Some Possible Process Configurations for Modern Wastewater Treatment Plants for Per- and Polyfluoroalkyl Substances (PFASs) Removal
Abstract
1. Introduction
2. Methodology
3. A Summary of Treatment Technologies for PFASs in Wastewater and Sludge
4. PFASs Removal in Full-Scale Wastewater Treatment Plants
5. Wastewater Treatment Plant Configurations for PFASs Removal
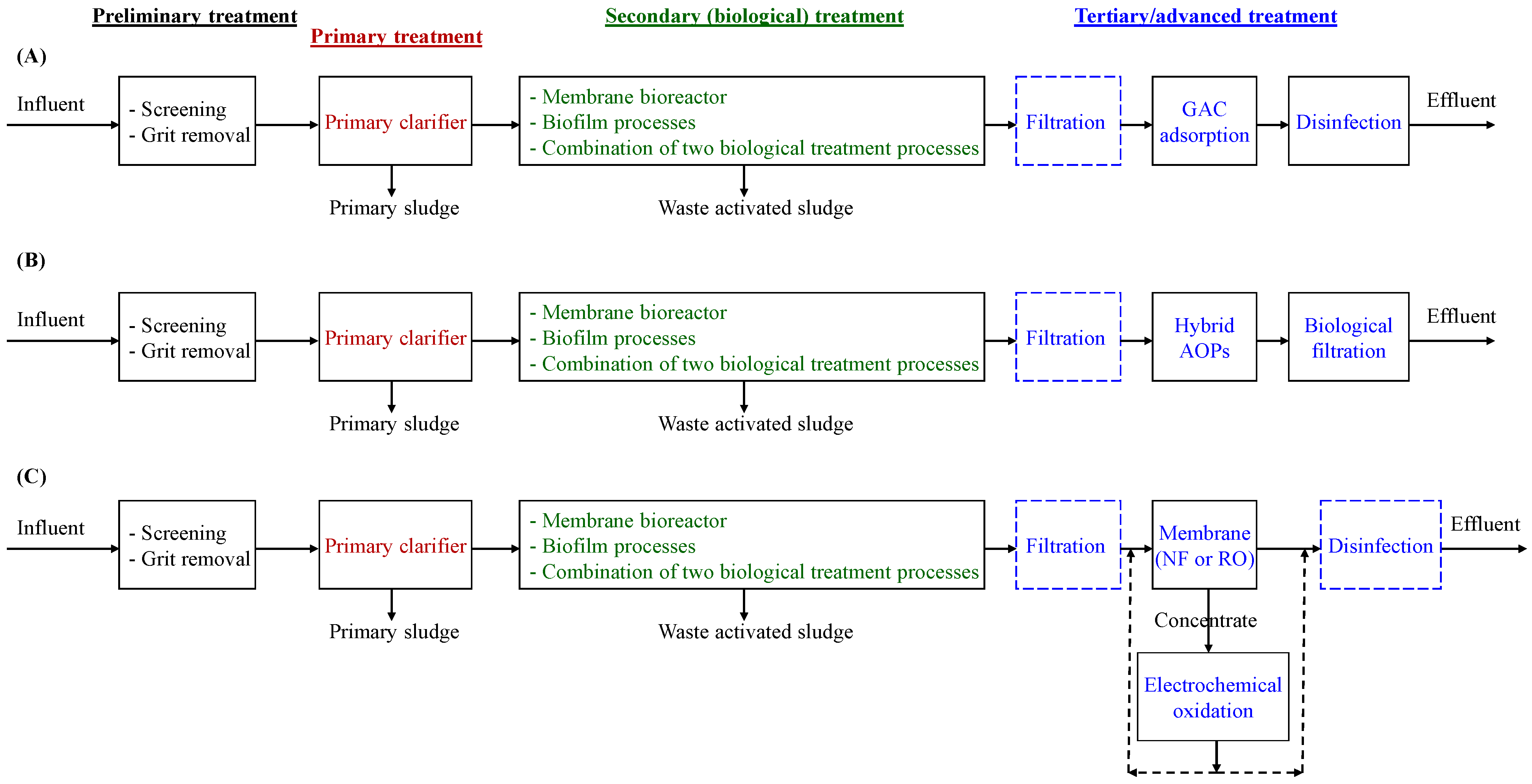
6. Conclusions and Future Perspectives
- The removal efficiencies and fate of PFASs in WWTPs may be influenced by influent wastewater source (i.e., industrial, domestic, urban runoff and/or agricultural) and characteristics (e.g., PFASs’ physicochemical characteristics, concentrations), design and/or type of applied treatment techniques, and process operating conditions (including temperature, flow rate, hydraulic and sludge retention time, mixed liquor suspended solids, etc.).
