Effects of Different Straw Incorporation Amounts on Soil Organic Carbon, Microbial Biomass, and Enzyme Activities in Dry-Crop Farmland
Abstract
1. Introduction
2. Materials and Methods
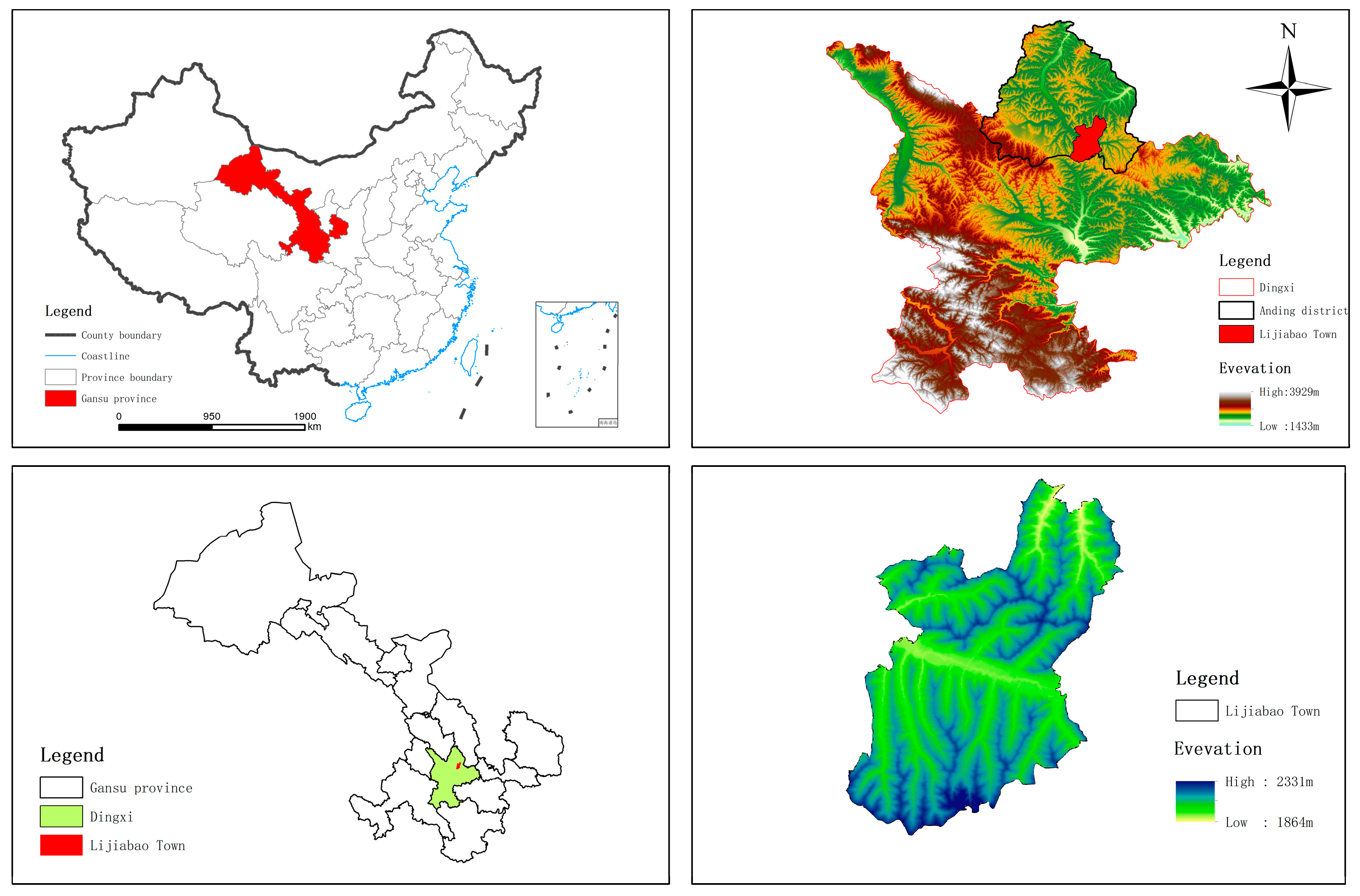
2.1. Overview of the Test Site
2.2. Experimental Methods and Sampling
2.3. Sample Determination and Methods
2.4. Calculation of Indices
2.5. Data Processing
3. Results
3.1. Effects of Straw Incorporation Amount on Soil Physicochemical Properties
3.1.1. Effects of Straw Incorporation Amount on Soil pH
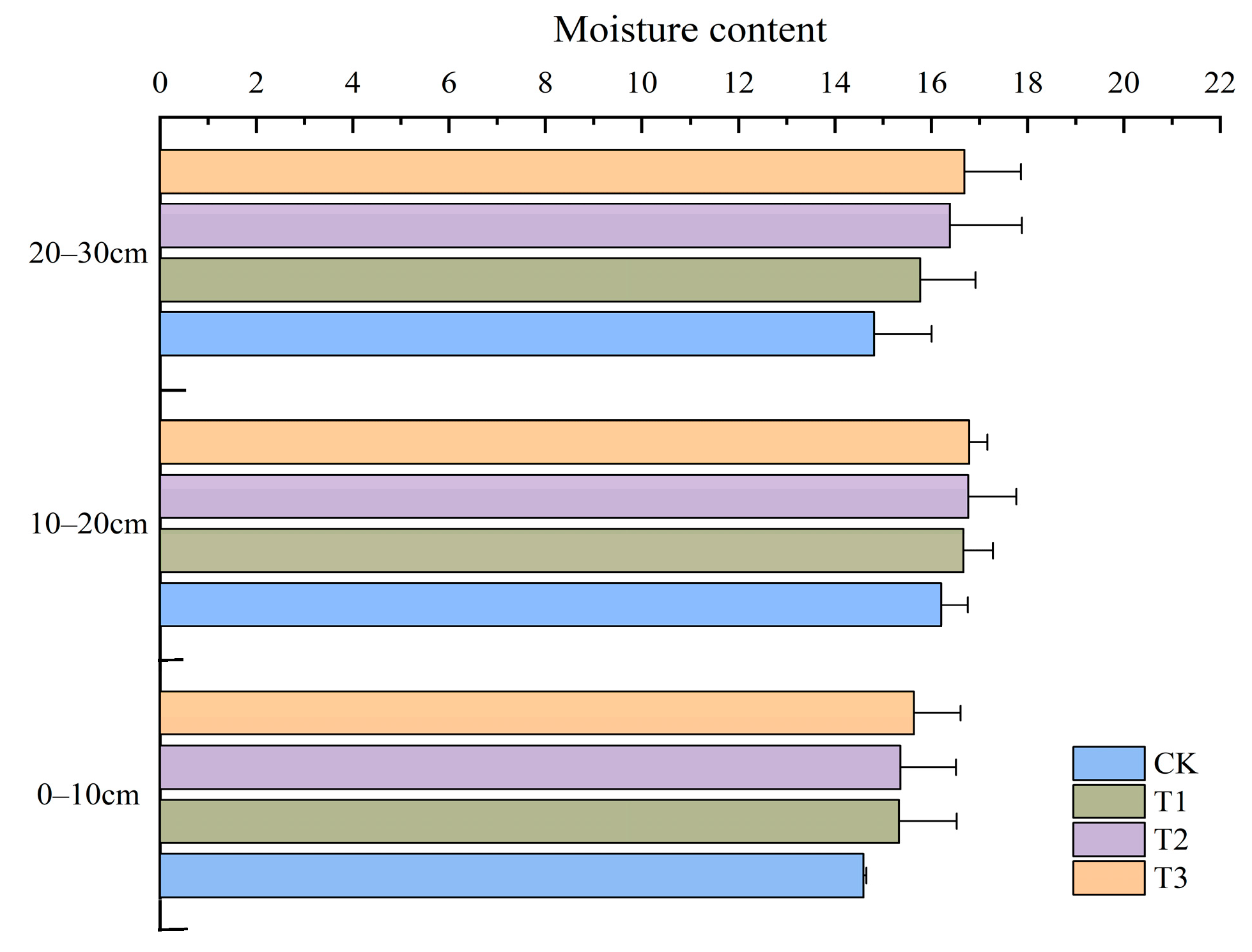
3.1.2. Effects of Straw Incorporation Amount on Soil Moisture
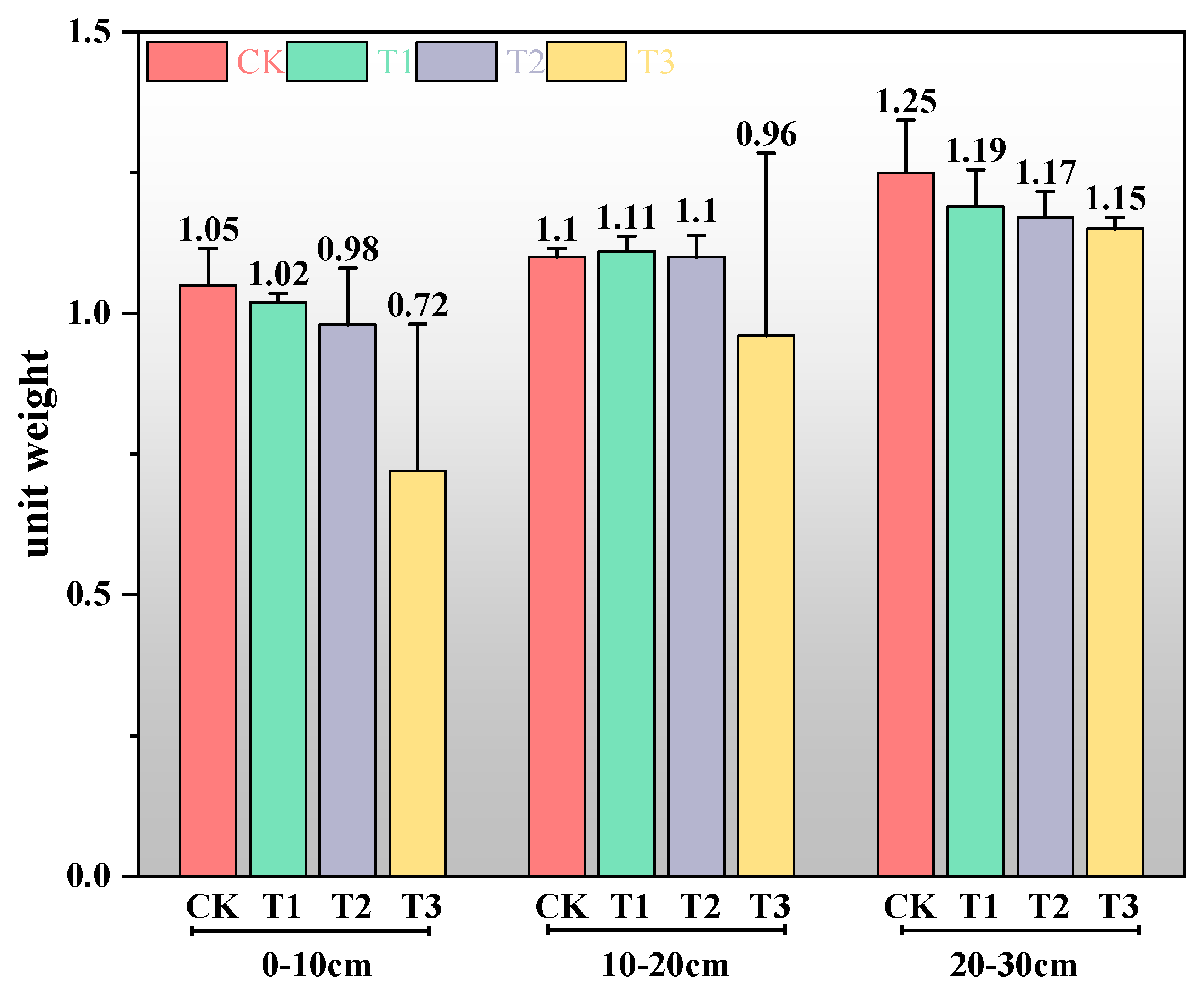
3.1.3. Effects of Straw Incorporation Amount on Soil Unit Weight
3.2. Effects of Straw Incorporation Amount on Soil Microorganisms
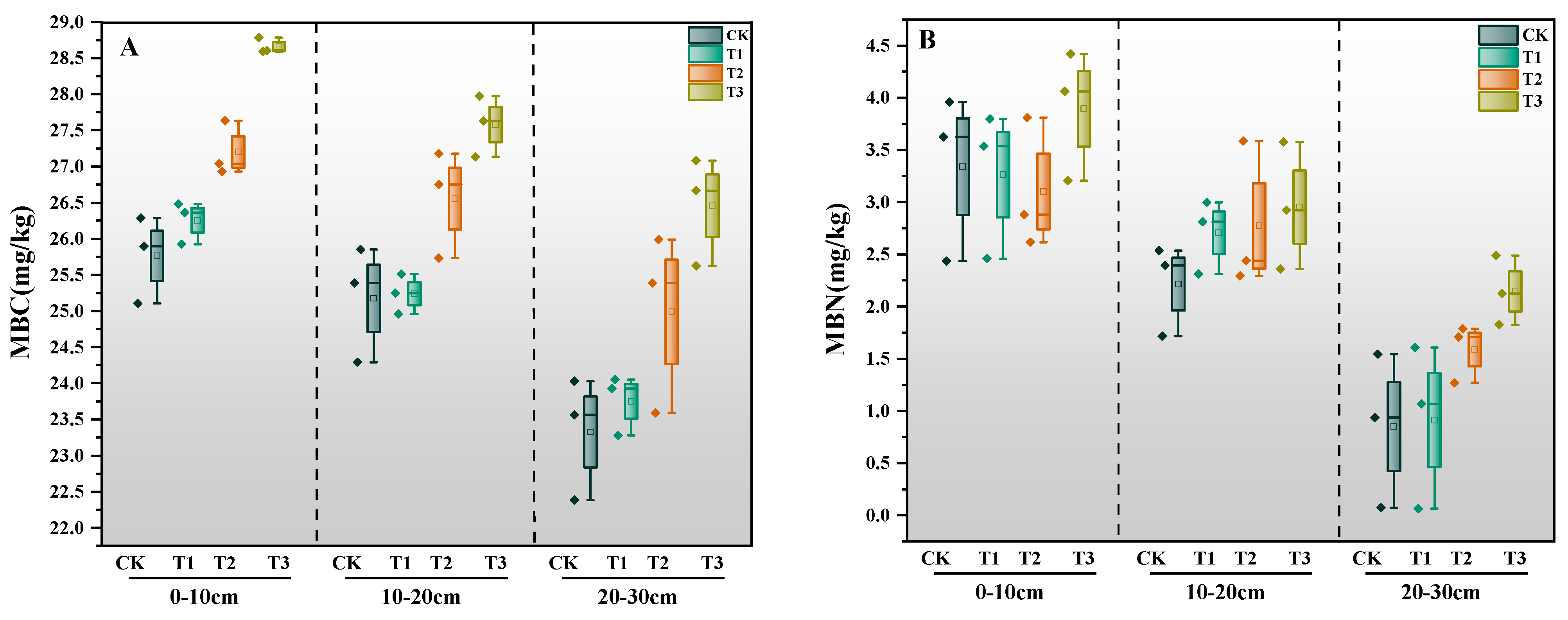
3.2.1. Effects of Straw Incorporation Amount on MBC and MBN
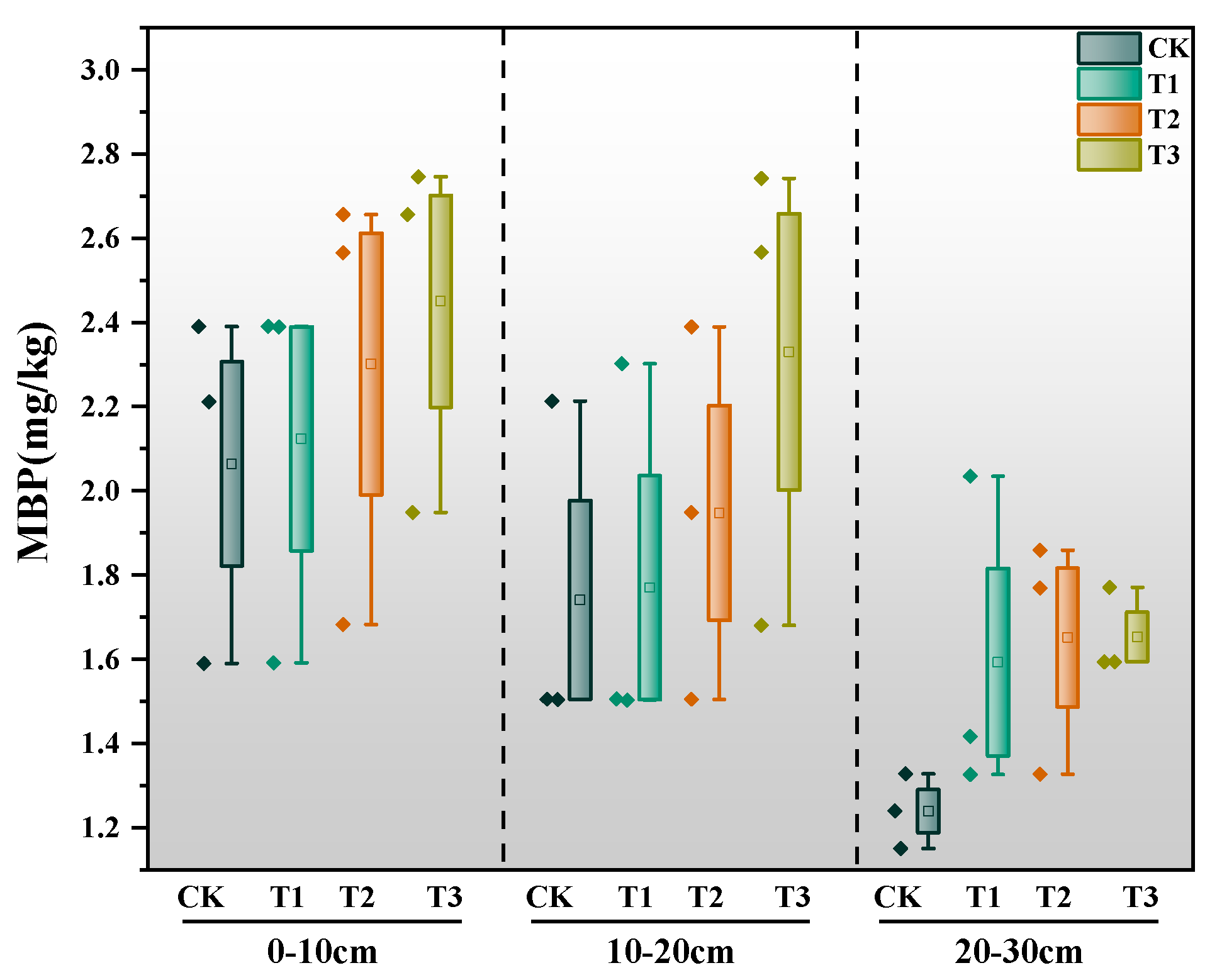
3.2.2. Effects of Straw Incorporation Amount on MBP
3.3. Effects of Straw Incorporation Amount on Soil Enzyme Activities
3.3.1. Effects of Straw Incorporation Amount on Soil Sucrase Activity
3.3.2. Effects of Straw Incorporation Amount on Soil Alkaline Phosphatase Activity
3.4. Effects of Straw Incorporation Amount on SOC
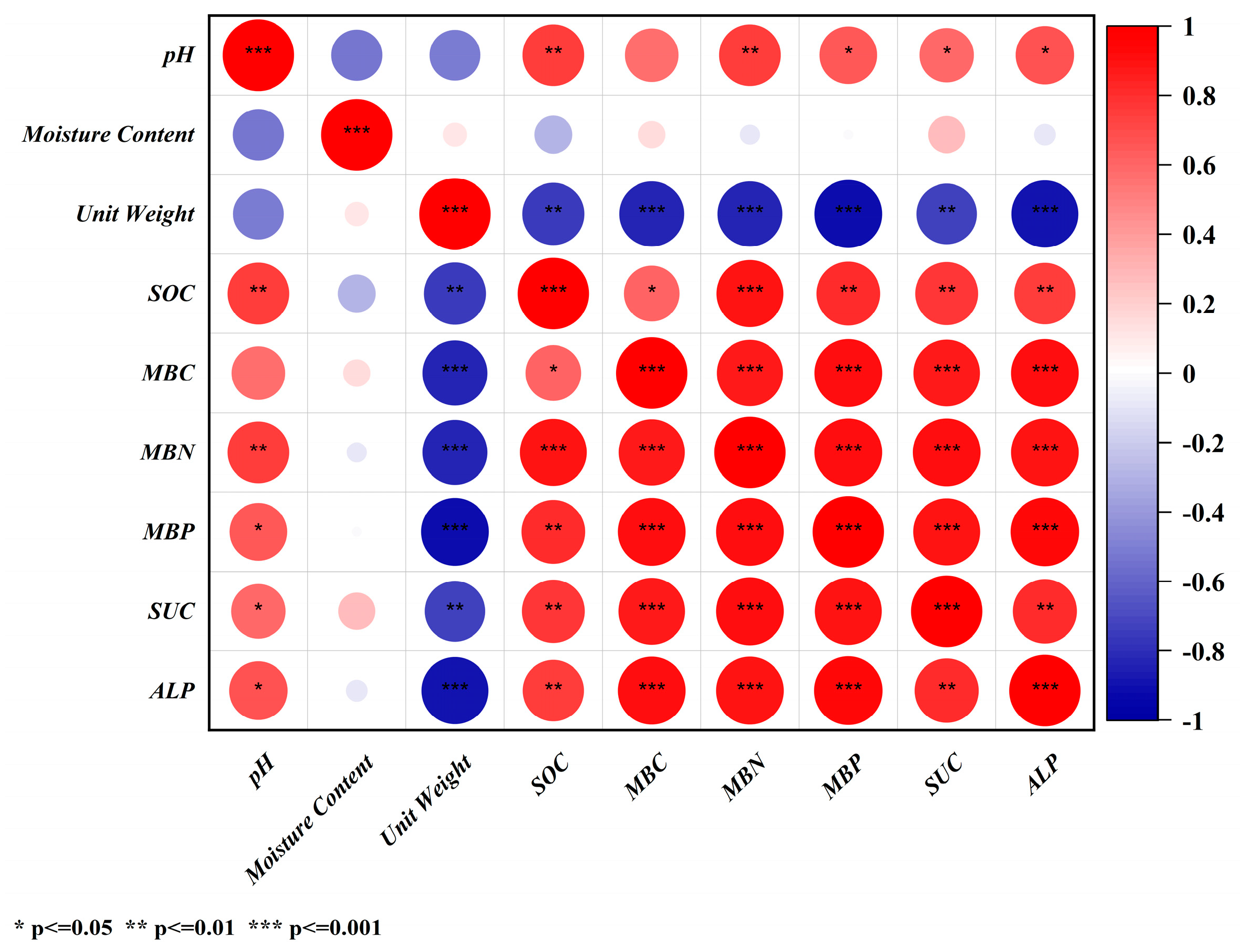
3.5. Correlation Analysis
