Effect of Silver Nanoparticles and Vermicompost on the Control of Longidorus elongatus (De Man, 1876) in Miscanthus × Giganteus and Its Growth and Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
- Control (only water was added);
- 1 L of solutions of Ag-NPs (dose 60 mg per 1 L soil);
- 20 L of vermicompost (Ve) produced by E. fetida with 150–200 earthworms.

2.2. Silver Nanoparticles Application
2.3. Vermicompost Preparation
2.4. Soil Analysis
2.5. Nematode Isolation and Analysis
2.6. Miscanthus × Giganteus Traits Measurments
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewandowski, I.; Clifton-Brown, J.; Kiesel, A.; Hastings, A.; Iqbal, Y. Miscanthus. In Perennial Grasses for Bioenergy and Bioproducts; Alexopoulou, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–59. [Google Scholar]
- Ben Fradj, N.; Rozakis, S.; Borzęcka, M.; Matyka, M. Miscanthus in the European Bio-Economy: A Network Analysis. Ind. Crops Prod. 2020, 148, 112281. [Google Scholar] [CrossRef]
- Borkowska, H.; Molas, R. Yield Comparison of Four Lignocellulosic Perennial Energy Crop Species. Biomass Bioenergy 2013, 51, 145–153. [Google Scholar] [CrossRef]
- Rusinowski, S.; Krzyzak, J.; Sitko, K. Cultivation of C4 perennial energy grasses on heavy metal contaminated arable land: Impact on soil, biomass, and photosynthetic traits. Environ. Pollut. 2019, 250, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Drazic, G.; Milovanovic, J.; Stefanovic, S.; Petric, I. Potential of Miscanthus × giganteus for heavy metals removing from industrial deposol. Acta Reg. Environ. 2017, 2, 56–58. [Google Scholar] [CrossRef][Green Version]
- Nsangawimana, F.; Pourrut, B.; Waterlot, C.; Louvel, B.; Bidar, G.; Labidi, S.; Fontaine, J.; Muchembled, J.; Lounes-Hadj, S.A.; Fiourrier, H.; et al. Metal accumulation and shoot yield of Miscanthus × giganteus growing in contaminated agricultural soils: Insights into agronomic practices. Agric. Ecosyst. Environ. 2015, 213, 61–71. [Google Scholar] [CrossRef]
- Nebeská, D.; Trögl, J.; Pidlisnyuk, V.; Popelka, J.; Veronesi Dáňová, P.; Usťak, S.; Honzík, R. Effect of Growing Miscanthus × giganteus on Soil Microbial Communities in Post-Military Soil. Sustainability 2018, 10, 4021. [Google Scholar] [CrossRef]
- Shah, T.M.; Khan, A.H.; Nicholls, C.; Sohoo, I.; Otterpohl, R. Using Landfill Sites and Marginal Lands for Socio-Economically Sustainable Biomass Production through Cultivation of Non-Food Energy Crops: An Analysis Focused on South Asia and Europe. Sustainability 2023, 15, 4923. [Google Scholar] [CrossRef]
- Mazur, A.; Kowalczyk-Juśko, A. The Assessment of the Usefulness of Miscanthus × giganteus to Water and Soil Protection against Erosive Degradation. Resources 2021, 10, 66. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public Health 2013, 2013, 58764. [Google Scholar] [CrossRef]
- Bourgeois, E.; Dequiedt, S.; Lelièvre, M.; van Oort, F.; Lamy, I.; Ranjard, L.; Maron, P.A. Miscanthus bioenergy crop stimulates nutrient-cycler bacteria and fungi in wastewater-contaminated agricultural soil. Environ. Chem. Lett. 2015, 13, 503–511. [Google Scholar] [CrossRef]