- Within the WWTPs, short-chain PFASs have a tendency to remain in aqueous streams, whereas long-chain PFASs dominate in sludge/biosolids.
- Biological treatment processes may be considered as high-potential technologies for PFASs remediation in sludge due to their low-cost and eco-friendly nature.
- Three theoretical configurations for the wastewater processing train of modern WWTPs (Figure 2A–C) were presented to remove PFASs. The tertiary/advanced treatment steps were in configuration A (filtration, GAC adsorption, and disinfection), configuration B (filtration, hybrid AOPs, and biological filtration), and configuration C (filtration, membrane (NF or RO), electrochemical oxidation for treatment of membrane concentrate, and disinfection (if applicable)). Note that each treatment train can provide a certain degree of PFASs removal, and performance and techno-economic assessments and optimization are required before selection/implementation.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Nguyen, T.M.H.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Nguyen, T.A.H. Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation. J. Water Process Eng. 2020, 36, 101393. [Google Scholar]
- Lenka, S.P.; Kah, M.; Padhye, L.P. A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants. Water Res. 2021, 199, 117187. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Mok, K.; Garrett, K.K.; Poudrier, G.; Brown, P.; Birnbaum, L.S.; Goldenman, G.; Miller, M.F.; Patton, S.; Poehlein, M.; et al. Presumptive contamination: A new approach to PFAS contamination based on likely sources. Environ. Sci. Technol. Lett. 2022, 9, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Sha, S.; Luo, J.; Huang, Z.; Jackie, X.Z. Treatment train approaches for the remediation of per- and polyfluoroalkyl substances (PFAS): A critical review. J. Hazard. Mater. 2020, 386, 121963. [Google Scholar] [CrossRef]
- Dey, D.; Shafi, T.; Chowdhury, S.; Dubey, B.K.; Sen, R. Progress and perspectives on carbon-based materials for adsorptive removal and photocatalytic degradation of perfluoroalkyl and polyfluoroalkyl substances (PFAS). Chemosphere 2024, 351, 141164. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Our Current Understanding of the Human Health and Environmental Risks of PFAS. 7 June 2023. Available online: https://www.epa.gov/pfas/our-current-understanding-human-health-and-environmental-risks-pfas (accessed on 25 June 2024).
