Mechanism of Carbon Monoxide (CO) Generation and Potential Human Health Hazard during Mechanized Tunnel Driving in Organic-Rich Rocks: Field and Laboratory Study
Abstract
1. Introduction
2. Materials, Experimental Set-Up and Methods
2.1. Materials
2.2. Experimental Set-Up
2.3. Analytical Methods
2.3.1. Solid-Phase Characterization
2.3.2. Fluid-Phase Characterization
3. Results
3.1. Field Study: Geology, Mechanized Tunnel Driving and CO Concentration in the Air
3.1.1. Geological Background
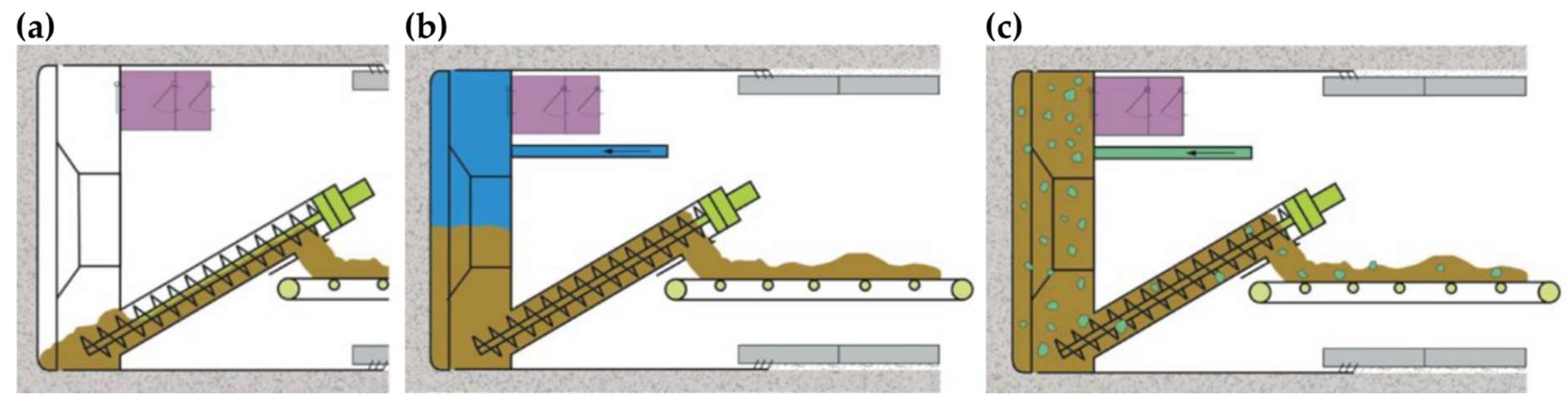
3.1.2. Mechanized Tunnel Driving
- The ‘open mode’ is used in the case of a rock with a mechanically stable working face and hence does not require the active support of the working face (Figure 2a).
- In the ‘semi-open mode’, the additional support of the working face is provided by the supply of excavated material, such as conditioned earth slurry or rock material, as well as compressed air to hold back water ingress from the surrounding host rocks (Figure 2b).
- The ‘closed mode’ is used when the working face is not stable or unclear geological conditions exist (e.g., presence of fault zones). In this case, the excavation chamber is completely filled with a mix of earth and slurry, which acts as working face support and ensures a closed environment during the tunneling process (Figure 2c).
