Abstract
The frequent occurrence of localized and seasonal droughts has caused severe economic losses in maize production in South China. To promote sustainable maize production, selecting and breeding drought-tolerant varieties is vital for addressing water scarcity. Drought stress affects all aspects of crop morphological performance. In this study, the morphological performance of 285 maize inbred lines under drought stress was investigated using D-value analysis, correlation analysis, principal component analysis, cluster analysis and stepwise regression analysis. All indicators were significantly different in the regular treatment compared to the drought treatment. Specifically, survival rate, root fresh weight, root dry weight, plant dry weight, root/crown ratio, and plant fresh weight were used as indicators for drought-tolerance evaluation. Furthermore, the drought-tolerant inbred line CML323 and the drought-sensitive inbred line CB2-49-1 were screened by comprehensively evaluating D values. The drought-tolerant inbred line CML323 exhibits higher leaf relative water content, chlorophyll content, proline content, and ascorbate peroxidase and peroxidase activity while having lower malondialdehyde content, consequently demonstrating excellent drought tolerance. This study provides valuable insights into drought-tolerance indicators and reference materials for breeding maize varieties.
1. Introduction
Maize has become China’s number one food crop and is prominent in developing the national economy [1]. Drought constrains the primary abiotic stressor for maize yield, and the frequency of drought events is a formidable obstacle in maize production [2,3]. In some areas of Guangxi, maize can be planted in two seasons a year. Frequent droughts in the early spring maize season significantly affect the emergence of maize seedlings and expected growth. Therefore, this study was carried out to screen for drought-tolerance identification indexes and drought-tolerant maize inbred lines. It may help us to better deal with the impacts of climate change and thus enhance the sustainable development of the maize seed industry in South China in the near future.
Drought, at the seedling stage, mainly affects the nutritional growth of maize, which later affects maize yield [4,5]. The root system is the main organ for absorbing water and nutrients and has a vital role in the developmental process of maize [6,7]. Under drought stress conditions, the root system enhances its adaptation to the water environment by changing and regulating its morphology, and higher root growth can increase water uptake capacity, thereby reducing drought damage to crops [8]. Muhammad et al. [9], in their study of drought tolerance in maize seedlings, found that growth parameters such as root length, root/crown ratio, and fresh seedling weight were drastically reduced. In addition, changes in the biomass of the above-ground portion of maize are a response mechanism to drought stress in maize [10]. Under drought conditions, the crop adapts to the external environment through its regulation, which is ultimately reflected in the external morphology of the plant, with changes in above-ground biomass being the most intuitive response to drought stress [11]. Crops under drought conditions show reduced growth potential, with a decreasing leaf area and number of leaves and a reduction in plant height and dry matter [12]. At the same time, drought increases the accumulation of free radicals in the leaves, which reduces the photosynthetic capacity [13]. The final growth of the crop depends on seedling growth [14], so it is necessary to estimate drought resilience for seedling growth under drought stress.
Studies conducted recently have identified several characteristics linked to drought tolerance in maize. However, various phenotypes, including yield components, plant height, dry matter weight, root length, ear height, filament spacing, and ear length [15], remain key traits for screening breeding materials for maize [16]. Bao et al. [17] and Nakhforoosh et al. [18] found that plant height and dry matter accumulation are critical indicators for screening drought-tolerant materials. Research has indicated that root features are sensitive to water stress and that different drought-tolerant types of maize have varied root properties. Thus, root traits may serve as valuable markers of drought resistance [19,20].
In physiology, crops can have various responses and adaptive mechanisms to resist stress [21]. When a crop is under adversity, the content of osmoregulatory substances in its body changes with the degree of drought [22]. The accumulation of proline can be mentioned, which reduces the osmotic potential and prevents excessive water loss, leading to death [23]. The final degradation product of the peroxidation reaction is malondialdehyde, and its content can determine the degree of peroxidation of the plasma membrane, thus reflecting the damage to the membrane system [24,25]. Plants under drought stress have higher concentrations of reactive oxygen species, which will hasten the process of membrane lipid peroxidation and lead to cell membrane destruction [26,27]. Under drought stress, the interaction of peroxidase and ascorbate peroxidase activities in crops can maintain the normal metabolism of plant reactive oxygen species, which is of great significance for crop stress resistance [28,29].
Seasonal and localized droughts can profoundly affect crop growth and development and, consequently, nutrient accumulation and maize yields [30]. Furthermore, the majority of research endeavors concentrate on physiological and biochemical components to examine representative indicators for assessing drought tolerance in maize. A limited number of these studies assessed drought tolerance in maize by combining a number of phenotypes. In field production, phenotypes can directly reflect maize’s drought tolerance. Moreover, the current regional inbred lines are not screened for better adaptation to the environment. Therefore, it is rational to screen out the inbred lines that are resistant to drought and better acclimatized to the climate. This study investigated the changes in the morphological traits of 285 inbred lines above and below ground under drought treatment. The physiological and biochemical studies of CML323 and CB2-49-1—inbred lines under drought stress—will provide valuable resources for the future breeding of drought-tolerant and high-yielding maize varieties. This work sheds light on the drought-tolerance markers of inbred lines of maize, which are essential for choosing and developing maize cultivars for climate adaptation.
2. Materials and Methods
2.1. Plant Material and Experimental Treatments
The 285 maize inbred lines were evaluated provided by the Guangxi Academy of Agricultural Sciences (Supplementary Table S1). The 285 maize inbred lines are germplasm resources of the Guangxi Academy of Agricultural Sciences and are important for broadening the basis of the sustainable development of maize germplasm in South China. The CML323 (drought-tolerant) and CB2-49-1 (drought-sensitive) inbred lines were chosen for subsequent testing due to their different performance under drought stress.
2.2. The First Experiment
In this study, 285 seedlings of maize inbred lines were tested for drought tolerance in a controlled greenhouse environment at the Guangxi Academy of Agricultural Sciences. Each pot (30 cm × 10 cm) was filled with 8.0 kg soil, and we planted five seeds in each pot. The design of the experiment was a completely randomized design (CRD) with three replications.
