Environmental Radioactivity, Ecotoxicology (238U, 232Th and 40K) and Potentially Toxic Elements in Water and Sediments from North Africa Dams
Abstract
1. Introduction
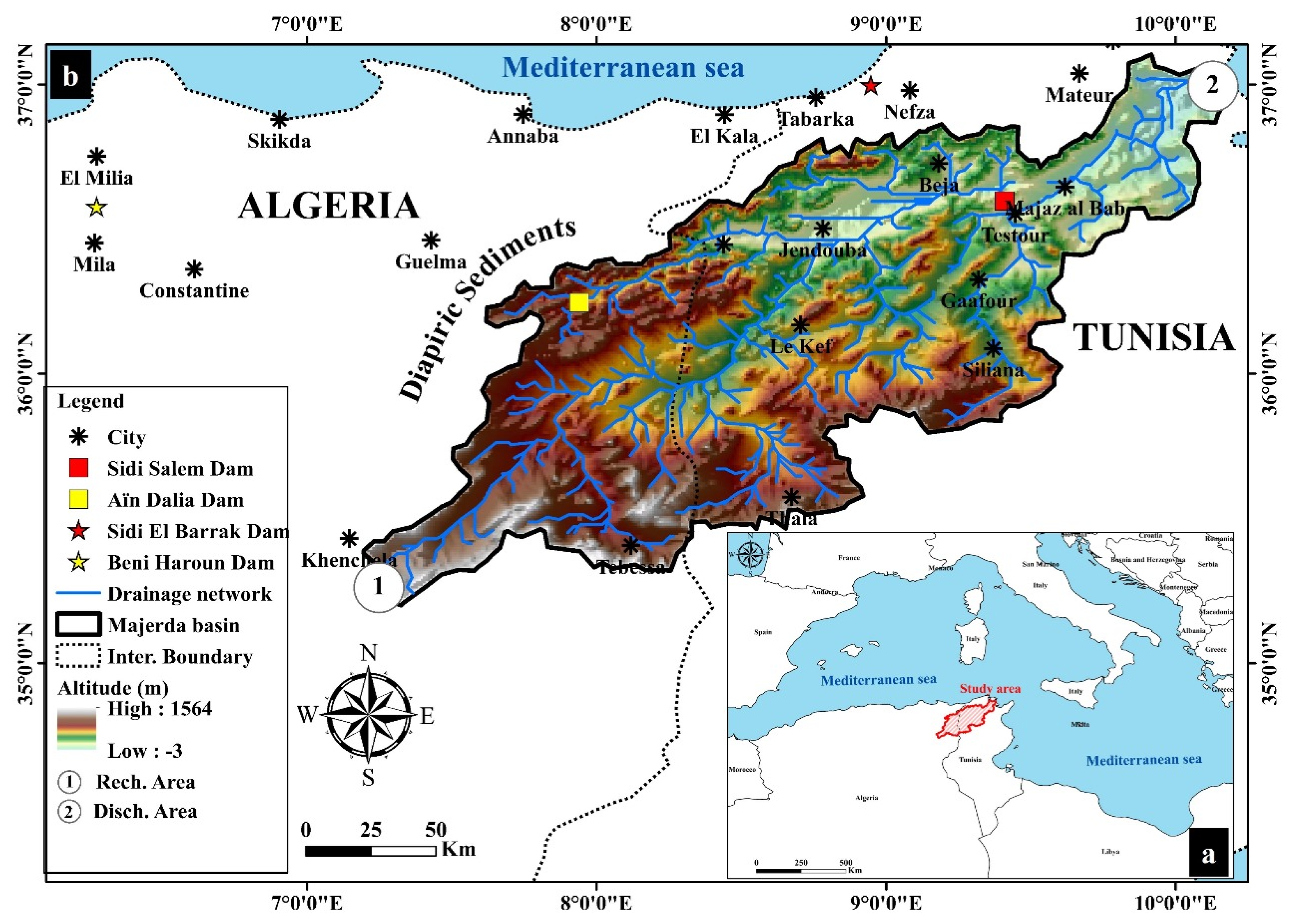
2. Study Area
3. Geological and Tectonic Setting
4. Materials and Methods
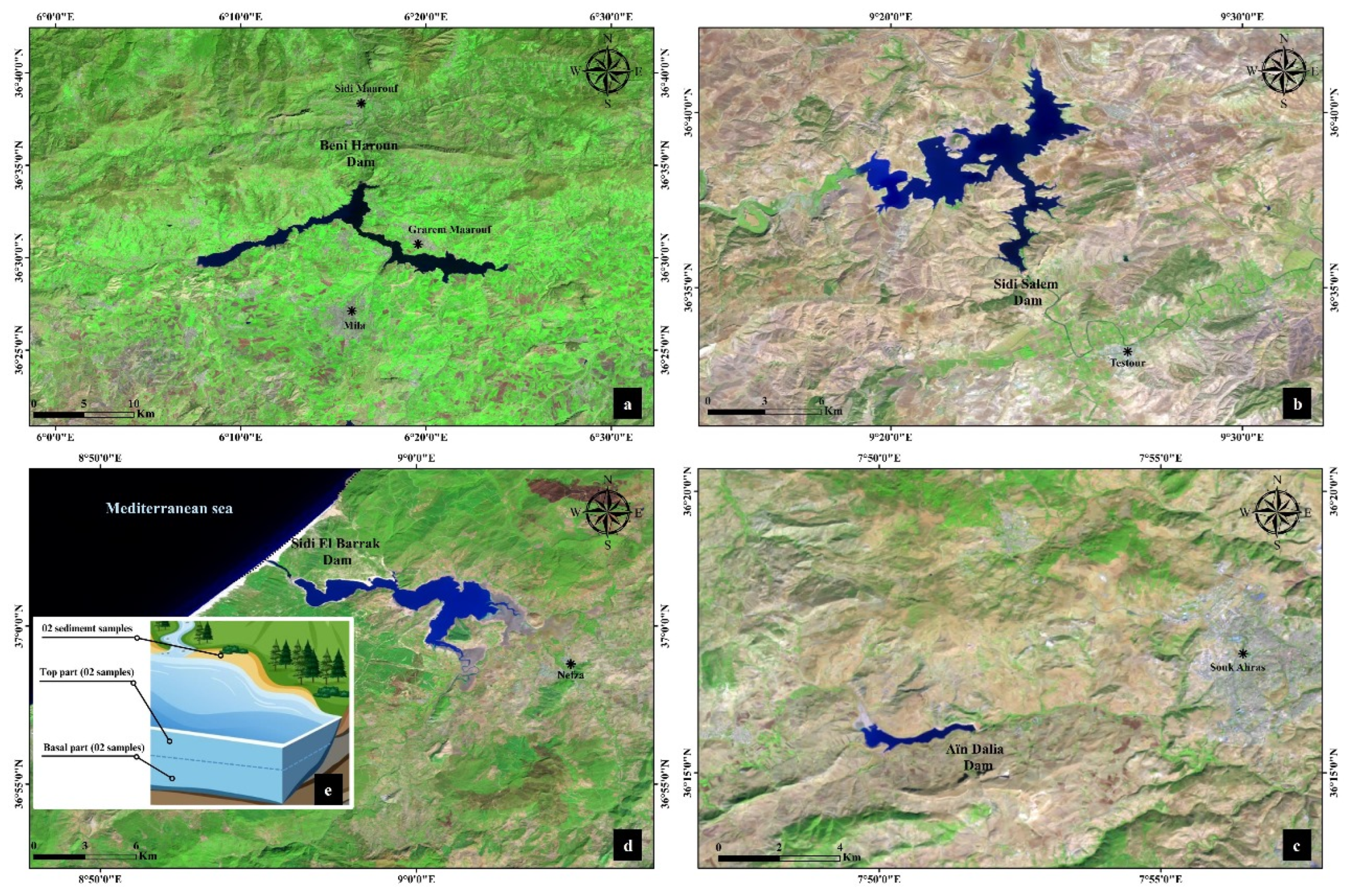
4.1. Sample Collection and Preparation
4.2. Geochemical Analysis
4.3. Ecotoxicological and Radiological Parameter Risks
D (nGy xh−1) = 0.462 CU + 0.604 CTH + 0.0417 CK
5. Results
6. Discussion
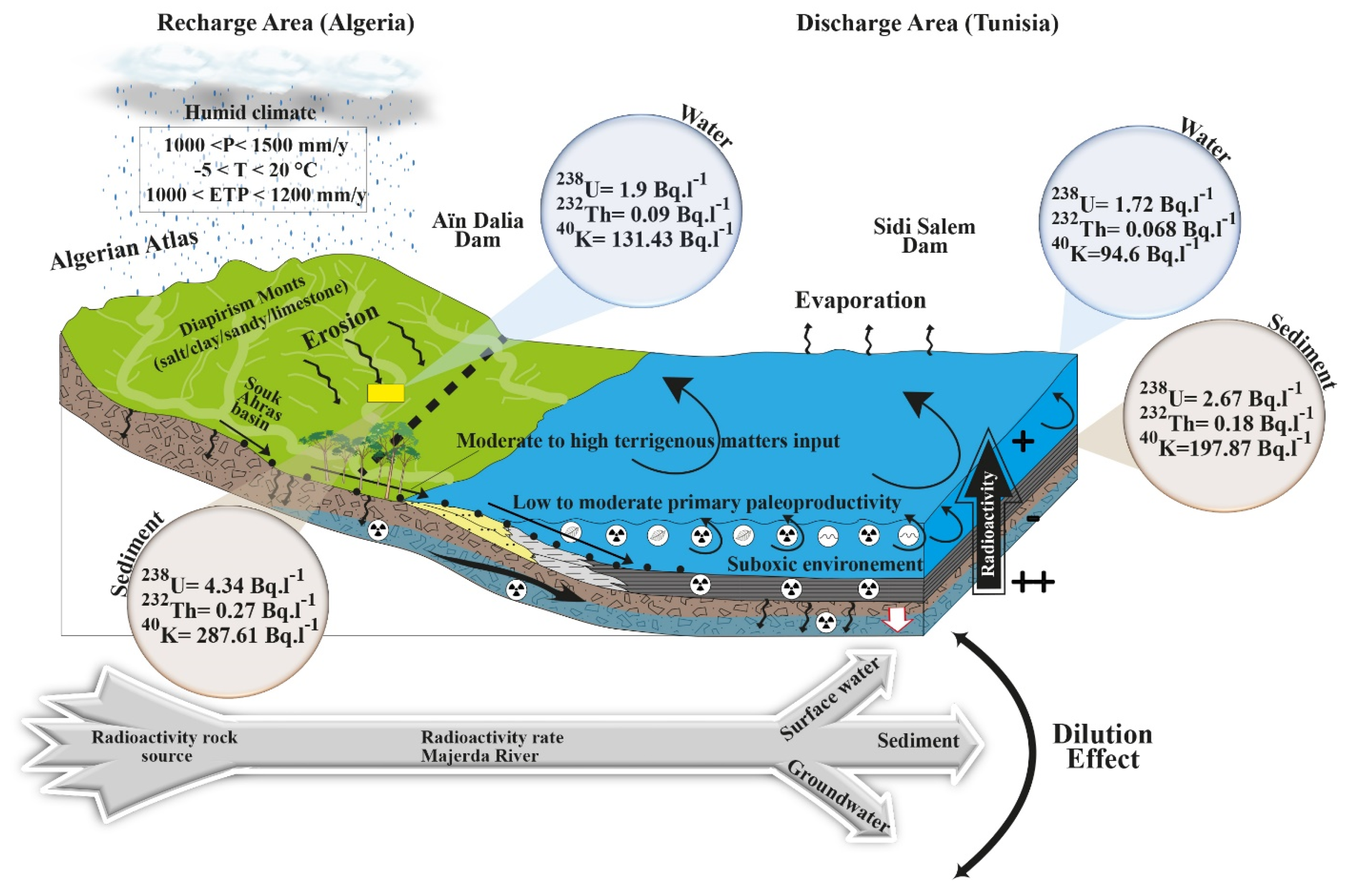
6.1. Spatial Distribution of the Radioactivity in the Study Area
234Th → 234Pa + 4He + Energy
234Pa → 234U + 4He + Energy
234U → 230Th + 4He + Energy
230Th → 226Ra + 4He + Energy
226Ra → 222Rn + 4He + Energy
6.2. Risk Map of the Radioactivity Distribution in the Study Area
6.3. Spatial Distribution of the Potentially Toxic Elements (PTEs)
6.4. Relationship between Radioactive Elements and PTEs
6.4.1. Pearson’s Correlation Matrix
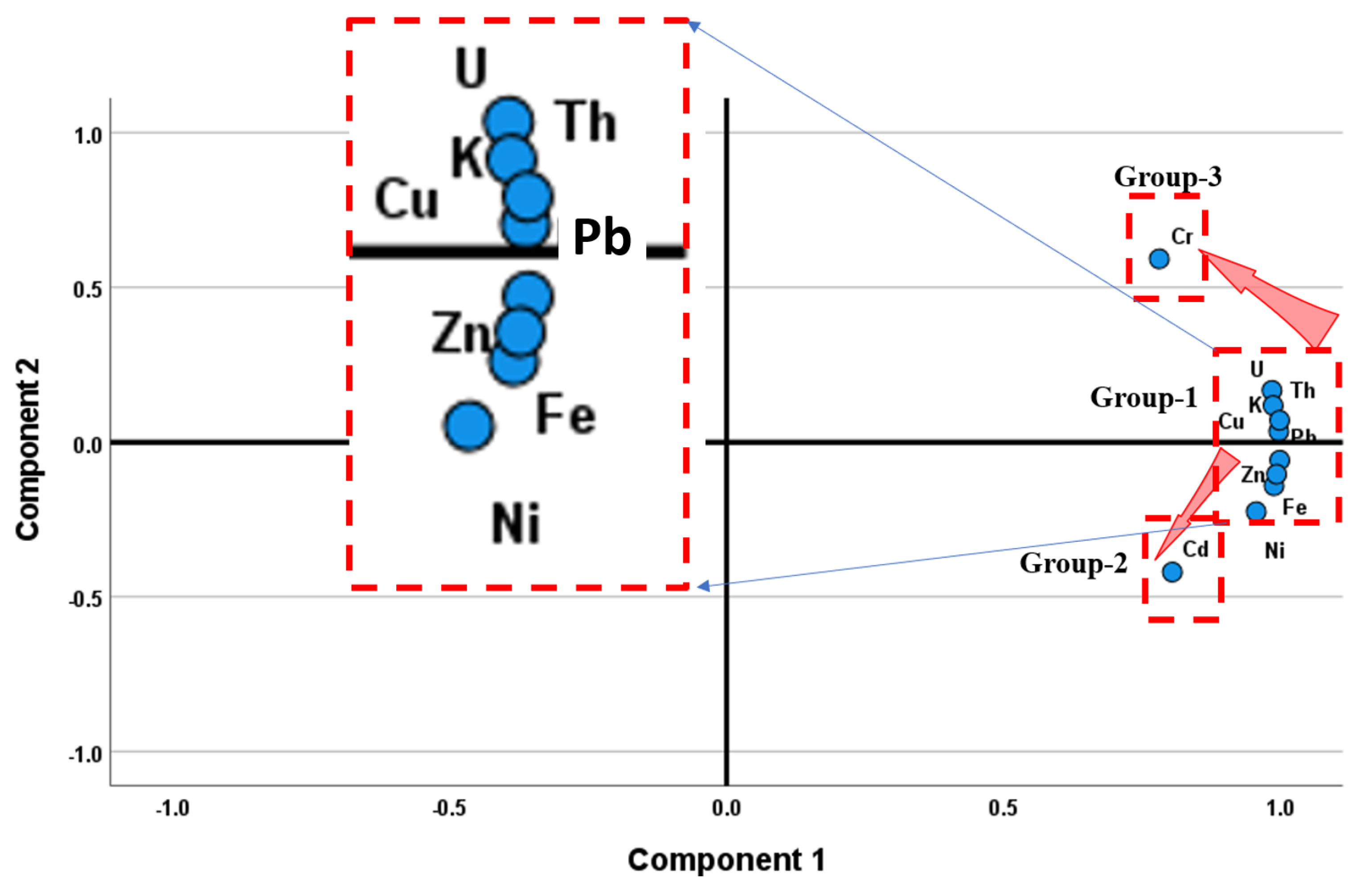
6.4.2. Principal Component Analysis (PCA)
7. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chimenti, M.; Natali, S.; Giannecchini, R.; Zanchetta, G.; Baneschi, I.; Doveri, M.; Isola, I.; Piccini, L. Hydrogeochemistry and Isotopic Composition of Waters in the Renella Cave (Central Italy): New Insights into Groundwater Dynamics. Water 2023, 15, 1764. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Y.; Meng, Y.; Liu, G.; Huang, X.; Chen, Y.; Chen, K. Stable isotopes reveal the surface water-groundwater interaction and variation in young water fraction in an urbanized river zone. Urban Clim. 2023, 51, 101641. [Google Scholar] [CrossRef]
- Dhaoui, O.; Antunes, I.M.H.R.; Boente, C.; Agoubi, B.; Kharroubi, A. Hydrogeochemical processes on inland aquifer systems: A combined multivariate statistical technique and isotopic approach. Groundw. Sustain. Dev. 2023, 20, 100887. [Google Scholar] [CrossRef]