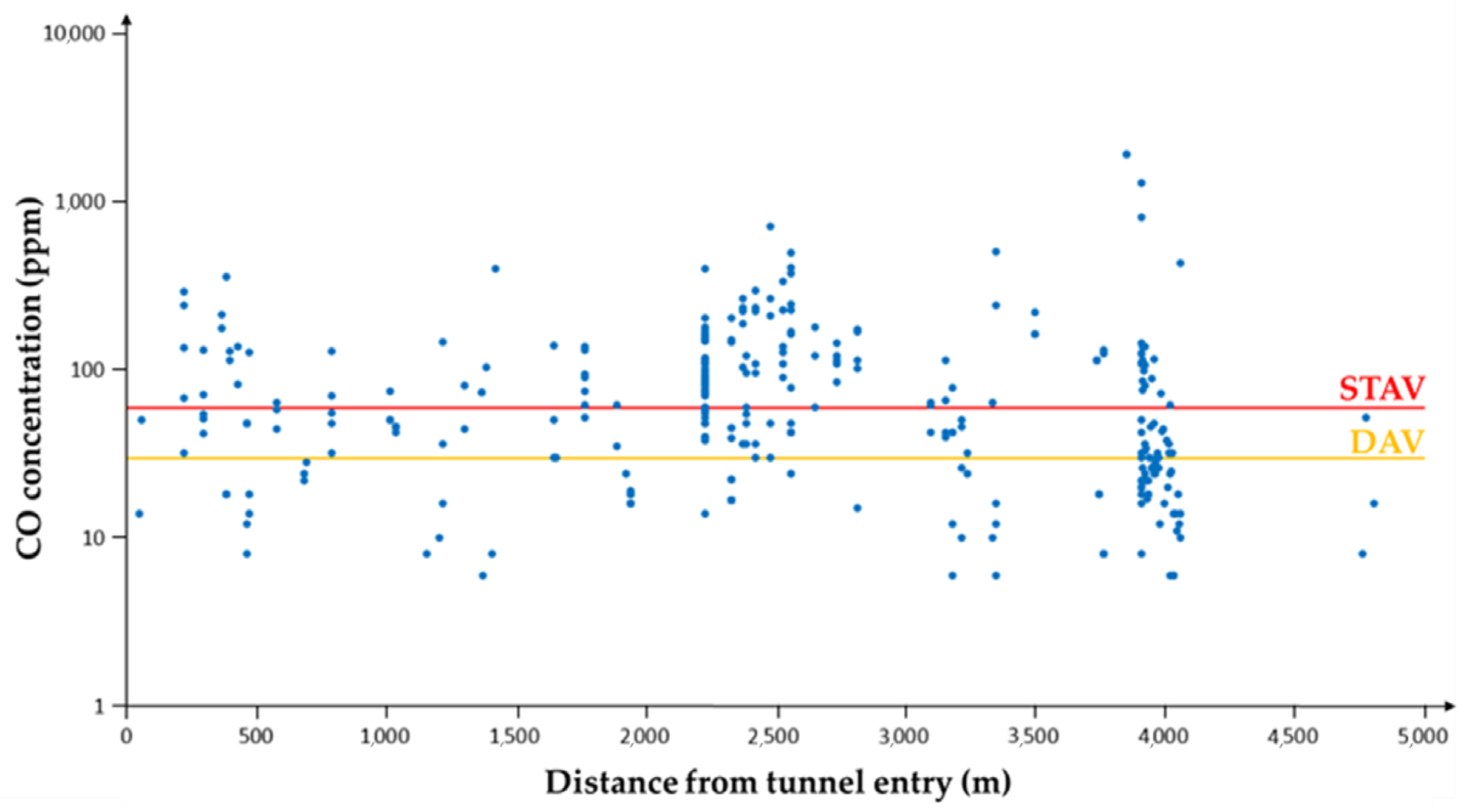
3.1.3. CO Concentration in the Tunnel Atmosphere
3.2. Laboratory Study: CO Generation in Organic-Rich Sedimentary Rocks
3.2.1. Host Rock Composition
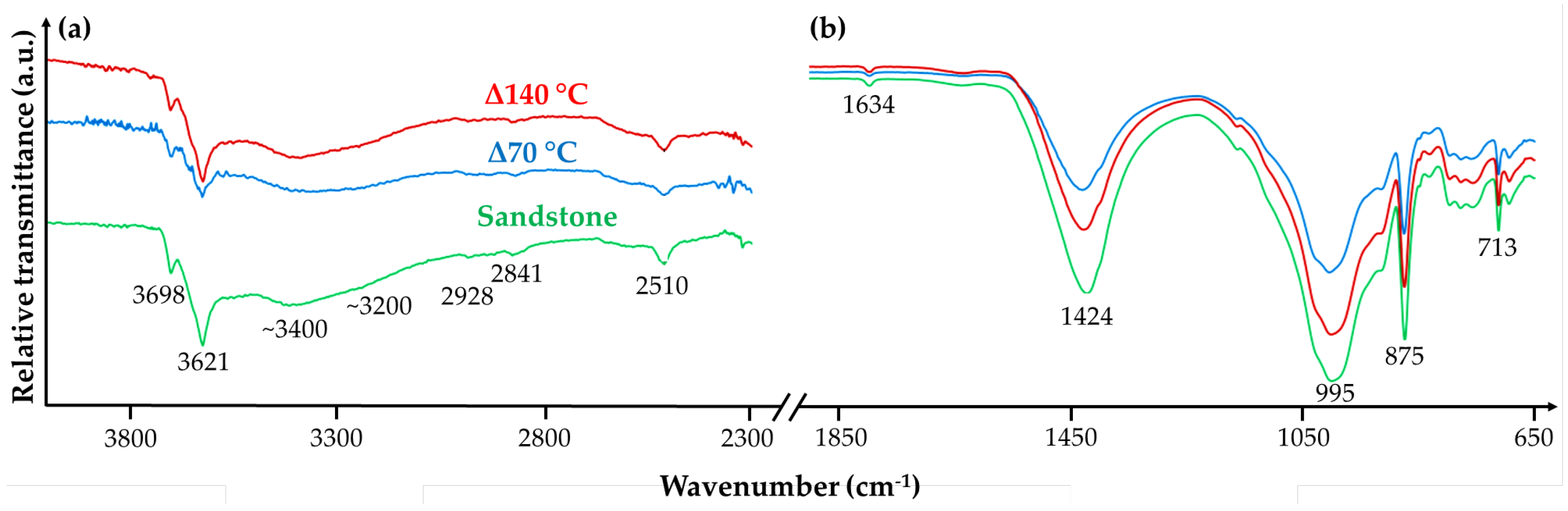
3.2.2. Alteration of Host Rocks upon Thermal Treatment
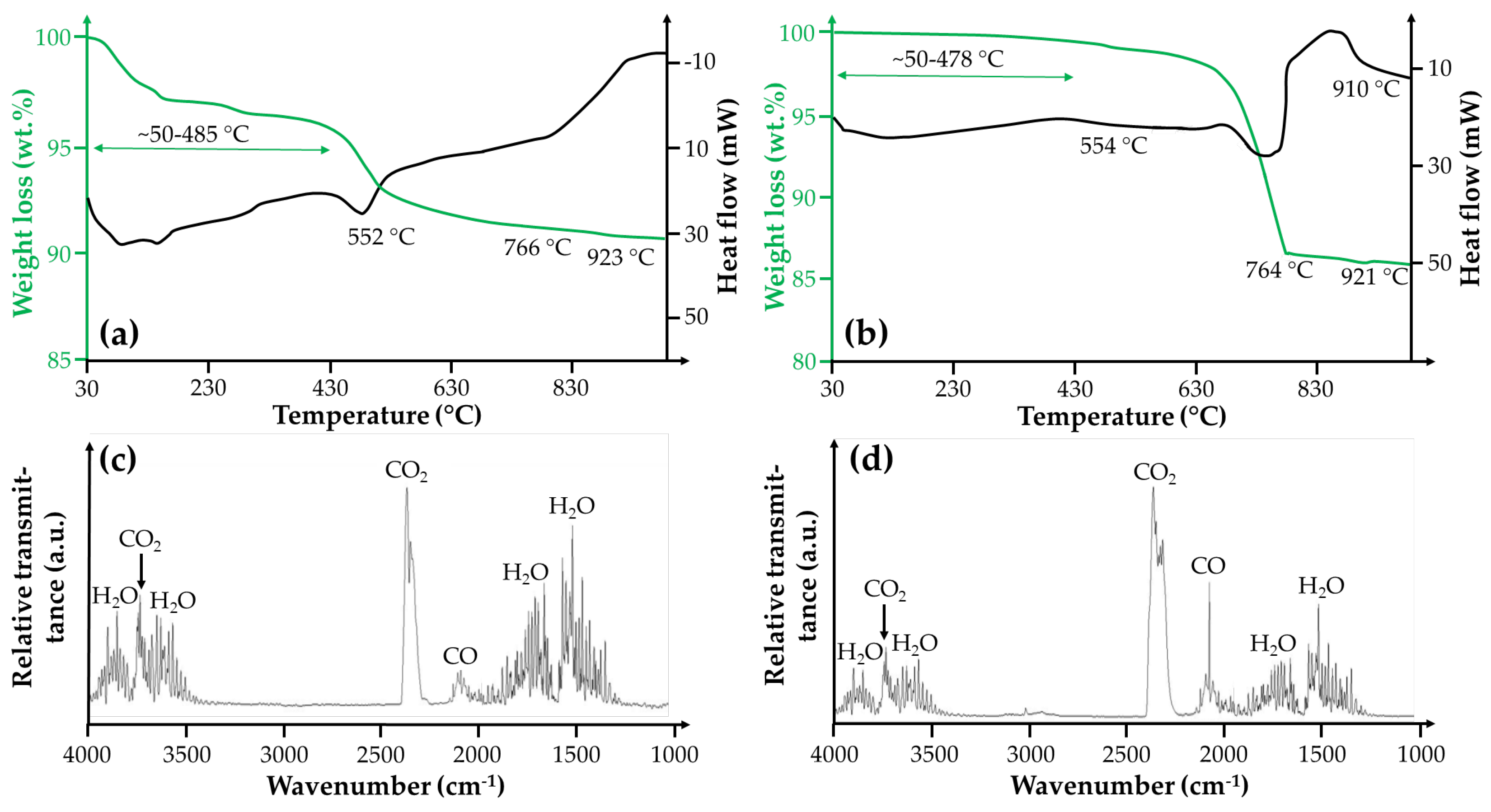
3.2.3. CO and CO2 Liberation from Host Rocks
4. Discussion
4.1. CO and CO2 Release Mechanisms
- The interaction of the TBM drill head (when operated in ‘closed’ mode; cf. Section 3.1.2) with the organic-rich Neogene sedimentary units (TOC: ~0.3 up to ~0.7 wt.%) could have caused particulate OM to incompletely decompose into CO and CO2 [65,66,67]. Such a thermal alteration process could have proceeded via frictional heat and ‘cold combustion’ at ~70 °C measured at the working face.
- Gaseous CO could have been temporarily generated during tunnel fires during the incomplete conversion of sedimentary OM into CO2 [13].
- The formation of CO-rich exhaust gases could have originated from tunneling machines with combustion engines [16].
4.2. Human Health Damage Assessment
5. Conclusions
- Sedimentary OM started to decompose at 50–70 °C and was subsequently converted into CO (5–1923 ppm) and then CO2 during mechanized tunnel driving.
- Frictional heat, cold combustion and the incomplete conversion of OM under oxygen-depleted, (semi-)closed conditions can act as important mechanisms for the release of CO to the tunnel atmosphere, especially if the OM is immature.
- Higher temperatures favor the maturation of OM and its thermal conversion into CO and CO2, as indicated by the thermal alteration of claystone and sandstone that were locally enriched in OM, but graphitic layers that are exposed in crystalline rocks can also bear a risk of CO liberation (up to ~200 ppm as documented in this tunnel).
- Further investigations, which may include the on-site monitoring of the tunnel atmosphere (e.g., using continuous data logging or remote transmission) and the analysis of the δ13C isotope signatures of host rock-associated OM and atmospheric CO and CO2 at selected hot spots, are necessary to quantify the carbon mass balances.
- Comparative studies with environmental and air monitoring on the complex enrichment mechanisms of CO in other tunnel projects under construction are required in order to achieve secure working conditions for tunnellers at exposed sites and to maintain the current sustainability and economic standards.
