Hydrophobic Coatings’ Efficiency and Limestones’ Resistance to Salt Crystallisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Building Stones and Coatings Application
2.2. Natural Stone Test Methods: Determination of Resistance to Salt Crystallisation through Modified EN 12370:2019 and EN 14147:2003
- (i)
- The immersion of 4 cm sides cubes in a 14% solution of sodium sulphate decahydrate (mirabilite) for 2 h;
- (ii)
- Drying at a temperature of 105 ± 5 °C for at least 15 h;
- (iii)
- Cooling at room temperature for 2 ± 0.5 h before re-immersion in mirabilite solution;
- (iv)
- After the 15th cycle, the specimens are stored for 24 ± 1 h in fresh water at (23 ± 5 °C); Finally, they are washed thoroughly with flowing water.
- (i)
- Soaking in a 14% solution of sodium sulphate decahydrate (i.e., Mirabilite mineral) for 2 h;
- (ii)
- Drying in an oven at a temperature of 40 °C for 22 h instead of the excessive 105 ± 5 °C imposed by EN 12370;
- (iii)
- Cooling at room temperature for 30 min before soaking in fresh mirabilite solution;
- (iv)
- After the 15th cycle, the specimens are removed from the oven and stored for 24 ± 1 h in water at (23 ± 5 °C). Finally, they are washed thoroughly with flowing water.
- (i)
- Spraying the NaCl salt fog for 4 h ± 15 min at 35 °C;
- (ii)
- Drying the specimens at 35 °C in the chamber for 8 h ± 15 min.
2.3. Stone Characterisation and Damage Assessment
3. Results
3.1. Stone Characterisations by Petrographic Investigations
3.2. Damage Assessment by Visual Inspection
3.3. Wettability and Static Contact Angle
- Branco results
- Lioz results
- Alpinina results
- Blue limestone results
3.4. Mass Variation and Open Porosity Variation
3.5. Ultrasound Propagation, Velocity Ratio Index, and Quality of Building Materials
- Branco results
- Lioz results
- Alpinina results
- Blue limestone results
3.6. Uniaxial Compressive Strength
- Branco results
- Lioz results
- Alpinina results
- Blue limestone results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardell, C.; Delalieux, F.; Roumpopoulos, K.; Moropoulou, A.; Auger, F.; Van Grieken, R. Salt-induced decay in calcareous stone monuments and buildings in a marine environment in SW France. Constr. Build. Mater. 2003, 17, 165–179. [Google Scholar] [CrossRef]
- Del Monte, M.; Sabbioni, C.; Zappia, G. The origin of calcium oxalates on historical buildings, monuments and natural outcrops. Sci. Total Environ. 1987, 67, 17–39. [Google Scholar] [CrossRef]
- Török, Á. Black crusts on travertine: Factors controlling development and stability. Environ. Geol. 2008, 56, 583–594. [Google Scholar] [CrossRef]
- Flatt, R.J. Salt damage in porous materials: How high supersaturations are generated. J. Cryst. Growth 2002, 242, 435–454. [Google Scholar] [CrossRef]
- Marić, M.K.; Ožbolt, J.; Balabanić, G.; Zhychkovska, O.; Gambarelli, S. Chloride Transport in Cracked Concrete Subjected to Wetting—Drying Cycles: Numerical Simulations and Measurements on Bridges Exposed to De-Icing Salts. Front. Built Environ. 2020, 6, 561897. [Google Scholar] [CrossRef]
- Andriani, G.F.; Walsh, N. The effects of wetting and drying, and marine salt crystallization on calcarenite rocks used as building material in historic monuments. Geol. Soc. Spec. Publ. 2007, 271, 179–188. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Belfiore, C.M.; Aloise, P.; Randazzo, L.; Rovella, N.; Pezzino, A.; Montana, G. Study of the effects of salt crystallization on degradation of limestone rocks. Period. Mineral. 2013, 82, 113–127. [Google Scholar] [CrossRef]
- Desarnaud, J.; Bonn, D.; Shahidzadeh, N. The Pressure induced by salt crystallization in confinement. Sci. Rep. 2016, 6, 30856. [Google Scholar] [CrossRef] [PubMed]
- Coussy, O. Deformation and stress from in-pore drying-induced crystallization of salt. J. Mech. Phys. Solids 2006, 54, 1517–1547. [Google Scholar] [CrossRef]
- Manohar, S.; Santhanam, M.; Chockalingam, N. Performance and microstructure of bricks with protective coatings subjected to salt weathering. Constr. Build. Mater. 2019, 226, 94–105. [Google Scholar] [CrossRef]
- Tiano, P.C.M.; Aoki, I.V. Corrosion protection of steel structures in industrial and marine atmospheres by waterborne acrylics DTM (direct to metal) paint system. In Proceedings of the European Corrosion Congress, Graz, Austria, 6–10 September 2015. [Google Scholar]
- López-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of protective coatings for high-corrosivity category atmospheres in offshore applications. Materials 2019, 12, 1325. [Google Scholar] [CrossRef]
- Pires, V.; Rosa, L.G.; Amaral, P.M.; Sim, J.A.R. The Susceptibility to Salt Fog Degradation of Stone Cladding Materials: A Laboratory Case Study on Two Limestones from Portugal. Heritage 2023, 6, 492–504. [Google Scholar] [CrossRef]
- EN 12370:2019; Natural Stone Test Methods—Determination of Resistance to Salt Crystallisation. European Standard: Brussels, Belgium, 2019.
