and (d) horizontal tubular.
Current research indicates the existence of microalgae adapted to low operating temperatures [37]. These microorganisms are primarily found in cold regions, such as the Arctic and Northern European countries, and can grow even in extremely low temperatures. These microorganisms’ adaptation includes higher concentrations of microalgal lipids’ polyunsaturated fatty acid profile [38]. These polar microalgae can generate valuable substances, including antioxidants, carotenoids, and phenolic compounds [39], which can be used not only as food supplements or feed but also for wastewater treatment in a similar way to conventional freshwater microalgae [34]. Polar microalgae are promising for use in regions with milder climates, including Central and Eastern Europe. According to laboratory studies, polar microalgae perform productively as conventional freshwater microalgae under extreme climatic conditions when temperatures exceed 20 °C [32]. During winter, polar microalgae strains could replace conventional microalgae species to extend the cultivation campaign. This replacement has the potential to improve the economic balance of farming systems. Cicci et al. [40] stated that light intensity plays a significant role in microalgae growth. During winter, areas in central or Eastern Europe have relatively low intensity of photosynthetically active radiation (PAR, 400–700 nm), significantly reducing the cultivation time during the year. Microalgae that can grow in low light intensities are highly sensitive to the effects of high light intensities. This is particularly noticeable at midday when sunlight intensity is at its highest. To cultivate polar microalgae efficiently, it is crucial to design systems that effectively utilize incident light and prevent direct sunlight at high intensities that could damage the produced microalgal biomass. Figure 6 demonstrates one of the first model rotary reactors for cultivating polar microalgae tested under extreme climatic conditions in the Arctic regions [41].

Figure 6.
A rotary photobioreactor for the cultivation of polar microalgae was installed in Longyearbyen, Svalbard. Source: own photo documentation.
The successful transfer of technical solutions and experimental results from laboratories and semi-operational facilities to operational commercial scale remains limited, mainly due to the high investment and operating costs, which are strongly influenced by the local conditions of the installation site. Most cultivation technologies are only commercially available in locations with more hours of sunlight and more favorable temperatures throughout the year (e.g., Southern or parts of Western Europe). Consequently, applying these technologies in Central and Eastern Europe is often more complex or infeasible. Unfortunately, these regions are frequently undeservedly criticized or even condemned. One possible approach to achieving efficient and economically viable microalgae production cultivation systems is to use polar microalgae strains adapted to the Arctic regions’ extreme climatic conditions. No rotary or stationary cultivation system exists that can withstand extreme operating conditions and allow scale-up of cultivation technologies to industrial scale.
3.2. Sustainable Renewable Energy and Green Products from Microalgae
Third-generation (3G) biorefineries use microbial cell factories to convert CO2 into algal biomass that can produce carbon-neutral food, feed, pharmaceuticals, chemicals, and biofuels. The economic viability of 3G biofuel production is questionable due to the low biomass productivity and the biofuel’s low efficiency. As scientists and industry tend to focus on producing high-value products such as pharmaceuticals and cosmetics and their economically viable applications, the production of 3G biofuels is often overlooked. The high-value-added products may improve the economics of 3G biorefineries [42]. One of the leading examples of value-added bioproducts is astaxanthin (the most powerful natural antioxidants, possessing hepato- and cardio-protective properties [43]) obtained from the cultivation of the microalgae Hematococcus pluvialis in tubular photo-bioreactors using LED lamps from AlgalifTM, Iceland [44]. Another high-value-added bioproduct of microalgae is phycocyanin (C-PC). Phycocyanin has therapeutic properties. These include antioxidant, anti-inflammatory, and anti-cancer properties [45]. The commercial value of phycocyanin can range from 25 EUR/mg for partially purified phycocyanin to 200 EUR/mg for phycocyanin, with a purity of 3.5 [46].
The scientific group of Prof. Ledakowicz [47,48] performed the research to optimize the biosynthesis process of thermostable C-PC from thermophilic cyanobacteria and to develop a series of efficient operations of down-stream processing (DSP) will allow the selection of an effective method for obtaining thermostable C-PC, combined with further biomass debris treatment by anaerobic digestion pyrolysis and gasification as presented in Figure 7, proposing a circular approach according to the principles of sustainable development. The thermophilic strain Synechococcus sp. PCC6715 was grown at 45 °C in an unsterile BG11 medium in two photobioreactors [47,48]. Centrifugation was used for the microalgal biomass harvesting. The thawed, thickened biomass was suspended in a PBS buffer. It was disrupted by 6 cycles of alternating freeze–thaw. The PBS buffer was extracted four times. The techniques used to purify thermostable C-PC from Synechococcus sp. PCC6715 included foam fractionation (F.F.): recovery 49%, purification factor PF = 1.472; two-phase aqueous extraction (ATPE) PEG 6000–phosphate salt: recovery 97%, PF = 1.466; ultrafiltration (Hydrosart 10 kDa membrane): recovery 92%, PF = 1.472; and fast protein liquid chromatography (FPLC) gave PF = 3.4 pharmaceutical quality [49]. Biomass debris was washed out with distilled water and frozen for further processing.
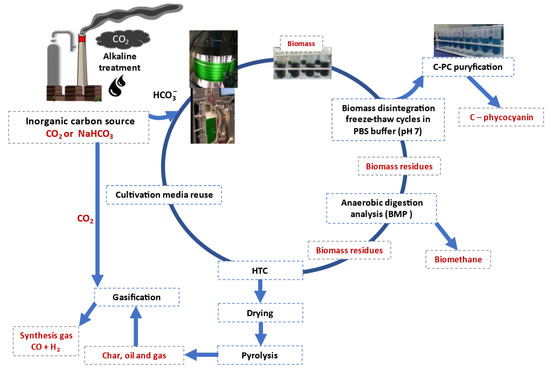
Figure 7.
The idea of technology to produce thermostable C-PC—circular approach. Source: adapted from [49,50,51].
3.2.1. Anaerobic Fermentation of Microalgal Residues
Wet waste biomass can be used as a substrate for an anaerobic process in which it is converted to biogas. Anaerobic digestion is a well-established technology for producing biogas that has been extensively researched and applied for over 60 years. Physical, chemical, and biological substrate pretreatment can increase biogas production yield [52]. The cell wall of algae contains poorly degradable compounds such as cellulose. The extraction of compounds from algae destroys the cell wall, increasing biogas production yield. Various microorganisms undergo anaerobic digestion, so providing an active inoculum is essential. Biogas production yield from algae is in a wide range (from 20 to 600 mL/gVS) [53]. The process has a low speed due to the limited hydrolysis step and the use of anaerobic microorganisms [54]. Fermentation of algae can generate some problems related to low algal concentration, low C/N ratio or short storage time. Because algae can contain levels of protein from 8 to 70%, in the fermentation process, ammonia can be produced, which inhibits the anaerobic digestion process [55]. Free ammonia is a toxic chemical for microalgae and nitrifying bacteria; the ammonia in the reactor must also controlled according to the free ammonia/inlet biomass ratio. The uninfluenced ammonia/biomass ratio (mg-NH3/mg-TSS or VSS) should be kept below 0.06 for high removal [56]. Carbon-rich biomass is added to the feedstock as a substrate for the anaerobic digestion process. Co-digestion of two or more substrates has a beneficial effect on increasing biogas production yield by up to 200% and improving the stability of the anaerobic digestion process [57]. Ferreira et al. [58] achieved the highest methane yield using a mixture of 75% food waste and 25% microalgae. This resulted in a methane yield of 514 mL/gVS and a synergy between the substrates that was 28% higher than the theoretical value. Anaerobic digestion of algae is mainly carried out under mesophilic conditions, i.e., at 35–40 °C. Approximately 50% of the organic matter is degraded in this process [59]. The remainder of the organic matter consists of compounds not degraded in anaerobic digestion, such as lignin. Anaerobic digestion of microalgae is carried out in the same design of microbial bioreactors as those used for anaerobic digestion of other biomass resources [57]. The effective geometrical set-up of the digester and impeller should be a prime focus during the scaleup of the lab-scale digesters, and mixing in real-scale digesters should be optimized.
