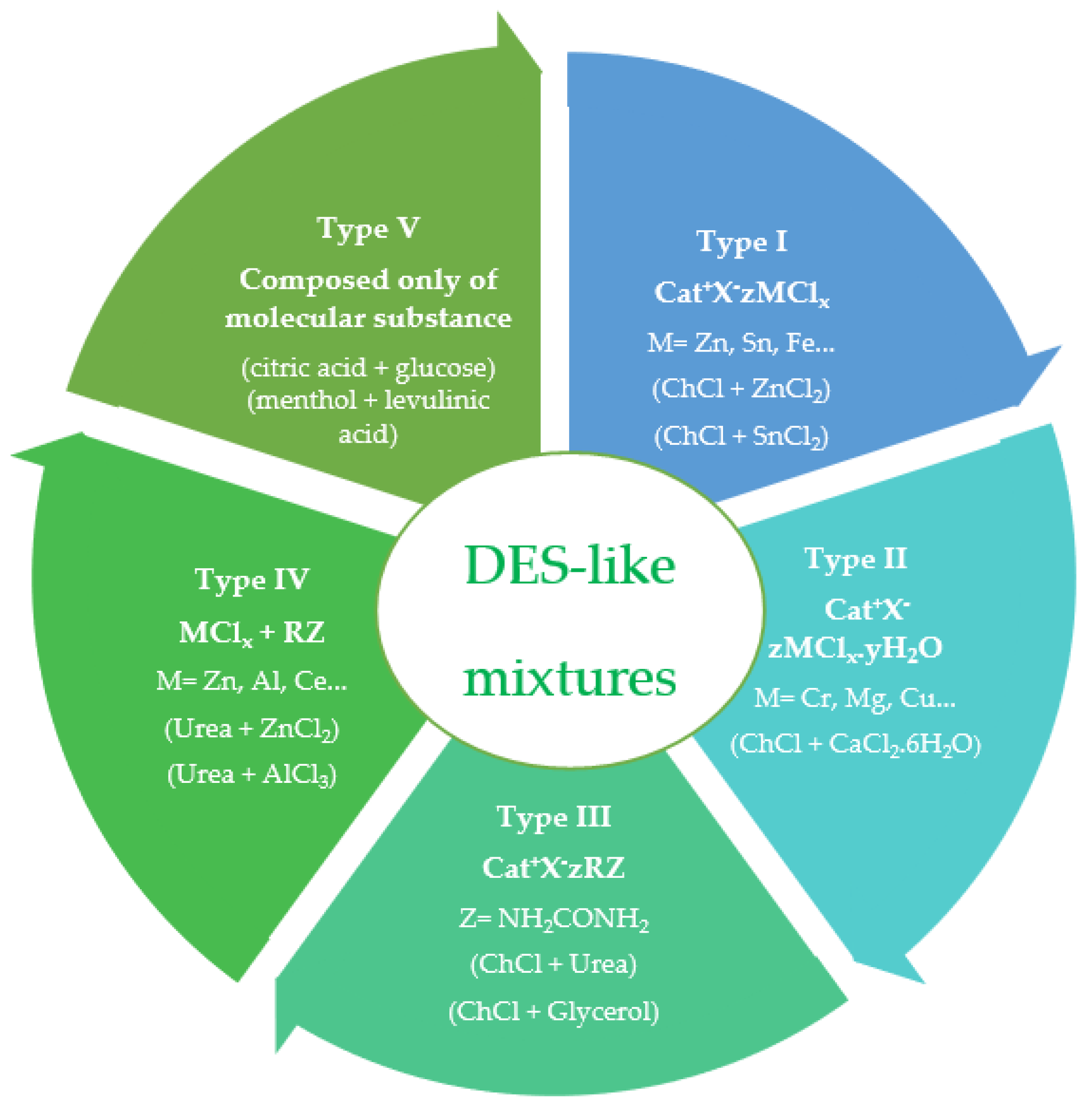
2.1. Structure of Lignin and Its Possible Transformations after the Application of DES-like Mixtures
The fractionation of lignocellulosic biomass with deep eutectic solvent-like mixtures (DES-like mixtures), also known as “green solvents”, has emerged as a potential pretreatment technique. The required characteristics of a green solvent should have performance comparable to conventional solvents but should be based on renewable raw material, that are available on a large scale, and are cost-effective, recyclable, available in good technical quality, atomically or energetically efficient, low toxicity, biodegradable, nonflammable, and thermally or electrochemically stable with easy transport and storage [
4].
For the valorization of biomass, pretreatment with DES-like mixtures is often used, which mainly results in the dissolution and removal of lignin from the biomass. By removing lignin, the residue after fractionation remains rich in cellulose, and the purpose of using these solvents is precisely to remove lignin from cellulose. However, in the valorization of biomass, hemicelluloses also have a fundamental influence, and by solubilizing them, it is possible to obtain high-value products. Regarding biomass fractionation using DES-like mixtures, it is important to solubilize lignin and hemicelluloses and, at the same time, conduct the process without changes in cellulose content [
15].
Lignin is among the three-dimensional aromatic polymers with a chemical structure formed by three precursors, called monolignols, which form the supporting layer of the plant cell. During the preparation of these monolignols, phenolic subunits are formed, namely guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H), which are connected to each other through aryl ether bonds, such as β–O–4 and α-O-4, or carbon–carbon bonds (bonds of type 5-5 and β–β), which form a strong complex that is difficult to disrupt [
3,
4]. The chemical bonds between the structural units of the lignin are diverse, among which the C-O bond containing the β–O–4 bond, 4-O-5 bond, and α-O-4 bond, and the C-C bond containing the β–β bond, β–5 bond, 5–5 bond, and β-1 bond are the most abundant. Lignin, as the main component that forms lignocellulosic materials with a content of approximately 10 to 35%, is strongly linked in biomass to carbohydrates using chemical bonds to form ligninsaccharide complexes. The essence of biomass pretreatment consists of breaking the chemical bonds within the lignin and thus splitting the bonds in the ligninpolysaccharide complex [
6]. During biomass fractionation, not only are the covalent bonds between lignin and hemicellulose broken and the hydrogen bonds that connect lignin and cellulose broken, but the application of DES-like mixtures to biomass relies primarily on the cleavage of the aryl ether (C–O) and carbon–carbon bonds (C–C) in lignin. Other chemical reactions that occur during biomass valorization, are hydroxylation, condensation, crosslinking, and demethoxylation [
1,
2,
3,
4].
Existing lignin degradation methods mainly include physical degradation, chemical degradation, and biodegradation. Promising routes for the fractionation and subsequent valorization of lignin have recently been shown by DES-like mixtures due to their attractive properties. The first and most important step in the recovery of lignin is the effective fractionation of lignin with favorable properties [
2,
3,
4]. For the transformation of lignin during pretreatment with DES-like mixtures, it is still necessary to investigate possible mechanisms in reactions with DES-like mixtures. In addition, the chemistry of lignin and the interactions between biomass and lignin are not fully understood, but many authors have studied lignin extractions, various depolymerizations of lignin into monomers, and possible lignin transformations and ongoing mechanisms after the application of DES-like mixtures [
6].