Two moisture factors were designed: well-watered (WW) and drought stress (DS), with three replicates per treatment. Drought stress was initiated at the three-leaf stage of the plants, and normal watering was applied in the WW treatment group. The degree of drought was determined by measuring leaf relative water content (Figure 1A), and data were collected when the leaf relative water content decreased from 98.8% (well-watered) to 59.73% (severely drought-prone) [31]. At the same time, the survival rate test was carried out. Each pot (60 cm × 36 cm) was filled with 16 kg of soil. Each pot was planted with 9 materials, and each material had 20 seeds. Soil water content was controlled by a weighing method. Water control was started at the three-leaf stage to control the target soil water content, maintaining it at 40–45% of the field water content, and drought stress for a total of 5 d. The pots were then re-watered to a field moisture content of 75–80% (Figure 1B), and survival was calculated after 5 d of re-watering. The experiment began on 27 April 2023 and ended on 3 June 2023.
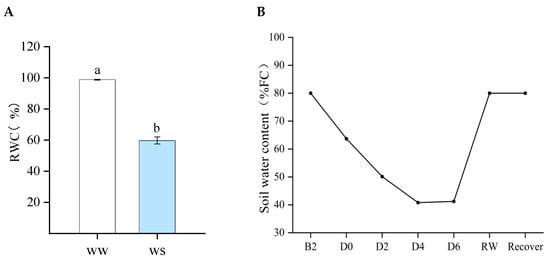
Figure 1.
(A) The relative water content (RWC) of leaves under normal water and drought-stress conditions; (B) monitoring of soil water content from initiation of drought treatment to rehydration. Bars shows standard error and different lowercase letters indicate significant differences (p < 0.05).
2.3. The Second Experiment
Through the comprehensive identification of drought resistance in the previous experiment, the drought-resistant inbred line CML323 and the sensitive inbred line CB2-49-1 were selected as the materials for the experiment. This experiment was conducted in a greenhouse at the Guangxi Academy of Agricultural Sciences. The design of the experiment was a completely randomized design (CRD) with three replications. The soil used for maize cultivation was field soil with a maximum field water holding capacity of 21.61%. Five seeds were sown in pots with an inner diameter of 21.5 cm and a height of 19 cm, each containing 6 kg. Three biological replicates were set up. Drought treatment was initiated when maize seedlings reached the three-leaf stage. The target soil water content was controlled at 40–45% of the field water capacity, and the date when the soil water content first dropped to 60% was recorded as 0 d. Drought stress was applied to seedlings for 5 d, and the soil water content was controlled by the weighing method. Samples were taken after 0 d, 1 d, 3 d, and 5 d of drought treatment, and the fourth leaf of the maize seedlings was taken for physiological and biochemical measurements. The experiment began on 4 March 2024 and ended on 6 April 2024.
2.4. Sampling and Measurements
2.4.1. Chlorophyll Contents
The SPAD values were determined using a Nissan SPAD-502 chlorophyll meter (Konica Minolta Inc., Tokyo, Japan) using non-isolated leaves, and the average values were taken after taking SPAD values using a Nissan SPAD-502 chlorophyll meter on non-isolated leaves. The average values were taken after three measurements from the tip, middle, and base of the first fully expanded leaf at the top of the maize plant.
2.4.2. Morphological Parameters
Plant height determination: Three plants of uniform length were collected from each treatment, and the length (cm) from the top of the longest seedling of maize to the node ground of the shoot sheath was measured with a straight edge.
Biomass determination: For sampling, three plants were randomly selected from each material of each treatment, and the above-ground and below-ground parts of the plants were cut. The plant fresh weight and root fresh weight were determined separately. Samples were packed into paper sample bags at 105 °C, oven-dried for 30 min, dried at a constant temperature of 80 °C to obtain a constant weight, and cooled. Then, we weighed the shoot and root dry weight (g) with an analytical balance, respectively.
Root length determination: A ruler was used to measure the node of the maize shoot sheath to the tip of the primary root.
Root/crown ratio determination: The ratio of root fresh weight to plant fresh weight was used.
Stem diameter and leaf area determination: Three maize seedlings of equal growth status were selected, and the stem diameter was measured using vernier calipers (Ningbo Deli Tools Co., Ltd., Ningbo, China). Leaf area/plant (LA) was measured with a ruler and is LA calculated as follows:
where 0.75 is the empirical correction coefficient for maize LA; m is the number of plants measured; n is the total number of leaves of the ith plant; and Lij and Wij are the maximum leaf length and maximum leaf width, respectively, of the jth leaf of the ith plant.
2.4.3. Relative Water Content
The third fully expanded leaf was taken from the plant and cut from the leaf base; then, we weighed the fresh weight (FW) and quickly inserted it into the water and soaked it in the dark for 24 h. The excess water on the surface of the leaf was wiped off, and the turgid weight (TW) was weighed. The leaf was dried to a constant weight to calculate the dry weight (DW). Finally, the following equation was used to calculate RWC (%) [32,33].
2.4.4. Photosynthetic Pigment
Fresh leaves were weighed to 0.5 g and put into 5 mL of 95% ethanol in a graduated test tube, before being immersed in the dark for 24 h until the leaves turned completely white. Then, 1 mL of the extract was taken in a glass cuvette, and the absorbance values at 663 nm and 645 nm were determined and recorded as A663 and A645, respectively.
where V is the volume of the extract, D is the dilution, and m is the sample mass.
2.4.5. Antioxidant Activity
We weighed 0.1 g of the sample and added 1 mL of extract for ice-bath homogenization. The sample was centrifuged at 15,000× g for 20 min at 4 °C. The supernatant was collected to measure peroxidase and ascorbate peroxidase activities using a kit (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China). The absorbance of ascorbate peroxidase at 290 nm was measured using a spectrophotometer at both 10 s and 130 s. Definition of ascorbate peroxidase activity units: one unit of enzyme activity per milligram of protein oxidizing 1 μmol of AsA per minute. Peroxidase absorbance values at 470 nm for 30 s and 1 min 30 s were determined using a spectrophotometer. Definition of peroxidase unit: 0.01 change in absorbance at 470 nm per minute is one unit of enzyme activity.
