Production of High-Value Green Chemicals via Catalytic Fast Pyrolysis of Eucalyptus urograndis Forest Residues
Abstract
1. Introduction
2. Materials and Methods Experimental
2.1. Biomass Preparation
2.2. Biomass Characterization
2.3. Preparation of Catalysts
2.4. Py-GC/MS Processes
3. Results and Discussion
3.1. Biomass Characterization
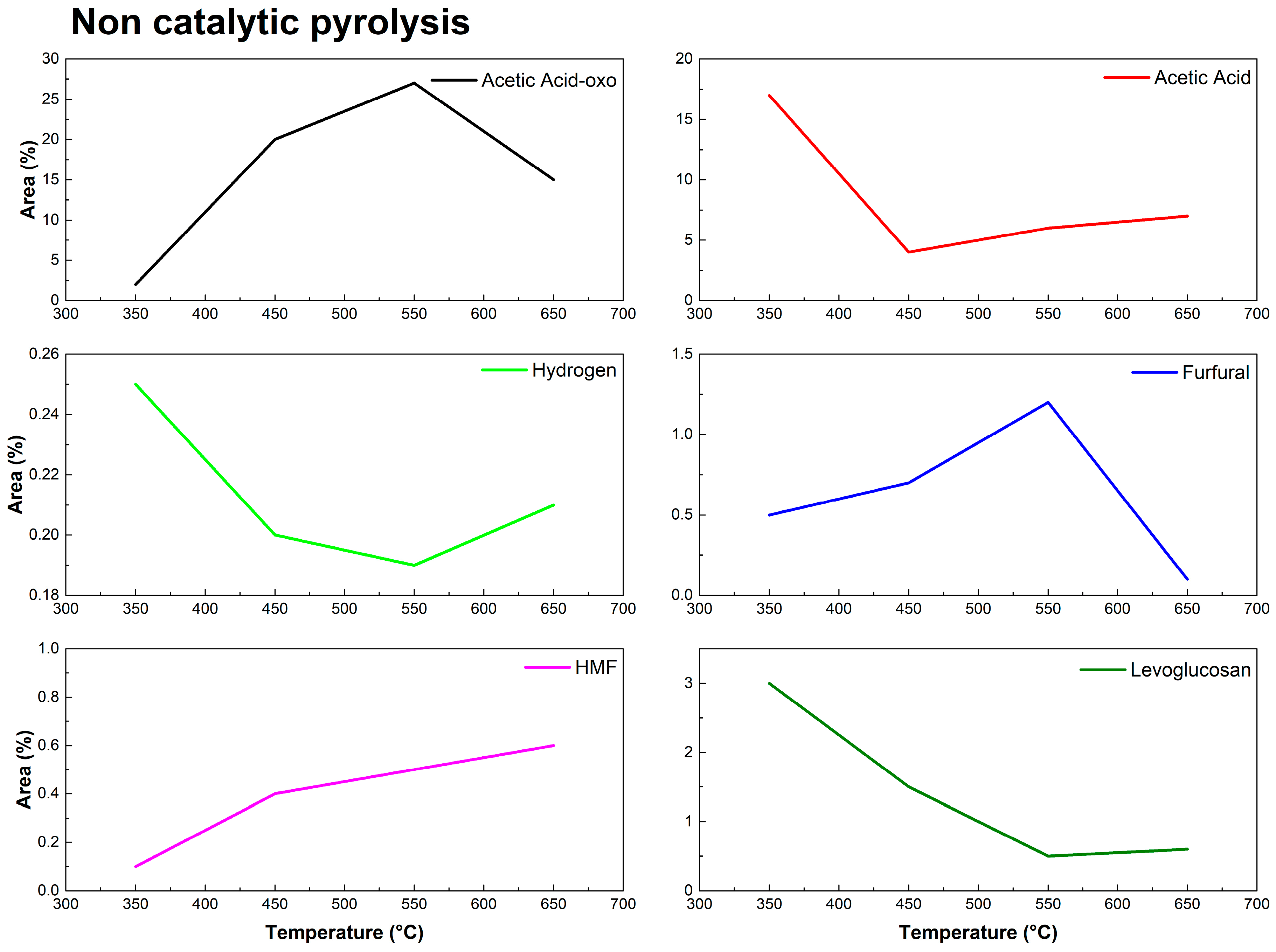
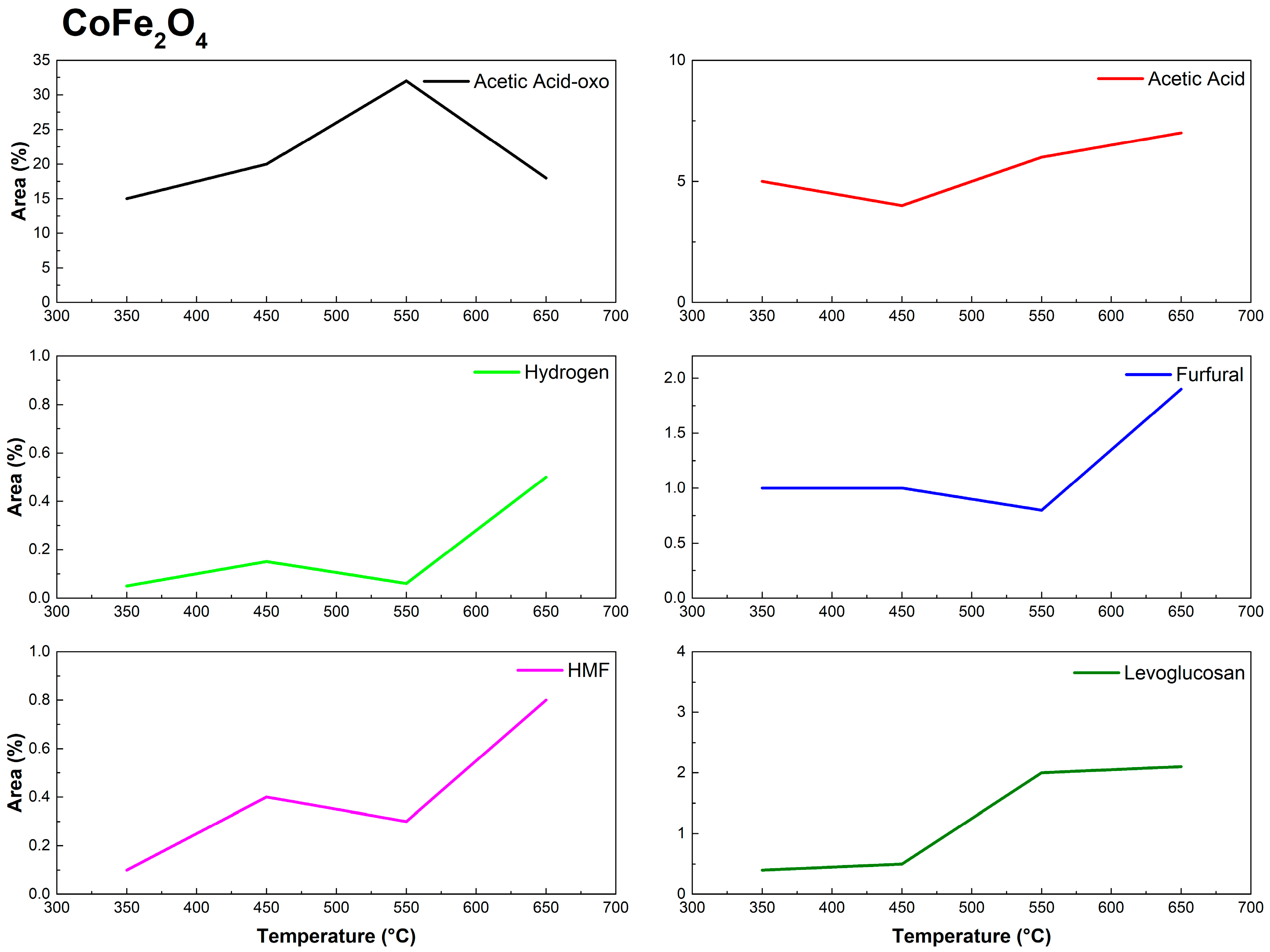
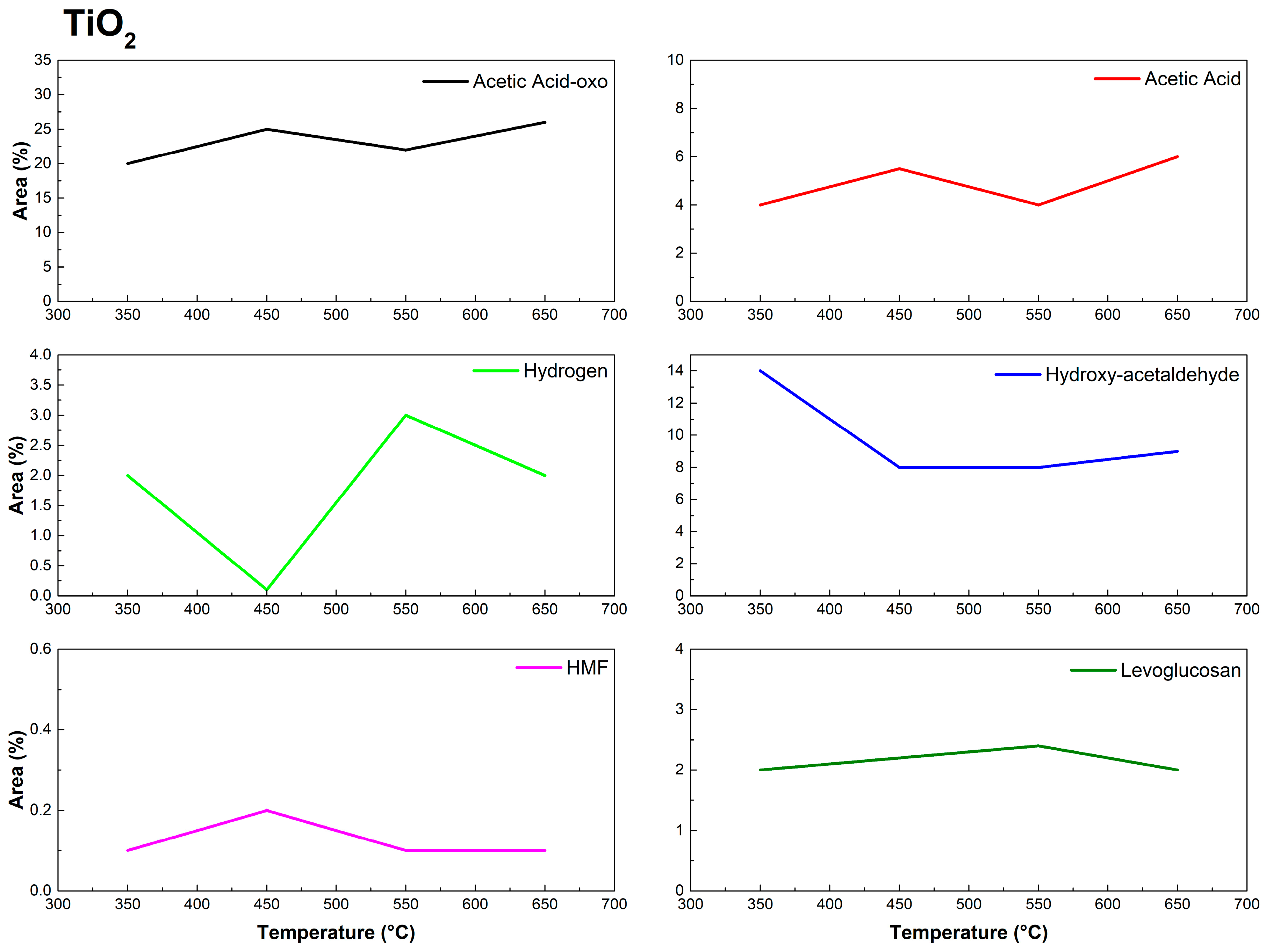
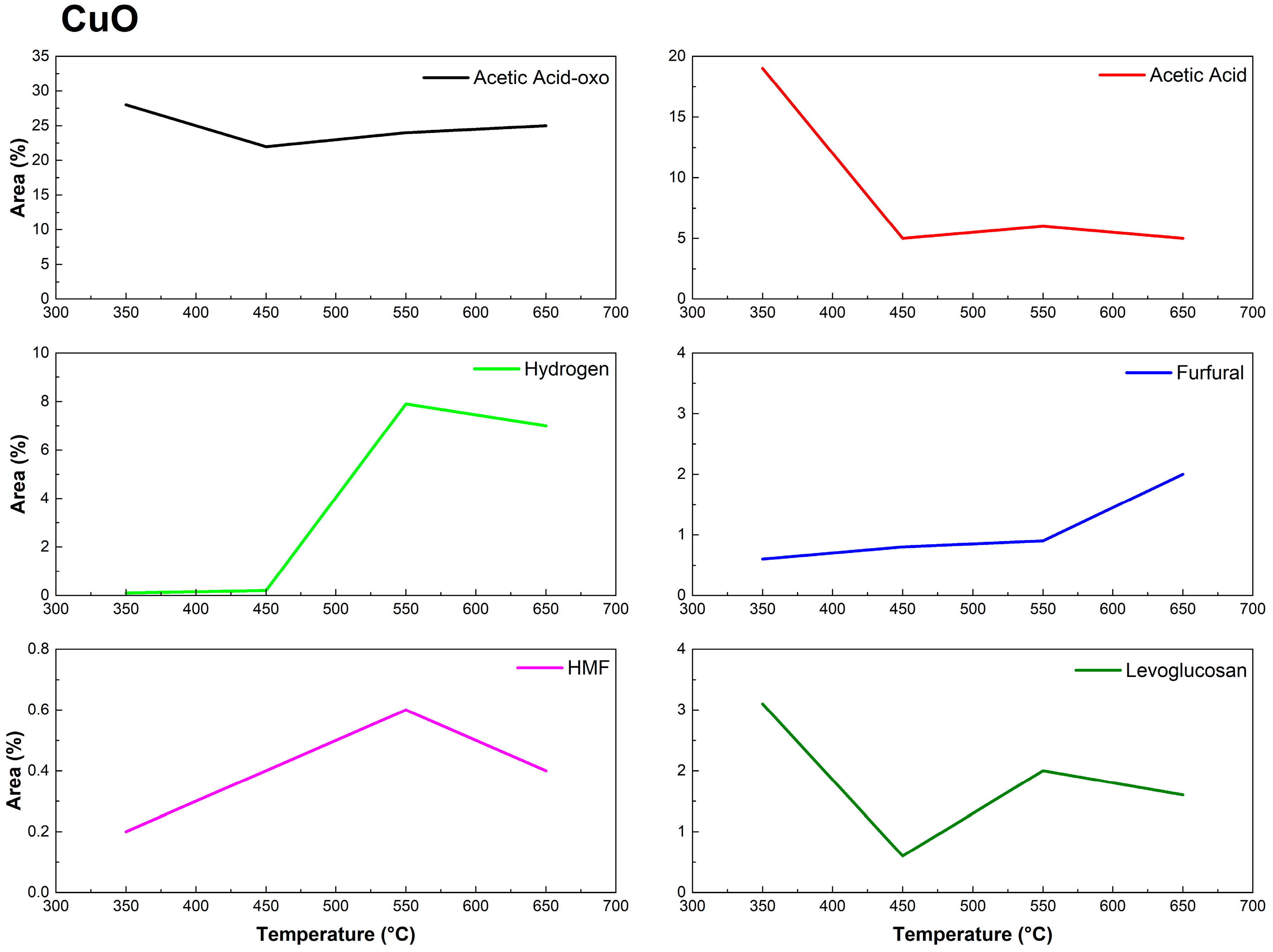
3.2. Py-GC/MS of Eucalyptus urograndis Canopy
3.3. Technical-Economic Assessment (TEA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naji, S.Z.; Tye, C.T.; Abd, A.A. State of the Art of Vegetable Oil Transformation into Biofuels Using Catalytic Cracking Technology: Recent Trends and Future Perspectives. Process Biochem. 2021, 109, 148–168. [Google Scholar] [CrossRef]
- Marques, A.V.; Pereira, H. Aliphatic Bio-Oils from Corks: A Py-GC/MS Study. J. Anal. Appl. Pyrolysis 2014, 109, 29–40. [Google Scholar] [CrossRef]
- Li, G.; Ma, S.; Ye, F.; Luo, Y.; Fan, S.; Lang, X.; Wang, Y.; Zhou, L. Permeation Characteristics of a T-Type Zeolite Membrane for Bio-Oil Pervaporation Dehydration. Microporous Mesoporous Mater. 2021, 315, 110884. [Google Scholar] [CrossRef]