- Ahmed, M.B.; Johir, M.A.H.; McLaughlan, R.; Nguyen, N.L.; Xu, B.; Nghiem, D.L. Per- and polyfluoroalkyl substances in soil and sediments: Occurrence, fate, remediation and future outlook. Sci. Total Environ. 2020, 748, 141251. [Google Scholar] [CrossRef]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Jin, W.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Podder, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.B.; Goel, R. Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef]
- Militao, I.M.; Roddick, F.A.; Bergamasco, R.; Fan, L. Removing PFAS from aquatic systems using natural and renewable material-based adsorbents: A review. J. Environ. Chem. Eng. 2021, 9, 105271. [Google Scholar] [CrossRef]
- Boone, J.S.; Vigo, C.; Boone, T.; Byrne, C.; Ferrario, J.; Benson, R.; Donohue, J.; Simmons, J.E.; Kolpin, D.W.; Furlong, E.T.; et al. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ. 2019, 653, 359–369. [Google Scholar] [CrossRef] [PubMed]
- EGagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, F.; Eriksson, U.; Kärrman, A.; Yeung, L.W.Y. Per- and polyfluoroalkyl substances (PFAS) in sludge from wastewater treatment plants in Sweden—First findings of novel fluorinated copolymers in Europe including temporal analysis. Sci. Total Environ. 2022, 846, 157406. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.E.; Hooper, J.L.; Strom, L.E.; Abusallout, I.; Dickenson, E.R.V.; Thompson, K.A.; Mohan, G.R.; Drennan, D.; Wu, K.; Guelfo, J.L. Occurrence of quantifiable and semi-quantifiable poly- and perfluoroalkyl substances in united states wastewater treatment plants. Water Res. 2023, 233, 119724. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodríguez-Hernandéz, J.A.; González-González, R.B.; Macias-Garbett, R.; Martínez-Ruiz, M.; Reyes-Pardo, H.; Hernández Martínez, S.A.; Parra-Arroyo, L.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; et al. Detection and tertiary treatment technologies of poly-and perfluoroalkyl substances in wastewater treatment plants. Front. Environ. Sci. 2022, 10, 864894. [Google Scholar] [CrossRef]
- Zhou, T.; Li, X.; Liu, H.; Dong, S.; Zhang, Z.; Wang, Z.; Li, J.; Nghiem, L.D.; Khan, S.J.; Wang, Q. Occurrence, fate, and remediation for per-and polyfluoroalkyl substances (PFAS) in sewage sludge: A comprehensive review. J. Hazard. Mater. 2024, 466, 133637. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Gobelius, L.; Glimstedt, L.; Olsson, J.; Wiberg, K.; Ahrens, L. Mass flow of per- and polyfluoroalkyl substances (PFAS) in a Swedish municipal wastewater network and wastewater treatment plant. Chemosphere 2023, 336, 139182. [Google Scholar] [CrossRef]
- Nzeribe, B.N.; Crimi, M.; Mededovic Thagard, S.; Holsen, T.M. Physico-chemical processes for the treatment of per-and polyfluoroalkyl substances (PFAS): A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Meng, J.; Wang, T. Seasonal and annual variations in removal efficiency of perfluoroalkyl substances by different wastewater treatment processes. Environ. Pollut. 2018, 242 Pt B, 2059–2067. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, M.; Lu, Y.; Meng, J.; Li, Q.; Lu, X. Removal of perfluoalkyl acids (PFAAs) through fluorochemical industrial and domestic wastewater treatment plants and bioaccumulation in aquatic plants in river and artificial wetland. Environ. Int. 2019, 129, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Kibambe, M.G.; Momba, M.N.B.; Daso, A.P.; Coetzee, M.A.A. Evaluation of the efficiency of selected wastewater treatment processes in removing selected perfluoroalkyl substances (PFASs). J. Environ. Manage. 2020, 255, 109945. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, D.; Curran, C. Evaluation of PFAS in Wastewater Treatment Plants (WWTPs) across the United States, AECOM. December 2021. Available online: https://publications.aecom.com/pfas/2021-12-31_AECOM_Final_PFAS_Technical_Memorandum_pck.pdf (accessed on 21 July 2024).