Biogas production has increased twofold in Europe between 2010 and 2020, so new biomass sources such as algae will be increasingly used [52]. The anaerobic digestion process generates biogas, mainly made up of CO2 and CH4. This biogas can be utilized to generate electricity and heat through cogeneration systems. Upgrading biogas to biomethane is one of the most active topics in the scientific literature. It is of great interest to the bioenergy industry due to renewable energy targets. The biomethane can be used as advanced biofuel or injected directly into the gas grid. At present, the most used technologies for this purpose are water washing, chemical or physical absorption, andsorption dominantly in pressure swing configuration [60] or innovative membrane permeation [61]. A commercial hollow polyimide membrane is often applied for CO2 removal from biogas for further effective reuse [62]. Carbon dioxide is removed from the gas using a separation technique such as a membrane to increase the calorific value of biogas. CO2 can be removed from biogas by cultivation of microalgae (methane is not toxic to microalgae) [48] or conversion of H2 and CO2 by lithoautotroph microorganisms into CH4 [63]. The performance evaluation by Ighalo et al. [64] has shown that microalgae can capture and sequester CO2 with an efficiency of 40% to 93.7%.
Research is required to identify alternatives to artificial nutrients to reduce the environmental impact and cost of nutrient supply. One promising strategy to increase sustainability and obtain valuable products is the cultivation of microalgae, especially digestate, in anaerobic wastewater [65]. Microalgae can also be grown on nutrient-rich digestate from anaerobic digestion, thereby providing simultaneous biogas upgrading and digestate bioremediation [66]. High nutrient levels, turbid digestate, biological contaminants, ammonia, and metal toxicity can also inhibit microalgal growth. Keeping nutrient levels adequate and reducing the occurrence of contaminants is a critical issue [67]. Another product of anaerobic digestion is digested sludge, which can be recycled as fertilizer.
3.2.2. Hydrothermal Carbonization of Microalgal Residues
Unfortunately, digested sludge cannot always be used as fertilizer. One method of processing digested sludge is hydrothermal carbonization (HTC), which produces hydrochar and liquid phases. One significant advantage of this hydrothermal process is that substrate containing water (more than 60%) can be utilized. It is carried out in the 180 to 250 °C temperature range under autogenous pressure (in subcritical water). The char production yield is a broad range between 20 and 70%. The hydrochar produced has hydrophobic properties, making it easy to de-water. It can potentially be used as fertilizer, an adsorbent or fuel. The char created by the HTC technology has a much lower specific surface area than that obtained by the pyrolysis process. It has a much higher adsorption capacity due to its capacity for ion exchange and complexation [68]. Both the H:C and O:C transmission ratios in hydrochar are lowered when the processing temperature is raised [69]. The liquid from the HTC process contains easily degradable substances that can be recycled as a medium for algae cultivation or as a substrate for anaerobic digestion. It has been observed that liquid can be produced after HTC for biochemical processes when the process temperature is around 180 °C [70]. On the contrary, liquid after the HTC process at 250 °C contains toxic substances, including cyclic oxygen-containing compounds. After the HTC process, the liquid phase can contain up to 80% of the nitrogen in the substrate before the process [71]. The acids produced by the HTC process have autocatalytic properties that increase the reaction rate and improve char properties (e.g., pore structure) [72]. The HTC process also produces CO2 with a yield of less than 5%.
3.2.3. Pyrolysis of Microalgal Residues
Drying is required for the further treatment of biomass residues. This process uses a lot of energy to evaporate the water. The hydrochar from the HTC is quickly separated in the sedimentation process, reducing the drying costs. After drying, the waste biomass can be pyrolyzed. Pyrolyzing algae is highly complex because algae contain lipids, proteins, and carbohydrates. The process is carried out under anaerobic conditions at temperatures between 300 and 800 °C, forming char, oil, and gas. Thermogravimetric analysis indicates that the most vigorous degradation of macro and microalgae occurs at 250–400 °C [73]. Raising the pyrolysis temperature increases the effectiveness of gas production and lowers the efficiency of char production. The pyrolysis process can take place in an N2, Ar or CO2 atmosphere. Using CO2 instead of N2 leads to lower output of char and oil, but increases the yields of gas production (especially CO) and acetic acid in the liquid phase. [74,75]. In addition, CO2 in the atmosphere during pyrolysis leads to the breakdown of volatile fatty acids, increased protein degradation, and reduced fat degradation [76]. Subject to temperature and heat-up rate, the pyrolysis procedure can be grouped into three different classes: slow, medium, and fast [77]. Fast pyrolysis (heating rate > 100 K/min) produces mainly oil, whereas slow pyrolysis (heating rate < 1 K/min) produces mainly char [78]. The use of microwaves as a heat source in the algae pyrolysis process significantly increases the yield of gas production, calorific value, and char properties for the gasification process [79,80]. The gas produced during pyrolysis contains H2, CH4, CO, CO2, and low hydrocarbons (CxHy). This gas can be used for heat and power generation as well as for chemical production. as being upgraded for chemical production [81]. Pyrolysis oil consists of acids, alcohols, aromatics, esters, furans, ketones, phenols, and sugars [82]. Oil from algae contains more oxygenous and nitrogenous compounds than oil produced from lignocellulosic biomass. Oxygen in oil can cause acidity, corrosion, and low calorific value [83], while nitrogen in oil can produce NOx in combustion [84]. The oil properties in the pyrolysis process are improved by adding a catalyst, e.g., zeolites [75,83]. Used oil as fuel or chemical needs to apply rectification or extraction process [83]. Char can be recycled as a fuel in combustion and gasification processes. It has a higher calorific value than the substrate. It also contains significant amounts of carbon and nitrogen, making it suitable for use as a fertilizer or adsorbent.
3.2.4. Gasification of Microalgal Residues in CO2 Atmosphere
Gasification is one way to recycle CO2, the gasifying agent in the Boudouard reaction by Equation (1). This reaction is endothermic and occurs at temperatures above 850 °C.