- Santhanabharathi, B.; Pradhoshini, K.P.; Suhail Ahmed, M.; Priyadharshini, M.; Shafeeka Parveen, M.H.; Alam, L.; Saiyad Musthafa, M. Source, fate and transfer of primordial radionuclides as potential contaminants in environmental matrices of high and low background radiation areas—A critical review. Int. J. Environ. Anal. Chem. 2023, 1–27. [Google Scholar] [CrossRef]
- Lovrenčić Mikelić, I.; Barišić, D. Natural and anthropogenic radionuclides in karstic coastal area (Kaštela Bay, Adriatic Sea, Croatia) exposed to anthropogenic activities: Distribution, sources, and influencing factors. Radiochim. Acta 2023, 111, 147–157. [Google Scholar] [CrossRef]
- Taher Hussien, M.; Salaheldin, G.; Salaheldin Mohamed, H.; Mansour, H. Distribution of natural radionuclides and their radiological risks on agricultural soil samples collected from Yemen. Pollution 2023, 9, 195–210. [Google Scholar]
- International Atomic Energy Agency (IAEA). Measurement of radionuclides in food and environmental. In A Guide Book; IAEA Technical Report; International Atomic Energy Agency: Vienna, Austria, 1989; Series No. 295. [Google Scholar] [CrossRef]
- Kaba, O.B.; Souissi, F.; Keita, D.; Filippov, L.O.; Conté, M.S.M.; Kanari, N. Mineral Weathering and Metal Leaching under Meteoric Conditions in F-(Ba-Pb-Zn) Mining Waste of Hammam Zriba (NE Tunisia). Materials 2023, 16, 7443. [Google Scholar] [CrossRef]
- Ayari, J.; Barbieri, M.; Boschetti, T.; Barhoumi, A.; Sellami, A.; Braham, A.; Charef, A. Major-and Trace-Element Geochemistry of Geothermal Water from the Nappe Zone, Northern Tunisia: Implications for Mineral Prospecting and Health Risk Assessment. Environments 2023, 10, 151. [Google Scholar] [CrossRef]
- Fathy, D.; Zakaly, H.M.; Lasheen, E.S.R.; Elsaman, R.; Alarifi, S.S.; Sami, M.; Ene, A. Assessing geochemical and natural radioactivity impacts of Hamadat phosphatic mine through radiological indices. PLoS ONE 2023, 18, e0287422. [Google Scholar] [CrossRef]