For further lignin valorization, it is necessary to know the number of β−O−4 bonds, because these bonds are among the basic and key factors controlling lignin valorization. Among the factors that influence the number of β−O−4 bonds in DES-like mixtures of lignin are mainly the nature of the components that make up DES-like mixtures, the water content, and, of course, the conditions of delignification, or rather the reaction conditions in general. The groups most used in DES-like mixtures, specifically HBDs, are carboxylic acids, which achieve high efficiency in lignin fractionation, thus leading to the complete cleavage of β−O−4 bonds, while DES-like mixtures based on polyalcohols can partially preserve β−O−4 bonds [
16]. In addition to the nature of the components, DES-like mixtures also have reaction conditions that can be used to adjust the adaptation of lignin with different amounts of β−O−4 bonds to the efficiency of fractionation of lignin. The amount of β−O−4 ether bonds decreases significantly with increasing temperature, and, therefore, if a higher temperature (approximately 145 °C) is used during lignin fractionation, the complete cleavage of β−O−4 bonds occurs, regardless of the input biomass or lignocellulosic material. On the contrary, the reaction time has little effect on the cleavage of β–O–4 bonds [
16,
17]. Regarding the addition of water, lignin with different contents of β−O−4 bonds can be varied and obtained by adding water. After the introduction of water, the catalytic activity of acid protons decreases as a result of their solvation by water molecules. Changing the amount of water causes the content of β–O–4 bonds to change accordingly. It follows that as the amount of added water increases, the number of retained β–O–4 bonds increases. By lowering the temperature, shortening the time, or adding water, the percentage of preserved β–O–4 bonds in lignin increases at the expense of the yield and purity of the lignin [
16,
17,
18]. Due to the change in the number of β−O−4 bonds during lignin fractionation using DES-like mixtures, specifically the breaking of β−O−4 bonds and demethylation of methoxy groups, the number of phenolic hydroxyl bonds can increase, so it should generally be true that in lignin from biomass after treatment with DES-like mixtures, the number of hydroxyl groups is higher compared to lignin in native biomass [
19].
Because of the heterogeneous and difficult-to-decompose structure of lignin, it was not possible to achieve extensive application use of lignin. The structure of lignin is based on the random crossing of the bonds of three monomers due to different interunit bonds, mainly β–O–4, β–β, β–5, and 5–5. The physicochemical properties of DES-like mixtures, either by changing the individual components that make up this mixture or by changing the molar ratio between HBAs and HBDs in DES-like mixtures, can be tuned and adapted for a given application area. It is known from the literature that the molar ratio between HBAs and HBDs, functional groups, or the length of the carbon chain influence the effectiveness of DES-like mixtures to dissolve lignin [
17,
18,
19,
20].
2.2. The Effect of DES-like Mixtures on Model Samples and Machine Learning Methods
Zhang et al. [
21] focused in their work on the investigation of DES-like mixtures based on amino groups (amino acids) for biomass fractionation and the associated dissolution of lignin. For the synthesis of DES-like mixtures, compounds such as
L-alanine,
L-arginine, and
L-proline were selected as HBAs, and, on the contrary, formic, acetic, lactic, and levulic acids were used as HBD-forming DES-like mixtures. These acids were used to investigate the effect of alkyl chain, hydroxyl, carbonyl, and carboxyl groups on the characterization and efficiency of DES-like mixtures to remove lignin from plant biomass. The results show that during the application of DES-like mixtures to the dealkaline lignin model, new hydrogen bonds were formed between the DES-like mixtures and the lignin, which resulted in the dissolution of the lignin. The type and number of functional groups within the DES-like mixtures (HBA/HBD) also determined the nature of the newly synthesized hydrogen bonds within the DES-like mixtures, and thus also the ability to form a hydrogen bond with lignin. Active protons that facilitated the proton-catalyzed cleavage of β–O–4 were provided by only one hydroxyl and one carboxyl group within the HBD. The excess of functional groups (guanidine, carbonyl) led to a more extensive and stronger network of hydrogen bonds in DES-like mixtures, which reduced the ability to dissolve lignin and led to the high viscosity of DES-like mixtures. From a spectroscopic point of view, a new bond between lignin and DES-like mixtures and thus a looser structure of lignin was revealed.
Wang et al. [
22] used models that represent hemicelluloses and lignin in naturally occurring plants. To observe the effect of DES-like mixtures, these models were mixed with the cellulose model to observe the difference and change in the action of DES-like mixtures in the two models, namely cellulose/hemicellulose and cellulose/lignin. To determine the optimal conditions for the interaction of DES-like mixtures with both models, the effect of temperature, water content, and different molar ratios of HBAs and HBDs in the synthesized DES-like mixtures were also monitored. The interactions between DES-like mixtures based on choline chloride/glycerol, choline chloride/lactic acid, and choline chloride/urea with the cellulose/hemicellulose and cellulose/lignin systems were monitored using a simulated calculation. Hemicellulose model structures were created from xylan monomers, and the veratrylglycerol-b-guaiacyl ether model was chosen as the lignin model. In this work, the number of hydrogen bonds, analysis of radial distribution functions, and interaction energy were analyzed. The results showed that the addition of DES-like mixtures can change the structure of hydrogen bonds, i.e., create hydrogen bonds mainly with cellulose, while there were fewer of them with lignin. DES-like mixtures based on choline chloride/urea produced the largest hydrogen bonds, 1.18 and 1.48 times more than choline chloride/glycerol and choline chloride/lactic acid. The energy of the interaction between the systems was monitored, and the results revealed that in the choline chloride/urea system, the energy of interaction with lignin was higher because of the presence of the amino group. The choline chloride/urea system removed 78.3% of hydrogen bonds (with the lignin model) and 68.4% (with the hemicellulose model). Finally, the addition of water to the systems promoted an increase in the effect of DES-like mixtures on biomass.
The separation of cellulose, hemicelluloses, and lignin is an important step for the utilization of biomass for high-value products. Pretreatment of lignocellulosic materials using green solvents, specifically DES-like mixtures, meets the conditions and principles of green chemistry and is currently receiving a lot of attention. The literature knows that the effectiveness of such biomass pretreatment using DES-like mixtures is influenced by various reaction mechanisms and certain variables during the process, which are related to the nature and composition of the biomass or the properties of the synthesized and used DES-like mixtures [
23,
24]. Xu et al. [
24] devoted themselves to machine learning methods to predict the ability of DES-like mixtures to fractionate biomass and its individual components. In this work, the effects of pretreatment were analyzed from reaction conditions, properties of DES-like mixtures, and properties of lignocellulosic materials. The results show that as far as the removal of lignin from the biomass is concerned, this process was mainly influenced by the delignification temperature, the hydrophilicity of DES-like mixtures, and, finally, the content of hemicelluloses in the raw biomass. In summary, temperature was the most important variable in biomass pretreatment using DES-like mixtures, but the physicochemical properties of DES-like mixtures, such as acidity, polarity, and hydrophilicity, also influenced lignin separation [
24].
