Effects of Aquatic Plant Diversity and Cipangopaludinas chinensis on Nitrogen Removal and Its Stability in Constructed Wetlands
Abstract
1. Introduction
2. Materials and Methods
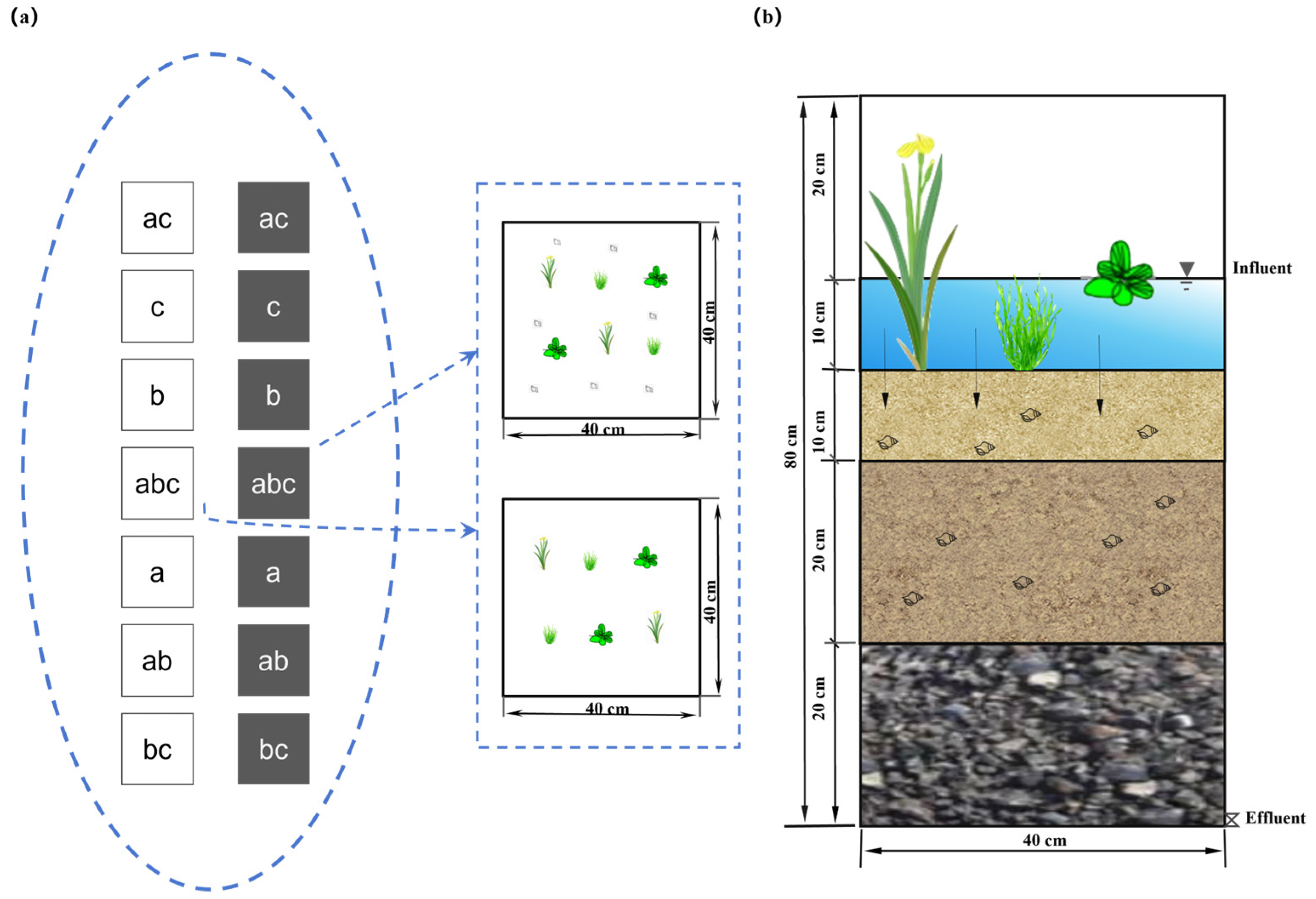
2.1. Experimental Design
2.2. Sample Collection and Measurements
2.3. Parameter Calculation
2.4. Statistical Analysis
3. Results
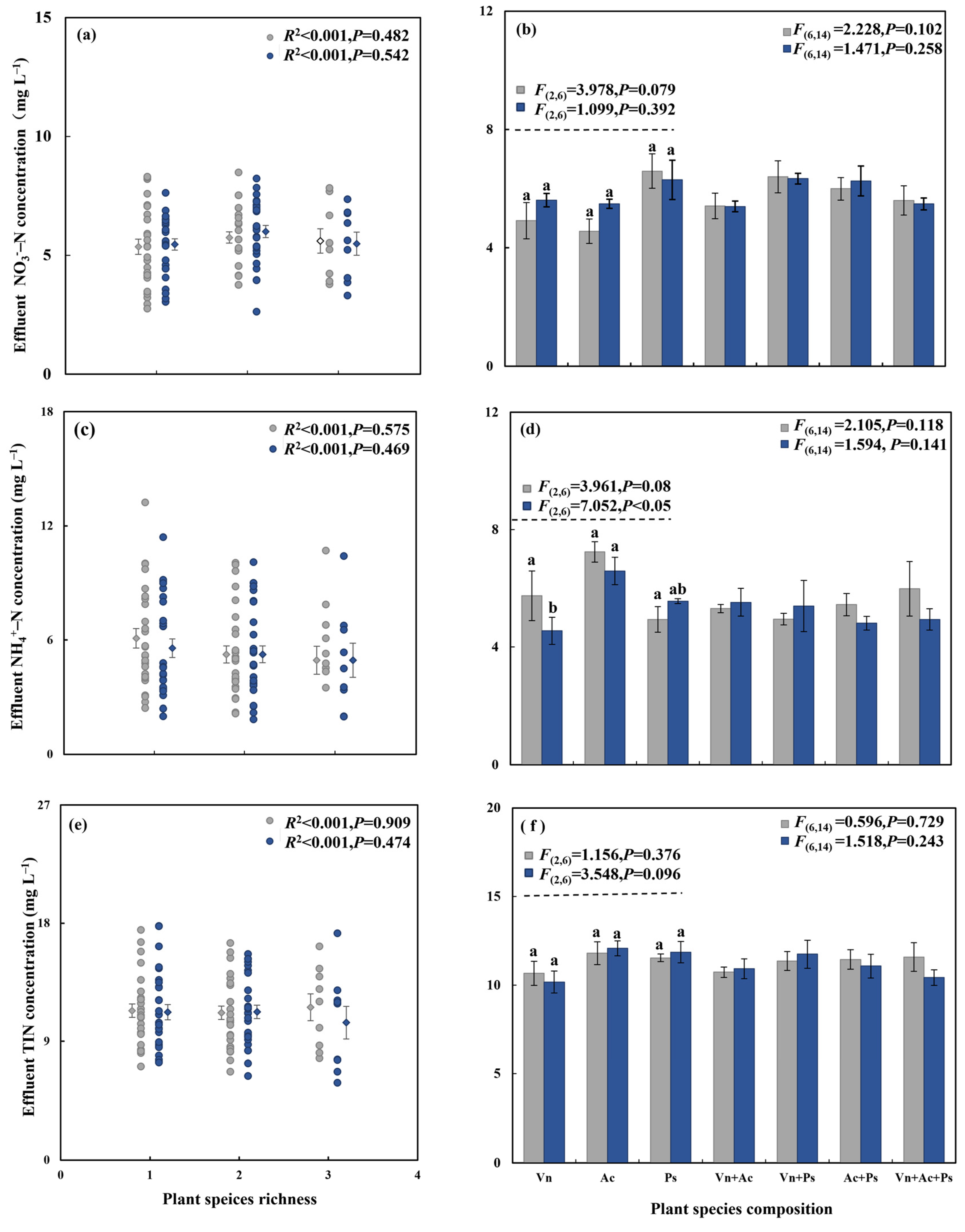
3.1. Effect of Aquatic Plant Species Diversity with Different Life Forms and C. chinensis on N Removal
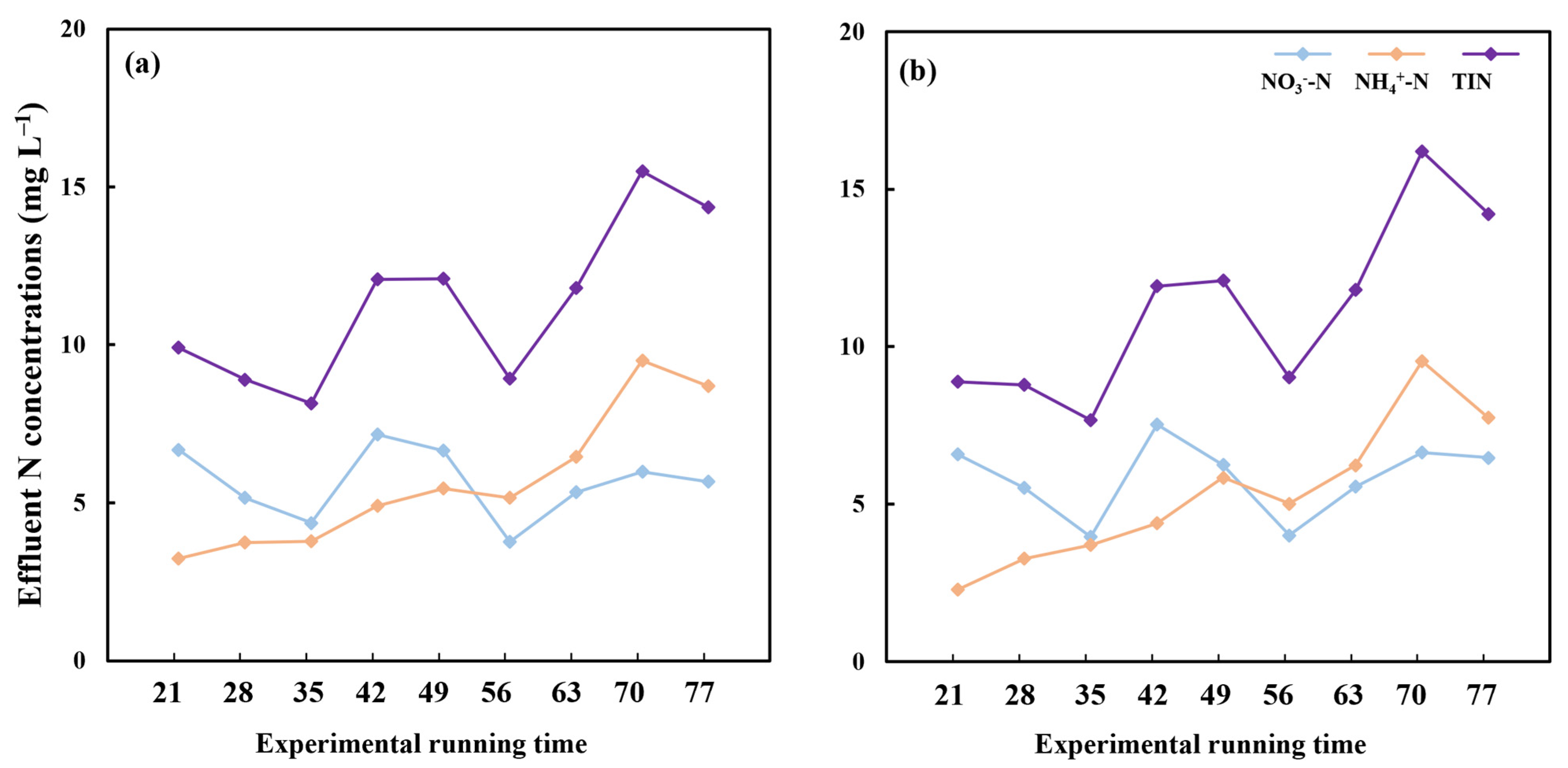
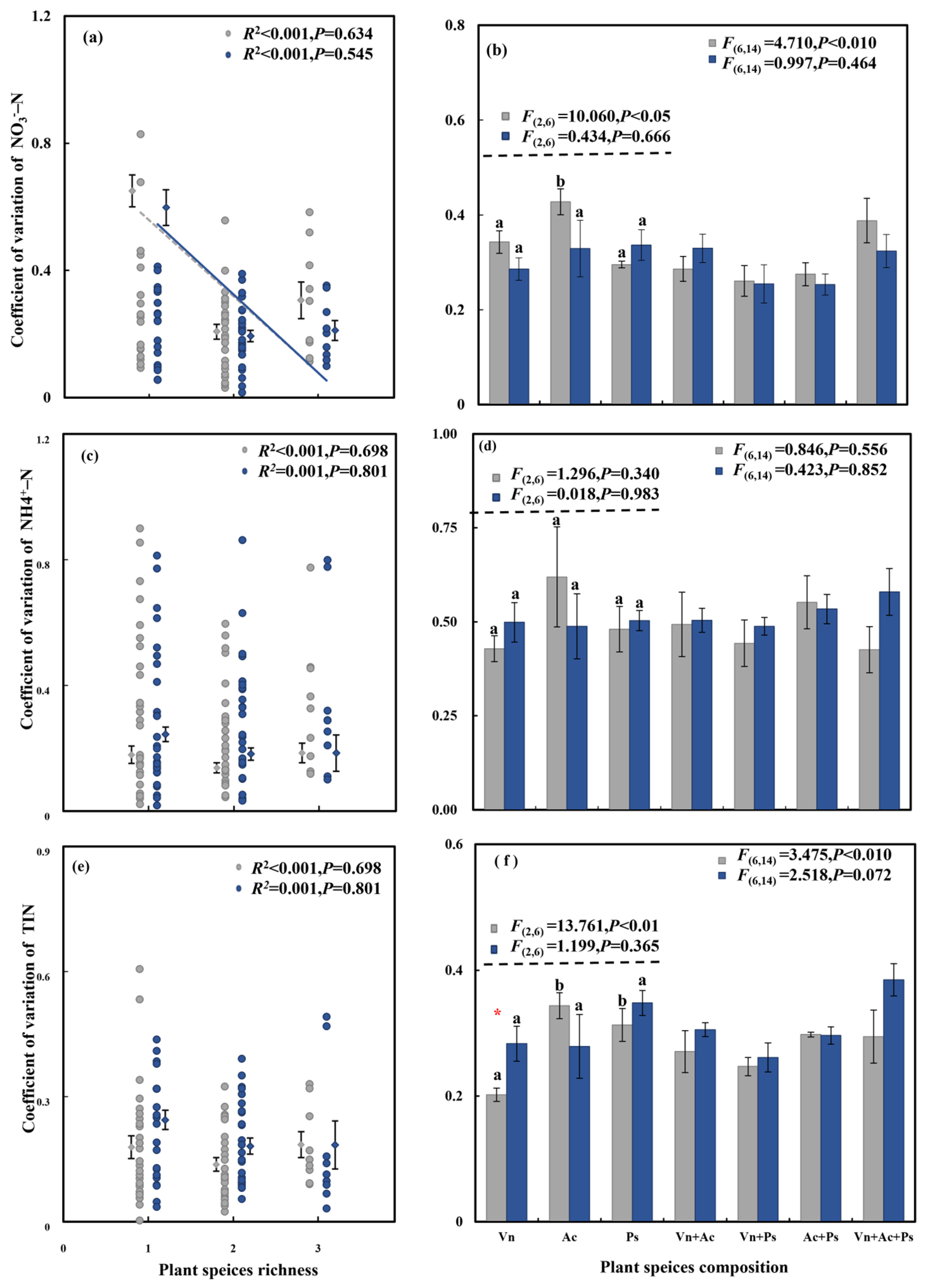
3.2. Effect of Aquatic Plant Species Diversity with Different Life Forms and C. chinensis on Temporal Stability of Effluent N Concentration
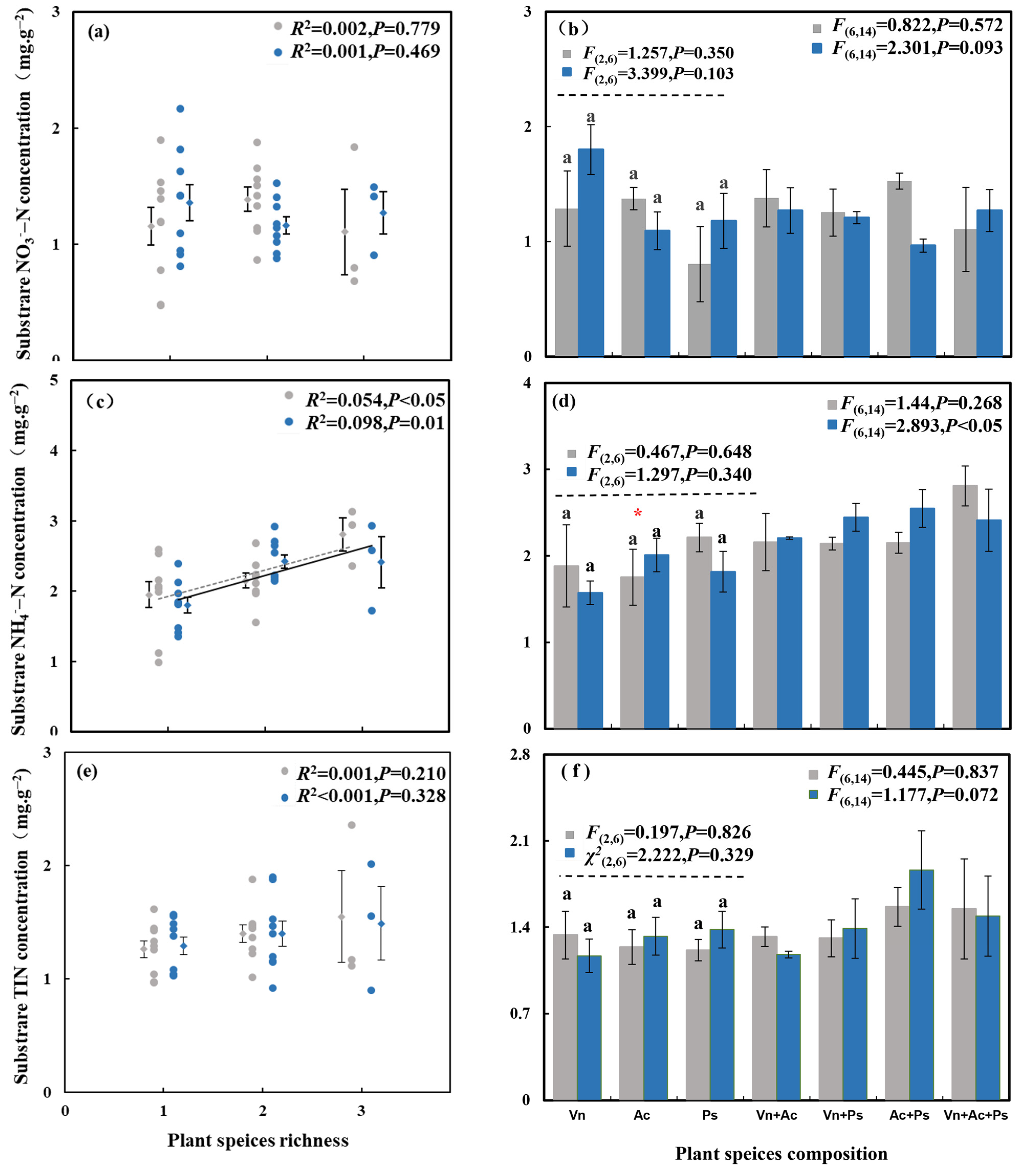
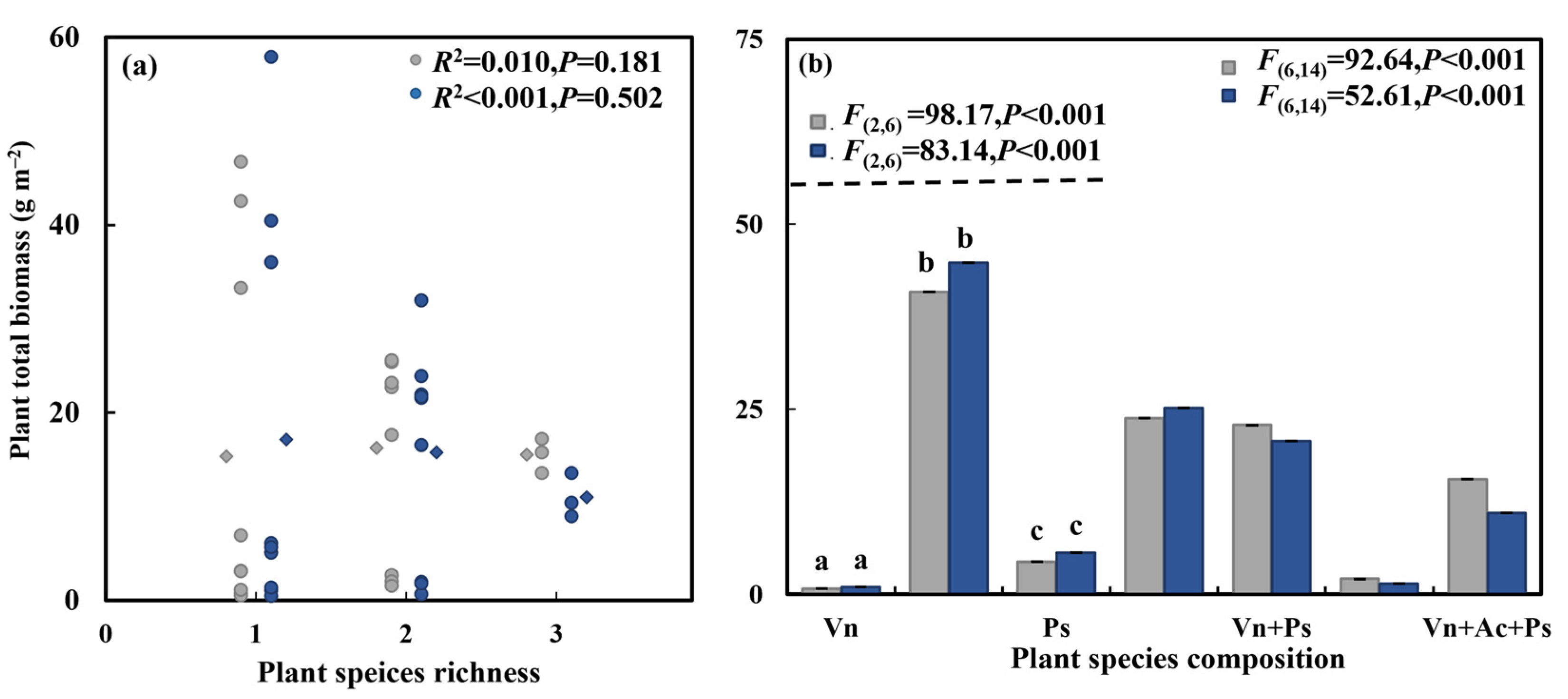
3.3. Effect of Aquatic Plant Species Diversity with Different Life Forms and C. chinensis on Substrate N Concentration and Total Plant Biomass
4. Discussion
4.1. Impact of Aquatic Plant Diversity and C. chinensis on Effluent N Concentration
4.2. Impact of Aquatic Plant Diversity and C. chinensis on Temporal Stability of N Concentration in Effluent
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pointet, T. The United Nations World Water Development Report 2022 on groundwater, a synthesis. Lhb-Hydrosci. J. 2022, 108. [Google Scholar] [CrossRef]
- Francis, J. The United Nations World Water Development Reports: A comparison and contrasts of 2016 and 2017 reports. J. Scientometr. Res. 2017, 6, 209–211. [Google Scholar] [CrossRef]
- Weerasekara, P. The United Nations World Water Development Report 2017 Wastewater: The Untapped Resource. Future Food-J. Food Agric. Soc. 2017, 5, 80–81. [Google Scholar] [CrossRef]