- Andrejić, G.; Šinžar-Sekulić, J.; Prica, M.; Gajić, G.; Dželetović, Ž.; Rakić, T. Assessment of the adaptive and phytoremediation potential of Miscanthus × giganteus grown in flotation tailings. Arch. Biol. Sci. 2019, 71, 687–696. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide Research on Plant Defense against Biotic Stresses as Improvement for Sustainable Agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef]
- Sikandar, A.; Wu, F.; He, H.; Ullah, R.M.K.; Wu, H. Growth, Physiological, and Biochemical Variations in Tomatoes after Infection with Different Density Levels of Meloidogyne enterolobii. Plants 2024, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Orlando, V.; Collett, R.L.; Moreira, D.; Costa, S.R.; Inácio, M.L. Linking Nematode Communities and Soil Health under Climate Change. Sustainability 2023, 15, 11747. [Google Scholar] [CrossRef]
- Singh, S.; Awasthi, L.P.; Jangre, A.; Nirmalkar, V.K. Transmission of Plant Viruses through Soil-Inhabiting Nematode Vectors. In Applied Plant Virology. Advances, Detection, and Antiviral Strategies; Awasthi, L.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 291–300. [Google Scholar]
- Peneva, V.; Urek, G.; Lazarova, S.; Širca, S.; Knapič, M.; Elshishka, M.; Brown, D. Longidoridae and Nepoviruses in Bulgaria and Slovenia. Helminthologia 2012, 49, 49–56. [Google Scholar] [CrossRef]
- Zhukov, O.; Yorkina, N.; Budakova, V.; Kunakh, O. Terrain and Tree Stand Effect on the Spatial Variation of the Soil Penetration Resistance in Urban Park. Int. J. Environ. Stud. 2015, 79, 485–501. [Google Scholar] [CrossRef]
- Stefanovska, T.; Skwiercz, A.; Zouhar, M.; Pidlisnyuk, V.; Zhukov, O. Plant-Feeding Nematodes Associated with Miscanthus × Giganteus and Their Use as Potential Indicators of the Plantations’ State. Int. J. Environ. Sci. Technol. 2021, 18, 57–72. [Google Scholar] [CrossRef]
- Stefanovska, T.; Skwiercz, A.; Flis, Ł.; Pidlisnyuk, V.; Zouhar, M. First Record of the Ectoparasitic Nematode Amplimerlinius Macrurus (Nematoda: Tylenchida) on the Perennial Grass Miscanthus × Giganteus (Angiosperms: Poaceae) in Ukraine. J. Nematol. 2021, 53, e2021-24. [Google Scholar] [CrossRef]
- Mekete, T.; Gray, M.E.; Niblack, T.L. Distribution, morphological description, and molecular characterization of Xiphinema and Longidorous spp. associated with plants, Miscanthus spp. and Panicum virgatum used for biofuels. Glob. Chang. Biol. Bioenergy 2009, 1, 257–266. [Google Scholar] [CrossRef]
- Mekete, T.; Reynolds, K.; Lopez-Nicora, H.D.; Gray, M.E.; Niblack, T.L. Distribution and diversity of root-lession nematode, Pratylenchus spp. associated with Miscanthus × giganteus and Panicum virgatum used for biofuels, and species identifcation in a multiplex polymerase chain reaction. Nematology 2011, 13, 673–686. [Google Scholar] [CrossRef]