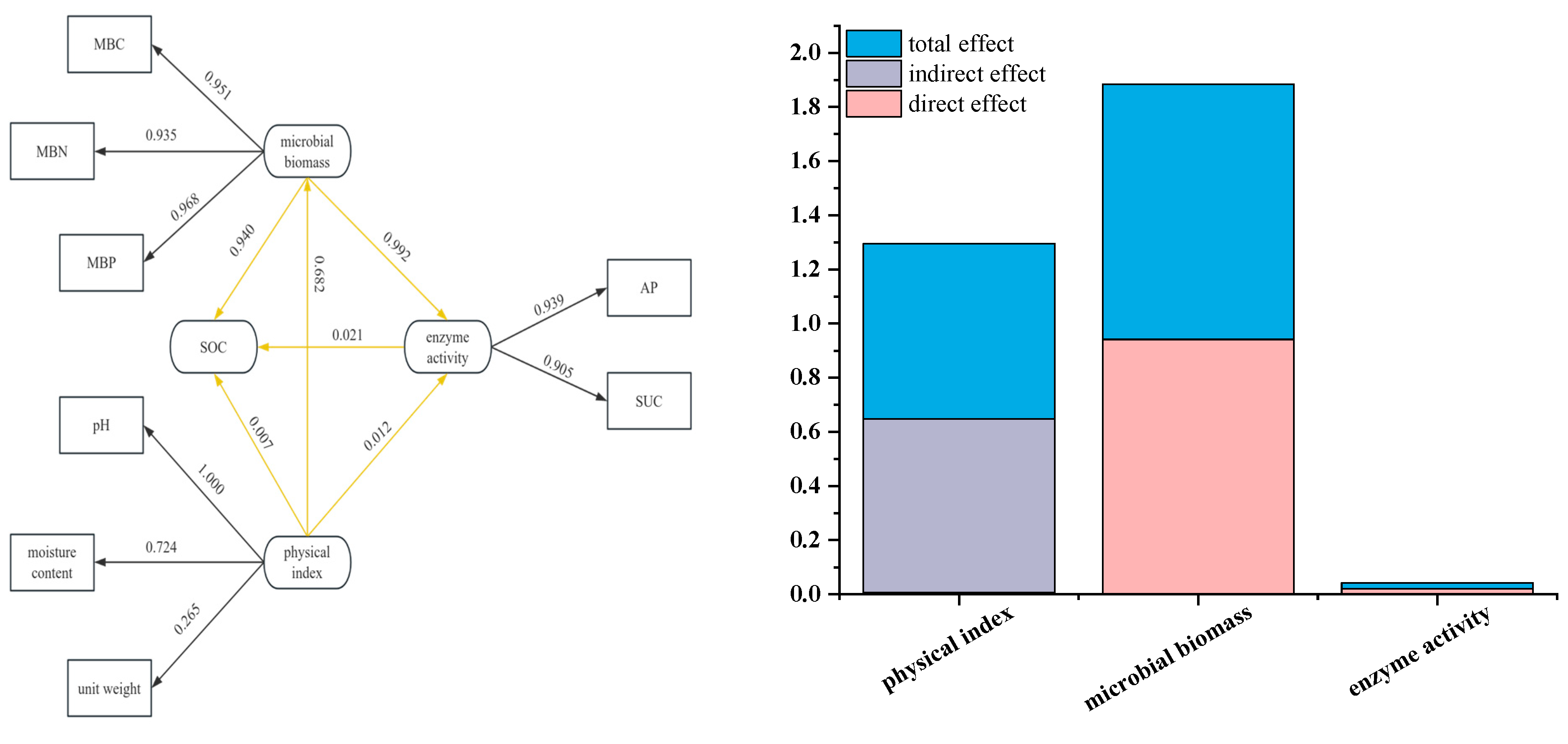
3.6. Structural Equation Modeling (SEM)
4. Discussion
4.1. Effects of Straw Incorporation Amount on Soil Physicochemical Properties
4.2. Effects of Straw Incorporation Amount on Microorganisms and Enzyme Activity
4.3. Effects of Straw Incorporation Amount on Soil SOC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powlson, D.S.; Prookes, P.C.; Christensen, B.T. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of Charcoal Quantity on Microbial Biomass and Activity in Temperate Soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Lao, X.; Wu, Z.; Gao, Y. Effect of Long-Term Inputing Straw to Soil on Soil Fertility. Trans. Chin. Soc. Agric. Eng. 2002, 18, 49–52. [Google Scholar]
- Angens, D.A.; Bissomette, N.; Legere, A.; Samson, N. Microbial and biochemical changes induced by rotation and tillage in soil under barley production. Can. J. Soil Sci. 1993, 73, 39–50. [Google Scholar] [CrossRef]
- Parkin, T.B. Spatial variability of microbial processes in soil: A view. J. Environ. Qual. 1993, 22, 409–417. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, W.; Wen, D.Z. Effects of no-tillage on soil biological properties in farmlands: A review. Chin. J. Soil Sci. 2004, 35, 347–351. [Google Scholar]
