Fish Viscera Hydrolysates and Their Use as Biostimulants for Plants as an Approach towards a Circular Economy in Europe: A Review
Abstract
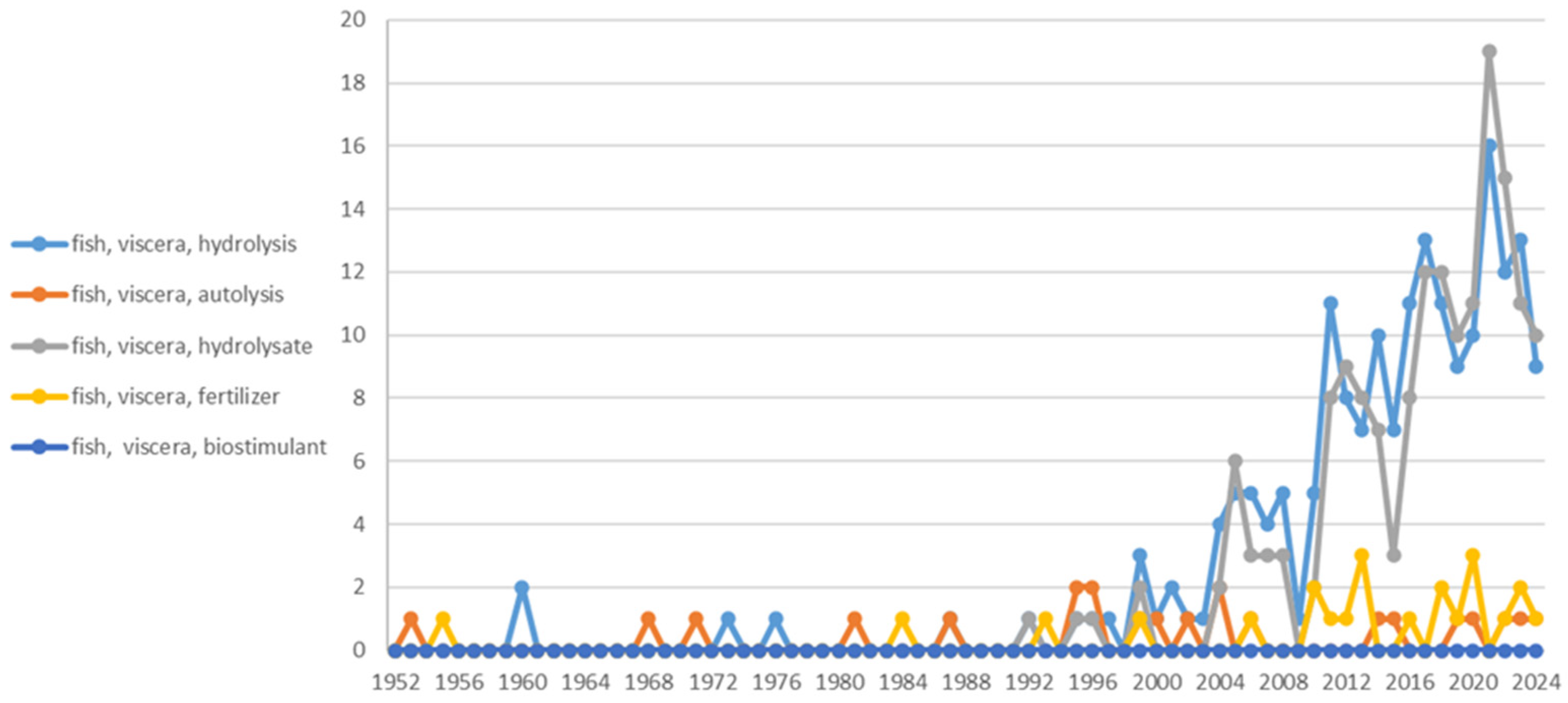
1. Introduction
2. Bio-Based Fertilizers for Plants
2.1. EU Fertilizer Product Regulation