- EN 14147:2003; Natural Stone Test Methods—Determination of Resistance to Ageing by Salt Mist. European Standard: Brussels, Belgium, 2003.
- Striani, R.; Corcione, C.E.; Anna, G.D.; Frigione, M. Progress in Organic Coatings Durability of a sunlight-curable organic—Inorganic hybrid protective coating for porous stones in natural and artificial weathering conditions. Prog. Org. Coat. 2016, 101, 1–14. [Google Scholar] [CrossRef]
- Al-dosari, M.A.; Darwish, S.; El-hafez, M.A.; Elmarzugi, N.; Mansour, S. Effects of Adding Nanosilica on Performance of Ethylsilicat (TEOS) as Consolidation and Protection Materials for Highly Porous Artistic Stone. J. Mater. Sci. Eng. A 2016, 6, 192–204. [Google Scholar] [CrossRef][Green Version]
- Bergamonti, L.; Alfieri, I.; Lorenzi, A.; Predieri, G.; Barone, G.; Gemelli, G.; Mazzoleni, P.; Raneri, S. Nanocrystalline TiO2 coatings by sol–gel: Photocatalytic activity on Pietra di Noto biocalcarenite. J. Sol-Gel Sci. Technol. 2015, 75, 141–151. [Google Scholar] [CrossRef]
- Belfiore, C.M.; Fichera, G.V.; Francesco, M.; Russa, L.; Pezzino, A.; Ruffolo, S.A.; Biologiche, S.; Sez, A. The Baroque architecture of Scicli (south-eastern Sicily): Characterization of degradation materials and testing of protective products. Period. Mineral. 2012, 81, 19–33. [Google Scholar] [CrossRef]
- Lisci, C.; Pires, V.; Sitzia, F.; Mirão, J. Limestones durability study on salt crystallisation: An integrated approach. Case Stud. Constr. Mater. 2022, 17, e01572. [Google Scholar] [CrossRef]
- Di Benedetto, C.; Bianchin, S.; Cappelletti, P.; Colella, A.; De Gennaro, M.; Favaro, M.; Gambirasi, A.; Langella, A.; Luca, G.; Soranzo, M. The neapolitan yellow tuff and the vicenza stone: Experimental investigations about effectiveness of antiswelling treatment. In Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone Columbia University, New York, NY, USA, 22–26 October 2012. [Google Scholar]
- Leal, N.; Simão, J.; Gartmann, C.; Silva, Z. Salt-fog experiments on consolidant and water-repellent treated dimension stones. In Proceedings of the Salt Weathering on Buildings and Stone Sculptures, Limassol, Cyprus, 19–22 October 2011; pp. 187–194. [Google Scholar]
- Celik, M.Y.; Sert, M.; Arsoy, Z. Investigation of the Effect of Protective Chemicals on the Deterioration of Andesite Used as Building Stone Due to Salt Mist. In Proceedings of the CivilTech International Symposium on Innovations in Civil Engineering and Technology, Afyon, Turkey, 23–25 October 2019; pp. 36–48. [Google Scholar]
- EN 16581:2014; Conservation of Cultural Heritage—Surface Protection for Porous Inorganic Materials—Laboratory Test Methods for the Evaluation of the Performance of Water Repellent Products. European Standard: Brussels, Belgium, 2014.
- EN 1936:2008; Natural Stone Test Methods—Determination of Real Density and Apparent Density, and of Total and Open Porosity. European Standard: Brussels, Belgium, 2008.
- EN 14579:2004; Natural Stone Test Methods—Determination of Sound Speed Propagation. European Standard: Brussels, Belgium, 2004.
- Kahraman, S.; Ulker, U.; Delibalta, M.S. A quality classification of building stones from P-wave velocity and its application to stone cutting with gang saws. J. S. Afr. Inst. Min. Metall. 2007, 107, 427–430. [Google Scholar] [CrossRef]
- Fais, S.; Cuccuru, F.; Ligas, P.; Casula, G.; Bianchi, M.G. Integrated ultrasonic, laser scanning and petrographical characterisation of carbonate building materials on na architectural structure of a historic building. Bull. Eng. Geol. Environ. 2014, 76, 71–84. [Google Scholar] [CrossRef]
- Cuccuru, F.; Ligas, P.; Fais, S. Dynamic elastic characterization of carbonate rocks used as building materials in the historical city centre of Cagliari (Italy). Q. J. Eng. Geol. Hydrogeol. 2014, 47, 259–266. [Google Scholar] [CrossRef]
- EN 1926:2008; Natural Stone Test Methods—Determination of Uniaxial Compressive Strength. European Standard: Brussels, Belgium, 2008.
- EN 15802:2010; Natural Stone Test Methods—Conservation of Cultural Property—Test Methods—Determination of Static Contact Angle. European Standard: Brussels, Belgium, 2010.