- The relevance of OM decomposition and gas phase developments (e.g., CO2, CO and CH4) in the course of tunneling should also not be underestimated with regard to the sustainable use of subsurface infrastructure, potentially causing unwanted mineral and biomass deposition in the drainage system or construction material alterations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence; Latest ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 1–702. [Google Scholar] [CrossRef]
- Berger, W.H.; Smetacek, V.S.; Wefer, G. Productivity of the Oceans: Present and Past; Latest ed.; Wiley-Interscience: New York, NY, USA, 1989; pp. 1–471. [Google Scholar] [CrossRef]
- Meyers, P.A. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem. Geol. 1994, 114, 289–302. [Google Scholar] [CrossRef]
- Hansell, D.A.; Carlson, C.A. Deep-ocean gradients in the concentration of dissolved organic carbon. Nature 1998, 395, 263–266. [Google Scholar] [CrossRef]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.D.; Zabel, M. Marine Geochemistry, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–574. [Google Scholar] [CrossRef]
- Regnier, P.; Resplandy, L.; Najjar, R.G.; Ciais, P. The land-to-ocean loops of the global carbon cycle. Nature 2022, 603, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hilton, R.G.; West, A.J. Mountains, erosion and the carbon cycle. Nat. Rev. Earth Environ. 2020, 1, 284–299. [Google Scholar] [CrossRef]
- Galan, I.; Baldermann, A.; Kusterle, W.; Dietzel, M.; Mittermayr, F. Durability of shotcrete for underground support—Review and update. Constr. Build. Mater. 2019, 202, 465–493. [Google Scholar] [CrossRef]
- Maie, N.; Anzai, S.; Tokai, K.; Kakino, W.; Taruya, H.; Ninomiya, H. Using oxygen/ozone nanobubbles for in situ oxidation of dissolved hydrogen sulfide at a residential tunnel-construction site. J. Environ. Manag. 2022, 302, 114068. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Shao, Z.; Xu, C.; Li, M.; Zhang, Y. Characteristics of methane-air explosions in large-scale tunnels with different structures. Tunn. Undergr. Space Technol. 2021, 109, 103767. [Google Scholar] [CrossRef]
- Luschnik, A. Bau des neuen Kaiser-Wilhelm-Tunnels unter schwierigen Verkehrsbedingungen. Bauportal 2011, 123, 17–21. [Google Scholar]
- Menéndez, J.; Merlé, N.; Fernández-Oro, J.M.; Galdo, M.; de Prado, L.Á.; Loredo, J.; Bernardo-Sánchez, A. Concentration, Propagation and Dilution of Toxic Gases in Underground Excavations under Different Ventilation Modes. Int. J. Environ. Res. Public Health 2022, 19, 7092. [Google Scholar] [CrossRef]
- Zamorategui-Molina, A.; Gutiérrez-Ortega, N.L.; Baltazar-Vera, J.C.; Del Ángel-Soto, J.; Tirado-Torres, D. Carbon Monoxide and Particulate Matter Concentrations inside the Road Tunnels of Guanajuato City, Mexico. Aerosol Air Qual. Res. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Luo, Y.; Shi, Z.; Ji, X.; Zhu, H. Study on the Annual Reduction Rate of Vehicle Emission Factors for Carbon Monoxide: A Case Study of Urban Road Tunnels in Shenzhen, China. Adv. Civ. Eng. 2020, 1686753, 1–17. [Google Scholar] [CrossRef]
- Yang, Q.; Shen, H.; Liang, Z. Analysis of particulate matter and carbon monoxide emission rates from vehicles in a Shanghai tunnel. Sustain. Cities Soc. 2020, 56, 102104. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines for Indoor Air Quality: Selected Pollutants; Latest ed.; University of California: Berkeley, CA, USA, 2010; pp. 1–454. [Google Scholar]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet Health 2022, 6, 535–547. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Cohen, A.; Smith, K.R. Addressing the Burden of Disease Attributable to Air Pollution in India: The Need to Integrate across Household and Ambient Air Pollution Exposures. Environ. Health Perspect. 2014, 122, 1–2. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). 2020 National Emissions Inventory (NEI) Technical Support Document (TSD); Latest ed.; EPA: Washington, DC, USA, 2020; Section 1–37.