2.2. Biostimulants
2.3. Amino Acid-Based Biostimulants
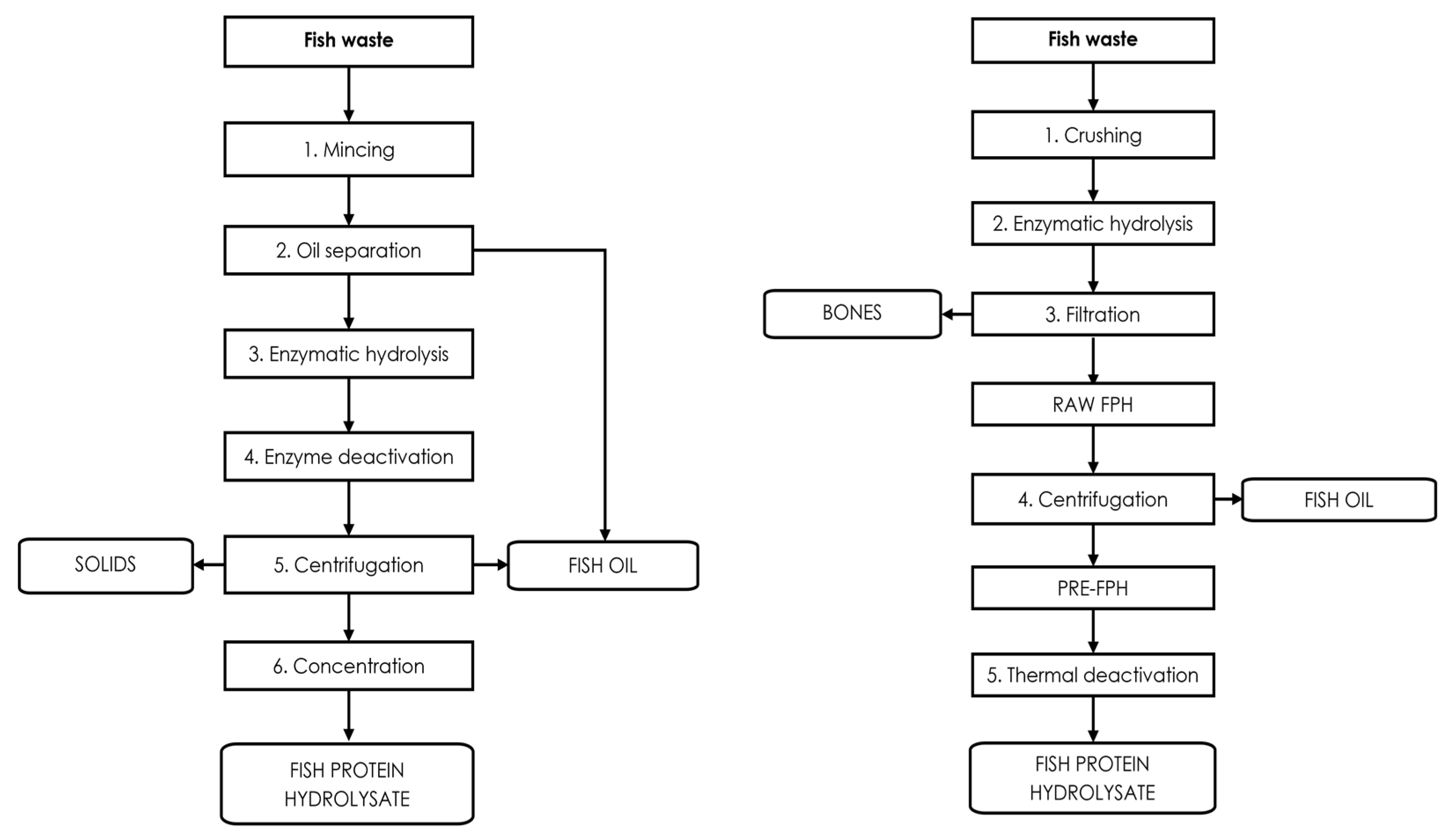
3. Fish Protein Hydrolysates
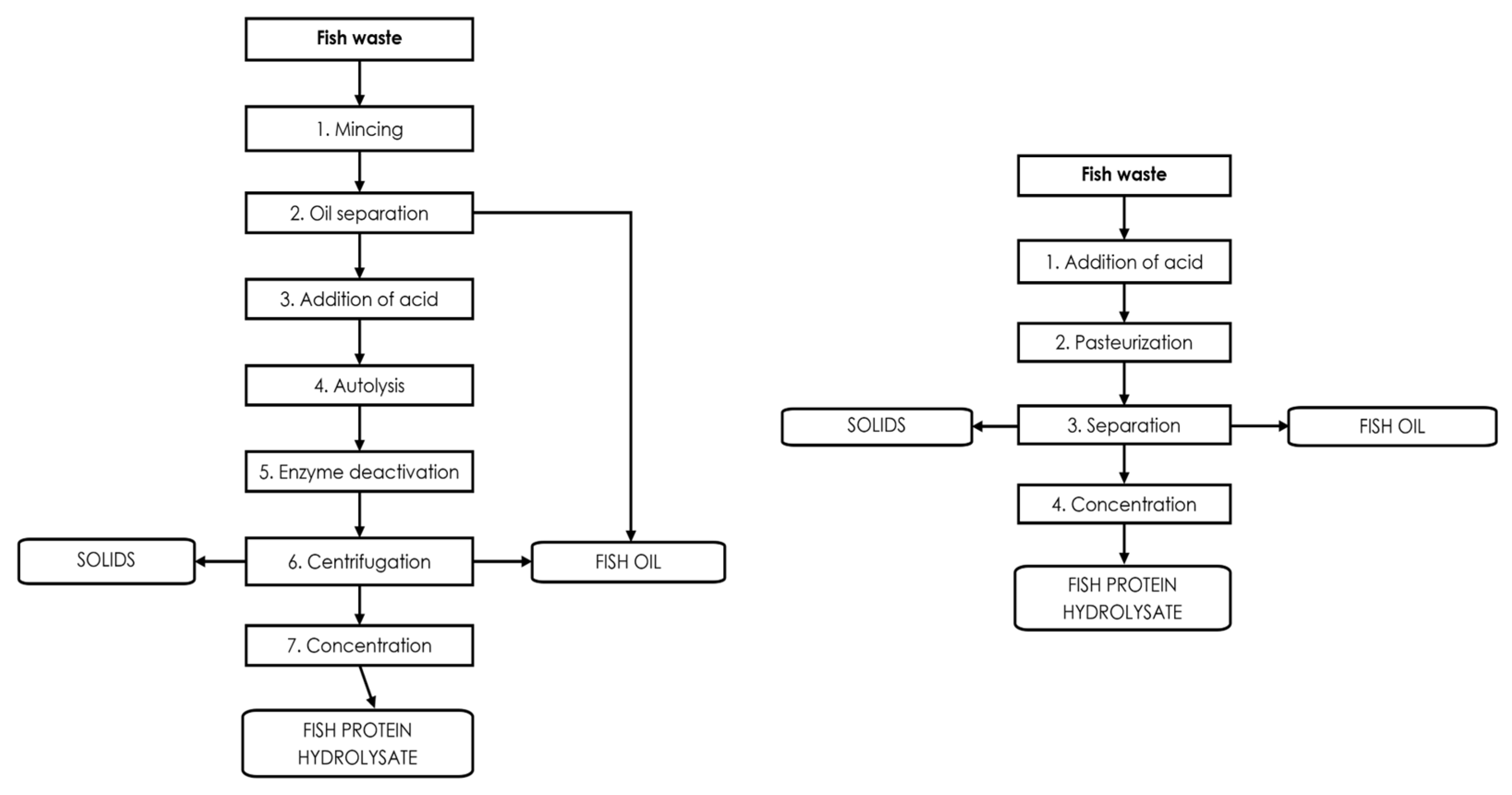
3.1. Chemical Hydrolysis
3.2. Enzymatic Hydrolysis
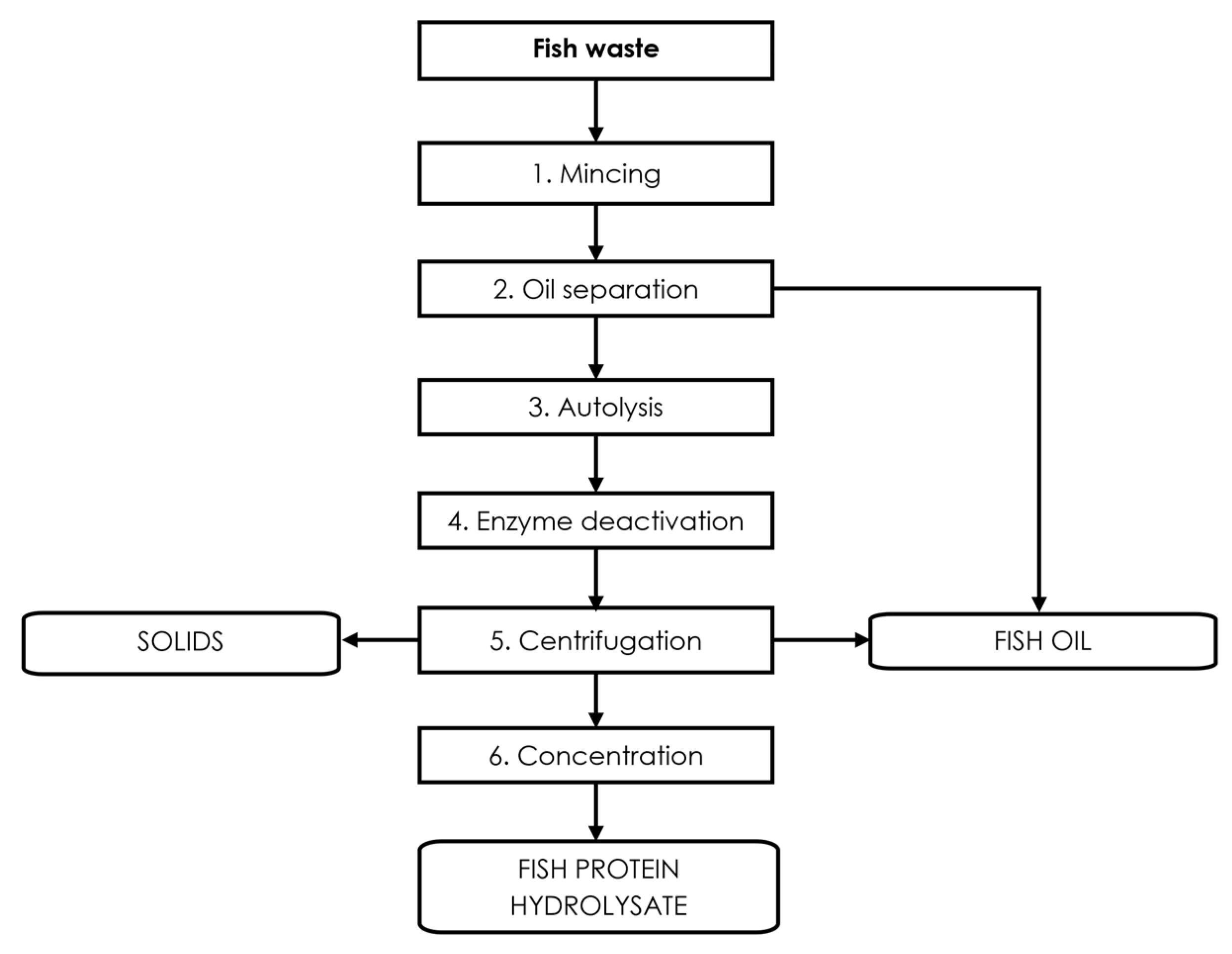
3.3. Endogenous Enzymes of Fish
3.4. Use of Fish Protein Hydrolysates in Agriculture
4. Fish Viscera
Fish Viscera Protein Hydrolysates
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture. 2022. Available online: https://www.fao.org/documents/card/en/c/cc0461en (accessed on 12 March 2024).