- Sasanelli, N.; Konrat, A.; Migunova, V.; Toderas, I.; Iurcu-Straistaru, E.; Rusu, S.; Bivol, A.; Andoni, C.; Veronico, P. Review on Control Methods against Plant Parasitic Nematodes Applied in Southern Member States (C Zone) of the European Union. Agriculture 2021, 11, 602. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Zapałowska, A.; Skwiercz, A.; Damszel, M.; Telesiński, A.; Sierota, Z.; Gorczyca, A. An Evaluation of Selected Chemical, Biochemical, and Biological Parameters of Soil Enriched with Vermicompost. Environ. Sci. Pollut. Res. 2021, 28, 8117–8127. [Google Scholar] [CrossRef] [PubMed]
- Przemieniecki, S.W.; Skwiercz, A.; Damszel, M.; Telesiński, A.; Zapałowska, A.; Sierota, Z.; Gorczyca, A. Ecology, Biology and Enzymatic Activity of the Rhizosphere Planted with Larix Decidua Seedlings after Addition of Vermicompost. Appl. Soil Ecol. 2021, 168, 104101. [Google Scholar] [CrossRef]
- Singh, A.; Singh, D.P.; Tiwari, R.; Kumar, K.; Singh, R.V.; Singh, S.; Prasanna, R.; Saxena, A.K.; Nain, L. Taxonomic and Functional Annotation of Gut Bacterial Communities of Eisenia foetida and Perionyx excavatus. Microbiol. Res. 2015, 175, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Zahoor, M.; Sher Khan, R.; Ikram, M.; Islam, N.U. The Impact of Silver Nanoparticles on the Growth of Plants: The Agriculture Applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef]
- Fouda, M.M.G.; Abdelsalam, N.R.; Gohar, I.M.A.; Hanfy, A.E.M.; Othman, S.I.; Zaitoun, A.F.; Allam, A.A.; Morsy, O.M.; El-Naggar, M. Utilization of High Throughput Microcrystalline Cellulose Decorated Silver Nanoparticles as an Eco-Nematicide on Root-Knot Nematodes. Colloids Surf. B Biointerfaces 2020, 188, 110805. [Google Scholar] [CrossRef]
- Daramola, F.; Lewu, N.; Nkiko, J.; Lewu, F. Nematicidal Effects of Silver Nanoparticles (AG-NPs) on the Root-Knot Nematode, Meloidogyne Javanica Associated with Swiss Chard (Beta vulgaris L.). Helminthologia 2023, 60, 189–195. [Google Scholar] [CrossRef]
- Silva, L.P.; Silveira, A.P.; Bonatto, C.C.; Reis, I.G.; Milreu, P.V. Silver Nanoparticles as Antimicrobial Agents: Past, Present, and Future. In Nanostructures for Antimicrobial Therapy; Nanostructures in Therapeutic Medicine Series; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–596. [Google Scholar]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Kabała, C.; Karczewska, A. Methodology for Laboratory Analysis of Soils and Plants, 8th ed.; Institute of Soil and Environmental Protection, University of Life Sciences: Wrocław, Poland, 2019. (In Polish) [Google Scholar]
- Skwiercz, A.; Zapałowska, A.; Flis, Ł.; Koc-Jurczyk, J.; Jurczyk, Ł.; Litwińczuk, W.; Puchalski, C. Plant Parasitic Nematodes on Paulownia tomentosa in Poland. J. Hortic. Res. 2022, 30, 31–40. [Google Scholar] [CrossRef]
- Brzeski, M.W. Nematodes of Tylenchinain Poland and Temperate Europe; Muzeum i Instytutu Zoologii, Polska Akademia Nauk (MiIZ PAN): Warsaw, Poland, 1998; Available online: https://miiz.waw.pl/pl/wydawnictwa/spis-artykulow/19-serie-wydawnicze-ksiki-dvd/597-nematodes (accessed on 1 December 2023).
- Andrassy, I. Free Living Nematodes of Hungary (Nematoda: Errantia), II; Hungarian Natural History Museum and Systematic Zoology, Group of the Hungarian Academy of Sciences: Budapest, Hungry, 2007; p. 469. Available online: https://revistaselectronicas.ujaen.es/index.php/jnms/article/view/610 (accessed on 1 December 2023).
