Unveiling the Allelopathic Potential of Wedelia Leaf Extract as a Bioherbicide against Purple Nutsedge: A Promising Strategy for Sustainable Weed Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
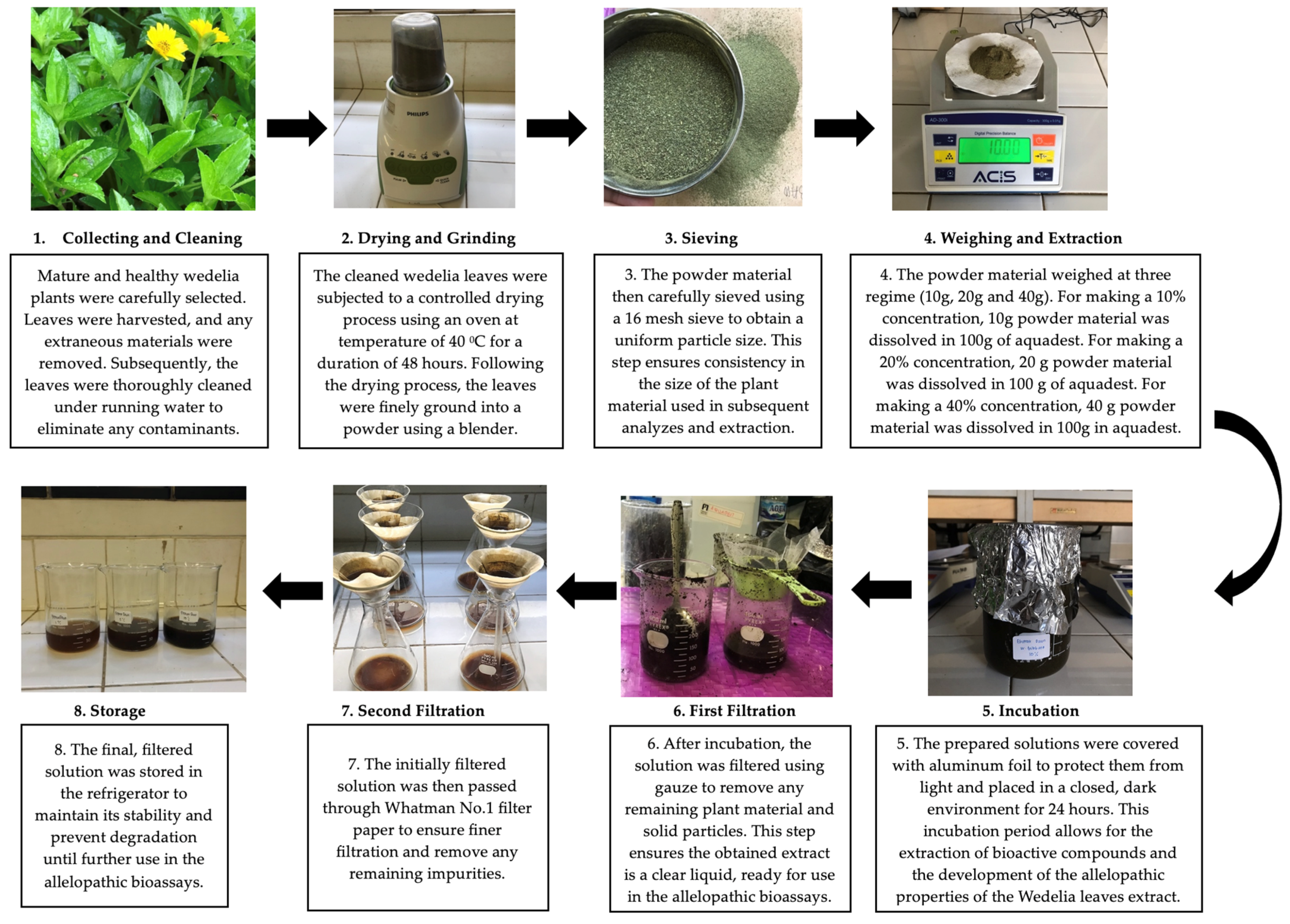
2.3. Extract Preparations
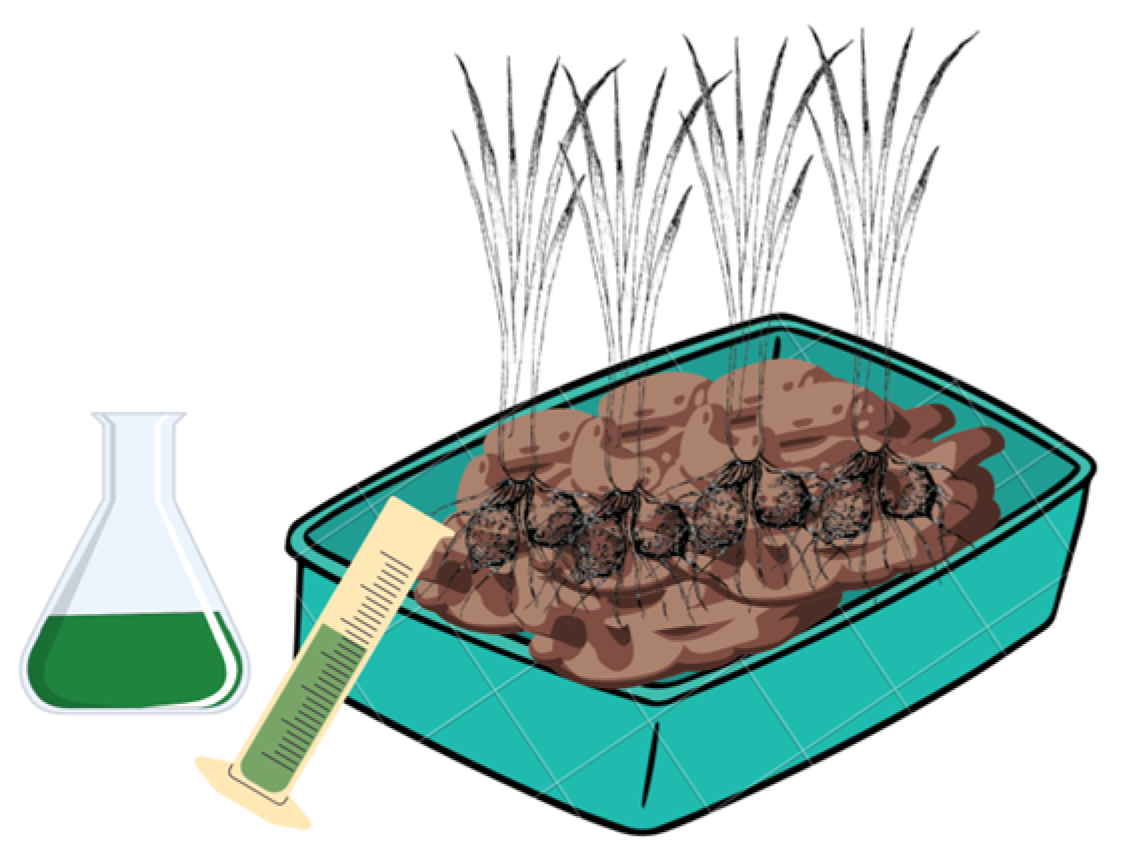
2.4. Tubers Planting
2.5. Extract Application
2.6. Observations
2.6.1. Microclimate Condition
2.6.2. Growth Parameters
2.6.3. Physiological Parameters
2.6.4. Biochemical Parameters
2.6.5. Tuber Anatomy
2.7. Statistical Analysis
3. Results
3.1. Microclimate Condition
3.2. Growth Parameters
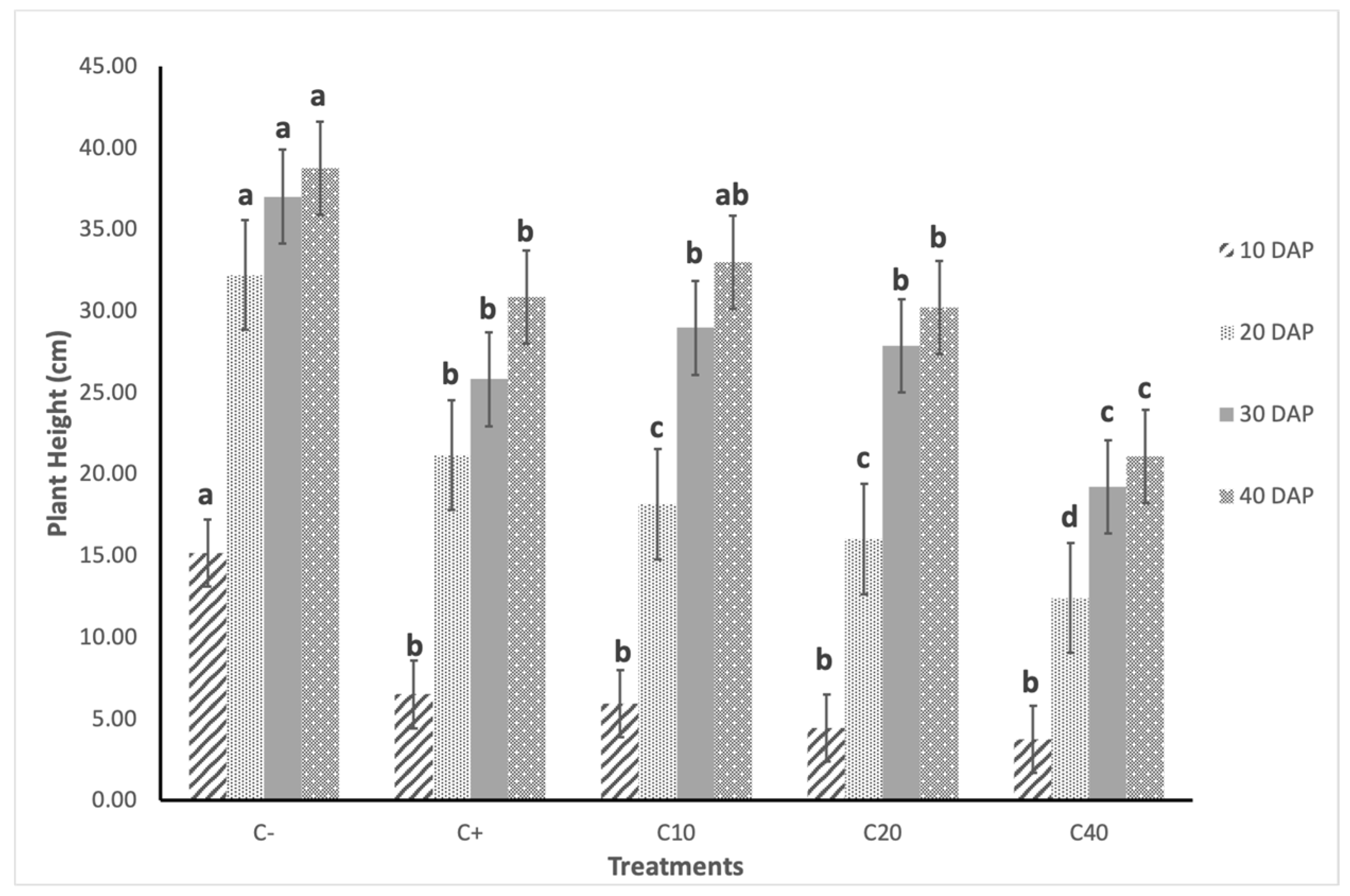
3.2.1. Plant Height
3.2.2. Number of Shoots and Leaves
3.2.3. Leaf and Root Areas
3.2.4. Total Root Length
3.2.5. Fresh and Dry Weights
3.3. Physiological Parameters
3.4. Biochemical Parameters
3.4.1. Lipid Peroxidation (MDA)
3.4.2. Hydrogen Peroxide
3.4.3. Peroxide
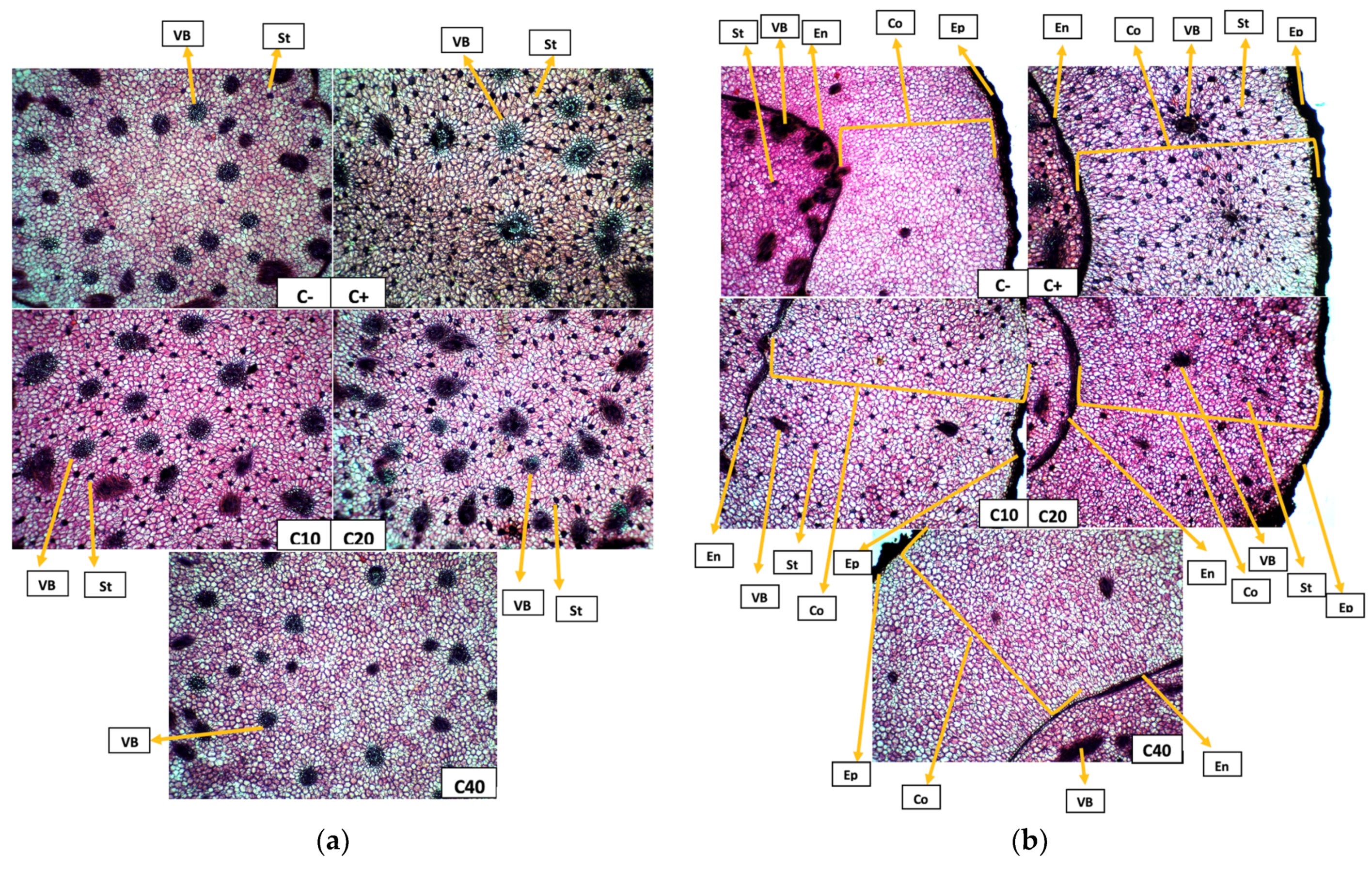
3.5. Tuber Anatomy
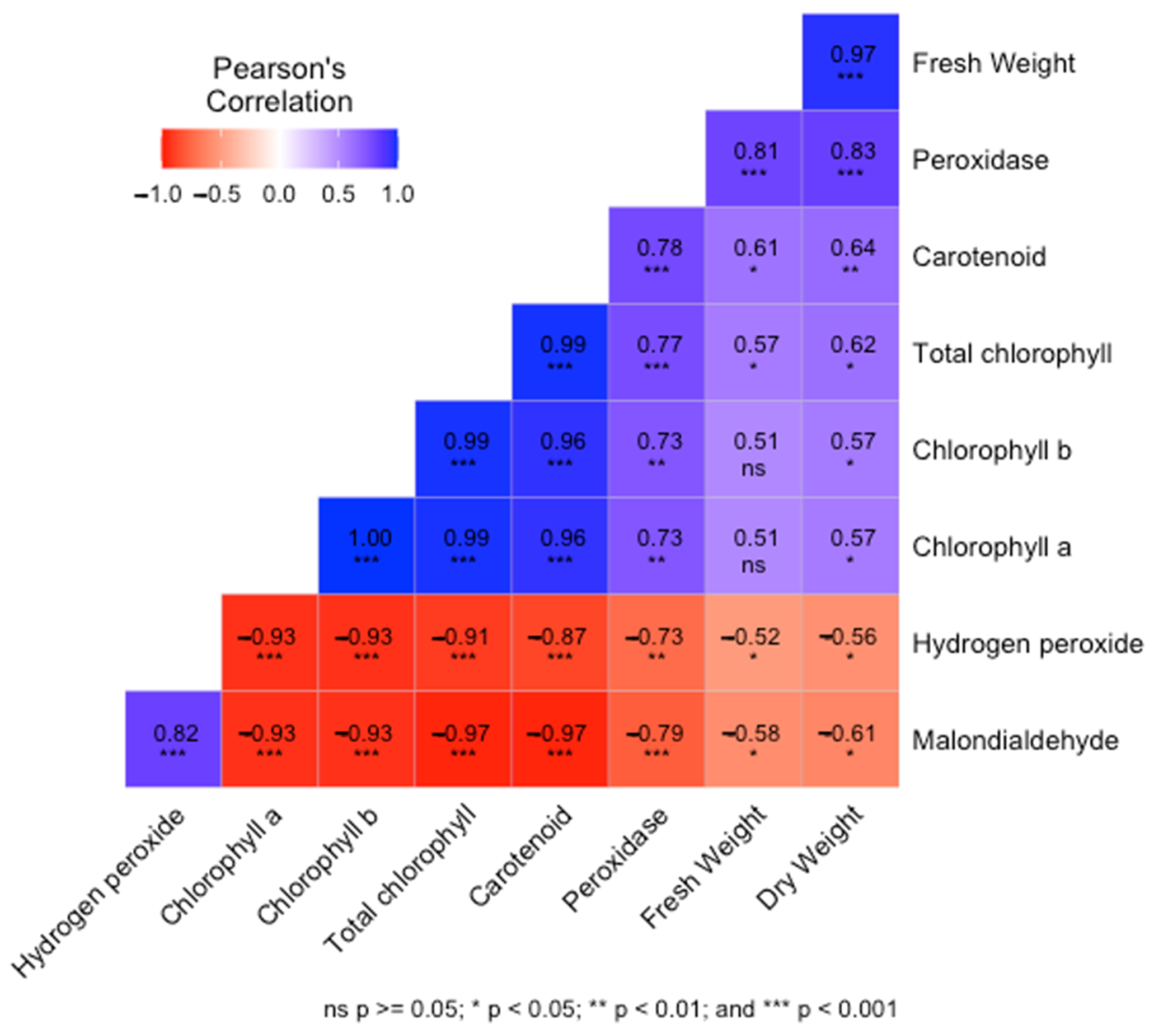
3.6. Correlation between Parameters
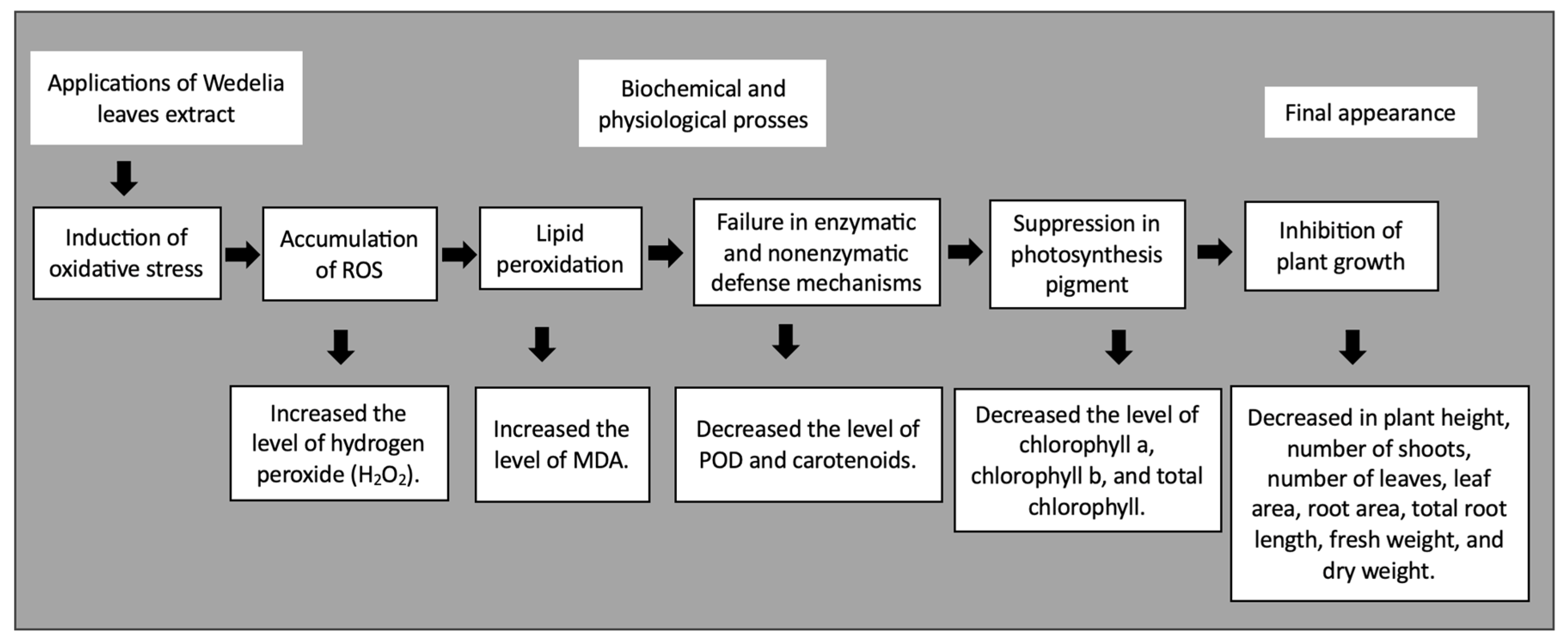
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mcfarlane, I.; Alyanak, L.; Jensen, J.; Kollodge, R.; Daldin, J.; Jayaram, T.; Ratcliffe, L.; Trautwein, C.; Baker, D.; Botev, N.; et al. State of World Population 2023 Billion Lives, Infinite Possibilities: The Case for Rights and Choices; United Nations Population Fund: New York, NY, USA, 2023. [Google Scholar]
- Singh, K.D.; Mobolade, A.J.; Bharali, R.; Sahoo, D.; Rajashekar, Y. Main Plant Volatiles as Stored Grain Pest Management Approach: A Review. J. Agric. Food Res. 2021, 4, 100127. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant Based Natural Products as Potential Ecofriendly and Safer Biopesticides: A Comprehensive Overview of Their Advantages over Conventional Pesticides, Limitations and Regulatory Aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef] [PubMed]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a Source of Bioherbicides: Challenges and Prospects for Sustainable Agriculture. Rev. Environ. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of Yield and Economic Losses in Agriculture Due to Weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H. Allelopathic Potential of Sesame Plant Leachate against Cyperus rotundus L. Ann. Agrar. Sci. 2017, 15, 141–147. [Google Scholar] [CrossRef]
- Roy, K. Nishimoto Purple Nutsedge Tuber Sprouting Copy. Weed Biol. Manag. 2001, 1, 203–208. [Google Scholar] [CrossRef]
- Peerzada, A.M. Biology, Agricultural Impact, and Management of Cyperus rotundus L.: The World’s Most Tenacious Weed. Acta Physiol. Plant 2017, 39, 270. [Google Scholar] [CrossRef]
- Morales-Payan, J.P.; Stall, W.M.; Shilling, D.G.; Charudattan, R.; Dusky, J.A.; Bewick, T.A. Above- and Belowground Interference of Purple and Yellow Nutsedge (Cyperus Spp.) with Tomato. Weed Sci. 2003, 51, 181–185. [Google Scholar] [CrossRef]
- Matloob, A.; Shareef, M.N.; Khaliq, A.; Farooq, M. Quantification of Allelopathic Potential of Different Crop Residues for the Purple Nutsedge Suppression Zahid Ata Cheema. Pak. J. Weed Sci. 2010, 16, 1–12. [Google Scholar]
- Chand, M.; Lal, R.; Khippal, A.; Singh, S. Integrated Weed Management in Sugarcane Ratoon. Indian J. Sugarcane Technol. 2010, 25, 17–19. [Google Scholar]
- Tuor, F.A.; Froud-Williams, R.J. Influence of Nitrogen on Competition between Purple Nutsedge, Maize and Soybean. Int. J. Pest. Manag. 2002, 48, 73–79. [Google Scholar] [CrossRef]
- El Sawi, S.A.; Ibrahim, M.E.; El-Rokiek, K.G.; El-Din, S.A.S. Allelopathic Potential of Essential Oils Isolated from Peels of Three Citrus Species. Ann. Agric. Sci. 2019, 64, 89–94. [Google Scholar] [CrossRef]