Other gasification agents, such as steam or oxygen, can be incorporated. Adding oxygen to the gasification agent means the gasification reaction can be exothermic [77]. Char from, e.g., pyrolysis processes, is utilized as a carbon source in the reaction by Equation (1). The gasification process is considerably slower than pyrolysis [85]. At temperatures above 850 °C, the gasification rate depends on the mass transfer. Raising the temperature does not significantly increase the gasification rate, as the process is controlled by diffusion [86,87]. The increased pressure and partial pressure of CO2 improve the gasification process, resulting in better heat and mass transfer phenomena [87]. Increasing the CO2 concentration above 50% in gasification processes does not always increase the gasification rate [85]. The average concentration of CO2 from industry is around 14%. The gasification process is a heterogeneous reaction. Its efficiency depends on the porosity and surface area of the char. A faster exchange of reactants on the char surface is achieved by increasing the flow rate of the gasification agent. The efficiency of the gasification process is also significantly influenced by particle size. Smaller char particles offer less mass and heat movement resistance, resulting in a faster reaction. The gasification process rate is affected by the ash content in char. The rate of gasification is accelerated by the addition of Mg, Na, Fe, K, and Ca, and retarded by the inclusion of Al and Si. Charcoal properties are strongly influenced by the pyrolysis process. The reactivity of the char is dependent on the temperature, the atmosphere, the pyrolysis time, the pressure, and the particle size, with the temperature being the most critical factor. It has been observed that char reactivity in the gasification process increases with higher temperatures during the pyrolysis process [88]. Increasing the pressure during the pyrolysis process leads to less reactivity of the char [89]. Pyrolysis and gasification can occur in the same atmosphere (in situ) or another (ex situ). The gas produced by in situ gasification has better properties than that produced by ex situ gasification [90]. A literature review shows that more research has been carried out on microalgae under different conditions and with other species than on macroalgae [91]. The kinetics of the gasification process are carried out in thermobalance and fixed-bed reactors. Large-scale gasification of algae is carried out in fluidized bed reactors or high-temperature downdraft gasifiers (HTDG).
Additional H2 can be obtained using the CO produced in the WGS reaction. This H2 can synthesize CH3OH, higher alcohols or DME [92]. Using CO2 as a gasification agent reduces syngas nitrogen compounds, increases calorific gas value, and lowers tar yield production [93]. Co-gasification of biomass and coal creates a synergistic effect [94]. The gasification process using CO2 is also carried out through CaO looping. The catalytic properties of CaO increase the concentration of H2 in the syngas produced by up to 10% [95].
3.3. Biochemical CO2-to-X Sustainability towards LCA Analysis
Generally known, climate neutrality, decarbonization, and CO2-to-X strategies deal with reaching low-carbon technologies, for which the upgrade of existing and development of novel technologies, infrastructure, and strong political and public support is needed [96]. Life cycle assessment of the product (LCA) represents an effective instrumentation tool ensuring the evaluation of technological pathways’ environmental impact in decarbonization strategies. This tool assesses the environmental footprint of carbon, determines carbon-intensive steps in the product supply chain, and highlights options for minimizing carbon emissions in the complex manufacturing process [97]. LCA analysis also helps avoid double counting, carbon leakage or greenwashing [98].
The available literature sources bring several LCA studies regarding microalgal cultivation with subsequent microalgae treatment. The studies usually evaluate environmental impact factors for the whole CO2 processing chain, i.e., CO2 to microalgae, harvesting, product separation, and cleaning, all with the implementation of conventional or renewable energy sources. Schneider et al. [99] found that microalgal cultivation in raceway ponds, centrifugal separation, and drying evinced the lowest environmental impact to fix CO2 to microalgal biomass against filtration, electrolocation, and flocculation with NaOH. No differences in carbon footprint were found when applying the airlift or paddle wheels configuration for mixing the cultivation batch. Microalgal cultivation and freeze-drying were identified as the dominant environmental footprint factors in the CO2 to microalgal powder pathway. These can be reduced by implementing renewable energy systems, environmentally friendly nutrients for culture medium, and cleaning solutions [100]. The carbon footprint for microalgae-derived biofuels is presented by the study of Bradley et al. [101], in which biodiesel was produced from Phaeodactylum and Nannochloropsis strains.
It has been found that microalgae-derived biofuels are not yet preferred over fossil-derived fuels. This is because fossil-derived diesel emits 8.84 × 10−2 kg CO2eq, while microalgae-derived biodiesel, which uses energy from solar systems, emits 1.48 × 10−1 kg CO2eq. The microalgal cultivation system needs to be improved regarding process efficiency and energy use because 0.99 MJ of energy for microalgal cultivation was required to produce 1 MJ of microalgae-derived biodiesel. Microalgal pigments, including chlorophylls, carotenoids, and phycobiliproteins, offer eco-friendly alternatives to synthetic pigments. Microalgae-derived pigments were found to have a higher environmental impact value than synthetic pigments [102]. Regarding astaxanthin production, electricity production is a significant ecological impact factor. Applying sunlight instead of artificial illumination sharply decreases the environmental impact factor [102]. López-Herrada et al. [103] serve a life-cycle analysis to create a fungicide based on amphidinols. The calculation proved the hypothesis that photosynthetic bioprocesses are net CO2 producers, ecotoxicity is reduced for microalgal fungicides compared to synthetic ones, and microalgal-derived fungicides evinced a lower environmental footprint than commercial ones. The environmental impact of wastewater treatment can be significantly reduced by combining it with microalgae cultivation. The advantages of this procedure are the low operational expenses, the recycling of the nutrient content of the effluent into high-value products, and the direct absorption of CO2 emissions [104]. Regarding microalgal fermentation processes, the microalgal heterotrophic fermentation in commercial fermenters with a volume of 60 m3 to produce bio-oils displayed 86% of the environmental impacts by the nutrient need and electricity requirement for fermentation. The CO2 emissions increased with the length of the fermentation time. At a residence time of 131 h, the daily GHG emissions were 1.92 × 103 CO2eq per day, producing one kilogram of bio-oil [105]. Climate change, fossil resource scarcity, human toxicity, freshwater, and marine ecotoxicity have been identified as phototrophic cascade fermenters’ most significant environmental impact [106]. Huang et al. [107] demonstrate a consolidated bioprocessing system in which pigment-extracted microalgal residues are co-fermented with biomass to produce ethanol. Life cycle assessments have revealed that such co-fermentation has a 2.7 to 10.7 times lower environmental impact than alternative ethanol production pathways using microalgal biomass. The environmental impact has been assessed to quantify the energy conversion characteristics and environmental effects of biohydrogen and biomethane industrial production two-stage fermentation from microalgae and food waste [108]. With 53.8% and 16.6% of the total energy input, the dominant energetic processes were biomass pretreatment and microalgae cultivation. The total greenhouse gas emissions were 124 g CO2eq per MJ. Microalgae photosynthesis absorbed 49 g CO2eq per MJ of carbon sources. The primary sources of emissions were electricity production (41.6%), CO2 release in pressurized water (27.8%), and energy recovery (19.8%).
Microalgal biomass and residues can also undergo thermochemical conversion via hydrothermal liquefaction, pyrolysis or gasification. LCA studies on microalgal thermochemical treatment concluded that sustainability and less impact on the environment can be reached only if energy-intensive drying processes are excluded [109]. Hydrothermal liquefaction (HTL) was found to be a promising transformation pathway to produce renewable or jet fuels. A significant decrease in GHG emission was observed for the microalgal-based jet fuel of 35.2 kg CO2eq per GJ compared to the fossil-based fuel of 86.5 kgCO2eq per GJ [110]. Hydrothermal liquefaction of wastewater-based microalgal biomass was more sustainable in most impact categories. The GHG emissions were reported for the HTL-based bio-oil production of 0.233 kg CO2eq MJ−1 from microalgal cultivation in wastewater compared to 21 times higher HTL-based bio-oil production of 4.7 kg CO2eq MJ−1 from microalgal cultivation applying synthetic nutrients [111]. Chen and Quinn [112] pointed out that biofuel from algal hydrothermal liquefaction performs worse than conventional fuels regarding ecotoxicity and eutrophication potential.