- Kim, J.; Xin, X.; Mamo, B.T.; Hawkins, G.L.; Li, K.; Chen, Y.; Huang, Q.; Huang, C.H. Occurrence and fate of ultrashort-chain and other per- and polyfluoroalkyl substances (PFAS) in wastewater treatment plants. ACS EST Water 2022, 2, 1380–1390. [Google Scholar] [CrossRef]
- Seay, B.A.; Dasu, K.; MacGregor, I.C.; Austin, M.P.; Krile, R.T.; Frank, A.J.; Fenton, G.A.; Heiss, D.R.; Williamson, R.J.; Buehler, S. Per- and polyfluoroalkyl substances fate and transport at a wastewater treatment plant with a collocated sewage sludge incinerator. Sci. Total Environ. 2023, 874, 162357. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, Z.; Hamza, R.A.; Suehring, R. The significance of fluorinated compound chain length, treatment technology, and influent composition on per- and polyfluoroalkyl substances removal in worldwide wastewater treatment plants. Integr. Environ. Assess. Manag. 2024, 20, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Metcalf and Eddy Inc. Wastewater Engineering: Treatment, Disposal and Reuse, 3rd ed.; Revised by George Tchobanoglous, Franklin L. Burton; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Jafarinejad, S. Cost estimation and economical evaluation of three configurations of activated sludge process for a wastewater treatment plant (WWTP) using simulation. Appl. Water Sci. 2017, 7, 2513–2521. [Google Scholar] [CrossRef]
- Jafarinejad, S. Economic analysis: Trickling filter/activated sludge or nitrifying trickling filter/activated sludge? Ecol. Chem. Eng. S 2019, 26, 345–356. [Google Scholar] [CrossRef]
- Jafarinejad, S. A framework for the design of the future energy-efficient, cost-effective, reliable, resilient, and sustainable full-scale wastewater treatment plants. Curr. Opin. Environ. Sci. Health 2020, 13, 91–100. [Google Scholar] [CrossRef]
- Jafarinejad, S. Comparison of the full-scale municipal wastewater treatment plant designs consisting of modified Bardenpho process with and without membrane bioreactor for nutrient removal: Cost analysis. In Sustainable Development of Water and Environment, Proceedings of the International Conference on Sustainable Development of Water and Environment (ICSDWE 2021), Virtual Conference, 13 March 2021; Jeon, H.Y., Ed.; Part of the Environmental Science and Engineering book series (ESE); Springer: Cham, Switzerland, 2021; pp. 47–63. [Google Scholar]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediation 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Merino, N.; Qu, Y.; Deeb, R.A.; Hawley, E.L.; Hoffmann, M.R.; Mahendra, S. Degradation and removal methods for perfluoroalkyl substances in water. Environ. Eng. Sci. 2016, 33, 615–649. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Alam, M.M.; Zhou, J.L.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Rahman, M.S.; Hossen, J.; Hasan, A.T.M.K.; Moni, M.A. Advanced treatment technologies efficacies and mechanism of perand poly-fluoroalkyl substances removal from water. Process Saf. Environ. Prot. 2020, 136, 1–14. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ozaki, N.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Occurrence of per-and polyfluoroalkyl substances in aquatic environments and their removal by advanced oxidation processes. Chemosphere 2023, 330, 138666. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Lin, T.; Zhang, X.; Jiang, F.; Chen, H. Impact of biological activated carbon filtration and backwashing on the behaviour of PFASs in drinking water treatment plants. J. Hazard. Mater. 2023, 446, 130641. [Google Scholar] [CrossRef]