- Messiha, N.K.; Ahmed, S.A.; El-Rokiek, K.G.; Dawood, M.G.; El-Masry, R.R. The Physiological Influence of Allelochemicals in Two Brassicaceae Plant Seeds on the Growth and Propagative Capacity of Cyprus rotundus and Zea mays L. World Appl. Sci. J. 2013, 26, 1142–1149. [Google Scholar]
- Rokiek, E.-M.; Kowthar, G.; Rafat, R.E.-M.; Messiha, N.K.; Ahmed, S.A. The Allelopathic Effect of Mango Leaves on the Growth and Propagative Capacity of Purple Nutsedge (Cyperus rotundus L.). J. Am. Sci. 2010, 6, 151–159. [Google Scholar]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowsk, A. Allelochemicals as Bioherbicides—Present and Perspectives; InTech: London, UK, 2013. [Google Scholar]
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Hoang Anh, L.; Van Quan, N.; Tuan Nghia, L.; Dang Xuan, T. Phenolic Allelochemicals: Achievements, Limitations, and Prospective Approaches in Weed Management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for Weed Control in Agricultural Systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Bajwa, A.A. Sustainable Weed Management in Conservation Agriculture. Crop Prot. 2014, 65, 105–113. [Google Scholar] [CrossRef]
- Azlan Azizan, K.; Ibrahim, S.; Haizun Abdul Ghani, N.; Firdaus Nawawi, M. LC-MS Based Metabolomics Analysis to Identify Potential Allelochemicals in Wedelia Trilobata. Nat. Prod. 2016, 10, 788–793. [Google Scholar]
- Shahena, S.; Rajan, M.; Chandran, V.; Mathew, L. Allelopathic Effect of Wedelia Trilobata L., on the Germination and Growth of Cicer Arietinum, Vigna Unguiculata, and Vigna Radiata Seedlings. J. Appl. Biol. Biotechnol. 2021, 9, 93–114. [Google Scholar] [CrossRef]
- Nie, C.; Luo, S.; Zeng, R.; Mo, M.; Li, H.; Lin, C. Allelopathic Potential of Wedelia trilobata L.: Effects on Germination, Growth and Physiological Parameters of Rice; The Regional Institute: Barton, Australia, 2005. [Google Scholar]
- Zhang, Z.H.; Hu, B.Q.; Hu, G. Assessment of Allelopathic Potential of Wedelia Trilobata on the Germination, Seedling Growth and Chlorophyll Content of Rape. Adv. Mat. Res. 2013, 807–809, 719–722. [Google Scholar] [CrossRef]