Similarly, Ge et al. [
25] focused on the prediction of the efficiency of delignification using DES-like mixtures and the structure of lignin using machine learning. They used various analyses to elucidate the relationship between 77 variables and the mechanism of removal of lignin from biomass. They monitored the influence of the composition of input lignocellulosic materials, reaction conditions, properties of DES-like mixtures, and the structure of lignin on several target variables such as β–O–4 bonds, β–β bonds, β–5 bonds, mass and number of the average molecular weight, ratio S/G units or hydroxyl, phenolic group content, and the delignification process itself. The results revealed the same effect as in the work by Xu et al. [
24], namely that temperature and parameters related to the HBD polarity and acidity of Lewis acids significantly contributed to the degree of lignin depolymerization. Other properties that influenced the effect of biomass pretreatment using DES-like mixtures include intermolecular bonds, and when synthesizing DES-like mixtures, attention should be paid to the physical and chemical properties of HBDs in DES-like mixtures [
25].
Chen et al. [
26] investigated the effect of more than 40 DES-like mixtures based on choline chloride on the solubilization of industrial lignin (alkaline lignin and sodium lignosulfate). For comparison, some DES-like mixtures were selected for the treatment of poplar wood and straw to investigate the effect on natural lignin. DES-like mixtures were synthesized by mixing choline chloride and the three main categories of HBDs (acidic, neutral, and alkaline). The results showed that the type and proportion of HBDs in the prepared DES-like mixtures have an important influence on their characteristic properties; for example, the presence of hydroxyl groups was shown to facilitate the formation of DES-like mixtures, that is, the increase in the HBD content facilitated the solubility of lignin by creating possibilities for hydrogen bonds formation [
25,
26,
27]. In general, HBDs containing carboxyl groups showed a greater lignin solubilization capacity compared to those containing hydroxyl, and amino/amide groups. Another observation found that the solubilization of lignin decreased with increasing carbon chain length in HBDs; that is, longer carbon chains inhibit the effect of these HBDs on lignin solubilization, while, in contrast, the presence of hydroxyl groups increased the solubility of lignin. The presence of carboxyl groups in the applied DES-like mixtures has a negative effect on the removal of lignin from biomass due to an increase in viscosity or the decarboxylation of these groups in polyacids (release of CO
2) [
28]. In summary, the solubility of lignin after the application of DES-like mixtures is primarily facilitated by the breakdown of hydrogen bonds due to HBDs in DES-like mixtures. Parameters such as the number of functional groups or the length of the carbon chain in HBDs have a negative effect on lignin removal. The presence of hydroxyl groups (that is, carboxyl groups, such as HBDs, in DES-like mixtures) promotes the solubility of lignin, and, in addition, high temperatures and the presence of water in DES-like mixtures can improve and increase the effect of DES-like mixtures on lignin removal [
26].
Han et al. [
29] proposed the application of acidic DES-like mixtures in the composition of choline chloride, betaine, and
L-carnitine as HBAs, and on the other hand, HBD compounds such as oxalic acid, benzoic acid, ethyl gallate, and 5-methoxysalicylic acid were used for the solubilization of different types of lignin (kraft lignin, alkali lignin, or dealkaline lignin). Alkali lignin was chosen as a model for determining the ability of DES-like mixtures to dissolve lignin. When the solubility of alkali lignin was analyzed, it was found that with an increase in the proportion of HBDs, the solubility of lignin increased because of the presence of more possibilities for generating hydrogen bonds with alkali lignin. Of all synthesized DES-like mixtures, DES-like mixtures based on choline chloride showed a good ability to dissolve the used lignin, and, on the contrary, benzoic acid and ethyl gallate proved to be suitable HBDs due to their suitable acidity. Furthermore, it was found that the solubility of lignins is influenced by the reaction temperature, i.e., with an increase in the temperature, the solubility of lignins also increased; on the contrary, with an increase in the temperature, the viscosity of the synthesized DES-like mixtures decreased, which was advantageous before the interaction between lignin and DES-like mixtures, and thus the solubilization of lignin was promoted. The dissolution in this work was influenced by the content of three monomers in lignin (syringyl, guaiacyl, and
p-hydroxyphenyl) and by the content of hydroxyl groups in lignin samples (different polarity). Effective DES-like mixtures were shown to have stronger acidity (α > 0.95) and appropriate polarity. In addition to these aspects, the pK
a value (HBD) is also important.
Various DES-like mixtures [
30] were tested in this work to dissolve alkaline lignin, and a microscope, FT-IR, and NMR were used for subsequent analysis of the lignin composition. Choline chloride and urea were selected as HBAs in this study, and lactic acid and butanoic acid were used as HBDs, and these compounds were used to synthesize DES-like mixtures in different molar ratios. The initial results showed that the solubility of alkaline lignin was significant when lactic acid was used as an HBD, which can be attributed to the hydroxyl groups that lactic acid contains, thus increasing the polarity of the system. FT-IR and NMR spectra showed that the structure of lignin in the aromatic regions of the nebula was not affected, but the strength of the signal attributed to the β–O–4 bonds was also weakened, indicating that the β–O–4 bonds were broken, and the lignin was depolymerized in a solvent (lactic acid). The use of lactic acid as a fractionation solvent is not considered if the purpose of the fractionation is to obtain a substrate to produce the lactic acid fermentation product. Choline chloride could form a bond with the dissolved parts of lignin, increasing the molecular weight.
Sosa et al. [
31] investigated the solubility of kraft lignin. This was improved by increasing the carbon chain length of the HBD or by changing the molar ratio, while the solvents in the composition of choline chloride with 1,6-hexanediol or maleic acid were the best DES-like mixtures for its dissolution. At the same time, they found that the addition of water was a negative factor. Thermal treatments with DES-like mixtures based on carboxylic acids at a temperature of 120 °C produce chemical modifications of sulfated lignin, including the breaking of several covalent C-O bonds, namely β–O–4, α-O-4, and α-O-α. Further research and investigation during biomass pretreatment using DES-like mixtures revealed that when mono- or dicarboxylic acids are used as HBDs, the synthesized DES-like mixtures are more effective compared to polyols as HBDs. Afonso et al. [
32] reported that the acidity of hydrogen bonds, which is given by the parameter α (KamletTaft parameters), had a positive and linear relationship with the fractionation efficiency of lignocellulosic materials [
33]. In addition to these DES-like mixtures based on various HBDs, there are currently interesting DES-like mixtures derived from lignin, specifically based on choline chloride and catechol, vanillin, or p-coumaric acid, and they achieve interesting results in terms of removing lignin from biomass. In summary, DES-like mixtures with a basic character produce condensed lignin of small particle size and of low purity compared to the lignin obtained with acidic DES-like mixtures. The contribution of HBDs in DES-like mixtures, namely the acidity of HBDs, plays a significant role not only in the dissolution of lignin but also in the appearance of the side reactions of lignin [
32,
33].