- Kumar, S.; Kumar, R.; Sood, P. Role of Microbial Enriched Vermicompost in Plant-Parasitic Nematode Management. In Nematodes-Recent Advances, Management and New Perspectives; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Walia, S.S.; Kaur, T. Role of Earthworms in Vermicomposting. In Earthworms and Vermicomposting; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Gabour, E.I.; Marahatta, S.P.; Lau, J.-W. Vermicomposting: A potential management approach for the reniform nematode, Rotylenchulus reniformis. Nematropica 2015, 45, 285–287. [Google Scholar]
- Edwards, C.A.; Arancon, N.Q.; Emerson, E.; Pulliam, R. Suppression of plant-parasitic nematodes and arthropod pests by vermicompost teas. Biocycle 2007, 48, 38–39. [Google Scholar]
- Rodríguez-Kábana, R. Organic and inorganic nitrogen amendments to soil as nematode suppressants. J. Nematol. 1986, 18, 129–135. [Google Scholar]
- Kerry, B. Fungal parasites of cysts nematodes. In Biological Interaction in Soils; Edwards, C.A., Stinner, B.R., Stinner, D., Rabatin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 293–306. [Google Scholar]
- Siddiqui, Z.A.; Mahmood, I. Role of bacteria in the management of plant-parasitic nematodes: A review. Bioresour. Technol. 1999, 69, 167–179. [Google Scholar] [CrossRef]
- Bilgrami, L. Evaluation of the predation abilities of the mite Hypoaspis calcuttaensis, predaceous on plant and soil nematodes. Fundam. Appl. Nematol. 1997, 20, 96–97. [Google Scholar]
- Heckmann, L.-H.; Hovgaard, M.B.; Sutherland, D.S.; Autrup, H.; Besenbacher, F.; Scott-Fordsmand, J.J. Limit-Test Toxicity Screening of Selected Inorganic Nanoparticles to the Earthworm Eisenia fetida. Ecotoxicology 2011, 20, 226–233. [Google Scholar] [CrossRef]
- Hu, C.W.; Li, M.; Cui, Y.B.; Li, D.S.; Chen, J.; Yang, L.Y. Toxicological Effects of TiO2 and ZnO Nanoparticles in Soil on Earthworm Eisenia fetida. Soil Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Van der Ploeg, M.J.C.; Baveco, J.M.; van der Hout, A.; Bakker, R.; Rietjens, I.M.C.M.; van den Brink, N.W. Effects of C60 Nanoparticle Exposure on Earthworms (Lumbricus rubellus) and Implications for Population Dynamics. Environ. Pollut. 2011, 159, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Shoults-Wilson, W.A.; Reinsch, B.C.; Tsyusko, O.V.; Bertsch, P.M.; Lowry, G.V.; Unrine, J.M. Role of Particle Size and Soil Type in Toxicity of Silver Nanoparticles to Earthworms. Soil Sci. Soc. Am. J. 2011, 75, 365–377. [Google Scholar] [CrossRef]