- Araújo, C.A.; Morgado, C.S.A.; Gomes, A.K.C.; Gomes, A.C.C.; Simas, N.K. Asteraceae Family: A Review of Its Allelopathic Potential and the Case of Acmella Oleracea and Sphagneticola Trilobata. Rodriguesia 2021, 72, 1–25. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Sharma, R. Allelopathic Effect of Ageratum Conyzoides on Chlorophyll Content in The Leaves of Mungbean. Int. J. Recent Sci. Res. 2016, 7, 13296–13298. [Google Scholar]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of Malondialdehyde (MDA) by Thiobarbituric Acid (TBA) Assay. In Plant-Microbe Interactions; Springer: Berlin/Heidelberg, Germany, 2021; pp. 103–105. [Google Scholar] [CrossRef]
- Albro, P.W.; Corbett, J.T.; Schroeder, J.L. Effects of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin on Lipid Peroxidation in Microsomal Systems in Vitro. Chem. Biol. Interact. 1986, 57, 301–313. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Guo, Z.; Tan, H.; Zhu, X. A Simple Colorimetric Method for Determination of Hydrogen Peroxide in Plant Tissues. Plant Growth Regul. 2006, 49, 113–118. [Google Scholar] [CrossRef]
- Alexander, A.G. Oxidizing Enzymes of Sugarcane: Peroxidase. J. Agric. Univ. Puerto Rico 1966, 50, 36–52. [Google Scholar] [CrossRef]
- Molisch, H. The Influence of One Plant on Another: Allelopathy; Narwal, S.S., Ed.; Scientific Publisheris (India): Jodhpur, India, 2001; ISBN 8172332858. [Google Scholar]
- Inderjit; Nilsen, E.T. Bioassays and Field Studies for Allelopathy in Terrestrial Plants: Progress and Problems. CRC Crit. Rev. Plant Sci. 2003, 22, 221–238. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and Crop Allelopathy. CRC Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Bogatek, R. Allelopathic in Teractions between Plants. Multi Site Action of Allelochemicals. Acta Physiol. Plantarium 2005, 27, 395–407. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oelmüller, R. Reactive Oxygen Species Generation and Signaling in Plants. Plant Signal Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.R.; Asaduzzaman, M.; Ueno, M.; Tanaka, H.; Asao, T. Alleviation of Allelochemical Stress-Induced Growth Inhibition and Oxidative Damage in Lettuce under Closed Hydroponics through Electro-Degradation. Hortic. Sci. 2020, 47, 53–68. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current Knowledge on Weed Control Mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- El-Tayeb, M.A. Response of Barley Grains to the Interactive Effect of Salinity and Salicylic Acid. Plant Growth Regul. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- Gill, S.S.; Nafees, K.A.; Naser, A.A.; Tuteja, N. Amelioration of Cadmium Stress in Crop Plants by Nutrient Management: Morphological, Physiological and Biochemical Aspects. Plant Stress 2011, 5, 1–15. [Google Scholar]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Qian, H.; Xu, X.; Chen, W.; Jiang, H.; Jin, Y.; Liu, W.; Fu, Z. Allelochemical Stress Causes Oxidative Damage and Inhibition of Photosynthesis in Chlorella Vulgaris. Chemosphere 2009, 75, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Romagni, J.G.; Meazza, G.; Nanayakkara, N.P.D.; Dayan, F.E. The Phytotoxic Lichen Metabolite, Usnic Acid, Is a Potent Inhibitor of Plant p-Hydroxyphenylpyruvate Dioxygenase. FEBS Lett. 2000, 480, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Ron, M. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Venkateshwarlu, G.; Ravindra, V.; Challa, P. Mangiferin: An Allelopathin from Mango (Mangifera indica L.) Leaves. Allelopath. J. 2001, 8, 221–224. [Google Scholar]