- Kumar, A.; Malyan, V.; Sahu, M. Air Pollution Control Technologies for Indoor Particulate Matter Pollution: A Review. Aerosol Sci. Eng. 2023, 7, 261–282. [Google Scholar] [CrossRef]
- Grba, N.; Baldermann, A.; Dietzel, M. Novel green technology for wastewater treatment: Geo-material/geopolymer applications for heavy metal removal from aquatic media. Int. J. Sediment Res. 2023, 38, 33–48. [Google Scholar] [CrossRef]
- Chen, R.; Yin, P.; Meng, X.; Liu, C.; Wang, L.; Xu, X.; Ross, J.A.; Tse, L.A.; Zhao, Z.; Kan, H.; et al. Fine Particulate Air Pollution and Daily Mortality. Am. J. Respir. Crit. Care Med. 2017, 196, 73–81. [Google Scholar] [CrossRef]
- Ni, K.; Carter, E.; Schauer, J.J.; Ezzati, M.; Zhang, Y.; Niu, H.; Lai, A.M.; Shan, M.; Wang, Y.; Yang, X.; et al. Seasonal variation in outdoor, indoor, and personal air pollution exposures of women using wood stoves in the Tibetan Plateau: Baseline assessment for an energy intervention study. Environ. Int. 2016, 94, 449–457. [Google Scholar] [CrossRef]
- Levy, R.L. Carbon monoxide pollution and neurodevelopment: A public health concern. Neurotoxicol. Teratol. 2015, 49, 31–40. [Google Scholar] [CrossRef]
- Seo, H.J.; Chung, Y.H.; Song, T.J. An Experimental Study for Deriving Fire Risk Evaluation Factors for Cables in Utility Tunnels. Fire 2023, 6, 342. [Google Scholar] [CrossRef]
- Li, L.; Zhu, D.; Gao, Z.; Xu, P.; Zhang, W. A study on longitudinal distribution of temperature rise and carbon monoxide concentration in tunnel fires with one opening portal. Case Stud. Therm. Eng. 2021, 28, 101535. [Google Scholar] [CrossRef]
- Kwiecień, J.; Szopińska, K. Mapping Carbon Monoxide Pollution of Residential Areas in a Polish City. Remote Sens. 2020, 12, 2885. [Google Scholar] [CrossRef]
- Ali, M.U.; Yu, Y.; Yousaf, B.; Munir, M.A.M.; Ullah, S.; Zheng, C.; Kuang, X.; Wong, M.H. Health impacts of indoor air pollution from household solid fuel on children and women. J. Hazard. Mater. 2021, 416, 126127. [Google Scholar] [CrossRef] [PubMed]
- Grenzwerteverordnung 2021, BGBL. II Nr. 253/2001 (vgl. RIS—Rechtsinformationssystem des Bundes) Siehe v.a. Anhang I Stoffliste inkl. MAK-Werte und TRK-Werte. Available online: https://www.ris.bka.gv.at/Dokumente/BgblPdf/2001_253_2/2001_253_2.pdf (accessed on 10 September 2024).
- Coburn, R.F. The partial pressure of carbon monoxide in human tissues calculated using a parallel capillary-tissue cylinder model. J. Appl. Physiol. 2018, 124, 761–768. [Google Scholar] [CrossRef]
- Painer, F.; Baldermann, A.; Gallien, F.; Eichinger, S.; Steindl, F.; Dohrmann, R.; Dietzel, M. Synthesis of Zeolites from Fine-Grained Perlite and Their Application as Sorbents. Materials 2022, 15, 4474. [Google Scholar] [CrossRef]
- Eichinger, S.; Boch, R.; Leis, A.; Baldermann, A.; Domberger, G.; Schwab, C.; Dietzel, M. Green inhibitors reduce unwanted calcium carbonate precipitation: Implications for technical settings. Wat. Res. 2022, 208, 117850. [Google Scholar] [CrossRef]
- Kralik, M.; Zieritz, I.; Grath, J.; Vincze, G.; Philippitsch, R.; Pavlik, H. Hydrochemische Karte Österreichs—Oberflächennaher Grundwasserkörper und Fließgewässer; Mittelwerte von Wassergüteerhebungsdaten (WGEV-DATEN) 1991–2001; 2. Überarbeitete Auflage, Umweltbundesamt: Wien, Austria, 2005; pp. 1–19. [Google Scholar]
- Pfleiderer, S.; Reitner, H.; Leis, A. Availability, dynamics and chemistry of groundwater in the Bucklige Welt region of Lower Austria. Austrian J. Earth Sci. 2017, 110, 1–13. [Google Scholar] [CrossRef]
- Baldermann, A.; Dietzel, M.; Reinprecht, V. Chemical weathering and progressing alteration as possible controlling factors for creeping landslides. Sci. Total Environ. 2021, 778, 146300. [Google Scholar] [CrossRef]
- Pepper, A.S.; Dodd, T.A. Simple kinetic models of petroleum formation. Part II: Oil-gas cracking. Mar. Petrol. Geol. 1995, 12, 321–340. [Google Scholar] [CrossRef]
- Adegoke, A.K.; Hakimi, M.H.; Abdullah, W.H.; Ibrahim, E.-K.; Yandoka, B.M.S.; Faifi, H.A.; Lashin, A. Geochemistry and oil-gas generation modeling of the Late Cretaceous shales from the Chad (Bornu) Basin, Northeast Nigeria. J. Nat. Gas Sci. Eng. 2020, 79, 103341. [Google Scholar] [CrossRef]
