Assessing the Impact of Arsenic on Benthic Estuarine Fauna Behavior: Implications for Ecosystem Sustainability
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Organisms: Collection and Acclimation
2.2. Experimental Conditions for the Behavioral Assays
2.2.1. Particle Reworking Activity
2.2.2. Post-Exposure Burrowing
2.3. Data Analysis
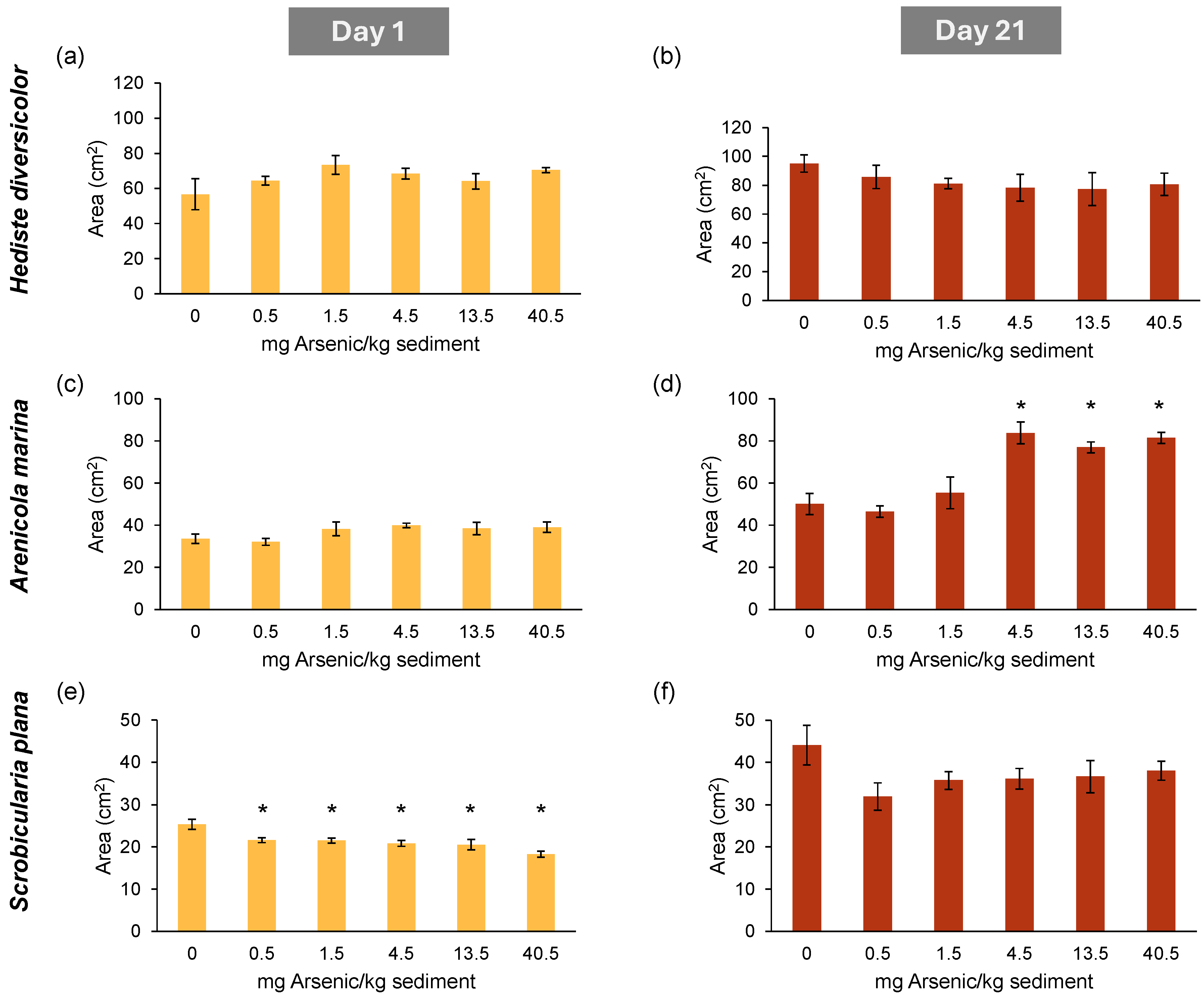
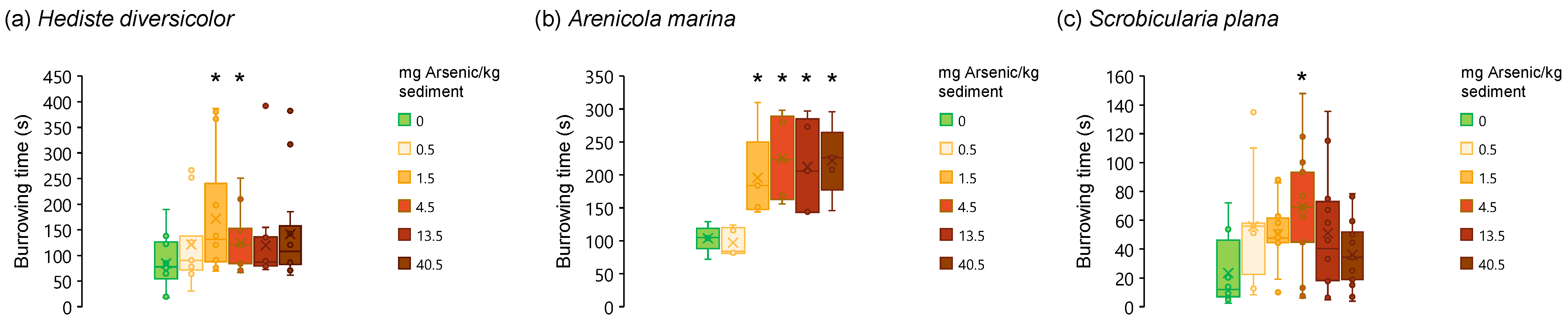
3. Results
3.1. Particle Reworking Activity
3.1.1. Remobilized Area
3.1.2. Maximum Depth Penetration
3.2. Post-Exposure Burrowing
3.3. Principal Coordinate Ordination (PCO)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals; Springer: New York, NY, USA, 2001; Volume 860. [Google Scholar]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [PubMed]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [PubMed]
- Bundschuh, J.; Schneider, J.; Alam, M.A.; Niazi, N.K.; Herath, I.; Parvez, F.; Tomaszewska, B.; Guilherme, L.R.G.; Maity, J.P.; López, D.L.; et al. Seven potential sources of arsenic pollution in Latin America and their environmental and health impacts. Sci. Total Environ. 2021, 780, 146274. [Google Scholar]
