Abstract
Although chemical fertilization has gained a lot of attention due to its ability to increase the yield of fruit trees, it has been known to cause numerous environmental problems such as soil deterioration, alleviating beneficial microorganisms, and reducing fruit quality and safety. Hence, today, we aim to reduce these problems by using eco-friendly and sustainable biostimulants to promote nutritional status, yield, and quality. The effect of wood vinegar (WV) on mango production has yet to be investigated. Therefore, a field trial was conducted during the 2023 and 2024 seasons to evaluate the regulatory effect of individual and combined application of wood vinegar (WV), seaweed extract (SW), and humic acid (HA) on the performance of mango (Mangifera indica L.) cv. Ewais. The results revealed that all treatments had a pronounced effect and significantly improved the total chlorophyll content (107.7 and 106.6%), leaf N (2.02 and 2.23%), P (0.38 and 0.4), and K (1.07 and 1.13%), as well as enhancing the quality of mango fruits by increasing fruit length (11.68 and 12.38 cm), fruit width (7.8 and 8.59 cm), total sugars (40 and 37.3%), and TSS (21.9 and 20.8%) while reducing the total acidity (64.3 and 69.0%) in the 2023 and 2024 seasons, respectively, compared with the control. Based on this study, the treatment of 2 L/ha seaweed + 2 L/ha humic acid + 2 L/ha wood vinegar combined had the greatest effect on enhancing Ewais mango fruit yield by up-regulating leaf mineral acquisition, antioxidant response, and sugar accumulation. This study supports the application of HA and SW in combination with WV to improve mango fruit yield and quality.
1. Introduction
Mango (Mangifera indica L.) is a long-season evergreen perennial plant belonging to the Anacardiaceae family [1]. It is a major fruit crop in tropical and warmer subtropical areas [2], renowned for its widespread cultivation and global popularity due to its significant contribution to increased income [3] through its excellent nutritional value, unique taste, and health benefits [4]. Despite robust blooming and an initial fruit set, mangos experience significant financial losses in fruit output [5]. The main causes of low mango productivity are often related to nutrition, such as soil degradation, which results in a low fruit set, irregular flowering, low fruit retention, and irregular bearing, all of which produce low yields and fruits of poor quality with short shelf lives [6]. Sustainable crop productivity depends on soil fertility; thus, there is a necessity for sustainable farming in which the use of low-cost renewable resources sustains soil fertility to improve crop yield [7]. Furthermore, food security is threatened by the rapidly increasing population and low productivity of crops [8]. Therefore, it is essential to restore and improve the damaged soil to cope with the food security issue caused by soil degradation and loss, thus ensuring a sustainable soil supply and productivity of crops [9].
Applying chemical fertilizers to manage soil fertility is crucial for efficiently raising crop yields, but overuse and insistence on their use have led to several environmental issues, including water pollution, soil degradation, loss of soil ecosystem, and decreased soil fertility, all of which hurt human health. [10,11]. So, to improve and sustain soil fertility, health, and food quality, biostimulants should be used instead of chemical fertilizers. [10,11]. Therefore, the use of plant biostimulants (PBs) is a novel, cutting-edge, and eco-friendly agronomic strategy [12] as a promising weapon in the arsenal of farmers to improve the yield of crops since they have the ability to enhance nutrient availability and induce morphological and physiological changes in plants [13].
Biostimulants are any microorganisms or substances applied to plants in order to increase environmental stress tolerance, nutrition efficiency, and/or crop quality properties [14]. These biostimulants achieve this through mechanisms that do not directly increase soil fertility [15] but rather improve plant metabolism without altering those processes [16]. Biostimulants encompass a wide range of substances, including botanical and algae extracts, microorganisms, bacteria, fungi, biochemicals, protein hydrolysates, amino acids, vitamins, antioxidants, anti-transpirants, and humic and fulvic acids, as well as their derivatives [17]. Biostimulants are usually classified into three major groups that include humic substances (HSs), such as humic acid; hormone-containing products, such as seaweed extracts; and amino acid-containing products, such as L-glycine and L-methionine [18]. Biostimulants are widely used as safe alternatives to some chemical fertilizers to stimulate plant growth and crop productivity under optimal or stressful conditions [19] due to their modulating hormonal, i.e., ‘auxin-like, gibberellin-like’, effects [20], which improve the growth of plants and enrich the overall quality attributes of crops [21]. Among these materials, wood vinegar, seaweed extract, and humic acid have attracted the most attention because of their superior capacity to boost yields and improve nutrient utilization efficiency as well as their clean and nonpolluting effects on the environment [18,22]. In recent years, advancements in the technologies for producing seaweed extract, urea humate, and wood vinegar, along with increased production efficiency, have led to a reduction in the costs associated with their application in agricultural production [23].
Wood vinegar (WV) is an acidic aqueous solution, considered a compound plant growth regulator analog, and is environmentally friendly [23]. Pyroligneous acid is the technical term referring to wood vinegar [23,24]. It is an organic liquid mixture condensed from carbonized flue gas to produce biochar from wood and its residues under high temperatures and hypoxia [25,26]. It contains water by 80–90%, dozens of major organic substances, and more than 200 chemical substances [26,27]. These organic compounds include aldehydes, ketones, alcohols, organic acids, benzene and its derivatives, heterocyclic compounds, phenols and their derivatives, carbohydrate derivatives, alkyl phenyl ethers, and nitrogen compounds [28,29,30]. The main components of wood vinegar are acetic acid and also phenolic and organic acids, alcohol, ester compounds, and alkane [31,32]. Wood vinegar compositions and yields are determined by the composition of the starting material and process conditions [33]. Wood vinegar can positively impact crop growth and yield. It helps improve soil quality, which can lead to increased crop production. Additionally, wood vinegar can boost the activity of antioxidant enzymes in plants, raise soluble protein levels, and significantly enhance photosynthesis, supporting healthier and more productive crops [26,34,35].
Seaweed extract (SW) is a cost-effective and natural source with various bio-stimulating components. These include different forms of carbohydrates and amino acids of organic matter and minerals (i.e., potassium, nitrogen, phosphorus, calcium, copper, manganese, magnesium, and zinc), phytohormones (i.e., polyamines, auxins, and cytokinins), and gibberellins. It also contains vitamins, polysaccharides, polyphenols, sterols, pigments, antioxidants, and antimicrobial agent osmoprotectants [36,37,38,39]. Consequently, seaweed acts as a commercial biostimulant that positively influences plant physiological and biochemical responses, as well as agronomic outcomes [40,41]. It enhances photosynthesis, balances the ratio of carbohydrates to nitrogen, and supports flowering, thereby improving fruit set and productivity [39]. Seaweed extract can enhance various plant aspects, including growth, nutritional status, vegetative development, flower formation quality, fruit set percentage, yield, and fruit longevity. It also helps plants resist unfavorable stresses [42,43].