To reveal the mechanism of solubilization of dealkaline lignin, Zhang et al. [
34] prepared and used DES-like mixtures based on amino acid/polyol.
L-arginine and
L-proline compounds were used as HBAs, and ethylene glycol and glycerol compounds were used as HBDs. For comparison, the influence of DES-like mixtures based on choline chloride was also monitored and evaluated. The results of this study showed that a higher value of α DES-like mixtures (Kamlet–Taft solvatochromic parameters; ability to donate hydrogen bond) resulted in the dissolution of lignin (the presence of a high content of β–O–4 bonds in lignin). Among all DES-like mixtures investigated (water content was 2% by weight), DES-like mixtures based on choline chloride/lactic acid with the highest α value (2.35) showed the best ability to dissolve lignin (29.09% by weight). Furthermore, various analyses (FT-IR, NMR, etc.) showed that the addition of water to the DES-like mixtures improved the mass transfer and activated the reaction sites in the DES-like mixtures. However, when DES-like mixtures (choline chloride/lactic acid) were applied, the addition of water had a negative effect as a result of the interaction of water with chloride anions. Electrostatic potential analysis showed that the higher the electropositivity of DES-like mixtures, the more favorable it is for lignin solubilization. In summary, the ability of DES-like mixtures to act as a cosolvent or hydrotrope was mainly determined by the characteristics and properties of the components forming the DES-like mixtures.
2.3. Fractionation of Lignocellulosic Materials Using DES-like Mixtures
In the work by Ee et al. [
10], acidic ternary DES-like mixtures based on lactic acid, alanine, and ethylene glycol were used to fractionate biomass and obtain high-purity lignin. To understand the mechanism of the effect of DES-like mixtures on the delignification of rice husks, they used nuclear magnetic resonance (NMR) spectroscopic analysis and various analytical characterizations or theoretical calculations. The biomass delignification efficiency was compared between ternary and binary DES-like mixtures (alanine/lactic acid). The input biomass contained 23.4% hemicelluloses, 36.5% cellulose, 27.1% lignin, and approximately 13% ash; on the other hand, the wheat biomass residues after the application of DES-like mixtures contained up to 80% of the content composed of cellulose together with hemicelluloses. Binary DES-like mixtures with a high lignin composition of 11.2 % but much lower lignin contents were achieved by applying ternary DES-like mixtures (5.6%); this shows that the addition of a third diol component to DES-like mixtures can improve lignin fractionation. As mentioned above, NMR analysis was applied to the chemistry and structure of isolated lignin, which revealed that pretreatment of DES-like mixtures resulted in fewer S, G, and H units compared to alkaline pretreatment. By further analysis, specifically 13C qNMR, the authors identified and quantified complex lignin-saccharide bonds. The absence of peaks (105.90 ppm) shows the pure fractionation of high-purity lignin, and the absence of ether bonds (90–72 ppm) corresponds to a possible dissolution mechanism through β–O–4 bond cleavage. Furthermore, the results showed that resinol structures formed specifically by β–β bonds during condensation reactions were present in lignin samples isolated by binary and ternary DES-like mixtures, indicating the simultaneous cleavage of β–O–4 aryl ether bonds.
The work by Yan et al. [
35] focuses on the separation of lignin from plant biomass and the subsequent production of lignin nanomaterials. For the synthesis of these lignin nanoparticles, the synthesis by means of the microwave fractionation of biomass with the participation of ternary DES-like mixtures was used. Binary and ternary DES-like mixtures were used and compared to monitor the ability to decompose biomass, and the result of this monitoring is the fact that from a visual point of view, the dissolution rate of lignin treated with ternary DES-like mixtures was better than the rate of treatment with binary DES-like mixtures, which was also confirmed by scanning electron microscopy (SEM) images (different surface of untreated and treated biomass when using binary and ternary DES-like mixtures). Regarding the numerical values, treatment with ternary DES-like mixtures removed 60.8% to 70.3%, while treatment with binary DES-like mixtures systems under the same conditions dissolved 7.6–36.7% of lignin. In the next part of the work, the authors focus on the structural changes of lignin during fractionation using DES-like mixtures with the help of Fourier-transform infrared spectroscopy (FT-IR) and two-dimensional heteronuclear single quantum coherence spectroscopy (2D-HSQC) NMR. Because of the strong interactions of hydrogen bonds and the network of covalent bonds in ligninsaccharide complexes, fractionation of lignin is difficult. However, the results of the analyses showed that some signals in the NMR spectrum disappeared from lignin after treatment with DES-like mixtures, indicating the cleavage of bonds between xylan and lignin. Furthermore, the signals corresponding to the bonds in lignin (β–O–4 bonds, β–β bonds, β–5 bonds) disappeared due to the destruction and removal of bonds in the structures analyzed. A signal appeared in the HSQC spectrum, which is attributed to the resinol structure (lignin condensation). The S/G unit ratio is an important parameter to monitor changes in the structure of lignin, and this ratio of S/G lignin in biomass increased from 1.28 to 2.76 after treatment with DES-like mixtures, indicating that G-type lignin mainly underwent fractionation during treatment. In summary, pretreatment using DES-like mixtures changed the structure of biomass due to the cleavage of β–O–4 bonds and fractionation of G-type lignin in biomass.
In the work by Nie et al. [
36], a more ecological degumming method was used using DES-like mixtures for the synthesis of kenaf fibers. In the work, DES-like mixtures based on choline chloride, as HBAs, were synthesized, and three types of compounds, as HBDs, were selected, namely urea, oxalic acid dihydrate, and ethylene glycol. The effect and mechanism of the three different DES-like mixtures on the degumming of kenaf lime were investigated and discussed. The results showed that fiber fractionation and the degumming effect increased with increasing temperature. For the treatment with DES-like mixtures based on choline chloride/ethylene glycol, because of the strong hydrogen bonds, a high reaction temperature and a longer reaction time were required. A large amount of non-cellulosic components was removed during the treatment with choline chloride/urea. The effects of three different DES-like mixtures on kenaf bran were characterized using SEM. As shown in the SEM images, treatment with choline chloride/oxalic acid dihydrate could remove most of the gummy components and alter the structure in crystalline cellulose under acidic conditions, and the β-1,4-glycosidic bonds in cellulose were broken under acidic conditions, resulting in reduced polymerization [
37]. Another part of the work was the investigation of the chemical composition of kenaf before and after treatment with DES-like mixtures. The results revealed that when the temperature reached 160 °C, the removal rates of hemicellulose and lignin reached 26.7% and 62.3% (choline chloride/urea). DES-like mixtures prepared with amide-based HBD achieved higher lignin removal rates than polyol-based HBD due to lignin removal by deprotonation of phenolic hydroxyl groups in lignin [
38].