- Boumaza, B.; Kechiched, R.; Chekushina, T.V.; Benabdeslam, N.; Senouci, K.; Merzeg, F.A.; Harizi, K. Geochemical distribution and environmental assessment of potentially toxic elements in farmland soils, sediments, and tailings from phosphate industrial area (NE Algeria). J. Hazard. Mater. 2023, 465, 133110. [Google Scholar] [CrossRef]
- Kosnett, M.J. Heavy metal intoxication and chelators. In Basic and Clinical Pharmacology; Katzung, B.G., Ed.; McGrawHill Professional: Chicago, IL, USA, 2007; ISBN 0-07-145153-6. [Google Scholar]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharm. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Liger, E.; Hernández, F.; Expósito, F.J.; Díaz, J.P.; Salazar-Carballo, P.Á.; Gordo, E.; González, C.; López-Pérez, M. Transport and Deposition of Radionuclides from Northern Africa to the Southern Iberian Peninsula and the Canary Islands During the Intense Dust Intrusions of March 2022. Available at SSRN 4653654.
- Gatea, M.A.; Jumaah, G.F.; Al Anbari, R.H.; Alsalhy, Q.F. Review on Decontamination Manners of Radioactive Liquids. Water Air Soil Pollut. 2023, 234, 652. [Google Scholar] [CrossRef]
- Hamed, Y.; Khelifi, F.; Houda, B.; Ben Sâad, A.; Ncibi, K.; Hadji, R.; Melki, A.; Hamad, A. Phosphate mining pollution in southern Tunisia: Environmental, epidemiological, and socioeconomic investigation. Environ. Dev. Sustain. 2022, 25, 13619–13636. [Google Scholar] [CrossRef]
- Hammami, F.; May, M.E. Mineralogical, geochemical, geotechnical and technological characterization of Tunisian Oued Zarga Clays: Industrial application in the ceramic industry. Arab. J. Geosci. 2023, 16, 76. [Google Scholar] [CrossRef]
- UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation, 2000, Volume I: Annex A. Dose Assessment Methodologies; United Nations: New York, NY, USA, 2000. [Google Scholar]
- Bachari, M.; Belkhiria, W.; Negra, M.H.; Grosheny, D.; Soltani, A. Tectonic controls on Late Cretaceous sedimentation on the southern Tethyan passive margin, Tunisia: New evidence of structural segmentation and early basin inversion. Int. Geol. Rev. 2023, 1–18. [Google Scholar] [CrossRef]
