Abstract
Mercury is classified as one of the world’s most toxic and dangerous pollutants as it tends to bioaccumulate and biomagnify within the trophic chain and is persistent. Various approaches are available to remediate Hg-affected sites including phytoremediation, which includes the use of plants to clean up contaminated environments. The phytoremediation of mercury contamination is attracting increasing attention because of its advantages: it is environmentally friendly, inexpensive, simple, and can improve soil fertility. In this report, VOSviewer (version 1.6.1) and Bibliometrix (version 4.16) software were used to analyze 457 and 697 documents published from 2000 to 2023, retrieved from the databases WoS and Scopus, respectively. China, India, the United States, and Spain were the top four most productive countries. The largest topic area was environmental sciences, and the Chinese Academy of Sciences was the organization that contributed the most to the overall number of publications. The keywords with the highest frequency excluding phytoremediation and mercury in WoS were heavy metals, accumulation, cadmium, soils, and phytoextraction. In Scopus, the most frequent keywords were bioremediation, heavy metals, soil pollution, bioaccumulation, biodegradation, and environmental. From the above analysis, we concluded that future research should focus on (1) finding native plants, (2) genetic engineering applications, (3) increasing remediation ability through assisted phytoremediation, and (4) the detoxification mechanism of mercury. This study provides insights into trending themes and serves as a reference for future research.
1. Introduction
Mercury (Hg) is a global pollutant because of its harmful effects on human and environmental health [1]. It is among the top 10 chemicals of concern and ranks third on the Agency for Toxic Substances and Disease Registry’s priority list of substances [2]. It is poisonous, threatens health and ecosystems, and can accumulate to a high level, especially in fish, and cause serious diseases; it has carcinogenic, teratogenic, neuteratogenic, neurotoxic, and genotoxicity effects. Acute exposure to this metal also causes psychological problems such as anxiety, sleep disorders, and depression [3]; its exposure in soils has caused kidney damage and even death [4].
This pollutant exists in elemental, organic, and inorganic forms. In gaseous form, mercury is emitted through the atmosphere and reaches long distances before settling in the soil. According to the United Nations Environment Program, mercury emissions amount to around 2200 tons per year, of which artisanal and small-scale mining activities account for almost 38% of global emissions [5,6]. Volatile mercury is persistent and can be easily taken up by the plant system, accumulating in the food chain and entering the human body. The presence of reactive Hg in the soil under acidic conditions, low redox potential, and a high concentration of organic matter leads to the formation of methylmercury (MeHg) [7,8,9]. MeHg, the most common organic form, bioaccumulates efficiently in organisms and biomagnifies in the food chain. Its bioaccumulation within organisms severely distorts the biological food chain and eventually enters ecosystems [10]. Exposure to MeHg in fish is often the main concern associated with Hg contamination sites, making fish consumption the main route of mercury exposure in human beings [11,12].
Mercury does not degrade in the environment, and because of its bioaccumulative nature, it is necessary to look for alternatives for the remediation of soils contaminated with this metal so that it can be separated from the contaminating media, or otherwise transformed into less toxic species [13]. There are physical, chemical, and biological remediation techniques, of these, the biological remediation approach has gained the most attention due to its environmentally sustainable nature and ability to treat heavy metal-contaminated soil. It comprises two methods: bioremediation and phytoremediation as well as a combination of the two [14]. Bioremediation uses living organisms to remove and neutralize contaminants and involves the use of microorganisms (e.g., bacteria, microalgae, yeasts) to remove/immobilize, transform, or detoxify heavy metals from the environment [15,16,17]. Phytoremediation refers to the use of plants to extract, reduce, transform, or immobilize contaminants (organic and inorganic) contained in soils, sediments, and groundwater [5,18,19]. Plants have developed strategies to address toxic elements through chelation, extrusion, regulatory distribution, and vascular sequestration [20]. In addition, this technology involves translocation, accumulation, transport, transformation, and volatilization processes [21,22,23].
A large portion of the research on the phytoremediation of mercury has focused on finding mercury accumulator or hyperaccumulator plants because, despite their phytotoxic characteristics, there are plant communities that grow on mercury-contaminated sites [24,25,26,27,28]. Hyperaccumulator plants can accumulate large amounts of heavy metals at concentrations 10 to 100 times higher than non-hyperaccumulator plants can tolerate [29]. The limit of mercury hyperaccumulation in plant shoots is estimated to be 10 ppm, although this threshold needs to be more clearly established [30,31]. Therefore, the search for mercury hyperaccumulators and their application in practice has become a research hotspot [1].
In recent years, researchers have found remediating plants for mercury-contaminated soils, reporting on the mechanisms associated with Hg tolerance and the improvement of phytoremediation techniques by chemical and/or microorganism-assisted remediation. For example, Cardamine violifolia has a phytoremediation effect on soils contaminated with different mercury concentrations, reaching concentrations of 6000 μg/g [32]. The addition of sodium thiosulfate (Na2S2O3) to mercury-contaminated pots showed that Oxalis corniculata could recover and remediate mercury-contaminated soils [33]. In the case of rhizobial associations, Lupinus albus L. plants were able to accumulate approximately 370 and 360 mg/kg Hg in the roots and nodules, respectively, and to maintain constant levels of photosynthetic pigments when inoculated with the mercury-tolerant Bradyrhizobium canariense L-7AH strain [34]. On the other hand, the PtABCC1 transporter gene from Populus trichocarpa was overexpressed in transgenic Arabidopsis and Populus tomentosa (poplar) plants, showing higher mercury tolerance than wild-type plants [35].
Bibliometric analysis identifies and summarizes the main research points relevant to expanding, publishing, and applying up-to-date knowledge on a subject. It was initially proposed by Pritchard (1969) to analyze books and other media [36]. It integrates mathematical, statistical, and bibliographic methods to quantitatively analyze the distribution structure and quantitative changes in the literature in a given field [37]. This popular and effective method is used to determine and suggest future research trends and essential problems based on historical publications [38,39]. Therefore, a comprehensive bibliometric review in the research field will help current and future researchers strategically identify gaps and leverage their studies [40].
In this paper, we used VOSviewer, and Bibliometrix to analyze publications related to the phytoremediation of mercury-contaminated sites in Scopus and WOS. We wanted to show: (1) the overlap between the two databases and the types of publications; (2) which countries had the highest number of publications and how countries cooperate; (3) which publications are the most cited and which subject area they belonged to; and (4) how the field of mercury contamination phytoremediation has advanced. The types of publication, citations, countries, thematic categories, and institutions were collected and analyzed. Document co-citation analysis was considered to establish the similarity between documents based on bibliographic references as well as the co-authorship and citation analysis of documents, giving information based on the number of documents in which they appeared together, the kinship among documents, and the number of times in which they cited each other. Finally, word co-occurrence analysis was considered, which measures the similarity of documents, relating them according to their degree of co-occurrence. Therefore, this study aimed to analyze the distribution of publications in the field of the phytoremediation of heavy metals, such as mercury, to report on their critical points and provide reference data for new research directions based on bibliometrics to analyze mapping and knowledge gaps.
2. Materials and Methods
A bibliometric analysis was performed to understand the trend of the phytoremediation of mercury contamination using papers published from January 2000 to June 2023 in the academic databases WoS and Scopus. Traditionally, WoS and Scopus have been the two most widely used databases for bibliometric analysis [41]. It is important to note that the selection of this period was made due to the low volume of publications (less than 20) before 2000.
In both databases, the search was performed in the subject field including article title, abstract, and keywords. The query sets used for the literature search were (“phytoremediation”) AND (“mercury” OR “Hg”) AND (“plant*” OR “phyto*” OR “vegetation” OR “biomass” OR “hyperaccumulator” OR *accumula*). All articles were individually screened to eliminate irrelevant documents (i.e., those dealing with heavy-metal phytoremediation in which mercury was not included). The search results indicated 515 articles for WoS and 813 for Scopus. After eliminating the documents that did not comply with the thematic work, it was reduced to 458 documents (88.9% of 515) for WoS and 698 (85.85% of 813) for Scopus. The documents included citation information, volume, year, authors, affiliations, abstracts and keywords, funding details, and other information such as conference information and references.
Different research methods have been proposed in the field of bibliometric analysis, with scientific mapping being a recent method, which not only reveals the laws of development but also graphically expresses the relationship and evolutionary laws of knowledge structure in related fields [42,43]. Currently, there are several tools for scientific mapping including knowledge graph analysis, which have advantages and characteristics of their own [44,45]. Bibliometrix is an open-source tool for quantitative research in scientometrics and bibliometrics developed by Massimo Aria and Corrado Cuccurullo. This tool includes the main bibliometric methods of analysis and allows for the construction of data matrices for co-citation, linkage, scientific collaboration analysis, and co-word analysis (https://www.bibliometrix.org) accessed on 31 August 2023. VoSviewer is software that is used to plot co-occurrence maps of authors, citations, keywords, and other data and was developed by Van Eck and Waltman [46,47]. In this article, these two tools (VoSviewer 1.6.16, Low Countries, and R-Studio’s Bibliometrix R package version 4.1) were used to perform a global network map of collaborating countries, an author co-citation analysis was conducted to analyze the relationships between highly cited references, co-authorship for the analysis of the most productive authors, keyword or term co-occurrence analysis, and the strongest citation keywords. In turn, the “Analyze results” option was used in both databases to extract information on annual research, countries, journals, funders, and thematic areas (i.e., metric information on journals and documents). Before the diagrams were made, the data were processed manually in an Excel matrix to merge synonyms or similar terms and eliminate meaningless words to avoid affecting the analysis of the results.
3. Results and Discussion
3.1. Overlap Between WoS and Scopus Publications
A total of 1156 documents were found in the databases together, distributed between 458 documents for WoS and 698 for Scopus, of which 27% (313) were duplicates (i.e., they belonged to both databases) (Figure 1). Scopus is the largest database of abstracts and literature citations, with a broader coverage of journals compared with the WoS, but the latter is more selective [41].
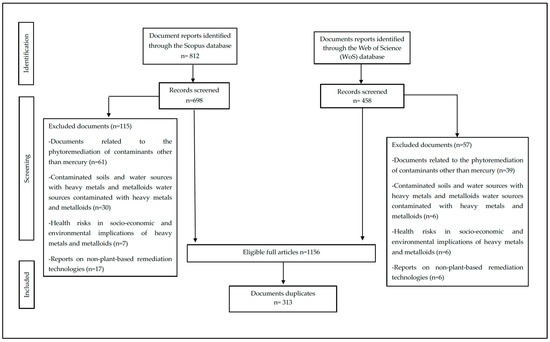
Figure 1.
Prisma diagram.
3.2. Annual Trends
The types of scientific publications related to the phytoremediation of mercury contamination are presented in Table 1. The papers included research articles, review articles, books, conference papers, and short surveys. In both databases, research articles were the most published (468; 67%) for Scopus and (396; 87%) for WoS, followed by review papers (15 and 11%, respectively).

Table 1.
Types of documents published in the period from 2000 to 2023.
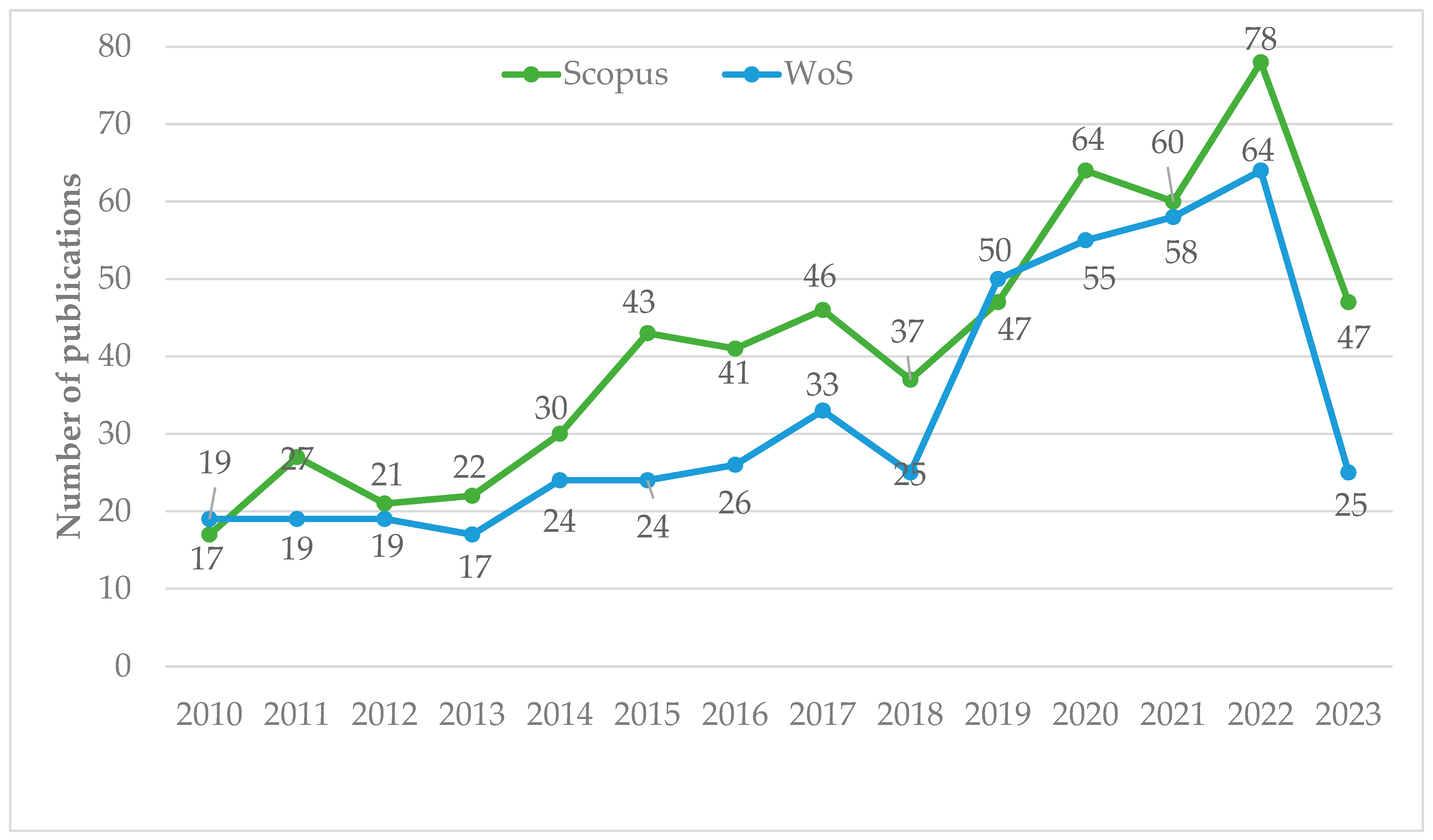
On the other hand, the annual production of publications is shown in Figure 2. Published research on the phytoremediation of mercury contamination show an increasing trend in both databases. Minimizing mercury contamination in soil is of great significance for ensuring the quality and safety of crops and maintaining ecological safety [32]. As far as the Scopus database is concerned, there was a significant increase in publications from 2011. Between 2015 and 2023, the number of published papers (463) more than doubled. In 2022, 78 articles were published, 2.88 times more than in 2011. Similarly, in WoS, the same trend continued, with 360 documents published between 2015 and 2023, which was 3.34 times more than in 2011. More publications are expected in the future due to the importance of phytoremediation not only in remediating contaminated land and water bodies, but also to discover Hg hyperaccumulators and aided techniques for the improvement of this ecological remediation technology [5,48,49].