Humic acid (HA) is a natural organic substance that results from either plant, animal, or microbial residue decomposition and the metabolic activity of microbes by microorganisms and is subsequently converted to a brown or dark brown macromolecular colloidal substance [15,44]. It contains a varied range of functional groups (methoxy, carboxyl, hydroxyl, quinone, and phenolic hydroxyl groups) and has notable chelate complexing and hydrophilic, redox, and adsorption properties. Accordingly, adding humic acid to farmland soil can successfully increase plant growth and resistance by inducing cell respiration, photosynthesis, water uptake, enzyme activation, protein synthesis, increased activity of plant enzymes/hormones, increased cell membrane permeability, cation exchange capacity, availability and transportation of nutrients, soil structure, and water reservation capability, as well as increased oxygen and growth, supplying root cells [23,45,46,47,48]. Moreover, studies showed that humic acid not only enhances root, leaf, and shoot growth and the formation of lateral roots, but also enhances increased plant growth, photosynthetic processes, the accumulation of biomass, productivity, and improvement of nutritional status [49,50].
Although a significant amount of published research has evaluated the individual role of humic acid, wood vinegar, and seaweed on the growth and productivity of many crops, their combined effects on the nutritional status, yield, and fruit quality of Ewais mango are limited. So, multifunctional wood vinegar, humic acid, and seaweed extract treatments are proposed as an easy, economical, safe, sustainable, eco-friendly, and promising agricultural practice for improving Ewais mango’s nutritional status, yield, and fruit quality. Additionally, the findings will offer helpful information to mango growers, particularly those who practice organic farming, to safely apply alternative fertilizers in mango orchards instead of chemical fertilizers.
2. Materials and Methods
2.1. Experimental Site and Plant Materials
The experiment was undertaken in two successive seasons, 2022/2023 and 2023/2024, for 8-year-old Ewais mango trees (Mangifera indica L.) budded on Sukkary rootstock. The trees in the orchard area had a height of approximately 4 × 6 m apart (400 trees/hectare). The trees were irrigated with Nile water with a surface drip irrigation system in private commercial orchards located in the El-Beheira Governorate, Egypt. The latitude is 30°43′37.1″ N; longitude 29°57′39.0″ E. The soil in the experimental region is sandy and has an average pH of 7.4–7.6. The irrigation systems comprised two drip lines per tree row with four drippers (discharge 8.0 L/h). All trees regularly received the same common monthly irrigation requirement applied with each fertigated treatment during the phenological periods according to Abdel-Sattar et al. [51], where the experiment was conducted in the same area. The amount of irrigation water application annually was 9520 m3 ha−1 in 2022/2023 (equating to 160, 160, 360, 480, 640, 800, 1280, 1920, 1920, 960, 480, and 360 L tree−1 in each month from December 2022 to November 2023, respectively) and 9448 m3 ha−1 in 2023/2024 (equating to 168, 168, 378, 504, 672, 880, 1408, 2112, 2112, and 1056 L tree−1 in each month from December 2023 to September 2024, respectively). Irrigation was applied on two, two, three, three, four, five, eight, twelve, twelve, six, three, and three occasions in each month from December to November, respectively. Every mango tree was cared for using conventional agricultural techniques, such as pruning, hoeing, pest management, and trimming, by Egypt’s Ministry of Agriculture recommendations. According to Alebidi et al. [6]’s method, the mango trees were fertilized using mineral and organic fertilizers for commercial fruit production. Table 1 specifies the soil parameters of the field area at the beginning of the selected orchard.

Table 1.
Soil characteristics of the experimental site.
2.2. Experimental Design and Treatments
In this experiment, one hundred and twenty-eight trees as uniform as possible in size, productivity, vigor, and appearance with no visual nutrient deficiency symptoms were selected. All the evenly chosen trees were designed and applied with eight fertigation treatments. These treatments were organized in a randomized complete block design with four replicates per treatment and four trees per replicate (i.e., eight treatments × four replicates × four trees per replicate = 128 trees). In both tested seasons, each treatment was applied at four distinct phenological stages (four times), including the beginning of shoot growth (first week of December, 311 BBCH scale), the beginning of bud swelling (first week of January, 511 BBCH scale), the beginning of flowering (15 April, 611 BBCH state), and the beginning of fruit set (701 BBCH state, 20 May). The treatments were added with irrigation water in the last quarter of an hour so that this quantity was placed each time as follows: T1, control; T2, humic acid at 6 L/ha; T3, seaweed extract at 6 L/ha; T4, wood vinegar at 6 L/ha; T5, 3 L/ha humic acid + 3 L/ha seaweed; T6, 3 L/ha humic acid + 3 L/ha wood vinegar; T7, 3 L/ha seaweed+ 3 L/ha wood vinegar; T8, 2 L/ha seaweed+ 2 L/ha+ 2 L/ha humic acid + 2 L/ha wood vinegar.
The wood vinegar used for the experiment was purchased from Kunyu Environmental Development Co., Ltd. (Kunming, China) and was created by the combined pyrolysis of grape, Chinese fir, and corn straw at a mass ratio of 1:1:1, with the main constituents being acids (57.45%), phenols (26.455%), aldehydes (3.80%), ketones (2.47%), esters (1.33%), and alcohols (3.20%), with a pH of 3.7. Seaweed extract (Ascophyllum nodsoum) was obtained from the commercial product Acadian produced by the Gulf Palace Fertilizer Factory, Second Industrial City, Riyadh, Kingdom of Saudi Arabia, containing 10.58% organic materials (i.e., cytokinins, auxins, betaines, gibberellin, etc.), macroelements (0.7% N, 1.5% P, 6% K, 0.02% Ca, 0.23% S, and 0.04% Mg), microelements (20–50 ppm Fe, 1–5 ppm Cu, 5–15 ppm Zn, 1–5 ppm Mn, and 20–30 ppm B), 7% carbohydrates (alginic acid, mannitol, and laminarin), and 0.1% amino acids. The source of humic acid was potassium humate, which was purchased from the Egypt Biotechnology Company, Egypt, with contents of 85% humic acid, 3% fulvic acid, 12% K2O, 10% K, 1% organic N, 1.72 ppm available N, 0.23 ppm available P, and 1.95 ppm available K with 5.64 pH.
2.3. Measurements and Determinations
2.3.1. Nutritional Status and Yield
A sample of 10 leaves was randomly selected from each replicate and dried at 70 °C to constant weight. Sulfuric acid and hydrogen peroxide were used to digest the samples, as defined by Evenhuis and De Waard [52]. The nitrogen concentration was evaluated using the micro-Kjeldahl method reported by Wang et al. [53]. Using a spectrophotometer (model UV-visible 1800, Tokyo, Japan) at a wavelength of 405 nm, the content of phosphorus was evaluated by using the vanadomolybdate method, as described by Wieczorek et al. [54]. Potassium was determined according to Asch et al. [55] using flame photometer (SKZ International Co., Ltd., Jinan, Shandong, China). The concentration of Ca, Mg, Fe, and Zn was performed with an atomic absorption spectrophotometer (ASS) iCE 3300 AA from Thermo Scientific according to Stafilov and Karadjova, [56]. There was a concentration of photosynthetic pigments, chlorophyll a and b, and total chlorophyll (%) according to Fadeel, [57], while total carbohydrates (mg/g. d.w) were determined according to Dubois et al. [58]. Additionally, fruit yield was obtained by the following equation: yield (Kg/tree) = the average of fruit weight × number of fruit/trees.