- Nickson, R.T.; McArthur, J.M.; Ravenscroft, P.; Burgess, W.G.; Ahmed, K.M. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl. Geochem. 2000, 15, 403–413. [Google Scholar]
- Chakraborti, D.; Basu, G.K.; Biswas, B.K.; Chowdhury, U.K.; Rahman, M.M.; Paul, K.; Chowdhury, T.R.; Chanda, C.R.; Lodh, D.; Ray, S.L. Characterization of arsenic bearing sediments in Gangetic delta of West Bengal-India. Arsen. Expo. Health Eff. 2001, 4, 27–52. [Google Scholar]
- Bai, J.; Zhao, Q.; Wang, W.; Wang, X.; Jia, J.; Cui, B.; Liu, X. Arsenic and heavy metals pollution along a salinity gradient in drained coastal wetland soils: Depth distributions, sources and toxic risks. Ecol. Indic. 2019, 96, 91–98. [Google Scholar]
- Ereira, T.; Coelho, J.P.; Duarte, A.C.; Pardal, M.A.; Pereira, M.E. Size-dependent arsenic accumulation in Scrobicularia plana in a temperate coastal lagoon (Ria de Aveiro, Portugal). Water Air Soil Pollut. 2015, 226, 213. [Google Scholar]
- Agency of Toxic Substances and Disease Registry (ATSDR). Available online: https://www.atsdr.cdc.gov (accessed on 29 July 2024).