- USEPA, US Environmental Protection Agency. Human Health Evaluation Manual: Part E, Supplemental Guidance for Dermal Risk Assessment). In Risk Assessment Guidance For Superfund; Report EPA/540/R/99/005; US Environmental Protection Agency: Washington, DC, USA, 2014; Volume I. [Google Scholar]
- El Arabi, A.M.; Ahmed, N.K.; Din, K.S. Natural radionuclides and dose estimation in natural water resources from Elba protective area, Egypt. Radiat. Prot. Dosim. 2006, 121, 284–292. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiological Protection (ICRP). Protection of the Public in Situations of Prolonged Radiation Exposure, ICRP Publication 82. Ann. ICRP 2000, 29, 1–2. [Google Scholar]
- ICRP, International Commission on Radiological Protection. Age-Dependent Doses to Members of the Public from Intake of Radionuclides: Part 5, Compilation of Ingestion and Inhalation Dose Coefficients; ICRP Publication 72; Pergamon Press: Oxford, UK, 1996. [Google Scholar]
- World Health Organization (WHO). Guidelines For Drinking-Water Quality, WHO Library Cataloguing-in-Publication Data NLM Classification, 4th ed.; WA 675; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Dhaouadi, S.; Ghannem, S.; Kanzari, S.; Ben Slimene, I.; Khadhar, S.; Ben Moussa, A. Assessment of Heavy Metals Contamination and Potential Ecological Risk in Surface Sediments of the El Bey Wadi, Tunisia. Soil Sediment Contam. Int. J. 2023, 1–12. [Google Scholar] [CrossRef]
- Salhi, R.; Durães, N.; Dhaoui, M.; Patinha, C.; da Silva, E.F.; Mlayah, A. Contamination assessment and availability of potential toxic elements from the Sidi Driss tailing pile (NW Tunisia) based on geochemical and geophysical methods. J. Afr. Earth Sci. 2023, 202, 104921. [Google Scholar] [CrossRef]
- Ncibi, K.; Mastrocicco, M.; Colombani, N.; Busico, G.; Hadji, R.; Hamed, Y.; Shuhab, K. Differentiating Nitrate Origins and Fate in a Semi-Arid Basin (Tunisia) via Geostatistical Analyses and Groundwater Modelling. Water 2022, 14, 4124. [Google Scholar] [CrossRef]
