Electrochemical Methods for Nutrient Removal in Wastewater: A Review of Advanced Electrode Materials, Processes, and Applications
Abstract
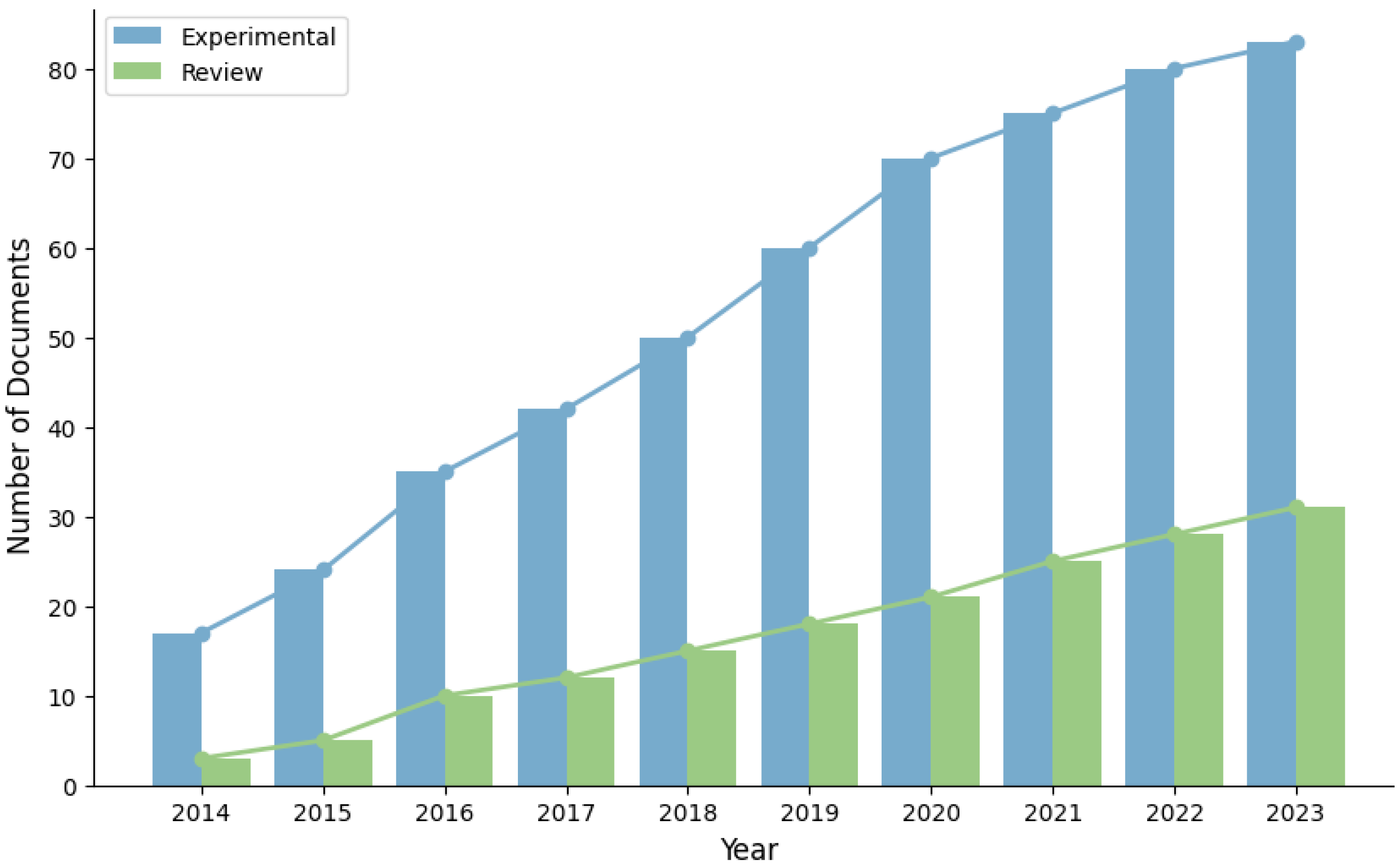
1. Introduction
2. Innovative Electrode Materials in Electrochemical Wastewater Treatment
3. Mechanisms of Nutrient Removal
3.1. Key Principles and Characteristics of Electrochemical Technology
3.2. Advanced Electrochemical Approaches for Nitrogenous and Phosphorus Compounds Removal
3.3. Electrochemical Ammonia Oxidation
3.4. Electrocoagulation for Phosphate Removal
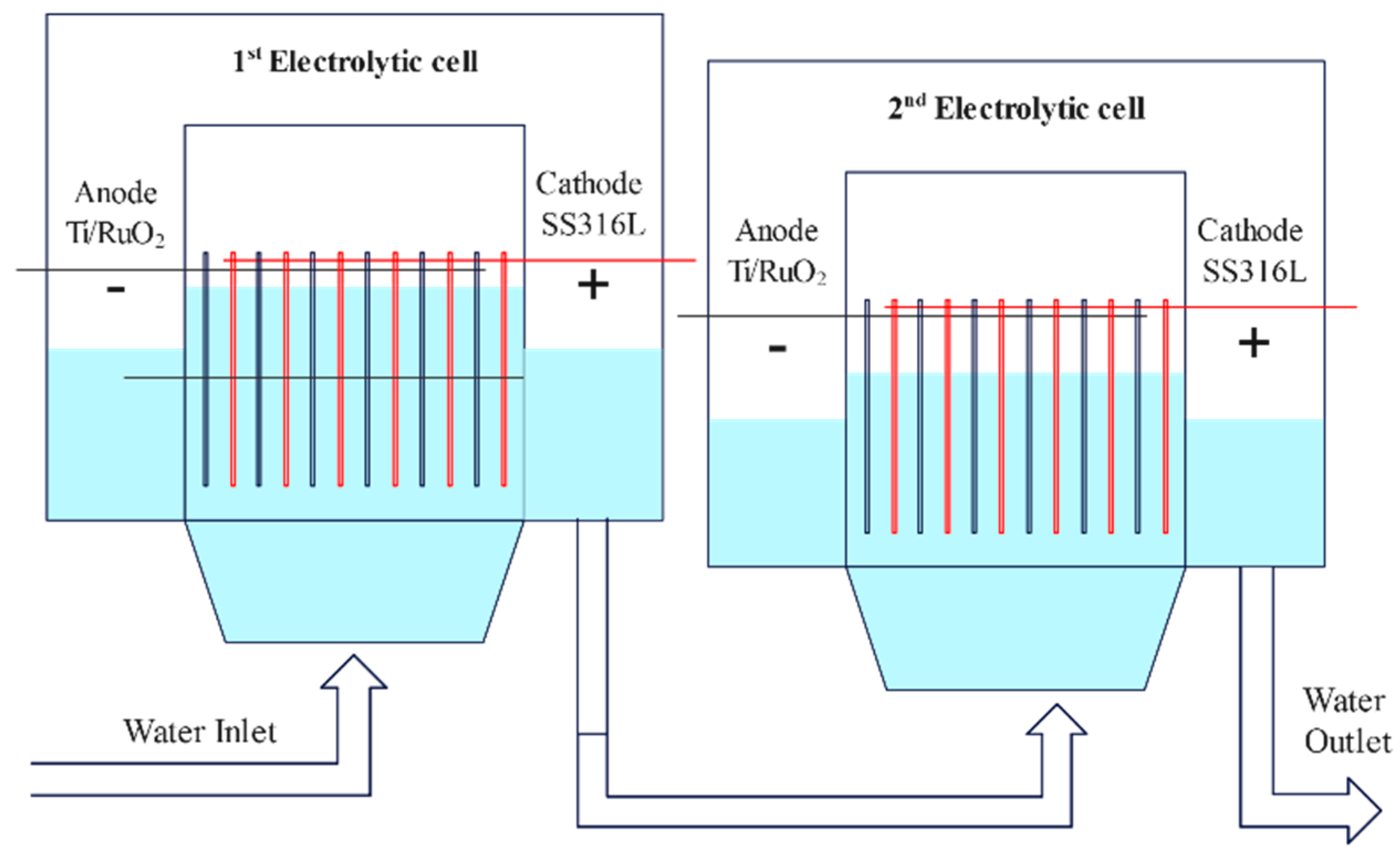
4. Practical Application Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devlin, M.; Brodie, J. Nutrients and Eutrophication. In Marine Pollution—Monitoring, Management and Mitigation; Reichelt-Brushett, A., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 75–100. ISBN 978-3-031-10127-4. [Google Scholar]
- Du, J.; Waite, T.D.; Feng, J.; Lei, Y.; Tang, W. Coupled Electrochemical Methods for Nitrogen and Phosphorus Recovery from Wastewater: A Review. Environ. Chem. Lett. 2023, 21, 885–909. [Google Scholar] [CrossRef]
- Moloantoa, K.M.; Khetsha, Z.P.; van Heerden, E.; Castillo, J.C.; Cason, E.D. Nitrate Water Contamination from Industrial Activities and Complete Denitrification as a Remediation Option. Water 2022, 14, 799. [Google Scholar] [CrossRef]
- Ansari, A.A.; Gill, S.S.; Khan, F.A. Eutrophication: Threat to Aquatic Ecosystems. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Singh Gill, S., Lanza, G.R., Rast, W., Eds.; SSBM Springer: Dordrecht, The Netherlands, 2010; pp. 143–170. ISBN 978-90-481-9625-8. [Google Scholar]
- Nthumbi, R.M.; Catherine Ngila, J.; Moodley, B.; Kindness, A.; Petrik, L. Application of Chitosan/Polyacrylamide Nanofibres for Removal of Chromate and Phosphate in Water. Phys. Chem. Earth Parts ABC 2012, 50–52, 243–251. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; He, P.-J.; Shao, L.-M. Insight into the Heavy Metal Binding Potential of Dissolved Organic Matter in MSW Leachate Using EEM Quenching Combined with PARAFAC Analysis. Water Res. 2011, 45, 1711–1719. [Google Scholar] [CrossRef]
- Kiedrzyńska, E.; Kiedrzyński, M.; Zalewski, M. Sustainable Floodplain Management for Flood Prevention and Water Quality Improvement. Nat. Hazards 2015, 76, 955–977. [Google Scholar] [CrossRef]
- European Commission Water Framework Directive—European Commission. Available online: https://ec.europa.eu/environment/water/water-framework/index_en.html (accessed on 27 July 2024).
- Wiering, M.; Boezeman, D.; Crabbé, A. The Water Framework Directive and Agricultural Diffuse Pollution: Fighting a Running Battle? Water 2020, 12, 1447. [Google Scholar] [CrossRef]
- Water Research Foundation Nutrient Removal Challenge. Available online: https://www.waterrf.org (accessed on 27 July 2024).
- Costa, J.M. Considerations on Electrochemical Technologies for Water Purification and Wastewater Treatment. Int. J. Environ. Res. Public Health 2023, 20, 6140. [Google Scholar] [CrossRef]