Unfortunately, some papers focus on LCA analysis of pyrolysis or gasification of microalgal residues. Catalytic pyrolysis offers the opportunity to transform microalgae into biofuels and fine chemicals. Microalgal biomass productivity makes pyrolysis less common than cellulosic biomass [113]. Life cycle assessment of microalgae cultivation, bio-oil extraction, and slow pyrolysis processing of microalgae residues show a net increase of up to 50% in the less impact on the environment and total life cycle impact [114]. Drying of microalgal biomass before pyrolysis has a significant effect on environmental impact factors. The carbon footprint can be improved using nutrients from waste streams, integrating renewable energy and heat recovery systems. Wang et al. [115] performed LCA analysis for pyrolysis treating microalgae and microalgal residues after bio-oil extraction. The microalgal pre-treatment and bio-oil produced resulted in an 11% reduction in energy use plus a 25% increase in greenhouse gas emissions compared to no oil extraction. Azadi et al. [116] analyzed algal syngas using a fluidized bed gasifier for LCA. A cradle-to-grave assessment determined that the carbon footprint ranged from 0.07 to 0.195 kg CO2eq MJ−1. In comparison, the carbon footprint of syngas produced by steam reforming of natural gas is around 0.1 kg CO2eq MJ−1. A solar system can reduce the carbon footprint to less than 0.04 g CO2eq MJ−1.
4. Discussion and Future Directions
It is generally known that decarbonization deals with the reduction of CO2, which is emitted by human activity. As presented in the introduction in Figure 3, almost seven energy and land-use systems are responsible for global emissions, i.e., energetics (electricity and heat generation), industries (steel, cement, chemical, and petrochemical), mobility (roads, aviation, maritime, and rails), buildings (heating and cooking), agriculture (farm needs and practices and fishing), forestry, and waste treatment (solid waste treatment, incineration, and wastewater treatment). To accelerate decarbonization, scientists and engineers of various professions must define sustainable technological pathways to reach net-zero emissions. The definition of sustainability or sustainable development was determined by the World Commission on Environment and Development in 1987, as follows [117]: “Sustainable development is a development that meets the needs of the present without compromising the ability of future generations to meet their own needs.” Emerging engineering sustainability in carbon capture and use strategies means that there is the necessity for multicriteria decision-making strategies concerning uncertainties to reach technological readiness level TRL 8–9 (system proven in operational environment) under favorable process and energy efficiencies respecting local climatic conditions, local power sector and industry structure, and local decision-making policies.
As mentioned above, microalgal biomass is a sustainable product that can be used to produce valuable green products, renewable methane or as a feedstock for pyrolysis or gasification to produce syngas as a renewable fuel or as a reactant for the Fisher–Tropsh synthesis to produce e-fuel. The paper reviewed the current state of scientific knowledge in microalgal cultivation systems, microalgal-based chemicals, anaerobic fermentation and pyrolysis of algal residues, and gasification of biomass residuals, all concerning the potential application under not climatically favorable conditions in Central and Eastern Europe or related world regions. The focus of the analysis was on the sustainability of the processes in terms of their benefits, constraints, and technology deployment known as technological readiness level (TRL), as presented in Table 2. TRL levels of investigated technology are usually higher than 7. Only the HTL process has the level TRL 6 (prototype demonstration). This means that reviewed technologies were tested by prototype in an operational environment at least and are ready for scale-up to their full functional scale. The profitability of 3G biofuel production remains uncertain. There are currently no industrial plants that produce biofuels from algae. In addition, the production efficiency of algae needs to be three times higher and the costs ten times lower to be profitable. At present, microalgae are the most economical source of nutraceuticals and biologically active medical and food products such as proteins, pigments, vitamins, etc. The high-value-added products can improve the economics of 3G biorefineries. Biofacades clean the atmosphere in cities by eliminating harmful CO2 and producing O2. Integrating biofacades into the biorefinery system is a primary objective [118,119]. Flat plate photobioreactors have been recognized to be advantageous in architectural design. The payback period ranges from 9 to 13 years. It is recommended that algae be grown in non-agricultural areas. Producing bio-diesel and bio-products from algae may be profitable in the future, pending the realization of expectations from geneticists and bioengineers at Viridos [120,121]. To make 3G biorefineries profitable, this study’s focus on algae should shift from biofuels to high value-added derivatives (e.g., phycocyanin or astaxanthin) and the integration of bio- and thermo-chemical platforms.

Table 2.
Sustainability of demonstrated CO2-to-X conversion pathways. Source: adapted as referenced by individual technologies.
A sustainable product portfolio, including biofuels, can be generated by implementing algae biorefineries. The research needs and challenges in Table 3 and the SWOT analysis in Table 4 still exist, which limits its implementation at the industrial scale. However, further research is required to determine the economic viability of producing different articles. Various processes can be coupled to reduce operating costs. Efficient biomass production is a prerequisite for constructing a successful algae biorefinery. Environmental factors such as CO2 and effluent utilization should be considered for a successful biorefinery. A successful algae biorefinery can be achieved by continuous biomass production and conversion into high-value products using effective separation methods and low energy consumption. In addition, data mining, process control, process optimization, and geometric configuration of reactors and bioreactors will require machine learning methods.

Table 3.
Research needs and challenges of technological pathways. Source: adapted as referenced by individual technologies.

Table 4.
SWOT analysis for demonstrated technological pathways. Source: adapted from [2,7,8,26,28,96].
5. Conclusions
Carbon capture and use strategies are being explored for worldwide energy sustainability and raw material security. The right choice of sustainable CO2-to-X transformation technique is dependent on multicriteria decision-making strategies concerning uncertainties to reach technological readiness level TRL 8–9 (system proven in operational environment) under favorable process and energy efficiencies respecting local climatic conditions, local power sector and industry structure, and local decision-making policies. Microalgal biorefineries are only commercially available in locations with more hours of sunlight and more favorable temperatures throughout the year (e.g., Southern or parts of Western Europe). Consequently, applying these technologies in Central and Eastern Europe is often more complex or infeasible. This review identified the new featured and sustainable directions of microalgal biorefinery pathways under harsh weather conditions by applying chemical process engineering analysis, systematic data synthesis, and critical technical evaluation. Currently, there are no commercially viable installations for producing economically attractive biofuels from microalgae. Microalgal production efficiency must be three times higher, and costs must be ten times lower to be profitable. To reduce operational costs, various processes can be coupled. Efficient biomass production is required to build a successful microalgal biorefinery. Environmental factors such as the use of CO2 and effluent should be considered for a successful biorefinery. A successful microalgal biorefinery can be quickly established by continuously producing biomass and converting it into high-value products using efficient separation methods and low energy consumption.