- Madyouni, H.; Almanza, V.; Benabdallah, S.; Joaquim-Justo, C.; Romdhane, M.S.; Habaieb, H.; Deliege, J.F. Assessment of Water Quality Variations and Trophic State of the Joumine Reservoir (Tunisia) by Multivariate Analysis. Water 2023, 15, 3019. [Google Scholar] [CrossRef]
- Moussaoui, Z.; Gentilucci, M.; Wederni, K.; Hidouri, N.; Hamedi, M.; Dhaoui, Z.; Hamed, Y. Hydrogeochemical and Stable Isotope Data of the Groundwater of a Multi-Aquifer System in the Maknessy Basin (Mediterranean Area, Central Tunisia). Hydrology 2023, 10, 32. [Google Scholar] [CrossRef]
- Khezami, F.; Gómez-Navarro, O.; Barbieri, M.V.; Khiari, N.; Chkirbene, A.; Chiron, S.; Pérez, S. Occurrence of contaminants of emerging concern and pesticides and relative risk assessment in Tunisian groundwater. Sci. Total Environ. 2024, 906, 167319. [Google Scholar] [CrossRef] [PubMed]
- Ghazouani, N.; Jelassi, H.; Messaoudi, S.; Gabtni, H.; Mlayah, A. An Integrated Assessment of Groundwater in an Anthropized Coastal Aquifer System Using Geochemical and Geophysical Methods (Eastern Tunisia, Mediterranean Region). Water Air Soil Pollut. 2023, 234, 655. [Google Scholar] [CrossRef]
- Farhat, B.; Chrigui, R.; Rebai, N.; Sebei, A. Analysis of hydrochemical characteristics and assessment of organic pollutants (PAH and PCB) in El Fahs plain aquifer, northeast of Tunisia. Environ. Sci. Pollut. Res. 2023, 1–23. [Google Scholar] [CrossRef]
- Ayari, J.; Ouelhazi, H.; Charef, A.; Barhoumi, A. Delineation of seawater intrusion and groundwater quality assessment in coastal aquifers: The Korba coastal aquifer (Northeastern Tunisia). Mar. Pollut. Bull. 2023, 188, 114643. [Google Scholar] [CrossRef]
- Akpolile, F.A.; Ugbede, F.O. Natural radioactivity study and radiological risk assessment in surface water and sediments from Tuomo river in Burutu, delta state Nigeria. J. Niger. Assoc. Math. Phys. (J. NAMP) 2019, 50, 267–274. [Google Scholar]
- Khandaker, M.U.; Uwatse, O.B.; Khairi, B.A.B.S.; Faruque, M.R.I.; Bradley, D.A. Terrestrial radionuclides in surface (dam) water and concomitant dose in metropolitan kuala Lumpur. Radiat. Prot. Dosim. 2019, 185, 343–350. [Google Scholar] [CrossRef]