- Pena-Vergara, G.; Castro, L.R.; Gasparetto, C.A.; Bizzo, W.A. Energy from Planted Forest and Its Residues Characterization in Brazil. Energy 2022, 239, 122243. [Google Scholar] [CrossRef]
- Vagia, E.C.; Lemonidou, A.A. Hydrogen Production via Steam Reforming of Bio-Oil Components over Calcium Aluminate Supported Nickel and Noble Metal Catalysts. Appl. Catal. A Gen. 2008, 351, 111–121. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Choi, W.; Jo, H.; Choi, J.W.; Suh, D.J.; Lee, H.; Kim, C.; Kim, K.H.; Lee, K.Y.; Ha, J.M. Stabilization of Acid-Rich Bio-Oil by Catalytic Mild Hydrotreating. Environ. Pollut. 2021, 272, 116180. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, W.Z.; Zhu, X.F. Overview of Fuel Properties of Biomass Fast Pyrolysis Oils. Energy Convers. Manag. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Urrutia, R.I.; Gutierrez, V.S.; Stefanazzi, N.; Volpe, M.A.; Werdin González, J.O. Pyrolysis Liquids from Lignocellulosic Biomass as a Potential Tool for Insect Pest Management: A Comprehensive Review. Ind. Crop. Prod. 2022, 177, 114533. [Google Scholar] [CrossRef]