2.4.6. Malondialdehyde Content
Malondialdehyde content was determined using a kit (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China). After weighing 0.1 g of the sample, 1 mL of extract was added, and the mixture was homogenized in an ice bath. The homogenate was then centrifuged at 8000× g for 10 min at 4 °C. Absorbance values were determined at 532 nm and 600 nm by a spectrophotometer, respectively.
2.4.7. Proline Content
Proline content was determined using a kit (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China). First, 0.1 g of sample was weighed, 1 mL of extraction solution was added for homogenization in an ice bath, and then the sample was extracted by shaking in a boiling water bath for 10 min. The absorbance value was determined by a spectrophotometer at 520 nm.
2.5. Tolerance Analysis
The tolerant coefficient (TC) is the ratio of the drought treatment value for each indicator to the value of each indicator [34]. The proposed formulas used to calculate the values of the affiliation function μ(Xi) and D values for each maize variety are as follows [35,36]:
2.6. Statistical Analysis
The experimental data were analyzed by using a two-way ANOVA in SPSS statistics v. 21 (IBM Inc., Armonk, NY, USA). Mean differences between treatments were assessed by using the least-significant-difference (DMRT) test, and PCA and correlation analyses were performed using SPSS software. Plotting was performed with OriginPro 2021 software (Origin Lab Corporation, Northampton, MA, USA), while cluster analysis was performed using two R packages: ggplot2 and ggtree [37].
3. Results
3.1. Response of Maize Seedling Traits to Drought Stress
Relative chlorophyll content, plant height, plant fresh weight, plant dry weight, root fresh weight, root dry weight, root length, and root/crown ratio were significantly different (p < 0.01) under drought stress compared to the normal treatment (Table 1). The six traits of SPAD, PH, PFW, PDW, RFW, and RDW decreased by 9.89%, 23.37%, 60.02%, 31.17%, 34.35%, and 27.19%, respectively, under drought stress compared with the normal conditions; however, the RL and RCR increased by 27.61% and 77.78%, which indicated that drought promotes the increase in RL and RCR.

Table 1.
Statistical analysis of the main indicators of maize under normal and drought treatments.
In terms of the degree of changes, all eight indicators varied under drought stress, demonstrating that the selected indicators effectively reflect maize seedling drought tolerance. The maximum coefficient of variation was 33.37% for RFW under normal treatment conditions and 40.32% for RCR under drought treatment conditions. The distribution of the mean values of drought-tolerance coefficients of the traits at the seedling stage ranged from 0.41 (PFW) to 1.79 (RCR), which showed a wide variation in the degree of sensitivity to drought among the traits. The analysis of the coefficient of variation (CV) of the drought-tolerance coefficient for different traits revealed that the maximum value of the CV of drought-tolerance coefficient was for the root/crown ratio (35.10%) and the minimum value of the CV of drought-tolerance coefficient was for the relative chlorophyll content (6.16%).
3.2. Comprehensive Evaluation of D Value
A single indicator cannot be used as a reliable indicator of drought tolerance in a crop, and it is through the D values of multiple traits that we can better go about evaluating drought tolerance in maize. The larger D value represents the greater drought resistance of the maize inbred lines. There were significant differences in drought tolerance among the 285 inbred lines, with the D values of the test inbred lines varying from 0.72 to 0.11 (Supplement Table S2). When subjected to drought stress CML323 (No. L022), the material had a higher D value (>0.70) than the other inbred lines and was the most drought-resistant of the batch; CB2-49-1 (No. L224) inbred lines had the lowest D value and were the least drought-resistant inbred lines of the batch.
3.3. Survival Rate Analysis
From the survival rate test, it was found that 40.70% of the 285 maize inbred lines supplied for the test had a survival rate between 0% and 20%, and 3.51% had a survival rate between 90 and 100% (Supplement Table S2). ShuangM9 (No. L106) and GRL152A (No. L171) achieved 100% survival and were ranked first and second in terms of survival rate; however, they ranked twelfth and eighth in terms of composite D value. The survival rate of the CML323 inbred line was 82.35% and ranked first in the D value. The CB2-49-1 inbred line had the least survival (0.00%) and had the lowest D value. As can be seen from Supplement Table S2, the larger the D value, the relatively higher the survival rate.
3.4. Principal Component Analysis
Principal component analysis extracted three eigenvalues greater than 1.0, labeled F1 to F3, which collectively accounted for 65.32% of the variance in the nine indicators (Table 2). In terms of constituent loads, F1 was mainly associated with below-ground traits such as root dry weight, root fresh weight, and root/crown ratio, suggesting that they play an important role in morphological parameters under drought stress. In F2, the main effects were on above-ground traits such as plant fresh weight, plant dry weight and plant height. The largest loadings of relative chlorophyll content and survival were found in F3, where plant dry weight had the smallest loadings, suggesting that this indicator had little effect on the drought tolerance of maize in F3 (Supplement Figure S1).

Table 2.
Loading matrix and the variance contribution rate of the principal component under drought stress.
A stepwise regression equation for drought-tolerance evaluation was established using the D value as the dependent variable and the drought-tolerance coefficients of each identification index as the independent variable. The optimal stepwise regression equation was derived: predicted D value = 0.141 + 0.335 × SR + 0.071 × RFW − 0.042 × PDW + 0.024 × RCR + 0.091 × PFW (R2 = 0.797; F = 180.041; p < 0.001). Through this equation, it was found that SR, RFW, RDW, PDW, RCR, and PFW among the nine indexes had a significant effect on the D value, which was the critical index for the comprehensive evaluation of maize drought tolerance.