- Al-Zahrani, K.H.; Baig, M.B. Water in the Kingdom of Saudi Arabia: Sustainable management options. J. Anim. Plant Sci. 2011, 21, 601–604. [Google Scholar]
- Olszewska-Wasiolek, M. Estimates of the Occupational Radiological Hazard in the Phosphate Fertilizers Industry in Poland. Radiat. Prot. Dosim. 1995, 58, 269–276. [Google Scholar] [CrossRef]
- Pfister, H.; Philipp, G.; Pauly, H. Population dose from natural radionuclides in phosphate fertilizers. Radiat. Environ. Biophys. 1976, 13, 247–261. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef]
- Guimond, R.J.; Hardin, J.M. Radioactivity released from phosphate-containing fertilizers and from gypsum. Int. J. Radiat. Appl. Instrum. Part. C Radiat. Phys. Chem. 1989, 34, 309–315. [Google Scholar] [CrossRef]
- Al Shaaibi, M.; Ali, J.; Duraman, N.; Tsikouras, B.; Masri, Z. Assessment of radioactivity concentration in intertidal sediments from coastal provinces in Oman and estimation of hazard and radiation indices. Mar. Pollut. Bull. 2021, 168, 112442. [Google Scholar] [CrossRef]
- Saad, H.R. Radioactivity concentrations in sediments and their correlation to the coastal structure in Kuwait. Appl. Radiat. Isot. 2002, 56, 991–997. [Google Scholar] [CrossRef]
- Darabi-Golestan, F.; Hezarkhani, A.; Zare, M.R. Assessment of 226Ra, 238U, 232Th, 137Cs and 40K activities from the northern coastline of Oman Sea (water and sediments). Mar. Pollut. Bull. 2017, 118, 197–205. [Google Scholar] [CrossRef]
- Özmen, S.F.; Cesur, A.; Boztosun, I.; Yavuz, M.E.L.E.K. Distribution of natural and anthropogenic radionuclides in beach sand samples from Mediterranean Coast of Turkey. Radiat. Phys. Chem. 2014, 103, 37–44. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Radionuclides Rule: A Quick Reference Guide. U.S. Environmental Protection Agency. 2000. Available online: https://www.epa.gov/dwreginfo/radionuclides-rule (accessed on 1 January 2024).