- Asaithambi, P.; Yesuf, M.B.; Govindarajan, R.; Hariharan, N.M.; Thangavelu, P.; Alemayehu, E. A Review of Hybrid Process Development Based on Electrochemical and Advanced Oxidation Processes for the Treatment of Industrial Wastewater. Int. J. Chem. Eng. 2022, 2022, 1105376. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Zhou, Z.; Sharma, V.K. Occurrence, Transportation, Monitoring and Treatment of Emerging Micro-Pollutants in Waste Water—A Review from Global Views. Microchem. J. 2013, 110, 292–300. [Google Scholar] [CrossRef]
- Qiao, J.; Xiong, Y. Electrochemical Oxidation Technology: A Review of Its Application in High-Efficiency Treatment of Wastewater Containing Persistent Organic Pollutants. J. Water Process Eng. 2021, 44, 102308. [Google Scholar] [CrossRef]
- Ken, D.S.; Sinha, A. Dimensionally Stable Anode (Ti/RuO2) Mediated Electro-Oxidation and Multi-Response Optimization Study for Remediation of Coke-Oven Wastewater. J. Environ. Chem. Eng. 2021, 9, 105025. [Google Scholar] [CrossRef]
- Merma, A.G.; Santos, B.F.; Rego, A.S.C.; Hacha, R.R.; Torem, M.L. Treatment of Oily Wastewater from Mining Industry Using Electrocoagulation: Fundamentals and Process Optimization. J. Mater. Res. Technol. 2020, 9, 15164–15176. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Yu, G.; Deng, S. Removal of Low Concentrations of Nickel Ions in Electroplating Wastewater by Combination of Electrodialysis and Electrodeposition. Chemosphere 2021, 263, 128208. [Google Scholar] [CrossRef] [PubMed]
- Yakamercan, E.; Bhatt, P.; Aygun, A.; Adesope, A.W.; Simsek, H. Comprehensive Understanding of Electrochemical Treatment Systems Combined with Biological Processes for Wastewater Remediation. Environ. Pollut. 2023, 330, 121680. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Huang, H.; Tan, Y.; Chen, G.; Hao, T. Emerging Electrochemistry-Based Process for Sludge Treatment and Resources Recovery: A Review. Water Res. 2022, 209, 117939. [Google Scholar] [CrossRef]
- García-Espinoza, J.D.; Robles, I.; Durán-Moreno, A.; Godínez, L.A. Photo-Assisted Electrochemical Advanced Oxidation Processes for the Disinfection of Aqueous Solutions: A Review. Chemosphere 2021, 274, 129957. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z.; Fang, S.; Song, B.; Cao, P.; Liu, H.; Yang, Y. Removal and Toxic Forecast of Microplastics Treated by Electrocoagulation: Influence of Dissolved Organic Matter. Chemosphere 2022, 308, 136309. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Hasan, M.N.; Altaf, M.M.; Khan, N.A.; Khan, A.H.; Khan, A.A.; Ahmed, S.; Kumar, P.S.; Naushad, M.; Rajapaksha, A.U.; Iqbal, J.; et al. Recent Technologies for Nutrient Removal and Recovery from Wastewaters: A Review. Chemosphere 2021, 277, 130328. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Li, C.; Tian, F.; Gao, N. Comparative Evaluation of Metoprolol Degradation by UV/Chlorine and UV/H2O2 Processes. Chemosphere 2020, 243, 125325. [Google Scholar] [CrossRef]
- International Water Association (IWA) 2024 IWA Nutrient Removal and Recovery Specialist Conference. Available online: https://iwa-network.org/ (accessed on 27 July 2024).