- Wright, D.; MacLeod, B.; Hammond, N.; Longnecker, N. Can grain growers and agronomists identify common leaf diseases and biosecurity threats in grain crops? An Australian example. Crop Prot. 2016, 89, 78–88. [Google Scholar] [CrossRef]
- Gao, F.; Cui, Z.; Sun, S. Effects of straw input on soil moisture, corn biological traits, and yield in the dry zone of east-central Gansu. Arid Reg. Agric. Res. 2016, 34, 74–78. [Google Scholar]
- Lu, W.; Jia, Z.; Gao, F. Effects of straw input on soil moisture and crop productivity in dry-crop farmland in Ningnan. J. Agric. Environ. Sci. 2011, 30, 93–99. [Google Scholar]
- Wu, F. Effects of Continuous Multi-Year Corn Stover Input on Soil Physicochemical Properties and Crop Growth. Master’s Thesis, China Agricultural University, Beijing, China, 2005. [Google Scholar]
- Sadet-Bourgeteau, S.; Djemiel, C.; Chemidlin Prévost-Bouré, N.; Feder, F. Dynamic of bacterial and archaeal diversity in a tropical soil over 6 years of repeated organic and inorganic fertilization. Front. Microbiol. 2022, 13, 943314. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Chemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 1999; pp. 106–110. [Google Scholar]
- Jenkinson, D.S.; Parry, L.C. The nitrogen cycle in the Broad balk wheat experiment: A model for the turnover of nitrogen through the soil microbial biomass. Soil Biol. Biochem. 1989, 21, 535–541. [Google Scholar] [CrossRef]