- Moreira, T.F.M.; Pessoa, L.G.A.; Seixas, F.A.V.; Ineu, R.P.; Gonçalves, O.H.; Leimann, F.V.; Ribeiro, R.P. Chemometric evaluation of enzymatic hydrolysis in the production of fish protein hydrolysates with acetylcholinesterase inhibitory activity. Food Chem. 2022, 367, 130728. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, R.M.; Macedo Viegas, E.M.; Carneiro, D.J. Amino acid composition of processed fish silage using different raw materials. Anim. Feed Sci. Technol. 2003, 105, 199–204. [Google Scholar] [CrossRef]
- Radziemska, M.; Vaverková, M.D.; Adamcová, D.; Brtnický, M.; Mazur, Z. Valorization of fish waste compost as a fertilizer for agricultural use. Waste Biomass Valoriz. 2019, 10, 2537–2545. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Ferrante, A.; Magnani, G. Application of biostimulants in floating system for improving rocket quality. J. Food Agric. Environ. 2005, 3, 86–88. [Google Scholar]
- Kurniawati, A.; Toth, G.; Ylivainio, K.; Toth, Z. Opportunities and challenges of bio-based fertilizers utilization for improving soil health. Org. Agric. 2023, 13, 335–350. [Google Scholar] [CrossRef]
- European Commission. Circular Economy: New Regulation to Boost the Use of Organic and Waste-Based Fertilisers; European Commission: Brussels, Belgium, 17 March 2016; Available online: https://ec.europa.eu/commission/presscorner/api/files/document/print/en/ip_16_827/IP_16_827_EN.pdf (accessed on 2 May 2024).
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]
- Tur-Cardona, J.; Bonnichsen, O.; Speelman, S.; Verspecht, A.; Carpentier, L.; Debruyne, L.; Marchand, F.; Jacobsen, B.H.; Buysse, J. Farmers’ reasons to accept bio-based fertilizers: A choice experiment in seven different European countries. J. Clean. Prod. 2018, 197, 406–416. [Google Scholar] [CrossRef]
- Jaies, I.; Qayoom, I.; Saba, F.; Khan, S. Fish wastes as source of fertilizers and manures. In Fish Wastes to Valuable Products, 1st ed.; Maqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 329–338. [Google Scholar]
- European Commission. Regulation 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations No 1069/2009 and repealing Regulation No 2003/2003. Off. J. Eur. Union 2019, 170, 1–114. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 8 April 2024).
- Madende, M.; Hayes, M. Fish by-product use as biostimulants: An overview of the current state of the art, including relevant legislation and regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar] [CrossRef]
- Drobek, M.; Frac, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 355. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Abbott, L.K.; Macdonald, L.M.; Wong, M.T.F.; Webb, M.J.; Jenkins, S.N.; Farrell, M. Potential roles of biological amendments for profitable grain production—A review. Agric. Ecosyst. Environ. 2018, 256, 34–50. [Google Scholar] [CrossRef]
- Pecha, J.; Fürst, T.; Kolomazník, K.; Friebrová, V.; Svoboda, P. Protein biostimulant foliar uptake modelling: The impact of climatic conditions. AIChE 2012, 58, 2010–2019. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- European Biostimulants Industry Council. 2018. Available online: https://biostimulants.eu/ (accessed on 24 April 2024).
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Ferrante, A. Effect of glutamic acid foliar applications on lettuce under water stress. Physiol. Mol. Biol. Plants 2021, 27, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.G.; Yu, C.; Yang, L.; Qin, S.J.; Ma, H.Y.; Du, G.D.; Liu, G.C.; Khanizadeh, S. Effects of Foliar-Applied L-Glutamic Acid on the Diurnal Variations of Leaf Gas Exchange and Chlorophyll Fluorescence Parameters in Hawthorn (Crataegus pinnatifida Bge.). Eur. J. Hortic. Sci. 2009, 74, 204–209. [Google Scholar]
- Shahrajabian, M.H.; Cheng, Q.; Sun, W. The effects of amino acids, phenols and protein hydrolysates as biostimulants on sustainable crop production and alleviated stress. Recent Pat. Biotechnol. 2022, 16, 319–328. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2014, 31, 1–17. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Skene, K.R.; Formanek, P. Non-protein amino acids: Plant, soil and ecosystem interactions. Plant Soil 2011, 342, 31–48. [Google Scholar] [CrossRef]
- Ashmead, H.D.; Ashmead, H.H.; Miller, G.W.; Hsu, H.H. Foliar Feeding of Plants with Amino Acid Chelates; Noyes Publications: Park Ridge, NJ, USA, 1986. [Google Scholar]
- Souri, M.K. Aminochelate fertilizers: The new approach to the old problem; a review. Open Agric. 2016, 1, 118–123. [Google Scholar] [CrossRef]
- Serna-Rodríguez, J.R.; Castro-Brindis, R.; Colinas-León, M.T.; Sahagún-Castellanos, J.; Rodríguez-Pérez, J.E. Aplicación foliar de ácido glutámico en plantas de jitomate (Lycopersicon esculentum Mill.). Rev. Chapingo. Ser. Hortic. 2011, 17, 9–13. [Google Scholar] [CrossRef]