- Folk, R.L. Practical petrographic classification of limestones. Am. Assoc. Pet. Geol. Bull. 1959, 43, 1–38. [Google Scholar]
- Hong, T.; Ridley, S.; Oreszczyn, I. A Hygrothermal Monitoring and Modeling of Historic Roof; International Building Performance Simulation Association: Eindhoven, The Netherlands, 2003; pp. 11–14. [Google Scholar]
- Sousa, L.; Siegesmund, S.; Wedekind, W. Salt weathering in granitoids: An overview on the controlling factors. Environ. Earth Sci. 2018, 77, 1–29. [Google Scholar] [CrossRef]
- Tomašić, I.; Lukić, D.; Peček, N.; Kršinić, A. Dynamics of capillary water absorption in natural stone. Bull. Eng. Geol. Environ. 2011, 70, 673–680. [Google Scholar] [CrossRef]
- El-Gohary, M. Physical deterioration of Egyptian limestone affected by saline water. Int. J. Conserv. Sci. 2013, 4, 447–458. [Google Scholar]
- Pellis, G.; Giussani, B.; Letardi, P.; Poli, T.; Rizzi, P.; Salvadori, B.; Sansonetti, A.; Scalarone, D. Improvement in the sustainability and stability of acrylic protective coatings for outdoor bronze artworks. Polym. Degrad. Stab. 2023, 218, 110575. [Google Scholar] [CrossRef]
- Silva, T.P.; de Oliveira, D.; Veiga, J.P.; Lisboa, V.; Carvalho, J.; Barreiros, M.A.; Coutinho, M.L.; Salas-Colera, E.; Vigário, R. Contribution to the Understanding of the Colour Change in Bluish-Grey Limestones. Heritage 2022, 5, 1479–1503. [Google Scholar] [CrossRef]
- Sawlowicz, Z. Pyrite framboids and their development: A new conceptual mechanism. Geol. Rundschau 1993, 82, 148–156. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Frigione, M. Novel Nano-Filled Coatings for the Protection of Built Heritage Stone Surfaces. Nanomaterials 2021, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Young, T. An essay on cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Clegg, C. Contact Angle Made Easy, Carl Clegg. 2013. Available online: https://books.google.pt/books?id=5bHNoAEACAAJ (accessed on 15 December 2023).


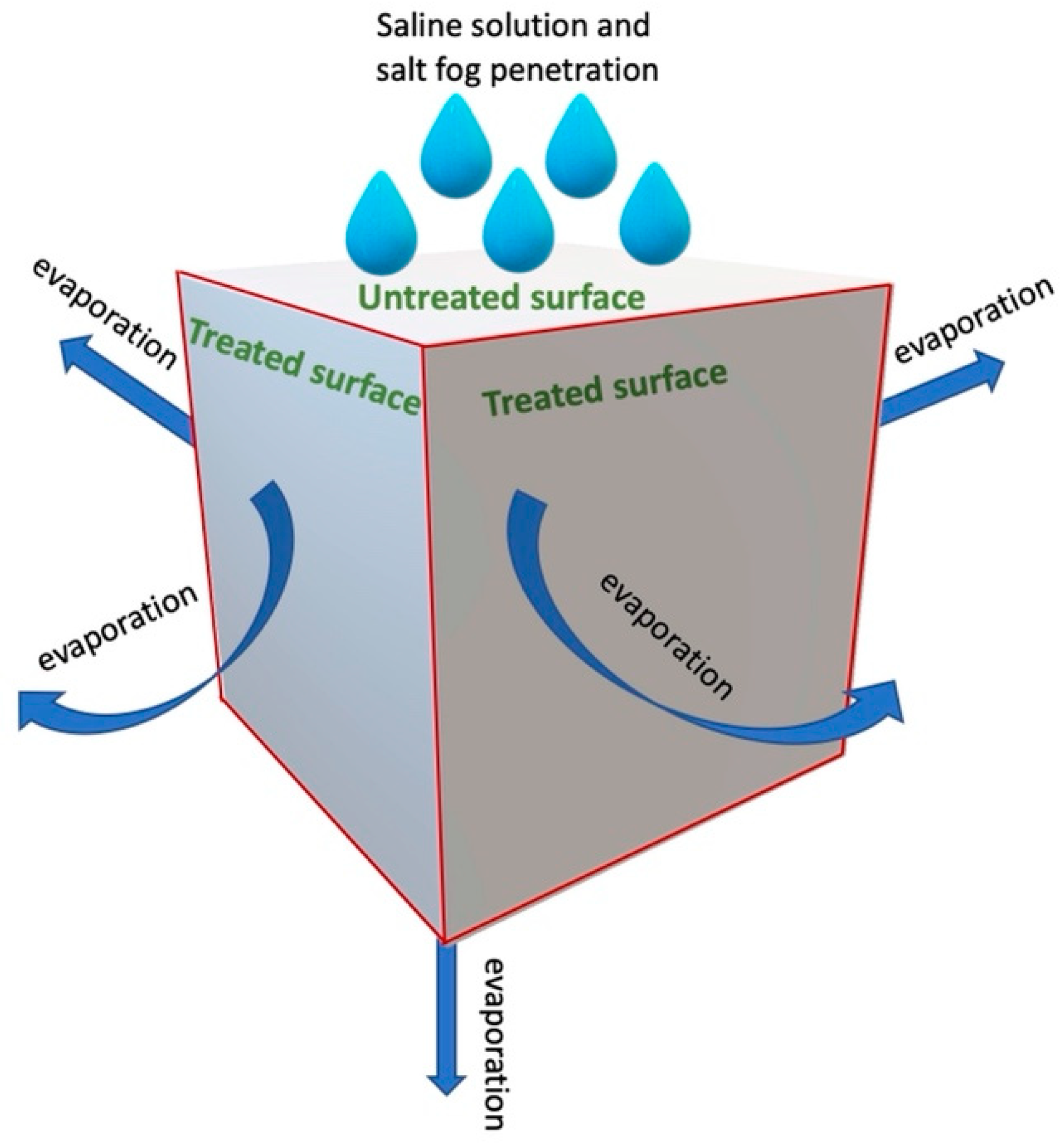

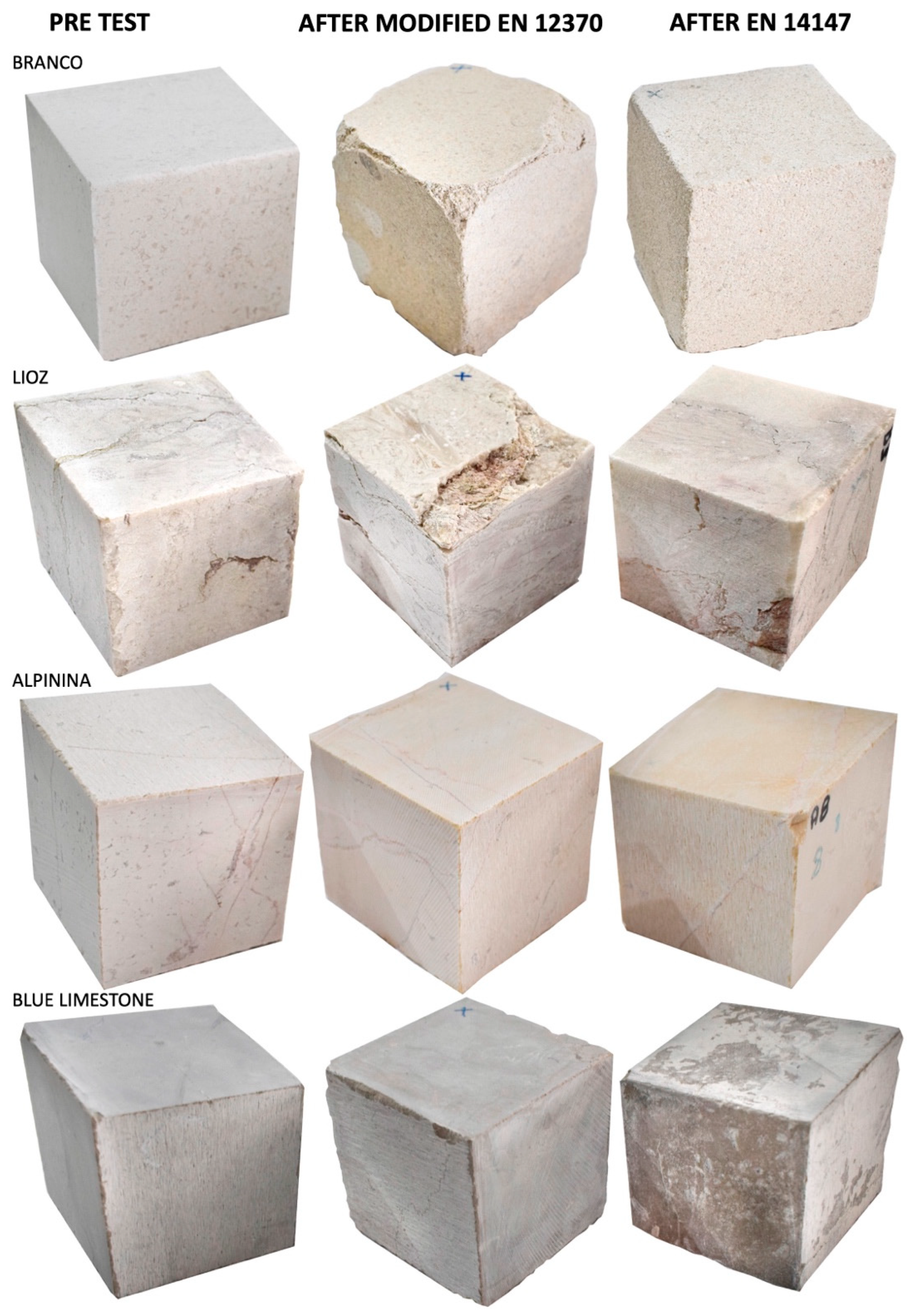

| References | Methodology | Samples | Substrate | Coating and Application Method | Outcome |
|---|---|---|---|---|---|
| Striani et al., 2016 [17] | EN 12370 | Cubic specimens of 4 cm sides | Lecce Stone: calcarenite | HYBRIDSUN: silane-based with acrylic component. Application using a brush on all surfaces of the cubes | Positive |
| Al-Dosari et al., 2016 [18] | EN 12370 | Cubic specimens of 3 cm sides | Sandstones of Kharga Oasis in Egypt | SILRES® BS OH 100: silica/polymer nanocomposites. Application using a brushing on all surfaces of the cubes | Positive |