- Zheng, Y.; Wan, Y.; Zhang, Y.; Huang, J.; Yang, Y.; Tsang, D.C.W.; Wang, H.; Chen, H.; Gao, B. Recovery of Phosphorus from Wastewater: A Review Based on Current Phosphorous Removal Technologies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1148–1172. [Google Scholar] [CrossRef] [PubMed]
- Brião, G.d.V.; da Costa, T.B.; Antonelli, R.; Costa, J.M. Electrochemical Processes for the Treatment of Contaminant-Rich Wastewater: A Comprehensive Review. Chemosphere 2024, 355, 141884. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Brillas, E. Remediation of Water Pollution Caused by Pharmaceutical Residues Based on Electrochemical Separation and Degradation Technologies: A Review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, P.; Li, J. Electrocoagulation Technology for Water Purification: An Update Review on Reactor Design and Some Newly Concerned Pollutants Removal. J. Environ. Manag. 2021, 296, 113259. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Matis, K.A. Electroflotation Process: A Review. J. Mol. Liq. 2016, 220, 657–664. [Google Scholar] [CrossRef]
- Sivasubramanian, P.; Kumar, M.; Kirankumar, V.S.; Samuel, M.S.; Dong, C.-D.; Chang, J.-H. Capacitive Deionization and Electrosorption Techniques with Different Electrodes for Wastewater Treatment Applications. Desalination 2023, 559, 116652. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Li, Y.; Yin, L. Application of Advanced Oxidation Processes for the Removal of Micro/Nanoplastics from Water: A Review. Chemosphere 2024, 346, 140636. [Google Scholar] [CrossRef]
- Ma, J.; Gao, M.; Shi, H.; Ni, J.; Xu, Y.; Wang, Q. Progress in Research and Development of Particle Electrodes for Three-Dimensional Electrochemical Treatment of Wastewater: A Review. Environ. Sci. Pollut. Res. Int. 2021, 28, 47800–47824. [Google Scholar] [CrossRef]
- Pearce, J.M. Photovoltaics—A Path to Sustainable Futures. Futures 2002, 34, 663–674. [Google Scholar] [CrossRef]
- Staff, C.B. Solar Is Now ‘Cheapest Electricity in History’, Confirms IEA. Available online: https://www.carbonbrief.org/solar-is-now-cheapest-electricity-in-history-confirms-iea/ (accessed on 23 July 2024).
- Pérez-Arriaga, I.J.; Batlle, C. Impacts of Intermittent Renewables on Electricity Generation System Operation. Econ. Energy Environ. Policy 2012, 1, 3–18. [Google Scholar] [CrossRef]
- Jing, X.; Wang, X.; Li, X.; Wang, D.; Xu, H.; Yan, W. Progress in the Preparation of Metal Oxide Electrodes for the Electrochemical Treatment of Organic Wastewater: A Short Review. Catalysts 2023, 13, 1096. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Loizidou, M.; Haralambous, K.-J. Stainless Steel in Thermal Desalination and Brine Treatment: Current Status and Prospects. Met. Mater. Int. 2020, 26, 1463–1482. [Google Scholar] [CrossRef]
- Ram, C.; Zaman, B.; Dhir, A. Study on Corrosion Investigations in Industrial Effluents: A Review. Corros. Rev. 2019, 37, 115–130. [Google Scholar] [CrossRef]
- Shestakova, M.; Sillanpää, M. Electrode Materials Used for Electrochemical Oxidation of Organic Compounds in Wastewater. Rev. Environ. Sci. Biotechnol. 2017, 16, 223–238. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Anglada, Á.; Urtiaga, A.; Ortiz, I. Contributions of Electrochemical Oxidation to Waste-Water Treatment: Fundamentals and Review of Applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Comninellis, C.; Chen, G. (Eds.) Electrochemistry for the Environment; Springer: New York, NY, USA, 2010; ISBN 978-0-387-36922-8. [Google Scholar]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical Oxidation of Organic Pollutants for the Wastewater Treatment: Direct and Indirect Processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Ramalho, A.M.Z.; Martínez-Huitle, C.A.; Silva, D.R. da Application of Electrochemical Technology for Removing Petroleum Hydrocarbons from Produced Water Using a DSA-Type Anode at Different Flow Rates. Fuel 2010, 89, 531–534. [Google Scholar] [CrossRef]
- Ellouze, S.; Panizza, M.; Barbucci, A.; Cerisola, G.; Mhiri, T.; Elaoud, S.C. Ferulic Acid Treatment by Electrochemical Oxidation Using a BDD Anode. J. Taiwan Inst. Chem. Eng. 2016, 59, 132–137. [Google Scholar] [CrossRef]
- Kuang, P.; Natsui, K.; Feng, C.; Einaga, Y. Electrochemical Reduction of Nitrate on Boron-Doped Diamond Electrodes: Effects of Surface Termination and Boron-Doping Level. Chemosphere 2020, 251, 126364. [Google Scholar] [CrossRef]
- Kang, W.; Yan, L.; Tang, J.; Wu, S.; Yu, H.; Li, Z. Electrochemical Activation of Graphite Electrode for Nitrate Reduction: Energetic Performance and Application Potential. Appl. Catal. B Environ. 2023, 329, 122553. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Sheikhshoaie, I.; Nugraha, A.S.; Jang, H.W.; Yamauchi, Y.; Shokouhimehr, M. Performance of Metal–Organic Frameworks in the Electrochemical Sensing of Environmental Pollutants. J. Mater. Chem. A 2021, 9, 8195–8220. [Google Scholar] [CrossRef]
- Li, B.; Meng, Y.; Lin, X.; Tu, D.; Shen, S.; He, T.; Li, Y.; Zhang, H. Enhanced Photo-Electro-Fenton Degradation Performance Using Graphene Structure-Oriented MOFs (2Fe/Co)/CNF Cathode Membrane. J. Clean. Prod. 2023, 401, 136782. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical Review of Electrochemical Advanced Oxidation Processes for Water Treatment Applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Castro, R.S.d.S.; Dória, A.R.; Ferreira, M.B.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Chapter Eight—Advancements in Mixed Metal Oxide Anodes for Efficient Electrochemical Treatment of Wastewater. In Advances in Chemical Pollution, Environmental Management and Protection; Kumar, A., Bilal, M., Ferreira, L.F.R., Eds.; Recent Advancements in Wastewater Management: Nano-Based Remediation; Elsevier: Amsterdam, The Netherlands, 2024; Volume 10, pp. 191–218. [Google Scholar]