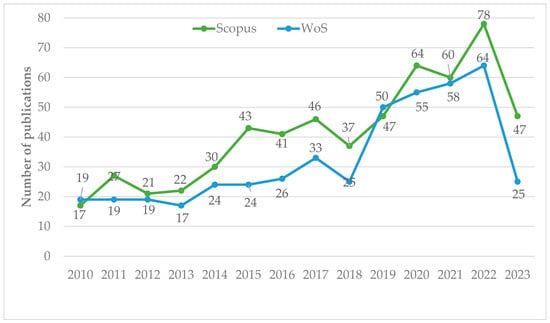
Figure 2.
Number of annual publications on the phytoremediation of mercury contamination included in the Scopus and WoS databases.
3.3. Main Subject Areas
The analysis by category or subject area indicated that in both databases, most of the documents belonged to the category of Environmental Sciences, represented by 33.5% for Scopus and 58.6% for WoS. It should be mentioned that in the Scopus database, the six areas that followed in order were Agricultural and Biological Sciences (16.5%), Biochemistry, Genetics, and Molecular Biology (9.9%), Medicine (5.7%), Engineering (5.6%), Chemistry (5.1%), and Chemistry Engineering (5.0%) (see Figure S1A). In the case of WoS, Environmental Engineering (13.1%), Plant Sciences (10.3%), Water Resources (6.1%), Toxicology (5.0%), Biotechnology Applied Microbiology (4.8%), and Chemistry Multidisciplinary (4.6%) predominated (see Figure S1B). In [50], the authors reported that Scopus has an orientation toward biomedical research and natural sciences, while engineering seems to be represented to a greater extent in WoS.
The results of this study are similar to those reported by [37,51] through a scientometric study on phytoremediation and heavy-metal hyperaccumulation using the WoS database, which reported that Environmental Sciences, Engineering Environmental, Water Resources, Plant Sciences, Toxicology, Soil Science, and Biotechnology Applied Microbiology were the most important categories. On the other hand, [52], in their article titled “Scientometric study of treatment technologies of soil pollution: Present and future challenges” reported that Environmental Sciences covered the largest number of publications, with 47.4% and 43.0% for WoS and Scopus, respectively. In this work, the authors present current technologies and their combinations for the remediation of contaminated soils, and also discuss different techniques and mechanisms for soil remediation including physical, chemical, and biological methods. Among these, the biological technique of phytoremediation was the keyword with the highest number of occurrences in the WoS and Scopus databases.
3.4. Most Productive Countries and Organizations
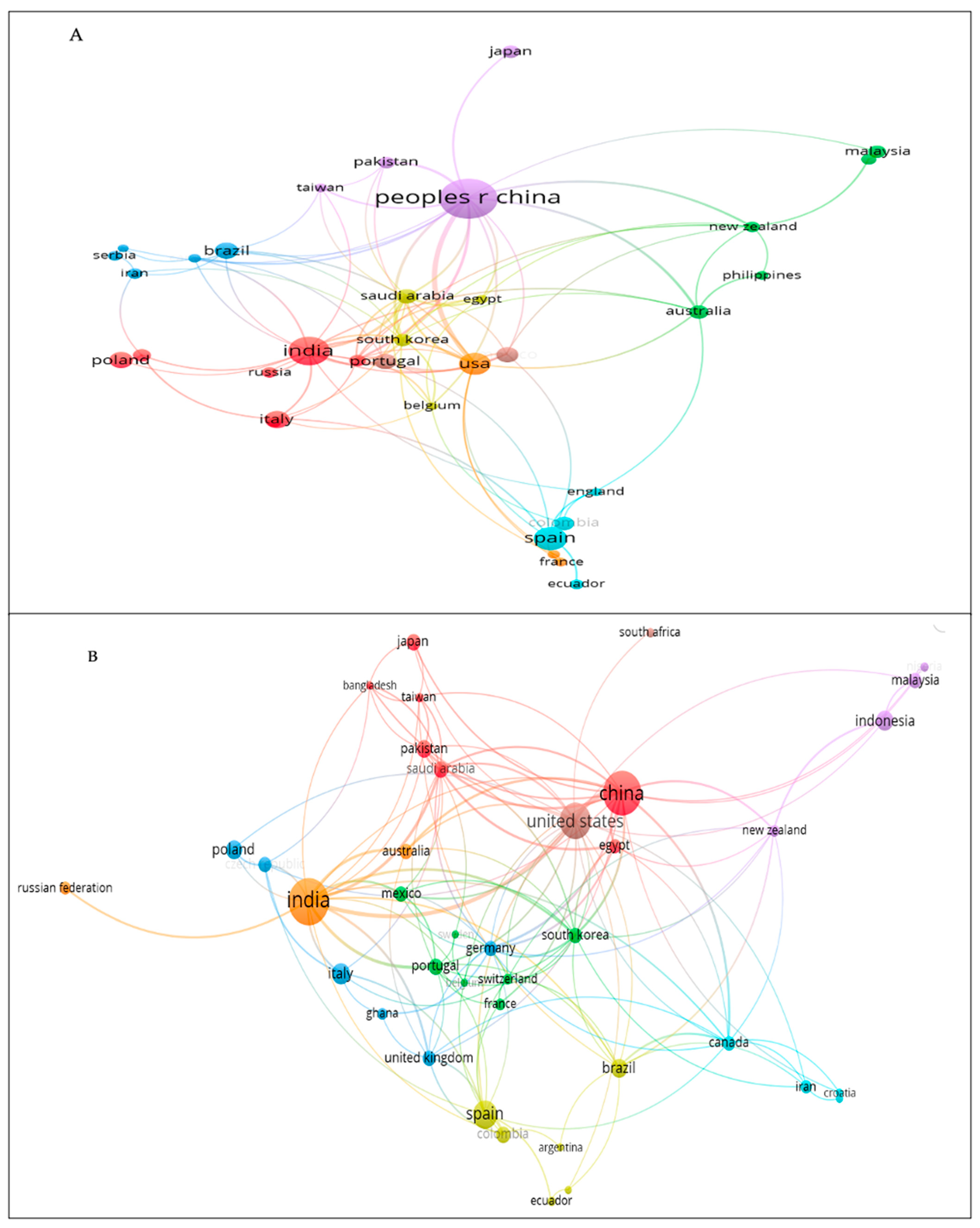
The remediation of mercury-contaminated sites is a global concern because of its bio-accumulative nature in food chains and the environment; hence, the intensity of the investigation may vary between countries. Figure S2A,B shows the number of papers published by each country in the WoS and Scopus databases. The intensity of the blue color is related to the countries with the highest number of published papers. In contrast, the gray color is related to the countries where no papers related to this topic have been published. Regarding the number of documents published by each country in the WoS database, 80 countries have published research papers related to this topic. China, India, and Spain were the countries with the highest production, with 115, 58, and 40 papers, respectively.
Regarding the number of citations, this is defined as the number of publications that cite the articles published each year [36]. Similarly, China and India had the highest number of citations (2784 and 1551), while Spain as in fifth place (867). In Scopus, 77 countries were found to have published papers on this topic. India (145), China (131), and the United States (86) had the highest production of documents. The highest number of citations by country was in China (2784), followed by India (1551), and the USA (1222). Out of 80 countries, 36 had at least 5 papers in WoS, while out of 77 countries in Scopus, 41 had at least 5 papers. In both databases, Africa had the fewest publications, followed by most of South America (except Brazil) and some Asian nations.
The co-authorship network was constructed with more than five articles for each database (Figure 3A,B). In WoS, the countries with the highest co-authorship were China, India, USA, Saudi Arabia, and South Korea, with total link strength (TLS) values of 52, 33, 33, 33, 26, and 23, respectively. Eight groups were distinguished, the most relevant being the first group formed by India, Italy, Germany, and Poland. Group 2 consisted of China, Japan, Taiwan, and Pakistan, while Group 3 included Spain, Colombia, Ecuador, and England. The USA was related to most countries. As in WoS, the most cooperative countries in Scopus were China, India, USA, Saudi Arabia, and South Korea, with TLS values of 59, 58, 53, 31, and 24, respectively. Eight groups were detected, of which four were the most relevant. China appeared in the first group and was related to most other countries. The second group mostly comprised European countries such as the Czech Republic, Germany, Italy, Poland, and the United Kingdom. The third group included South American countries (Argentina, Colombia, Peru, Brazil, Ecuador) and Spain. Finally, the fourth group comprised the United States and South Africa.
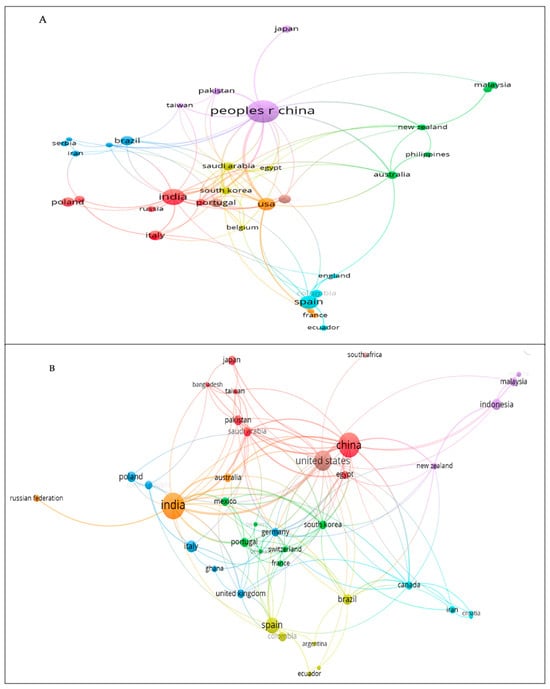
Figure 3.
Map of country cooperation: (A) WoS and (B) Scopus.
In general, global emissions are mostly caused by human activity, the static burning of fossil fuels, and biomass, accounting for about 24% of emissions. However, small-scale artisanal gold mining represents nearly 38% of total global emissions and was the main source of emissions in South America and Sub-Saharan Africa [6]. In addition, mercury mines are one of the most persistent anthropogenic sources of environmental contamination. Historical mining and cinnabar ore retorting released many elemental Hg (Hg0) and water-soluble Hg compounds into nearby surroundings [53,54] and generated numerous wastelands composed of mercury-enriched mine tailings (calcines) adjacent to abandoned retorts and smelters [5].
China was the country with the most publications related to the phytoremediation of mercury pollution. This could be attributed to the fact that, despite being rich in plant resources and preserving various ancient plants including more than 14,000 plant species, a significant number of mercury-enriched wastelands can be found in the Wanshan mining region. Mercury concentrations in calcines have been recorded as high as 4400 μg/g [36], continuously releasing mercury into surrounding ecosystems even after the mercury mines had been abandoned for several decades, emitting Hg0 and secondary Hg compounds through natural weathering and runoff into the air, soil, and water, entering the biota [9,55,56]. Due to the large cinnabar ore reserves and production of elemental Hg, Wanshan was once called the “Mercury Capital” of China. Thus, the abundant plant resources and high mercury concentrations have provided the basis for the evolution of accumulator plants [26]. Furthermore, the Chinese government has been actively improving the environmental quality in recent years and has invested significant financial and human resources in remediating contaminated soils [57,58]. Furthermore, the most prolific institutions and authors came from this country (Table 2 and Table S1). It is also worth noting that the three main sources of funding for research related to the phytoremediation of mercury contamination came from China: the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Key Research and Development Program of China.

Table 2.
Top 15 most prolific institutions in the phytoremediation of mercury contamination.
Similarly, countries such as the United States and Spain have large-scale mercury mineralization zones distributed along the world’s plate margins. For example, the New Almaden mine in the United States and the Spanish Almaden mine (the largest mercury mine in the world) with approximately 34,000 mg/kg of mercury, where the smelting process discharges a large amount of Hg into the environment [5,59]. Furthermore, the United States was the first country to actively participate in this field of study, indicating that it was a pioneer in this area [60,61].
It is also important to mention that in countries such as Colombia, Brazil, Southeast Asian countries, and some African countries, gold mining also causes severe mercury contamination [5,62,63].
Therefore, due to the remediation of large amounts of mercury-contaminated farmlands, phytoremediation, as an eco-friendly and efficient technology for the treatment of contaminated soils, has received significant attention and application in different countries.
On the other hand, 742 and 160 institutions for WoS and Scopus, respectively, contributed to the topic of the phytoremediation of mercury-contaminated sites. Institutions were ranked according to the total number of publications. In WoS and Scopus, six organizations had at least 10 papers. There as a greater diversity of institutions in WoS. In both databases, the Chinese Academy of Sciences contributed the most documents on this research topic with 29 for WoS and 39 for Scopus (Table 2).
3.5. Most Productive Authors
Determining the scientific production of the authors allowed us to analyze one of the most important structures that make up the scientific community on the phytoremediation of mercury-contaminated sites, reflecting the existing relationships among the members of this community. A total of 2476 authors had publications related to this topic in the Scopus database, whereas 1973 authors were registered in WoS. Table S1 shows the 10 authors with the highest number of publications related to the phytoremediation of mercury-contaminated sites, totaling 77 articles for WoS and 100 for Scopus.