| Bergamonti et al., 2015 [19] | EN 12370 | Cubic specimens of 4 cm sides | Noto Stone: biocalcarenite | Nanocrystalline TiO2-based coatings. Application using a brushing on all surfaces of the cubes | Positive |
| Belfiore et al., 2012 [20] | EN 12370 | Cubic specimens of 5 cm sides | Scicli calcarenite | Paraloid B72: acrylic resin ethylmethacrylate methylacrylate copolymer. Silo 111: organosiloxane oligomer. PVA K40: vinyl acetate homopolymer. Application via brushing on only one face of the cubes | Only Silo 111 was the most appropriate |
| Lisci et al., current study | Modified EN 12370 according to Lisci et al., 2021 [21] | 3 Cubic specimens of 5 cm sides for each lithology and each treatment. | Branco, Lioz, Alpinina, Blue limestone: português limestones | Aminopropyltriethoxysilane, aminefluorosilane, methylmethoxysilane, Application via brushing on 5 faces of the cubes. | Positive |
| Di Benedetto et al., 2012 [22] | EN 12370 | Cubic specimens of 4 cm sides | Neapolitan Yellow volcanic tuff; Vicenza Stone limestone | Tetramethylenediammonium dichloride. Application via immersion | Negative in terms of chemical compatibility with the tuff; Positive for Vicenza Stone |
| Di Benedetto et al., 2012 [22] | EN 14147 | Cubic specimens of 5 cm sides | Neapolitan Yellow volcanic tuff; Vicenza Stone biodetrial carbonate | Tetramethylenediammonium dichloride. Application via immersion | Positive |
| Leal et al., 2011 [23] | EN 14147 | Cubic specimens of 4 cm sides | Semi Rijo. Moleanos and Cinzento azulado: bioclastic limestones. Cinzento Monchique and Cinzento azulado de Alpalhão: coarse-grained nepheline syenite and fine-grained biotitic granite, respectively. | Silane and siloxane-based in water emulsion. Products are applied by manually spraying on the stone surface | Generally positive, mainly for silicate stones |
| Celik et al., 2019 [24] | EN 14147 | Cubic specimens of 5 cm sides | Andesits of Afyonkarahisar region (Turkey) | Siloxane-based water repellent. Application via brushing | Positive |
| Lisci et al., current study | EN 14147 | 3 Cubic specimens of 5 cm sides for each lithology and treatment. | Branco, Lioz, Alpinina, Blue limestone: português limestones | Aminopropyltriethoxysilane. aminefluorosilane. methylmethoxysilane. Application via rolling on 5 faces of the cubes. | Positive |
| Standard Reference | Modified EN 12370:2019 | EN 14147:2003 |
|---|---|---|
| Test type | Determination of resistance to salt crystallisation. | Natural stone test methods: determination of resistance to ageing by salt mist. |
| Type of salt | Saline solution of mirabilite (Na2SO4•10H2O) | Saline fog of sodium chloride (NaCl) |
| Number of specimens |
|
|