3.5. Correlation Analysis
Correlation analysis can reveal whether there is a dependency between traits, as well as the direction and strength of the correlation. Pearson’s correlation analysis showed (Figure 2) that there was no significant correlation (p > 0.05) between the relative chlorophyll content and each trait index. The correlation between plant height and fresh weight, plant dry weight, and root/crown ratio reached highly significant levels (p < 0.01), with a negative relationship with the root/crown ratio. The correlation between plant fresh weight, plant dry weight, and root fresh weight also reached highly significant levels (p < 0.01), and the differences in root dry weight and root length were not significant (p > 0.05). Plant dry weight and root fresh weight existed at substantial levels (p < 0.05) with root dry weight, root length, and root/crown ratio, respectively. In addition to this, there was a highly significant negative relationship between RCR and PH, PFW, and PDW (p < 0.01). In summary, the correlations among the indicators are somewhat different, and the effects of plant fresh weight, plant dry weight, root fresh weight, and root dry weight on the drought resistance of maize should be emphasized when selecting drought-resistance indicators for maize.
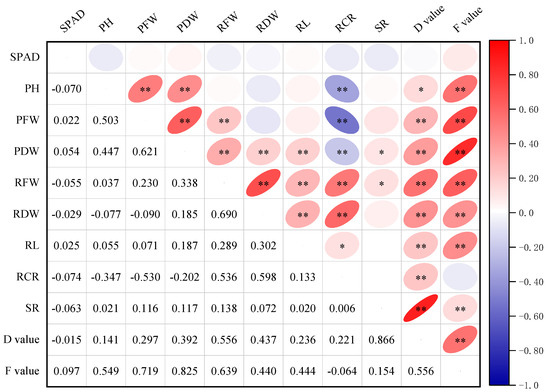
Figure 2.
Correlation analysis between all trait indicators. *, p < 0.05; **, p < 0.01; SPAD, chlorophyll relative content; PH, plant height; PFW, plant fresh weight; PDW, plant dry weight; RFW, root fresh weight; RDW, root dry weight; RL, root length; RCR, root/crown ratio; SR, survival rate of two maize inbred lines CML323 and CB2-49-1 under drought stress.
As shown in Figure 2, there were significant (p < 0.05) differences between survival and plant dry weight and root fresh weight, and non-significant (p > 0.05) differences with relative chlorophyll content, plant height, plant fresh weight, root dry weight, root length, and root/crown ratio. There was a highly significant correlation between survival rate and the D value and F value, with survival rate having the highest correlation with the D value at 0.87. This indicates that survival rate can be used as an indicator for drought evaluation, and the drought resistance of maize is determined by several indicators. Therefore, it is necessary to combine different related indicators to evaluate more meaningfully.
The D value was significantly and positively correlated (p < 0.05) with the other indicators, except for the relative chlorophyll content. There was a highly significant positive relationship (p < 0.01) between the F value and root length, root dry weight, root fresh weight, plant dry weight, plant fresh weight, and plant height, and a non-significant negative relationship (p > 0.05) with root/crown ratio. The D and F values indicate that plant height, plant fresh weight, plant dry weight, root fresh weight, and root dry weight are closely related to the drought tolerance of maize, and they can be used as important indexes for the evaluation of drought tolerance of maize.
3.6. Cluster Analysis
Cluster analysis of the inbred lines was performed using the sum of squared deviations of the Euclidean distances based on the D values. The 285 maize inbred lines were classified into five groups: extremely drought-tolerant, drought-tolerant, intermediate, sensitive, and extremely sensitive (Figure 3). Cluster I contained the inbred lines with the highest D values in the experiment, with 24 inbred lines, among which CML323 had the most considerable D value and was highly tolerant to drought stress; cluster II contains 67 inbred lines; cluster III contains 57 inbred lines; cluster IV contains 55 inbred lines; and cluster V contained 82 inbred lines, of which CB2-49-1, CT1-217, and D006 were the least drought-tolerant to drought stress, with the lowest D values and the highest sensitivities. The ranking of drought tolerance of each group was as follows: I > II > III > IV > V.
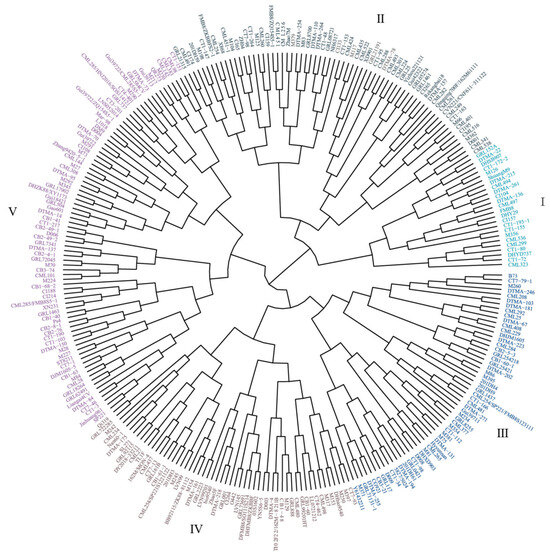
Figure 3.
Clustering analysis of 285 materials based on composite evaluation value. Cluster I, extremely drought-tolerant; cluster II, drought-tolerant; cluster III, intermediate; cluster IV, sensitive; cluster V, extremely sensitive.
3.7. Phenotypic Differences in Drought Response between Two Maize Inbred Lines
Plant height in both maize inbred lines increased gradually under continuous drought stress, although a decrease was observed at 5 days. As shown in Table 3, the drought-resistant inbred line (CML323) exhibited a 27.32% increase, while the drought-sensitive inbred line (CB2-49-1) showed a 13.73% increase in plant height at 5 days compared to day 0. There was no significant difference (p > 0.05) between the drought-stressed CML323 inbred line at 0 d compared to 1 d, and there was a significant difference between it and all other treatments; there was also a significant difference (p < 0.05) between the drought-stressed CB2-49-1 inbred line at 0 d and all treatments. The CML323 and CB2-49-1 inbred lines showed an increasing trend in stem thickness until 3 d of drought stress; however, they showed a decreasing trend at 5 d. The CB2-49-1 inbred line had a smaller stem thickness than CML323. The leaf area of CML323 was not significantly different (p > 0.05) among the drought treatments, where treatment at 5 d showed a 14.70% decrease in leaf area compared to 0 d. A significant difference was observed in the leaf area of the CB2-49-1 self-compatible line at 0 d as compared to 5 d, where treatment at 5 d showed a decrease of 18.57% in leaf area compared to 0 d.

