1. Introduction
The concept of soil quality was initially defined as “the capacity of soil to function within an ecosystem and under various land uses in such a way that it sustains biological productivity, maintains water and air quality, and promotes the health of animals and plants” [
1]. Larson and Pierce [
2] expanded on this by suggesting that a soil’s physical, chemical, and biological characteristics enable it to perform three essential functions: (1) provide a medium for plant growth, (2) control and regulate water flow in the environment, and (3) serve as an environmental filter.
While the concept of soil quality may seem straightforward, its definition and quantification pose considerable challenges [
3]. Some researchers contend that the term “quality” is difficult to apply to soil, given its dynamic, complex, and variable nature [
4,
5,
6]. However, a growing number of studies underscore the critical role of soil quality in environmental sustainability and human well-being [
7,
8,
9]. Soil quality provides a comprehensive framework for examining the interactions among the biological, chemical, and physical properties of soil, which is essential for sustainable land use and effective soil management of non-renewable soil resources [
1,
3,
4,
5,
6,
7,
8,
9,
10,
11]. For this reason, Lal [
12] recently suggested that restoring soil quality in agricultural lands could help mitigate soil degradation.
Evaluating soil quality requires an analysis of both the inherent and dynamic characteristics of soil. In any region, soil quality assessment is influenced by a combination of factors, including management practices like crop rotation and manure application, as well as climate and soil type [
9]. The initial step in assessing soil quality involves identifying suitable soil quality indicators (SQIs) to create a minimum dataset (MDS) for assessment [
13]. Selecting indicators that encompass a wide range of physical, chemical, and biological attributes is crucial for an accurate evaluation of soil quality. Additionally, it is important to ensure that the chosen parameters effectively convey the information offered by all relevant indicators [
14].
Many soil characteristics that influence soil quality are often highly correlated, interacting with other soil properties [
2,
11,
14]. Due to these correlations, a more robust evaluation of soil quality can be achieved using statistical approaches that take these relationships into account. Multivariate statistical analyses, for example, allow for the examination of multiple correlated variables at once, revealing patterns that might be missed when variables are assessed independently [
15]. Numerous studies have utilized multivariate methods to identify a smaller set of soil quality indicators, an MDS that can effectively describe changes in soil quality [
16,
17,
18,
19], and these have been applied to various land use types, including coastal areas [
20], agricultural zones [
21], and grasslands [
19]. Zhou et al. [
22] used ANOVA and factor analysis to identify a subset of 4 key soil indicators from an initial group of 26 to build an MDS for evaluating soil quality in wheat-producing regions of China. Similarly, Brejda et al. [
21] employed principal component and discriminant analyses to identify sensitive soil indicators at a regional scale.
In the Mediterranean region, only a few studies have developed specific sets of soil quality indicators for specific land uses like forest [
23,
24] and agricultural land uses e.g., [
10,
11,
25,
26,
27], and even fewer have incorporated biological parameters [
10,
11,
26]. Reis and Dintaroglou [
28] employed Principal Component Analysis to evaluate dynamic soil quality in a semi-arid Mediterranean watershed for different land uses. Navaro et al. [
29] used multivariate methods to select the most appropriate indicators for a soil quality index in Mediterranean ecosystems.