- Shoults-Wilson, W.A.; Zhurbich, O.I.; McNear, D.H.; Tsyusko, O.V.; Bertsch, P.M.; Unrine, J.M. Evidence for Avoidance of Ag Nanoparticles by Earthworms (Eisenia fetida). Ecotoxicology 2011, 20, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska, M.; Skwiercz, A.; Wesołowska, A.; Kozacki, D.; Przewodowski, W.; Kulpa, D. Effects of Silver, Gold, and Platinum Nanoparticles on Selected Nematode Trophic Groups. J. Hortic. Res. 2023, 31, 23–34. [Google Scholar] [CrossRef]
- Baronia, R.; Kumar, P.; Singh, S.P.; Walia, R.K. Silver Nanoparticles as a Potential Nematicide against Meloidogyne graminicola. J. Nematol. 2020, 52, e2020-02. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.C. Potential Nematicidial Activity of Silver Nanoparticles Against the Root-Knot Nematode (Meloidogyne incognita). Online J. Complement. Altern. Med. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Zuhair, R.; Moustafa, Y.T.A.; Mustafa, N.S.A.; El-Dahshouri, M.F.; Zhang, L.; Ageba, M.F. Efficacy of Amended Vermicompost for Bio-Control of Root Knot Nematode (RKN) Meloidogyne Incognita Infesting Tomato in Egypt. Environ. Technol. Innov. 2022, 27, 102397. [Google Scholar] [CrossRef]
- Renčo, M.; Kováčik, P. Assessment of the Nematicidal Potential of Vermicompost, Vermicompost Tea, and Urea Application on the Potato-Cyst Nematodes Globodera Rostochiensis and Globodera Pallida. J. Plant Prot. Res. 2015, 55, 187–192. [Google Scholar] [CrossRef][Green Version]
- Rostami, M.; Karegar, A.; Taghavi, S.M. Biocontrol Potential of Bacterial Isolates from Vermicompost and Earthworm against the Root-Knot Nematode Meloidogyne Javanica Infecting Tomato Plants. Egypt. J. Biol. Pest Control. 2021, 31, 36. [Google Scholar] [CrossRef]
- Tikoria, R.; Kaur, A.; Ohri, P. Amelioration of Oxidative Stress and Growth Enhancement by Application of Vermicompost via Modulating Phyto-Constituents in Tomato Plants during Nematode Stress. J. Soil Sci. Plant Nutr. 2023, 23, 3944–3960. [Google Scholar] [CrossRef]
- Gorczyca, A.; Przemieniecki, S.W.; Oćwieja, M. Comparative effect of silver nanoparticles on maize rhizoplane microbiome in initial phase of plants growth. Int. Agrophys. 2024, 38, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Pallavi; Mehta, C.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Seif, S.M.; Sorooshzadeh, A.H.; Rezazadeh, S.; Naghdibadi, H.A. Effect of nano silver and silver nitrate on seed yield of borage. J. Med. Plants Res. 2011, 5, 706–710. [Google Scholar] [CrossRef]