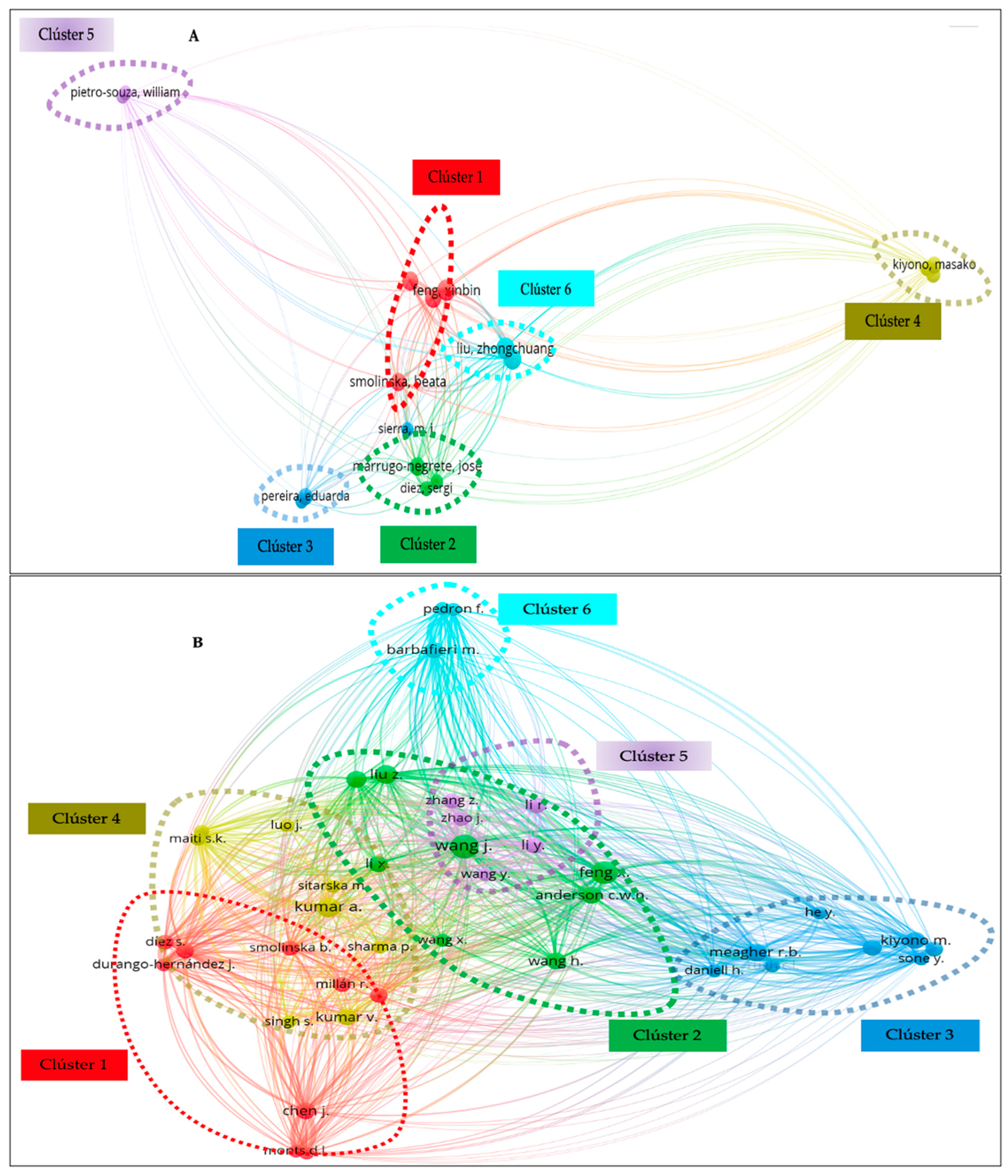
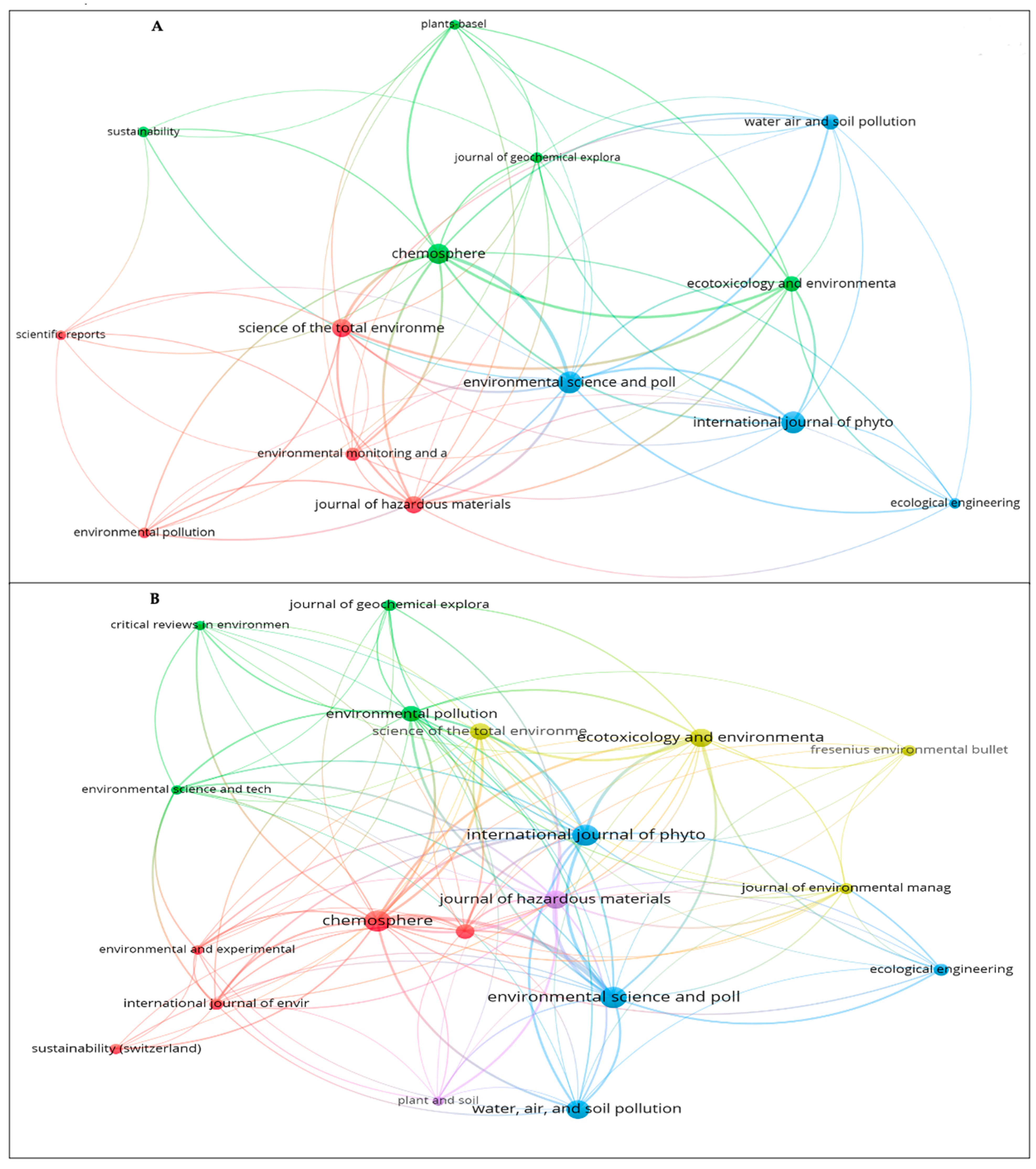
Similarly, Figure 4 shows the authors who to date have contributed the largest number of publications on the phytoremediation of mercury contamination. Each circle in the network in Figure 4A,B symbolizes a researcher; the closer the circles are together, the more closely the researchers collaborate. The extent of the co-citation link’s strength is shown by the size of the circle. Collaboration improves efficiency by making better use of available resources and transferring knowledge [64]. Seventeen authors produced at least five papers in WoS (Figure 4A). The top five authors were Liu Z. (10 publications), Feng X. (10 publications), Wang L. (9 publications), Wang J. (8), and Anderson C.W.N. (8). Three of the top writers were represented in cluster 1 of the six clusters (six colors). For Scopus, 41 authors met the requirement of at least 5 papers (Figure 4B). According to Scopus, the top five writers were Wang J. with 16 papers published, Feng X. (14), Kumar A. (11), Liu Z. (10), and Meagher R.B. (9). Three of the top authors were represented in group 1 of the five groups that were produced. From the Institute of Geochemistry, author Feng X. with 88 and 133 total links in WoS and Scopus, respectively, had the highest total link strength.
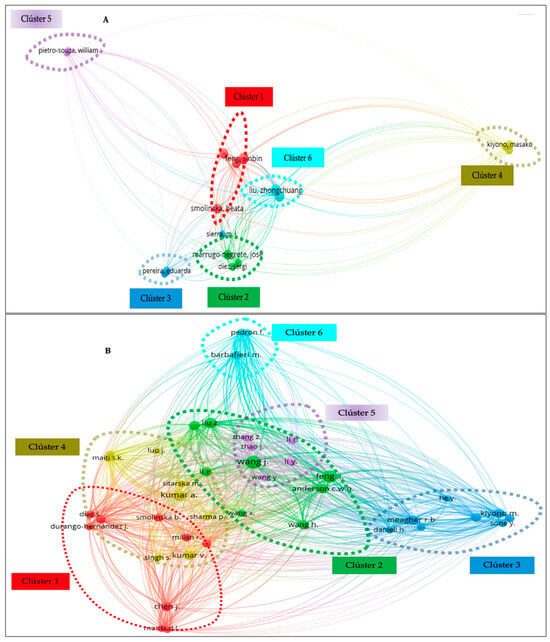
Figure 4.
The main authors that contributed to the publication of scientific papers related to mercury phytoremediation: (A) WoS and (B) Scopus.
It should be emphasized that Feng X, Wang J, and Anderson C.W.N have together investigated Brassica juncea L. plants, evaluating mercury accumulation and its exposure to different chelating agents, in which ammonium thiosulfate and sodium thiosulfate, EDTA, among others, were used to improve the phytoextraction of this heavy metal in the plant as well as the leaching of bioavailable mercury caused by chelating agents [65,66].
In a recent publication, Feng X and Wang J et al. reported that the plant Cadamine violifolia could be considered as a mercury hyperaccumulator due to the high concentrations of mercury that accumulated in its shoots and roots, translocation factors higher than 1.0, and a bioconcentration between 1.8 and 223 [32].
3.6. Most Recognized Journals
Regarding the publications, the analysis of journals in each research area can help researchers to accurately understand the top journals in the field of that research; in addition, it can guide bibliographic queries, data collection, and article writing [67,68]. In WoS, 197 journals were found, 14 of which had 5 or more published papers (Figure 5A). However, in Scopus, 359 sources were found, but only 20 journals matched the requirement of 5 or more published articles (Figure 5B).
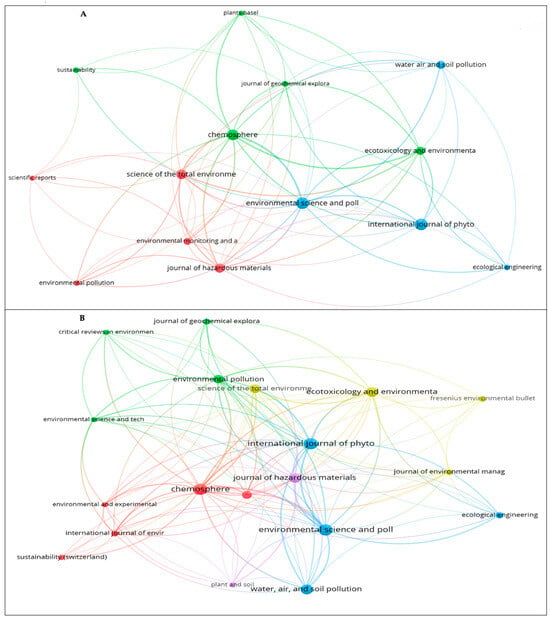
Figure 5.
Research sources: (A) WoS and (B) Scopus.
The top 10 journals in WoS accounted for 35.4% of the total (Table S2). These journals included Environmental Science and Pollution Research with 26 papers (5.7% of publications), International Journal of Phytoremediation with 26 papers (5.7%), and Chemosphere with 23 (5.0%). As for Scopus, the top 10 journals accounted for 26.7%. The top three journals, with a combined 26 published papers (3.7%), were Chemosphere, Environmental Science and Pollution Research, and International Journal of Phytoremediation (Table S3).
Oliveira Fernandes et al. (2021), in a scientometric analysis of research on mercury in soil, found that the 10 most cited articles included four from the journal Science of the Total Environment and one from Chemosphere [7]. It should be mentioned that [52], in their bibliometric analysis on hyperaccumulators for potentially toxic elements in the WoS database, reported that International Journal of Phytoremediation ranked first, followed by Environmental Science and Pollution Research, and Chemosphere, coinciding with the information reported in this study. Meanwhile, [37], in their study on the phytoremediation of heavy-metal pollution, reported that the top three journals were Environmental Science and Pollution Research, International Journal of Phytoremediation, and Ecotoxicology and Environmental Safety.
The WoS database showed that Science of the Total Environment received the most citations (1551), followed by Chemosphere (875), Ecotoxicology and Environmental Safety, and other journals with citation counts between 875 and 1551. Five journals were in the top quartile (Q1), while six journals had an H-index of at least 150. Four journals are published in the Netherlands and three in the United Kingdom. The subject areas of Environmental Science, Medicine, and Agricultural and Biological Sciences were related (Table S2). The journal with the most citations in Scopus was Chemosphere (2031), followed by Science of the Total Environment (1582), and Ecotoxicology and Environmental Safety (1500). Seven journals had an H-index of 150 or more, and six of them were in the first quartile (Q1). Notably, four of the top ten most productive journals are published in the Netherlands, while three are published in the United Kingdom. Environmental Science, Medicine, Chemistry, and Agricultural and Biological Sciences were the subject areas associated with the phytoremediation of mercury exposure (Table S3). Both databases indexed all journals, which have been categorized under the Environmental Science category.