2.3.2. Fruit Physico–Chemical Characteristics
In each replicate, a sample of eight fruits was randomly selected from the side of the four directions of the tree to evaluate fruit weight (g), seed weight(g), pulp weight (g), and pulp/fruit (%). Moreover, fruit dimensions (length and width) (using a digital caliper, cm, Mitutoyo 500-197-20, Kawasaki, Japan) and fruit shape index (fruit length/fruit width) were calculated according to Alebidi et al. [6] at the stage of maturity (green peel and soft in touch with gray pedicle). At room temperature, total soluble solids (TSS) were estimated as Brix % by using a digital refractometer (DR 6000, A. Kruss Optronic GmbH, Hamburg, Germany). Total acidity was estimated as grams of citric acid/100 mL fruit juice by using 0.1 N sodium hydroxide in the presence of Phenolphethalene indicator [59]. Moreover, the ratio of TSS/acidity was determined. V.C. (ascorbic acid) was estimated by the oxidation of ascorbic acid with 2,6-dichlorophenolindophenol dye and the value was expressed as mg/100 mL juice according to AOAC [60]. As well, fruit total sugars percent were performed by using phenol sulfuric acid method according to Malik and Singh [61] and Nielsen [62]. Fruit-reducing sugars were measured colorimetrically using the Lane and Eynon method, as described by Egan et al. [63]. The difference between total and reducing sugars was used to compute non-reducing sugars. Using a spectrophotometer at a wavelength of 440 nm, the carotenoid content was determined by Moran and Porath [64], and the values were expressed as mg/100 g fruit weight (f.w.).
2.4. Statistical Analysis
The data underwent analysis of variance (ANOVA) following Gomez and Gomez’s [65] methodology. Treatment means were compared using the least significant difference (LSD) test. All statistical analyses were conducted using SAS version 9.13 (SAS Statistical Package [66]) at the 5% significance level.
3. Results
3.1. Nutritional Status
The obtained data revealed that all applied treatments significantly enhanced the nutrient acquisition in Ewais leaf compared to the control (Table 2). The highest values were recorded with HA application in combination with SW and WV. It significantly (p < 0.05) improved the content of N, P, K, Ca, Mg, Fe, and Zn in the 2023 and 2024 seasons compared with the control treatment. Applying HA and SW alone or in combination with WV induced a significant increase in the concentration of chlorophyll a and b, total chlorophyll, and total carbohydrates (Figure 1). Particularly, HA in conjugation with SW and WV produced the highest improvement. It significantly (p < 0.05) improved the chlorophyll a and b, total chlorophyll, and total carbohydrate percentages by 84.6, 179, 107.7, and 35.6% in the 2023 season and 91.8, 146.4, 106.6, and 36.6% in the 2024 season, respectively, as compared with the control.

Table 2.
Response of mango trees cv. Ewais to soil application of humic acid (HA), seaweed extract (SW), and wood vinegar (WV) on the leaf mineral contents in the 2023 and 2024 seasons.
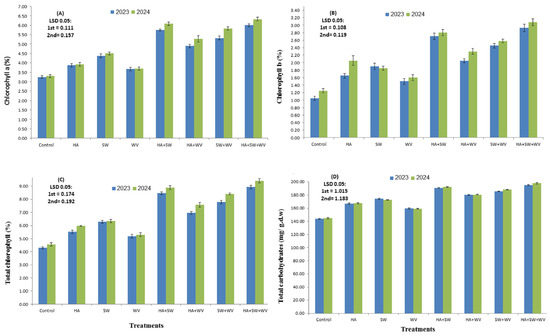
Figure 1.
The impact of humic acid (HA), seaweed extract (SW), and wood vinegar (WV) applied to leaf chlorophyll (chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and total carbohydrate (D) content of Ewais mango trees assessed for the 2023 and 2024 seasons. Data are presented as means ± SE.
3.2. Yield (Kg/Tree)
The data listed in Figure 2 demonstrated that all tested treatments significantly increased the yield (kg/tree) as compared with the control in the 2023 and 2024 seasons. Briefly, the fruits treated with HA along with SW and WV scored almost 2.1 and 2.2 times higher than that of the control, producing the highest yield (65.72 and 71.79 kg/tree in 2023 and 2024 seasons, respectively), followed by treatment with HA and SW in both seasons. However, the control trees exhibited the lowest yield weight (31.49 and 32.35 kg/tree) in the 2023 and 2024 seasons, respectively.
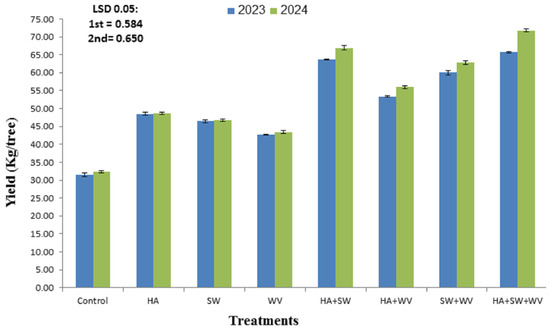
Figure 2.
Effect of applying humic acid (HA), seaweed extract (SW), and wood vinegar (WV) to soil on the yield of Ewais mango trees in the 2023 and 2024 seasons. Data are presented as means ± SE.
3.3. Fruit Physical Characteristics
The data represented in (Table 3) showed that the physical characteristics of Ewais mango fruits were markedly increased by adding HA, SW, and WV either alone or in combination, compared with the control in both seasons, particularly HA. Application in conjunction with SW and WV recorded the highest values. It significantly (p < 0.05) enhanced the fruit weight, pulp weight, pulp/fruit ratio, fruit length, and fruit shape as compared with the control. Additionally, the current findings also revealed that applying HA, SW, and WV resulted in a significant reduction in seed and peel weight when compared with the control. Adding SW, in particular, achieved the lowest values when combined with HA and WV. In comparison with the control, it significantly reduced the seed weight and peel weight by 32.2 and 15.8% in the 2023 season and 32.5 and 16.5% in the 2024 season, respectively.

Table 3.
Response of mango trees cv. Ewais to soil application of humic acid (HA), seaweed extract (SW), and wood vinegar (WV) on fruit physical characteristics in the 2023 and 2024 seasons.
3.4. Fruit Chemical Characteristics
The present data clearly showed that all applied treatments significantly changed the mango fruit’s internal characteristics in comparison with the control (Figure 3). HA application combined with SW and WV had a pronounced effect and significantly (p < 0.05) enhanced the TSS, TSS/acidity, V.C., total sugars, and reducing and non-reducing sugars, as well as total carotenoids, by 21.9, 101, 42.4, 40, 23.1, 48.7, and 67.8% in the 2023 season and 20.8, 105.3, 41.8, 37.3, 23.5, 43.9 and 64.8% in the 2024 season, respectively. The second highest values in these parameters were noticed with HA and SW treatment in both seasons. The results also showed that the application of HA, SW, and WV resulted in a significant reduction in acidity content (64.3–69.0%) when compared with the control. The usage of HA combined with SW or WV was more effective than using them individually.
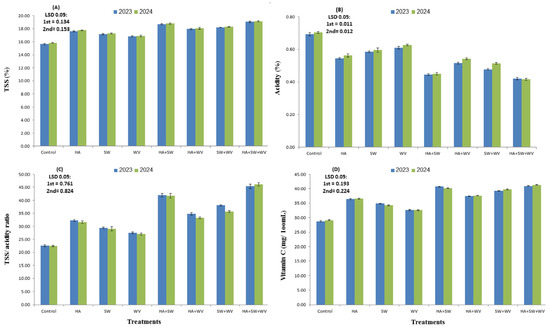
Figure 3.