- Hardman, D.J.; McEldowney, S.; Waite, S. Pollution, Ecology and Biotreatment; Longman Scientific & Technical: Harlow, UK, 1994. [Google Scholar]
- Milivojevic, J.; Krstic, D.; Šmit, B.; Djekic, V. Assessment of heavy metal contamination and calculation of its pollution index for Uglješnica River, Serbia. Bull. Environ. Contam. Toxicol. 2016, 97, 737–742. [Google Scholar] [CrossRef]
- Barbee, J.Y.J.; Prince, T.S. Acute respiratory distress syndrome in a welder exposed to metal fumes. South. Med. J. 1999, 92, 510–520. [Google Scholar] [CrossRef]
- Aleksandra, D.; Urszula, B. The impact of Nickel on human health. J. Elementol. 2008, 13, 685–696. [Google Scholar]
- Adimalla, N.; Wu, J. Groundwater quality and associated health risks in a semi-arid region of south India: Implication to sustainable groundwater management. Hum. Ecol. Risk Assess Int. J. 2019, 25, 191–216. [Google Scholar] [CrossRef]
- Boudabra, B.; Agoubi, B. A groundwater risk assessment for irrigation purpose based on salinity indicators: Applied to southeastern Tunisia. Environ. Monit. Assess. 2023, 195, 38. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd ed.; J. Wiley & Sons: New York, NY, USA, 1981; 780p. [Google Scholar]




| Age Group | 0–1 | 1–2 | 2–7 | 7–12 | 12–17 | 17–25 | 25–45 | 45–65 | >65 |
|---|---|---|---|---|---|---|---|---|---|
| Upper side (mSv·y−1) | 1.22 | 1.59 | 1.83 | 2.14 | 3.66 | 4.45 | 5.12 | 6.34 | 7.04 |
| Lower side (mSv·y−1) | 0.84 | 1.09 | 1.26 | 1.47 | 2.52 | 3.06 | 3.76 | 4.12 | 5.02 |
| World standard value [24] | 0.12 mSv·y−1 | ||||||||
| Location/Country | D (nGy·h−1) | Raeq (Bq·kg−1) | Hext | Hint | AGED (μSv·y−1) |
|---|---|---|---|---|---|
| Aïn Dalia dam/Algeria | 14.12 | 36.79 | - | - | 104.53 |
| Sidi Salem dam/Tunisia | 9.59 | 18.16 | - | - | 71.13 |
| World standard value [24] | 57.00 | 370.00 | 1.00 | 1.00 | 1.00 Sv·y−1 |
| Region/Country | 238U, Bq·kg−1 | 232Th, Bq·kg−1 | 40K, Bq·kg−1 | References |
|---|---|---|---|---|
| Sidi Salem dam (Water)/Tunisia | 1.72 | 0.068 | 94.6 | Present study |
| Sidi Salem dam (Sediment) /Tunisia | 2.67 | 0.18 | 197.87 | Present study |
| * Sidi El Barrak dam (Water)/Tunisia | 0.82 | 0.032 | 67.98 | Present study |
| * Sidi El Barrak dam (Sediment)/Tunisia | 0.45 | 0.024 | 45.62 | Present study |
| Aïn Dalia dam (Water)/Algeria | 1.9 | 0.09 | 131.43 | Present study |
| Aïn Dalia dam (Sediment)/Algeria | 4.34 | 0.27 | 286.61 | Present study |
| * Beni Haroun dam (Water)/Algeria | 0.7 | 0.04 | 98.7 | Present study |
| * Beni Haroun dam (Sediment)/Algeria | 0.52 | 0.03 | 75.4 | Present study |
| Gafsa Phos. Rock/Tunisia | 702 | 75 | 90 | [16] |
| Tunisia | - | 29 | 32 | [37] |
| Egypt | 686 | 5.7 | 68.6 | [21] |
| Morocco | - | 20 | 10 | [38] |
| Algeria | - | 64 | 22 | [39] |
| Saudi Arabia | - | 17–39 | 242–2453 | [36] |
| Germany | - | 15 | 720 | [38] |
| USA | - | 49 | 200 | [40] |
| India | - | 65 | 2624 | [39] |
| Jordan | - | 2 | 8 | [37] |