- Kataki, S.; Chatterjee, S.; Vairale, M.; Dwivedi, S.; Gupta, D. Constructed wetland, an eco-technology for wastewater treat ment: A review on types of wastewater treated and components of the technology (macrophyte, biolfilm and substrate). J. Environ. Manag. 2021, 283. [Google Scholar] [CrossRef]
- Wu, H.; Wang, R.; Yan, P.; Wu, S.; Chen, Z.; Zhao, Y.; Cheng, C.; Hu, Z.; Zhuang, L.; Guo, Z.; et al. Constructed wetlands for pollution control. Nat. Rev. Earth Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Wang, W.; Yan, W.; Wen, Y.; Ling, S.; Xiao, Z.; Qi, J.; Xin, C.; Ping, Q.; Ying, X. Improving the efficacy of different life-form macrophytes in phytoremediation of artificial eutrophic water by combined planting. Environ. Sci. Pollut. Res. 2023, 30, 67621–67633. [Google Scholar] [CrossRef]
- Ma, Y.; Gu, X.; Zhang, Y.; Yan, P.; Zhang, M.; Sun, S.; Ren, Y.; Tang, L.; He, S. Unveiling the microplastic perturbation on surface flow constructed wetlands with macrophytes of different life forms: Responses of nitrogen removal and sensory quality. J. Hazard. Mater. 2024, 477, 135283. [Google Scholar] [CrossRef] [PubMed]
- van Gerven, L.; de Klein, J.; Gerla, D.; Kooi, B.; Kuiper, J.; Mooij, W. Competition for light and nutrients in layered communities of aquatic plants. Am. Nat. 2015, 186, 72–83. [Google Scholar] [CrossRef]
- Olesen, A.; Jensen, S.; Alnoee, A.; Baattrup-Pedersen, A.; Lauridsen, T.; Sorrell, B.; Riis, T. Nutrient kinetics in submerged plant beds: A mesocosm study simulating constructed drainage wetlands. Ecol. Eng. 2018, 122, 263–270. [Google Scholar] [CrossRef]
- Knops, J.; Bradley, K.; Wedin, D. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol. Lett. 2002, 5, 454–466. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Choudhury, I.; McKie, G.; Hallin, S.; Ecke, F. Mixtures of macrophyte growth forms promote nitrogen cycling in wetlands. Sci. Total Environ. 2018, 635, 1436–1443. [Google Scholar] [CrossRef]