- Sillen, W.M.; Thijs, S.; Abbamondi, G.R.; Janssen, J.; Weyens, N.; White, J.C.; Vangronsveld, J. Effects of silver nanoparticles on soil microorganisms and maize biomass are linked in the rhizosphere. Soil Biol. Biochem. 2015, 91, 14–22. [Google Scholar] [CrossRef]
- Sable, S.V.; Ranade, S.; Joshi, S. Role of AgNPs in the Enhancement of Seed Germination and Its Effect on Plumule and Radicle Length of Pennisetum Glaucum. IET Nanobiotechnol. 2018, 12, 922–926. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; Brooks, M.; Gerfen, J.; Wang, Q.; Fotis, C.; Sparer, A.; Ma, X.; Berg, R.; Geisler, M. Reproductive Toxicity and Life History Study of Silver Nanoparticle Effect, Uptake and Transport in Arabidopsis Thaliana. Nanomaterials 2014, 4, 301–318. [Google Scholar] [CrossRef]
- Ebrayat, J.M.; Moudilou, E.N.; Lapied, E. Harmful effects of nanoparticles on animals. J. Nanotechnol. 2015, 2015, 861092. [Google Scholar] [CrossRef]
- Kucharska, K.; Pezowicz, E.; Tumialis, D.; Barkowska, M. Effect of silver nanoparticles on the mortality and pathogenicity of entomopathogenicnematodes. Ecol. Chem. Eng. 2011, 18, 1065–1070. [Google Scholar]
- Kucharska, K.; Zajdel, B.; Pezowicz, E.; Jarmuł-Pietraszczyk, J.; Mazurkiewicz, A.; Tumialis, D. Control of thelesser mealworm Alphitobius diaperinus using entomopathogenic nematodes (EPNs) combined withnanoparticles. Ann. Wars. Univ. LifeSci. SGGW Anim. Sci. 2016, 55, 57–67. [Google Scholar]
- Tha, E.H.; Abo-Shady, N.M. Effect of silver nanoparticles on the mortality pathogenicity and re-productivity of entomopathogenic nematodes. Int. J. Zool. Res. 2016, 12, 47–50. [Google Scholar] [CrossRef]
- Rehman, S.U.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: Enhancing Plant Growth and Combating Abiotic and Biotic Stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Zapałowska, A.; Skwiercz, A.; Puchalski, C.; Malewski, T. Influence of Eisenia fetida on the Nematode Populations during Vermicomposting Process. Appl. Sci. 2024, 14, 1576. [Google Scholar] [CrossRef]
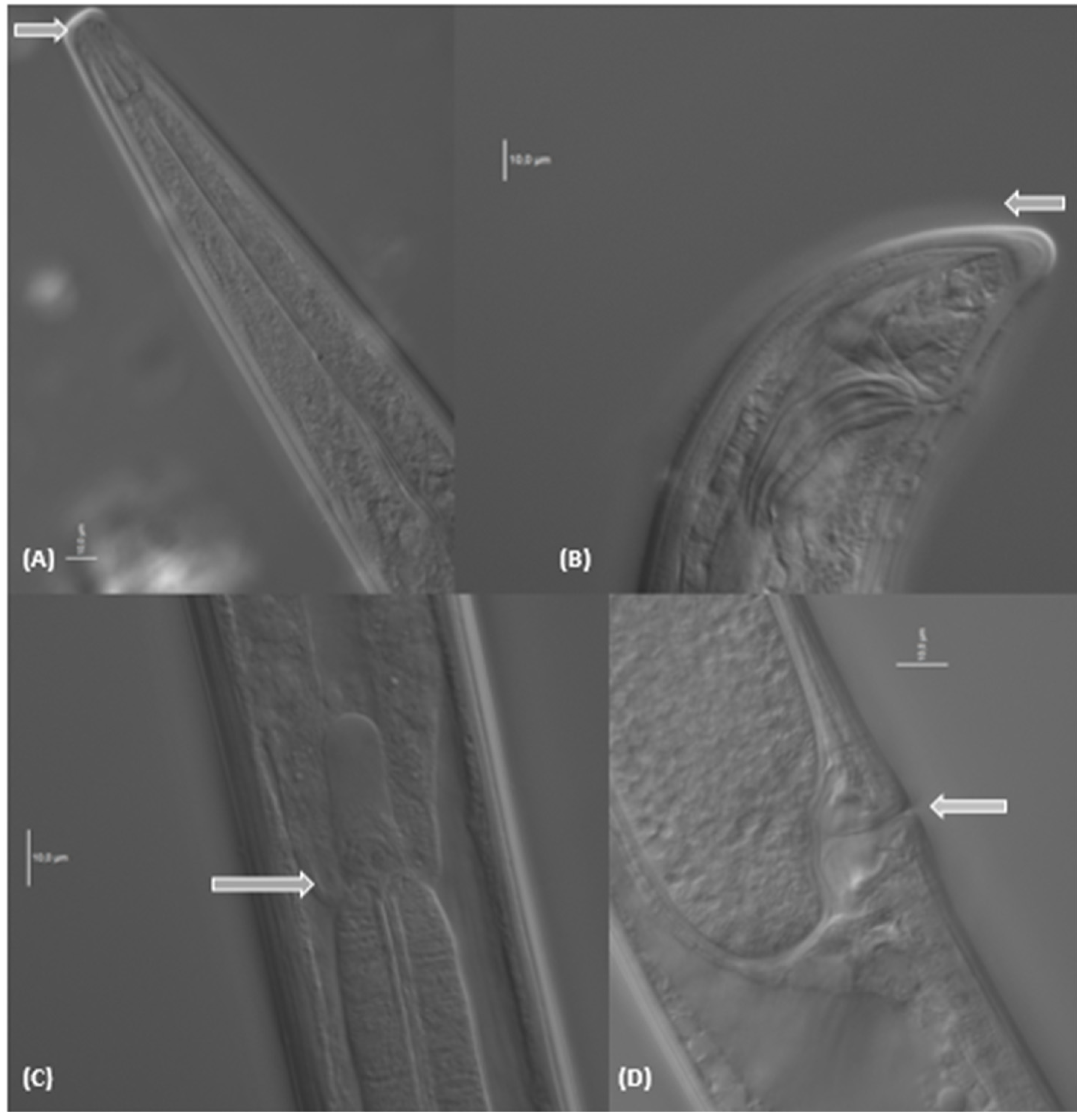
| Location: Kosakowo, Poland (54°37′02.00″ N 18°26′05.10″ E) Host Plant: Miscantus × giganteus | ||
|---|---|---|
| Character | Female | Male |
| n * | 50 | 50 |
| L | 5.25 ± 0.56 (4.88–6.80) | 5.15 ± 0.51 (4.29–5.94) |
| a | 97.5 ± 5.80 (89.4–109.4) | 101.2 ± 6.06 (95.3–108.3) |
| b | 12.6 ± 1.74 (10.8–18.2) | 11.8 ± 1.65 (9.3–13.4) |
| c | 112.5 ± 16.79 (96.2–160.0) | 94.3 ± 14.1 (82.3–110.2) |
| c’ | 1.19 ± 0.10 (1.00–1.39) | 1.40 ± 0.19 (1.24–1.52) |
| V/Spicules length | 48.5 ± 1.8 (44.2–52.4) | 59 ± 2.1 (49–61) |
| Odontostylet length | 85.2 ± 6.1 (78–95) | 82.5 ± 5.7 (80–89) |