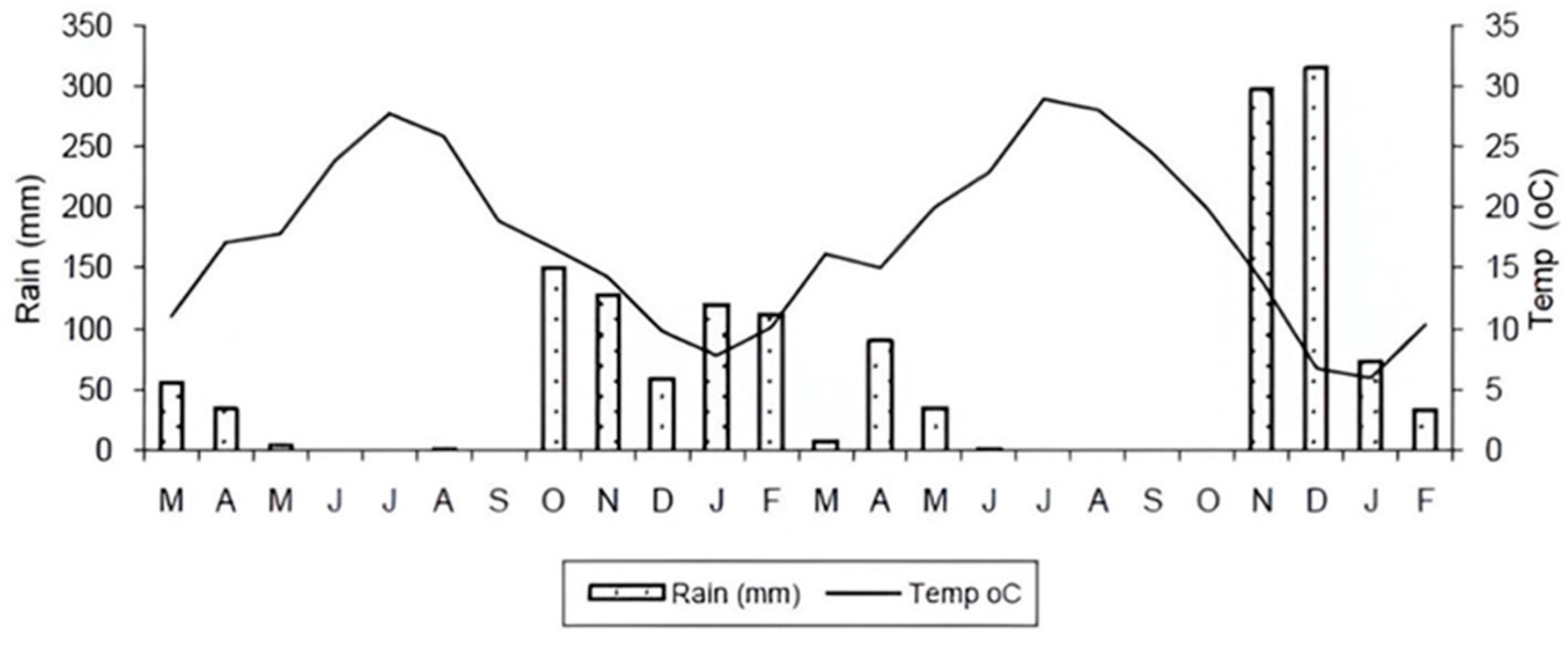
Most studies aimed at identifying MDS do not account for the seasonal variability of soil quality indicators, as soil sampling is typically conducted during one specific season. Soil function is significantly influenced by seasonal variations in temperature and moisture, as well as by management practices in agricultural systems. This is particularly important in Mediterranean regions, which are characterized by a pronounced seasonal contrast in temperature and rainfall between winter and summer. Soil quality indicators in Mediterranean agroecosystems often exhibit significant seasonal variability that is frequently overlooked in efforts to establish MDS at a regional scale [
30,
31]. Furthermore, Mediterranean agroecosystems are notable for their highly variable soil cover, spatial diversity, and long history of continuous human settlement and intensive cultivation [
32], which further influence soil quality.