- Li, Q.; Deng, H.; He, R.; Hu, S.; Sun, L.; Li, M.; Wu, Q.L.; Zeng, J. Effects of different emergent macrophytes on methane flux and rhizosphere microbial communities in wetlands. Sci. Total Environ. 2024, 932. [Google Scholar] [CrossRef]
- Puche, E.; Cruz, D.; Delgado, P.; Rosinska, J.; Rodrigo, M. Changes in submerged macrophyte diversity, coverage and biomass in a biosphere reserve site after 20 years: A plea for conservation efforts. Biol. Conserv. 2024, 294, 110607. [Google Scholar] [CrossRef]
- Shen, K.; Cao, H.; Tao, J.; Zheng, X.; Han, W. Adding Cipangopaludina chinensis altered the effects of submerged macrophyte diversity on water turbidity and nitrogen concentration. Water Air Soil Pollut. 2024, 235. [Google Scholar] [CrossRef]
- Grimm, V.; Wissel, C. Babel, or the ecological stability discussions: An inventory and analysis of terminology and a guide for avoiding confusion. Oecologia 1997, 109, 323–334. [Google Scholar] [CrossRef]
- Wang, S.; Lamy, T.; Hallett, L.; Loreau, M. Stability and synchrony across ecological hierarchies in heterogeneous meta communities: Linking theory to data. Ecography 2019, 42, 1200–1211. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.; Cadotte, M. Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 1996, 379, 718–720. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.M. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef]
- Wang, X.; Luo, B.; Wang, L.; Zhao, Y.; Wang, Q.; Li, D.; Gu, B.; Min, Y.; Chang, S.; Ge, Y.; et al. Plant diversity improves the effluent quality and stability of floating constructed wetlands under increased ammonium/nitrate ratio in influent. J. Environ. Manag. 2020, 266. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Long, Z.; Wang, H.; Zhang, G. Functions of constructed wetland animals in water environment protection—A critical review. Sci. Total Environ. 