- Coppola, F.; Almeida, Â.; Henriques, B.; Soares, A.M.; Figueira, E.; Pereira, E.; Freitas, R. Biochemical responses and accumulation patterns of Mytilus galloprovincialis exposed to thermal stress and Arsenic contamination. Ecotoxicol. Environ. Saf. 2018, 147, 954–962. [Google Scholar]
- Velez, C.; Teixeira, M.; Wrona, F.J.; Soares, A.M.; Figueira, E.; Freitas, R. Clam Ruditapes philippinarum recovery from short-term exposure to the combined effect of salinity shifts and Arsenic contamination. Aquat. Toxicol. 2016, 173, 154–164. [Google Scholar]
- Coppola, F.; Pires, A.; Velez, C.; Soares, A.M.; Pereira, E.; Figueira, E.; Freitas, R. Biochemical and physiological alterations induced in Diopatra neapolitana after a long-term exposure to Arsenic. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 189, 1–9. [Google Scholar]
- Casado-Martinez, M.C.; Duncan, E.; Smith, B.D.; Maher, W.A.; Rainbow, P.S. Arsenic toxicity in a sediment-dwelling polychaete: Detoxification and arsenic metabolism. Ecotoxicology 2012, 21, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Vieira, K.S.; Delgado, J.F.; Lima, L.S.; Souza, P.F.; Crapez, M.A.C.; Correa, T.R.; Aguiar, V.M.C.; Neto, J.B.; Fonseca, E.M. Human health risk assessment associated with the consumption of mussels (Perna perna) and oysters (Crassostrea rhizophorae) contaminated with metals and arsenic in the estuarine channel of Vitória Bay (ES), Southeast Brazil. Mar. Pollut. Bull. 2021, 172, 112877. [Google Scholar] [CrossRef] [PubMed]
- Cuccaro, A.; De Marchi, L.; Oliva, M.; Sanches, M.V.; Freitas, R.; Casu, V.; Monni, G.; Miragliotta, V.; Pretti, C. Sperm quality assessment in Ficopomatus enigmaticus (Fauvel, 1923): Effects of selected organic and inorganic chemicals across salinity levels. Ecotoxicol. Environ. Saf. 2021, 207, 111219. [Google Scholar] [CrossRef] [PubMed]
- Hellou, J. Behavioural ecotoxicology, an “early warning” signal to assess environmental quality. Environ. Sci. Pollut. Res. 2011, 18, 1–11. [Google Scholar] [CrossRef]
- Araújo, C.V.; Roque, D.; Blasco, J.; Ribeiro, R.; Moreira-Santos, M.; Toribio, A.; Aguirre, E.; Barro, S. Stress-driven emigration in complex field scenarios of habitat disturbance: The heterogeneous multi-habitat assay system (HeMHAS). Sci. Total Environ. 2018, 644, 31–36. [Google Scholar] [CrossRef]
- Venâncio, C.; Ribeiro, R.; Lopes, I. Active emigration from climate change-caused seawater intrusion into freshwater habitats. Environ. Pollut. 2020, 258, 113805. [Google Scholar] [CrossRef]
- Islam, M.A.; Lopes, I.; Domingues, I.; Silva, D.C.; Blasco, J.; Pereira, J.L.; Araújo, C.V. Behavioural, developmental and biochemical effects in zebrafish caused by ibuprofen, irgarol and terbuthylazine. Chemosphere 2023, 344, 140373. [Google Scholar] [CrossRef]
- Curley, E.A.; Thomas, R.; Adams, C.E.; Stephen, A. Behavioural and metabolic responses of Unionida mussels to stress. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 3184–3200. [Google Scholar] [CrossRef]
- Ford, A.T.; Ågerstrand, M.; Brooks, B.W.; Allen, J.; Bertram, M.G.; Brodin, T.; Dang, Z.; Duquesne, S.; Sahm, R.; Hoffmann, F.; et al. The role of behavioral ecotoxicology in environmental protection. Environ. Sci. Technol. 2021, 55, 5620–5628. [Google Scholar] [CrossRef]
- Diepens, N.J.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. Modeling of Bioaccumulation in Marine Benthic Invertebrates Using a Multispecies Experimental Approach. Environ. Sci. Technol. 2015, 49, 13575–13585. [Google Scholar] [CrossRef]
- Scola, S.; Blasco, J.; Campana, O. “Nanosize effect” in the metal-handling strategy of the bivalve Scrobicularia plana exposed to CuO nanoparticles and copper ions in whole-sediment toxicity tests. Sci. Total Environ. 2021, 760, 143886. [Google Scholar] [CrossRef]