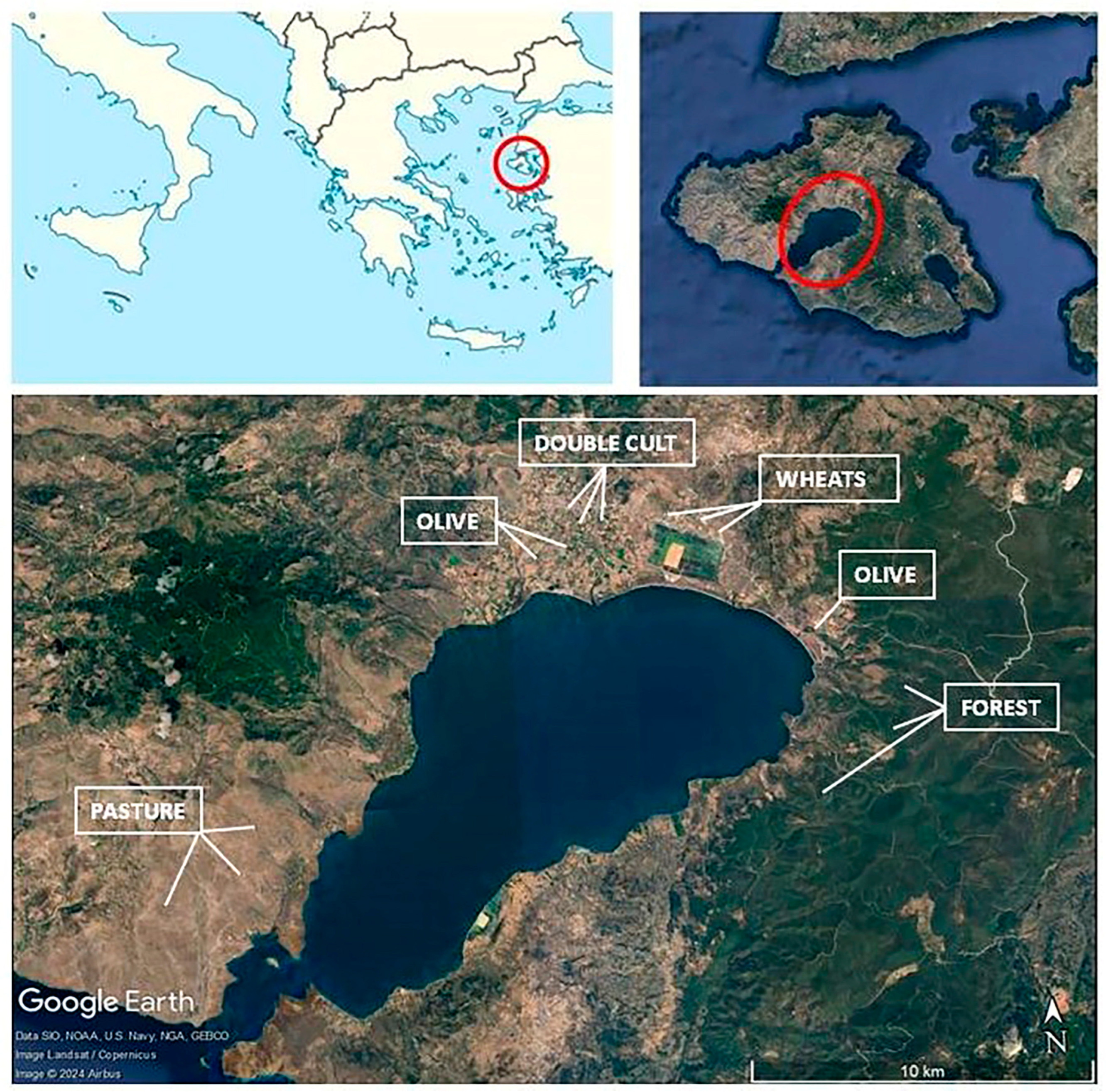
To address these challenges, the present study introduces a statistics-based methodology for identifying an MDS for soil quality assessment, incorporating seasonal samplings of soils, across five different land use types, over two consecutive years. The study aimed to: (i) identify regional-scale soil quality “factors” from a set of 23 biological, chemical, and physical soil quality indicators, (ii) determine which soil quality factors exhibit significant variation based on land use, and (iii) pinpoint soil properties that can serve as reliable indicators for monitoring soil quality on a regional scale, taking into account the seasonal variation of soil functions in Mediterranean agroecosystems.