2021, 760. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, J.; Xie, H.; Hu, Z.; He, H.; Wang, W. Effects of Misgurnus anguillicaudatus and Cipangopaludina cathayensis on pollutant removal and microbial community in constructed wetlands. Water 2015, 7, 2422–2434. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, J.; Xie, H.; Guo, Z.; Ngo, H.; Guo, W.; Liang, S. Enhanced nutrient removal and mechanisms study in benthic fauna added surface-flow constructed wetlands: The role of Tubifex tubifex. Bioresour. Technol. 2017, 224, 157–165. [Google Scholar] [CrossRef]
- Yang, L.; Shen, K.; Xu, X.; Xiao, D.; Cao, H.; Lin, Y.; Zheng, X.; Zhao, M.; Han, W. Adding Corbicula fluminea altered the effect of plant species diversity on greenhouse gas emissions and nitrogen removal from constructed wetlands in the low-temperature season. Sci. Total Environ. 2024, 907. [Google Scholar] [CrossRef]
- Hu, H.; Ao, S.; Yang, E.; Li, Q. Treating eutrophic water for nutrient reduction using an aquatic macrophyte (Ipomoea aquatica Forsskal) in a deep flow technique system. Agric. Water Manag. 2008, 95, 607–615. [Google Scholar] [CrossRef]
- Venail, P.; Gross, K.; Oakley, T.; Narwani, A.; Allan, E.; Flombaum, P.; Isbell, F.; Joshi, J.; Reich, P.; Tilman, D.; et al. Species richness, but not phylogenetic diversity, influences community biomass production and temporal stability in a re-examination of 16 grassland biodiversity studies. Funct. Ecol. 2015, 29, 615–626. [Google Scholar] [CrossRef]
- Yu, Q.; Duan, X.; Gu, Y.; Li, J.; Zhang, X.; Chen, C.; Zhao, D. Increasing chemical oxygen demand and nitrogen removal efficiencies of surface-flow constructed wetlands in macrophyte-dominant seasons by adding artificial macrophytes. Bioresour. Technol. 2022, 348, 126755. [Google Scholar] [CrossRef]