- Kong, W.; Shen, B.; Ma, J.; Kong, J.; Feng, S.; Wang, Z.; Xiong, L. Pyrolysis of Spirulina Platensis, Tetradesmus Obliquus and Chlorella Vulgaris by TG-FTIR and Py-GC/MS: Kinetic Analysis and Pyrolysis Behaviour. Energy 2022, 244, 123165. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A Review of Pyrolysis Technologies and Feedstock: A Blending Approach for Plastic and Biomass towards Optimum Biochar Yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Muñoz, R.; Palomar, C.R.; García-Alvaro, A.; de Godos, I.; de Almeida Guimarães, V.; Hedo, E.B. Biofuels in Low Carbon Economies and Societies. In Biofuels in the Circular Economy; Springer Nature: Singapore, 2023; pp. 31–58. ISBN 9789811958373. [Google Scholar]
- Deng, R.; Huang, D.; Wan, J.; Xue, W.; Wen, X.; Liu, X.; Chen, S.; Lei, L.; Zhang, Q. Recent Advances of Biochar Materials for Typical Potentially Toxic Elements Management in Aquatic Environments: A Review. J. Clean. Prod. 2020, 255, 119523. [Google Scholar] [CrossRef]
- Soria, R.I.; Rolfe, S.A.; Betancourth, M.P.; Thornton, S.F. The Relationship between Properties of Plant-Based Biochars and Sorption of Cd(II), Pb(II) and Zn(II) in Soil Model Systems. Heliyon 2020, 6, E05388. [Google Scholar] [CrossRef] [PubMed]
- Merckel, R.D.; Heydenrych, M.D.; Sithole, B.B. Pyrolysis Oil Composition and Catalytic Activity Estimated by Cumulative Mass Analysis Using Py-GC/MS EGA-MS. Energy 2021, 219, 119428. [Google Scholar] [CrossRef]