4. Discussion
In this study, the dataset used in the multivariate analyses incorporates the effects of both land use and season on the examined SQIs. Land use within the same climate and soil type influences soil function through management practices such as tillage, irrigation, fertilization, and biomass removal. Additionally, vegetation cover plays a role, as this determines the quantity and quality of plant residues entering the soil system. Although land use impacts individual soil quality indicators (SQIs), it has a more substantial effect on the overall set of indices (physical, chemical, and biological), grouping soil functions into distinct categories for each land use. For instance, soil functions in crop lands, while differing between crop types, are more pronounced compared to those in forests and pastures.
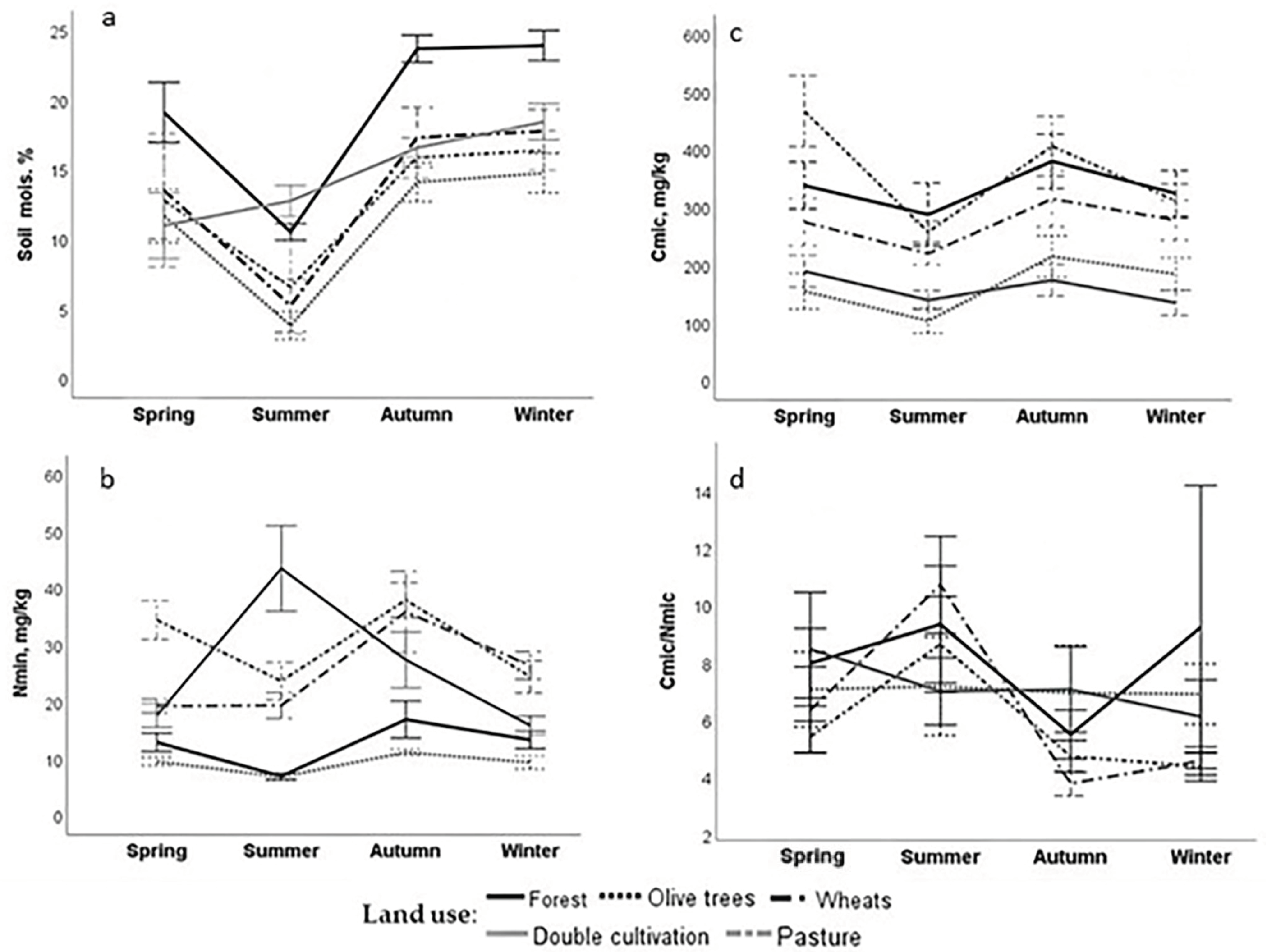
Seasonal variability of SQIs is a crucial factor in understanding soil function and has been investigated in the study area, specifically for soil microbial biomass properties [
31]. In Mediterranean regions, the hot and dry summer conditions are likely to have a more pronounced impact on the temporal changes of many SQIs compared to other factors. For example, soil microbial biomass often shows a decline during the summer, followed by an increase in autumn as rainfall returns. This pattern is consistent across various land uses, despite differences in vegetation, management practices, or Corg levels in Mediterranean agroecosystems. However, the magnitude of these seasonal shifts—from spring to summer and summer to autumn—varies significantly depending on land use [
31]. Furthermore, the timing of management practices, such as irrigation, soil cultivation, and fertilization, strongly influences the variability of SQIs, including nutrient availability, electrical conductivity (EC), and soil microbial biomass indices.
PCA highlighted NO
3-N and P as key indicators distinguishing soil functions across different land uses. The observed differences may be linked to soil management practices, such as the regular application of chemical fertilizers (e.g., 11-15-15 type) that increase inorganic phosphorus levels, as animal excretions in pastures that increase nitrate nitrogen, and the closed nutrient cycling in forests. Two other indicators, Cmic and Cact, representing labile organic carbon in soils [
52,
53,
54,