Table 3.
CML323 and CB2-49-1 phenotypic traits at different times under drought stress.
3.8. Differences in Physiological Indices between Two Maize Inbred Lines under Drought Stress
It can be seen (Figure 4) that under drought conditions, the leaf water content of the two inbred lines showed a decreasing trend, with CB2-49-1 showing a greater change than CML323. The drought treatment of CML323 and CB2-49-1 inbred lines decreased the relative leaf water content by 2.41% and 25.86% at 5 d compared to 0 d, respectively. The chlorophyll content of the two inbred lines crosses showed an increasing and then decreasing trend, peaking at day 3 of drought treatment. Chlorophyll content increased by 8.90% and 12.67% in the drought-stress treatment at 3 d compared to 0 d, and it decreased by 9.37% and 5.33% in the drought treatment at 5 d compared to 3 d in the drought-tolerant inbred line (CML323) and sensitive inbred line (CB2-49-1), respectively.
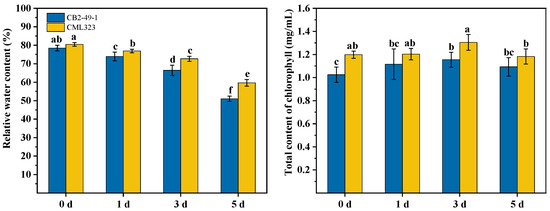
Figure 4.
Changes in relative water content and chlorophyll content of CML323 and CB2-49-1 inbred lines under different drought stress times (days). Bars shows standard error and different lowercase letters indicate significant differences (p < 0.05).
Under drought stress (Figure 5), the ascorbate peroxidase activity (APX) of the two inbred lines showed an increasing trend, in which the drought treatment of the sensitive inbred lines (CB2-49-1) increased by 13.25%, 32.67%, and 42.16% at 1 d, 3 d, and 5 d compared to 0 d, respectively. The differences between the 3 d and 5 d treatments and 0 d reached a significant level (p < 0.05). Drought-tolerant inbred lines (CML323) increased by 30.97%, 57.85% and 92.04% in drought treatments at 1 d, 3 d and 5 d compared to 0 d, respectively, with significant differences (p < 0.05) between treatments. Changes in peroxidase activity showed an upward trend, with no significant difference (p > 0.05) among treatments in the sensitive inbred lines (CB2-49-1), and a 19.07% increase in the drought treatment at 5 d compared to 0 d. The drought treatment of drought-tolerant inbred lines (CML323) increased peroxidase activity by 0.66%, 18.22% and 46.13% at 1 d, 3 d and 5 d compared to 0 d, respectively. The proline (Pro) content of the drought-resistant (CML323) and sensitive (CB2-49-1) inbred lines did not differ significantly in the early stage of the stress treatment and differed significantly in the late stage of the stress (p < 0.05), where the drought-treated CB2-49-1 and CML323 inbred lines showed increases of 24.63% and 70.45%, respectively, at 5 d compared with 0 d. CB2-49-1 malondialdehyde (MDA) content was not significantly different (p < 0.05) among the treatments during the 5 days of drought stress, in which the drought treatment increased by 20.89% at 5 d as compared to 0 d, respectively, and the drought treatment of the CML323 inbred line showed a significant difference between the drought treatments at 5 d and 0 d (p < 0.05), as well as an increase of 7.05%.
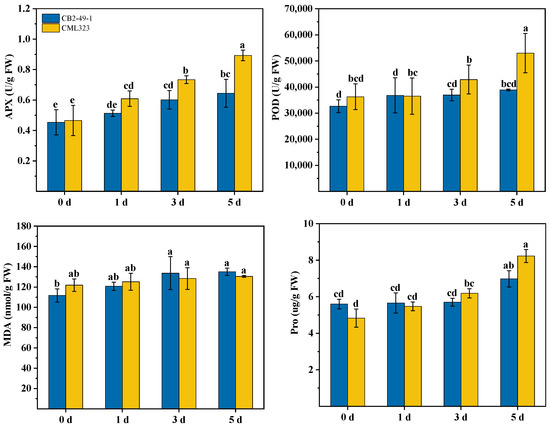
Figure 5.
Physiological and biochemical traits of CML323 and CB2-49-1 inbred lines under different drought stress times (days). APX, ascorbate peroxidase; POD, peroxidase; Pro, proline; MDA, malondialdehyde. Bars shows standard error and different lowercase letters indicate significant differences (p < 0.05).
4. Discussion
An in-depth study was conducted on the drought-resistant inbred line CML323 and the sensitive inbred line CB2-49-1. The results showed that stem thickness and leaf area were more affected by drought in the sensitive inbred line (CB2-49-1) and only slightly in the drought-tolerant inbred line (CML323). Jungklang et al. [38] suggested that drought-tolerant varieties of maize have higher relative leaf water content to maintain relatively stable cellular water content and reduce cellular breakage under drought stress. The relative water content of leaves of the CML323 inbred line was higher than that of CB2-49-1 under drought stress, suggesting that the CML323 inbred line has a strong osmotic adjustment ability. Drought stress has been reported to reduce chlorophyll content, with drought-tolerant genes being able to maintain higher chlorophyll content under drought stress [39,40]. The results showed that the chlorophyll content of the CML323 inbred line was higher than that of CB2-49-1 under dry stress, and the chlorophyll content decreased more slowly.