- Hong, G.; Son, Y.; Kim, G. Effects of interspecific competition on the growth and competitiveness of five emergent macrophytes in a constructed lentic wetland. Paddy Water Environ. 2014, 12, S193–S202. [Google Scholar] [CrossRef]
- Johns, C.; Ramsey, M.; Bell, D.; Vaughton, G. Does increased salinity reduce functional depth tolerance of four non-halophytic wetland macrophyte species? Aquat. Bot. 2014, 116, 13–18. [Google Scholar] [CrossRef]
- Han, W.; Ge, Y.; Ren, Y.; Luo, B.; Du, Y.; Chang, J.; Wu, J. Removal of metals and their pools in plant in response to plant diversity in microcosms of floating constructed wetlands. Ecol. Eng. 2018, 113, 65–73. [Google Scholar] [CrossRef]
- Han, W.; Luo, G.; Luo, B.; Yu, C.; Wang, H.; Chang, J.; Ge, Y. Effects of plant diversity on greenhouse gas emissions in microcosms simulating vertical constructed wetlands with high ammonium loading. J. Environ. Sci. 2019, 77, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Culicov, O.A.; Yurukova, L. Comparison of element accumulation of different moss-and lichen-bags, exposed in the city of Sofia (Bulgaria). J. Atmos. Chem. 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Cristina, R.; Sara, B.; Rossano, B.; Romane, D.; Marco, B. Macrophyte growth forms and hydrological connectivity affect greenhouse gas concentration in small eutrophic wetlands. Aquat. Bot. 2023, 188, ttps. [Google Scholar]
- Karatayev, A.; Burlakova, L.; Karatayev, V.; Padilla, D. Introduction, distribution, spread, and impacts of exotic freshwater gastropods in Texas. Hydrobiologia 2009, 619, 181–194. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, L.; Shi, C.; Fan, C. Influence of Chironomid Larvae on oxygen and nitrogen fluxes across the sediment-water interface (Lake Taihu, China). J. Environ. Sci. 2013, 25, 978–985. [Google Scholar] [CrossRef]