55], also distinguish soil functions among land uses. These indicators are influenced by management practices such as tillage and biomass removal in crops, animal excretions in pastures, and nutrient cycling in forests, which affect labile carbon pools in the soil. Crop residues serve as a crucial source of energy and nutrients for microbial proliferation, contributing to the formation of soil organic carbon. Labile/active organic carbon pools represent a small part of soil organic carbon but serve as sensitive indicators of soil biogeochemical processes under agricultural management [
56] that must be further studied.
In the current study, six key soil quality factors were identified: organic matter, microbial biomass, nutrients, compaction, C/N ratio, and Cact/Corg ratio. Each of these factors plays a role in supporting one or more essential soil functions.
The
organic matter factor, in particular, reflects both long-term and short-term changes associated with land use change [
57]. Soil organic matter (SOM) underpins crucial ecosystem services, such as food production, climate regulation, water filtration, erosion control, nutrient cycling, and providing energy for soil organisms [
58,
59]. It is widely regarded as a vital indicator of soil quality for the Mediterranean agroecosystems [
27,
60,
61,
62]. SOM also plays a key role in enhancing the resilience and adaptability of soils to environmental pressures [
1]. The loss of soil organic carbon, often observed during the conversion of natural ecosystems to agricultural systems [
63,
64], is associated with reduced inputs of organic materials, decreased natural protection of organic carbon due to tillage, shifts in soil moisture and temperature that accelerate decomposition rates, and increased soil erosion [
65]. Tillage, in particular, involves the physical disruption of the upper soil layers, which reduces soil aggregation and influences the turnover of aggregates, thereby impacting the soil carbon balance [
66]. Conservation tillage techniques, such as reduced tillage, have been shown to increase total organic carbon in the surface layer, promoting microaggregation, and enhancing aggregate stability [
67]. These practices can be effective alternatives for improving soil quality by increasing organic matter in cultivated soils and have to be incorporated into Mediterranean agroecosystem management. Additionally, crop rotations that include legumes, along with the application of organic amendments like animal residues and organic waste, can enhance carbon storage in soils [
68]. Finally, if a significant portion of the diminishing soil organic matter could be restored through appropriate management practices, it might even be possible to mitigate some of the annual increases in atmospheric CO
2 levels [
69].