- Ghysels, S.; Rathnayake, D.; Maziarka, P.; Mašek, O.; Sohi, S.; Ronsse, F. Biochar Stability Scores from Analytical Pyrolysis (Py-GC-MS). J. Anal. Appl. Pyrolysis 2022, 161, 105412. [Google Scholar] [CrossRef]
- Mattonai, M.; Licursi, D.; Antonetti, C.; Raspolli Galletti, A.M.; Ribechini, E. Py-GC/MS and HPLC-DAD Characterization of Hazelnut Shell and Cuticle: Insights into Possible Re-Evaluation of Waste Biomass. J. Anal. Appl. Pyrolysis 2017, 127, 321–328. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Wang, Y.; Yuan, H.; Chen, Y.; Luo, B. Effect of Potassium on the Pyrolysis of Biomass Components: Pyrolysis Behaviors, Product Distribution and Kinetic Characteristics. Waste Manag. 2021, 121, 255–264. [Google Scholar] [CrossRef] [PubMed]
- David, G.F.; de Pereira, S.P.S.; Fernandes, S.A.; Cubides-Roman, D.C.; Siqueira, R.K.; Perez, V.H.; Lacerda, V. Fast Pyrolysis as a Tool for Obtaining Levoglucosan after Pretreatment of Biomass with Niobium Catalysts. Waste Manag. 2021, 126, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Biswas, B.; Saini, K.; Kumar, A.; Kumar, J.; Krishna, B.B.; Bhaskar, T. Py-GC/MS Study of Prot Lignin with Cobalt Impregnated Titania, Ceria and Zirconia Catalysts. Renew. Energy 2021, 172, 121–129. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, C.L.; Lee, K.T.; Lam, S.S.; Ong, H.C.; Ok, Y.S.; Saeidi, S.; Sharma, A.K.; Hsieh, T.H. Catalytic Level Identification of ZSM-5 on Biomass Pyrolysis and Aromatic Hydrocarbon Formation. Chemosphere 2021, 271, 129510. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, S.; Su, Y.; Shi, L.; Zhang, S.; Xiong, Y. Simultaneous Production of Aromatics-Rich Bio-Oil and Carbon Nanomaterials from Catalytic Co-Pyrolysis of Biomass/Plastic Wastes and in-Line Catalytic Upgrading of Pyrolysis Gas. Waste Manag. 2021, 121, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Ortiz, F.J.; Alonso-Fariñas, B.; Campanario, F.J.; Kruse, A. Life Cycle Assessment of the Fischer-Tropsch Biofuels Production by Supercritical Water Reforming of the Bio-Oil Aqueous Phase. Energy 2020, 210, 118648. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Antonakou, E.V.; Karakoulia, S.A.; Vasalos, I.A.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic Conversion of Biomass Pyrolysis Products by Mesoporous Materials: Effect of Steam Stability and Acidity of Al-MCM-41 Catalysts. Chem. Eng. J. 2007, 134, 51–57. [Google Scholar] [CrossRef]
- ABNT. NBR 8633; Charcoal: Determination of Calorific Value. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 1984.