- Stockhausen, M.; Galimberti, R.; Elias, R.; Di Paolo, L.; Schwark, L. Expulsinator assessment of oil/gas generation and expulsion characteristics of different source rocks. Mar. Petrol. Geol. 2021, 129, 105057. [Google Scholar] [CrossRef]
- Baldermann, A.; Stamm, F.M.; Farkaš, J.; Löhr, S.; Ratz, B.; Letofsky-Papst, I.; Dietzel, M. Precipitation of short-range order hydroxy aluminosilicate (HAS) and hydrous ferric silicate (HFS) at ambient temperature: Insights into mineral formation pathways, crystal chemistry and solubility-stability relationships. Chem. Geol. 2024, 646, 121911. [Google Scholar] [CrossRef]
- Han, S.; Löhr, S.C.; Abbott, A.N.; Baldermann, A.; Farkaš, J.; McMahon, W.; Milliken, K.L.; Rafiei, M.; Wheeler, C.; Owen, M. Earth system science applications of next-generation SEM-EDS automated mineral mapping. Front. Earth Sci. 2022, 10, 956912. [Google Scholar] [CrossRef]
- Baldermann, A.; Dohrmann, R.; Kaufhold, S.; Nickel, C.; Letofsky-Papst, I.; Dietzel, M. The Fe-Mg-saponite solid solution series—A hydrothermal synthesis study. Clay Miner. 2014, 49, 391–415. [Google Scholar] [CrossRef]
- Grba, N.; Grengg, C.; Petronijević, M.; Dietzel, M.; Baldermann, A. Substantial Copper (Cu2+) Uptake by Metakaolin-Based Geopolymer and Its Resistance to Acid Leaching and Ion Exchange. Polymers 2023, 15, 1971. [Google Scholar] [CrossRef]
- Oberhollenzer, S.; Hauser, L.; Baldermann, A.; Marte, R.; Tschuchnigg, F.; Schweiger, H.F.; Nachtnebel, M.; Dietzel, M. Microstructure development in lacustrine, fine-grained sediments traced by in situ and laboratory testing. Int. J. Environ. Sci. Technol. 2024, 21, 1–16. [Google Scholar] [CrossRef]
- Pettauer, M.; Baldermann, A.; Eder, S.; Dietzel, M. Hydration of MgO: Reaction Kinetics and pH Control on Brucite Crystal Morphology. Cryst. Growth Des. 2024, 24, 3085–3092. [Google Scholar] [CrossRef]
- Baldermann, A.; Mittermayr, F.; Bernasconi, S.M.; Dietzel, M.; Grengg, C.; Hippler, D.; Kluge, T.; Leis, A.; Lin, K.; Wang, X.; et al. Fracture dolomite as an archive of continental palaeo-environmental conditions. Commun. Earth Environ. 2020, 1, 35. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3-A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol. Surv. Tech. Methods 2013, 6, 497. [Google Scholar]
- Voigt, M.; Pearce, C.R.; Fries, D.M.; Baldermann, A.; Oelkers, E.H. Magnesium isotope fractionation during hydrothermal seawater-basalt interaction. Geochim. Cosmochim. Acta 2020, 272, 21–35. [Google Scholar] [CrossRef]
- Sellner, P.; Janotta, M.; Koinig, J. Systematic Drainage Measures in the Tunnel Construction—Experiences in the Neogene Sections of the Investigation Tunnels for the Koralm Tunnel. Geomech. Tunn. 2008, 1, 287–293. [Google Scholar] [CrossRef]
- Moritz, B.; Wagner, H.; Mussger, K.; Handke, D.; Harer, G. Criteria for the selection of tunnelling method through the example of the Koralm Tunnel. Geomech. Tunn. 2011, 4, 305–315. [Google Scholar] [CrossRef]
- Herg, A.; Stüwe, K. Tectonic interpretation of the metamorphic field gradient south of the Koralpe in the Eastern Alps. Austrian J. Earth Sci. 2018, 111/2, 155–170. [Google Scholar] [CrossRef]
- Wehrmeyer, G. Multi-Mode-TBM—Flexible Maschinentechnik für stark variierende Baugründe. In Innovationen im Spezialtiefbau: Fachseminar am 05. Dezember 2013 an der Technischen Universität Berlin; Universitätsverlag der TU Berlin: Berlin, Germany, 2013; pp. 55–74. Available online: https://api-depositonce.tu-berlin.de/server/api/core/bitstreams/e773622c-39e2-4a86-9dfc-1b3b4df1e2b9/content (accessed on 10 September 2024).
- Lasaga, A.C. Chemical kinetics of water-rock interactions. J. Geophys. Res. Solid Earth 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Luhmann, A.J.; Kong, X.-Z.; Saar, M.O.; Seyfried, W.E., Jr. CO2 sequestration in feldspar-rich sandstone: Coupled evolution of fluid chemistry, mineral reaction rates, and hydrogeochemical properties. Geochim. Cosmochim. Acta 2015, 160, 132–154. [Google Scholar] [CrossRef]
- Qin, F.; Beckingham, L.E. The impact of mineral reactive surface area variation on simulated mineral reactions and reaction rates. Appl. Geochem. 2021, 124, 104852. [Google Scholar] [CrossRef]