Effect of applying humic acid (HA), seaweed extract (SW), and wood vinegar (WV) to soil on TSS (A), acidity (B), TSS/acidity ratio (C), and vitamin C (D) of Ewais mango trees in the 2023 and 2024 seasons.
4. Discussion
Sustainable agriculture aims to create and implement technologies that ensure safe and nutritious food production while protecting soil health and the environment [67]. Healthy soil is essential for addressing food and nutrition insecurity as well as poverty. This investigation illustrates for the first time the impact of applying WV alone or coupled with HA and SW on mango nutritional status, fruit quality, and yield. In this present study, it was found that the application of HA combined with SW and/or WV improved Ewais leaf nutrients (Table 2) as reported by many authors. Diab and El-Hmied [68], Mosa et al. [11], and Alsudays et al. [50] concluded that the application of HA improved leaf nutrient content. This effect is primarily due to humic acid (HA) enhancing root growth, accelerating the uptake of mineral ions from root surfaces, and increasing the rate at which these ions are transported into plant tissue cells [49]. It was reported that humic acid (HA) improves leaf N, P, K, and zinc of mango [69], olive cv. Koroneiki [70], and sweet orange seedlings [71]. Abobaker et al. [72] also reported that using the lowest concentration of SW produced a quick method of supplying minerals to plants, which stimulates a variety of physiological plant responses and enhances the nutritional status of Valencia orange trees. Along the same line, the application of WV was shown to improve nutritional status, as widely found by many authors [73,74,75]. Wood vinegar has a complex composition, primarily consisting of acids and phenols, which are known for their strong biological activity and ability to enhance plant growth. Its acidic properties can help adjust soil pH [34], increase nutrient availability [76], reduce ammonium loss [77], and decrease N2O and CH4 emissions [78]. Moreover, WV can help the soil by increasing the population of beneficial microbes enhancing plant root growth [79]. In addition, the nutritional component in WV attracts microbes, including bacteria and fungus to plant roots [34], which enhances the leaf mineral content due to N added by bacteria and P coming from mycorrhiza [24]. The possible mechanisms for these enhancements include improved nutrient uptake due to enhanced root growth and the potential activation of nutrient transporter genes [80].
Photosynthetic pigments such as chlorophyll a, chlorophyll b, and carotenoids play an important part in chlorophyll biosynthesis [67,72]. In this research, applying HA and SW alone or in combination with WV induced a significant increase in the concentration of chlorophyll a and b, total chlorophyll, and total carbohydrates (Figure 1). According to Yang et al. [81], SW and HA application enhances the plant uptake of nutrients such as N, P, and K, which are involved in chlorophyll synthesis and chloroplast formation in tree leaves. SW contains Mg, which is an important element for chlorophyll synthesis [82], enhancing photosynthesis processes, and leaf chlorophyll content, which leads to improved carbohydrate content [83,84]. The result from the application of WV-improved photosynthetic pigments might suggest that WV has a very complex chemical composition, which usually includes a very high concentration of polyphenol, which is probably the most important for its antioxidant, antimicrobial, and growth enhancer properties [77]. Moreover, polyphenols are able to increase chlorophyll a, chlorophyll b, and total chlorophyll content in the plant [85].
The application of biostimulants is an effective way to stimulate plant growth and crop productivity and improve and enrich the overall quality attributes of crops [21,86,87]. In this present study, the application of WV either alone or combined with SW and/or HA-enhanced mango fruit weight, pulp weight, fruit length and width, fruit shape index, and yield (Figure 2 and Table 3). This may be a result of the positive effect of these treatments on the aggregation of health-beneficial phytochemicals like phytohormones, minerals, and amino acids [25,26,41,88,89]. The results acquired from this study coincide with the findings of Refs. [38,90]. The noticeable effect was attributed to the identification of karrikins in WV, which transformed its application in fruit production due to their signaling and physiological effects [91,92], which resemble known plant hormones. Karrikins have been shown to promote plant growth [93]. Similarly, the increase in fruit productivity and fruit growth owing to the application of SW can be explained by the fact that SW is characterized by high contents of gibberellin, cytokinin, mineral nutrients, vitamins, and polysaccharides [94], which results in increased cell elongation and cell division [95,96]. In the case of the olive tree, SW application led to the enhancement of fruit set and productivity [95]. When HA is applied to plants, it frequently causes changes in the primary and secondary metabolism as well as the activation of plasma membrane H+ -ATPase, which leads to an increase in root development, nutrient uptake, and photosynthetic rate [97]. Also, after being added to the rhizosphere, HA can affect both the transcription and activity of some plant hormones [98]. This improvement could be because HA can work as a semi-hormonal compound such as IAA, cytokinin, and gibberellin.
The current findings manifested a significant improvement in the percentages of TSS, TSS/acid, vitamin C, total sugar, reducing and non-reducing sugars, and total carotenoids in Ewais mango fruit treated by WV, SW, and HA, implying a direct effect on the quality of mango fruit (Figure 3 and Figure 4). This effect can be attributed to their roles in enhancing fruit growth and mineral content, as well as regulating various metabolic processes such as photosynthetic activity, which promotes fruit quality [39,99,100,101]. It was reported that the active principles in WV behave in a way similar to that of other plant growth regulators [102]. The application of WV can influence a plant’s internal hormonal balance in several ways: supplementing low hormone levels, deactivating existing hormones, interacting with hormone synthesis, or boosting antioxidant activity [26,35]. Moreover, evidence has shown that sugar accumulation can be induced by WV application by improving the nutritional quality of the soil by making more nutrients available, such as NH4, Mg, K, and Fe, where Mg can improve the activation of sugars [103]. Likewise, Fe availability promotes the production of vitamin C [104]. Interestingly, the components of WV (acids, alcohols, esters, and some minerals) could also have contributed to better fruit growth, production, and quality [105]. The significant impact of SW consists of a variety of micronutrients that have a role in the creation of amino acids, proteins, carbohydrates, and other compounds, as well as the micronutrients absorbed by the leaves, possibly leading to an increase in photo-assimilate production. Increasing total soluble solids (TSS), acidity, and the TSS/acid ratio, as well as reducing, non-reducing, and overall sugar content comes from the movement of photo-assimilates to the fruit via the phloem. This is influenced by the physiological actions of major and minor nutrients, amino acids, vitamins, and growth regulators that impact cellular metabolism [15,39,81]. Studies have indicated that lower acidity in mangoes may result from the accumulation of refined sugars, which aids in the delivery of sugars to the fruit tissues and the conversion of organic acids to sugars [106]. Moreover, the application of SW enhances the chlorophyll content in leaves, thus boosting photosynthesis [43]. The improvement in total sugars and reducing sugars could be attributed to a faster rate of photosynthesis, which might have resulted in more carbohydrate buildup in fruits [107,108]. Conventionally, Vitamin C, sugars, carotenoids, organic acid, and phenols are plant metabolites whose contents are regulated by environmental factors and plant genetics [99,100]. Many investigations showed that humic acid as an environmental factor can affect plant metabolism by inducing protease synthesis. Recently, it was shown that the HA hormone-like effect, particularly IAA, has been documented in sugar content, TSS, and acidity in Kesar mango [109], Zebda mango [110], and ‘Le Conte’ pear [11]. The enhanced effect of HA on TSS may be due to the accumulation of photosynthesis rate, phytohormone activity, N uptake assimilation, and stimulation of photosynthetic pigment [111]. In the same line, the study by Hermans et al. [112] suggested that HA can improve total sugar content due to the enhancement of carbohydrate accumulation in leaf and fruit tissues, which ultimately is converted to sucrose and glucose, as well as the breakdown of starch into sugars during ripening.