3.7. Most Cited Documents
In both databases, a document citation analysis was performed. The citation visualization network of documents is shown in Figure S3, with documents with at least 30 citations serving as the starting point. The papers “Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability” [69], “The Phytochelatin Transporters AtABCC1 and AtABCC2 Mediate Tolerance to Cadmium and Mercury” [70], and “A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques” [71] had the most citations in WoS, with 814 (9.2%), 382 (4.3%), and 277 (3.1%), respectively. As for Scopus, the papers “Plant Responses to Abiotic Stresses: Heavy Metal-induced Oxidative Stress and Protection by Mycorrhization” [72] (1766; 5.7%), “Remediation Technologies for Metal-Contaminated Soils and Groundwater: An Evaluation” [73] (1235; 4.0%), and “Trace Elements in Agroecosystems and Impacts on the Environment” [74] (1171; 3.8%) were the most cited papers in the works considered for this study. In most of these papers, Hg was discussed with other toxic metals. Tables S4 and S5 list the 20 papers with the most citations in the WoS and Scopus databases.
The articles that were most frequently cited in WoS can be categorized into four groups. (1) Reviews of various remediation techniques for cleaning up heavy metal-contaminated sites. Among these, phytoremediation is a promising technique for cleaning up large sites with relatively low contaminant concentrations at shallow depths. It is considered cost-effective, ecologically beneficial, and has high public acceptance. (2) Studies that explore the mechanisms used by plants to accumulate, tolerate, and overexpress transporters associated with mercury detoxification. (3) Articles focused on the use of constructed wetlands to remove metals and metalloids from contaminated waters. (4) Studies investigating the health risks associated with heavy-metal contamination in soil, particularly mercury and cadmium. In Scopus, the most cited papers could be divided into six categories. (1) Molecular mechanisms associated with the role of antioxidants as protection against heavy-metal stress in roots, fungi, and mycorrhizae. (2) Selection of remediation methods for heavy-metal contaminated soils and sediments, which depend on the site characteristics, concentration, types of contaminants to be removed, and end use of the contaminated medium. (3) Improvements in the phytoremediation process such as plant-microorganism synergy. (4) Use of wetlands in their restoration processes. (5) Use of transgenic plants in the process of phytoremediation of heavy metals, in case mercury, transporters, and inclusion of mers-type genes are cited.
In WoS, more than 50% of the 20 highly cited papers came from the following five countries: China (15%), India (15%), USA (10%), Spain (10%), and Italy (10%). According to Table S4, reviews comprised 60% of the papers, whereas research articles comprised 40%. A total of 50% of the most cited papers were published in the journals with the highest scientific production (Table S2), predominantly Ecotoxicology and Environmental Safety, Chemosphere, and Science of the Total Environmental Safety. In contrast, the 20 most often cited reviews in Scopus were written by authors from the United States (25%), China (105), India (10%), Germany (10%), and the United Kingdom (10%) (Table S5). Major journals published only 25% of the manuscripts (Table S3), while China, the United States, and India worked closely together on both databases (Figure 3A,B). Information about a country was based on data provided by the corresponding author.
It should be noted that China, the United States, and India have focused on the accumulation of heavy metals by aquatic plants in aquatic matrices [75,76,77]. The potential to remove mercury from sediments was established by research on the aquatic plants Carex heterostachya, C. brevicuspis, A. selengensis, Polygonum hydropiper, and G. longituba [78]. According to Ulaganathan, E. crassipes, A. cristata, and S. natans are three more prospective candidate aquatic species for the phytoremediation of hazardous metals such as Pb, As, Hg, Cd, and Ni [77]. In China, mercury-contaminated croplands account for 1.6% of all croplands contaminated with heavy metals, resulting in mercury accumulation in the food chain. For example, rice (Oryza sativa L.) has been shown to absorb and accumulate mercury from the soil and transfer it to edible parts of plants, with mercury and methylmercury concentrations between 7.5 and 608 μg/kg and 7.37–62.3 μg/kg, respectively. Methylmercury absorbed by rice accounted for more than 30% of the total mercury. These results exceed the 20 μg/kg permitted limit for food safety [79,80]. Likewise, the effect of soil microorganisms on mercury uptake in rice plants was examined to determine possible soil phytoremediation agents, and it was observed that inoculations of arbuscular mycorrhizal fungi (AMF) significantly reduced the concentration of the metal in rice. Therefore, this study suggested the possibility of using microorganisms for the remediation of heavy-metal contamination in soil and provided valuable information on the reduction in human exposure to mercury through rice consumption [81].
On the other hand, recent studies in both databases on mercury phytoremediation have focused on the use of microorganisms to remediate mercury contamination, and good results have been obtained [82,83]. For example, the phytoremediation potential of Boehmeria nivea L. Gaud and the response of its rhizosphere soil microbiome to mercury contamination were evaluated, and Proteobacteria, Actinobacteria, Gemmatimonadota, Latescibacterota, and NB1-j were identified as potential mercury-tolerant taxa [84]. Furthermore, [85] demonstrated that the endophytic fungi Westerdykella aquatica P71 and Pseudomonodictys pantanalensis nov. A73 stimulated mercury uptake and promoted its accumulation in plant tissues, preferably in roots, mitigating the effects of metal phytotoxicity. Sharma et al. performed the phytoremediation of heavy metals using Brevundimonas sp. in the rhizosphere zone of Saccharum munja L, revealing that heavy metals such as Fe, Mn, Pb, Cd, Cr, Cu, Zn, Ni, As, and Hg were uptaken and translocated [86]. Bacterial isolates identified as Jeotgalicococcus huakuii and Bacillus amyloliquefaciens from C. dactylon and E. indica plants, respectively, showed the complete detoxification and bioaccumulation of mercury, in addition to indole acetic acid (IAA) production, ammonium production, and siderophore production [87]. On the other hand, many scholars have tried to find a hyperaccumulator of mercury, and more than 200 plant species have been used to test their ability to accumulate and transfer mercury [24,26]. Cardamine violifolia could be considered as a mercury hyperaccumulator as it accumulated concentrations of up to 6000 μg/g in its roots and upper parts [32].
In general, articles in recent years have focused on finding phytoremediation plants for mercury contamination from their natural habitats, on the improvement of crops for safer food through phytoremediation, the use of chelating ligands and plant–microorganism symbiotic relationships to strengthen the phytoremediation capacity of plants, the use of constructed wetlands to improve the phytoremediation of aquatic ecosystems as well as studies at the molecular level related to metal sequestration, detoxification, uptake and transport in plants, and omics approaches related to the analysis of proteins and genes expressed in plants.
3.8. Author Keyword Trend Analysis
Keywords are terms or short phrases that classify and address entries in indexing and information retrieval systems in databases for a particular manuscript or subject area. Keywords have become an essential bidirectional tool for people who write and people who search for information about related manuscripts or subject areas [52,53,88,89].
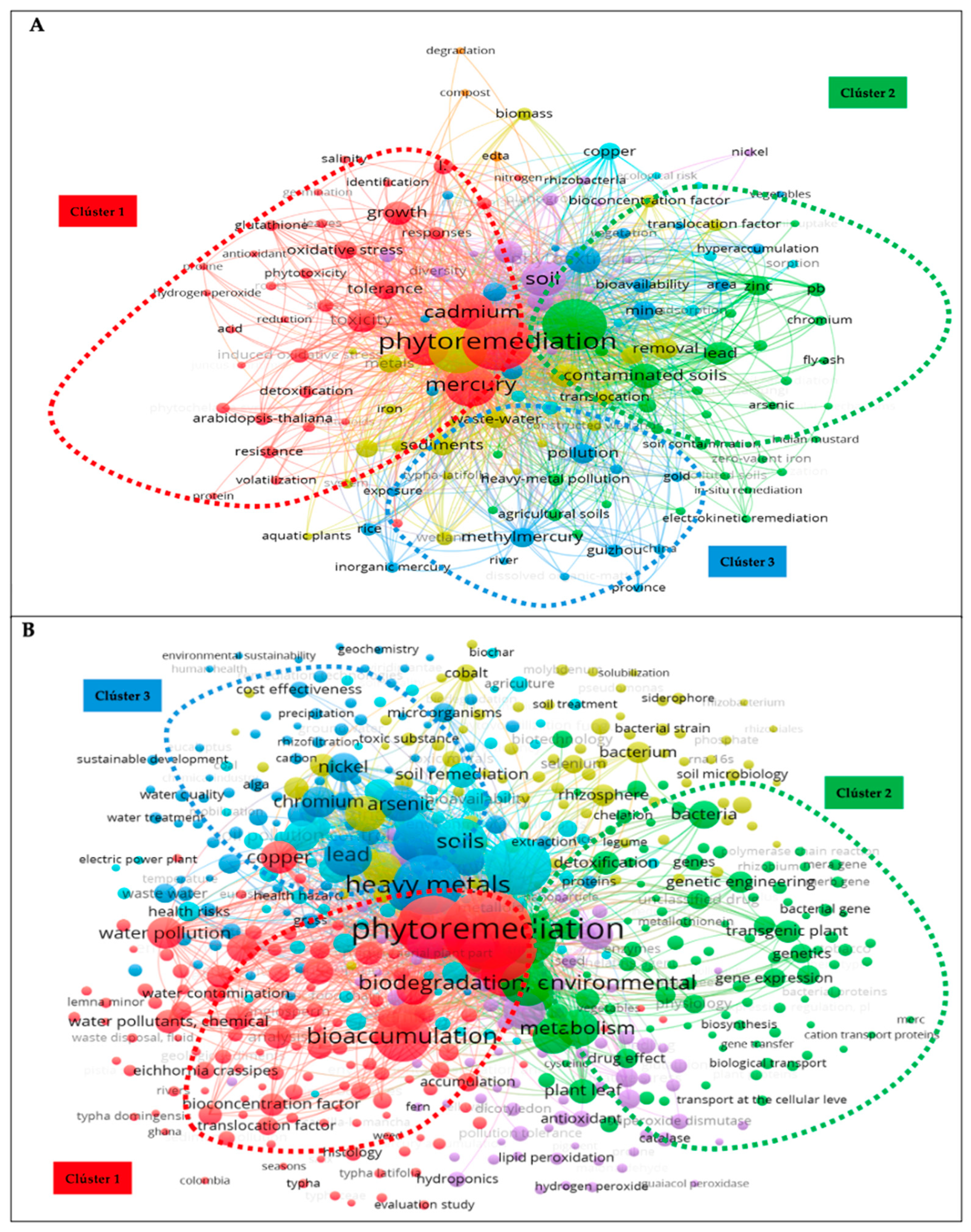
A criterion of selecting keywords with a minimum of five occurrences was applied. In WoS, the frequency of keywords in 457 documents was evaluated, and 188 keywords were found (Figure 6A). Meanwhile, 666 words were found in Scopus out of 697 analyzed (Figure 6B).
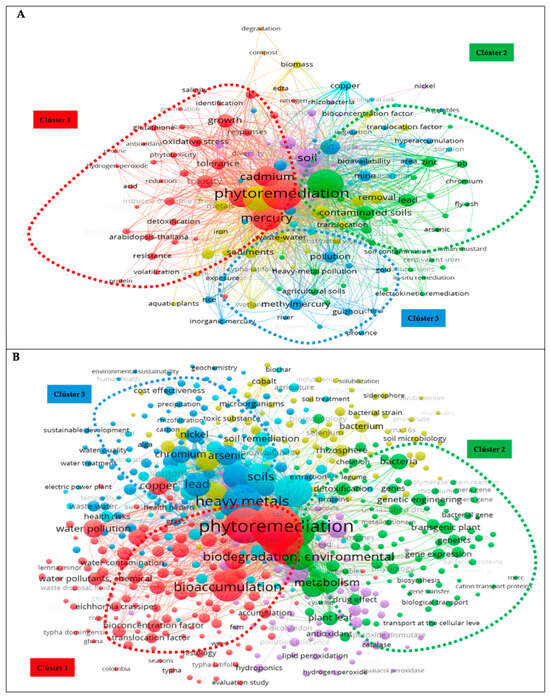
Figure 6.
Keyword co-occurrence analysis: (A) WoS and (B) Scopus.
Excluding the terms phytoremediation and mercury in both databases, the most used keywords in WoS were heavy metals, with 242 (7.2%) occurrences, accumulation (196, 5.8%), cadmium (122; 3.6%), soils (115; 3.4%), and phytoextraction (70; 2.1%). In Scopus, excluding the words nonhuman and article, the keywords with the highest number of occurrences were bioremediation (333; 2.6%), heavy metals (299; 2.3%), soil pollution (277; 2.2%), bioaccumulation (198; 1.5%), and biodegradation and environmental (175; 1.4%). Even though they were not included in the descriptor, these keywords had a high co-occurrence strength, indicating that they were highly important and received a lot of attention.
In addition, Figure 6A displays the co-occurrence of keywords in the WoS database, categorizing them into eight clusters. Among the three major clusters, the red cluster, which used the term phytoremediation, was the largest. It connected phrases such as “oxidative stress”, “detoxification”, “tolerance”, and “heavy metals such as cadmium and mercury” and contained the keywords antioxidant, growth, tolerance, resistance, glutathione, proteins, and phytotoxicity. Group 2 (green) was more related to soil contamination by heavy metals, their accumulation, and remediation and included the term heavy metals and heavy metals such as arsenic, chromium, lead, and zinc. It appeared in the cluster of agricultural soils, spatial distribution, and other remediation techniques such as electrokinetic remediation. All keywords in this cluster had a close conceptual link. Group 3 (blue cluster) contained different forms of mercury: atmospheric mercury, inorganic mercury, and methylmercury. Words such as exposure, extraction, fractionation, and speciation were part of this cluster. The Scopus database findings regarding the co-occurrence of terms were divided into six groups or clusters (Figure 6B), with the following being the most significant. The first cluster (red) focused on phytoremediation. The effectiveness of a plant with phytoremediation potential was defined by the terms bioaccumulation, translocation, bioconcentration factor, and translocation as well as bioremediation. The same was true for this cluster of connections between phytoremediation and aquatic settings including aquatic macrophytes, aquatic ecosystems and environments, wetlands, water pollution, and aquatic plants of the genus Typha. Group two (green color) involved genetic engineering associated with phytoremediation, gene and protein expression, genetically modified plants, mers genes, and other processes related to plant detoxification and tolerance to heavy metals such as mercury. In addition, this group contained several mercury salts and compounds including methylmercury and mercury chloride. Cluster three (blue) included environmental pollution by heavy metals and their removal processes. It also included phytoremediation processes such as phytoextraction, phytovolatilization, phytodegradation, phytostabilization, and hyperaccumulation, which define the effectiveness of phytoremediation. Heavy metals such as arsenic, cadmium, chromium, lead, and nickel, were found in this group. In general, the research topic can be expressed using keywords. Through these studies, it is possible to comprehend the key research areas and upcoming developments in the field [43].