Author Contributions
Conceptualization, L.K., S.L., P.P., Z.S. (Zoltán Siménfalvi) and R.Š.; writing, resources, and investigation for the introduction L.K., P.P. and M.P. (Marián Peciar); biochemical transformation pathways, S.L. and R.S.; microalgal cultivation V.B. and T.J.; renewable energy and green products from microalgae, S.L. and R.S.; sustainability towards LCA analysis, L.K., M.P. (Máté Petrik) and Z.S. (Zoltán Szamosi); discussion, L.K., T.J., R.Š., M.P. (Marián Peciar), S.L. and Z.S. (Zoltán Siménfalvi); visualization, L.K.; editing, L.K.; project administration, L.K.; and funding acquisition, L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the International Visegrad Fund, grant number 22120032, and by the infrastructure provided by the Ministry of Education, Youth and Sports of the Czech Republic, grant number CZ.02.1.01/0.0/0.0/16_019/0000753.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Statista Annual Carbon Dioxide (CO2) Emissions Worldwide from 1940 to 2022. Available online: https://www.statista.com/statistics/276629/global-co2-emissions (accessed on 23 November 2023).
- Krátký, L. Carbon Capture and Utilization Technologies: A Technical State of Art. Acta Sci. Pol. Biotechnol. 2018, 17, 59–68. [Google Scholar]
- Our World in Data Share of Global CO2 Emissions. Available online: https://ourworldindata.org/grapher/annual-share-of-co2-emissions (accessed on 23 November 2023).
- Statista Carbon Dioxide Emissions in the European Union by Country. Available online: https://www.statista.com/statistics/1171389/co2-emissions-european-union/ (accessed on 28 November 2023).
- Our World in Data CO2 Emissions per Capita by Country. Available online: https://ourworldindata.org/grapher/co2-per-capita-marimekko (accessed on 23 November 2023).
- Our World in Data Emissions by Sector. Available online: https://ourworldindata.org/emissions-by-sector (accessed on 23 November 2023).
- National Energy Technology Laboratory CO2 Utilization Focus Area. Available online: https://netl.doe.gov/sites/default/files/2021-10/FY21-Carbon-Utilization-Peer-Review-Overview-Report_07262021.pdf (accessed on 23 November 2023).
- Mennicken, L.; Janz, A.; Roth, S. The German R&D Program for CO2 Utilization—Innovations for a Green Economy. Environ. Sci. Pollut. Res. 2016, 23, 11386–11392. [Google Scholar]
- Thakur, I.S.; Kumar, M.; Varjani, S.J.; Wu, Y.; Gnansounou, E.; Ravindran, S. Sequestration and Utilization of Carbon Dioxide by Chemical and Biological Methods for Biofuels and Biomaterials by Chemoautotrophs: Opportunities and Challenges. Bioresour. Technol. 2018, 256, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Itakura, K.I. A State-of-the-Art Review on Technology for Carbon Utilization and Storage. Energies 2023, 16, 3992. [Google Scholar] [CrossRef]
- Alok, A.; Shrestha, R.; Ban, S.; Devkota, S.; Uprety, B.; Joshi, R. Technological Advances in the Transformative Utilization of CO2 to Value-Added Products. J. Environ. Chem. Eng. 2022, 10, 106922. [Google Scholar]
- Rafiee, A.; Rajab Khalilpour, K.; Milani, D.; Panahi, M. Trends in CO2 Conversion and Utilization: A Review from Process Systems Perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar]
- Valluri, S.; Claremboux, V.; Kawatra, S. Opportunities and Challenges in CO2 Utilization. J. Environ. Sci. 2022, 113, 322–344. [Google Scholar] [CrossRef]
- Meylan, F.D.; Moreau, V.; Erkman, S. CO2 Utilization in the Perspective of Industrial Ecology, an Overview. J. CO2 Util. 2015, 12, 101–108. [Google Scholar] [CrossRef]
- Zhi, K.; Li, Z.; Wang, B.; Klemeš, J.J.; Guo, L. A Review of CO2 Utilization and Emissions Reduction: From the Perspective of the Chemical Engineering. Process Saf. Environ. Prot. 2023, 172, 681–699. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.; Nasser, G.A.; Adamu, H.; Muraza, O.; Saleh, T.A. CO2 Utilization in Syngas Conversion to Dimethyl Ether and Aromatics: Roles and Challenges of Zeolites-Based Catalysts. J. Energy Chem. 2023, 79, 418–449. [Google Scholar] [CrossRef]
- Alper, E.; Yuksel Orhan, O. CO2 Utilization: Developments in Conversion Processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Szima, S.; Cormos, C.C. CO2 Utilization Technologies: A Techno-Economic Analysis for Synthetic Natural Gas Production. Energies 2021, 14, 1258. [Google Scholar] [CrossRef]
- Parvez, A.M.; Afzal, M.T.; Victor Hebb, T.G.; Schmid, M. Utilization of CO2 in Thermochemical Conversion of Biomass for Enhanced Product Properties: A Review. J. CO2 Util. 2020, 40, 101217. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Padhi, D.; Sen, R.; Nayak, M. Bio-Inspired CO2 Capture and Utilization by Microalgae for Bioenergy Feedstock Production: A Greener Approach for Environmental Protection. Bioresour. Technol. Rep. 2022, 19, 101116. [Google Scholar] [CrossRef]
- Chen, J.; Dai, L.; Mataya, D.; Cobb, K.; Chen, P.; Ruan, R. Enhanced Sustainable Integration of CO2 Utilization and Wastewater Treatment Using Microalgae in Circular Economy Concept. Bioresour. Technol. 2022, 366, 128188. [Google Scholar] [CrossRef] [PubMed]
- Theofanidis, S.A.; Antzaras, A.N.; Lemonidou, A.A. CO2 as a Building Block: From Capture to Utilization. Curr. Opin. Chem. Eng. 2023, 39, 100902. [Google Scholar] [CrossRef]
- Li, N.; Mo, L.; Unluer, C. Emerging CO2 utilization Technologies for Construction Materials: A Review. J. CO2 Util. 2022, 65, 102237. [Google Scholar] [CrossRef]
- Ho, H.J.; Iizuka, A. Mineral Carbonation Using Seawater for CO2 Sequestration and Utilization: A Review. Sep. Purif. Technol. 2023, 307, 122855. [Google Scholar] [CrossRef]
- Styring, P.; Jansen, D.; de Coninick, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy: Using CO2 to Manufacture Fuel, Chemicals and Materials; Centre for Low Carbon Futures; York Science Park: York, UK, 2011; ISBN 0957258801. [Google Scholar]
- Aresta, M.; Dibenedetto, A. Industrial Utilization of Carbon Dioxide (CO2). In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Woodhead Publishing: Cambridge, UK, 2010; Volume 2, pp. 377–410. [Google Scholar] [CrossRef]
- Naims, H. Economics of Carbon Dioxide Capture and Utilization—A Supply and Demand Perspective. Environ. Sci. Pollut. Res. 2016, 23, 22226–22241. [Google Scholar] [CrossRef] [PubMed]
- Kratky, L. Lignocellulosic Waste Treatment in Biorefinery Concept: Challenges and Opportunities. In Zero Waste Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 59–94. [Google Scholar]
- Alam, F.; Mobin, S.; Chowdhury, H. Third Generation Biofuel from Algae. Procedia Eng. 2015, 105, 763–768. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed Photobioreactors for Production of Microalgal Biomasses. Biotechnol. Adv. 2012, 30, 904–912. [Google Scholar] [CrossRef]
- Cheregi, O.; Ekendahl, S.; Engelbrektsson, J.; Strömberg, N.; Godhe, A.; Spetea, C. Microalgae Biotechnology in Nordic Countries—The Potential of Local Strains. Physiol. Plant 2019, 166, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 23 November 2023).