- Balushkina, E. Assessment of the Neva Estuary ecosystem state on the basis of structural characteristics of benthic animal communities in 1994–2005. Inland Water Biol. 2009, 2, 355–363. [Google Scholar] [CrossRef]
- MacArthur, R. Fluctuations of animal populations and a measure of community stability. Ecology 1955, 36, 533–536. [Google Scholar] [CrossRef]
- Richardson, D.; Elton, C.S. 1958: The ecology of invasions by animals and plants. London: Methuen. Prog. Phys. Geogr. 2007, 31, 659–666. [Google Scholar] [CrossRef]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Schippers, P.; Joenje, W. Modelling the effect of fertiliser, mowing, disturbance and width on the biodiversity of plant communities of field boundaries. Agric. Ecosyst. Environ. 2002, 93, 351–365. [Google Scholar] [CrossRef]
- Dong, B.; Wang, G.; Yu, H. Oxygen, pH, and Eh microprofiles around submerged macrophyte Vallisneria natans response to growing stages. IOP Conf. Ser. Earth Environ. Sci. 2017, 82, 012026. [Google Scholar] [CrossRef]
- Li, W.; Xufa, M.; Jun, C. Do submerged macrophyte species influence crustacean zooplankton functional group richness and their resource use efficiency in the low-light environment? Front. Plant Sci. 2023, 14, 1185947. [Google Scholar]
- Guo, Y.; Xie, H.; Zhang, J.; Wang, W.; Ngo, H.; Guo, W.; Kang, Y.; Zhang, B. Improving nutrient removal performance of surface flow constructed wetlands in winter using hardy submerged plant-benthic fauna systems. RSC Adv. 2018, 42179–42188. [Google Scholar] [CrossRef]
| V. natans | A. calamus | P. stratiotes | ||||
|---|---|---|---|---|---|---|
| Presence | Absence | Presence | Absence | Presence | Absence | |
| (a) | ||||||
| TIN | 11.080 ± 0.974 | 11.590 ± 0.730 | 11.386 ± 0.945 | 11.181 ± 0.814 | 11.477 ± 0.806 | 11.060 ± 0.954 |