- Lu, R. Soil and Agro-Chemical Analytical Methods; China Agriculture Press: Beijing, China, 1999. [Google Scholar]
- Nsabimana, D.; Haynes, R.J.; Wallis, F.M. Size activity and catabolic diversity of the soil microbial biomass as affected by land use. Appl. Soil Ecol. 2004, 26, 81–92. [Google Scholar] [CrossRef]
- Juma, N.G.; Paul, E.A. Mince ralizable soil nitrogen: Amounts and ex-tractability ratios. Soil Sci. Am. J. 1984, 48, 76–80. [Google Scholar] [CrossRef]
- Wu, J.-S.; Lin, Q.-M.; Huang, Q.-Y.; Xiao, H.A. Determine of Soilmicrobial Biomass and Its Application; Meteorological Press: Beijing, China, 2006; pp. 65–74. [Google Scholar]
- Zhang, G.S.; Chan, K.Y.; Li, G.D.; Huang, G.B. Effect of straw and plastic film management under contrasting tillage practices on the physical properties of an erodible loess soil. Soil Tillage Res. 2008, 98, 113–119. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Li, T. Effects of tillage practices on soil properties and winter wheat yield in dry farmland in Northwest China. Northwest J. Agric. 2019, 28, 1411–1418. [Google Scholar]
- Zhong, Z. Experiments on the determination of soil moisture content. Rural Econ. Technol. 2016, 27, 2–3. [Google Scholar]