In summary, DES-like mixtures based on oxalic acid can be assessed to have a significant effect in the removal of lignin and hemicelluloses due to proton-catalyzed bond cleavage; i.e., protons from carboxylic acids can facilitate bond cleavage reactions (ether bonds, glycosidic bonds, and lignin–saccharide bonds). FT-IR images revealed that the structure of cellulose did not change during treatment with DES-like mixtures; on the contrary, the absorption peak at 1734 cm
−1 decreased (bent and stretched in hemicellulose), which indicates the removal of hemicellulose, and the characteristic peaks for lignin were weakened after pretreatment due to a reduction in lignin content [
36].
Corn cob fractionation and lignin extraction were used by Yang et al. [
39] with Brønsted acid DES-like mixtures. In this study, two acidic polyol-based DES-like mixtures, namely ethylene glycol/oxalic acid and glycerol/oxalic acid, were synthesized. To investigate the effect and mechanism of DES-like mixtures on corn cob fractionation, changes in biomass structure, structural properties of prepared lignin, or the effect of the polyol component in DES-like mixtures on β–O–4 bonds were monitored. The results show that the efficiency of delignification was directly related to the character of DES-like mixtures and the reaction temperature (a higher temperature contributed to the destruction of plant cell walls with the subsequent splitting of hydrogen and covalent bonds in the biomass). Deviations in the efficiency of delignification between the two used DES-like mixtures may be related to their physicochemical properties (viscosity, KamletTaft solvatochromic parameters). The structural properties of lignin (after application of DES-like mixtures) were investigated using NMR spectroscopy, the results of which showed that the total content of β–O–4 bonds decreased slightly after increasing the temperature; that is, pretreatment with the DES-like mixtures achieved lignin dissociation with substantial preservation of β–O–4 bonds. Regarding the fractionation mechanism, acidic DES-like mixtures promoted carbocation intermediates (dehydration to C-α lignin), and then these intermediates were coupled with a polyol to form a polyol-alkylated lignin [
40]. In summary, under mild conditions, more than 53% of lignin was obtained, and these lignins, after treatment with DES-like mixtures, were modified with polyol (α-alkylation), which allowed the preservation of β–O–4 substructures (73 to 99%) and resulted in the suppression of condensation processes.
Zhao et al. [
41] applied a series of several diol-based DES-like mixtures to poplar wood. As part of this work, the effect of DES-like mixtures on the fractionation of poplar wood was investigated from the point of view of whether and how different DES-like mixtures, and their alkalinity, temperature, and presoaking time affect the effectiveness of the pretreatment of the input biomass. The study revealed that the pretreatment mechanism was carried out by series of analyses (FT-IR, SEM, X-ray diffraction analysis (XRD)). Compounds such as sodium hydroxide, potassium hydroxide, and lithium hydroxide were used as HBAs, while ethylene glycol, polyethylene glycol, 1,4-butyl glycol, and 1,2-propylene glycol were used as HBDs. Lignin removal ranged from 56.6% to 76.4% (DES-like mixtures based on diol monomers), while DES-like mixtures based on polyethylene glycol removed 37.6% to 56.2%. The best pretreatment effect was achieved using a mixture of potassium hydroxide/1,2-propylene glycol for 7 h at 130 °C, which removed 89.2% of lignin and 71.6% of hemicellulose while retaining 97.5% of cellulose. As part of the pH measurement, it was shown that all synthesized DES-like mixtures had an alkaline character and thus could dissolve lignin and hemicellulose, but only a little cellulose, and in addition, the higher the pH values of the prepared DES-like mixtures, the more lignin and hemicelluloses were removed [
41,
42]. The results on the effect of temperature on biomass fractionation, that is, lignin removal, showed that with increasing temperature, lignin removal gradually increased from 48.1% to 97.4%. As part of this influence, it is assumed that the viscosity of DES-like mixtures decreased with increasing temperature, and thus the dissolved substance in the solvent is more diffuse, which increases the possibilities of contact between the biomass and the solvent [
41,
42,
43]. Temperature in this process has been shown to have little effect on the effectiveness of pretreatment.
Zhao et al. [
41] further reported that X-ray diffraction analysis (XRD) revealed a change in the crystalline structure of cellulose (change in the crystallinity index) before application (48.3) and after application (57.1) of DES-like mixtures (potassium hydroxide/1,2-propylene glycol) of poplar biomass; thus, crystallinity increased during the process due to the removal of amorphous structures (lignin and hemicellulose). FT-IR analysis revealed a change in peaks at 1737 cm
−1 (C=O stretching vibration peak in carbonyl or ester groups), 1602 cm
−1, 1510 cm
−1, and 1245 cm
−1 (vibration peaks of the aromatic ring skeleton of lignin), and their disappearance in the FT-IR spectrum of poplar wood treated with DES-like mixtures indicates the removal of the carbonyl group in hemicellulose and the cancelation of the ester bond between lignin and hemicellulose and the disappearance of the aromatic structure of lignin [
44]. Finally, SEM analysis showed differences between the surface of untreated poplar wood and wood treated with DES-like mixtures (porous structures and fiber bundles) due to the removal of hemicelluloses and lignin.
Provost et al. [
45] tested and investigated the changes related to the pretreatment of softwood and brewer’s draff with DES-like mixtures to characterize the obtained lignin. The synthesized DES-like mixtures were a mixture of choline chloride as HBAs and two HBDs based on lactic acid or glycerol. When applying DES-like mixtures to the biomass used, moderate reaction temperatures of 60 and 80 °C were applied (
Table 1). Lignin characterization was performed using FT-IR, size exclusion chromatography, and 2D-HSQC NMR analysis. Regarding the effect of temperature and the DES-like mixtures used, the study results revealed that the lignin extraction yield for both biomasses increased with increasing temperature, which can be explained by the decrease in viscosity of the DES-like mixtures, resulting in better heat and mass transfer, i.e., better efficiency of DES-like mixtures [
28]. When using DES-like mixtures based on choline chloride/lactic acid for the extraction of lignin from softwood, a higher lignin extraction (56.7–77.7%) was achieved than when extracting lignin from brewer’s draff (34.5–39.3%), which may be due to the difference in % lignin in the input biomass, the complexity of the structure of lignin, or the different presence of lignin subunits (G, H, and S). Furthermore, the results revealed that acidic DES-like mixtures with a carboxylic acid, such as HBDs (lactic acid; pK
a = 3.08), achieved a higher extraction efficiency than another type of HBD (glycerol; pK
a = 14.15) [
30]. FT-IR images revealed that DES-like mixtures based on choline chloride/lactic acid were able to extract lignin from biomass but did not cause adverse reactions by forming esters by binding through phenolic hydroxyl groups of lignin subunits by grafting. The evolution of the molecular weights of lignin fractions after the application of DES-like mixtures revealed that the degradation reaction became the primary reaction during the pretreatment of DES-like mixtures, as smaller lignin fragments were obtained when the temperature increased. Among the different conditions tested and for both biomasses, the extraction of lignin with a high ratio of β–O–4 aryl ether linkages was carried out by acidic DES-like mixtures based on choline chloride/lactic acid at 80 °C.