| Odontophore length | 65.6 ± 3.1 (59–73) | 68 ± 3.2 (67–69) |
| Total stylet length | 148.6 ± 43.5 (138–162) | 150.5 ± 43.6 (147–158) |
| Anterior end to guide ring | 32.0 ± 1.2 (30–34) | 32.8 ± 1.2 (31–34) |
| Pharyngeal bulb length | 113.4 ± 5.8 (104–122) | 115 ± 5.9 (107–120) |
| Pharyngeal bulb width | 20.0 ± 2.0 (18–22) | 18.7 ± 1.8 (17–21) |
| Tail length | 47.0 ± 4.0 (42–56) | 55.3 ± 4.7 (48–67) |
| Hyaline part of tail length | 11.3 ± 1.7 (9–15) | 13.5 ± 2.0 (12–16) |
| Width at level of: | ||
| Lips | 14.5 ± 0.5 (14–15) | 14.8 ± 0.5 (14–15) |
| Guide ring | 22.5 ± 0.5 (22–23) | 22 ± 0.5 (21–23) |
| Base of pharynx | 44.4 ± 1.7 (42–48) | 43.5 ± 1.7 (41–48) |
| Vulva or mid-body | 53.7 ± 3.6 (49–61) | 50.8 ± 3.4 (45–54) |
| Anus | 39.6 ± 1.5 (37–43) | 39.5 ± 1.5 (36–43) |
| Parameter | Vermicompost (Imput) |
|---|---|
| pH-H2O | 7.17 |
| pH-KCl | 7.0 |
| Electrical conductivity (mS·cm−1) | 2.41 |
| Nitrate (mg·kg−1) | 200 |
| Ammonium (mg·kg−1) | 68.7 |
| Phosphorus (mg·kg−1) | 617 |
| Potassium (mg·kg−1) | 1795 |
| Calcium (mg·kg−1) | 1410 |
| Magnesium (mg·kg−1) | 369 |
| Chlorine (mg·kg−1) | 189 |
| Organic carbon (%) | 5.08 |
| Total nitrogen (%) | 0.23 |
| C/N ratio | 21/1 |
| Variant | Properties of Soil | |||||||
|---|---|---|---|---|---|---|---|---|
| Salinity | N-NO3 | P | K | Mg | Ca | N-NH4 | Corg | |
| [NaClg·L−1] | Available form [mg·kg−1 Soil] | [%] | ||||||
| R2 = 0.78, p < 0.001 | R2 = 0.74, p < 0.001 | R2 = 0.59, p < 0.007 | R2 = 0.96, p < 0.001 | R2 = 0.84, p < 0.001 | R2 = 0.08, p = 0.27 | R2 = 0.73, p < 0.001 | R2 = 0.81, p < 0.001 | |
| Control | 0.20 ± 0.01 | 27.75 ± 1.71 | 245.8 ± 8.7 | 87.8 ± 6.3 | 147.5 ± 6.5 | 2212.5 ± 85.4 | 477.5 ± 63.4 | 3.36 ± 0.04 |
| Ve | 0.27 ± 0.02 * | 35.25 ± 2.22 * | 267.5 ± 6.5 * | 150.3 ± 4.6 * | 189.5 ± 8.8 * | 2351.8 ± 128.2 | 325.8 ± 21.1 * | 3.73 ± 0.13 * |
| Ag-NPs | 0.22 ± 0.01 | 28.50 ± 2.08 | 247.5 ± 6.5 | 120.5 ± 4.2 * | 188.0 ± 10.3 * | 2353.8 ± 171.9 | 366.3 ± 12.5 * | 3.41 ± 0.09 |
| Variant | RR Trait | Stem Height [cm] | Stem Thickness [cm] | Root Length [cm] | |||
|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | ||
| Control | Mean | 95.0 ± 8.9 | 172.5 ± 31.2 | 4.0 ± 0.6 | 6.2 ± 0.5 | 13.4 ± 1.6 | 15.2 ± 1.75 |
| Ve | 105.0 ± 9.1 | 185.0 ± 11.5 | 4.2 ± 0.4 | 7.6 ± 0.7 | 16.4 ± 1.8 * | 17.4 ± 2.02 * | |
| Ag-NPs | 102.0 ± 8.2 | 182.0 ± 19.8 | 4.7 ± 1.1 | 7.8 ± 0.5 | 14.8 ± 1.73 | 16.8 ± 1.25 * | |
| Control | Minimum | 52.0 ± 7.3 | 86.0 ± 13.5 | 4.5 ± 0.5 | 6.3 ± 0.9 | 12.2 ± 1.51 | 14.2 ± 1.27 |