- Lopes, M.L.; Rodrigues, J.P.; Crespo, D.; Dolbeth, M.; Calado, R.; Lillebø, A.I. Functional traits of a native and an invasive clam of the genus Ruditapes occurring in sympatry in a coastal lagoon. Sci. Rep. 2018, 8, 16901. [Google Scholar] [CrossRef]
- Pires, A.; Martins, R.; Magalhes, L.; Soares, A.M.V.M.; Figueira, E.; Quintino, V.; Rodrigues, A.M.; Freitas, R. Expansion of lugworms towards southern European habitats and their identification using combined ecological, morphological and genetic approaches. Mar. Ecol. Prog. Ser. 2015, 533, 177–190. [Google Scholar] [CrossRef]
- Dolbeth, M.; Babe, O.; Costa, D.A.; Mucha, A.P.; Cardoso, P.G.; Arenas, F. Benthic estuarine communities’ contribution to bioturbation under the experimental effect of marine heatwaves. Sci. Rep. 2021, 11, 11422. [Google Scholar] [CrossRef]
- Bonnard, M.; Roméo, M.; Amiard-Triquet, C. Effects of copper on the burrowing behavior of estuarine and coastal invertebrates, the polychaete Nereis diversicolor and the bivalve Scrobicularia plana. Hum. Ecol. Risk Assess. 2009, 15, 11–26. [Google Scholar] [CrossRef]
- Shen, H.; Thrush, S.F.; Wan, X.; Li, H.; Qiao, Y.; Jiang, G.; Sun, R.; Wang, L.; He, P. Optimization of hard clams, polychaetes, physical disturbance and denitrifying bacteria of removing nutrients in marine sediment. Mar. Pollut. Bull. 2016, 110, 86–92. [Google Scholar] [CrossRef]
- Buffet, P.E.; Tankoua, O.F.; Pan, J.F.; Berhanu, D.; Herrenknecht, C.; Poirier, L.; Amiard-Triquet, C.; Amiard, J.C.; Bérard, J.B.; Risso, C.; et al. Behavioural and biochemical responses of two marine invertebrates Scrobicularia plana and Hediste diversicolor to copper oxide nanoparticles. Chemosphere 2011, 84, 166–174. [Google Scholar] [CrossRef]
- Wiesebron, L.E.; Steiner, N.; Morys, C.; Ysebaert, T.; Bouma, T.J. Sediment bulk density effects on benthic macrofauna burrowing and bioturbation behavior. Front. Mar. Sci. 2021, 8, 707785. [Google Scholar] [CrossRef]
- Mouneyrac, C.; Mastain, O.; Amiard, J.C.; Amiard-Triquet, C.; Beaunier, P.; Jeantet, A.Y.; Smith, B.D.; Rainbow, P.S. Trace-metal detoxification and tolerance of the estuarine worm Hediste diversicolor chronically exposed in their environment. Mar. Biol. 2003, 143, 731–744. [Google Scholar] [CrossRef]
- Urban-Malinga, B.; Jakubowska, M.; Hallmann, A.; Dąbrowska, A. Do the graphene nanoflakes pose a potential threat to the polychaete Hediste diversicolor? Chemosphere 2021, 269, 128685. [Google Scholar] [CrossRef]
- Venâncio, C.; Wijewardene, L.; Ribeiro, R.; Lopes, I. Combined effects of two abiotic stressors (salinity and temperature) on a laboratory-simulated population of Daphnia longispina. Hydrobiologia 2023, 850, 3197–3208. [Google Scholar] [CrossRef]
- Figueiredo, M.J.; Venâncio, C.; Cardoso, P.; Marques, P.; Figueira, E.; Pires, A. Potential advantage of invasive estuarine worms over native species under exposure to relevant concentrations of graphene oxide: Behavioral and biochemical insights. Mar. Environ. Res. 2024, 202, 106821. [Google Scholar] [CrossRef]
- Gray, J.S.; Elliott, M. Ecology of Marine Sediments: From Science to Management; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Thrush, S.F.; Hewitt, J.E.; Cummings, V.J.; Dayton, P.K. The role of disturbance in sustaining small-scale heterogeneity across scales in soft sediment habitats. Ecology 2006, 87, 1039–1044. [Google Scholar]
- Petersen, K.; Kristensen, E.; Bjerregaard, P. Influence of bioturbating animals on flux of cadmium into estuarine sediment. Mar. Environ. Res. 1998, 45, 403–415. [Google Scholar] [CrossRef]
- Gebhardt, C.; Forster, S. Size-selective feeding of Arenicola marina promotes long-term burial of microplastic particles in marine sediments. Environ. Pollut. 2018, 242, 1777–1786. [Google Scholar]