Ma et al. [
46] focused on the degumming of hemp fibers using DES-like mixtures based on choline chloride and urea with the participation of alkaline pretreatment (H
2O
2-hydrogen peroxide). As part of their work, various operating conditions such as temperature, time, biomass ratio, and solvents were investigated for the degumming effect of hemp fibers and the mechanism of degumming. The results of the chemical composition showed that the lignin content after the application of DES-like mixtures (choline chloride/urea) reached the lowest amount (3.5%) at a reaction temperature of 130 °C due to the lower viscosity of DES-like mixtures (higher speed of the movement of molecules in the system, easier penetration into hemp fibers). The values of the degree of polymerization of the fiber after degumming increased with the reaction temperature at the same time as the temperature increased to 150 °C, and this value decreased due to the recondensation reactions of lignin. The residual rubber content and the lignin content of the degummed fiber decreased significantly from about 18.5 to 12.5% and 5.1 to 3.5%, respectively, when the reaction time increased from 60 min to 120 min. For the ratio of DES-like mixtures and input biomass (bath ratio), the residual rubber content first decreased and then remained at a constant value (if the bath ratio increased). After subsequent treatment with alkaline H
2O
2, the lignin content decreased to 1.94%. To understand the structural changes of lignin after degumming hemp fibers, NMR analysis was used, which showed that the main interunit bonds in lignin were bonds β–O–4, β–5, and β–β(
Table 1). In summary, most β–O–4 linkages and all β–5 linkages were degraded after degumming [
46,
47].
Chen et al. [
48] proposed ternary DES-like mixtures based on choline chloride/glycerol with the participation of metal salts forming acidic, alkaline, and neutral solvents (K
2CO
3, FeCl3, KCl, AlCl
3, ZnCl
2, and MgCl
2) for the fractionation of sugarcane bagasse. By analyzing the physicochemical properties of DES-like mixtures and the interactions of hydrogen bonds, the mechanism of delignification and the function of DES-like mixtures were identified. The physicochemical properties of DES-like mixtures such as pH, viscosity, KamletTaft solvatochromic parameters, and hydrogen bond interaction may have some connection with lignocellulosic fractionation and its efficiency [
39]. The results indicated that acidic DES-like mixtures based on choline chloride/glycerol/FeCl
3 and choline chloride/glycerol/AlCl
3 removed 47.6–61.9% of lignin, while alkaline choline chloride/glycerol/K
2CO
3 resulted in 72% delignification. This indicates that the hydrogen bond donor acidity (α value) and acceptor basicity (β value) of the solvents can be positively related to the biomass fractionation process. Furthermore, it was shown that the increased temperature promoted the removal of lignin fractions in a short time. Regarding the mechanism of biomass delignification using acidic ternary DES-like mixtures based on choline chloride/glycerol/FeCl
3, the dissolution of polymers, such as lignin and hemicellulose, is closely related to the acidity of the solvent. If the effect of Fe
3+ and Cl
− is combined with subsequent proton catalysis, the intramolecular network of H-bonds of lignin-saccharide complexes can be degraded by this process, and then the ether or ester bonds in lignin–saccharide complexes are split [
48,
49]. Otherwise, proton-catalyzed bond cleavage is the main driving force during fractionation under acidic conditions, in which the destruction of β–O–4 bonds is the most important step [
50]. During this process, protonation of the benzylic carbon (C
α) occurs to form a benzylcarbenium ion by the removal of the hydroxyl group in the α position, and this carbenium ion can be converted to the enol ether structure during the delignification process by either the protonation of C
β or deprotonation of C
α. Finally, the enol ether is hydrated and deprotonated to C
β in an acidic environment to form ketone and phenolic groups [
48,
49,
50].
Liu et al. [
51] applied DES-like mixtures based on carboxylic acid with the participation of microwave radiation for the fractionation of corn cobs. The work examined the effects of DES-like mixtures on the main structural characteristics of lignin. Three types of carboxylic acids were chosen as DES-like mixtures, namely formic, lactic, and acetic acids, which were synthesized together with choline chloride. Ground wood lignin was isolated from biomass, namely corn cob, which was treated with DES-like mixtures followed by heating in a microwave reactor. The results of the characterization of DES-like mixtures of lignin showed that the removal ratio and the recovery yield of this lignin were in the order of choline chloride/formic acid < choline chloride/lactic acid < choline chloride/acetic acid. The purity of lignin from both types of pretreatments was approximately 90%. In summary, it can be said that microwave heating increases the ionic characteristics of DES-like mixtures and the polarity of DES-like mixtures, thereby facilitating the synthesis of protons to cleave ether or ester bonds in lignin–carbohydrate complexes, improving the extraction performance of lignin from biomass [
51,
52]. Regarding the microwave pretreatment of DES-like mixtures, the signal intensity of acetyl groups in DES-like mixture lignin samples significantly increased, indicating that DES-like mixture lignin samples were acetylated. NMR analysis showed that signals between 90 and 72 ppm were not present due to the removal of ether bonds in DES-like mixtures of lignin, and these samples were mainly composed of S and G units. It is hypothesized that the microwave pretreatment of DES-like mixtures could promote the destruction of the β–O–4 bond in lignin and the release of phenolic hydroxyl groups [
51].
A cheap and recyclable solvent (DES-like mixtures) based on choline chloride/oxalic acid and ethylene glycol was used in [
53] for the pretreatment of seed casings (
Eucommia ulmoides) and subsequent value-added lignin conversion. The results showed that at 100 °C for 2 h, 78.7% of hemicellulose and 62.1% of lignin were removed, while 84.0% of cellulose was retained. The regeneration yields of solid residue and lignin decreased significantly as the temperature increased from 70 °C to 120 °C. For the molar ratio of components in DES-like mixtures, the content of oxalic acid was positively correlated with the rate of lignin removal. FT-IR images showed that the peak characteristics of the aromatic structures of lignin decreased and decreased more significantly with increasing temperature. Furthermore, the absorption peak attributed to lignin decreased with increasing oxalic acid content and time. In addition, the results revealed that the retention rate of β–O–4 bonds was negatively correlated with pretreatment time and oxalic acid content in DES-like mixtures.
In another study, Wang et al. [
54] investigated the destruction of the cell wall of biomass, specifically poplar wood, using DES-like mixtures for lignin valorization. An atomic force microscope showed that the pretreatment of DES-like mixtures based on choline chloride/lactic acid removed non-cellulosic components, i.e., after application, β–O–4 bonds between lignin units were broken, which led to a decrease in molecular weight (
Table 2). However, the long pretreatment time led to the repolymerization of the regenerated lignin samples. NMR analysis revealed that pretreatment with DES-like mixtures completely removed β–1 bonds (Cα−Hα in spirodienone substructures). In addition, the signals belonging to β–O–4, β–β, and β–5 linkages gradually decreased with time of pretreatment with DES-like mixtures, indicating the disruption of β–O–4 linkages and aryl ether linkages by the acidic system of DES-like mixtures. According to further analysis, it was found that the number of β–O–4 aryl ether linkages and linkages decreased significantly with treatment time and treatment temperature. In addition, the ratio of units in lignin (S/G) increased due to the condensation reaction.