The
microbial biomass factor governs ecological processes that drive carbon and nutrient cycles, making it a sensitive measure of soil management impacts. Soil microbial biomass has been extensively recognized as a critical soil quality indicator [
70,
71,
72] and has been reported among the most important ecological indicators of soil quality in the Mediterranean ecosystem [
30]. Microbial biomass serves as both a source of mobile nutrients and a key player in the cycling and transformation of organic matter and plant nutrients in the soil [
73]. Understanding microbial properties—such as the quantity, diversity, and activity of microbial biomass—is crucial for gaining deeper insights into the factors that contribute to soil health [
74]. As a property that can predict future shifts in the amount of total organic matter [
75], soil microbial biomass monitoring is a valuable tool for understanding and anticipating long-term changes in soil conditions. Many management practices in agroecosystems have been linked to the reduction of soil organic matter, leading to declines in soil biological fertility and resilience [
76]. This issue is particularly pronounced in the rainfed agricultural systems of Mediterranean climates, where high summer temperatures and the alternation of wet and dry soil conditions contribute to high annual mineralization rates of organic matter [
77]. The variability of abiotic factors in the Mediterranean agroecosystems is more extreme compared to temperate regions [
78], and thus the synchronization of soil fauna and flora activity with the dynamics of certain chemical soil properties, influenced by seasonal climate changes, is a defining characteristic of Mediterranean-type ecosystems [
79].
The nutrients factor affects nutrient availability, while the compaction factor influences water retention, aeration, and soil physical, chemical, and biological properties.
The
C/N and
Cact/Corg factors, while unidimensional in factor analysis, represent complex and dynamics soil functions. The C/N ratio is a key indicator of the quality of organic substrates available for decomposition [
80,
81], while the Cact/Corg ratio reflects the mineralization dynamics of organic matter [
41]. Together, these factors provide a comprehensive assessment of soil quality in Mediterranean agroecosystems.
The differentiation of the six quality factors based on land use reflects dynamic soil qualities [
14] and assesses the impact of land use and management practices on soil quality. The
organic matter and
compaction factors have been previously recognized by other researchers [
21,
81]. In this study, four additional factors are identified:
microbial biomass,
nutrients,
C/N, and
Cact/Corg. Evaluating these soil quality factors identifies five of the six as significant for assessing changes in soil quality due to land use changes. The
Cact/Corg factor appears less important, as it does not effectively express soil quality dynamics.
In general, soil quality factors exhibit similar behavior across different land uses, consistent with the dominant indicators that comprise them. The
organic matter factor is significantly affected by the land use and associated soil management like cultivation practices. The
microbial biomass factor is influenced by carbon and nitrogen incorporation into microbial biomass, while the
nutrients factor is impacted by nitrogen availability (via fertilization or mineralization). Soil management practices affect the
compaction factor, and the
C/N factor is influenced by the quantity and composition of plant residues. Among these, the
organic matter and
C/N factors, which reflect the quantity and quality of soil organic matter, appear to be the most crucial determinants of soil quality in Mediterranean agroecosystems. Changes in soil quality, resulting from land use and management practices, are reflected in all components of each factor [
82].
The impact of land use on soil function is closely linked to the intensity of land management. While different crops generally have a similar characteristic effect on overall soil function, they also exhibit differences due to variations in the intensity and type of cultivation practices associated with each crop. Specifically, in this study, land use influences the content of soil organic matter, its quality, and the active pools of organic carbon. It seems that the quality of organic matter and the characteristics of active carbon pools, rather than the overall concentration of soil organic matter, play a dominant role in determining nutrient availability in soils.