- Ben Osman, Y.; Hajjar-Garreau, S.; Berling, D.; Akrout, H. Elaboration of Highly Modified Stainless Steel/Lead Dioxide Anodes for Enhanced Electrochemical Degradation of Ampicillin in Water. Separations 2023, 10, 5. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Ghasemian, S.; Zhang, N.; Yagar, M.; de Lannoy, C.-F. Mixed Metal Oxide Anodes Used for the Electrochemical Degradation of a Real Mixed Industrial Wastewater. Chemosphere 2022, 286, 131600. [Google Scholar] [CrossRef]
- El-Kacemi, S.; Zazou, H.; Oturan, N.; Dietze, M.; Hamdani, M.; Es-Souni, M.; Oturan, M.A. Nanostructured ZnO-TiO2 Thin Film Oxide as Anode Material in Electrooxidation of Organic Pollutants. Application to the Removal of Dye Amido Black 10B from Water. Environ. Sci. Pollut. Res. 2017, 24, 1442–1449. [Google Scholar] [CrossRef]
- Cheng, S.; Xiao, D.; Sun, Y. Efficient Treatment of High-Concentration Sulfurous Wastewater by Using Electrochemical Oxidation Process with Ti/SnO2–Sb Anode and Air Cathode. SN Appl. Sci. 2020, 2, 517. [Google Scholar] [CrossRef]
- De Luna, Y.; Bensalah, N. Review on the Electrochemical Oxidation of Endocrine-Disrupting Chemicals Using BDD Anodes. Curr. Opin. Electrochem. 2022, 32, 100900. [Google Scholar] [CrossRef]
- Labiadh, L.; Barbucci, A.; Carpanese, M.P.; Gadri, A.; Ammar, S.; Panizza, M. Comparative Depollution of Methyl Orange Aqueous Solutions by Electrochemical Incineration Using TiRuSnO2, BDD and PbO2 as High Oxidation Power Anodes. J. Electroanal. Chem. 2016, 766, 94–99. [Google Scholar] [CrossRef]
- Urtiaga, A.; Fernandez-Castro, P.; Gómez, P.; Ortiz, I. Remediation of Wastewaters Containing Tetrahydrofuran. Study of the Electrochemical Mineralization on BDD Electrodes. Chem. Eng. J. 2014, 239, 341–350. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.H.; Park, H. Electrocatalytic Activity of Metal-Doped SnO2 for the Decomposition of Aqueous Contaminants: Ta-SnO2 vs. Sb-SnO2. Chem. Eng. J. 2021, 409, 128175. [Google Scholar] [CrossRef]
- Yang, S.Y.; Choo, Y.S.; Kim, S.; Lim, S.K.; Lee, J.; Park, H. Boosting the Electrocatalytic Activities of SnO2 Electrodes for Remediation of Aqueous Pollutants by Doping with Various Metals. Appl. Catal. B Environ. 2012, 111–112, 317–325. [Google Scholar] [CrossRef]
- Duan, X.; Ma, F.; Yuan, Z.; Chang, L.; Jin, X. Electrochemical Degradation of Phenol in Aqueous Solution Using PbO2 Anode. J. Taiwan Inst. Chem. Eng. 2013, 44, 95–102. [Google Scholar] [CrossRef]
- Pereira, G.F.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electrochemical Degradation of the Herbicide Picloram Using a Filter-Press Flow Reactor with a Boron-Doped Diamond or β-PbO2 Anode. Electrochim. Acta 2015, 179, 588–598. [Google Scholar] [CrossRef]
- Abdulgani, I.; Escalona-Durán, F.; de Araújo, D.M.; dos Santos, E.V.; Barbosa Segundo, I.D.; Martínez-Huitle, C.A. The Role of Saline-Related Species in the Electrochemical Treatment of Produced Water Using Ti/IrO2-Ta2O5 Anode. J. Electroanal. Chem. 2022, 910, 116163. [Google Scholar] [CrossRef]
- da Silva, A.J.C.; dos Santos, E.V.; de Oliveira Morais, C.C.; Martínez-Huitle, C.A.; Castro, S.S.L. Electrochemical Treatment of Fresh, Brine and Saline Produced Water Generated by Petrochemical Industry Using Ti/IrO2–Ta2O5 and BDD in Flow Reactor. Chem. Eng. J. 2013, 233, 47–55. [Google Scholar] [CrossRef]
- Aquino Neto, S.; de Andrade, A.R. Electrooxidation of Glyphosate Herbicide at Different DSA® Compositions: pH, Concentration and Supporting Electrolyte Effect. Electrochim. Acta 2009, 54, 2039–2045. [Google Scholar] [CrossRef]
- Panakoulias, T.; Kalatzis, P.; Kalderis, D.; Katsaounis, A. Electrochemical Degradation of Reactive Red 120 Using DSA and BDD Anodes. J. Appl. Electrochem. 2010, 40, 1759–1765. [Google Scholar] [CrossRef]
- Rabiee, H.; Ge, L.; Zhang, X.; Hu, S.; Li, M.; Yuan, Z. Gas Diffusion Electrodes (GDEs) for Electrochemical Reduction of Carbon Dioxide, Carbon Monoxide, and Dinitrogen to Value-Added Products: A Review. Energy Environ. Sci. 2021, 14, 1959–2008. [Google Scholar] [CrossRef]
- Molla Nadali Pishnamaz, H.; Farimaniraad, H.; Baghdadi, M.; Aminzadeh Goharrizi, B.; Mahpishanian, S. Application of Nickel Foam Cathode Modified by Single-Wall Carbon Nanotube in Electro-Fenton Process Coupled with Anodic Oxidation: Enhancing Organic Pollutants Removal. J. Electroanal. Chem. 2023, 929, 117130. [Google Scholar] [CrossRef]
- Zhou, W.; Rajic, L.; Chen, L.; Kou, K.; Ding, Y.; Meng, X.; Wang, Y.; Mulaw, B.; Gao, J.; Qin, Y.; et al. Activated Carbon as Effective Cathode Material in Iron-Free Electro-Fenton Process: Integrated H2O2 Electrogeneration, Activation, and Pollutants Adsorption. Electrochim. Acta 2019, 296, 317–326. [Google Scholar] [CrossRef]
- Ramesh, K.; Gnanavel, B.; Shkir, M. Enhanced Visible Light Photocatalytic Degradation of Bisphenol A (BPA) by Reduced Graphene Oxide (RGO)–Metal Oxide (TiO2, ZnO and WO3) Based Nanocomposites. Diam. Relat. Mater. 2021, 118, 108514. [Google Scholar] [CrossRef]
- El-Ghenymy, A.; Alsheyab, M.; Khodary, A.; Sirés, I.; Abdel-Wahab, A. Corrosion Behavior of Pure Titanium Anodes in Saline Medium and Their Performance for Humic Acid Removal by Electrocoagulation. Chemosphere 2020, 246, 125674. [Google Scholar] [CrossRef]
- Singh, T.S.A.; Ramesh, S.T. An Experimental Study of CI Reactive Blue 25 Removal from Aqueous Solution by Electrocoagulation Using Aluminum Sacrificial Electrode: Kinetics and Influence of Parameters on Electrocoagulation Performance. Desalination Water Treat. 2014, 52, 2634–2642. [Google Scholar] [CrossRef]