| NO3−-N | 5.583 ± 0.913 | 5.716 ± 1.078 | 5.393 ± 0.803 | 5.969 ± 1.111 | 6.147 ± 0.810 ** | 4.965 ± 0.782 |
| NH4+ -N | 5.496 ± 0.988 | 5.873 ± 1.131 | 5.992 ± 1.075 | 5.212 ± 0.879 | 5.330 ± 0.898 | 6.095 ± 1.119 |
| (b) | ||||||
| TIN | 10.811 ± 1.056 | 11.666 ± 0.918 | 11.121 ± 1.038 | 11.253 ± 1.056 | 11.271 ± 0.806 | 11.053 ± 1.093 |
| NO3−-N | 5.708 ± 0.476 | 6.013 ± 0.786 | 5.656 ± 0.461 | 6.082 ± 0.467 | 6.094 ± 0.810 * | 5.498 ± 0.283 |
| NH4+ -N | 5.103 ± 0.901 | 5.652 ± 0.846 | 5.464 ± 1.015 | 5.171 ± 0.901 | 5.176 ± 0.898 | 5.554 ± 1.060 |
| V. natans | A. calamus | P. stratiotes | ||||
|---|---|---|---|---|---|---|
| Presence | Absence | Presence | Absence | Presence | Absence | |
| (a) | ||||||
| TIN | 0.253 ± 0.052 ** | 0.318 ± 0.033 | 0.301 ± 0.048 | 0.254 ± 0.052 | 0.288 ± 0.044 | 0.272 ± 0.066 |
| NO3−-N | 0.319 ± 0.068 | 0.332 ± 0.074 | 0.344 ± 0.079 | 0.299 ± 0.047 | 0.304 ± 0.066 | 0.352 ± 0.068 |
| NH4+ -N | 0.447 ± 0.093 | 0.550 ± 0.144 | 0.522 ± 0.148 | 0.450 ± 0.079 | 0.475 ± 0.102 | 0.513 ± 0.154 |
| (b) | ||||||
| TIN | 0.308 ± 0.056 | 0.307 ± 0.054 | 0.316 ± 0.054 | 0.297 ± 0.056 | 0.322 ± 0.047 | 0.289 ± 0.049 |
| NO3−-N | 0.298 ± 0.055 ** | 0.206 ± 0.069 | 0.344 ± 0.068 | 0.299 ± 0.055 | 0.291 ± 0.054 | 0.314 ± 0.061 |
| NH4+ -N | 0.517 ± 0.073 | 0.508 ± 0.082 | 0.522 ± 0.089 | 0.450 ± 0.073 | 0.526 ± 0.072 | 0.496 ± 0.086 |
| V. natans | A. calamus | P. stratiotes | ||||
|---|---|---|---|---|---|---|
| Presence | Absence | Presence | Absence | Presence | Absence | |
| (a) | ||||||
| TIN | 1.379 ± 0.100 | 1.299 ± 0.120 | 1.481 ± 0.107 | 1.286 ± 0.077 | 1.409 ± 0.109 | 1.299 ± 0.074 |
| NO3−-N | 1.256 ± 0.128 | 1.235 ± 0.148 | 1.346 ± 0.108 | 1.115 ± 0.165 | 1.173 ± 0.138 | 1.346 ± 0.368 |
| NH4+-N | 2.248 ± 0.168 | 2.039 ± 0.131 | 2.218 ± 0.160 | 2.079 ± 0.154 | 2.328 ± 0.107 * | 1.932 ± 0.200 |