Sekmen et al. [41] found that M-503 (drought-tolerance genotype) was associated with elevated APX and POD activities. Sarker et al. [42] found a slight increase in APX activity in the sensitive variety VA15 under drought stress. In contrast, the increase in APX activity in the drought-tolerant variety VA13 was large and significant. Ascorbate peroxidase (APX) and peroxidase (POD) activities were induced by drought stress in CML323 and CB2-49-1. However, the drought-tolerant inbred line CML323 showed a significantly higher increase in APX and POD activities than the sensitive inbred line CB2-49-1. Proline (Pro) and malondialdehyde (MDA) accumulate in plants under drought-stress conditions. Pro is mainly used to regulate osmotic pressure in plants so that they can absorb water from the outside world despite drought conditions, and various metabolic activities can be carried out normally [43]. Current research by national and international scholars suggests that changes in Pro content are related to drought stress [44]. Pro content gradually increased with increasing drought stress in this experiment. A sensitive inbred line (CB2-49-1) and a drought-tolerant inbred line (CML323) under drought treatment increased by 7.05% and 20.89% at 5 d compared to 0 d, respectively. MDA is the end product of cell membrane lipid peroxidation, and the level of its content can reflect the degree of cell membrane peroxidation [45,46]. In this study, CML323 showed lower MDA content than CB2-49-1 under drought stress. In summary, with CB2-49-1, higher leaf relative water content, chlorophyll content, proline content, malondialdehyde content, and higher antioxidant capacity showed that CML323 was highly drought-tolerant.
Tolerance to drought is a complicated quantitative attribute influenced by many agronomic traits [17]. Drought stress reduces various aspects of crop performance such as morphology, physiology, and yield, among which the most intuitive change presented is morphological alteration, and morphological indicators can and directly reflect the magnitude of the drought-tolerance ability of maize varieties [47,48]. Drought stress inhibits maize’s capacity for metabolism, lowers the amount of biomass and leaf area, and lowers the rate of photosynthetic respiration by lowering the amount of chlorophyll in the leaves. All of these effects eventually result in a reduction in maize output [49,50]. Shahzad et al. [51] found significant interactions between root length, root dry weight, and root density in maize under drought stress, and these changes could well differentiate germplasm response under stress. In this study, under drought stress, SPAD and pH were suppressed, and PFW, PDW, RFW, and RDW biomass accumulation was significantly reduced. On the contrary, RL and RCR were elevated considerably when subjected to stress (Table 1). The drought-resistant material outperformed the drought-sensitive material in terms of plant height, plant fresh weight, and plant dry weight among the 285 inbred lines of maize. These were some of the benefits of the drought-resistant material. Drought stress directly affects the survival of maize, and the ability of maize to survive under drought stress is one of the most direct and practical indicators of drought tolerance [52]. The findings demonstrated that increased drought tolerance is associated with higher survival rates.
The comprehensive evaluation of crop drought tolerance requires the selection of appropriate evaluation indices and evaluation methods [53]. Previous studies have found that comprehensive evaluation using multiple indicators and methods is more accurate [54]. Most crop drought-tolerance evaluation methods use the complete drought-tolerance coefficient as the criterion to rank the tested inbred lines for drought tolerance to evaluate crop drought tolerance [55]. The D value was calculated using an affiliation function and the weights of different indicators to assess the drought tolerance of maize in a more comprehensive and precise manner. This method was employed in this study to compute the D value, which was then utilized to perform cluster analysis. The D value was categorized into five groups based on the outcomes of the cluster analysis. The very drought-resistant inbred lines were identified as CML323, CT1-80, and CML299, and the most drought-sensitive inbred lines were CB2-49-1, CT1-217, and D006. On the other hand, the re-watering survival test was consistent with the D-value results. However, deviations were observed in some materials. This is possibly due to differences in ambient temperatures for drought stresses, as well as the basis for judging survival under drought stress, which has an impact on the results of the evaluation.
In addition, principal components can minimize useful missing information by converting many potentially relevant variables into fewer representative ones [56,57]. A single indicator was transformed into four principal components through principal component analysis, cumulatively explaining 74.68% of the information in the original data. Moreover, the correlation analysis is consistent with that conclusion. The D value was positively and significantly related to indicators other than relative chlorophyll content, and the F value was positively and significantly associated with indicators other than relative chlorophyll content and root/crown ratio (p < 0.05). Using stepwise regression analysis, we obtained six indicators closely related to the D value, including SR, RFW, RDW, PDW, RCR, and PFW, which can be used as effective evaluation indexes for identifying drought resistance in maize seedlings. This result provides important help for the future selection of drought-resistant varieties in South China, as well as a scientific basis for the physiological response of maize under drought stress.
5. Conclusions
This experiment evaluated 285 maize inbred lines for drought tolerance. The region’s maize inbred lines’ capacity to withstand drought was assessed using D-value analysis, correlation analysis, principal component analysis, cluster analysis, and stepwise regression analysis. All traits were significantly affected by drought stress. The principal component analysis identified four main components, effectively explaining maize’s drought tolerance. Combined with stepwise regression analysis, six indicators were selected: SR, RFW, RDW, PDW, RCR, and PFW. Furthermore, cluster analysis was conducted using the D value of the comprehensive evaluation of drought tolerance. This resulted in the classification of 285 inbred lines into five groups: extremely drought-tolerant, drought-tolerant, intermediate, sensitive, and extremely sensitive. Among them, CML323 is representative of a highly drought-tolerant type, and CB2-49-1 is representative of a sensitive type. We phenotypically and physiologically compared the drought-tolerant inbred line CML323 with the sensitive inbred line CB2-49-1 under drought treatment. Compared with CB2-49-1, CML323 had better phenotypic, physiological, and biochemical indices—higher leaf relative water content, chlorophyll content, proline content, and ascorbate peroxidase and peroxidase activity—which indicate that CML323 is more drought-tolerant. The maize inbred line CML323 can be used as a valuable reference material for the future selection and breeding of drought-tolerant varieties of maize and is of great significance for promoting the sustainable development of maize in drought-tolerance adaptation. This study’s selection of drought-tolerance indicators was based on phenotypic indicators. Although phenotypic indicators are the most intuitive response to drought tolerance in maize in field production, the lack of relevant combined physiological indicators has some limitations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16177366/s1, Table S1: Maize materials used in the experiment; Table S2: Survival and drought tolerance D-value of test materials under drought stress; Figure S1: Loading matrix and the variance contribution rate of the principal component under drought stress.