- Govindan, K.; Angelin, A.; Kalpana, M.; Rangarajan, M.; Shankar, P.; Jang, A. Electrocoagulants Characteristics and Application of Electrocoagulation for Micropollutant Removal and Transformation Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 1775–1788. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Zhang, C. Generation and Application of Reactive Chlorine Species by Electrochemical Process Combined with UV Irradiation: Synergistic Mechanism for Enhanced Degradation Performance. Sci. Total Environ. 2020, 712, 136501. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.Y.; Yoon, J. The Role of Reactive Oxygen Species in the Electrochemical Inactivation of Microorganisms. Environ. Sci. Technol. 2006, 40, 6117–6122. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Qian, A.; Yuan, S.; Xie, S.; Tong, M.; Zhang, P.; Zheng, Y. Oxidizing Capacity of Iron Electrocoagulation Systems for Refractory Organic Contaminant Transformation. Environ. Sci. Technol. 2019, 53, 12629–12638. [Google Scholar] [CrossRef]
- Luo, R.; Wang, C.; Yao, Y.; Qi, J.; Li, J. Insights into the Relationship of Reactive Oxygen Species and Anions in Persulfate-Based Advanced Oxidation Processes for Saline Organic Wastewater Treatment. Environ. Sci. Water Res. Technol. 2022, 8, 465–483. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key Role of Persistent Free Radicals in Hydrogen Peroxide Activation by Biochar: Implications to Organic Contaminant Degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [Google Scholar] [CrossRef]
- Massima Mouele, E.S.; Fatoba, O.O.; Babajide, O.; Badmus, K.O.; Petrik, L.F. Review of the Methods for Determination of Reactive Oxygen Species and Suggestion for Their Application in Advanced Oxidation Induced by Dielectric Barrier Discharges. Environ. Sci. Pollut. Res. 2018, 25, 9265–9282. [Google Scholar] [CrossRef]
- Islam, S.M.D.-U. Electrocoagulation (EC) Technology for Wastewater Treatment and Pollutants Removal. Sustain. Water Resour. Manag. 2019, 5, 359–380. [Google Scholar] [CrossRef]
- Zhang, G.; Ruan, J.; Du, T. Recent Advances on Photocatalytic and Electrochemical Oxidation for Ammonia Treatment from Water/Wastewater. ACS EST Eng. 2021, 1, 310–325. [Google Scholar] [CrossRef]
- Wittbrodt, B.T.; Squires, D.A.; Walbeck, J.; Campbell, E.; Campbell, W.H.; Pearce, J.M. Open-Source Photometric System for Enzymatic Nitrate Quantification. PLoS ONE 2015, 10, e0134989. [Google Scholar] [CrossRef]
- Hu, Q.; He, L.; Lan, R.; Feng, C.; Pei, X. Recent Advances in Phosphate Removal from Municipal Wastewater by Electrocoagulation Process: A Review. Sep. Purif. Technol. 2023, 308, 122944. [Google Scholar] [CrossRef]
- Yuan, J.; Cheng, X.; Wang, H.; Lei, C.; Pardiwala, S.; Yang, B.; Li, Z.; Zhang, Q.; Lei, L.; Wang, S.; et al. A Superaerophobic Bimetallic Selenides Heterostructure for Efficient Industrial-Level Oxygen Evolution at Ultra-High Current Densities. Nano-Micro Lett. 2020, 12, 104. [Google Scholar] [CrossRef]
- Wallace, S.W.; McCrum, I.T.; Janik, M.J. Ammonia Electro-Oxidation Mechanism on the Platinum (100) Surface. Catal. Today 2021, 371, 50–57. [Google Scholar] [CrossRef]
- Jarrah, N.; Mu’azu, N.D. Simultaneous Electro-Oxidation of Phenol, CN−, S2− and NH4+ in Synthetic Wastewater Using Boron Doped Diamond Anode. J. Environ. Chem. Eng. 2016, 4, 2656–2664. [Google Scholar] [CrossRef]
- Hashim, K.S.; Al Khaddar, R.; Jasim, N.; Shaw, A.; Phipps, D.; Kot, P.; Pedrola, M.O.; Alattabi, A.W.; Abdulredha, M.; Alawsh, R. Electrocoagulation as a Green Technology for Phosphate Removal from River Water. Sep. Purif. Technol. 2019, 210, 135–144. [Google Scholar] [CrossRef]
- Takabe, Y.; Fujiyama, M.; Yamasaki, Y.; Masuda, T. Influences of Electrode Distance and Electrolysis Time on Phosphorus Precipitation and Composition during Electrolysis of Anaerobic Digestion Effluent. Sci. Total Environ. 2022, 803, 150114. [Google Scholar] [CrossRef]
- Omwene, P.I.; Kobya, M. Treatment of Domestic Wastewater Phosphate by Electrocoagulation Using Fe and Al Electrodes: A Comparative Study. Process Saf. Environ. Prot. 2018, 116, 34–51. [Google Scholar] [CrossRef]
- He, S.-L.; Huang, Q.; Zhang, Y.; Nie, Y.-L. Evaluation of the Performance of Different Anodes in the Electrochemical Oxidation of Ammonia. Water. Air. Soil Pollut. 2015, 226, 89. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, Y.; Chen, H.M.; Hu, Z.; Hung, S.; Ma, N.; Dai, J.; Lin, H.; Chen, C.; Zhou, W.; et al. An Amorphous Nickel–Iron-Based Electrocatalyst with Unusual Local Structures for Ultrafast Oxygen Evolution Reaction. Adv. Mater. 2019, 31, 1900883. [Google Scholar] [CrossRef]
- Paul, R.; Zhu, L.; Chen, H.; Qu, J.; Dai, L. Recent Advances in Carbon-Based Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, 1806403. [Google Scholar] [CrossRef]
- Kearney, D.; Dugauguez, O.; Bejan, D.; Bunce, N.J. Electrochemical Oxidation for Denitrification of Ammonia: A Conceptual Approach for Remediation of Ammonia in Poultry Barns. ACS Sustain. Chem. Eng. 2013, 1, 190–197. [Google Scholar] [CrossRef]
- Kim, K.-W.; Kim, Y.-J.; Kim, I.-T.; Park, G.-I.; Lee, E.-H. Electrochemical Conversion Characteristics of Ammonia to Nitrogen. Water Res. 2006, 40, 1431–1441. [Google Scholar] [CrossRef]
- Cho, K.; Qu, Y.; Kwon, D.; Zhang, H.; Cid, C.A.; Aryanfar, A.; Hoffmann, M.R. Effects of Anodic Potential and Chloride Ion on Overall Reactivity in Electrochemical Reactors Designed for Solar-Powered Wastewater Treatment. Environ. Sci. Technol. 2014, 48, 2377–2384. [Google Scholar] [CrossRef]