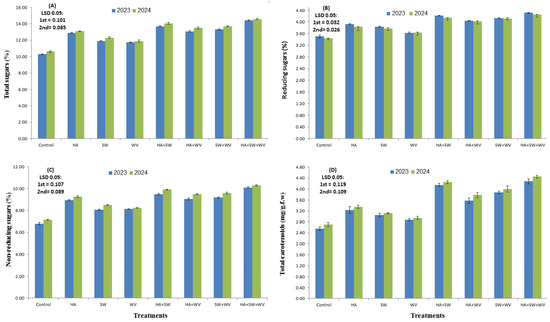
Figure 4.
Effect of applying humic acid (HA), seaweed extract (SW), and wood vinegar (WV) to soil on total sugar (A), reducing sugar (B), non-reducing sugar (C), and total carotenoids (D) of Ewais mango trees in the 2023 and 2024 seasons.
It is important to distinguish between the direct and indirect effects of biostimulants on plant growth. The main ways that indirect impacts manifest themselves are through attributes like improved soil structure, increased microbial population, enhanced cation exchange capacity, and nutrient enrichment in the soil in conjunction with recommended fertilization programs. The direct effects are primarily hormonal in origin and involve a variety of biochemical actions that are applied in the cell wall, membrane, or cytoplasm. In contrast, biostimulants are not significant sources of nitrogen, phosphorus, and potassium that can be relied upon without the addition of recommended fertilization programs, especially in fruit trees on poor soils.
5. Conclusions
Our findings showed that the application of WV, SW, and HA might be applied as a safe and eco-friendly substitute to decrease the chemical fertilization of mango trees to maintain fruit production and growth. This study lays the groundwork for future investigations into the mechanisms of WV, SW, and HA modification in mango, which has the potential to significantly advance the scientific community. Based on the findings of this study, it is recommended that a combination of 2 L seaweed + 2 L humic acid + 2 L wood vinegar per hectare in the fertigation of mango trees be applied four times, as this treatment seemed to result in the best outcomes in terms of productivity. This will improve and increase the final yield of the trees, in addition to enhancing the physico–chemical properties of the fruit.
Author Contributions
Conceptualization, M.A.-S. and L.Y.M.; methodology, M.A.-S. and L.Y.M.; software, M.A.-S. and L.Y.M.; validation, M.A.-S. and H.Z.R.; formal analysis, M.A.-S. and L.Y.M.; investigation, M.A.-S. and L.Y.M.; resources M.A.-S., L.Y.M. and H.Z.R.; data curation, M.A.-S. and L.Y.M.; writing—original draft preparation, M.A.-S., H.Z.R. and L.Y.M.; writing—review and editing, M.A.-S., H.Z.R. and L.Y.M.; visualization, M.A.-S. and H.Z.R.; supervision, M.A.-S.; project administration, M.A.-S.; funding acquisition, M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project (number: RSPD2024R707), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mukherjee, S.K.; Litz, R.E. Botany and Importance. In The Mango: Botany, Production and Uses; Litz, R.E., Ed.; CABI: Cambridge, MA, USA, 2009; pp. 1–18. [Google Scholar]
- Liu, X.; Xiao, Y.; Zi, J.; Yan, J.; Li, C.; Du, C.; Wan, J.; Wu, H.; Zheng, B.; Wang, S.; et al. Differential effects of low and high temperature stress on pollen germination and tube length of mango (Mangifera indica L.) genotypes. Sci. Rep. 2023, 13, 611. [Google Scholar] [CrossRef]
- Wang, J.; Elbagory, M.; He, Y.; Zhang, X.; Hui, Y.; Eissa, M.A.; Ding, Z.; El-Nahrawy, S.; Omara, A.E.-D.; Zoghdan, M.G.; et al. Modeling of P-Loss Risk and Nutrition for Mango (Mangifera indica L.) in Sandy Calcareous Soils: A 4-Years Field Trial for Sustainable P Management. Horticulturae 2022, 8, 1064. [Google Scholar] [CrossRef]
- Makhasha, E.; Al-Obeed, R.S.; Abdel-Sattar, M. Responses of Nutritional Status and Productivity of Timor Mango Trees to Foliar Spray of Conventional and/or Nano Zinc. Sustainability 2024, 16, 6060. [Google Scholar] [CrossRef]
- Kundu, A.K.; Tarai, R.K.; Nayak, A.; Senapati, B. Influence of plant growth regulators on fruit drop, fruit retention and fruit yield of mango (Mangifera indica L.) cv. Amrapali under west central table land zone of Odisha. Plant Sci. Today 2024, 1, 79–84. [Google Scholar] [CrossRef]
- Alebidi, A.; Abdel-Sattar, M.; Mostafa, L.Y.; Hamad, A.S.A.; Rihan, H.Z. Synergistic Effects of Applying Potassium Nitrate Spray with Putrescine on Productivity and Fruit Quality of Mango Trees cv. Ewais. Agronomy 2023, 13, 2717. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Almutairi, K.F.; Aboukarima, A.M.; El-Mahrouky, M. Impact of organic manure on fruit set, fruit retention, yield, and nutritional status in pomegranate (Punica granatum L. “Wonderful”) under water and mineral fertilization deficits. PeerJ 2021, 9, e10979. [Google Scholar] [CrossRef]
- Webb, N.P.; Marshall, N.A.; Stringer, L.C.; Reed, M.S.; Chappell, A.; Herrick, J.E. Land degradation and climate change: Building climate resilience in agriculture. Front. Ecol. Environ. 2017, 15, 450–459. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Z.; Meki, K.; Wang, X.; Liu, B.; Zheng, H.; You, X.; Li, F. Effect of co-application of wood vinegar and biochar on seed germination and seedling growth. J. Soils Sediments 2019, 19, 3934–3944. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, S.; Wang, Y. What could promote farmers to replace chemical fertilizers with organic fertilizers? J. Clean. Prod. 2018, 199, 882–890. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Abd EL-Megeed, N.A.; Ali, M.M.; Abada, H.S.; Ali, H.M.; Siddiqui, M.H.; Sas-Paszt, L. Preharvest Foliar Applications of Citric Acid, Gibberellic Acid and Humic Acid Improve Growth and Fruit Quality of ‘Le Conte’ Pear (Pyrus communis L.). Horticulturae 2022, 8, 507. [Google Scholar] [CrossRef]
- Chaski, C.; Petropoulos, S.A. The Alleviation Effects of Biostimulants Application on Lettuce Plants Grown under Deficit Irrigation. Horticulturae 2022, 8, 1089. [Google Scholar] [CrossRef]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M.A. High-Throughput Physiological Functional Phenotyping System for Time- and Cost-Effective Screening of Potential Biostimulants. bioRxiv 2019. [Google Scholar] [CrossRef]
- Lau, S.-E.; Teo, W.F.A.; Teoh, E.Y.; Tan, B.C. Microbiome Engineering and Plant Biostimulants for Sustainable Crop Improvement and Mitigation of Biotic and Abiotic Stresses. Discov. Food 2022, 2, 9. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in Agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef]