- Sonwani, R.K.; Swain, G.; Giri, B.S.; Singh, R.S. Bioresource Technology Biodegradation of Congo Red Dye in a Moving Bed Bio Fi Lm Reactor: Performance Evaluation and Kinetic Modeling. Bioresour. Technol. 2020, 302, 122811. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xiong, Q.; Jiang, L.; Wang, Y.; Tang, Q.; He, J.; Wang, J. Carbon Supported Copper Catalyst Prepared in Situ by One-Pot Pyrolysis of Bougainvillea Glabra: An Efficient and Stable Catalyst for Selective Oxidation of Cyclohexane. Appl. Surf. Sci. 2022, 576, 151833. [Google Scholar] [CrossRef]
- Fabbri, D.; Fabbri, F.; Falini, G.; Baravelli, V.; Magnani, A.; Torri, C.; Maskrot, H.; Leconte, Y. The Activity of Nanopowder and Mesoporous Titanium Catalysts for the Analysis of Fatty Acids in Triglycerides by Pyrolysis Methylation a with Dimethyl Carbonate. J. Anal. Appl. Pyrolysis 2008, 82, 248–254. [Google Scholar] [CrossRef]
- Wei, X.; Yang, H.; Liu, B.; Yu, H.; Wang, C.; Wu, S.; Jia, Z.; Han, W. Synthesis of Titanium Oxyfluoride with Oxygen Vacancy as Novel Catalysts for Pyrolysis of Fluorinated Greenhouse Gasses to Hydrofluoroolefins. J. Taiwan Inst. Chem. Eng. 2021, 129, 189–196. [Google Scholar] [CrossRef]
- Khanh Tran, Q.; Vu Ly, H.; Anh Vo, T.; Tae Hwang, H.; Kim, J.; Kim, S.S. Highly Selective Hydrodeoxygenation of Wood Pallet Sawdust Pyrolysis Oil to Methyl Phenol Derivatives Using Cobalt and Iron on Activated Carbon Supported Catalysts. Energy Convers. Manag. X 2022, 14, 100184. [Google Scholar] [CrossRef]
- Wang, J.; Cui, J.; Lv, P.; Song, X.; Bai, Y.; Su, W.; Yu, G.; Ma, Y. Synergistic Effect between Coal and Iron-Based Waste Catalyst Containing Wax from Fisher-Tropsch Synthesis during Their Co-Pyrolysis. J. Anal. Appl. Pyrolysis 2022, 162, 105461. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Zhang, T.; Wang, Q. Hydrogen-Rich Syngas Production from Biomass Pyrolysis and Catalytic Reforming Using Biochar-Based Catalysts. Fuel 2022, 313, 123006. [Google Scholar] [CrossRef]
- Qian, L.; Liu, S.; Zhang, W.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.; Chen, M. Enhanced Reduction and Adsorption of Hexavalent Chromium by Palladium and Silicon Rich Biochar Supported Nanoscale Zero-Valent Iron. J. Colloid Interface Sci. 2019, 533, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Alberton, K.S.; Moraes, A.B.R.; Anderson, P.R.; Stein, Z.T.R.; Stoffes Junior, M.J.; Stein, C.R. Síntese e Caracterização Morfológica e Estrutural de Nanopartículas Magnéticas de Ferrita de Cobalto. Braz. J. Dev. 2020, 6, 39371–39378. [Google Scholar] [CrossRef]
- Demuner, I.F.; Gomes, F.J.B.; Coura, M.R.; Gomes, J.S.; Demuner, A.J.; Carvalho, A.M.M.L.; Silva, C.M. Determination of Chemical Modification of Eucalypt Kraft Lignin after Thermal Treatment by Py-GC–MS. J. Anal. Appl. Pyrolysis 2021, 156, 105158. [Google Scholar] [CrossRef]
- Oudia, A.; Mészáros, E.; Simões, R.; Queiroz, J.; Jakab, E. Pyrolysis-GC/MS and TG/MS Study of Mediated Laccase Biodelignification of Eucalyptus Globulus Kraft Pulp. J. Anal. Appl. Pyrolysis 2007, 78, 233–242. [Google Scholar] [CrossRef]
- Nakai, D.K. Avaliação do Potencial Energético de Eucalyptus spp. Em Gaseificador do tipo Contracorrente Diogo Keiji Nakai Avaliação do Potencial Energético de Eucalyptus spp. Em Gaseificador do tipo Contracorrente; University of Brasilia: Brasília, Brazil, 2014. [Google Scholar]