- Jasper, J.T.; Shafaat, O.S.; Hoffmann, M.R. Electrochemical Transformation of Trace Organic Contaminants in Latrine Wastewater. Environ. Sci. Technol. 2016, 50, 10198–10208. [Google Scholar] [CrossRef]
- Yang, Y.; Shin, J.; Jasper, J.T.; Hoffmann, M.R. Multilayer Heterojunction Anodes for Saline Wastewater Treatment: Design Strategies and Reactive Species Generation Mechanisms. Environ. Sci. Technol. 2016, 50, 8780–8787. [Google Scholar] [CrossRef]
- Yang, Y.; Kao, L.C.; Liu, Y.; Sun, K.; Yu, H.; Guo, J.; Liou, S.Y.H.; Hoffmann, M.R. Cobalt-Doped Black TiO2 Nanotube Array as a Stable Anode for Oxygen Evolution and Electrochemical Wastewater Treatment. ACS Catal. 2018, 8, 4278–4287. [Google Scholar] [CrossRef]
- Yuan, M.; Sun, J.; Wu, Y.; Zheng, M.; Sheng, C.; Wu, C.; Wang, Q. Enhanced Electroreduction of Low-Concentration Nitrate Using a Cu-Pd Bimetallic Cathode System: Performance Evaluation, Mechanism Analysis, and Potential Applications. J. Environ. Chem. Eng. 2024, 12, 113031. [Google Scholar] [CrossRef]
- Little, D.J.; Milton, R.; Smith, I.I.I.; Hamann, T.W. Electrolysis of Liquid Ammonia for Hydrogen Generation. Energy Environ. Sci. 2015, 8, 2775–2781. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical Technologies for Wastewater Treatment and Resource Reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Mao, R.; Shi, Y.; Lin, S.; Qiao, M.; Zhao, X. Removal of Phosphate in Secondary Effluent from Municipal Wastewater Treatment Plant by Iron and Aluminum Electrocoagulation: Efficiency and Mechanism. Sep. Purif. Technol. 2022, 286, 120439. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Qudah, Y.; Assirey, E. Combined Biological Wastewater Treatment with Electrocoagulation as a Post-Polishing Process: A Review. Sep. Sci. Technol. 2020, 55, 2334–2352. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Y.; Cotterill, S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water 2023, 15, 1455. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Hamdani, M. Removal of Persistent Organic Pollutants (POPs) from Water and Wastewater by Adsorption and Electrocoagulation Process. Groundw. Sustain. Dev. 2021, 13, 100575. [Google Scholar] [CrossRef]
- Abdulhadi, B.; Kot, P.; Hashim, K.; Shaw, A.; Muradov, M.; Al-Khaddar, R. Continuous-Flow Electrocoagulation (EC) Process for Iron Removal from Water: Experimental, Statistical and Economic Study. Sci. Total Environ. 2021, 760, 143417. [Google Scholar] [CrossRef]
- Sari-Erkan, H. Wastewater Treatment from the Biodiesel Production Using Waste Cooking Oil by Electrocoagulation: A Multivariate Approach. Water Sci. Technol. 2019, 79, 2366–2377. [Google Scholar] [CrossRef]
- Criado, S.P.; Gonçalves, M.J.; Ballod Tavares, L.B.; Bertoli, S.L. Optimization of Electrocoagulation Process for Disperse and Reactive Dyes Using the Response Surface Method with Reuse Application. J. Clean. Prod. 2020, 275, 122690. [Google Scholar] [CrossRef]
- Ni’am, M.F.; Othman, F. Experimental Design of Electrocoagulation and Magnetic Technology for Enhancing Suspended Solids Removal from Synthetic Wastewater. Int. J. Sci. Eng. 2014, 7, 178–192. [Google Scholar] [CrossRef][Green Version]
- Rahman, N.A.; Jol, C.J.; Linus, A.A.; Ismail, V. Emerging Application of Electrocoagulation for Tropical Peat Water Treatment: A Review. Chem. Eng. Process.-Process Intensif. 2021, 165, 108449. [Google Scholar] [CrossRef]
- Bakshi, A.; Verma, A.K.; Dash, A.K. Electrocoagulation for Removal of Phosphate from Aqueous Solution: Statistical Modeling and Techno-Economic Study. J. Clean. Prod. 2020, 246, 118988. [Google Scholar] [CrossRef]
- Zeng, J.; Ji, M.; Zhao, Y.; Helmer Pedersen, T.; Wang, H. Optimization of Electrocoagulation Process Parameters for Enhancing Phosphate Removal in a Biofilm-Electrocoagulation System. Water Sci. Technol. 2021, 83, 2560–2574. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A Comprehensive Review of Electrocoagulation for Water Treatment: Potentials and Challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Al-Raad, A.A.; Hanafiah, M.M. Removal of Inorganic Pollutants Using Electrocoagulation Technology: A Review of Emerging Applications and Mechanisms. J. Environ. Manag. 2021, 300, 113696. [Google Scholar] [CrossRef]
- Ghaffarian Khorram, A.; Fallah, N. Comparison of Electrocoagulation and Photocatalytic Process for Treatment of Industrial Dyeing Wastewater: Energy Consumption Analysis. Environ. Prog. Sustain. Energy 2020, 39, 13288. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical Advanced Oxidation Processes: Today and Tomorrow. A Review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Muddemann, T.; Neuber, R.; Haupt, D.; Graßl, T.; Issa, M.; Bienen, F.; Enstrup, M.; Möller, J.; Matthée, T.; Sievers, M.; et al. Improving the Treatment Efficiency and Lowering the Operating Costs of Electrochemical Advanced Oxidation Processes. Processes 2021, 9, 1482. [Google Scholar] [CrossRef]
- Pervez, M.d.N.; Balakrishnan, M.; Hasan, S.W.; Choo, K.-H.; Zhao, Y.; Cai, Y.; Zarra, T.; Belgiorno, V.; Naddeo, V. A Critical Review on Nanomaterials Membrane Bioreactor (NMs-MBR) for Wastewater Treatment. NPJ Clean Water 2020, 3, 43. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z.; Vu, M.T.; Mohammed, J.A.H.; Pathak, N.; Nghiem, L.D.; Nguyen, L.N. Membrane Bioreactor for Wastewater Treatment: Current Status, Novel Configurations and Cost Analysis. In Cost-Efficient Wastewater Treatment Technologies: Engineered Systems; Nasr, M., Negm, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 147–167. ISBN 978-3-031-12902-5. [Google Scholar]
- Clematis, D.; Delucchi, M.; Panizza, M. Electrochemical Technologies for Wastewater Treatment at Pilot Plant Scale. Curr. Opin. Electrochem. 2023, 37, 101172. [Google Scholar] [CrossRef]