- Meng, Q.-F.; Li, D.-W.; Zhang, J.; Zhou, L.-R.; Wang, H.-Y.; Wang, G.-C. Soil properties and corn (Zea mays L.) production under manure application combined with deep tillage management in solonetzic soils of Songnen Plain, Northeast China. J. Integr. Agric. 2016, 15, 879–890. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Liu, J.; Yuan, Z.; Hu, S.; Sun, C.; Du, J.; Ji, W.; Cao, G.; Wang, Z. Effects of Straw Returning on Drought Tolerance and Growth Status of Maize Under Drought Stress in the Cold and Arid Regions of Northern China. Agronomy 2024, 14, 2580. [Google Scholar] [CrossRef]
- Maizels, M. Effect of sodium content on sodium efflux from human red cells suspended in sodium-free media containing potassium, rubidium, cesium, or lithium chloride. J. Physiol. 1968, 195, 657–679. [Google Scholar] [CrossRef]
- Ye, W.-P.; Xie, X.-L.; Wang, K.-R.; Li, Z.-G. Effects of rice straw manuring in different periods on growth and yield of rice. Chin. J. Rice Sci. 2008, 22, 65–70. [Google Scholar]
- Chen, C.-Q.; Hu, Q.-Y.; Sun, B.; Zhang, J.-B. Simulating trends in soil organic carbon in calcareous flavor aquic soil under long-term fertilization using the DNDC model. Plant Nutr. Fertil. Sci. 2010, 16, 1410–1417. [Google Scholar]
- Ren, W.-J.; Huang, Y.; Wu, J.-X.; Liu, D.-Y.; Yang, W.-Y. Effects of no-till agenda stubble-remaining on soil enzyme activities in broadcasting rice seedlings paddy field. J. Appl. Ecol. 2011, 22, 2913–2918. [Google Scholar]