- Vatankhah, H.; Tajdini, B.; Milstead, R.P.; Clevenger, E.; Murray, C.; Knappe, D.; Remucal, C.K.; Bellona, C. Impact of ozone-biologically active filtration on the breakthrough of Perfluoroalkyl acids during granular activated carbon treatment of municipal wastewater effluent. Water Res. 2022, 223, 118988. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Hand, S.; Cusick, R.D. Electrochemical disinfection in water and wastewater treatment: Identifying impacts of water quality and operating conditions on performance. Environ. Sci. Technol. 2021, 55, 3470–3482. [Google Scholar] [CrossRef]
- Soriano, A.; Schaefer, C.; Urtiaga, A. Enhanced treatment of perfluoroalkyl acids in groundwater by membrane separation and electrochemical oxidation. Chem. Eng. J. Adv. 2020, 4, 100042. [Google Scholar] [CrossRef]
- Veciana, M.; Braunig, J.; Farhat, A.; Pype, M.L.; Freguia, S.; Carvalho, G.; Keller, J.; Ledezma, P. Electrochemical oxidation processes for PFAS removal from contaminated water and wastewater: Fundamentals, gaps and opportunities towards practical implementation. J. Hazard. Mater. 2022, 434, 128886. [Google Scholar] [CrossRef]
- Franke, V.; McCleaf, P.; Lindegren, K.; Ahrens, L. Efficient removal of per- and polyfluoroalkyl substances (PFASs) in drinking water treatment: Nanofiltration combined with active carbon or anion exchange. Environ. Sci. Water Res. Technol. 2019, 5, 1836–1843. [Google Scholar] [CrossRef]
- Soriano, A.; Gorri, D.; Urtiaga, A. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Res. 2017, 112, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tow, E.W.; Ersan, M.S.; Kum, S.; Lee, T.; Speth, T.F.; Owen, C.; Bellona, C.; Nadagouda, M.N.; Mikelonis, A.M.; Westerhoff, P.; et al. Managing and treating per-and polyfluoroalkyl substances (PFAS) in membrane concentrates. AWWA Water Sci. 2021, 3, e1233. [Google Scholar] [CrossRef] [PubMed]
- Gewurtz, S.B.; Auyeung, A.S.; De Silva, A.O.; Teslic, S.; Smyth, S.A. Per- and polyfluoroalkyl substances (PFAS) in Canadian municipal wastewater and biosolids: Recent patterns and time trends 2009 to 2021. Sci. Total Environ. 2024, 912, 168638. [Google Scholar] [CrossRef] [PubMed]
- Grgas, D.; Petrina, A.; Štefanac, T.; Bešlo, D.; Landeka Dragičević, T.A. A review: Per- and polyfluoroalkyl substances-Biological degradation. Toxics 2023, 11, 446. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Biswas, J.K.; Datta, R. Biodegradation of per- and polyfluoroalkyl substances (PFAS): A review. Bioresour. Technol. 2022, 344, 126223. [Google Scholar] [CrossRef]

| Media | Treatment Type | Treatment Technologies | References |
|---|---|---|---|
| Wastewater | Separation/concentration |
| Vo et al. [1] and Wanninayake [19] |
| Destruction |
| ||
| Sludge | Physical |
| Zhou et al. [18] |
| Chemical |
| ||
| Biological |
|
| WWTP Processes | Type | PFASs Removal Results | References |
|---|---|---|---|
| Wastewater processing train: grids, screening, aerated sand traps, primary sedimentation with pre-precipitation of ferric chloride addition, biological treatment with activated sludge in aerated and anoxic basins, and chemical treatment with ferric chloride Sludge processing train: thickening, anaerobic digestion, and dewatering (by centrifuges) | Domestic (may receive a mixture of domestic, industrial, and other commercial wastewater) | Average removal efficiency of 10 ± 68% for individual PFAS | Gobelius et al. [20] |
| Wastewater processing train: bar rack, grit chamber, primary clarifiers, aeration basins, secondary clarifiers, and chlorine contact tanks Sludge processing train: gravity thickener, dewatering by centrifuge, sewage sludge incinerator, ventury/tray scrubber, and wet ash lagoon | Domestic | PFASs destruction and removal efficiency of 51% at the sewage sludge incinerator | Seay et al. [27] |
| Screening, grit removal, primary clarifier, biological treatment (anaerobic/anoxic/aerobic), secondary clarifier, ultrafiltration/granular media filter, pre-ozonation, biological activated carbon filter, and post-ozonation | Domestic | 191.3% increase of perfluoroalkyl acids (PFAAs) after biological treatment | Kim et al. [26] |