- Urtiaga, A.; Rueda, A.; Anglada, Á.; Ortiz, I. Integrated Treatment of Landfill Leachates Including Electrooxidation at Pilot Plant Scale. J. Hazard. Mater. 2009, 166, 1530–1534. [Google Scholar] [CrossRef]
- Durán, F.E.; de Araújo, D.M.; do Nascimento Brito, C.; Santos, E.V.; Ganiyu, S.O.; Martínez-Huitle, C.A. Electrochemical Technology for the Treatment of Real Washing Machine Effluent at Pre-Pilot Plant Scale by Using Active and Non-Active Anodes. J. Electroanal. Chem. 2018, 818, 216–222. [Google Scholar] [CrossRef]
- Tawabini, B.S.; Plakas, K.V.; Karabelas, A.J. A Pilot Study of BTEX Removal from Highly Saline Water by an Advanced Electrochemical Process. J. Water Process Eng. 2020, 37, 101427. [Google Scholar] [CrossRef]
- Bugueño-Carrasco, S.; Monteil, H.; Toledo-Neira, C.; Sandoval, M.Á.; Thiam, A.; Salazar, R. Elimination of Pharmaceutical Pollutants by Solar Photoelectro-Fenton Process in a Pilot Plant. Environ. Sci. Pollut. Res. 2021, 28, 23753–23766. [Google Scholar] [CrossRef]
- Alcaide, F.; Álvarez, G.; Guelfi, D.R.V.; Brillas, E.; Sirés, I. A Stable CoSP/MWCNTs Air-Diffusion Cathode for the Photoelectro-Fenton Degradation of Organic Pollutants at Pre-Pilot Scale. Chem. Eng. J. 2020, 379, 122417. [Google Scholar] [CrossRef]
- dos Santos, A.J.; Cabot, P.L.; Brillas, E.; Sirés, I. A Comprehensive Study on the Electrochemical Advanced Oxidation of Antihypertensive Captopril in Different Cells and Aqueous Matrices. Appl. Catal. B Environ. 2020, 277, 119240. [Google Scholar] [CrossRef]
- Roccamante, M.; Salmerón, I.; Ruiz, A.; Oller, I.; Malato, S. New Approaches to Solar Advanced Oxidation Processes for Elimination of Priority Substances Based on Electrooxidation and Ozonation at Pilot Plant Scale. Catal. Today 2020, 355, 844–850. [Google Scholar] [CrossRef]
- Salmerón, I.; Rivas, G.; Oller, I.; Martínez-Piernas, A.; Agüera, A.; Malato, S. Nanofiltration Retentate Treatment from Urban Wastewater Secondary Effluent by Solar Electrochemical Oxidation Processes. Sep. Purif. Technol. 2021, 254, 117614. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.; Picos-Benítez, A.; Bravo-Yumi, N.; Perez-Segura, T.; Bandala, E.R.; Peralta-Hernández, J.M. Electro-Fenton Mineralization of Diazo Dye Black NT2 Using a Pre-Pilot Flow Plant. J. Electroanal. Chem. 2021, 895, 115492. [Google Scholar] [CrossRef]
- Meng, X.; Zeng, P.; Lin, S.; Bao, H.; Wu, M.; Yang, L.; Jing, G.; Han, H.; Zhang, C.; Jiang, X.; et al. Removal of Chemical Oxygen Demand and Ammonia Nitrogen from High Salinity Tungsten Smelting Wastewater by One-Step Electrochemical Oxidation: From Bench-Scale Test, Pilot-Scale Test, to Industrial Test. J. Environ. Manag. 2023, 340, 117983. [Google Scholar] [CrossRef] [PubMed]
- Mishima, I.; Hama, M.; Tabata, Y.; Nakajima, J. Long-Term Investigation of Phosphorus Removal by Iron Electrocoagulation in Small-Scale Wastewater Treatment Plants. Water Sci. Technol. 2018, 78, 1304–1311. [Google Scholar] [CrossRef]
- Devlin, T.R.; di Biase, A.; Wei, V.; Elektorowicz, M.; Oleszkiewicz, J.A. Removal of Soluble Phosphorus from Surface Water Using Iron (Fe–Fe) and Aluminum (Al–Al) Electrodes. Environ. Sci. Technol. 2017, 51, 13825–13833. [Google Scholar] [CrossRef]
| Process | Application | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Electrocoagulation | Colloidal and suspended particles | Minimal chemical consumption Easy operation Low sludge production High removal efficiency Possibility of automation | Corrosion of the anode material Ineffective removal of complex pollutants Sludge generation | [18,29] |
| Electroflotation | Colloidal particles and suspended solids | Reduction in chemical consumption | Sludge generation Risk of corrosion Significant energy consumption | [30] |
| Electrodeionization | Ions and other charged species | Nonstop process Ultrapure water production | Formation of concentrate solution Sensitivity to feed water quality | [31] |
| Electrochemical Oxidation | Organic and recalcitrant compounds | Rapid reaction rate Small footprint Mineralization of organics Low need for additional chemical reagents or catalysts | High cost and safety requirements due to reactive compounds Large amount of sludge produced | [32] |
| Electrode Material | Key Properties and Advantages | Drawbacks and Issues | Efficiency /Applications | Ref. |
|---|---|---|---|---|
| Boron-Doped Diamond (BDD) |
|
| 96% COD removal for methyl orange; effective for refractory pollutants. | [57,58,59] |
| Tin dioxide (SnO2) |
|
| 85–90% COD removal; common in industrial wastewater treatment. | [60,61] |
| Lead dioxide (PbO2) |
|
| 79% TOC removal for dyes; used in chemical industry effluents. | [62,63] |
| Ti/IrO2–Ta2O5 |
|
| 79% COD removal in petrochemical wastewater. | [64,65] |
| Dimensionally Stable Anodes (DSA) |
|
| Used in industrial wastewater with complex organics. | [66,67] |
| Pt/Ti (Platinized Titanium) |
|
|
| [2,18] |
| Pt/Nb (Platinum on Niobium) |
|
|
| [2,18] |
| Electrode Material | Key Properties and Advantage | Drawbacks and Issues | Ref. |
|---|---|---|---|
| Gas Diffusion Electrode (GDE) |
|
| [68] |
| Carbon Nanotube (CNT) |
|
| [69] |
| Activated Carbon (AC) |
|
| [59,70] |
| Reduced Graphene Oxide (rGO) |
|
| [71] |
| Metal–Organic Frameworks (MOFs) |
|
| [50] |
| Pollutant | Anode Material | Electrolyte | Operating Conditions | Results | Ref. |
|---|---|---|---|---|---|
| Ammonia | BDD | 0.1 M Na2SO4 | Electrodes: BDD (7 cm2), Pt (7 cm2), and GC. pH: 6. Temperature: 25 °C Methods: Cyclic voltammetry and galvanostatic mode | Pt (100) showed faster ammonia oxidation due to the lower barrier of N2H4 formation via NH2 dimerization, compared to Pt (111). | [87] |