- Alahmad, K.; Noman, A.; Xia, W.; Jiang, Q.; Xu, Y. Influence of the Enzymatic Hydrolysis Using Flavourzyme Enzyme on Functional, Secondary Structure, and Antioxidant Characteristics of Protein Hydrolysates Produced from Bighead Carp (Hypophthalmichthys nobilis). Molecules 2023, 28, 519. [Google Scholar] [CrossRef]
- Domínguez, H.; Iñarra, B.; Labidi, J.; Mendiola, D.; Bald, C. Comparison of amino acid release between enzymatic hydrolysis and acid autolysis of rainbow trout viscera. Heliyon 2024, 10, e27030. [Google Scholar] [CrossRef]
- Opheim, M.; Slizyte, R.; Sterten, H.; Provan, F.; Larssen, E.; Kjos, N.P. Hydrolysis of Atlantic salmon (Salmo salar) rest raw materials—Effect of raw material and processing on composition, nutritional value, and potential bioactive peptides in the hydrolysates. Process Biochem. 2015, 50, 1247–1257. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Heyser, J.W.; De Bruin, D.; Kincaid, M.L.; Johnson, R.Y.; Rodriguez, M.M.; Robinson, N.J. Inhibition of NaCI-induced proline biosynthesis by exogenous proline in halophilic Distichlis spicata suspension cultures. J. Exp. Bot. 1989, 40, 225–232. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 1998, 36, 767–772. [Google Scholar] [CrossRef]
- Chrominski, A.; Halls, S.; Weber, D.J.; Smith, B.N. Proline affects ACC to ethylene conversion under salt and water stresses in the halophyte, Allenrolfea occidentalis. Environ. Exp. Bot. 1989, 29, 359–363. [Google Scholar] [CrossRef]
- Flores, T.; Todd, C.D.; Tovar-Mendez, A.; Dhanoa, P.K.; Correa-Aragunde, N.; Hoyos, M.E.; Brownfield, D.M.; Mullen, R.T.; Lamattina, L.; Polacco, J.C. Arginase-negative mutants of arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol. 2008, 147, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Fraguas, J.; Mirón, J.; Valcárcel, J.; Pérez-Martín, R.I.; Antelo, L.T. Valorisation of fish discards assisted by enzymatic hydrolysis and microbial bioconversion: Lab and pilot plant studies and preliminary sustainability evaluation. J. Clean. Prod. 2020, 246, 119027. [Google Scholar] [CrossRef]
- Gao, M.T.; Hirata, M.; Toorisaka, E.; Hano, T. Acid-hydrolysis of fish wastes for lactic acid fermentation. Bioresour. Technol. 2006, 97, 2414–2420. [Google Scholar] [CrossRef]
- Wisuthiphaet, N.; Kongruang, S. Production of fish protein hydrolysates by acid and enzymatic hydrolysis. J. Med. Bioeng. 2015, 4, 466–470. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Aspmo, S.I.; Horn, S.J.; Eijsink, V.G.H. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem. 2005, 40, 1957–1966. [Google Scholar] [CrossRef]
- Garofalo, S.F.; Cavallini, N.; Demichelis, F.; Savorani, F.; Mancini, G.; Fino, D.; Tommasi, T. From tuna viscera to added-value products: A circular approach for fish-waste recovery by green enzymatic hydrolysis. Food Bioprod. Process. 2023, 137, 155–167. [Google Scholar] [CrossRef]
- Ramakrishnan, V.V.; Ghaly, A.E.; Brooks, M.S.; Budge, S.M. Extraction of proteins from mackerel fish processing waste using Alcalase enzyme. Bioprocess. Biotech. 2013, 3, 130. [Google Scholar] [CrossRef]
- Valcarcel, J.; Sanz, N.; Vázquez, J.A. Optimization of the enzymatic protein hydrolysis of by-products from seabream (Sparus aurata) and seabass (Dicentrarchus labrax), chemical and functional characterization. Foods 2020, 9, 1503. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez Amado, I.; Valcarcel, J. Valorization of aquaculture by-products of salmonids to produce enzymatic hydrolysates: Process optimization, chemical characterization and evaluation of bioactives. Mar. Drugs 2019, 17, 676. [Google Scholar] [CrossRef]
- Korkmaz, K.; Tokur, B. Optimization of hydrolysis conditions for the production of protein hydrolysates from fish wastes using response surface methodology. Food Biosci. 2022, 45, 101312. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish waste: From problem to valuable resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Vannabun, A.; Ketnawa, S.; Phongthai, S.; Benjakul, S.; Rawdkuen, S. Characterization of acid and alkaline proteases from viscera of farmed giant catfish. Food Biosci. 2014, 6, 9–16. [Google Scholar] [CrossRef]
- Sriket, C. Proteases in fish and shellfish: Role on muscle softening and prevention. Int. Food Res. J. 2014, 21, 433–445. [Google Scholar]
- Martin, A.M. (Ed.) Fisheries Processing, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Morrissey, M.T.; Okada, T. Marine enzymes from seafood by-products. In Maximising the Value of Marine by-Products, 1st ed.; Shahidi, F., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 374–396. [Google Scholar]
- Olsen, R.; Toppe, J. Fish silage hydrolysates: Not only a feed nutrient, but also a useful feed additive. Trends Food Sci. Technol. 2017, 66, 93–97. [Google Scholar] [CrossRef]
- Richardsen, R.; Nystøyl, N.; Strandheim, G.; Viken, A. Analyse Marint Restråstoff. 2014. Available online: https://sintef.brage.unit.no/sintef-xmlui/handle/11250/2454964?locale-attribute=en (accessed on 9 April 2024).