| Quality of Building Materials |
|---|
| VRI < 0.25 Very poor |
| 0.25 < VRI < 0.50 Poor |
| 0.50 < VRI < 0.75 Fair |
| 0.75 < VRI < 0.90 Good |
| VRI > 0.90 Very good |
| Pre-Test (Θ°) | After Modified EN 12370 (Θ°) | After EN 14147 (Θ°) | ||||
|---|---|---|---|---|---|---|
| Sample | Min. | Max. | Min. | Max. | Min. | Max. |
| Branco Untreated | 0 | 0 | 0 | 0 | 0 | 0 |
| Branco COATING 1 | 98 | 105 | 0 | 84 | 93 | 104 |
| Branco COATING 2 | 122 | 125 | 60 | 75 | 81 | 94 |
| Branco COATING 3 | 130 | 137 | 64 | 84 | 91 | 96 |
| Sample | ||||||
| Lioz Untreated | 0 | 0 | 0 | 0 | 0 | 0 |
| Lioz COATING 1 | 99 | 103 | 89 | 100 | 85 | 102 |
| Lioz COATING 2 | 123 | 125 | 97 | 108 | 104 | 107 |
| Lioz COATING 3 | 130 | 134 | 107 | 112 | 72 | 76 |
| Sample | ||||||
| Alpinina Untreated | 0 | 0 | 0 | 0 | 0 | 0 |
| Alpinina COATING 1 | 110 | 116 | 77 | 109 | 85 | 87 |
| Alpinina COATING 2 | 124 | 127 | 83 | 125 | 102 | 115 |
| Alpinina COATING 3 | 133 | 137 | 106 | 117 | 101 | 105 |
| Sample | ||||||
| Blue limestone Untreated | 0 | 0 | 0 | 0 | 0 | 0 |
| Blue limestone COATING 1 | 110 | 115 | 70 | 78 | 42 | 70 |
| Blue limestone COATING 2 | 120 | 126 | 102 | 116 | 53 | 91 |
| Blue limestone COATING 3 | 132 | 135 | 120 | 123 | 96 | 120 |
| ΔMass (%) | Δ Open Porosity (%) | ||||
|---|---|---|---|---|---|
| Sample | Modified EN 12370 | EN 14147 | Modified EN 12370 | EN 14147 | |
| Branco Untreated | average | −0.35 | 0.22 | 0.01 | −0.12 |
| st.dev. | 1.04 | 0.10 | 0.02 | 0.06 | |
| Branco COATING 1 | average | 0.52 | 0.01 | 0.31 | −0.09 |
| st.dev. | ±0.51 | ±0.12 | ±0.08 | ±0.09 | |
| Branco COATING 2 | average | −0.57 | −0.11 | 0.07 | −0.03 |
| st.dev. | ±1.36 | ±0.06 | ±0.08 | ±0.05 | |
| Branco COATING 3 | average | −0.05 | −0.21 | 0.08 | −0.05 |
| st.dev. | ±0.45 | ±0.12 | ±0.06 | ±0.02 | |
| All Branco samples | average | −0.11 | −0.02 | 0.12 | −0.07 |
| st.dev | ±0.89 | ±0.19 | ±0.13 | ±0.06 | |
| ΔMass (%) | Δ Open Porosity (%) | ||||
| Sample | Modified EN 12370 | EN 14147 | Modified EN 12370 | EN 14147 | |
| Lioz Untreated | average | −0.55 | −0.03 | 0.08 | 0.13 |
| st.dev. | ±0.91 | ±0.02 | ±0.21 | ±0.14 | |
| Lioz COATING 1 | average | −0.02 | −0.02 | 0.31 | 0.14 |
| st.dev. | ±0.04 | ±0.01 | ±0.08 | ±0.11 | |
| Lioz COATING 2 | average | −0.65 | −0.02 | 0.15 | 0.32 |
| st.dev. | ±1.08 | ±0.01 | ±0.16 | ±0.35 | |
| Lioz COATING 3 | average | 0.01 | −0.03 | 0.03 | 0.67 |
| st.dev. | ±0.01 | ±0.04 | ±0.20 | ±0.48 | |
| All Lioz samples | average | −0.30 | −0.02 | 0.14 | 0.32 |
| st.dev | ±0.68 | ±0.02 | ±0.18 | ±0.35 | |
| Δ Mass (%) | Δ Open Porosity (%) | ||||
| Sample | Modified EN 12370 | EN 14147 | Modified EN 12370 | EN 14147 | |