- Rabinowitch, E.I. The Role of Chlorophyll in Photosynthesis. Sci. Am. 1965, 213, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Silla, F.; González-Gil, A.; González-Molina, M.E.; Mediavilla, S.; Escudero, A. Estimation of Chlorophyll in Quercus Leaves Using a Portable Chlorophyll Meter: Effects of Species and Leaf Age. Ann. For. Sci. 2010, 67, 108. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. A Chlorophyll Fluorescence Analysis of Photosynthetic Efficiency, Quantum Yield and Photon Energy Dissipation in PSII Antennae of Lactuca sativa L. Leaves Exposed to Cinnamic Acid. Plant Physiol. Biochem. 2011, 49, 1290–1298. [Google Scholar] [CrossRef]
- Hejl, A.M.; Koster, K.L. The Allelochemical Sorgoleone Inhibits Root H +-ATPase and Water Uptake. J. Chem. Ecol. 2004, 30, 2181–2191. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Allelochemical Stress Inhibits Growth, Leaf Water Relations, PSII Photochemistry, Non-Photochemical Fluorescence Quenching, and Heat Energy Dissipation in Three C 3 Perennial Species. J. Exp. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef]
- Bornman, J.F.; Vogelmann, T.C. Effect of UV-B Radiation on Leaf Optical Properties Measured with Fibre Optics. J. Exp. Bot. 1991, 42, 547–554. [Google Scholar] [CrossRef]
- Björkman, O. Responses to Different Quantum Flux Densities. In Physiological Plant Ecology I; Springer: Berlin/Heidelberg, Germany, 1981; pp. 57–107. [Google Scholar] [CrossRef]
- Md Khan, S.I.; Kaium, A.; Sarkar, B.K.; Begum, R.; Begum, N.; Islam, M.A.; Md Chowdhury, T.I.; Habib, M.; Md. Hakim, A. Potencies of Justicia adhatoda L. for Its Possible Phytotoxic Activity. Plant Sci. Today 2021, 8, 146–149. [Google Scholar] [CrossRef]
- Islam Khan, M.S.; Kato-Noguchi, H. Assessment of Allelopathic Potential of Couroupita Guianensis Aubl. Plant Omics 2016, 9, 115–120. [Google Scholar] [CrossRef]
- White, R.H.; Worsham, A.D.; Blum, U. Allelopathic Potential of Legume Debris and Aqueous Extracts. Weed Sci. 1989, 37, 674–679. [Google Scholar] [CrossRef]

| DAP | Microclimate Condition | |
|---|---|---|
| Temperature (°C) | Air Humidity (%) | |
| 10 | 30.4 | 69% |