- Rossel, P.E.; Stubbins, A.; Rebling, T.; Koschinsky, A.; Hawkes, J.A.; Dittmar, T. Thermally altered marine dissolved organic matter in hydrothermal fluids. Org. Geochem. 2017, 110, 73–86. [Google Scholar] [CrossRef]
- Seewald, J.S.; Seyfried, W.E., Jr.; Thornton, E.C. Organic-rich sediment alteration: An experimental and theoretical study at elevated temperatures and pressures. Appl. Geochem. 1990, 5, 193–209. [Google Scholar] [CrossRef]
- Zhou, Z.; Cao, X.; Schmidt-Rohr, K.; Olk, D.C.; Zhuang, S.; Zhou, J.; Cao, Z.; Mao, J. Similarities in chemical composition of soil organic matter across a millennia-old paddy soil chronosequence as revealed by advanced solid-state NMR spectroscopy. Biol. Fertil. Soils 2014, 50, 571–581. [Google Scholar] [CrossRef]
- Derkowski, A.; Kuligiewicz, A. Rehydroxylation in smectites and other clay minerals observed in-situ with a modified thermogravimetric system. Appl. Clay Sci. 2017, 136, 219–229. [Google Scholar] [CrossRef]
- Barros, N.; Salgado, J.; Feijόo, S. Calorimetry and soil. Thermochim. Acta 2007, 458, 11–17. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Occasional Papers of the Geological Institute of Hungary: Budapest, Hungary, 2011; pp. 1–180. [Google Scholar]
- Che, C.; Glotch, T.D.; Bish, D.L.; Michalski, J.R.; Xu, W. Spectroscopic study of the dehydration and/or dehydroxylation of phyllosilicate and zeolite minerals. J. Geophys. Res. 2011, 116, E05007. [Google Scholar] [CrossRef]
- Azzolina-Jury, F.; Thibault-Starzyk, F. Mechanism of Low Pressure Plasma-Assisted CO2 Hydrogenation Over Ni-USY by Microsecond Time-resolved FTIR Spectroscopy. Top Catal. 2017, 60, 1709–1721. [Google Scholar] [CrossRef]
- Abelsohn, A.; Sanborn, M.D.; Jessiman, B.J.; Weir, E. Identifying and managing adverse environmental health effects: 6. Carbon monoxide poisoning. Can. Med. Assoc. J. 2002, 166, 1685–1690. [Google Scholar] [PubMed] [PubMed Central]
- Zuo, Y.; Jones, R.D. Photochemistry of natural dissolved organic matter in lake and wetland waters—Production of carbon monoxide. Wat. Res. 1997, 31, 850–858. [Google Scholar] [CrossRef]
- Koppmann, R.; von Czapiewski, K.; Reid, J.S. A review of biomass burning emissions, part I: Gaseous emissions of carbon monoxide, methane, volatile organic compounds, and nitrogen containing compounds. Atmos. Chem. Phys. Discuss. 2005, 5, 10455–10516. [Google Scholar] [CrossRef]
- Hellebrand, H.J.; Schade, G.W. Carbon Monoxide from Composting due to Thermal Oxidation of Biomass. J. Environ. Qual. 2008, 37, 592–598. [Google Scholar] [CrossRef]
- Barker, C.; Smith, M.P. Mass Spectrometric Determination of Gases in Individual Fluid Inclusions in Natural Minerals. Anal. Chem. 1986, 58, 1330–1333. [Google Scholar] [CrossRef]
- Fantke, E.P.; Bijster, M.; Guignard, C.; Hauschild, M.; Huijbregts, M.; Jolliet, O.; Kounina, A.; Magaud, V.; Margni, M.; Mckone, T.; et al. UNEP/SETAC scientific consensus model for characterizing human toxicological and ecotoxicological impacts of chemical emissions in life cycle assessment. In USEtox® 2.0 Documentation; Version 1.1; USEtox®; International Center Hosted at the Technical University of Denmark: Lyngby, Demark, 2017. [Google Scholar]
- Du, C.; Kulay, L.; Cavalett, O.; Dias, L.; Freire, F. Life cycle assessment addressing health effects of particulate matter of mechanical versus manual sugarcane harvesting in Brazil. Int. J. Life Cycle Assess. 2018, 23, 787–799. [Google Scholar] [CrossRef]
- Yan, Q.; Yang, K.; Wu, W.; Wang, F.; He, F. Prevention and control of gas hazards in a tunnel under construction: A case study. Environ. Earth Sci. 2020, 79, 317. [Google Scholar] [CrossRef]
- Reid, J.M.; Cripps, J.C. Geochemical lessons from Carsington Dam failure of 1984 and reconstruction. Q. J. Eng. Geol. Hydrogeol. 2019, 52, 414–424. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Yin, F.; Wang, G.; Chen, H.; He, C.; Xu, Y. Supercritical water oxidation of oil-based drill cuttings. J. Hazard. Mater. 2017, 332, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Yang, Y.; Zhang, H.; Zhang, J.; Huang, Y. A critical review of the possible effects of physical and chemical properties of subcritical water on the performance of water-based drilling fluids designed for ultra-high temperature and ultra-high pressure drilling applications. J. Petrol. Sci. Eng. 2020, 187, 106795. [Google Scholar] [CrossRef]