- Moody, J.W.; McGinty, C.M.; Quinn, J.C. Global Evaluation of Biofuel Potential from Microalgae. Proc. Natl. Acad. Sci. USA 2014, 111, 8691–8696. [Google Scholar] [CrossRef] [PubMed]
- Kratky, L.; Jirout, T.; Belohlav, V. Economic Feasibility Study for Artificial Lighting of Microalgal Flat-Panel Photobioreactors. Int. J. Environ. Sci. Technol. 2023, 20, 12089–12100. [Google Scholar] [CrossRef]
- Bitog, J.P.; Lee, I.-B.; Lee, C.-G.; Kim, K.-S.; Hwang, H.-S.; Hong, S.-W.; Seo, I.-H.; Kwon, K.-S.; Mostafa, E. Application of Computational Fluid Dynamics for Modeling and Designing Photobioreactors for Microalgae Production: A Review. Comput. Electron. Agric. 2011, 76, 131–147. [Google Scholar] [CrossRef]
- Lyon, B.R.; Mock, T. Polar Microalgae: New Approaches towards Understanding Adaptations to an Extreme and Changing Environment. Biology 2014, 3, 56–80. [Google Scholar] [CrossRef]
- Hulatt, C.J.; Berecz, O.; Egeland, E.S.; Wijffels, R.H.; Kiron, V. Polar Snow Algae as a Valuable Source of Lipids? Bioresour. Technol. 2017, 235, 338–347. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; León, R.; Vigara, J.; Funk, C. Exploring Nordic Microalgae as a Potential Novel Source of Antioxidant and Bioactive Compounds. New Biotechnol. 2023, 73, 1–8. [Google Scholar] [CrossRef]
- Cicci, A.; Stoller, M.; Bravi, M. Analysis of Microalgae Growth in Residual Light: A Diagnostics Tool for Low-Cost Alternative Cultural Media. Chem. Eng. Trans. 2014, 38, 79–84. [Google Scholar] [CrossRef]
- Bělohlav, V.; Jirout, T.; Elster, J.; Liška, J.; Nedbalová, L.; Kvíderová, J. Cultivation of Polar Microalgae in a Rotating Flat-Panel Photobioreactor. Chem. Listy 2023, 117, 613–618. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, M.; Kamangar, S. Strategies to Produce Cost-Effective Third-Generation Biofuel from Microalgae. Front. Energy Res. 2021, 9, 749968. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Algalif in the Media. Available online: https://algalif.is/category/algalif-in-the-media/ (accessed on 23 November 2023).
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef]
- Gluszcz, P.; Klepacz-Smółka, A.; Ledakowicz, S. Experimental Evaluation of a Helical Laboratory Photobioreactor for Cultivation of Thermophilic Cyanobacteria—Hydrodynamics and Mass Transfer Studies. Chem. Process Eng.-Inz. Chem. Proces. 2018, 39, 457–473. [Google Scholar] [CrossRef]
- Heubeck, S.; Craggs, R.J.; Shilton, A. Influence of CO2 Scrubbing from Biogas on the Treatment Performance of a High Rate Algal Pond. Water Sci. Technol. 2007, 55, 193–200. [Google Scholar] [CrossRef]
- Antecka, A.; Klepacz-Smółka, A.; Szeląg, R.; Pietrzyk, D.; Ledakowicz, S. Comparison of Three Methods for Thermostable C-Phycocyanin Separation and Purification. Chem. Eng. Process. Process Intensif. 2022, 171, 108563. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Slezak, R. From Biofuels to High Added Value Bioproducts in the Development of 3G Biorefineries. Available online: https://ccuv4.fs.cvut.cz/events/the-ccuv4-workshop-in-prague/ (accessed on 16 January 2024).
- Klepacz-Smolka, A.; Shah, M.R.; Jiang, Y.; Zhong, Y.; Chen, P.; Pietrzyk, D.; Szelag, R.; Ledakowicz, S.; Daroch, M. Microalgae Are Not an Umbrella Solution for Power Industry Waste Abatement but Could Play a Role in Their Valorization. Crit. Rev. Biotechnol. 2023, 1–29. [Google Scholar] [CrossRef]
- Abusweireh, R.S.; Rajamohan, N.; Sonne, C.; Vasseghian, Y. Algae Biogas Production Focusing on Operating Conditions and Conversion Mechanisms—A Review. Heliyon 2023, 9, e17757. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic Digestion of Algae Biomass: A Review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Huang, Q.-S.; Ni, B.-J. Methane Production from Algae in Anaerobic Digestion: Role of Corncob Ash Supplementation. J. Clean. Prod. 2021, 327, 129485. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Kaushik, P.; Malik, A.; Vijay, V.K. Phycoremediation Coupled Production of Algal Biomass, Harvesting and Anaerobic Digestion: Possibilities and Challenges. Biotechnol. Adv. 2013, 31, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, S.; Cuevas-Rodríguez, G.; Toda, T. Effect of Ammonia Concentration on a Microalgal-Nitrifying Bacterial Photobioreactor Treating Anaerobic Digester Effluent. Biochem. Eng. J. 2021, 173, 108057. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from Microalgae: Technologies, Challenges and Opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Ferreira, L.O.; Astals, S.; Passos, F. Anaerobic Co-digestion of Food Waste and Microalgae in an Integrated Treatment Plant. J. Chem. Technol. Biotechnol. 2022, 97, 1545–1554. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Jeon, J.-K.; Kim, J.; Ryoo, R.; Jeong, K.-E.; Park, Y.-K. Highly Valuable Chemicals Production from Catalytic Upgrading of Radiata Pine Sawdust-Derived Pyrolytic Vapors over Mesoporous MFI Zeolites. Appl. Catal. B 2010, 95, 365–373. [Google Scholar] [CrossRef]
- Bahrun, M.H.V.; Bono, A.; Othman, N.; Zaini, M.A.A. Carbon Dioxide Removal from Biogas through Pressure Swing Adsorption—A Review. Chem. Eng. Res. Des. 2022, 183, 285–306. [Google Scholar] [CrossRef]
- Yan, S.; He, Q.; Zhao, S.; Wang, Y.; Ai, P. Biogas Upgrading by CO2 Removal with a Highly Selective Natural Amino Acid Salt in Gas–Liquid Membrane Contactor. Chem. Eng. Process. Process Intensif. 2014, 85, 125–135. [Google Scholar] [CrossRef]
- Koutsiantzi, C.; Kampylafka, A.; Zouboulis, A.; Mitrakas, M.; Kikkinides, E.S. Theoretical and Experimental Study of CO2 Removal from Biogas Employing a Hollow Fiber Polyimide Membrane. Sustain. Chem. Pharm. 2023, 35, 101221. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Dulta, K.; Kurniawan, S.B.; Omoarukhe, F.O.; Ewuzie, U.; Eshiemogie, S.O.; Ojo, A.U.; Abdullah, S.R.S. Progress in Microalgae Application for CO2 Sequestration. Clean. Chem. Eng. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects. Appl. Sci. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Integration of Anaerobic Digestion and Microalgal Cultivation for Digestate Bioremediation and Biogas Upgrading. Bioresour. Technol. 2019, 290, 121804. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic Digestate as a Low-Cost Nutrient Source for Sustainable Microalgae Cultivation: A Way Forward through Waste Valorization Approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef]