- Garg, S.; Nain, P.; Kumar, A.; Joshi, S.; Punetha, H.; Sharma, P.K.; Siddiqui, S.; Alshaharni, M.O.; Algopishi, U.B.; Mittal, A. Next generation plant biostimulants & genome sequencing strategies for sustainable agriculture development. Front. Microbiol. 2024, 15, 1439561. [Google Scholar] [CrossRef]
- Mackiewicz-Walec, E.; Olszewska, M. Biostimulants in the Production of Forage Grasses and Turfgrasses. Agriculture 2023, 13, 1796. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Gómez-Leyva, J.F.; Sánchez-Hernández, C.V.; Ocampo-Álvarez, H.; Ramírez-Romero, R.; Palmeros-Suárez, P.A. Seaweed extract ameliorates salt stress in tomato plants by enhancing the antioxidant system and expression of stress-responsive genes. J. Appl. Phycol. 2024, 36, 2269–2282. [Google Scholar] [CrossRef]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Khan, R.I.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of Seaweed Extract on Productivity and Quality Attributes of Four Onion Cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 22, 353–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Sun, K.; Luo, X.; Li, F. Comparative study of individual and co-application of biochar and wood vinegar on blueberry fruit yield and nutritional quality. Chemosphere 2020, 246, 125699. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, K.; Shen, Y.; Li, R.; Su, Y.; Deng, Y.; Xia, Y.; Zhang, N. Physiological and Biochemical Mechanisms of Wood VinegarInduced Stress Response against Tomato Fusarium Wilt Disease. Plants 2024, 13, 157. [Google Scholar] [CrossRef]
- Sun, X.; Guo, Y.; Zeng, L.; Li, X.; Liu, X.; Li, J.; Cui, D. Combined Urea Humate and Wood Vinegar Treatment Enhances Wheat–Maize Rotation System Yields and Nitrogen Utilization Efficiency Through Improving the Quality of Saline–Alkali Soils. J. Soil Sci. Plant Nutr. 2021, 21, 1759–1770. [Google Scholar] [CrossRef]
- Sirivardena, B.P.; Subasinghe, S.; Vidanapathirena, N.P.; Kumarasingha, H.K.M.S.; Dhanushka, T.G.B. Effects of pyroligneous acids (wood vinegar) produced from different wood species on vegetative growth of eggplant (Solanum melongena L.). Int. J. Minor Fruits Med. Aromat. Plants 2020, 6, 25–29. [Google Scholar]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood Vinegar as a Complex Growth Regulator Promotes the Growth, Yield, and Quality of Rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Sun, H.; Xue, L.; Zhou, B.; Yang, L.; Liu, J.; Xing, B. Wood vinegar and biochar co-application mitigates nitrous oxide and methane emissions from rice paddy soil: A two-year experiment. Environ. Pollut. 2020, 267, 115403. [Google Scholar] [CrossRef]
- Qin, W.; Ma, X.; Zhao, Z.; Zhang, S.; Liu, S. Antioxidant activities and chemical profiles of pyroligneous acids from walnut shell. J. Anal. Appl. Pyrolysis 2010, 88, 149–154. [Google Scholar]
- Ma, X.; Wei, Q.; Zhang, S.; Shi, L.; Zhao, Z. Isolation and bioactivities of organic acids and phenols from walnut shell pyroligneous acid. J. Anal. Appl. Pyrolysis 2011, 91, 338–343. [Google Scholar] [CrossRef]
- Ma, C.; Song, K.; Yu, J.; Yang, L.; Zhao, C.; Wang, W.; Zu, G.; Zu, Y. Pyrolysis process and antioxidant activity of pyroligneous acid from Rosmarinus officinalis leaves. J. Anal. Appl. Pyrolysis 2013, 104, 38–47. [Google Scholar] [CrossRef]
- Jothityangkoon, D.; Koolachart, R.; Wanapat, S.; Wongkaew, S.; Jogloy, S. Using wood vinegar in enhancing peanut yield and in controlling the contamination of aflatoxin producing fungus. Int. Crop Sci. 2008, 4, 253. [Google Scholar]
- Pan, X.; Zhang, Y.; Wang, X.; Liu, G. Effect of adding biochar with wood vinegar on the growth of cucumber. IOP Conf. Series Earth Environ. Sci. 2017, 261, 012149. [Google Scholar] [CrossRef]
- Ratanapisit, J.; Apiraksakul, S.; Rerngnarong, A.; Chungsiriporn, J.; Bunyakarn, C. Preliminary evaluation of production and characterization of wood vinegar from rubberwood. Songklanakarin J. Sci. Technol. 2009, 31, 343–349. [Google Scholar]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar–Manure Compost in Conjunction with Pyroligneous Solution Alleviated Salt Stress and Improved Leaf Bioactivity of Maize in a Saline Soil from Central China: A 2-Year Field Experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- Simma, B.; Polthanee, A.; Goggi, A.S. Wood vinegar seed priming improves yield and suppresses weeds in dryland direct-seeding rice under rainfed production. Agron. Sustain. Dev. 2017, 37, 56. [Google Scholar] [CrossRef]
- El Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Yarşı, G. Effects of seaweed fertilizer and wood vinegar on nutrient uptake, plant growth and yield of cucumber (Cucumis sativus L) grown in a greenhouse. J. Elem. 2023, 28, 937–948. [Google Scholar] [CrossRef]
- Alebidi, A.; Abdel-Sattar, M. Synergistic effect of seaweed extract and boric acid and/or calcium chloride on productivity and physico-chemical properties of Valencia orange. PeerJ 2024, 12, e17378. [Google Scholar] [CrossRef]
- Goni, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Laribi, B.; Annabi, H.A.; Bettaieb, T. Effects of Ulva intestinalis Linnaeus seaweed liquid extract on plant growth, photosynthetic performance and water status of two hydroponically grown Lamiaceae species: Peppermint (Mentha × piperita L.) and purple basil (Ocimum basilicum var. purpurascens Benth). S. Afr. J. Bot. 2023, 158, 63–72. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Rana, V.S.; Sharma, V.; Sharma, S.; Rana, N.; Kumar, V.; Sharma, U.; Almutairi, K.F.; Avila-Quezada, G.D.; Abd-Allah, E.F.; Gudeta, K. Seaweed extract as a biostimulant agent to enhance the fruit growth, yield, and quality of kiwifruit. Horticulturae 2023, 9, 432. [Google Scholar] [CrossRef]
- Buurman, P.; Nierop, K.G.J.; Kaal, J.; Senesi, N. Analytical pyrolysis and thermally assisted hydrolysis and methylation of EUROSOIL humic acid samples—A key to their source. Geoderma 2009, 150, 10–22. [Google Scholar] [CrossRef]
- Selim, E.M.; Mosa, A.A.; El-Ghamry, A.M. Evaluation of humic substances fertigation through surface and subsurface drip irrigation systems on potato grown under Egyptian sandy soil conditions. Agric. Water Manag. 2009, 96, 1218–1222. [Google Scholar] [CrossRef]