3.9. Keywords with the Strongest Citation Bursts
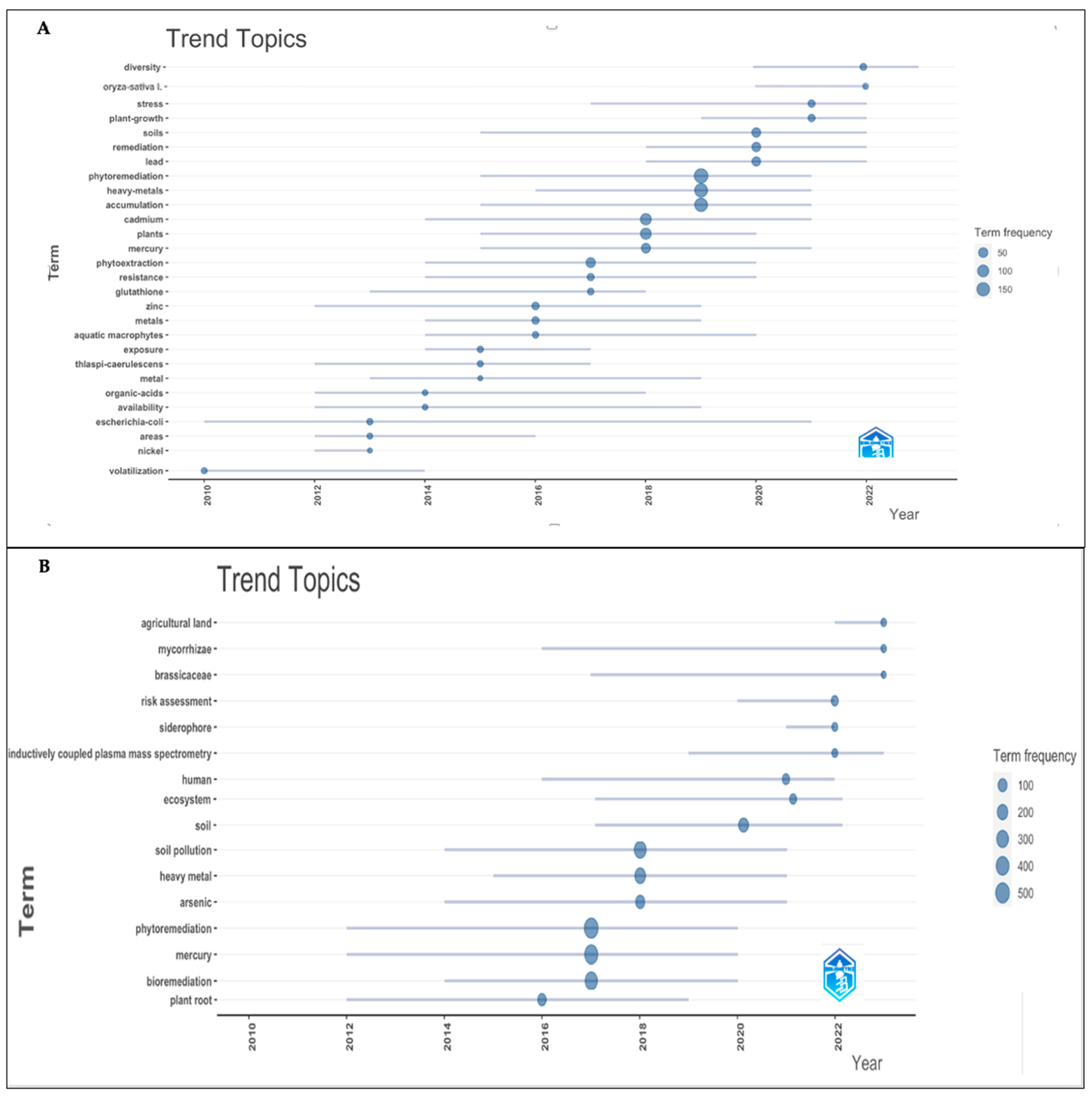
To obtain more information about the phytoremediation of mercury contamination, burst analysis in Bibliometrix software was used to perform a retrospective keyword analysis. Keyword analysis can be used to examine development trends and research frontiers. In the Bibliometrix software, the size of the circle indicates the strongest words, and the lines indicate the period. The program identified 28 and 18 main keywords with a minimum frequency of five for WoS and Scopus, respectively (Figure 7A,B, Supplementary Tables S6 and S7). In WoS, the keywords could be divided into the following main aspects:
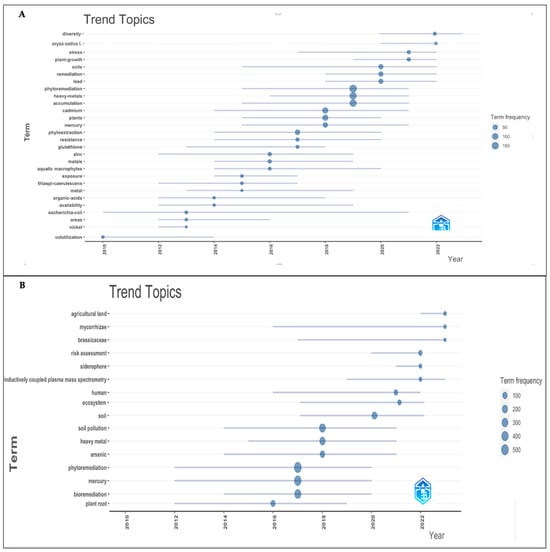
Figure 7.
Top keywords with the strongest citation bursts: (A) WoS and (B) Scopus.
- -
- Phytoremediation of heavy metals. The remediation of “heavy metals” (2016–2021), “metals” (2014–2019), and specific heavy metals such as “lead” (2018–2022), “cadmium” (2014–2021), “mercury” (2015–2021), “zinc” (2012–2019), and “nickel” (2012–2013) was investigated, with mercury and cadmium being the metals with the highest frequency. This group also included terms like “Remediation” (2018–2022), “Phytoremediation” (2015–2021), “Phytoextraction” (2014–2020), and “Volatilization” (2010–2014).
- -
- Exploration of mechanisms by which plants accumulate metals. Exploring the mechanisms by which plants absorb, accumulate, resist, stress, and translocate metal helps in understanding the process of mercury detoxification in plants. This group included “Diversity” (2020–2023), “Plant-Growth” (2019–2022), “Stress” (2017–2022), “Accumulation” (2015–2021), “Resistance” (2014–2020), “Availability” (2012–2019), “Organic-Acids” (2012–2018), “Glutathione” (2013–2018), and “Exposure” (2014–2017). Among them, “Accumulation” showed the highest frequency, which could indicate ongoing research into how plants accumulate mercury, since although plants show a well-developed mechanism for the absorption, translocation, and detoxification of mercury, there are still knowledge gaps in the molecular understanding of these processes [1].
- -
- Rice (Oryza sativa) (2020–2022) is one of the most important cereal crops, feeding nearly half of the world’s population. Mercury concentrations in rice grains can reach up to 460 μg/kg when grown in Hg-contaminated soils [90]. Thus, the accumulation levels of mercury in rice have been studied [91]. Through genetic engineering, E. coli expressed four metallothioneins from rice (Oryza sativa L.), which conferred enhanced mercury tolerance, metal binding, and sequestration [92].
- -
- Aquatic macrophytes (2014–2020) have been widely explored due to concern about the ecological water environment, and research has been conducted on the ability of several aquatic plants to absorb heavy metals [93,94].
Regarding the Scopus database, the keywords could be divided into the following aspects:
- -
- Phytoremediation of heavy metals. The remediation of “heavy metals” (2015–2021) and individual heavy metals like “mercury” (2012–2020) and “arsenic” (2014–2021) were investigated. This group also included terms such as “Bioremediation” (2014–2020), “Phytoremediation” (2012–2020), and “Phytostabilization” (2015–2022).
- -
- Search for strategies to improve phytoremediation efficiency. This group included “Siderophore” (2021–2022), “plant root” (2012–2019), and “Mycorrhizae”, which began in 2016 and continues to date, indicating that it is a research hotspot.
- -
- Identified hyperaccumulator families. There were 721 hyperaccumulator species divided into 52 families and 130 genera, ranging from annual grasses to perennial shrubs and trees, with the Brassicaceae family (2017–2023) being the largest [95,96,97].
- -
- Risk assessment. This group included “Risk Assessment” (2020–2022), “Human” (2016–2022), “agricultural land” (2022–2023), “ecosystem” (2016–2022), and “soil pollution” (2014–2021). It is known that mercury is highly toxic to living organisms, and its presence in agricultural land poses another route of exposure. Therefore, phytoremediation combined with risk assessment is a topic that requires attention.
- -
- China (2017–2022) is one of the top three countries publishing papers on the phytoremediation of mercury pollution and is a keyword with the highest citation frequency.
3.10. Points of Interest in the Research
Considering the keyword analysis, research on the phytoremediation of mercury contamination also informs on the degree of contamination in soils, sediments, and watersheds [24,98,99]. Thus, knowledge about local plant screening in mercury-contaminated sites will help to obtain a better effect on ecosystem remediation. In general, the keywords covered four research directions: (1) finding native plants, (2) genetic engineering applications (3) increasing remediation ability through assisted phytoremediation, and (4) the detoxification mechanism of Hg.
- (1)
- Identification of mercury phytoremediation plants
Some plants have adapted to survive in mercury-contaminated sites thanks to evolution in molecular tolerance mechanisms, so they can behave as mercury accumulators or hyperaccumulators [98]. When the plant absorbs mercury, it accumulates in the vacuoles, which have low metabolic activity, keeping heavy metals away from important cellular metabolic processes. This may serve as a tolerance mechanism in plants [100]. More than 700 plant species have been identified with the ability to hyperaccumulate heavy metals, ranging from annual grasses to perennial shrubs and trees [96]. In terms of research uniqueness, researchers have reported different plant species identified in mercury-contaminated sites. For example, in China, Quian et al. found a total of 259 plants belonging to species in 29 families that grew on mercury-rich wastelands composed of cinnabar mine tailings (calcines) in the Wanshan region of southwest China, the third-largest Hg mining district in the world. Of these plant species, Arthraxon hispidus, Eremochloa ciliaris, Fallopia multiflora, and Ixeris sonchifolia exhibited higher concentrations of this metal in their roots and shoots compared to other species [26]. Similarly, Marrugo-Negrete et al., in a former mining district in Colombia, identified 24 plant species, grouped into 16 families, and the five species with the highest concentrations of the metal in their roots were Jatropha curcas, Thalia geniculata, Piper marginathum, Cyperus ferax, and Ricinus communis [24]. Xun et al., in the mountainous area of Dehua and Wanshan in China (highly mercury-contaminated sites), collected a total of 14 dominant wild plants, among which were Cyrtomium macrohyllum, Woodwardia unigemmata, Dennstaedtia hirsute, Ageratum conyzoide, and Conyza canadensis. All of these plants exhibited high resistance to mercury toxicity [25]. However, although some species are considered as potential mercury hyperaccumulators, no mercury hyperaccumulator has been reported to date. Therefore, discovering mercury hyperaccumulators is highly desirable [5,26,32].
It should be said that at present, many studies on the phytoremediation of mercury contamination are still in the laboratory stage [25,32], and very few studies have been conducted at contaminated sites [101]. These studies often overlook the complex dynamics of open fields such as climatic conditions, geographic factors, and the heterogeneity of mercury contamination (Table S8). Therefore, further research is required to verify the effectiveness of the phytoremediation process on a larger scale and develop site-specific protocols that account for the variability in plant behavior. Overall, the conditions of the contaminated site and the plant communities present must be considered to improve phytoremediation in mercury-affected sites. It is also necessary to investigate the mercury content in plant species that have not been studied yet [5]. Native plants are suitable for managing both contamination and the environment [37].
- (2)
- Applications of Genetic Engineering (OK)
Currently, genetic engineering allows for the integration of genes from other organisms to enhance the capacity and accumulation of heavy metals in plants, thus improving the phytoremediation conditions. For example, genetically modified Spartina alterniflora for mercury phytoremediation is resistant to high concentrations of this metal [102]. Similarly, genetic engineering can also be used to change the storage location of heavy metals and reduce the damage to plants caused by stress to these pollutants [103]. Most genetically modified plants for mercury phytoremediation are based on the mer operon gene, and these genes are involved in the process of translocating mercury (Hg2+) into the cell. They play a significant role in the biogeochemistry and bioremediation of mercury by converting reactive inorganic and organic forms of mercury into relatively inert, volatile, monoatomic forms [104]. For instance, transgenic rice (Oryza sativa) with the merA gene is able to withstand higher mercury concentrations compared to plants without the gene [105]. Additionally, the overexpression of PtABCC1 in Populus trichocarpa has been shown to be effective in A. thaliana and Populus tormentosa, promoting growth at certain mercury concentrations and increasing mercury accumulation in both the roots and shoots compared to wild-type plants [35,106].
In general, genetically modified plants for mercury resistance have provided valuable insights, enabling significant steps toward achieving more successful phytoremediation methods [98]. Multi-omics methods such as transcriptomics, proteomics, and metabolomics are required to reveal the functional roles of genes, proteins, and associated metabolites in mercury accumulation.
- (3)
- Assisted phytoremediation
- -
- Chelating agentsSeveral approaches have been widely used to improve the phytoremediation capacity in the field of heavy-metal pollution. Sometimes, the availability of metals in the soil is insufficient, and consequently, no active uptake of the metal by the root is generated. Mercury negatively interferes with plant growth as well as with their metabolic process, so some research has focused on the use of assisted enhancements to improve the efficiency of phytoremediation. It has been shown that sodium thiosulfate (Na2S2O3), ammonium thiosulfate (NH4)2S2O3, ethylenediaminetetraacetic acid (EDTA), diethylaminapentaacetic acid (DTPA), (NH4)2SO4, NH4Cl, NaNO3, (NH4)2S2O3, HCl, KI, and citric acid are related to the phytoremediation of mercury-contaminated soils, helping to improve their efficiency [66,69,107,108,109].Previous studies conducted on Oxalis corniculata and Brassica juncea L. found that chelating agents increased the concentration of mercury in the plant tissues of plants, achieving higher translocation and bioconcentration factors, which could be associated with the fact that the soluble complexes chelated by them can easily pass through the endodermis and enter the xylem [66,69].On the other hand, the use of organic amendments has also been used to increase the adsorption capacity of heavy metals. For example, the use of biochar (MB2) and attapulgite (MA2) showed that they can improve the growth capacity in Solanum nigrum L., a favorable feature for phytoremediation as well as remove the soil contaminant and increase the concentrations of metals in plant tissues [110]. Likewise, the addition of compost (derived from green waste) to the contaminated soil decreased the concentration of total mercury, where the higher the compost content, the lower the mercury contamination in soils [111].The application of selenium (Se) fertilizers has been used as a cost-effective strategy to ameliorate mercury accumulation and the noxious effects of mercury [112]. For example, the development of apoplastic barriers in the root endodermis of Oryza sativa crops in hydroponic solutions contaminated with mercury and methylmercury (MeHg) improved with the addition of selenium and also showed a remarkable decrease in Hg and MeHg concentrations [113].Therefore, the inclusion of these chelating agents or the development of new ones to improve the ability of plants to remove mercury from the soil is an important goal to consider.