| Alpinina Untreated | average | −0.004 | 0.003 | 0.12 | 0.05 |
| st.dev. | ±0.01 | ±0.02 | ±0.11 | ±0.13 | |
| Alpinina COATING 1 | average | 0.005 | 0.004 | 0.08 | 0.13 |
| st.dev. | ±0.01 | ±0.02 | ±0.15 | ±0.06 | |
| Alpinina COATING 2 | average | 0.01 | −0.005 | 0.08 | 0.10 |
| st.dev. | ±0.01 | ±0.01 | ±0.03 | ±0.08 | |
| Alpinina COATING 3 | average | <0.001 | −0.01 | −0.004 | −0.03 |
| st.dev. | ±0.01 | ±0.01 | ±0.09 | ±0.04 | |
| All Alpinina samples | average | 0.001 | 0.001 | 0.07 | 0.06 |
| st.dev | ±0.01 | ±0.02 | ±0.10 | ±0.11 | |
| Δ Mass (%) | Δ Open Porosity (%) | ||||
| Sample | Modified EN 12370 | EN 14147 | Modified EN 12370 | EN 14147 | |
| Blue limestone Untreated | average | 0.13 | −0.08 | 0.04 | 0.43 |
| st.dev. | ±0.03 | ±0.01 | ±0.13 | ±0.14 | |
| Blue limestone COATING 1 | average | 0.13 | −0.04 | 0.07 | 0.43 |
| st.dev. | ±0.005 | ±0.04 | ±0.04 | ±0.22 | |
| Blue limestone COATING 2 | average | 0.16 | −0.10 | −0.004 | 0.50 |
| st.dev. | ±0.02 | ±0.01 | ±0.02 | ±0.08 | |
| Blue limestone COATING 3 | average | 0.12 | −0.03 | −0.03 | 0.49 |
| st.dev. | ±0.01 | ±0.12 | ±0.12 | ±0.20 | |
| All Blue limestone samples | average | 0.14 | −0.06 | 0.02 | 0.46 |
| st.dev | ±0.02 | ±0.06 | ±0.09 | ±0.15 | |
| MODIFIED EN 12370 | EN 14147 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Vp Pre Test (m/s) | Vp Post Test (m/s) | ΔVp (%) | QBM | Vp Pre Test (m/s) | Vp after Test (m/s) | ΔVp (%) | QBM | |
| Branco Untreated | average | 4196 | 3572 | −14.8 | 0.92 | 4017 | 3704 | −7.8 | 1 |
| st.dev | ±382 | ±332 | ±4.11 | ±0.02 | ±104 | ±70 | ±1.86 | ±0.01 | |
| Branco COATING 1 | average | 4056 | 3594 | −11.4 | 0.94 | 4041 | 3745 | −4 | 0.96 |
| st.dev | ±261 | ±309 | ±4.1 | ±0.02 | ±279 | ±159 | ±2.81 | ±0.01 | |
| Branco COATING 2 | average | 4026 | 3627 | −9.92 | 0.95 | 4254 | 3785 | −11 | 0.94 |
| st.dev | ±108 | ±94 | ±0.73 | ±0.004 | ±89 | ±63 | ±0.38 | ±0.002 | |
| Branco COATING 3 | average | 3807 | 3377 | −11.2 | 0.94 | 4012 | 3754 | −6.4 | 0.97 |
| st.dev | ±134 | ±60 | ±4.16 | ±0.02 | ±59 | ±80 | ±3 | ±0.01 | |
| All Branco samples | average | 4021 | 3543 | −12 | 0.94 QBM = very good | 4081 | 3747 | −8.2% | 0.96 QBM = very good |
| st.dev | ±256 | ±224 | ±4 | ±176 | ±94 | ±5 | |||
| Lioz Untreated | average | 4904 | 4855 | −1 | 0.99 | 5825 | 4792 | −17.7 | 0.91 |
| st.dev | ±5 | ±54 | ±1.02 | ±0.01 | ±83 | ±98 | ±1.63 | ±0.01 | |
| Lioz COATING 1 | average | 4988 | 4910 | −1.58 | 0.99 | 5763 | 4779 | −17.1 | 0.91 |
| st.dev | ±57 | ±77 | ±0.92 | ±0.005 | ±28 | ±77 | ±1.39 | ±0.01 | |
| Lioz COATING 2 | average | 5170 | 4951 | −4 | 0.98 | 5443 | 4703 | −13.6 | 0.93 |