- Neiva, P.S.; Furtado, D.B.; Finzer, J.R.D. Capacidade Térmica E Poder Calorifico De Biomassa Eucalipto. II Encontro Desenvolv. Process. Agroind. 2018, 1–8, 1–8. [Google Scholar]
- Florestais, T.D.P. Universidade de São Paulo Escola Superior de Agricultura “Luiz de Queiroz” Caracterização de Resíduos Madeireiros Reprocessados Para Uso Energético Juliana Rodrigues Siviero Dos Santos Piracicaba. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2020. [Google Scholar]
- de Félix, C.R.O.; de Azevedo Júnior, A.F.; Freitas, C.C.; Pires, C.A.d.M.; Teixeira, V.; Frety, R.; Brandão, S.T. Pirólise Rápida de Biomassa de Eucalipto Na Presença de Catalisador Al-MCM-41. Rev. Mater. 2017, 22, 11915. [Google Scholar] [CrossRef]
- Fabbri, D.; Torri, C.; Spokas, K.A. Analytical Pyrolysis of Synthetic Chars Derived from Biomass with Potential Agronomic Application (Biochar). Relationships with Impacts on Microbial Carbon Dioxide Production. J. Anal. Appl. Pyrolysis 2012, 93, 77–84. [Google Scholar] [CrossRef]
- Díez, C.; Martínez, O.; Calvo, L.F.; Cara, J.; Morán, A. Pyrolysis of Tyres. Influence of the Final Temperature of the Process on Emissions and the Calorific Value of the Products Recovered. Waste Manag. 2004, 24, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Lei, T.; Li, Z.; Zhang, Z.; Yang, S.; Xin, X.; Zhang, M.; He, X.; Zhang, Q.; Zhang, L. Catalytic Co-Pyrolysis of Corn Stalk and Polypropylene over Zn-Al Modified MCM-41 Catalysts for Aromatic Hydrocarbon-Rich Oil Production. Ind. Crop. Prod. 2021, 171, 113843. [Google Scholar] [CrossRef]
- Aboul-enein, A.A.; Awadallah, A.E.; El-desouki, D.S.; Aboul-gheit, N.A.K. Catalytic Pyrolysis of Sugarcane Bagasse by Zeolite Catalyst for the Production of Multi-Walled Carbon Nanotubes. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2021, 49, 1421–1434. [Google Scholar] [CrossRef]
- Huo, E.; Lei, H.; Liu, C.; Zhang, Y.; Xin, L.; Zhao, Y.; Qian, M.; Zhang, Q.; Lin, X.; Wang, C.; et al. Jet Fuel and Hydrogen Produced from Waste Plastics Catalytic Pyrolysis with Activated Carbon and MgO. Sci. Total Environ. 2020, 727, 138411. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, N.; Kim, J.; Lee, J. Co-Pyrolysis of Food Waste and Wood Bark to Produce Hydrogen with Minimizing Pollutant Emissions. Environ. Pollut. 2021, 270, 116045. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.P.; De Oliveira, D.M.; Da Silva, L.D.M.; Giménez, J.; Esplugas, S.; De Oliveira, S.C.; Dantas, R.F.; Sans, C.; Machulek, A. Evaluation of the Main Active Species Involved in the TiO2photocatalytic Degradation of Ametryn Herbicide and Its By-Products. J. Environ. Chem. Eng. 2021, 9, 105109. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper Catalysed Ullmann Type Chemistry: From Mechanistic Aspects to Modern Development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef]
- Sarchami, T.; Batta, N.; Berruti, F. Production and Separation of Acetic Acid from Pyrolysis Oil of Lignocellulosic Biomass: A Review. Biofuels, Bioprod. Biorefining 2021, 15, 1912–1937. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Sinha, A.S.K. Catalyst Modification Strategies to Enhance the Catalyst Activity and Stability during Steam Reforming of Acetic Acid for Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 12983–13010. [Google Scholar] [CrossRef]