| Ve | 72.0 ± 7.5 * | 120.0 ± 9.1 * | 4.3 ± 0.2 | 7.1 ± 1.0 | 14.2 ± 1.53 * | 15.2 ± 1.78 * | |
| Ag-NPs | 51.5 ± 7.2 | 101.5 ± 4.1 * | 4.2 ± 0.1 | 7.1 ± 1.3 | 13.5 ± 1.17 | 14.5 ± 1.06 | |
| Control | Maximum | 187.5 ± 15.5 | 210.0 ± 12.2 | 4.7 ± 0.2 | 6.1 ± 1.7 | 14.4 ± 1.62 | 15.8 ± 1.65 |
| Ve | 195.0 ± 26.1 | 220.0 ± 10.8 | 5.4 ± 0.7 | 8.1 ± 0.4 * | 17.8 ± 1.77 * | 19.8 ± 2.46 * | |
| Ag-NPs | 195.5 ± 16.5 | 211.5 ± 13.4 | 5.5 ± 0.6 | 8.1 ± 0.3 * | 16.3 ± 1.63 * | 18.8 ± 1.96 * | |
| Variant F = 14.6 p < 0.001 | Year F = 133.8 p < 0.001 | Wet Yield [g·m−2] | Dry Yield [g·m−2] |
|---|---|---|---|
| Control | 2021 | 355.0 ± 42.0 | 161.3 ± 8.5 |
| 2022 | 967.5 ± 85.0 | 452.5 ± 41.1 | |
| Ve | 2021 | 431.3 ± 62.0 * | 361.3 ± 42.9 * |
| 2022 | 1142.5 ± 123.4 * | 928.8 ± 124.9 * | |
| Ag-NPs | 2021 | 427.5 ± 61.8 | 361.3 ± 8.5 * |
| 2022 | 975.0 ± 126.1 | 855.0 ± 65.6 * |
| Variant | L. elongatus | |||
|---|---|---|---|---|
| 2021 (R2 = 0.95, p < 0.001) | 2022 (R2 = 0.99, p < 0.001) | |||
| Pf | Rf | Pf1 | Rf1 * | |
| Control | 198.8 ± 17.5 | 0.99 ± 0.09 | 221.3 ± 8.5 | 1.11 ± 0.04 |
| Ve | 80.1 ± 6.3 * | 0.40 ± 0.03 | 40.1 ± 9.1 * | 0.20 ± 0.05 |
| Ag-NPs | 75.0 ± 7.0 * | 0.38 ± 0.04 | 52.1 ± 7.3 * | 0.26 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skwiercz, A.; Stefanovska, T.; Zhukov, O.; Zapałowska, A.; Masłoń, A. Effect of Silver Nanoparticles and Vermicompost on the Control of Longidorus elongatus (De Man, 1876) in Miscanthus × Giganteus and Its Growth and Development. Sustainability 2024, 16, 8093. https://doi.org/10.3390/su16188093
Skwiercz A, Stefanovska T, Zhukov O, Zapałowska A, Masłoń A. Effect of Silver Nanoparticles and Vermicompost on the Control of Longidorus elongatus (De Man, 1876) in Miscanthus × Giganteus and Its Growth and Development. Sustainability. 2024; 16(18):8093. https://doi.org/10.3390/su16188093
Chicago/Turabian StyleSkwiercz, Andrzej, Tatyana Stefanovska, Olexander Zhukov, Anita Zapałowska, and Adam Masłoń. 2024. "Effect of Silver Nanoparticles and Vermicompost on the Control of Longidorus elongatus (De Man, 1876) in Miscanthus × Giganteus and Its Growth and Development" Sustainability 16, no. 18: 8093. https://doi.org/10.3390/su16188093
APA StyleSkwiercz, A., Stefanovska, T., Zhukov, O., Zapałowska, A., & Masłoń, A. (2024). Effect of Silver Nanoparticles and Vermicompost on the Control of Longidorus elongatus (De Man, 1876) in Miscanthus × Giganteus and Its Growth and Development. Sustainability, 16(18), 8093. https://doi.org/10.3390/su16188093