- Jian, X.; Zhuang, X.; Li, B.; Xu, X.; Wei, Z.; Song, Y.; Jiang, E. Comparison of Characterization and Adsorption of Biochars Produced from Hydrothermal Carbonization and Pyrolysis. Environ. Technol. Innov. 2018, 10, 27–35. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Benavente, V.; Weidemann, E.; Gentili, F.G.; Jansson, S. Influence of Hydrothermal Carbonization Conditions on the Porosity, Functionality, and Sorption Properties of Microalgae Hydrochars. Sci. Rep. 2023, 13, 8562. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira Castro, J.; Assemany, P.P.; de Oliveira Carneiro, A.C.; Ferreira, J.; de Jesus Júnior, M.M.; de Ávila Rodrigues, F.; Calijuri, M.L. Hydrothermal Carbonization of Microalgae Biomass Produced in Agro-Industrial Effluent: Products, Characterization and Applications. Sci. Total Environ. 2021, 768, 144480. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Jader, L.R.; Harned, L.A.; Sadowsky, M.J.; Schendel, F.J.; Lefebvre, P.A.; von Keitz, M.G.; Valentas, K.J. Hydrothermal Carbonization of Microalgae II. Fatty Acid, Char, and Algal Nutrient Products. Appl. Energy 2011, 88, 3286–3290. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Pires, E.; Fraile, J.M. Parametric Study of the Hydrothermal Carbonization of Cellulose and Effect of Acidic Conditions. Carbon 2017, 123, 421–432. [Google Scholar] [CrossRef]
- Ślęzak, R.; Nawrot, P.; Ledakowicz, S. Pyrolysis of Micro- and Macroalgae in Thermobalance Coupled with Mass Spectrometer. Algal Res. 2022, 66, 102782. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass Fast Pyrolysis in a Fluidized Bed Reactor under N2, CO2, CO, CH4 and H2 Atmospheres. Bioresour. Technol. 2011, 102, 4258–4264. [Google Scholar] [CrossRef]
- Kwon, E.E.; Jeon, Y.J.; Yi, H. New Candidate for Biofuel Feedstock beyond Terrestrial Biomass for Thermo-Chemical Process (Pyrolysis/Gasification) Enhanced by Carbon Dioxide (CO2). Bioresour. Technol. 2012, 123, 673–677. [Google Scholar] [CrossRef]
- Hong, Y.; Xie, C.; Chen, W.; Luo, X.; Shi, K.; Wu, T. Kinetic Study of the Pyrolysis of Microalgae under Nitrogen and CO2 Atmosphere. Renew. Energy 2020, 145, 2159–2168. [Google Scholar] [CrossRef]
- Díaz-Rey, M.R.; Cortés-Reyes, M.; Herrera, C.; Larrubia, M.A.; Amadeo, N.; Laborde, M.; Alemany, L.J. Hydrogen-Rich Gas Production from Algae-Biomass by Low Temperature Catalytic Gasification. Catal. Today 2015, 257, 177–184. [Google Scholar] [CrossRef]
- Slezak, R.; Unyay, H.; Szufa, S.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Reactors Torrefaction vs. Biomass Pyrolizers—Part 2. Energies 2023, 16, 2212. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, W.; Luo, X.; Pang, C.; Lester, E.; Wu, T. Microwave-Enhanced Pyrolysis of Macroalgae and Microalgae for Syngas Production. Bioresour. Technol. 2017, 237, 47–56. [Google Scholar] [CrossRef]
- Wu, C.; Budarin, V.L.; Wang, M.; Sharifi, V.; Gronnow, M.J.; Wu, Y.; Swithenbank, J.; Clark, J.H.; Williams, P.T. CO2 Gasification of Bio-Char Derived from Conventional and Microwave Pyrolysis. Appl. Energy 2015, 157, 533–539. [Google Scholar] [CrossRef]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of Sewage Sludge in a Screw-Feeding Reactor: Products Characterization and Ecological Risk Assessment of Heavy Metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Xiao, R.; Yang, W. Influence of Temperature on Organic Structure of Biomass Pyrolysis Products. Renew. Energy 2013, 50, 136–141. [Google Scholar] [CrossRef]
- Xu, W.; Ding, K.; Hu, L. A Mini Review on Pyrolysis of Natural Algae for Bio-Fuel and Chemicals. Processes 2021, 9, 2042. [Google Scholar] [CrossRef]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic Pyrolysis of Microalgae to High-Quality Liquid Bio-Fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- Slezak, R.; Krzystek, L.; Ledakowicz, S. CO2 Gasification of Char from Spent Mushroom Substrate in TG-MS System. J. Therm. Anal. Calorim. 2020, 140, 2337–2345. [Google Scholar] [CrossRef]
- Tanner, J.; Bhattacharya, S. Kinetics of CO2 and Steam Gasification of Victorian Brown Coal Chars. Chem. Eng. J. 2016, 285, 331–340. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Liu, Q.; Yang, J. Experimental and Kinetic Studies of Coal–CO2 Gasification in Isothermal and Pressurized Conditions. RSC Adv. 2017, 7, 2193–2201. [Google Scholar] [CrossRef]
- Wang, L.; Alsaker, N.; Skreiberg, Ø.; Hovd, B. Effect of Carbonization Conditions on CO2 Gasification Reactivity of Biocarbon. Energy Procedia 2017, 142, 932–937. [Google Scholar] [CrossRef]
- Bui, H.-H.; Wang, L.; Tran, K.-Q.; Skreiberg, Ø. CO2 Gasification of Charcoals Produced at Various Pressures. Fuel Process. Technol. 2016, 152, 207–214. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Shao, R.; Wang, Y.; Yu, J.; Xu, G. Isothermal Gasification of in Situ/Ex Situ Coal Char with CO2 in a Micro Fluidized Bed Reaction Analyzer. Energy Fuels 2015, 29, 4795–4802. [Google Scholar] [CrossRef]
- Atikah, M.S.N.; Taufiq Yap, Y.H.; Ilyas, R.A.; Harun, R. Optimization of Algae Residues Gasification: Experimental and Theoretical Approaches. J. Phys. Conf. Ser. 2022, 2259, 012012. [Google Scholar] [CrossRef]
- Roncancio, R.; Gore, J.P. CO2 Char Gasification: A Systematic Review from 2014 to 2020. Energy Convers. Manag. X 2021, 10, 100060. [Google Scholar] [CrossRef]
- Pohořelý, M.; Jeremiáš, M.; Svoboda, K.; Kameníková, P.; Skoblia, S.; Beňo, Z. CO2 as Moderator for Biomass Gasification. Fuel 2014, 117, 198–205. [Google Scholar] [CrossRef]
- Massoudi Farid, M.; Jeong, H.J.; Hwang, J. Co-Gasification of Coal–Biomass Blended Char with CO2 and H2O: Effect of Partial Pressure of the Gasifying Agent on Reaction Kinetics. Fuel 2015, 162, 234–238. [Google Scholar] [CrossRef]