- Olsen, R.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Prabha, J.; Narikimelli, A.; Sajini, M.I.; Vincent, S. Optimization for autolysis assisted production of fish protein hydrolysate from underutilized fish Pellona ditchela. Int. J. Sci. Eng. Res. 2013, 4, 1863–1869, ISSN 2229-5518. [Google Scholar]
- van’t Land, M.; Vanderperren, E.; Raes, K. The effect of raw material combination on the nutritional composition and stability of four types of autolyzed fish silage. Anim. Feed Sci. Technol. 2017, 234, 284–294. [Google Scholar] [CrossRef]
- Arason, S.; Thoroddsson, G.; Valdimarsson, G. The production of silage from waste and industrial fish: The Icelandic experience. In Marketing Profit Out of Seafood Wastes, Proceedings of the International Conference on Fish By-Products, Anchorage, AK, USA, 25–27 April 1990; Keller, S., Ed.; University of Alaska Fairbanks: Fairbanks, AK, USA, 1990; pp. 79–85. [Google Scholar]
- Cao, W.; Zhang, C.; Hong, P.; Ji, H. Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem. 2008, 109, 176–183. [Google Scholar] [CrossRef]
- Domínguez, H.; Iñarra, B.; Labidi, J.; Bald, C. Optimization of the autolysis of rainbow trout viscera for amino acid release using response surface methodology. Open Res. Eur. 2024, 4, 141. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Noori, F.; Gheshlaghi, S.P. Autolysis of rainbow trout by-products: Enzymatic activities, lipid and protein oxidation, and antioxidant activity of protein hydrolysates. LWT—Food Sci. Technol. 2021, 140, 110702. [Google Scholar] [CrossRef]
- Ahuja, I.; Dauksas, E.; Remme, J.F.; Richardsen, R.; Løes, A.K. Fish and fish waste-based fertilizers in organic farming—With status in Norway: A review. Waste Manag. 2020, 115, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 141–174. [Google Scholar]
- Cerdán, M.; Sánchez-Sánchez, A.; Oliver, M.; Juárez, M.; Sánchez-Andreu, J.J. Effect of foliar and root applications of amino acids on iron uptake by tomato plants. Acta Hortic. 2009, 830, 481–488. [Google Scholar] [CrossRef]
- Corte, L.; Dell’Abate, M.T.; Magini, A.; Migliore, M.; Felici, B.; Roscini, L.; Sardella, R.; Tancini, B.; Emiliani, C.; Cardinali, G.; et al. Assessment of safety and efficiency of nitrogen organic fertilizers from animal-based protein hydrolysates—A laboratory multidisciplinary approach. J. Sci. Food Agric. 2014, 94, 235–245. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2021 (FishStatJ). FAO Fisheries and Aquaculture Division [online]. Version 4.01.0, Rome. Update 2023. Available online: www.fao.org/fishery/es/statistics/software/fishstatj (accessed on 14 March 2024).
- FAO. Fishery and Aquaculture Statistics. Global Catches 1950–2021 (FishStatJ). FAO Fisheries and Aquaculture Division. Version 4.01.0, Rome. Update 2023. Available online: www.fao.org/fishery/es/statistics/software/fishstatj (accessed on 14 March 2024).
- APROMAR (La Acuicultura en España, 2023). Available online: https://apromar.es/notas-de-prensa/apromar-publica-su-informe-anual-la-acuicultura-en-espana-2023/ (accessed on 17 March 2024).
- Singh, A.; Soottawat, B. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef]
- SCOPUS Database. Available online: https://www2.scopus.com/search/form.uri?display=basic#basic (accessed on 17 June 2024).
- Venslauskas, K.; Navickas, K.; Nappa, M.; Kangas, P.; Mozūraitytė, R.; Šližytė, R.; Župerka, V. Energetic and Economic Evaluation of Zero-Waste Fish Co-Stream Processing. Int. J. Environ. Res. Public Health 2021, 18, 2358. [Google Scholar] [CrossRef]
- Gaviria, I.S.; Camano, J.A.; Zapata, J.E. Evaluation of the environmental impact of dry chemical silage obtained from the viscera of red tilapia (Oreochromis spp.) using ecological footprint methodology. Heliyon 2021, 7, e07337. [Google Scholar] [CrossRef]
- Bhaskar, N.; Benila, T.; Radha, C.; Lalitha, R.G. Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour. Technol. 2008, 99, 335–343. [Google Scholar] [CrossRef]
| Product Function Category (PFC) |
|---|
|
| (a) Organic |
| (b) Organo-mineral |
| (c) Inorganic |
|
|
|
|
|
|