| Kuala Lumpur/Malaysia | - | 1.2 | 35.1 | [35] |
| Buruta/Nigeria | - | 26.9 | 61 | [34] |
| Oman | - | 2.26 | 44.83 | [41] |
| Kuwait | - | 6 | 227 | [42] |
| Iran | - | 17.61 | 361.6 | [43] |
| Turkey | - | 9.0 | 157.7 | [44] |
| Contaminant | Maximum Contaminant Levels |
|---|---|
| Uranium | 30 microg·L−1 |
| Combined 226Rn and 228Ra | 5 pCi/L (0.185 Bq·L−1) |
| Gross alpha (excluding Rn and U but including 226Ra) | 15 pCi/L (0.555 Bq·L−1) |
| Beta particle and photon radioactivity | 40 mrem/y (0.04 mSv·y−1) |
| 238U | 232Th | 40K | Fe | Pb | Zn | Ni | Cu | Cr | Cd | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dalw 2 | 1.90 | 0.09 | 131.43 | 5.430 | 0.0980 | 0.087 | 0.024 | 0.063 | 0.015 | 0.025 |
| Dals 3 | 4.34 | 0.27 | 287.61 | 201.9 | 5.88 | 5.046 | 0.216 | 0.441 | 0.06 | 0.20 |
| Dtnw 4 | 1.72 | 0.068 | 94.6 | 9.700 | 0.065 | 0.061 | 0.018 | 0.043 | BDL 1 | 0.130 |
| Dtns 5 | 2.67 | 0.18 | 197.87 | 136.7 | 3.41 | 3.22 | 0.182 | 0.213 | BDL 1 | 0.15 |
| WHO limits | 1.0 | 0.1 | 10.0 | 0.01 | 0.07 | 2.00 | 0.05 | 0.003 |
| U | Th | K | Fe | Pb | Zn | Ni | Cu | Cr | Cd | |
|---|---|---|---|---|---|---|---|---|---|---|
| U | 1.000 | |||||||||
| Th | 0.980 | 1.000 | ||||||||
| K | 0.982 | 0.996 | 1.000 | |||||||
| Fe | 0.943 | 0.985 | 0.965 | 1.000 | ||||||
| Pb | 0.969 | 0.995 | 0.981 | 0.996 | 1.000 | |||||
| Zn | 0.954 | 0.990 | 0.974 | 0.999 | 0.998 | 1.000 | ||||
| Ni | 0.889 | 0.959 | 0.938 | 0.989 | 0.973 | 0.985 | 1.000 | |||
| Cu | 0.994 | 0.995 | 0.991 | 0.974 | 0.991 | 0.982 | 0.935 | 1.000 | ||
| Cr | 0.880 | 0.781 | 0.816 | 0.672 | 0.737 | 0.698 | 0.574 | 0.822 | 1.000 | |
| Cd | 0.748 | 0.751 | 0.692 | 0.821 | 0.810 | 0.810 | 0.781 | 0.775 | 0.463 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed, Y.; Ayadi, Y.; Hadji, R.; Ben Saad, A.; Gentilucci, M.; Elaloui, E. Environmental Radioactivity, Ecotoxicology (238U, 232Th and 40K) and Potentially Toxic Elements in Water and Sediments from North Africa Dams. Sustainability 2024, 16, 490. https://doi.org/10.3390/su16020490
Hamed Y, Ayadi Y, Hadji R, Ben Saad A, Gentilucci M, Elaloui E. Environmental Radioactivity, Ecotoxicology (238U, 232Th and 40K) and Potentially Toxic Elements in Water and Sediments from North Africa Dams. Sustainability. 2024; 16(2):490. https://doi.org/10.3390/su16020490
Chicago/Turabian StyleHamed, Younes, Yosra Ayadi, Rihab Hadji, Amina Ben Saad, Matteo Gentilucci, and Elimame Elaloui. 2024. "Environmental Radioactivity, Ecotoxicology (238U, 232Th and 40K) and Potentially Toxic Elements in Water and Sediments from North Africa Dams" Sustainability 16, no. 2: 490. https://doi.org/10.3390/su16020490
APA StyleHamed, Y., Ayadi, Y., Hadji, R., Ben Saad, A., Gentilucci, M., & Elaloui, E. (2024). Environmental Radioactivity, Ecotoxicology (238U, 232Th and 40K) and Potentially Toxic Elements in Water and Sediments from North Africa Dams. Sustainability, 16(2), 490. https://doi.org/10.3390/su16020490