- -
- Growth-promoting microorganismsGrowth-promoting rhizobacteria help plants cope with stressful environments in addition to forming beneficial host–plant relationships, which can increase the phytoavailability of metals while reducing their toxic effects. They stimulate the ability of the host plant to generate more biomass while storing significant concentrations of metals and decreasing toxicity [114,115,116]. Bacteria, fungi, and other microorganisms living in the rhizosphere can increase the availability of heavy metals by either changing the soil pH, releasing chelators, or having redox reactions with heavy-metal ions. Rhizosphere microorganisms in mercury-contaminated soils can reduce Hg2+ to less toxic volatile Hg0 through enzymatic reduction [15]. There is a belief that beneficial fungi in the rhizosphere stimulate root proliferation and enhance plant response to soil chemistry through plant–microbe association [117,118]. It should be added that rhizobacterial fungi and plant growth-promoting rhizobacteria (PGPR) induce heavy-metal tolerance responses [119]. For example, the symbiotic association between Lolium perenne roots and arbuscular mycorrhizal fungi (Glomus sp., Acaulospora sp., Entrophospora sp., Scutellosospora sp. and Glomus sp., Acaulospora sp., Entrophospora sp., Giaspora sp.) showed that there was enhanced mercury uptake. Arbuscular mycorrhizae play an important role in heavy-metal tolerance and have several extracellular and intracellular defense mechanisms such as plant tissue growth mechanisms and biological mechanisms of nitrogen fixation [120]. Likewise, the high mercury accumulation in Erato polymnioides could be based on a core of highly infectious strains of arbuscular mycorrhizal fungi (AMF) that are capable of improving the nutritional status of the plant and making mercury bioavailable species [31]. Therefore, the identification and characterization of heavy-metal tolerant microorganisms in soil and the development of microbial agents is a key research objective.
- (4)
- Mercury detoxification mechanisms
Plants that accumulate heavy metals have adopted strategies to cope with their harmful effects by carrying them from the source where they are found to their roots, either through the plasma membrane of root endodermal cells or by movement through the free space between the cell wall [121,122], where they can be stored or translocated to shoots through xylem vessels, where they are deposited in vacuoles. The travel of heavy metals to vacuoles is controlled and regulated by molecules involved in cross-membrane transport or their complexation and subsequent sequestration. ATP-binding cassette (ABC) transporters have been shown to transport As, Cd, and Hg [37]; it is also considered that Zn, Cu, or Fe transporters may be possible candidates to facilitate Hg influx [123].
It is worth mentioning that the formation of reactive oxygen species (ROS) is produced as by-products of several metabolic pathways that are located in different cellular compartments (chloroplasts, mitochondria). It is held that the Hg level in leaves interferes with mitochondrial activity and triggers ROS generation, malondialdehyde accumulation resulting from lipid peroxidation, and causes cell disruption [124]. Just as ROS can damage cells, they can also initiate responses related to gene overexpression. The accumulation of ROS can be counteracted by antioxidant enzymes including a variety of detoxifiers such as superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione peroxidase (GPX), glutathione-S-transferase (GST), catalase (CAT), and non-enzymatic low molecular weight enzymes such as ascorbic acid (ASH), glutathione (GSH), α-tocopherol, carotenoids, and flavonoids [125]. SOD, together with GSH, can scavenge reactive oxygen species and prevent lipid overoxidation in membranes [25]. SOD is considered to be the most efficient of the intracellular enzymes and is important in the plant’s tolerance to all types of stresses because they provide the first line of defense against the effects of elevated ROS levels [125]. Increased SOD at low Hg concentrations can scavenge ROS and alleviate plant damage, while increased GSH at high Hg concentrations, aside from helping to scavenge ROS, can also induce the formation of phytochelatins and an increase in proline, an intracellular substance important for an osmotic adjustment that can regulate cellular osmotic homeostasis disturbed by heavy-metal stress and protect the structural and functional integrity of cells [25,126,127,128]. Thus, for example, Cyrtomium macrophyllun, exposed to concentrations up to 1000 mg kg−1 of Hg, presented high resistance to mercury stress due to the increase in activities such as SOD, GSH, and proline compared to the control. Phytotoxic effects such as chlorosis and necrosis were low [25]. The transcripts and activities of SOD, POD, and CAT were pronouncedly enhanced in transgenic Arabidopsis compared to the control when exposed to HgCl2 [129].
Therefore, the synthesis of antioxidant and non-enzymatic enzymes in plant tissues may be an important mechanism for the establishment of the defense systems of reactive oxygen species under heavy-metal toxic stress [130].
3.11. Problems and Challenges
Significant progress has been made in addressing mercury contamination in soil through the use of emerging technologies such as phytoremediation, which remains a particularly attractive option. However, there are some issues to consider:
- -
- The practice of phytoremediation can take years for the various contaminated fields, so the discovery of local species is necessary, as screening for locally tolerant species is becoming an alternative approach for soil restoration [121] including native species that have not been previously studied.
- -
- Most studies have been conducted at the laboratory scale using nutrient cultures and pot experiments on soil contaminated with mercurial inorganic salts. These investigations should be transferred to field experiments because these experiments are not likely to be able to define a hyperaccumulator. In addition, the tests performed in the field or in the laboratory show significant differences. For example, van der Ent et al. pointed out that hyperaccumulators should be recorded from natural habitats and did not consider the extreme accumulation achieved by laboratory work with acidified and artificially modified soils or water to be hyperaccumulation [60].
- -
- Plant growth and the bioavailability of metals are very important in the phytoremediation process. For plants, high biomass, environmental adaptability, response to Hg tolerance, root characteristics (roots with high root zone), and root protection, as it is the main organ affected by mercury, are factors to be considered. Most of the accumulator plants found so far have short cycles, with low biomass and present phytotoxic effects as the concentrations of the metal increase. Regarding the bioavailability of metals, the conditions, physicochemical properties, and characteristics of the medium (water, soil, mud, sediment, etc.) all have influences or impacts on the phytoremediation potential of plants.
- -
- Phytoremediation assisted by chelating agents or microorganisms has been shown to enhance the phytoremediation process. Some microorganisms can promote plant growth and tolerate Hg, therefore, the rational screening of microorganisms should be carried out, and microbial taming techniques should be used to help plants ameliorate mercury-contaminated environments, so the mechanism of microbial symbiosis is worth exploring further. Studies conducted with chelating agents or amendments have shown an increase in the rate of accumulation, so there is an option to further investigate all soil amendments such as biochar, compost, and biosolids in the future.
- -
- With the development of genetic engineering and molecular biology, improvements in Hg phytoremediation processes have been found. The inclusion of transporters and genes can improve the uptake, translocation, and transformation of Hg into less toxic forms. Researchers can use multi-omics methods to report on the different processes involved in gene overexpression, mercury oxidative stress, and metal translocation.
- -
- After the phytoremediation process in mercury-accumulating plants, its removal should be considered. The question of how to treat plants with mercury after harvest should be studied. The release of atmospheric mercury from plants can also cause air pollution. Methods such as composting and pyrolysis have been used for the disposal of toxic plant residues after phytoremediation [131].
4. Conclusions and Perspectives
Conclusions
This study provided knowledge mapping of the phytoremediation of mercury contamination. Publications on this topic are increasing, with a total of 457 publications in WoS and 697 in Scopus, with higher growth in the last five years. In terms of research categories, Environmental Sciences was the most frequent subject area in both databases, followed by Agricultural and Biological Sciences in Scopus, and Environmental Engineering in WoS. China and India were the countries with the most publications in WoS and Scopus, respectively, followed by the United States, Spain, and Italy. Chinese organizations were the main drivers of research, with the Chinese Academy of Sciences being the most productive institution in this field of study, with these studies being mainly funded by the Natural Science Foundation of China. It also houses the most prolific authors.
The most prominent journals in this field were Chemosphere, International Journal of Phytoremediation, and Environmental Science and Pollution Research. The 20 most cited publications in Scopus were reviews, while in WoS, 60% were reviews and 40% were research articles. The most cited article in Scopus was from Germany, while the most cited in WoS was from the United States.
The co-occurrence and trending themes of the keywords made it possible to identify the critical points of the research and its evolution over time. For example, the terms with the highest number of occurrences were bioaccumulation and phytoextraction, which define the efficacy of a plant with phytoremediation potential. Genetic engineering, gene and protein expression, and genetically modified plants influence the process of assisted measures for improvements in the phytoremediation process. Similarly, more research should be conducted on the synergistic role between plants and microorganisms and on phytoremediation and chemical-assisted amendments. More research should also be conducted on aquatic plants for the remediation of ecosystems and aquatic environments contaminated with heavy metals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16219408/s1, Figure S1. Documents by subject area: (A) Scopus and (B) WoS; Figure S2. Countries’ scientific production: (A) WoS and (B) Scopus; Figure S3. Highly cited articles: (A) WoS and (B) Scopus; Table S1. Top 10 authors with the highest number of articles in WoS and Scopus on the phytoremediation of mercury contamination; Table S2. Journals with the greatest scientific production in WoS; Table S3. Journals with the greatest scientific production in Scopus; Table S4. Most cited papers on the phytoremediation of mercury pollution in WoS [69,70,71,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147]; Table S5. Most cited papers on the phytoremediation of mercury pollution in Scopus [3,14,22,69,70,71,72,73,74,148,149,150,151,152,153,154,155,156,157,158]; Table S6. Top 31 keywords with the strongest citation bursts in WoS; Table S7. Top 24 keywords with the strongest citation bursts in Scopus; Table S8. Plants used in mercury phytoremediation [16,24,25,28,32,35,101,123,124,159,160,161,162,163,164,165,166].
Author Contributions
Conceptualization, L.M.C. and P.N.C.; Methodology; L.M.C. and D.P.C.; Writing—original draft preparation, L.M.C. and P.N.C.; Writing—review and editing, L.M.C., D.P.C., A.L.G., P.N.C. and C.A.A.; Supervision, D.P.C. and P.N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by Universidad Tecnológica del Chocó, Colombia, Universidad Tecnológica de Pereira, Colombia, the Ministry of Science, and Technology and Innovation—MINCIENCIAS (Bicentennial Doctoral Excellence Scholarship Program), Colombia, and Aarhus University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, A.; Khanna, K.; Kour, J.; Dhiman, S.; Bhardwaj, T.; Devi, K.; Sharma, N.; Kumar, P.; Kapoor, N.; Sharma, P.; et al. Critical review on biogeochemical dynamics of mercury (Hg) and its abatement strategies. Chemosphere 2023, 319, 137917. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. ATSDR Agency for Toxic Substances and Disease Registry. ATSDR’s Substance Priority List. August 2022. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 25 July 2023).
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, K.R.; Krishnan, K.; Naidu, R.; Andrews, S.; Megharaj, M. Mercury toxicity to terrestrial biota. Ecol. Indic. 2017, 74, 451–462. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, B.; Wang, L.A.O.; Urbanovich, O.; Nagorskaya, L.; Li, X.; Tang, L. A review on phytoremediation of mercury-contaminated soils. J. Hazard. Mater. 2020, 400, 123138. [Google Scholar] [CrossRef]
- The United Nations Environment Programme (UN Environment, 2019). Available online: https://www.unep.org (accessed on 25 July 2023).