| Screening, grit removal, primary clarifier, biological treatment (anoxic/anaerobic/aerobic), aerobic membrane bioreactors, and UVdisinfection | Domestic | 185.1% increase of PFAAs after biological treatment | Kim et al. [26] |
| Wastewater processing train: screening, grit removal, primary clarifier, aeration tank, secondary clarifier, and UV disinfection Sludge processing train: sludge storage tanks and centrifuges | Domestic | Total PFASs of 97 ng/L in final effluent and total PFASs of 104 µg/kg in cake | Bogdan and Curran [25] |
| Screening, grit removal, primary clarifier, activated sludge aeration with nitrogen removal, secondary clarifier, dual-media pressure filter, and chlorine contact tanks | Domestic | Total PFASs of 78–136 ng/L in final effluent | Bogdan and Curran [25] |
| Primary sedimentation, activated sludge aeration with NDH process, secondary sedimentation, insert media gravity filtration, and chlorine contact tanks | Domestic | Total PFASs of 56–96 ng/L in final effluent | Bogdan and Curran [25] |
| Wastewater processing train: equalization tanks, screening, grit removal, sequencing batch reactors, post-equalization, disk filters, and UVdisinfection Sludge processing train: sludge storage tanks, rotary drum thickener, and thickened sludge tanks | Domestic | Total PFASs of 44 ng/L in final effluent and total PFASs of 10 µg/kg in thickened sludge | Bogdan and Curran [25] |
| Wastewater processing train: screening, grit removal, primary clarification, trickling filters, SCT tanks, secondary clarification, nitrifying trickling filters, denitrification filters, and disinfection Sludge processing train: dissolved aeration flotation tanks, anaerobic digestion, sludge storage tanks, and centrifuges | Domestic | Total PFASs of 80 ng/L in final effluent and total PFASs of 65–66 µg/kg in cake | Bogdan and Curran [25] |
| Coarse and fine grid, aerated grit chamber, first sedimentation, anaerobic–anoxic–oxic (A2O), second sedimentation, flocculation, third sedimentation, filtration, and UVdisinfection | Domestic | Removal efficiency of 69% for PFBA, 54% for PFBS, and 43% for all the 12 PFAAs; while PFOA and PFOS all increased | Wang et al. [23] |
| Coarse and fine grid, aerated grit chamber, first sedimentation, A2O (enhanced by adding carbon), second sedimentation, flocculation, third sedimentation, filtration, and UVdisinfection | Domestic | No removal efficiency for ∑PFAAs | Wang et al. [23] |
| Coarse grid, fine grid, main reaction tank and first sedimentation, upflow hydrolysis tank, A2O, moving bed biofilm reactor, second sedimentation, ozone contact tank, biological aerated filter, upflow sludge bed, filtration, and UVdisinfection | Industrial | Removal efficiency of 55% for ∑PFAAs, including 45% for PFBA, 58% for PFOA, 65% for PFBS, and 93% for PFOS | Wang et al. [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafarinejad, S. Some Possible Process Configurations for Modern Wastewater Treatment Plants for Per- and Polyfluoroalkyl Substances (PFASs) Removal. Sustainability 2024, 16, 8109. https://doi.org/10.3390/su16188109
Jafarinejad S. Some Possible Process Configurations for Modern Wastewater Treatment Plants for Per- and Polyfluoroalkyl Substances (PFASs) Removal. Sustainability. 2024; 16(18):8109. https://doi.org/10.3390/su16188109
Chicago/Turabian StyleJafarinejad, Shahryar. 2024. "Some Possible Process Configurations for Modern Wastewater Treatment Plants for Per- and Polyfluoroalkyl Substances (PFASs) Removal" Sustainability 16, no. 18: 8109. https://doi.org/10.3390/su16188109
APA StyleJafarinejad, S. (2024). Some Possible Process Configurations for Modern Wastewater Treatment Plants for Per- and Polyfluoroalkyl Substances (PFASs) Removal. Sustainability, 16(18), 8109. https://doi.org/10.3390/su16188109