- Gao, N.; Śliz, M.; Quan, C.; Bieniek, A.; Magdziarz, A. Biomass CO2 Gasification with CaO Looping for Syngas Production in a Fixed-Bed Reactor. Renew. Energy 2021, 167, 652–661. [Google Scholar] [CrossRef]
- Grenz, J.; Cerdas, F.; Herrmann, C. LCA Based Analysis of Product Portfolios—Towards Decarbonization. Procedia CIRP 2022, 105, 519–524. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC) (Ed.) Climate Change 2022—Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2023; ISBN 9781009157926. [Google Scholar]
- Bach, V. Life Cycle Assessment in the Context of Decarbonization and Carbon Neutrality. Int. J. Life Cycle Assess. 2023, 28, 741–745. [Google Scholar] [CrossRef]
- de Souza Schneider, R.D.C.; de Moura Lima, M.; Hoeltz, M.; de Farias Neves, F.; John, D.K.; de Azevedo, A. Life Cycle Assessment of Microalgae Production in a Raceway Pond with Alternative Culture Media. Algal Res. 2018, 32, 280–292. [Google Scholar] [CrossRef]
- Porcelli, R.; Dotto, F.; Pezzolesi, L.; Marazza, D.; Greggio, N.; Righi, S. Comparative Life Cycle Assessment of Microalgae Cultivation for Non-Energy Purposes Using Different Carbon Dioxide Sources. Sci. Total Environ. 2020, 721, 137714. [Google Scholar] [CrossRef]
- Bradley, T.; Rajaeifar, M.A.; Kenny, A.; Hainsworth, C.; del Pino, V.; del Valle Inclán, Y.; Povoa, I.; Mendonça, P.; Brown, L.; Smallbone, A.; et al. Life Cycle Assessment of Microalgae-Derived Biodiesel. Int. J. Life Cycle Assess. 2023, 28, 590–609. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life Cycle Assessment of the Production of the Red Antioxidant Carotenoid Astaxanthin by Microalgae: From Lab to Pilot Scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef]
- López-Herrada, E.; Gallardo-Rodríguez, J.J.; López-Rosales, L.; Cerón-García, M.C.; Sánchez-Mirón, A.; García-Camacho, F. Life-Cycle Assessment of a Microalgae-Based Fungicide under a Biorefinery Approach. Bioresour. Technol. 2023, 383, 129244. [Google Scholar] [CrossRef]
- Sangma, C.B.K.; Chalie-u, R. Life Cycle Assessment of Wastewater Treatment by Microalgae. In Valorization of Microalgal Biomass and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 137–178. [Google Scholar]
- Lu, Y.; Mu, D.; Xue, Z.; Xu, P.; Li, Y.; Xiang, W.; Burnett, J.; Bryant, K.; Zhou, W. Life Cycle Assessment of Industrial Production of Microalgal Oil from Heterotrophic Fermentation. Algal Res. 2021, 58, 102404. [Google Scholar] [CrossRef]
- Wimmerova, L.; Keken, Z.; Solcova, O.; Vavrova, K. A Comparative Analysis of Environmental Impacts of Operational Phases of Three Selected Microalgal Cultivation Systems. Sustainability 2022, 15, 769. [Google Scholar] [CrossRef]
- Huang, X.; Bai, S.; Liu, Z.; Hasunuma, T.; Kondo, A.; Ho, S.-H. Fermentation of Pigment-Extracted Microalgal Residue Using Yeast Cell-Surface Display: Direct High-Density Ethanol Production with Competitive Life Cycle Impacts. Green Chem. 2020, 22, 153–162. [Google Scholar] [CrossRef]
- Sun, C.; Xia, A.; Liao, Q.; Fu, Q.; Huang, Y.; Zhu, X. Life-Cycle Assessment of Biohythane Production via Two-Stage Anaerobic Fermentation from Microalgae and Food Waste. Renew. Sustain. Energy Rev. 2019, 112, 395–410. [Google Scholar] [CrossRef]
- Ubando, A.T.; Rivera, D.R.T.; Chen, W.-H.; Culaba, A.B. A Comprehensive Review of Life Cycle Assessment (LCA) of Microalgal and Lignocellulosic Bioenergy Products from Thermochemical Processes. Bioresour. Technol. 2019, 291, 121837. [Google Scholar] [CrossRef]
- Fortier, M.-O.P.; Roberts, G.W.; Stagg-Williams, S.M.; Sturm, B.S.M. Life Cycle Assessment of Bio-Jet Fuel from Hydrothermal Liquefaction of Microalgae. Appl. Energy 2014, 122, 73–82. [Google Scholar] [CrossRef]
- Naaz, F.; Samuchiwal, S.; Dalvi, V.; Bhattacharya, A.; Kishore Pant, K.; Malik, A. Hydrothermal Liquefaction Could Be a Sustainable Approach for Valorization of Wastewater Grown Algal Biomass into Cleaner Fuel. Energy Convers. Manag. 2023, 283, 116887. [Google Scholar] [CrossRef]
- Chen, P.H.; Quinn, J.C. Microalgae to Biofuels through Hydrothermal Liquefaction: Open-Source Techno-Economic Analysis and Life Cycle Assessment. Appl. Energy 2021, 289, 116613. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of Microalgae: A Critical Review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Grierson, S.; Strezov, V.; Bengtsson, J. Life Cycle Assessment of a Microalgae Biomass Cultivation, Bio-Oil Extraction and Pyrolysis Processing Regime. Algal Res. 2013, 2, 299–311. [Google Scholar] [CrossRef]
- Wang, X.; Guo, F.; Li, Y.; Yang, X. Effect of Pretreatment on Microalgae Pyrolysis: Kinetics, Biocrude Yield and Quality, and Life Cycle Assessment. Energy Convers. Manag. 2017, 132, 161–171. [Google Scholar] [CrossRef]
- Azadi, P.; Brownbridge, G.; Mosbach, S.; Inderwildi, O.; Kraft, M. Simulation and Life Cycle Assessment of Algae Gasification Process in Dual Fluidized Bed Gasifiers. Green Chem. 2015, 17, 1793–1801. [Google Scholar] [CrossRef]
- Klemeš, J.J. Assessing and Measuring Environmental Impact and Sustainability; Butterwort-Heinemann: Oxford, UK, 2015; ISBN 978-0-12-799968-5. [Google Scholar]
- Archdaily Algae Green Loop Influx Studio. Available online: https://www.archdaily.com/191229/algae-green-loop-influx-studio (accessed on 28 November 2023).
- Medl, A.; Stangl, R.; Florineth, F. Vertical Greening Systems—A Review on Recent Technologies and Research Advancement. Build. Environ. 2017, 125, 227–239. [Google Scholar] [CrossRef]
- Viridos Executes Agreement with ExxonMobil to Help Scale Algae Biofuels. Available online: https://www.businesswire.com/news/home/20211119005272/en/Viridos-Executes-Agreement-with-ExxonMobil-to-Help-Scale-Algae-Biofuels (accessed on 28 November 2023).
- Viridos Viridos Technology. Available online: https://www.viridos.com/technology (accessed on 28 November 2023).
- Kunjapur, A.M.; Eldridge, R.B. Photobioreactor Design for Commercial Biofuel Production from Microalgae. Ind. Eng. Chem. Res. 2010, 49, 3516–3526. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G. Mass Cultivation of Freshwater Microalgae. In Encyclopedia of Ecology; Elsevier: Oxford, UK, 2008. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).