2.4. Properties of DES-like Mixtures and Their Influence in Lignin Removal from Biomass
Because of the strong and compact structure, which is formed by covalent bonds and hydrogen bonds between the three main components (cellulose, lignin, and hemicelluloses), the fractionation of biomass using DES-like mixtures represents a complex process because of the different dissolution efficiency of these main components in DES-like mixtures. The effect of fractionation using DES-like mixtures was influenced by the properties of HBDs, and the hydrogen bonding interaction was weakened because of the competition of chloride ions during the treatment of DES-like mixtures. The most important mechanism of delignification is the selective breaking of ether bonds without the influence of bonds between carbons [
36,
55]. In the work by Nie et al. [
36], treatment with DES-like mixtures based on choline chloride/urea achieved significant effects on lignin removal, and the H
+ proton can catalyze the breaking of ether and ester bonds between lignin and hemicelluloses, resulting in the depolymerization of lignin from plant biomass; therefore, a significant and main reaction during pretreatment with DES-like mixtures is the depolymerization of lignin with the subsequent breaking of β–O–4 bonds [
56].
To understand the effect of DES-like mixtures, knowledge about the solubility of lignin during these delignification processes is necessary because DES-like mixtures have the greatest effect on lignin fraction. As reported by Yu et al. and Zhang et al. [
20,
21], delignification efficiencies vary with temperature, type of DES-like mixtures, type of HBA and HBD, and their molar ratio, as lower viscosities aid in lignin removal [
20]. Xu et al. [
57] determined that the acidity coefficient (pKa), hydrophilicity, and number of hydrogen bond donors are the most important characteristics of HBDs that can positively influence the efficiency of the application of DES-like mixtures. The mechanism of delignification is initiated by the fact that H
+ protons dissociated from HBDs (from DES-like mixtures) can cause, and at the same time accelerate, the splitting of ether and ester bonds between the polysaccharide portion (cellulose and hemicelluloses) and lignin, resulting in the removal of lignocellulosic materials [
27,
58].
During the application of acidic DES-like mixtures, the cleavage of β–O–4 bonds occurs, which includes the protonation of benzyl carbon (C
α) by acidolysis with subsequent dehydration and the formation of benzylic carbocations. This is followed by the promotion of the cleavage of the ether bond by the loss of the CH
2OH group in the C
γ position and the formation of α-β unsaturated compounds and formaldehyde [
58]. Another possible way of biomass delignification is the elimination reaction between carbocation C
α and hydrogen in C
β with subsequent hydrolysis of enol ethers in an acidic environment and the cleavage of β–O–4 (the formation of Hibbert ketones). Through the formation of covalent bonds between C
α (benzyl carbocation) and electron-rich positions (aromatic rings), lignin repolymerization occurs. In addition to these possible reactions; that is, the cleavage of β–O–4 bonds and condensation of lignin during the application of DES-like mixtures, other reactions also occur, namely acylation and demethoxylation [
56].
DES-like mixtures provide selective solubility of lignin compared to cellulose and hemicelluloses, which are supported by hydrogen bonds and a structure with strong interaction with the lignin molecule. After the separation of the lignin from the saccharide part, molecules formed by DES-like mixtures form hydrogen bonds with lignin molecules (with their fragments) and then dissolve them [
59]. Ji et al. [
60] reported that the halogen anion in choline chloride promotes the cleavage of ester and ether bonds between lignin and hemicelluloses, thereby providing protons to form additional hydrogen bonds with hydroxyl groups of lignin. Furthermore, DES-like mixtures based on acidic compounds effectively induce the cleavage of ester and ether bonds in the lignin molecule and, in addition, degrade the C–C bond in lignin. In the process of removing lignin from plant biomass, other reactions can also occur, such as the esterification reaction (between γ–OH in lignin and –COOH in DES-like mixtures) or acylation, sulfonation, demethoxylation, or dehydration reactions [
61].
The addition of lignin value is provided by the pretreatment of lignocellulosic materials, but it is challenging to achieve an intact lignin structure during the fractionation or delignification process. Liu et al. [
30] reported that if choline chloride is used as an HBA and lactic acid is used as an HBD, this system can initiate H-bonding, polarity, π–π, and ionic or charge interactions with lignin. Lactic acid can further promote the hydrolysis of hemicelluloses and thus the extraction of lignin. The mechanism of biomass delignification using DES-like mixtures represents the series of cleavages of various aryl ether and ester bonds in lignin, whereas acidic DES-like mixtures catalyze the cleavage of esters and ethers between lignin and the saccharide fraction, which results in the extraction and depolymerization of lignin. In addition, hydrogen bonds between OH–Cl in DES-like mixtures are stronger than those between OH–O bonds in lignin. The presence of many chloride ions represents the potential for the cleavage of lignocellulosic materials and ether bonds in lignin [
55]. In addition to extracting lignin, DES-like mixtures can also degrade lignin and cleave the ether bond in two ways. The removal of C
α alcohols and the formation of reactive benzyl carbons with subsequent disruption of the C
α double bond and conjugated structure are the first paths and represent the known mechanism of lignin depolymerization in acidic DES-like mixtures. The second way is by oxidation of the C
α position and acylation of the C
γ position (by oxidation of the OH group in the C
α position). A C
α ketone is necessary to facilitate the cleavage of the β–O–4 bond, and the cleavage of the C–O bond involves formylation, elimination, and hydrolysis. In addition to the cleavage of the β–O–4 bonds, possible reactions such as dehydration, acylation, demethoxylation, and condensation have also been observed during the pretreatment of DES-like mixtures [
30].
To understand the effect of DES-like mixtures on lignin, it is important to know the structure–process relationship between solvents and lignin. In general, the number of β–O–4 bonds in DES-like mixtures of lignin is enriched by the components of DES-like mixtures, the reaction conditions, and the addition of water. Individual components forming DES-like mixtures play a significant role in the number of β–O–4 bonds. DES-like mixtures based on acidified aqueous DES-like mixtures based on polyalcohols can largely partially preserve β–O–4 bonds; on the contrary, DES-like mixtures based on carboxylic acids lead to complete cleavage of β–O–4 bonds [
29,
59].
Due to the presence and increasing amount of choline chloride within DES-like mixtures, the β–O–4 bonds are more susceptible to breakage. The cleavage of intermediates formed between chlorine ions and lignin model compounds, promoted by the presence of a strong acid component, accelerates the cleavage of β–O–4. As is already known, the number of ether bonds decreases with increasing temperature, so the synthesis of lignin with different number of β–O–4 bonds can be adjusted by changing the reaction conditions. Of all bonds in lignin, the β–O–4 bond is the most susceptible to cleavage due to low bond energy [
31].