- Kumari, S.; Chhillar, H.; Chopra, P.; Khanna, R.R.; Khan, M.I.R. Potassium: A track to develop salinity tolerant plants. Plant Physiol. Biochem. 2021, 167, 1011–1023. [Google Scholar] [CrossRef]
- Mahdi, A.H.A.; Badawy, S.A.; Abdel Latef, A.A.H.; El Hosary, A.A.A.; Abd El Razek, U.A.; Taha, R.S. Integrated Effects of Potassium Humate and Planting Density on Growth, Physiological Traits and Yield of Vicia faba L. Grown in Newly Reclaimed Soil. Agronomy 2021, 11, 461. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Abd El-Mageed, T.A.A.; Saudy, H.S.; Al-Otaibi, H.H.; Mahmoud, M.A.A. The changes in various physio-biochemical parameters and yield traits of faba bean due to humic acid plus 6-benzylaminopurine application under deficit irrigation. Agronomy 2023, 131, 1227. [Google Scholar] [CrossRef]
- Zhou, L.; Monreal, C.M.; Xu, S.; McLaughlin, N.B.; Zhang, H.; Hao, G.; Liu, J. Effect of bentonite-humic acid application on the improvement of soil structure and maize yield in a sandy soil of a semi-arid region. Geoderma 2019, 338, 269–280. [Google Scholar] [CrossRef]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.S.; Alzuaibr, F.M.; Gharib, H.S.; et al. Applications of humic and fulvic acid under saline soil conditions to improve growth and yield in barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Al-Obeed, R.S.; Makhasha, E.; Mostafa, L.Y.; Abdelzaher, R.A.E.; Rihan, H.Z. Improving mangoes’ productivity and crop water productivity by 24-epibrassinosteroids and hydrogen peroxide under deficit irrigation. Agric. Water Manag. 2024, 298, 108860. [Google Scholar] [CrossRef]
- Evenhuis, B.; De Waard, P.W. Principles and practices in plant analysis. FAO Soils Bull. 1980, 38, 152–163. [Google Scholar]
- Wang, H.; Pampati, N.; McCormick, W.M.; Bhattacharyya, L. Protein nitrogen determination by Kjeldahl digestion and ion chromatography. J. Pharm. Sci. 2016, 105, 1851–1857. [Google Scholar] [CrossRef]
- Wieczorek, D.; Żyszka-Haberecht, B.; Kafka, A.; Lipok, J. Determination of phosphorus compounds in plant tissues: From colourimetry to advanced instrumental analytical chemistry. Plant Methods 2022, 18, 22. [Google Scholar] [CrossRef]
- Asch, J.; Johnson, K.; Mondal, S.; Asch, F. Comprehensive assessment of extraction methods for plant tissue samples for determining sodium and potassium via flame photometer and chloride via automated flow analysis. J. Plant Nutr. Soil Sci. 2022, 185, 308–316. [Google Scholar] [CrossRef]
- Stafilov, T.; Karadjova, I. Atomic absorption spectrometry in wine analysis. Maced J. Chem. Chem. Eng. 2009, 281, 7–31. [Google Scholar] [CrossRef]
- Fadeel, A.A. Location and properties of chloroplasts and pigment determination in roots. Physiol. Plant 1962, 15, 130–146. [Google Scholar] [CrossRef]
- Dubois, M.; Smith, F.; Gilles, K.A.; Hamilton, J.K.; Robers, P.A. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Sadler, G.D.; Murphy, P.A. pH and titratable acidity. In Food Analysis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 219–238. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Method of Analysis, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Malik, C.P.; Singh, M.B. Plant Engymology and Histo-Engymology; A Text Manual; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Nielsen, S.S. Phenol-sulfuric acid method for total carbohydrates. In Food Analysis Laboratory Manual; Food Analysis Texts Series; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar] [CrossRef]
- Egan, H.; Kirk, R.S.; Sawyer, R. Pearson’s Chemical Analysis of Food; Churchill Livingstone: Minneapolis, MN, USA, 1981; p. 591. [Google Scholar]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N, N-Dimethylformamide. Plant Physiol. 1980, 65, 479. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A. Statistical Procedures for Agricultural Research, 1st ed.; John Willey & Sons: New York, NY, USA, 1984. [Google Scholar]
- SAS Statistical Package, The SAS System for Windows, Version 9.13; SAS Institute Inc.: Cary, NC, USA, 2009.
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; et al. Biostimulant Application with a Tropical Plant Extract Enhances Corchorus olitorius Adaptation to Sub-Optimal Nutrient Regimens by Improving Physiological Parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Diab, S.M.; El-hmied, S.A.A. Improving Mango Productivity by Spraying Some Natural Extracts and Adding Humic Under the Conditions of Newly Reclaimed Lands. Middle East J. Agric. Res. 2022, 11, 719–732. [Google Scholar]
- Al-Marsoumi, F.S.H.; Al-Hadethi, M.E.A. Effect of Humic acid and Seaweed extract spray in leaf mineral content of mango seedlings. Plant Arch. 2020, 20, 827–830. [Google Scholar]
- Sotiropoulos, S.; Chatzissavvidis, C.; Papadakis, I.E.; Kavvadias, V.; Paschalidis, C.; Antonopoulou, C.; Kiriakopoulos, S. Enhancing the Yield of Mature Olive Trees via Comparative Fertilization Strategies, including a Foliar Application with Fulvic and Humic Acids, in Non-Irrigated Orchards with Calcareous and Non-Calcareous Soils. Horticulturae 2024, 10, 167. [Google Scholar] [CrossRef]
- Huang, W.; Shen, Q.; Yang, H.; Chen, X.; Huang, W.; Wu, H.; Lai, N.; Yang, L.; Huang, Z.; Chen, L. Effects of Humic Acid-Copper Interactions on Growth, Nutrient Absorption, and Photosynthetic Performance of Citrus sinensis Seedlings in Sand Culture. J. Plant Growth Regul. 2024, 43, 3920–3938. [Google Scholar] [CrossRef]
- Abobaker, A.M.; Bound, S.A.; Swarts, N.D.; Barry, K.M. Effect of fertilizer type and mycorrhizal inocula-tion on growth and development of sunflower (Helianthus annuus L.). Rhizosphere 2018, 6, 11–19. [Google Scholar] [CrossRef]
- Narwal, S.S. Allelopathic interactions in multiple cropping systems. In Allelopathy in Ecological Agriculture and Forestry; Narwal, S.S., Hoagland, R.E., Dilday, R.H., Reigosa, M.J., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 141–157. [Google Scholar]
- FFTC, Food & Fertilizer Technology Center. Wood Vinegar. 2005. Available online: http://www.fftc.agnet.org/library/pt/2005025/ (accessed on 2 April 2024).