| st.dev | ±355 | ±115 | ±5.45 | ±0.03 | ±82 | ±34 | ±1.84 | ±0.01 | |
| Lioz COATING 3 | average | 5008 | 4908 | −2 | 0.99 | 5759 | 4704 | −18.3 | 0.90 |
| st.dev | ±44 | ±64 | ±1.92 | ±0.01 | ±73 | ±89 | ±2.54 | ±0.01 | |
| All Lioz samples | average | 5018 | 4906 | −2.1 | 0.99 QBM = very good | 5698 | 4744 | −16.7 | 0.91 QBM = very good |
| st.dev | ±184 | ±77 | ±2.79 | ±168 | ±80 | ±3 | |||
| Alpinina Untreated | average | 5152 | 5136 | −0.31 | 0.99 | 5915 | 5104 | −13.72 | 0.93 |
| st.dev | ±37 | ±41 | ±0.51 | ±0.003 | ±47 | ±187 | ±2.48 | ±0.01 | |
| Alpinina COATING 1 | average | 5118 | 5122 | 0.08 | 1 | 5914 | 5148 | −12.96 | 0.93 |
| st.dev | ±141 | ±140 | ±0.05 | ±0.003 | ±28 | ±5 | ±0.49 | ±0.003 | |
| Alpinina COATING 2 | average | 5212 | 5158 | −1.04 | 0.99 | 6111 | 5094 | −16.45 | 0.91 |
| st.dev | ±17 | ±24 | ±0.71 | ±0.004 | ±347 | ±44 | ±5.10 | ±0.03 | |
| Alpinina COATING 3 | average | 5169 | 5168 | −0.01 | 1.00 | 6045 | 5124 | −15.23 | 0.92 |
| st.dev | ±21 | ±13 | ±0.19 | ±0.001 | ±90 | ±47 | ±0.57 | ±0.003 | |
| All Alpinina samples | average | 5163 | 5146 | −0.32% | 0.99 QBM = very good | 5996 | 5118 | −14.7% | 0.92 QBM = very good |
| st.dev | ±41 | ±36 | ±1 | ±178 | ±87 | ±3 | |||
| Blue limestone Untreated | average | 4919 | 4853 | −1.33 | 0.99 | 5616 | 4885 | −13.01 | 0.93 |
| st.dev | ±5 | ±23 | ±0.40 | ±0.002 | ±32 | ±31 | ±0.64 | ±0.003 | |
| Blue limestone COATING 1 | average | 4957 | 4894 | −1.27 | 0.99 | 5641 | 4863 | −13.79 | 0.93 |
| st.dev | ±50 | ±67 | ±0.89 | ±0.004 | ±33 | ±23 | ±0.19 | ±0.001 | |
| Blue limestone COATING 2 | average | 4902 | 4896 | −0.12 | 0.99 | 5604 | 4906 | −12.44 | 0.93 |
| st.dev | ±55 | ±25 | ±0.66 | ±0.003 | ±105 | ±29 | ±1.13 | ±0.006 | |
| Blue limestone COATING 3 | average | 4926 | 4796 | −2.64 | 0.99 | 5619 | 4890 | −12.97 | 0.93 |
| st.dev | ±27 | ±137 | ±2.68 | ±0.01 | ±14 | ±41 | ±0.52 | ±0.003 | |
| All Blue limestone samples | average | 4926 | 4860 | −1.34% | 0.99 QBM = very good | 5620 | 4886 | −13% | 0.93 QBM = very good |
| st.dev | ±28 | ±78 | ±2 | ±51 | ±31 | ±1 | |||
| Sample | Rc (MPa) Modified EN 12370 | σC (MPa) According to EN 14147 | σC (MPa) and ΔσC (%) after Modified EN 12370 and after EN 14147 | Sample | σC (MPa) Modified EN 12370 | σC (MPa) According to EN 14147 | σC (MPa) and ΔσC (%) after Modified EN 12370 and after EN 14147 | ||
|---|---|---|---|---|---|---|---|---|---|
| Branco Untreated | average | 30 | 57 | 37 ± 10 ΔσC = −7% after modified EN 12370 ΔσC = 37% after EN 14147 | Lioz Untreated | average | 112 | 101 | |
| st.dev | ±3 | ±2 | st.dev | ±17 | ±7 | 80 ± 19 ΔσC = 30% after modified EN 12370 ΔσC = 26% after EN 14147 | |||