Some authors stated [
50,
62] that the delignification of biomass using DES-like mixtures takes place by a mechanism that is governed by the formation of a hydrogen bond between DES-like mixtures (halogen ions) and lignin. These halogen ions in DES-like mixtures can form hydrogen bonds with hydroxyl groups in lignin and thus solubilize lignin. Thus, the effect of biomass fractionation relates to the ability of the electronegative halogen anion in DES-like mixtures to form a hydrogen bond with lignin. The second way, as already mentioned, can be the catalyzed cleavage of glycosidic, ether, and lignin–carbohydrate bonds. Thus, it follows that the dissociation constant (pK
a) of DES-like mixtures should be an indicator for estimating delignification performance. From this, it can be concluded that a lower pK
a represents a higher acidity, which should achieve a better performance in the extraction of lignin [
4]. In addition, DES-like mixtures with high values of Kamlet–Taft parameters (α, β, π*), especially α and β, have a better and more efficient performance in lignin fractionation. DES-like mixtures with a high α parameter can provide a hydrogen proton, and conversely, with a high β parameter, they have the ability to accept this proton [
4,
62].
The application of various acidic and alkaline DES-like mixtures for the fractionation of grapevine by-products and the removal of lignin was the aim of Zhu et al. [
63]. Their results revealed that the application of acidic DES-like mixtures based on choline chloride/lactic acid and choline chloride/malic acid removed approximately 53 to 58% of lignin, respectively. The better performance of DES-like mixtures based on choline chloride/lactic acid is attributed to its high conductivity, low viscosity, and low pH, and this higher acidity results in the cleavage of ester and ether bonds in the biomass cell walls. SEM analysis revealed the same result as Zhao et al. [
41], who found that the surface of vines treated with DES-like mixtures was rough and porous with a disturbed arrangement, which evokes that the application of DES-like mixtures disturbed the lignin–hemicellulose bonds. The results of FT-IR analysis show that the characteristic peaks of lignin and their intensities were weakened after pretreatment due to the removal of lignin from the biomass. NMR analysis was used to detect the substructure and chemical composition. The signal of γ-acetylated β–O–4 bonds was observed after the application of DES-like mixtures based on choline chloride/lactic acid, which is attributed to the acylation of carboxylic groups in lactic acid after pretreatment. All β–O–4 groups of lignin treated with the acid system (choline chloride/lactic acid) showed a weak signal trend. The obtained lignin yields and purity values of these lignins are shown in
Table 3.
The fractionation of biomass using the choline chloride/glycerol/K
2CO
3 system represents a typical alkali-catalyzed reaction in which the cleavage of the β–O–4 bond is controlled and varies depending on phenolic and non-phenolic compounds [
48]. The assumption is that phenolic units (free hydroxyl groups) undergo a depolymerization process; on the contrary, non-phenolic units, such as etherified phenolic hydroxyl groups, form epoxy intermediates. In the formation of a quinone methide (the removal of the hydroxyl group at C
α), the assumption is that the compound becomes aromatic and susceptible to nucleophilic attack. K
2CO
3 is considered a nucleophile and together with Cl
−, the formation of unstable intermediates or lignin compounds from lignin repolymerization is preferred rather than quinone methide formation. In addition, epoxy intermediates in the α and β positions (quinonemethide) can form glycerol-grafted lignin through an esterification reaction [
48,
64].
The degradation of lignin by DES-like mixtures involves a combination of solvation, the breaking of intermolecular bonds, and chemical transformations. Lignin is a complex and heterogeneous biopolymer found in plant cell walls, and its degradation is of interest for various applications, including biofuel production, chemical synthesis, and waste valorization. While the exact mechanisms can vary based on the specific DES-like mixtures and reaction conditions used, here is a general overview of how DES-like mixtures can facilitate the degradation of lignin. DES-like mixtures have the ability to solvate lignin and disrupt its complex three-dimensional structure. The hydrogen bond donor and acceptor components of DES-like mixtures interact with lignin’s functional groups, leading to the swelling and opening of lignin’s macromolecular structure. They can promote the depolymerization of lignin, breaking down the lignin polymer into smaller fragments. The solvation and disruption of lignin’s structure make its chemical bonds more susceptible to cleavage. DES-like mixtures can cleave these ether bonds, resulting in the fragmentation of lignin molecules into smaller phenolic compounds. DES-like mixtures can selectively degrade these units into simpler aromatic compounds and phenolic compounds (depolymerization of lignin units). However, solvents can promote cleavage reactions, and they can also facilitate condensation reactions. These reactions involve the recombination of lignin fragments to form new linkages or polymer structures. The balance between cleavage and condensation depends on reaction conditions. Some DES-like mixtures may facilitate redox reactions that lead to the degradation of lignin. The hydrogen bond donor and acceptor components of DES-like mixtures can participate in electron transfer processes, leading to the formation of radicals and subsequent bond cleavage. The mechanism of lignin degradation by DES-like mixtures can be influenced by factors such as the composition of the DES-like mixtures, temperature, reaction time, and the source of lignin [
60,
61,
62,
63,
64].
Jančíková et al. [
65] investigated the delignification of wheat straw using DES-like mixtures based on choline chloride and lactic acid in a molar ratio of 1:5. The work also included the evaluation of delignification under different conditions according to a rotation experiment. The authors investigated three factors affecting delignification, namely temperature, time, and the ratio of biomass and solvent used. The limiting parameters of the factors studied were as follows: temperature in the range of 80–160 °C, a time of 60–240 min, and a ratio of wheat straw to solvent of 1:10 to 1:60. A sub-objective of the work was to determine the optimum delignification conditions in terms of lignin yield and delignification efficiency after the application of DES-like mixtures. An interesting finding was that the lignin content of the original biomass was 27.89%, but after delignification, the lignin content ranged from 16.13 to 35.49%, which might have been due to lignin readsorption onto the fibers during the fractionation process. The optimum lignin content after delignification should be 16.44% at a temperature of 111 °C, a time of 60 min, and a biomass-to-solvent ratio of 1:27.
Jančíková et al. [
66] summarized the use of several possible combinations to create DES-like mixtures with the potential to be used in the processing of different types of biomasses. The results of the studies suggest that DES-like mixtures may have a significant ability to dissolve lignin molecules from plants and may realize a mild catalytic mechanism (acid base) that activates the controlled cleavage of unstable ether linkages between phenylpropane units. DES-like mixtures are used to delignify various types of lignocelluloses (softwoods, pulps, grasses, annual plants and herbs, and other lignocellulosic materials). DES-like mixtures have the ability to selectively cleave ether bonds without affecting the C-C bonds in lignin. The main mechanism is the cleavage of ether linkages, which leads to depolymerization of lignin, facilitating lignin extraction from biomass. DES-like mixtures can provide a mild acid-base catalytic mechanism to initiate the controlled cleavage of labile ether linkages between phenylpropane units. Separation processes should be selected based on the properties of the DES-like mixtures, the number of DES-like mixtures that could be obtained, the nature of the process, the properties of the target compound/product, the use of toxicants, the amount of energy required, and the cost of the equipment.