- Haider, F.; Sokolov, E.P.; Sokolova, I.M. Effects of mechanical disturbance and salinity stress on bioenergetics and burrowing behavior of the soft-shell clam Mya arenaria. J. Exp. Biol. 2018, 221, jeb172643. [Google Scholar]
- Bertrand, C.; Devin, S.; Mouneyrac, C.; Giambérini, L. Eco-physiological responses to salinity changes across the freshwater-marine continuum on two euryhaline bivalves: Corbicula fluminea and Scrobicularia plana. Ecol. Indic. 2017, 74, 334–342. [Google Scholar]
- Kalman, J.; Bonnail-Miguel, E.; Smith, B.D.; Bury, N.R.; Rainbow, P.S. Toxicity and the fractional distribution of trace metals accumulated from contaminated sediments by the clam Scrobicularia plana exposed in the laboratory and the field. Sci. Total Environ. 2015, 506, 109–117. [Google Scholar] [CrossRef]
- Castro, B.B.; Silva, C.; Macário, I.P.E.; Oliveira, B.; Gonçalves, F.; Pereira, J.L. Feeding inhibition in Corbicula fluminea (OF Muller, 1774) as an effect criterion to pollutant exposure: Perspectives for ecotoxicity screening and refinement of chemical control. Aquat. Toxicol. 2018, 196, 25–34. [Google Scholar] [CrossRef]
- Ray, A.; Gautam, A.; Das, S.; Pal, K.; Das, S.; Karmakar, P.; Ray, M.; Ray, S. Effects of copper oxide nanoparticle on gill filtration rate, respiration rate, hemocyte associated immune parameters and oxidative status of an Indian freshwater mussel. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108855. [Google Scholar]
- Patel, K.S.; Pandey, P.K.; Martín-Ramos, P.; Corns, W.T.; Varol, S.; Bhattacharya, P.; Zhu, Y. A review on arsenic in the environment: Contamination, mobility, sources, and exposure. RSC Adv. 2023, 13, 8803–8821. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.K.; Buchwalter, D.B.; Kerby, J.L.; LeFauve, M.K.; Varian-Ramos, C.W.; Swaddle, J.P. Integrative behavioral ecotoxicology: Bringing together fields to establish new insight to behavioral ecology, toxicology, and conservation. Curr. Zool. 2017, 63, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). p. 22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0056 (accessed on 6 October 2024).

| Comparison | As (mg/kg Sediment) Treatment | DF | Difference of Means | t | p Value |
|---|---|---|---|---|---|
| Hediste diversicolor Day 1 vs. Day 21 | 0 | 4 | - | - | 0.057 |
| 0.5 | 4 | - | - | 0.100 | |
| 1.5 | 4 | - | - | 0.091 | |
| 4.5 | 4 | - | - | 0.377 | |
| 13.5 | 4 | - | - | 0.266 | |
| 40.5 | 4 | - | - | 0.229 | |
| Arenicola marina Day 1 vs. Day 21 | 0 | 4 | 16.55 | 3.16 | 0.030 |
| 0.5 | 4 | 14.42 | 4.52 | 0.010 | |
| 1.5 | 4 | 17.12 | 2.78 | 0.049 | |
| 4.5 | 4 | 2.824 | 8.90 | <0.001 | |
| 13.5 | 4 | 38.50 | 41.6 | <0.001 | |
| 40.5 | 4 | 2.784 | 14.4 | <0.001 | |
| Scrobicularia plana Day 1 vs. Day 21 | 0 | 4 | 18.73 | 3.65 | 0.022 |
| 0.5 | 4 | 0.977 | 3.82 | 0.019 | |
| 1.5 | 4 | 1.335 | 7.57 | 0.002 | |
| 4.5 | 4 | 15.33 | 6.94 | 0.002 | |
| 13.5 | 4 | 16.14 | 5.12 | 0.007 | |
| 40.5 | 4 | 19.75 | 8.61 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venâncio, C.; Degara, L.; Pires, A. Assessing the Impact of Arsenic on Benthic Estuarine Fauna Behavior: Implications for Ecosystem Sustainability. Sustainability 2024, 16, 9728. https://doi.org/10.3390/su16229728
Venâncio C, Degara L, Pires A. Assessing the Impact of Arsenic on Benthic Estuarine Fauna Behavior: Implications for Ecosystem Sustainability. Sustainability. 2024; 16(22):9728. https://doi.org/10.3390/su16229728
Chicago/Turabian StyleVenâncio, Cátia, Letizia Degara, and Adília Pires. 2024. "Assessing the Impact of Arsenic on Benthic Estuarine Fauna Behavior: Implications for Ecosystem Sustainability" Sustainability 16, no. 22: 9728. https://doi.org/10.3390/su16229728
APA StyleVenâncio, C., Degara, L., & Pires, A. (2024). Assessing the Impact of Arsenic on Benthic Estuarine Fauna Behavior: Implications for Ecosystem Sustainability. Sustainability, 16(22), 9728. https://doi.org/10.3390/su16229728