| biomass | 15.759 ± 0.822 | 15.800 ± 2.131 | 20.586 ± 1.250 | 9.363 ± 1.173 | 11.232 ± 0.758 | 21.836 ± 1.974 |
| (b) | ||||||
| TIN | 1.306 ± 0.100 | 1.327 ± 0.120 | 1.464 ± 0.128 | 1.312 ± 0.097 | 1.451 ± 0.108 | 1.224 ± 0.064 |
| NO3−-N | 1.388 ± 0.104 * | 1.080 ± 0.183 | 1.150 ± 0.078 | 1.267 ± 0.138 | 1.156 ± 0.074 | 1.389 ± 0.143 |
| NH4+-N | 2.158 ± 0.137 | 1.982 ± 0.178 | 2.292 ± 0.116 | 1.944 ± 0.158 | 2.304 ± 0.138 * | 1.928 ± 0.115 |
| biomass | 14.463 ± 0.852 | 17.298 ± 2.388 | 20.602 ± 1.495 | 9.113 ± 1.015 | 9.702 ± 0.647 | 23.645 ± 2.231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, G.; Tao, J.; Zheng, X.; Yang, L.; Shen, K.; Xu, X.; Xiao, D.; Zhao, M.; Han, W. Effects of Aquatic Plant Diversity and Cipangopaludinas chinensis on Nitrogen Removal and Its Stability in Constructed Wetlands. Sustainability 2024, 16, 9492. https://doi.org/10.3390/su16219492
Kuang G, Tao J, Zheng X, Yang L, Shen K, Xu X, Xiao D, Zhao M, Han W. Effects of Aquatic Plant Diversity and Cipangopaludinas chinensis on Nitrogen Removal and Its Stability in Constructed Wetlands. Sustainability. 2024; 16(21):9492. https://doi.org/10.3390/su16219492
Chicago/Turabian StyleKuang, Guangqian, Jingwen Tao, Xiangyong Zheng, Luping Yang, Kai Shen, Xile Xu, Derong Xiao, Min Zhao, and Wenjuan Han. 2024. "Effects of Aquatic Plant Diversity and Cipangopaludinas chinensis on Nitrogen Removal and Its Stability in Constructed Wetlands" Sustainability 16, no. 21: 9492. https://doi.org/10.3390/su16219492
APA StyleKuang, G., Tao, J., Zheng, X., Yang, L., Shen, K., Xu, X., Xiao, D., Zhao, M., & Han, W. (2024). Effects of Aquatic Plant Diversity and Cipangopaludinas chinensis on Nitrogen Removal and Its Stability in Constructed Wetlands. Sustainability, 16(21), 9492. https://doi.org/10.3390/su16219492