| Branco COATING 1 | average | 38 | 53 | Lioz COATING 1 | average | 88 | 107 | ||
| st.dev | ±3 | ±6 | st.dev | ±6 | ±1 | ||||
| Branco COATING 2 | average | 36 | 50 | Lioz COATING 2 | average | 106 | 94 | ||
| st.dev | ±6 | ±7 | st.dev | ±15 | ±2 | ||||
| Branco COATING 3 | average | 34 | 42 | Lioz COATING 3 | average | 108 | 101 | ||
| st.dev | ±3 | ±6 | st.dev | ±13 | ±12 | ||||
| All Branco samples | average | 34 | 51 | All Lioz samples | average | 104 | 101 | ||
| st.dev | ±5 | ±8 | st.dev | ±15 | ±8 | ||||
| Sample | σC (MPa) Modified EN 12370 | σC (MPa) According to EN 14147 | σC (MPa) and ΔσC (%) after Modified EN 12370 and after EN 14147 | Sample | σC (MPa) Modified EN 12370 | σC (MPa) According to EN 14147 | σC (MPa) and ΔσC (%) after Modified EN 12370 and after EN 14147 | ||
| Alpinina Untreated | average | 135 | 154 | 100 ± 16 ΔσC = 30% after modified EN 12370 ΔσC = 36% after EN 14147 | Blue limestone Untreated | average | 170 | 229 | 133 ± 27 ΔσC = 35% after modified EN 12370 ΔσC = 50% after EN 14147 |
| st.dev | ±18 | ±30 | st.dev | ±40 | ±36 | ||||
| Alpinina COATING 1 | average | 123 | 159 | Blue limestone COATING 1 | average | 194 | 194 | ||
| st.dev | ±28 | ±30 | st.dev | ±53 | ±49 | ||||
| Alpinina COATING 2 | average | 130 | 113 | Blue limestone COATING 2 | average | 185 | 137 | ||
| st.dev | ±31 | ±48 | st.dev | ±39 | ±89 | ||||
| Alpinina COATING 3 | average | 133 | 118 | Blue limestone COATING 3 | average | 139 | 202 | ||
| st.dev | ±27 | ±59 | st.dev | ±70 | ±38 | ||||
| All Alpinina samples | average | 130 | 136 | All Blue limestone samples | average | 172 | 190 | ||
| st.dev | ±23 | ±43 | st.dev | ±49 | ±60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisci, C.; Galhano, C.; Simão, J.; Pires, V.; Sitzia, F.; Mirão, J. Hydrophobic Coatings’ Efficiency and Limestones’ Resistance to Salt Crystallisation. Sustainability 2024, 16, 816. https://doi.org/10.3390/su16020816
Lisci C, Galhano C, Simão J, Pires V, Sitzia F, Mirão J. Hydrophobic Coatings’ Efficiency and Limestones’ Resistance to Salt Crystallisation. Sustainability. 2024; 16(2):816. https://doi.org/10.3390/su16020816
Chicago/Turabian StyleLisci, Carla, Carlos Galhano, Joaquim Simão, Vera Pires, Fabio Sitzia, and José Mirão. 2024. "Hydrophobic Coatings’ Efficiency and Limestones’ Resistance to Salt Crystallisation" Sustainability 16, no. 2: 816. https://doi.org/10.3390/su16020816
APA StyleLisci, C., Galhano, C., Simão, J., Pires, V., Sitzia, F., & Mirão, J. (2024). Hydrophobic Coatings’ Efficiency and Limestones’ Resistance to Salt Crystallisation. Sustainability, 16(2), 816. https://doi.org/10.3390/su16020816