- Fernandes, I.O.; Gomes, L.F.; Monteiro, L.C.; Dórea, J.G.; Bernardi, J.V.E. A Scientometric Analysis of Research on World Mercury (Hg) in Soil (1991–2020). Water Air Soil Pollut. 2021, 232, 254. [Google Scholar] [CrossRef]
- He, M.; Tian, L.; Braaten, H.F.V.; Wu, Q.; Luo, J.; Cai, L.M.; Lin, Y. Mercury–Organic Matter Interactions in Soils and Sediments: Angel or Devil? Bull. Environ. Contam. Toxicol. 2019, 102, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, T.; Matsuyama, A.; Imura, R.; Kodamatani, H.; Miyamoto, J.; Kono, Y.; Kocman, D.; Kotnik, J.; Fajon, V.; Horvat, M. The distribution of total and methylmercury concentrations in soils near the Idrija mercury mine, Slovenia, and the dependence of the mercury concentrations on the chemical composition and organic carbon levels of the soil. Environ. Earth Sci. 2012, 65, 1309–1322. [Google Scholar] [CrossRef]
- Cai, X.; Cai, B.; Zhang, H.; Chen, L.; Zheng, C.; Tong, P.; Lin, H.; Zhang, Q.; Liu, M.; Tong, Y.; et al. Establishment of High-Resolution Atmospheric Mercury Emission Inventories for Chinese Cement Plants Based on the Mass Balance Method. Environ. Sci. Technol. 2020, 54, 13399–13408. [Google Scholar] [CrossRef] [PubMed]
- Eckley, C.S.; Gilmour, C.C.; Janssen, S.; Luxton, T.P.; Randall, P.M.; Whalin, L.; Austin, C. The assessment and remediation of mercury contaminated sites: A review of current approaches. Sci. Total Environ. 2020, 707, 136031. [Google Scholar] [CrossRef]
- Gutiérrez-Mosquera, H.; Marrugo-Negrete, J.; Díez, S.; Morales-Mira, G.; Montoya-Jaramillo, L.J.; Jonathan, M.P. Distribution of chemical forms of mercury in sediments from abandoned ponds created during former gold mining operations in Colombia. Chemosphere 2020, 258, 127319. [Google Scholar] [CrossRef]
- Mishra, B.; Chandra, M. Evaluation of phytoremediation potential of aromatic plants: A systematic review. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100405. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, H.N.; Ashraf, S. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Kumari, S.A.; Jamwal, R.; Mishra, N.; Singh, K. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100283. [Google Scholar] [CrossRef]
- Cepoi, L.; Zinicovscaia, I.; Valuta, A.; Codreanu, L.; Rudi, L.; Chiriac, T.; Yushin, N.; Grozdov, D.; Peshkova, A. Bioremediation capacity of edaphic cyanobacteria Nostoc linckia for chromium in association with other heavy-metals-contaminated soils. Environments 2021, 9, 1. [Google Scholar] [CrossRef]
- Raklami, A.; Meddich, A.; Oufdou, K.; Baslam, M. Plants—Microorganisms-Based Bioremediation for Heavy Metal Cleanup: Recent Developments, Phytoremediation Techniques, Regulation Mechanisms, and Molecular Responses. Int. J. Mol. Sci. 2022, 23, 5031. [Google Scholar] [CrossRef] [PubMed]
- Pratush, A.; Kumar, A.; Hu, Z. Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: A review. Int. Microbiol. 2018, 21, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Podar, D.; Maathuis, F.J.M. The role of roots and rhizosphere in providing tolerance to toxic metals and metalloids. Plant Cell. Environ. 2022, 45, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221, 1–18. [Google Scholar] [CrossRef]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci. Total Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef]
- Qian, X.; Wu, Y.; Zhou, H.; Xu, X.; Xu, Z.; Shang, L.; Qiu, G. Total mercury and methylmercury accumulation in wild plants grown at wastelands composed of mine tailings: Insights into potential candidates for phytoremediation. Environ. Pollut. 2018, 239, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Petelka, J.; Abraham, J.; Bockreis, A.; Deikumah, J.P.; Zerbe, S. Soil Heavy Metal(loid) Pollution and Phytoremediation Potential of Native Plants on a Former Gold Mine in Ghana. J. Water Air Soil Pollut. 2019, 230, 267. [Google Scholar] [CrossRef]
- Muryani, E.; Sajidan, S.; Budiastuti, M.T.S.; Pranoto, P. Diversity and potential of herbaceous plants as mercury (Hg) hyperaccumulators in small-scale gold mining sites in Pancurendang, Banyumas, Indonesia. J. Biol. Divers. 2023, 24, 3364–3372. [Google Scholar] [CrossRef]
- Kukier, U.; Peters, C.A.; Chaney, R.L.; Angle, J.S.; Roseberg, R.J. The Effect of pH on Metal Accumulation in Two Alyssum Species. J. Environ. Qual. 2004, 33, 2090–2102. [Google Scholar] [CrossRef]
- Santos, G.C.G.D.; Rodella, A.A.; Abreu, C.A.D.; Coscione, A.R. Vegetable species for phytoextraction of heavy metals Vegetable species for phytoextraction of boron, copper, lead, manganese and zinc from contaminated soil. Sci. Agric. 2010, 67, 713–719. [Google Scholar] [CrossRef]
- Chamba-Eras, I.; Griffith, D.M.; Kalinhoff, C.; Ramírez, J.; Gázquez, M.J. Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon. Plants 2022, 11, 1186. [Google Scholar] [CrossRef]
- Cui, L.; Tian, X.; Xie, H.; Cong, X.; Cui, L.; Wu, H.; Wang, J.; Li, B.; Zhao, J.; Cui, Y.; et al. Cardamine violifolia as a potential Hg hyperaccumulator and the cellular responses. Sci. Total Environ. 2023, 863, 160940. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Ding, S.; Xiao, H. Enhancer assisted-phytoremediation of mercury-contaminated soils by Oxalis corniculata L., and rhizosphere microorganism distribution of Oxalis corniculata L. Ecotoxicol. Environ. Saf. 2018, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.A.; Ruiz-Díez, B.; Fajardo, S.; López-Berdonces, M.A.; Higueras, P.L.; Fernández-Pascual, M. Lupinus albus plants acquire mercury tolerance when inoculated with an Hg-resistant Bradyrhizobium strain. Plant Physiol. Biochem. 2013, 73, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ma, Y.; Wang, H.; Huang, W.; Wang, X.; Han, L.; Sun, W.; Han, E.; Wang, B. Overexpression of PtABCC1 contributes to mercury tolerance and accumulation in Arabidopsis and poplar. Biochem. Biophys. Res. Commun. 2018, 497, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Shi, T.; Zhang, S.; Crittenden, J.; Guo, S.; Du, H. Bibliometric analysis of insights into soil remediation. J. Soils Sediments. 2018, 18, 2520–2534. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Yang, Y.; Li, S.; Wang, T.; Oleksak, P.; Chrienova, Z.; Wu, Q.; Nepovimova, E.; Zhang, X.; et al. Phytoremediation of heavy metal pollution: Hotspots and future prospects. Ecotoxicol. Environ. Saf. 2022, 234, 113403. [Google Scholar] [CrossRef]
- Armah, F.A.; Obiri, S.; Yawson, D.O.; Onumah, E.E.; Yengoh, G.T.; Afrifa, E.K.A.; Odoi, J.O. Anthropogenic sources and environmentally relevant concentrations of heavy metals in surface water of a mining district in Ghana: A multivariate statistical approach. J. Environ. Sci. Health Part A 2010, 45, 1804–1813. [Google Scholar] [CrossRef]
- Han, R.; Zhou, B.; Huang, Y.; Lu, X.; Li, S.; Li, N. Bibliometric overview of research trends on heavy metal health risks and impacts in 1989–2018. J. Clean. Prod. 2020, 276, 123249. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.-H.; Chang, J.-S. Microalgal biosorption of heavy metals: A comprehensive bibliometric review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, P.; Karmakar, M.; Leta, J.; Mayr, P. The journal coverage of Web of Science, Scopus and Dimensions: A comparative analysis. Scientometrics 2021, 126, 5113–5142. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Ho, Y.-S. Bibliometric analysis of tsunami research. Scientometrics 2007, 73, 3–17. [Google Scholar] [CrossRef]
- Shi, D.; Xie, C.; Wang, J.; Xiong, L. Changes in the structures and directions of heavy metal-contaminated soil remediation research from 1999 to 2020: A bibliometric & scientometric study. Environ. Res. Public Health 2021, 18, 7358. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, K.; Yu, Y.; Yang, B. Mapping of water footprint research: A bibliometric analysis during 2006–2015. J. Clean Prod. 2017, 149, 70–79. [Google Scholar] [CrossRef]
- Hood, W.W.; Wilson, C.S. The Literature of Bibliometrics, Scientometrics, and Informetrics. Scientometrics 2001, 52, 291–314. [Google Scholar] [CrossRef]
- van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 2, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qiu, D. Exploring coal spontaneous combustion by bibliometric analysis. Process Saf. Environ. Prot. 2019, 132, 1–10. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis: Heavy metal tolerance in hyperaccumulators. Environ. Chall. 2021, 4, 100197. [Google Scholar] [CrossRef]
- Mongeon, P.; Paul-Hus, A. The journal coverage of Web of Science and Scopus: A comparative analysis. Scientometrics 2016, 106, 213–228. [Google Scholar] [CrossRef]
- Zhang, D.; Dyck, M.; Filipović, L.; Filipović, V.; Lv, J.; He, H. Hyperaccumulators for potentially toxic elements: A scientometric analysis. Agronomy 2021, 11, 1729. [Google Scholar] [CrossRef]
- Valdiviezo Gonzales, L.G.; Castañeda-Olivera, C.A.; Cabello-Torres, R.J.; García Ávila, F.F.; Munive Cerrón, R.V.; Alfaro, J. Scientometric study of treatment technologies of soil pollution: Present and future challenges. Appl. Soil Ecol. 2023, 182, 104695. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Wang, S.; Shang, L. Mercury and methylmercury in riparian soil, sediments, mine-waste calcines, and moss from abandoned Hg mines in east Guizhou province, southwestern China. Appl. Geochem. 2005, 20, 627–638. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Meng, B.; Zhang, C.; Gu, C.; Du, B.; Lin, Y. Environmental geochemistry of an abandoned mercury mine in Yanwuping, Guizhou Province, China. Environ. Res. 2013, 125, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gosar, M.; Žibret, G. Mercury contents in the vertical profiles through alluvial sediments as a reflection of mining in Idrija (Slovenia). J. Geochem. Explor. 2011, 110, 81–91. [Google Scholar] [CrossRef]
- Kocman, D.; Kanduč, T.; Ogrinc, N.; Horvat, M. Distribution and partitioning of mercury in a river catchment impacted by former mercury mining activity. Biogeochemistry 2011, 104, 183–201. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Wang, Y.; Chang, J.; Liu, X. Trace element contamination in urban topsoil in China during 2000–2009 and 2010–2019: Pollution assessment and spatiotemporal analysis. Sci. Total Environ. 2021, 758, 143647. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Corella, J.P.; Valero-Garcés, B.L.; Wang, F.; Martínez-Cortizas, A.; Cuevas, C.A.; Saiz-Lopez, A. 700 years reconstruction of mercury and lead atmospheric deposition in the Pyrenees (NE Spain). Atmos. Environ. 2017, 155, 97–107. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil. 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Kumar, P.N.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The Use of Plants To Remove Heavy Metals from Soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef]
- Palacios-Torres, Y.; Caballero-Gallardo, K.; Olivero-Verbel, J. Mercury pollution by gold mining in a global biodiversity hotspot, the Choco biogeographic region, Colombia. Chemosphere 2018, 193, 421–430. [Google Scholar] [CrossRef]
- Chen, G.Q.; Li, J.S.; Chen, B.; Wen, C.; Yang, Q.; Alsaedi, A.; Hayat, T. An overview of mercury emissions by global fuel combustion: The impact of international trade. Renew. Sustain. Energy Rev. 2016, 65, 345–355. [Google Scholar] [CrossRef]
- Gutiérrez, J.K.R.; Velasco, N.Y.G. Redes de coautoría como herramienta de evaluación de la producción científica de los grupos de investigación. Rev. Gen. Inf. Doc. 2017, 27, 279–297. [Google Scholar] [CrossRef]
- Wang, J.; Xia, J.; Feng, X. Screening of chelating ligands to enhance mercury accumulation from historically mercury-contaminated soils for phytoextraction. J. Environ. Manag. 2017, 186, 233–239. [Google Scholar] [CrossRef]
- Wang, J.; Anderson, C.W.N.; Xing, Y.; Fan, Y.; Xia, J.; Shaheen, S.M.; Rinklebe, J.; Feng, X. Thiosulphate-induced phytoextraction of mercury in Brassica juncea: Spectroscopic investigations to define a mechanism for Hg uptake. Environ. Pollut. 2018, 242, 86–993. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, L.; Wu, H. Experimental study of the effects of soil pH and ionic species on the electro-osmotic consolidation of kaolin. J. Hazard. Mater. 2019, 368, 885–893. [Google Scholar] [CrossRef]
- Su, H.-N.; Lee, P.-C. Mapping knowledge structure by keyword co-occurrence: A first look at journal papers in Technology Foresight. Scientometrics 2010, 85, 65–79. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Schutzendubel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace. Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Malar, S.; Sahi, S.V.; Favas, P.J.C.; Venkatachalam, P. Mercury heavy-metal-induced physiochemical changes and genotoxic alterations in water hyacinths [Eichhornia crassipes (Mart.)]. Environ. Sci. Pollut. Res. 2015, 22, 4597–4608. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Ulaganathan, A.; Robinson, J.S.; Rajendran, S.; Geevaretnam, J.; Shanmugam, S.; Natarajan, A.; Abdulrahman, I.A.; Karthikeyan, P. Potentially toxic elements contamination and its removal by aquatic weeds in the riverine system: A comparative approach. Environ. Res. 2022, 206, 112613. [Google Scholar] [CrossRef]
- Peng, D.; Chen, M.; Su, X.; Liu, C.; Zhang, Z.; Middleton, B.A.; Lei, T. Mercury accumulation potential of aquatic plant species in West Dongting Lake, China. Environ. Pollut. 2023, 324, 121313. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Li, P.; Wang, S.; Li, G.; Shang, L.; Fu, X. Methylmercury Accumulation in Rice (Oryza sativa L.) Grown at Abandoned Mercury Mines in Guizhou, China. J. Agric. Food. Chem. 2008, 56, 2465–2468. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Qiu, G.; Wang, S.; Li, G.; Shang, L.; Meng, B.; Jiang, H.; Bai, W.; Li, Z.; et al. Human Exposure To Methylmercury through Rice Intake in Mercury Mining Areas, Guizhou Province, China. Environ. Sci. Technol. 2008, 42, 326–332. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Shi, F.; Meng, B.; Liu, J.; Mi, Y.; Dong, C.; Su, H.; Liu, X.; Wang, F.; et al. Influence of arbuscular mycorrhizal fungi on mercury accumulation in rice (Oryza sativa L.): From enriched isotope tracing perspective. Ecotoxicol. Environ. Saf. 2023, 255, 114776. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; López, J.E.; Díaz-García, L.; Montoya-Ruiz, C. Changes in Lolium perenne L. rhizosphere microbiome during phytoremediation of Cd- and Hg-contaminated soils. Environ. Sci. Pollut. Res. 2023, 30, 49498–49511. [Google Scholar] [CrossRef]