In this study, TN is highlighted as critical for determining shifts in soil quality within the soil
organic matter factor. Its significance as a fundamental property for soil quality is noted in numerous studies [
83,
84,
85] due to its incorporation of a large portion of the information related to interacting soil parameters. In this study, forest soils show the highest TN stocks, followed by grasslands and croplands, similar to findings in other studies [
85,
86]. TN was significantly correlated with soil moisture, clay, Corg, Cmic, and Cact, indicating its influence on both labile and stable forms of soil organic matter. TN is a SQI that incorporates soil organic matter dynamics, and furthermore, is a significant and direct contributor to plant nitrogen nutrition, even in agricultural contexts [
87]. TN has been reported by Zhao et al. [
88] for other types of climatic zones as a sensitive SQI among different land uses.
Soil C/N ratio emerged as a second crucial factor for assessing changes in soil quality. Its significance lies in its ability to reflect the dynamics of organic matter decomposition, which plays a pivotal role in overall soil quality. Soil C/N ratio has long been recognized as a key indicator of organic matter quality and nitrogen mineralization-immobilization processes [
89]. Shifts in soil C/N stoichiometry are known to significantly influence carbon dynamics in agroecosystems [
80]. Microorganisms use labile carbon as an energy source to produce extracellular enzymes, facilitating nitrogen extraction from soil organic matter (SOM) and leading to SOM mineralization [
90,
91]. Thus, the C/N ratio, though often underestimated, plays a fundamental role in regulating soil organic matter decomposition, indirectly impacting soil quality. The C/N ratio also serves as a common proxy for organic matter stability [
92], offering insights into soil quality changes. While interpreting shifts in C/N ratios in bulk soils is complex, especially in response to land use or climate change, it is essential for understanding potential soil organic carbon (SOC) sequestration or losses, as well as nutrient cycling and availability in agroecosystems. This makes the C/N ratio a valuable indicator for tracking soil quality changes in agricultural systems.
From a set of 23 physical, chemical, and biological SQIs, this study identifies soil properties of total nitrogen and C/N ratio as sensitive key indicators, capturing most of the variability across all 23 SQIs among land uses. These two indicators provide valuable insights into soil quality changes resulting from land use changes or the application of specific management practices. The proposed indicators are sufficient for assessing long-term soil quality changes.
Although this research focuses on a specific region of the Mediterranean, the findings related to soil quality factors are likely applicable to other regions with Mediterranean-type climates, where moisture and temperature patterns are the primary drivers of fundamental soil functions. Many of the soil quality indicators used in this study demonstrate similar behavior in response to land use across regions with Mediterranean climates, such as California [
93,
94]. This study’s examination of how soil quality changes with land use change and cultivation practices in Mediterranean ecosystems underscores the need for further research. The significant role of the quantity and quality of soil organic matter, along with its components, in soil functions and nutrient availability highlights the importance of investigating organic matter dynamics using indicators that best reflect the parameters being studied. Additional research on sensitive indicators that can accurately predict changes in organic matter, such as active carbon and microbial biomass properties, would be particularly useful for the early detection of soil quality degradation.
As mentioned, the comparative evaluation of soil quality with changes in land use or different cultivation practices can be effectively conducted using a limited number of indicators. However, soil quality cannot be accurately assessed through one or two properties alone, as it is determined by a combination of physical, chemical, and biological characteristics. The cost and time required for comprehensive soil analysis make it impractical for farmers to regularly assess and monitor changes in soil quality. Therefore, using the selected indicators of TN and C/N that capture condensed information of a broader set of physical, chemical, and biological SQIs can serve as a practical tool for evaluating the sustainability of soil resources. These indicators allow producers and land managers to quickly and efficiently track changes in soil quality following land use change or the adoption of new farming practices, facilitating the re-evaluation of management strategies.