Author Contributions
Formal analysis, X.X. and L.Q.; Investigation, W.C. and H.X.; Resources, H.Z., X.Y. and Y.J.; Writing—original draft, Z.Z.; Writing—review and editing, M.A.N.; Supervision, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Innovation Project of Guangxi Graduate Education (YCSW2024107), the Key R&D Projects of Guangxi (Guike AB21238004), the Fundamental Scientific Research of Guangxi Academy of Agricultural Sciences (Guinongke 2021YT017), the Major Science and Technology Projects of Guangxi (Guike AA22068095-6, Guike AA22068095-7) and Construction of Core Germplasm Bank of Maize in Guangxi and Mining of Disease Resistance Genes (2021GXNSFAA196003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The entire dataset used in the study is available from the corresponding author.
Acknowledgments
We thank Mingqiu Dai, Xiaopeng Sun, and Lei Du of Huazhong Agricultural University for their constructive comments and technical support in identifying maize drought stress in this manuscript. Shahid Ali for carefully revised the language throughout paper.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
SPAD, relative chlorophyll content; PH, plant height; PFW, plant fresh weight; PDW, plant dry weight; RFW, root fresh weight; RDW, root dry weight; RL, root length; RCR, root/crown ratio; WW, well water; DS, drought stress; CV, coefficient of variation; TC, tolerant coefficient; SR, survival rate; RWC, relative water content; APX, ascorbate peroxidase; POD, peroxidase; MDA, malondialdehyde; Pro, proline.
References
- Yao, Z.; Zhang, W.; Wang, X.; Lu, M.; Chadwick, D.; Zhang, Z.; Chen, X. Carbon footprint of maize production in tropical/subtropical region: A case study of Southwest China. Environ. Sci. Pollut. Res. 2021, 28, 28680–28691. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Ding, R.; Du, S.; Kang, S.; Tong, L.; Li, S. Stomatal conductance drives variations of yield and water use of maize under water and nitrogen stress. Agric. Water Manag. 2022, 268, 107651. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Hu, Y.X.; Tung, S.A.; Yang, L.; Wang, Y.; Zhou, X.B. Evaluating the effects of water-nitrogen interactions on carbon and nitrogen accumulation as well as related metabolic enzymes activity in autumn maize. J. Soil Sci. Plant Nutr. 2023, 23, 5245–5256. [Google Scholar] [CrossRef]
- Ahmadi, A.; Emam, Y.; Pessarakli, M. Biochemical changes in maize seedlings exposed to drought stress conditions at different nitrogen levels. J. Plant Nutr. 2010, 33, 541–556. [Google Scholar] [CrossRef]
- Wajhat-Un-Nisa; Sandhu, S.; Ranjan, R.; Sharda, R. Root plasticity: An effective selection technique for identification of drought tolerant maize (Zea mays L.) inbred lines. Sci. Rep. 2023, 13, 5501. [Google Scholar] [CrossRef]
- Dar, I.A.; Sofi, P.A.; Dar, Z.A.; Kamaluddin, X.; Lone, A.A. Screening of maize genotypes for drought tolerance related trait variability. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 668–682. [Google Scholar] [CrossRef]
- Wang, G.Y.; Ahmad, S.; Wang, Y.; Wang, B.W.; Huang, J.H.; Jahan, M.S.; Zhou, X.B.; Dar Shi, C.Q. Multivariate analysis compares and evaluates drought and flooding tolerances of maize germplasm. Plant Physiol. 2023, 193, 339–355. [Google Scholar] [CrossRef]
- Zeng, W.; Peng, Y.; Zhao, X.; Wu, B.; Chen, F.; Ren, B.; Zhuang, Z.; Gao, Q.; Ding, Y. Comparative proteomics analysis of the seedling root response of drought-sensitive and drought-tolerant maize varieties to drought stress. Int. J. Mol. Sci. 2019, 20, 2793. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Liang, Q.P.; et al. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 2023, 191, 2301–2315. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2014, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Gheysari, M.; Mirlatifi, S.M.; Bannayan, M.; Homaee, M.; Hoogenboom, G. Interaction of water and nitrogen on maize grown for silage. Agric. Water Manag. 2009, 96, 809–821. [Google Scholar] [CrossRef]
- Romdhane, L.; Radhouane, L.; Farooq, M.; Dal Cortivo, C.; Panozzo, A.; Vamerali, T. Morphological and biochemical changes in maize under drought and salinity stresses in a semi-arid environment. Plant Biosyst. 2020, 154, 396–404. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Bai, Y.; Camberato, J.; Xue, J.; Zhang, R. Effects of drought stress on the photosynthesis in maize. Russ. J. Plant Physiol. 2018, 65, 849–856. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, A.; Omrani, S.; Omrani, A.; Shojaei, S.H.; Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Nagy, J. Response of maize hybrids in drought-stress using drought tolerance indices. Water 2022, 14, 1012. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, Y.; Zhang, J. Identification of QTLs and Meta-QTLs for seven agronomic traits in multiple maize populations under well-watered and water-stressed conditions. Crop Sci. 2018, 58, 507–520. [Google Scholar] [CrossRef]
- Bao, X.; Hou, X.; Duan, W.; Yin, B.; Ren, J.; Wang, Y.; Liu, X.; Gu, L.; Zhen, W. Screening and evaluation of drought resistance traits of winter wheat in the North China Plain. Front. Plant Sci. 2023, 14, 1194759. [Google Scholar] [CrossRef] [PubMed]
- Nakhforoosh, A.; Bodewein, T.; Fiorani, F.; Bodner, G. Identification of water use strategies at early growth stages in durum wheat from shoot phenotyping and physiological measurements. Front. Plant Sci. 2016, 7, 1155. [Google Scholar] [CrossRef]