- Tancho, A. Applied Natural Farming; Distributed in Thailand by NSTDA Book Center (National Science and Technology Development Agency); Mae Jo Natural Farming Information Center and National Science and Technology Development Agency: Pathom Thani, Thailand, 2013; ISBN 978-974-229-867-8. [Google Scholar]
- Lashari, M.S.; Liu, Y.; Li, L.; Pan, W.; Fu, J.; Pan, G.; Zheng, J.; Zheng, J.; Zhang, X.; Yu, X. Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Food Crop Res. 2013, 144, 113–118. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Sun, H.; Feng, Y.; Ji, Y.; Shi, W.; Yang, L.; Xing, B. N2O and CH4 emissions from N-fertilized rice paddy soil can be mitigated by wood vinegar application at an appropriate rate. Atmos. Environ. 2018, 185, 153–158. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Wang, G.; Wang, J.; Gu, A.; Xue, X.; Chen, R. Effects of seaweed-extract-based organic fertilizers on the levels of mineral elements, sugar-acid components and hormones in fuji apples. Agronomy 2023, 13, 969. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Ali, M.M.; Ben Hifaa, A.B.; Mosa, W.F. Influence of spraying some biostimulants on yield, fruit quality, oil fruit content and nutritional status of olive (Olea europaea L.) under salinity. Horticulturae 2023, 9, 825. [Google Scholar] [CrossRef]
- Asadi, M.; Rasouli, F.; Amini, T.; Hassanpouraghdam, M.B.; Souri, S.; Skrovankova, S.; Mlcek, J.; Ercisli, S. Improvement of Photosynthetic Pigment Characteristics, Mineral Content, and Antioxidant Activity of Lettuce (Lactuca sativa L.) by Arbuscular Mycorrhizal Fungus and Seaweed Extract Foliar Application. Agronomy 2022, 12, 1943. [Google Scholar] [CrossRef]
- Prasad, K.; Das, A.K.; Oza, M.D.; Brahmbhatt, H.; Siddhanta, A.K.; Meena, R.; Eswaran, K.; Rajyaguru, M.R.; Ghosh, P.K. Detection and quantification of some plant growth regulators in a seaweed-based foliar spray employing a mass spectrometric technique sans chromatographic separation. J. Agric. Food Chem. 2010, 58, 4594–4601. [Google Scholar] [CrossRef]
- Vannini, A.; Moratelli, F.; Monaci, F.; Loppi, S. Effects of wood distillate and soy lecithin on the photosynthetic performance and growth of lettuce (Lactuca sativa L.). SN Appl. Sci. 2021, 3, 113. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Martínez-Lorente, S.E.; Martí-Guillén, J.M.; Pedreño, M.Á.; Almagro, L.; Sabater-Jara, A.B. Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress. Antioxidants 2024, 13, 318. [Google Scholar] [CrossRef]
- Tung-Yunn, H.O.; Quigg, A.; Finkel, Z.V.; Milligan, A.J.; Wgman, K.; Falkowski, P.G.; Morel, F.M.M. The elemental composition of some marine phytoplankton. J. Phycol. 2003, 39, 10–20. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Koç, I.; Yildiz, Ş.; Yardim, E.N. Effect of some pesticides and wood vinegar on soil nematodes in a wheat agro-ecosytem. Kahramanmaraş Sütçü İmam Univ. J. Agric. Nat. 2020, 23, 621–633. [Google Scholar]
- Dixon, K.W.; Merritt, D.J.; Flematti, G.R.; Ghisalberti, E.L. Karrikinolide—A phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Hortic. 2009, 813, 155–170. [Google Scholar] [CrossRef]
- Chiwocha, S.D.S.; Dixon, K.W.; Flematti, G.R.; Ghisalberti, E.L.; Merritt, D.J.; Nelson, D.C.; Riseborough, J.-A.M.; Smith, S.M.; Stevens, J.C. Karrikins: A new family of plant growth regulators in smoke. Plant Sci. 2009, 177, 252–256. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Ascough, G.D.; Verschaeve, L.; Baeten, K.; Arruda, M.P.; Van Staden, J. Effect of smoke-water and a smoke-isolated butenolide on the growth and genotoxicity of commercial onion. Sci. Hortic. 2010, 124, 434–439. [Google Scholar] [CrossRef]
- Spinelli, F.; Giovanni, F.; Massimo, N.; Mattia, S.; Guglielmo, C. Perspectives on the use of a seaweed extract to moderate the negative effects of alternate bearing in apple trees. J. Hortic. Sci. Biotechnol. 2009, 17, 131–137. [Google Scholar] [CrossRef]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The effects of a seaweed extract in addition to nitrogen and boron fertilization on productivity, fruit maturation, leaf nutritional status and oil quality of the olive (Olea europaea L.) cultivar Koroneiki. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef]
- Jindo, K.; Goron, T.L.; Pizarro-Tobías, P.; Sánchez-Monedero, M.A.; Audette, Y.; Deolu-Ajayi, A.O.; van der Werf, A.; Teklu, M.G.; Shenker, M.; Pombo Sudré, C.; et al. Application of biostimulant products and biological control agents in sustainable viticulture: A review. Front. Plant Sci. 2022, 13, 932311. [Google Scholar] [CrossRef]
- Souza, A.C.; Olivares, F.L.; Peres, L.E.P.; Piccolo, A.; Canellas, L.P. Plant hormone crosstalk mediated by humic acids. Chem. Biol. Technol. Agric. 2022, 9, 29. [Google Scholar] [CrossRef]
- Obata, T. Metabolons in plant primary and secondary metabolism. Phytochem. Rev. 2019, 18, 1483–1507. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A. Transcription factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Williams, A.; Gamir, J.; Gravot, A.; Pétriacq, P. Chapter Three—Untangling plant immune responses through metabolomics. Adv. Bot. Res. 2021, 98, 73–105. [Google Scholar] [CrossRef]
- Daws, M.I.; Davies, J.; Pritchard, H.W.; Brown, N.A.; Van Staden, J. Butenolide from plant-derived smoke enhances germination and seedling growth of arable weed species. Plant Growth Regul. 2007, 51, 73–82. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Michel, L.; Pena, A.; Pastenes, C.; Berríos, P.; Rombol, A.D.; Covarrubias, J.I. Sustainable strategies to prevent iron deficiency, improve yield and berry composition in blueberry (Vaccinium spp.). Front. Plant Sci. 2019, 10, 255. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, J.; Luo, T.; Zhang, K.; Khan, Z.; Zhou, Y.; Cheng, T.; Yuan, B.; Peng, X.; Hu, L. Wood Vinegar Impact on the Growth and Low-Temperature Tolerance of Rapeseed Seedlings. Agronomy 2022, 12, 2453. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelserer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef]
- Roshdy, K.A. Effect of spraying silicon and seaweed extract on growth and fruiting of grandnaine banana. Egypt. J. Agric. Res. 2014, 92, 979–991. [Google Scholar] [CrossRef]
- Alebidi, A.; Almutairi, K.; Merwad, M.; Mostafa, E.; Saleh, M.; Ashour, N.; Al-Obeed, R.; Elsabagh, A. Effect of Spraying Algae Extract and Potassium Nitrate on the Yield and Fruit Quality of Barhee Date Palms. Agronomy 2021, 11, 922. [Google Scholar] [CrossRef]
- Ngullie, C.R.; Tank, R.V.; Bhander, D.R. Effect of salicylic acid and humic acid on flowering, fruiting, yield and quality of mango (Mangifera indica L.) cv. KESAR. Adv. Res. J. Crop Improv. 2014, 5, 136–139. [Google Scholar] [CrossRef]
- El-Hoseiny, H.M.; Helaly, M.N.; Elsheery, N.I.; Alam-Eldeinry, S.H.M. Humic Acid and Boron to Minimize the Incidence of Alternate Bearing and Improve the Productivity and Fruit Quality of Mango Trees. HortScience 2020, 55, 1026–1037. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.R.; El-Greadly, N.; Helmy, Y.I.; Singer, S.M. Response of tomato plants to different rates of humic based fertilizer and NPK fertilization. J. Appl. Sci. Res. 2007, 3, 169–174. [Google Scholar]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).