- Zhou, W.; Chen, D.; Bu, Y.; Tu, N.M. Effects of rice straw inputing to the field on the metabolic diversity of soil microbial communities. Acta Sci. Circumstantiae 2008, 28, 326–330. [Google Scholar]
- Chen, D.; Yi, Z.; Zhou, W.; Tu, N. Effects of straw input on soil nutrients and microorganisms in late rice under different soil tillage systems. Acta Sci. Circumstantiae 2010, 30, 1722–1728. [Google Scholar]
- Lu, W.-T.; Jia, Z.-K.; Zhang, P.; Wang, W.; Hou, X.-Q.; Yang, B.-P.; Li, Y.-P. Effects of straw re-turning on soil labile organic carbon and enzyme activity in semi-arid areas of southern Ning Xia, China. J. Agro Environ. Sci. 2011, 30, 522–528. [Google Scholar]
- Han, W.; He, M. The application of exogenous cellulase to improve soil fertility and plant growth due to the acceleration of straw decomposition. Bioresour. Technol. 2010, 101, 3724–3731. [Google Scholar] [CrossRef]
- Tian, J.; Sun, C.; Yang, M.; Zhang, X. Studies on the Activities of Three Kinds of Soil Enzyme, Organic Matters, Microbes, and the Yields and Quality of Apples in Different Tree-aged Apple Orchards in Loess Plateau. J. Agric. Sci. Technol. 2012, 14, 115–122. [Google Scholar]
- Zhang, P.; Chen, X.; Wei, T.; Yang, Z.; Jia, Z.; Yang, B.; Han, Q.; Ren, X. Effects of straw incorporation on the soil nutrient contents, enzyme activities, and crop yield in a semiarid region of China. Soil Tillage Res. 2016, 160, 65–72. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Kunzová, E.; Hejcman, M. Yield development of winter wheat over 50 years of FYM, N, P and K fertilizer application on black earth soil in the Czech Republic. Field Crops Res. 2009, 111, 226–234. [Google Scholar] [CrossRef]
- García Sanchez, D. Decision making and the energy path towards carbon neutrality. The Case of Costa Rica. In Proceedings of the Berlin Conference on Human Dimensions of Global Environmental Change, Berlin, Germany, 5–6 October 2012. [Google Scholar] [CrossRef]
- Li, Z.P.; Han, F.X.; Su, Y.; Zhang, T.L.; Sun, B.; Monts, D.L.; Plodinec, M.J. Assessment of soil organic and carbonate carbon storage in China. Geoderma 2007, 138, 119–126. [Google Scholar] [CrossRef]


| Processing Code | Treatment | Amount of Straw Input to the Field (kg/hm2) |
|---|---|---|
| CK | Straw Not Input to Fields | 0 kg/hm2 |
| T1 | 1× Straw Input to Fields | 3500 kg/hm2 |
| T2 | 2× Straw Input to Fields | 7000 kg/hm2 |
| T3 | 4× Straw Input to Fields | 14,000 kg/hm2 |
| Treatment | 0–10 cm | 10–20 cm | 20–30 cm |
|---|---|---|---|
| CK | 4.33 ± 0.13 abcd | 4.22 ± 0.18 bcd | 3.35 ± 0.40 f |
| T1 | 4.45 ± 0.16 abc | 4.37 ± 0.16 abcd | 3.79 ± 0.08 e |
| T2 | 4.49 ± 0.10 abc | 4.47 ± 0.11 abc | 4.07 ± 0.14 de |
| T3 | 4.58 ± 0.07 a | 4.54 ± 0.03 ab | 4.20 ± 0.01 cd |