| Component | Claystone | Δ70 °C | Δ140 °C | Sandstone | Δ70 °C | Δ140 °C |
|---|---|---|---|---|---|---|
| Quartz | 31 | 2 | 3 | 57 | 1 | 3 |
| Muscovite | 21 | 1 | 4 | 6 | 2 | 3 |
| Orthoclase | 2 | 0 | −2 | 1 | 0 | −1 |
| Plagioclase | 3 | 0 | 0 | 6 | 0 | −1 |
| Kaolinite | 10 | 0 | −1 | 4 | −1 | −1 |
| Smectite | 25 | −2 | −3 | 8 | −1 | −1 |
| Calcite | 1 | 0 | 0 | 9 | 0 | −1 |
| Dolomite | 1 | 0 | 0 | 5 | 0 | 0 |
| Chlorite | 5 | −1 | −1 | 4 | −1 | −1 |
| SUM | 100 | Δ0 | Δ0 | 100 | Δ0 | Δ0 |
| TOC | 0.3 | 0.1 | 0.1 | 0.7 | 0.2 | 0.1 |
| OM | 0.6 | 0.2 | 0.2 | 1.5 | 0.4 | 0.2 |
| Component | Claystone | Δ70 °C | Δ140 °C | Sandstone | Δ70 °C | Δ140 °C |
|---|---|---|---|---|---|---|
| SiO2 | 48.6 | −0.9 | −1.2 | 58.6 | −0.8 | −1.4 |
| Al2O3 | 24.1 | −0.1 | −0.2 | 7.6 | −0.1 | −0.2 |
| Fe2O3 | 11.3 | −0.9 | −1.1 | 2.1 | −0.1 | −0.2 |
| MgO | 2.4 | <0.1 | <0.1 | 1.0 | <0.1 | <0.1 |
| K2O | 2.3 | 0.4 | 0.5 | 0.9 | 0.4 | 0.3 |
| CaO | 1.0 | −0.1 | −0.1 | 15.7 | −0.1 | −1.0 |
| Na2O | 0.3 | <0.1 | <0.1 | 0.7 | <0.1 | <0.1 |
| P2O5 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| SO3 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| TiO2 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| MnO | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| LOI | 9.9 | 1.6 | 2.2 | 13.4 | 0.7 | 2.5 |
| SUM | 100.0 | Δ0.0 | Δ0.0 | 100.0 | Δ0.0 | Δ0.0 |
| Claystone | Sandstone | |||
|---|---|---|---|---|
| Component | Δ70 °C | Δ140 °C | Δ70 °C | Δ140 °C |
| Na (mg/L) | 233.4 | 296.7 | 240.0 | 263.3 |
| K (mg/L) | 15.1 | 15.7 | 13.7 | 19.4 |
| Mg (mg/L) | 5.9 | 5.6 | 2.3 | 3.2 |
| Ca (mg/L) | 36.6 | 50.6 | 25.8 | 34.2 |
| Cl (mg/L) | 354.3 | 458.4 | 360.1 | 459.2 |
| HCO3 (mg/L) | 223.4 | 433.8 | 242.4 | |
| SO4 (mg/L) | 5.1 | 16.0 | 1.0 | 2.2 |
| Al (µg/L) | 318 | 350 | 175 | 225 |
| Ba (µg/L) | 171 | 397 | 8 | 24 |
| Co (µg/L) | 1 | 1 | 1 | 1 |
| Cr (µg/L) | 1 | 1 | 7 | 17 |
| Cu (µg/L) | 1 | 1 | 1 | 1 |
| Fe (µg/L) | 22 | 80 | 18 | 111 |
| Li (µg/L) | 260 | 633 | 23 | 47 |
| Mn (µg/L) | 89 | 454 | 3 | 5 |
| Ni (µg/L) | 1 | 1 | 5 | 6 |
| Si (µg/L) | 3465 | 5476 | 4687 | 6789 |
| Sr (µg/L) | 349 | 491 | 66 | 69 |
| Zn (µg/L) | 14 | 21 | 3 | 13 |
| pH (-) | 6.5 | 5.8 | 6.6 | 5.8 |
| EC (mol/kg) | 1.38·10−2 | 1.64·10−2 | 1.31·10−2 | 1.48·10−2 |
| ICB (%) | 1.45 | 2.61 | −0.99 | 0.22 |
| Claystone | Sandstone | |||
|---|---|---|---|---|
| SI (-) | Δ70 °C | Δ140 °C | Δ70 °C | Δ140 °C |
| Albite | –2.3 | –2.7 | –2.1 | –2.7 |
| Anorthite | –3.7 | –3.6 | –3.9 | –4.1 |
| Calcite | –0.6 | –1.2 | –0.7 | –1.5 |
| Clinochlore | –5.4 | –8.3 | –6.9 | –10.3 |
| Gibbsite | 1.9 | 1.8 | 1.6 | 1.5 |
| Hematite | 13.6 | 15.4 | 13.6 | 15.6 |
| Illite | 1.2 | 0.6 | 1.0 | 0.2 |
| K-Feldspar | –1.3 | –2.0 | –1.1 | –2.0 |
| Montmorillonite | –0.3 | –0.8 | –0.3 | –1.1 |
| Muscovite | 4.3 | 3.6 | 3.9 | 3.1 |
| Quartz | –0.9 | –0.9 | –0.8 | –0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldermann, A.; Boch, R.; Reinprecht, V.; Baldermann, C. Mechanism of Carbon Monoxide (CO) Generation and Potential Human Health Hazard during Mechanized Tunnel Driving in Organic-Rich Rocks: Field and Laboratory Study. Sustainability 2024, 16, 8107. https://doi.org/10.3390/su16188107
Baldermann A, Boch R, Reinprecht V, Baldermann C. Mechanism of Carbon Monoxide (CO) Generation and Potential Human Health Hazard during Mechanized Tunnel Driving in Organic-Rich Rocks: Field and Laboratory Study. Sustainability. 2024; 16(18):8107. https://doi.org/10.3390/su16188107
Chicago/Turabian StyleBaldermann, Andre, Ronny Boch, Volker Reinprecht, and Claudia Baldermann. 2024. "Mechanism of Carbon Monoxide (CO) Generation and Potential Human Health Hazard during Mechanized Tunnel Driving in Organic-Rich Rocks: Field and Laboratory Study" Sustainability 16, no. 18: 8107. https://doi.org/10.3390/su16188107
APA StyleBaldermann, A., Boch, R., Reinprecht, V., & Baldermann, C. (2024). Mechanism of Carbon Monoxide (CO) Generation and Potential Human Health Hazard during Mechanized Tunnel Driving in Organic-Rich Rocks: Field and Laboratory Study. Sustainability, 16(18), 8107. https://doi.org/10.3390/su16188107