| 20 | 32.6 | 59% |
| 30 | 30.1 | 63% |
| 40 | 32.2 | 55% |
| Average | 31.32 | 61.5% |
| Parameters | DAP | Treatments | ||||
|---|---|---|---|---|---|---|
| C− | C+ | C10 | C20 | C40 | ||
| Number of shoots | 20 | 83.25 ± 4.717 a | 73.50 ± 5.000 a | 73.00 ± 3.266 a | 60.50 ± 7.594 b | 60.25 ± 5.058 b |
| 40 | 90.00 ± 4.243 a | 77.75 ± 4.646 ab | 84.50 ± 5.802 ab | 81.75 ± 11.615 ab | 69.75 ± 7.544 b | |
| Number of leaves | 20 | 7.85 ± 0.191 a | 5.60 ± 0.432 b | 5.80 ± 0.163 b | 5.40 ± 0.712 b | 5.35 ± 0.619 b |
| 40 | 9.00 ± 0.283 a | 8.05 ± 0.915 ab | 8.55 ± 0.252 ab | 7.80 ± 0.432 b | 7.55 ± 0.551 b | |
| Leaf area (cm2) | 20 | 31.28 ± 5.096 a | 25.65 ± 1.955 a | 17.11 ± 4.871 b | 14.81 ± 2.975 b | 10.03 ± 1.450 b |
| 40 | 89.53 ± 22.346 a | 46.06 ± 13.295 b | 41.05 ± 1.101 b | 39.03 ± 16.636 b | 27.93 ± 3.936 b | |
| Root area (cm2) | 20 | 17.76 ± 3.716 a | 15.33 ± 3.190 ab | 12.37 ± 2.919 ab | 11.70 ± 2.934 ab | 9.28 ± 1.654 b |
| 40 | 29.51 ± 6.043 a | 16.80 ± 4.436 b | 14.93 ± 5.753 b | 12.72 ± 3.935 b | 9.93 ± 3.436 b | |
| Total root length (cm) | 20 | 32.20 ± 16.409 a | 21.15 ± 11.505 b | 18.15 ± 9.360 c | 16.00 ± 5.883 c | 12.40 ± 1.242 d |
| 40 | 38.75 ± 7.810 a | 30.85 ± 14.385 b | 32.95 ± 10.373 ab | 30.20 ± 2.031 b | 21.08 ± 7.736 c | |
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| C− | C+ | C10 | C20 | C40 | |
| No. of VBs | 33.00 ± 1.73 a | 13.00 ± 1.73 c | 17.00 ± 2.00 c | 27.00 ± 2.00 b | 24.00 ± 2.00 b |
| Fresh Weight (g) | 1.33 ± 0.456 a | 0.62 ± 0.172 b | 0.59 ± 0.208 b | 0.59 ± 0.052 b | 0.57 ± 0.152 b |
| Dry Weight (g) | 0.36 ± 0.116 a | 0.19 ± 0.058 ab | 0.18 ± 0.060 b | 0.18 ± 0.022 b | 0.15 ± 0.005 b |
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| C− | C+ | C10 | C20 | C40 | |
| Chlorophyll a (mg g−1 FW) | 0.63 ± 0.004 a | 0.63 ± 0.001 a | 0.53 ± 0.003 b | 0.50 ± 0.005 c | 0.43 ± 0.003 d |
| Chlorophyll b (mg g−1 FW) | 1.13 ± 0.007 a | 1.13 ± 0.003 a | 0.95 ± 0.005 b | 0.92 ± 0.008 c | 0.80 ± 0.005 d |