| Treatment | 0–10 cm | 10–20 cm | 20–30 cm |
|---|---|---|---|
| CK | 0.28 ± 0.01 abcd | 0.26 ± 0.01 cde | 0.25 ± 0.01 e |
| T1 | 0.27 ± 0.01 abcd | 0.27 ± 0.01 bcde | 0.25 ± 0.01 de |
| T2 | 0.29 ± 0.03 ab | 0.26 ± 0.00 cde | 0.25 ± 0.01 cde |
| T3 | 0.30 ± 0.01 a | 0.28 ± 0.01 abc | 0.27 ± 0.00 bcde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ren, X.; Cai, L. Effects of Different Straw Incorporation Amounts on Soil Organic Carbon, Microbial Biomass, and Enzyme Activities in Dry-Crop Farmland. Sustainability 2024, 16, 10588. https://doi.org/10.3390/su162310588
Zhang X, Ren X, Cai L. Effects of Different Straw Incorporation Amounts on Soil Organic Carbon, Microbial Biomass, and Enzyme Activities in Dry-Crop Farmland. Sustainability. 2024; 16(23):10588. https://doi.org/10.3390/su162310588
Chicago/Turabian StyleZhang, Xinyi, Xiaoyan Ren, and Liqun Cai. 2024. "Effects of Different Straw Incorporation Amounts on Soil Organic Carbon, Microbial Biomass, and Enzyme Activities in Dry-Crop Farmland" Sustainability 16, no. 23: 10588. https://doi.org/10.3390/su162310588
APA StyleZhang, X., Ren, X., & Cai, L. (2024). Effects of Different Straw Incorporation Amounts on Soil Organic Carbon, Microbial Biomass, and Enzyme Activities in Dry-Crop Farmland. Sustainability, 16(23), 10588. https://doi.org/10.3390/su162310588