| Component Material Category (CMC) |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Contaminant | Limit Value (mg/kg Dry Matter) |
|---|---|
| Mercury (Hg) | 1 |
| Cadmium (Cd) | 1.5 |
| Hexavalent chromium (Cr VI) | 2 |
| Inorganic arsenic (As) | 40 |
| Nickel (Ni) | 50 |
| Lead (Pb) | 120 |
| Copper (Cu) | 600 |
| Zinc (Zn) | 1500 |
| Raw Material | Enzyme | Dose | pH | Temperature (°C) | Time (Hours) | Reference |
|---|---|---|---|---|---|---|
| Seabream heads Seabass heads | Alcalase | 0.2% * | 8.2 8.5 | 57.1 58.4 | 3 3 | Valcarcel et al. [44] |
| Tuna viscera | Alcalase | 1% * | 8.5 | 55 | 2 | Garofalo et al. [42] |
| Rainbow trout frames and trimmings Salmon heads | Alcalase Alcalase | 0.1% * 0.2% * | 8.3 9 | 56 64 | 3 3 | Vázquez et al. [45] |
| Cod viscera | Alcalase | 1 g/100 g of sample | Unadjusted | 55 | 24 | Aspmo et al. [41] |
| Mackerel waste | Alcalase | 0.5% * | 7.5 | 55 | 1 | Ramakrishnan et al. [43] |
| Rainbow trout viscera | Alcalase + Protana Prime | 1% * | 7 | 60 | 7 | Domínguez et al. [30] |
| Trout waste | Alkaline protease | 1% (enzyme /substrate) | 8 | 60 | 1 | Korkmaz et al. [46] |
| Cod viscera | Protamex | 1 g/100 g of sample | Unadjusted | 55 | 24 | Aspmo et al. [41] |
| Bighead carp | Flavourzyme | 4% (enzyme /substrate) | 6.5 | 50 | 6 | Alahmad et al. [29] |
| Country | Captures | Estimated Range of by-Products Weight | Estimated Range of Viscera Weight | Aquaculture | Estimated Range of by-Products Weight | Estimated Range of Viscera Weight |
|---|---|---|---|---|---|---|
| Albania | 7589 | 4553–5312 | 607–1138 | 8048 | 4829–5634 | 644–1207 |
| Austria | 350 | 210–245 | 28–53 | 4875 | 2925–3413 | 390–731 |
| Belgium | 13,805 | 8283–9664 | 1104–20,171 | 223 | 134–156 | 18–33 |
| Belarus | 605 | 363–424 | 48–91 | 8504 | 5102–5953 | 680–1276 |
| Bosnia-Herzegovina | 305 | 183–214 | 24–46 | 3819 | 2291–2673 | 305–573 |
| Bulgaria | 55,484 | 33,291–38,839 | 4439–8323 | 12,565 | 7539–8796 | 1005–1885 |
| Croatia | 59,960 | 35,976–41,982 | 4797–8994 | 25,970 | 15,582–18,179 | 2078–3896 |
| Cyprus | 1357 | 814–950 | 109–204 | 7845 | 4707–5492 | 628–1177 |
| Czechia | 3314 | 1988–2320 | 265–497 | 20,991 | 12,595–14,694 | 1679–3149 |
| Denmark | 415,261 | 249,157–290,683 | 33,221–62,289 | 32,100 | 19,260–22,470 | 2568–4815 |
| Estonia | 63,189 | 37,913–44,232 | 5055–9478 | 849 | 509–594 | 68–127 |
| Faroe Islands | 532,282 | 319,369–372,597 | 42,583–79,842 | 115,650 | 69,390–80,955 | 9252–17,348 |
| Finland | 124,835 | 74,901–87,384 | 9987–18,725 | 14,399 | 8639–10,079 | 1152–2160 |
| France | 362,379 | 217,427–253,665 | 28,990–54,357 | 47,910 | 28,746–33,537 | 3833–7187 |
| Germany | 176,847 | 106,108–123,793 | 14,148–26,527 | 18,294 | 10,976–12,806 | 1464–2744 |
| Greece | 46,764 | 28,058–32,735 | 3741–7015 | 130,171 | 78,103–91,120 | 10,414–19,526 |
| Hungary | 4601 | 2761–3221 | 368–690 | 17,847 | 10,708–12,493 | 1428–2677 |
| Iceland | 1,027,250 | 616,350–719,075 | 82,180–154,088 | 53,136 | 31,882–37,195 | 4251–7970 |
| Ireland | 184,761 | 110,857–129,333 | 14,781–27,714 | 13,381 | 8029–9367 | 1070–2007 |
| Italy | 94,016 | 56,410–65,811 | 7521–14,102 | 60,484 | 36,290–42,339 | 4839–9073 |
| Latvia | 116,413 | 69,848–81,489 | 9313–17,462 | 901 | 541–631 | 72–135 |
| Liechtenstein | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuania | 91,253 | 54,752–63,877 | 7300 | 5135 | 3081–3595 | 411–770 |
| Luxembourg | 0 | 0 | 0 | 0 | 0 | 0 |
| Moldova | 0 | 0 | 0 | 12,900 | 7740–9030 | 1032–1935 |
| Malta | 2353 | 1412–1647 | 188–353 | 16,433 | 9860–11,503 | 1315–2465 |
| Montenegro | 753 | 452–527 | 60–113 | 640 | 384–448 | 51–96 |
| Netherlands | 261,571 | 156,943–183,100 | 20,926–39,236 | 5540 | 3324–3878 | 443–831 |
| North Macedonia | 514 | 308–360 | 41–77 | 3169 | 1901–2218 | 254–475 |
| Norway | 2,115,496 | 1,269,298–1,480,847 | 169,240–317,324 | 1,662,675 | 997,605–1,163,873 | 133,014–249,401 |
| Poland | 201,321 | 120,793–140,925 | 16,106–30,198 | 44,786 | 26,872–31,350 | 3583–6718 |
| Portugal | 156,076 | 93,646–109,253 | 12,486–23,411 | 8671 | 5203–6070 | 694–1301 |
| Romania | 3476 | 2086–2433 | 278–521 | 11,714 | 7028–8200 | 937–1757 |
| Serbia | 2354 | 1412–1648 | 188–353 | 7308 | 4385–5116 | 585–1096 |