- Chen, G.; Tao, J.; Liu, C.; Yan, B.; Li, W.; Li, X. Hydrogen Production via Acetic Acid Steam Reforming: A Critical Review on Catalysts. Renew. Sustain. Energy Rev. 2017, 79, 1091–1098. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Zhou, Z.; Yuan, H.; Zheng, T.; Chen, Y. Effect of Carbon Structure on Hydrogen Release Derived from Different Biomass Pyrolysis. Fuel 2020, 271, 117638. [Google Scholar] [CrossRef]
- Tran, T.K.; Kim, N.; Leu, H.J.; Pham, M.P.; Luong, N.A.; Vo, H.K. The Production of Hydrogen Gas from Modified Water Hyacinth (Eichhornia Crassipes) Biomass through Pyrolysis Process. Int. J. Hydrogen Energy 2021, 46, 13976–13984. [Google Scholar] [CrossRef]
- Terry, L.M.; Li, C.; Chew, J.J.; Aqsha, A.; How, B.S.; Loy, A.C.M.; Chin, B.L.F.; Khaerudini, D.S.; Hameed, N.; Guan, G.; et al. Bio-Oil Production from Pyrolysis of Oil Palm Biomass and the Upgrading Technologies: A Review. Carbon Resour. Convers. 2021, 4, 239–250. [Google Scholar] [CrossRef]
- Mazar, A.; Ajao, O.; Benali, M.; Jemaa, N.; Wafa Al-Dajani, W.; Paleologou, M. Integrated Multiproduct Biorefinery for Furfural Production with Acetic Acid and Lignin Recovery: Design, Scale-Up Evaluation, and Technoeconomic Analysis. ACS Sustain. Chem. Eng. 2020, 8, 17345–17358. [Google Scholar] [CrossRef]
- Xiang, W.; Ji, Q.; Xu, C.; Guo, Y.; Liu, Y.; Sun, D.; Zhou, W.; Xu, Z.; Qi, C.; Yang, S.; et al. Accelerated Photocatalytic Degradation of Iohexol over Co3O4/g-C3N4/Bi2O2CO3 of p-n/n-n Dual Heterojunction under Simulated Sunlight by Persulfate. Appl. Catal. B Environ. 2021, 285, 119847. [Google Scholar] [CrossRef]



| Parameters | Content | |
|---|---|---|
| Elemental analysis (%) | Carbon | 50.2 |
| Hydrogen | 6.34 | |
| Oxygen | 43.2 | |
| Nitrogen | 0.15 | |
| Sulfur | 0.11 | |
| Inorganics (%) | 0.78 | |
| HCl Insoluble (%) | 0.43 | |
| Metals (mg/kg) | Ca | 1.90 |
| Mg | 2.31 | |
| Fe | 0.51 | |
| Cu | 0.01 | |
| Mn | 1.36 | |
| Na | 4.61 | |
| K | 7.32 | |
| Lower heating value (LHV, MJ/kg) | 17.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bittencourt, R.d.C.; Guimarães, T.; Costa, M.M.d.; Silva, L.S.; Barbosa, V.O.d.P.; Arêdes, S.C.d.L.; Alves, K.S.; Carvalho, A.M.M.L. Production of High-Value Green Chemicals via Catalytic Fast Pyrolysis of Eucalyptus urograndis Forest Residues. Sustainability 2024, 16, 8294. https://doi.org/10.3390/su16198294
Bittencourt RdC, Guimarães T, Costa MMd, Silva LS, Barbosa VOdP, Arêdes SCdL, Alves KS, Carvalho AMML. Production of High-Value Green Chemicals via Catalytic Fast Pyrolysis of Eucalyptus urograndis Forest Residues. Sustainability. 2024; 16(19):8294. https://doi.org/10.3390/su16198294
Chicago/Turabian StyleBittencourt, Ricardo de C., Tiago Guimarães, Marcelo M. da Costa, Larissa S. Silva, Verônica O. de P. Barbosa, Stéphani Caroline de L. Arêdes, Krisnna S. Alves, and Ana Márcia M. L. Carvalho. 2024. "Production of High-Value Green Chemicals via Catalytic Fast Pyrolysis of Eucalyptus urograndis Forest Residues" Sustainability 16, no. 19: 8294. https://doi.org/10.3390/su16198294
APA StyleBittencourt, R. d. C., Guimarães, T., Costa, M. M. d., Silva, L. S., Barbosa, V. O. d. P., Arêdes, S. C. d. L., Alves, K. S., & Carvalho, A. M. M. L. (2024). Production of High-Value Green Chemicals via Catalytic Fast Pyrolysis of Eucalyptus urograndis Forest Residues. Sustainability, 16(19), 8294. https://doi.org/10.3390/su16198294