| 1100 mg/L Na2SO4 | Batch electrochemical reactor with BDD anode, graphite cathode, 100 mg/L phenol, 30 mA/cm2 current density, pH 4.8, 1100 mg/L Na2SO4, 210 min at 23 °C. | Maximum 100% TOC removal for single phenol, decreased to 90.8%, 87.49%, 82.35% in binary matrices. Presence of S2−, CN−, and NH4+ lowered TOC removal efficiency. | [88] | ||
| Phosphate | Aluminum baffle plates with perforations. | 150 mg/L K2HPO4 | Initial pH: 6 Interelectrode distance: 0.5 cm Current density: 6 mA/cm2 Electrolysis time: 60 min Temperature: 20 ± 1 °C | Phosphate removal efficiency > 99% at pH 4–8 for Al and Fe electrodes. Al electrodes showed higher efficiency and lower energy consumption compared to Fe. | [89] |
| Platinum-coated titanium | anaerobic digestion effluent | Batch electrolysis with Pt-coated Ti electrodes, 5L reactor, 4.0 A current (317 A/m2), 10, 5, and 1 mm electrode distance, 2 h electrolysis time, stirring. | P removal efficiency reduced with time. Initial precipitation as amorphous calcium phosphate (ACP), transforming to hydroxyapatite (HAP) over time. | [90] | |
| Aluminum (Al) and Iron (Fe) | Synthetic domestic wastewater | Initial pH: 4–7 Current density: 10–40 A/m2 EC time: 0–100 min Initial phosphorus concentration: 5.01–52.13 mg/L | Al electrodes more effective than Fe, achieving 99.9% removal at lower EC times and energy consumption. High P removal efficiency (>99%) at lower initial concentrations. | [91] |
| Approaches | Methodology Summary | Key Results | Ref. |
|---|---|---|---|
| Pt (100) facets for ammonia electrooxidation | DFT calculations on Pt (100) promote N-N bond formation, accelerating ammonia oxidation. | Demonstrated a lower energy barrier for NH4⁺ dimerization. | [87] |
| Electrochemical setup with platinum, palladium, or nickel electrodes | Setup in KOH/NaOH with controlled pH and temperature, using cyclic voltammetry and CA/CP analysis. | Identified key oxidation peaks and measured ammonia removal efficiency. | [101] |
| Electrolysis of liquid ammonia with platinum electrodes | Electrolysis in N,N-dimethylformamide with platinum electrodes under varying concentrations. | Observed electrode poisoning and hydrogen generation at the cathode. | [102] |
| Technology | Cost | Scalability | Nutrient Removal Efficiency | Energy Consumption (kWh/m3) |
|---|---|---|---|---|
| Electrochemical Advanced Oxidation (EAOPs) | Moderate to High | High (with optimization) | >90% COD removal, effective for diverse pollutants | 0.5–2.5 |
| Activated Sludge | Low | High | Moderate for nutrient removal | 0.3–1.0 |
| Membrane Bioreactors (MBRs) | High (Membrane costs) | Medium to High | High, >90% for organic removal | 1.0–2.5 |
| Ozone/UV/H2O2 | High (Chemical costs) | Medium | High (effective for organics) | 0.5–2.5 |
| Chemical Coagulation | Low | High | Moderate (limited to certain pollutants) | 0.2–0.8 |
| Anaerobic Digestion | Low | High | Effective for organic matter | 0.1–0.3 |
| Study | Year | Anode Material | Target Contaminants | Key Findings | Energy Consumption | Ref. |
|---|---|---|---|---|---|---|
| Anglada et al. | 2009 | BDD | Landfill leachates | Almost complete removal of organic matter and ammonium nitrogen | 35 kWh/m3 (120 kWh/kg COD) | [123] |
| Duran et al. | 2019 | Ti/Pt and BDD | Washing machine effluent | BDD showed >90% TOC removal; effective micropollutant remediation with low energy requirements | Not specified | [124] |
| Tawabini et al. | 2020 | BDD | BTEX, phenol in high salinity waters | Effective BTEX removal under optimal conditions | 5.92 kWh/m3 | [125] |
| Bugueño-Carrasco | 2020 | DSA | Antibiotics and NSAIDs | 99.2% TOC removal after 100 min; effective solar photoelectro-Fenton process | 7.15 kWh/m3 | [126] |
| Brillas Working Group | Multiple | RuO2-based, air-diffusion cathode, BDD | Urban wastewater with carbofuran | PEF more effective than EF or EO-H2O2; superior organic removal and mineralization | Not specified | [127,128] |
| Malato et al. | Multiple | GDE (cathode) and BDD (anode) | Model pesticides | Over 50% pesticide removal in 5 min under optimal conditions | Not specified | [129,130] |
| Villaseñor-Basulto et al. | 2020 | BDD and EF | Black NT2 dye | Paired BDD–electro-Fenton showed higher degradation rates; optimal conditions identified for EF process | 10.88 kWh/m3 | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Antonini, G.; Al-Omari, A.; Muller, C.; Mathew, J.; Bell, K.; Pearce, J.M.; Santoro, D. Electrochemical Methods for Nutrient Removal in Wastewater: A Review of Advanced Electrode Materials, Processes, and Applications. Sustainability 2024, 16, 9764. https://doi.org/10.3390/su16229764
Lee J, Antonini G, Al-Omari A, Muller C, Mathew J, Bell K, Pearce JM, Santoro D. Electrochemical Methods for Nutrient Removal in Wastewater: A Review of Advanced Electrode Materials, Processes, and Applications. Sustainability. 2024; 16(22):9764. https://doi.org/10.3390/su16229764
Chicago/Turabian StyleLee, Juwon, Giorgio Antonini, Ahmed Al-Omari, Christopher Muller, Jithin Mathew, Katherine Bell, Joshua M. Pearce, and Domenico Santoro. 2024. "Electrochemical Methods for Nutrient Removal in Wastewater: A Review of Advanced Electrode Materials, Processes, and Applications" Sustainability 16, no. 22: 9764. https://doi.org/10.3390/su16229764
APA StyleLee, J., Antonini, G., Al-Omari, A., Muller, C., Mathew, J., Bell, K., Pearce, J. M., & Santoro, D. (2024). Electrochemical Methods for Nutrient Removal in Wastewater: A Review of Advanced Electrode Materials, Processes, and Applications. Sustainability, 16(22), 9764. https://doi.org/10.3390/su16229764