| Total chlorophyll (mg g−1 FW) | 1.05 ± 0.004 a | 1.02 ± 0.007 b | 0.76 ± 0.005 c | 0.70 ± 0.003 d | 0.60 ± 0.006 e |
| Carotenoid (mg g−1 FW) | 0.29 ± 0.0003 a | 0.27 ± 0.0029 b | 0.20 ± 0.0036 c | 0.19 ± 0.0013 c | 0.17 ± 0.0017 d |
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| C− | C+ | C10 | C20 | C40 | |
| Malondialdehyde (nmol g−1 FW) | 14.20 ± 0.704 b | 14.36 ± 0.276 b | 19.87 ± 0.496 a | 21.18 ± 0.918 a | 21.27 ± 0.650 a |
| Hydrogen peroxide (mol g−1 FW) | 19.29 ± 6.402 d | 28.74 ± 4.275 cd | 39.62 ± 7.237 bc | 48.40 ± 6.830 b | 68.64 ± 3.841 a |
| Peroxidase (U min−1 g−1 FW) | 0.006 ± 0.0025 a | 0.003 ± 0.0005 ab | 0.002 ± 0.0008 ab | 0.002 ± 0.0008 b | 0.001 ± 0.0005 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyun, Q.; Respatie, D.W.; Indradewa, D. Unveiling the Allelopathic Potential of Wedelia Leaf Extract as a Bioherbicide against Purple Nutsedge: A Promising Strategy for Sustainable Weed Management. Sustainability 2024, 16, 479. https://doi.org/10.3390/su16020479
Uyun Q, Respatie DW, Indradewa D. Unveiling the Allelopathic Potential of Wedelia Leaf Extract as a Bioherbicide against Purple Nutsedge: A Promising Strategy for Sustainable Weed Management. Sustainability. 2024; 16(2):479. https://doi.org/10.3390/su16020479
Chicago/Turabian StyleUyun, Qurrotul, Dyah Weny Respatie, and Didik Indradewa. 2024. "Unveiling the Allelopathic Potential of Wedelia Leaf Extract as a Bioherbicide against Purple Nutsedge: A Promising Strategy for Sustainable Weed Management" Sustainability 16, no. 2: 479. https://doi.org/10.3390/su16020479
APA StyleUyun, Q., Respatie, D. W., & Indradewa, D. (2024). Unveiling the Allelopathic Potential of Wedelia Leaf Extract as a Bioherbicide against Purple Nutsedge: A Promising Strategy for Sustainable Weed Management. Sustainability, 16(2), 479. https://doi.org/10.3390/su16020479