| Slovakia | 1815 | 1089–1271 | 145–272 | 2304 | 1382–1613 | 184–346 |
| Slovenia | 241 | 145–169 | 19–36 | 1256 | 754–879 | 100–188 |
| Spain | 743,530 | 446,118–520,471 | 59,482–111,530 | 70,285 | 42,171–49,200 | 5623–10,543 |
| Sweden | 155,925 | 93,555–109,148 | 12,474–23,389 | 11,796 | 7078–8257 | 944–1769 |
| Switzerland | 1486 | 892–1040 | 119–223 | 2334 | 1400–1634 | 187–350 |
| Ukraine | 34,507 | 20,704–24,155 | 2761–5176 | 16,882 | 10,129–11,817 | 1351–2532 |
| United Kingdom | 523,488 | 314,093–366,442 | 41,879–78,523 | 219,198 | 131,519–153,439 | 17,536–32,880 |
| TOTAL | 7,587,527 | 4,552,516–5,311,269 | 607,002–1,138,129 | 2,700,987 | 1,620,592–1,890,691 | 216,079–405,148 |
| Countries of Europe, 2021 | Carp (Tonnes) | Seabass (Tonnes) | Gilt-Head Bream (Tonnes) | Salmon (Tonnes) | Trout (Tonnes) | Turbot (Tonnes) |
|---|---|---|---|---|---|---|
| Albania | - | 2463 | 3724 | - | 1861 | - |
| Austria | 666 | - | - | 8 | 3205 | - |
| Belgium | 7557 | - | - | - | 127 | - |
| Belarus | 356.2 | 54 | 82 | - | 3317 | - |
| Bosnia-Herzegovina | - | - | - | - | - | - |
| Bulgaria | 5986 | - | - | 1 | 5468 | - |
| Croatia | 3630 | 9039 | 7519 | - | 350 | - |
| Cyprus | - | 2680 | 5097 | - | 52 | - |
| Czechia | 18,709 | - | - | - | 1070 | - |
| Denmark | - | 14 | - | 1668 | 28,476 | - |
| Estonia | - | - | - | - | 712 | - |
| Faroe Islands | - | - | - | 115,650 | - | - |
| Finland | - | - | - | - | 13,551 | - |
| France | 1470 | 2290 | 1850 | - | 38,800 | - |
| Germany | 4610 | - | - | - | 8725 | - |
| Greece | 1 | 51,232 | 66,891 | - | 1911 | - |
| Hungary | 12,707 | - | - | - | 74 | - |
| Iceland | - | - | - | 46,458 | 6341 | - |
| Ireland | - | - | - | 12,844 | 537 | - |
| Italy | 199 | 7394 | 8176 | - | 41,971 | 30 |
| Latvia | 564 | - | - | - | 183 | - |
| Liechtenstein | - | - | - | - | - | - |
| Lithuania | 3734 | - | - | - | 131 | - |
| Luxembourg | - | - | - | - | - | - |
| Moldova | - | 221 | 2640 | - | - | - |
| Malta | 10,580 | - | - | - | - | - |
| Montenegro | - | 43 | 36 | - | 561 | - |
| Netherlands | - | - | - | - | 50 | 100 |
| North Macedonia | 299 | - | - | - | 2828 | - |
| Norway | - | - | - | 1,562,415 | 97,774 | - |
| Poland | 18,941 | - | - | - | 19,298 | - |
| Portugal | - | 834 | 3091 | - | 857 | 3538 |
| Romania | 7369 | - | - | - | 2747 | - |
| Serbia | 5649 | - | - | - | 1556 | - |
| Slovakia | 740 | - | - | - | 800 | - |
| Slovenia | 131 | - | - | - | 921 | - |
| Spain | - | 23,037 | 7823 | - | - | 7629 |
| Sweden | - | - | - | - | 11,703 | - |
| Switzerland | - | - | - | 162 | 1230 | - |
| Ukraine | 13,450 | - | - | - | 312 | - |
| United Kingdom | 168 | - | - | 205,000 | 13,253 | - |
| Total fish weight | 117,516 | 99,301 | 106,929 | 1,944,206 | 310,751 | 11,297 |
| Estimated range of by-products weight | 70,510–82,261 | 59,581–69,511 | 64,157–74,850 | 1,166,524–1,360,944 | 186,451–217,526 | 6778–7908 |
| Estimated range of viscera weight | 9401–17,627 | 7944–14,895 | 8554–16,039 | 155,536–291,631 | 24,860–46,613 | 904–1695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, H.; Iñarra, B.; Labidi, J.; Bald, C. Fish Viscera Hydrolysates and Their Use as Biostimulants for Plants as an Approach towards a Circular Economy in Europe: A Review. Sustainability 2024, 16, 8779. https://doi.org/10.3390/su16208779
Domínguez H, Iñarra B, Labidi J, Bald C. Fish Viscera Hydrolysates and Their Use as Biostimulants for Plants as an Approach towards a Circular Economy in Europe: A Review. Sustainability. 2024; 16(20):8779. https://doi.org/10.3390/su16208779
Chicago/Turabian StyleDomínguez, Haizea, Bruno Iñarra, Jalel Labidi, and Carlos Bald. 2024. "Fish Viscera Hydrolysates and Their Use as Biostimulants for Plants as an Approach towards a Circular Economy in Europe: A Review" Sustainability 16, no. 20: 8779. https://doi.org/10.3390/su16208779
APA StyleDomínguez, H., Iñarra, B., Labidi, J., & Bald, C. (2024). Fish Viscera Hydrolysates and Their Use as Biostimulants for Plants as an Approach towards a Circular Economy in Europe: A Review. Sustainability, 16(20), 8779. https://doi.org/10.3390/su16208779