- Guo, Y.; Sommer, N.; Martin, K.; Rasche, F. Rhizophagus irregularis improves Hg tolerance of Medicago truncatula by upregulating the Zn transporter genes ZIP2 and ZIP6. Mycorrhiza 2023, 33, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhao, L.; Wang, Z.; Teng, Y.; Luo, Y. Mercury Enrichment Characteristics and Rhizosphere Bacterial Community of Ramie (Boehmeria nivea L. Gaud.) in Mercury-Contaminated Soil. Sustainability 2023, 15, 6009. [Google Scholar] [CrossRef]
- Senabio, J.A.; de Campos Pereira, F.; Pietro-Souza, W.; Sousa, T.F.; Silva, G.F.; Soares, M.A. Enhanced mercury phytoremediation by Pseudomonodictys pantanalensis sp. nov. A73 and Westerdykella aquatica P71. Braz. J. Microbiol. 2023, 54, 949–964. [Google Scholar] [CrossRef]
- Sharma, P.; Chaturvedi, P.; Chandra, R.; Kumar, S. Identification of heavy metals tolerant Brevundimonas sp. from rhizospheric zone of Saccharum munja L. and their efficacy in in-situ phytoremediation. Chemosphere 2022, 295, 133823. [Google Scholar] [CrossRef]
- Ustiatik, R.; Nuraini, Y.; Suharjono, S.; Jeyakumar, P.; Anderson, C.W.N.; Handayanto, E. Mercury resistance and plant growth promoting traits of endophytic bacteria isolated from mercury-contaminated soil. Bioremediat. J. 2022, 26, 208–227. [Google Scholar] [CrossRef]
- Valdiviezo Gonzales, L.G.; García Ávila, F.F.; Cabello Torres, R.J.; Castañeda Olivera, C.A.; Alfaro Paredes, E. Scientometric study of drinking water treatments technologies: Present and future challenges. Cogent Eng. 2021, 8, 1929046. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, S.; Han, X.; Gao, X.; He, G.; Zhao, Y.; Li, H. A bibliometrics review of water footprint research in China: 2003-2018. Sustainability 2019, 11, 5082. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Larsen, T.; Shang, L.; Li, P. Bioaccumulation of Methylmercury versus Inorganic Mercury in Rice (Oryza sativa L.) Grain. Environ. Sci. Technol. 2010, 44, 4499–4504. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, F.; Yi, J.; Yan, H.; He, Z.; Li, X. Transcriptomic and physio-biochemical features in rice (Oryza sativa L.) in response to mercury stress. Chemosphere 2022, 309, 136612. [Google Scholar] [CrossRef]
- Shahpiri, A.; Mohammadzadeh, A. Mercury removal by engineered Escherichia coli cells expressing different rice metallothionein isoforms. Ann. Microbiol. 2018, 68, 145–152. [Google Scholar] [CrossRef]
- Gomes, M.V.T.; de Souza, R.R.; Teles, V.S.; Mendes, É.A. Phytoremediation of water contaminated with mercury using Typha domingensis in constructed wetland. Chemosphere 2014, 103, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Enamorado-Montes, G.; Durango-Hernández, J.; Pinedo-Hernández, J.; Díez, S. Removal of mercury from gold mine effluents using Limnocharis flava in constructed wetlands. Chemosphere 2017. 167, 188–192. [CrossRef]
- Dushenkov, S. Trends in phytoremediation of radionuclides. Plant Soil. 2003, 249, 167–175. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; Ent, A. van der. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. Available online: https://www.jstor.org/stable/90019919 (accessed on 23 September 2024). [CrossRef]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Tiodar, E.D.; Văcar, C.L.; Podar, D. Phytoremediation and microorganisms-assisted phytoremediation of mercury-contaminated soils: Challenges and perspectives. Int. J. Environ. Res. Public. Health. 2021, 18, 2435. [Google Scholar] [CrossRef]
- Gutiérrez-Mosquera, H.; Marrugo-Negrete, H.S.; Díez, S.; Morales–Mira, G.; Montoya-Jaramillo, L.J.; Jonathan, M.P. Mercury distribution in different environmental matrices in aquatic systems of abandoned gold mines, Western Colombia: Focus on human health. J. Hazard. Mater. 2012, 4040, 124080. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Marrugo–Madrid, S.; Turull, M.; Montes, G.E.; Pico, M.V.; Marrugo–Negrete, J.L.; Díez, S. Phytoremediation of mercury in soils impacted by gold mining: A case study of Colombia. Bioremediation Environ. Sustain. Toxic. Mech. Contam. Degrad. Detoxif. Chall. 2021, 2021, 145–160. [Google Scholar] [CrossRef]
- Czako, M.; Feng, X.; He, Y.; Liang, D.; Marton, L. Transgenic Spartina alterniflora for phytoremediation. Environ. Geochem. Health. 2006, 28, 103–110. [Google Scholar] [CrossRef]
- Yang, R.Y.; Li, T.T.; Yang, T.H.; Li, Y.P.; Liu, H.; Wang, L.; Lu, N.Q. Advances in enhanced phytoremediation by genetic engineering technology for heavy metal pollution in soil. Res. Environ. Sci. 2019, 32, 1294–1303. [Google Scholar]
- Priyadarshanee, M.; Chatterjee, S.; Rath, S.; Dash, H.R.; Das, S. Cellular and genetic mechanism of bacterial mercury resistance and their role in biogeochemistry and bioremediation. J. Hazard. Mater. 2022, 423, 26985. [Google Scholar] [CrossRef] [PubMed]
- Heaton, A.C.; Rugh, C.L.; Kim, T.; Wang, N.J.; Meagher, R.B. Toward detoxifying mercury-polluted aquatic sediments with rice genetically engineered for mercury resistance. Environ. Toxicol. Chem. 2003, 22, 2940–2947. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhou, Y.; Gong, T.; Wang, J.; Ge, Y. Enhanced phytoremediation of mixed heavy metal (mercury)—Organic pollutants (trichloroethylene) with transgenic alfalfa co-expressing glutathione S-transferase and human P450 2E1. J. Hazard. Mater. 2013, 260, 1100–1107. [Google Scholar] [CrossRef]
- Rodríguez, L.; Alonso-Azcárate, J.; Villaseñor, J.; Rodríguez-Castellanos, L. EDTA and hydrochloric acid effects on mercury accumulation by Lupinus albus. Environ. Sci. Pollut. Res. 2016, 23, 24739–24748. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Occurrence, toxic effects and removal of metformin in the aquatic environments in the world: Recent trends and perspectives. Sci. Total Environ. 2020, 702, 134924. [Google Scholar] [CrossRef] [PubMed]
- Amir, W.; Farid, M.; Ishaq, H.K.; Farid, S.; Zubair, M.; Alharby, H.F.; Ali, S. Accumulation potential and tolerance response of Typha latifolia L. under citric acid assisted phytoextraction of lead and mercury. Chemosphere 2020, 257, 127247. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, X.; Cui, Z. Phytoremediation of multi-metal contaminated mine tailings with Solanum nigrum L. and biochar/attapulgite amendments. Ecotoxicol. Environ. Saf. 2019, 180, 517–525. [Google Scholar] [CrossRef]
- Smolinska, B.; Leszczynska, J. Influence of combined use of iodide and compost on Hg accumulation by Lepidium sativum L. J. Environ. Manage. 2015, 150, 499–507. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, F.; Evans, R.D.; Zhong, H.; Zhao, J.; Zhou, D. Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: The key role of antagonism in soil. Sci. Rep. 2016, 6, 19477. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.; Wang, H.; Wang, L. Thiosulphate-induced mercury accumulation by plants: Metal uptake and transformation of mercury fractionation in soil—Results from a field study. Plant Soil 2014, 375, 21–33. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant-microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Ruley, J.A.; Tumuhairwe, J.B.; Amoding, A.; Westengen, O.T.; Vinje, H. Rhizobacteria Communities of Phytoremediation Plant Species in Petroleum Hydrocarbon Contaminated Soil of the Sudd Ecosystem, South Sudan. Int. J. Microbiol. 2020, 2020, 6639118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: An update. Bioresour. Technol. 2021, 328, 124835. [Google Scholar] [CrossRef]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead tolerance in plants: Strategies for phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161. [Google Scholar] [CrossRef]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2018, 41, 1201–1232. [Google Scholar] [CrossRef]
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H.J.B.A. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef]
- Leudo, A.M.; Cruz, Y.; Montoya-Ruiz, C.; Delgado, M.d.P.; Saldarriaga, J.F. Mercury phytoremediation with Lolium perenne—Mycorrhizae in contaminated soils. Sustainability 2020, 12, 3795. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, C.; Wang, G.; Guan, D.; Zhang, R.; Chen, Y.; Dai, J. Influencing pathways of soil microbial attributes on accumulation of heavy metals in brassica (Brassica campestris L. ssp. chinensis var. utilis Tsen et Lee) leaves. Environ. Pollut. 2020, 262, 114215. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 2016, 188, 206. [Google Scholar] [CrossRef]
- Esteban, E.; Moreno, E.; Penalosa, J.; Cabrero, J.I.; Millán, R.; Zornoza, P. Short and long-term uptake of Hg in white lupin plants: Kinetics and stress indicators. Environ. Exp. Bot. 2008, 62, 316–322. [Google Scholar] [CrossRef]
- Ibraheem, F.; Al-Hazmi, N.; El-Morsy, M.; Mosa, A. Ecological risk assessment of potential toxic elements in salt marshes on the east coast of the red sea: Differential physiological responses and adaptation capacities of dominant halophytes. Sustainability 2021, 13, 11282. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.D.; Parlak, K.U. Nickel-induced changes in lipid peroxidation, antioxidative enzymes, and metal accumulation in Lemma gibba. Int. J. Phytorem. 2011, 13, 805–817. [Google Scholar] [CrossRef]
- Manara, A. Plant Responses to Heavy Metal Toxicity. In Plants Heavy Metals; Springer: Dordrecht, The Netherlands, 2012; pp. 27–53. [Google Scholar] [CrossRef]
- Szalai, G.; Krantev, A.; Yordanova, R.; Popova, L.P.; Janda, T. Influence of salicylic acid on phytochelatin synthesis in Zea mays during Cd stress. Turk. J. Bot. 2013, 37, 708–714. [Google Scholar] [CrossRef]
- Xu, J.; Bravo, A.G.; Lagerkvist, A.; Bertilsson, S.; Sjöblom, R.; Kumpiene, J. Sources and remediation techniques for mercury contaminated soil. Environ. Int. 2015, 74, 42–53. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotoxicol. Environ. Saf. 2017, 135, 209–215. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Li, Y.; Wu, Y.; Zeng, Z.; Xu, R.; Zhang, J. Changes of heavy metal fractions during co-composting of agricultural waste and river sediment with inoculation of Phanerochaete chrysosporium. J. Hazard. Mater. 2019, 378, 120757. [Google Scholar] [CrossRef]
- Marchand, L.; Mench, M.; Jacob, D.L.; Otte, M.L. Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review. Environ. Pollut. 2010, 158, 3447–3461. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H. Aquatic arsenic: Phytoremediation using floating macrophytes. Chemosphere 2011, 83, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Vijayakumar, S.; Pathak, H. Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2020, 699, 134330. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.; Rinklebe, J.; O’Connor, D. Remediation of mercury-contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef] [PubMed]
- OB, A.; Muchie, M. Remediation of heavy metals in drinking water and wastewater treatment systems: Processes and applications. Int. J. Phys. Sci. 2010, 5, 1807–1817. [Google Scholar]
- Arao, T.; Ishikawa, S.; Murakami, M.; Abe, K.; Maejima, Y.; Makino, T. Heavy metal contamination of agricultural soil and countermeasures in Japan. Paddy Water Environ. 2010, 8, 247–257. [Google Scholar] [CrossRef]
- Gaur, N.; Flora, G.; Yadav, M.; Tiwari, A. A review with recent advancements on bioremediation-based abolition of heavy metals. Environ. Sci. Process. Impacts 2014, 16, 180–193. [Google Scholar] [CrossRef]
- Singh, J.S.; Abhilash, P.C.; Singh, H.B.; Singh, R.P.; Singh, D.P. Genetically engineered bacteria: An emerging tool for environmental remediation and future research perspectives. Gene 2011, 480, 1–9. [Google Scholar] [CrossRef]
- Bonanno, G. Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef]
- Li, K.; Ramakrishna, W. Effect of multiple metal-resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J. Hazard. Mater. 2011, 189, 531–539. [Google Scholar] [CrossRef]
- Monterroso, C.; Rodríguez, F.; Chaves, R.; Diez, J.; Becerra-Castro, C.; Kidd, P.S.; Macías, F. Heavy metal distribution in mine soils and plants growing in a Pb/Zn-mining area in NW Spain. Appl. Geochem. 2014, 44, 3–11. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutkowski, P.; Rissmann, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Bonanno, G.; Cirelli, G.L. Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicol. Environ. Saf. 2017, 143, 92–99. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F. Health risk and significance of mercury in the environment. Environ. Sci. Pollut. Res. 2015, 22, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 939161. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 14, 277–281. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Patra, M.; Sharma, A. Mercury toxicity in plants. The Botanical Review. 2000, 66, 379–422. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, J.; Lombi, E. Phytoremediation of metals, metalloids, and radionuclides. Adv. Agron. 2002, 75, 1–56. [Google Scholar] [CrossRef]
- Krämer, U. Phytoremediation: Novel approaches to cleaning up polluted soils. Curr. Opin. Biotechnol. 2005, 16, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Eapen, S.; D’souza, S.F. Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol. Adv. 2005, 23, 97–114. [Google Scholar] [CrossRef]
- Lebeau, T.; Braud, A.; Jézéquel, K. Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils: A review. Environ. Pollut. 2008, 153, 497–522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Cao, X.; Li, X.; Li, T.; Zhang, H.; Cui, X.; Cui, Z. Ecological toxicity of Cd, Pb, Zn, Hg and regulation mechanism in Solanum nigrum L. Chemosphere 2023, 313, 137447. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Enamorado-Montes, G.; Díez, S. Mercury uptake and effects on growth in Jatropha curcas. Environ. Sci. 2016, 48, 120–125. [Google Scholar] [CrossRef]
- Durante-Yánez, E.V.; Martínez-Macea, M.A.; Enamorado-Montes, G.; Combatt Caballero, E.; Marrugo-Negrete, J. Phytoremediation of soils contaminated with heavy metals from gold mining activities using Clidemia sericea D. Don. Plants. 2022, 11, 597. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.A.; Xu, J.; Ding, S.; Feng, X.; Xiao, H. Effects of different concentrations of mercury on accumulation of mercury by five plant species. Ecol. Eng. 2017, 106, 273–278. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, J.; Wu, S.C.; Zhang, S.; Christie, P.; Liang, P. Responses of the grass Paspalum distichum L. to Hg stress: A proteomic study. Ecotoxicol. Environ. Saf. 2019, 183, 109549. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ordiales, E.; Roqueñí, N.; Loredo, J. Mercury bioaccumulation by Juncus maritimus grown in a Hg contaminated salt marsh (northern Spain). Mar. Chem. 2020, 226, 103859. [Google Scholar] [CrossRef]
- Shiyab, S.; Chen, J.; Han, F.X.; Monts, D.L.; Matta, F.B.; Gu, M.; Su, Y. Phytotoxicity of mercury in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2009, 72, 619–625. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).