- Tuberosa, R.; Sanguineti, M.C.; Landi, P.; Michela Giuliani, M.; Salvi, S.; Conti, S. Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Physiol. Mol. Biol. Plants 2002, 48, 697–712. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, Q.; Jessup, K.E.; Hou, X.; Hao, B.; Marek, T.H.; Xu, W.; Evett, S.R.; O’Shaughnessy, S.A.; Brauer, D.K. Shoot and root traits in drought tolerant maize (Zea mays L.) hybrids. J. Integr. Agric. 2018, 17, 1093–1105. [Google Scholar] [CrossRef]
- Ghassemi, S.; Delangiz, N.; Asgari, L.B.; Saghafi, D.; Maggi, F. Review and future prospects on the mechanisms related to cold stress resistance and tolerance in medicinal plants. Acta Ecol. Sinica 2021, 1, 120–129. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front. Plant Sci. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, E.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinar, H.C.; Marur, C.J.; Vieira, L.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef]
- Shahana, T.; Rao, P.A.; Ram, S.S.; Sujatha, E. Mitigation of drought stress by 24-epibarassinolide and 28-homobrassinolide in pigeon pea seedlings. Int. J. Multidiscipl. Current. Res. 2015, 3, 904–911. [Google Scholar]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Sayed, M.A.; Hassan, L. Discerning of rice landraces (Oryza sativa L.) for morpho-physiological, antioxidant enzyme activity, and molecular’ markers’ responses to induced salt stress at the seedling stage. J. Plant Growth Regul. 2020, 39, 41–59. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.F.; Fu, W.; Guo, W.Q.; Ren, N.; Zhao, Y.N.; Ye, Y.L. Efficient physiological and nutrient use efficiency responses of maize leaves to drought stress under different field nitrogen conditions. Agronomy 2020, 10, 523. [Google Scholar] [CrossRef]
- Kamanga, R.M.; Mbega, E.; Ndakidemi, P. Drought tolerance mechanisms in plants: Physiological responses associated with water deficit stress in Solanum lycopersicum. Adv. Crop Sci. Technol. 2018, 6, 1000362. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Impa, S.S.; Nadaradjan, S.; Jagadish, S.V.K. Drought stress induced reactive oxygen species and antioxidants in plants. In Abiotic Stress Responses in Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 131–147. [Google Scholar]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Slatyer, R.O.; Markus, D.K. Plant-water relationships. Soil Sci. 1968, 106, 478. [Google Scholar] [CrossRef]
- Quevedo, Y.M.; Moreno, L.P.; Barragan, E. Predictive models of drought tolerance indices based on physiological morphological and biochemical markers for the selection of cotton (Gossypium hirsutum L.) varieties. J. Integr. Agric. 2022, 21, 1310–1320. [Google Scholar] [CrossRef]
- Shahrokhi, M.; Khorasani, S.K.; Ebrahimi, A. Evaluation of drought tolerance indices for screening some of super sweet maize (Zea mays L. var. saccharata) inbred lines. Agrivita J. Agric. Sci. 2020, 42, 435–448. [Google Scholar] [CrossRef]
- Yu, R.; Wang, G.; Yu, X.; Li, L.; Li, C.; Song, Y.; Xu, Z.; Zhang, J.; Guan, C. Assessing alfalfa (Medicago sativa L.) tolerance to salinity at seedling stage and screening of the salinity tolerance traits. Plant Biol. 2021, 23, 664–667. [Google Scholar] [CrossRef]
- Sakariyahu, S.; Indabo, S.S.; Aliyu, A.; Muhammad, H.U.; Ahmed, H.O.; Mohammed, S.B.; Adamu, A.K.; Aliyu, R.E. Cowpea landraces in northern Nigeria: Overview of seedling drought tolerance. Biologia 2024, 79, 381–392. [Google Scholar] [CrossRef]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Chi, Y.X.; Zeeshan, M.; Nasar, J.; Zhou, X.B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Rajarajan, K.; Ganesamurthy, K.; Raveendran, M. Differential responses of sorghum genotypes to drought stress revealed by physio-chemical and transcriptional analysis. Mol. Biol. Rep. 2021, 48, 2453–2462. [Google Scholar] [CrossRef]
- Sekmen, A.H.; Ozgur, R.; Uzilday, B.; Turkan, I. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 2014, 99, 141–149. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, P.M.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Wu, S.; Tian, J.; Ren, Y.; Wang, Y. Osmotic adjustment and antioxidant system regulated by nitrogen deposition improve photosynthetic and growth performance and alleviate oxidative damage in dwarf bamboo under drought stress. Front. Plant Sci. 2022, 13, 819071. [Google Scholar] [CrossRef]
- Claeys, H.; Inzé, D. The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef]
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Gao, W.; Qu, Y. Screening of key drought tolerance indices for cotton at the flowering and boll setting sage using the dimension reduction method. Front. Plant Sci. 2021, 12, 619926. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, L.; Lai, J.; Zhao, H.; Song, W. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Laskari, M.; Menexes, G.; Kalfas, I.; Gatzolis, I.; Dordas, C. Water stress effects on the morphological, physiological characteristics of maize (Zea mays L.), and on environmental cost. Agronomy 2022, 12, 2386. [Google Scholar] [CrossRef]
- Shahzad, A.; Gul, H.; Ahsan, M.; Wang, D.; Fahad, S. Comparative genetic evaluation of maize inbred lines at seedling and maturity stages snder drought stress. J. Plant Growth Regul. 2022, 42, 989–1005. [Google Scholar] [CrossRef]
- Jing, L.; Weng, B.; Yan, D.; Yuan, F.; Zhang, S.; Bi, W. Assessment of resilience in maize suitable planting areas under drought stress. Agric. Water Manag. 2023, 277, 108096. [Google Scholar] [CrossRef]
- Li, J.; Abbas, K.; Wang, L.; Gong, B.; Hou, S.; Wang, W.; Dai, B.; Xia, H.; Wu, X.; Lü, G.; et al. Drought resistance index screening and evaluation of lettuce under water deficit conditions on the basis of morphological and physiological differences. Front. Plant Sci. 2023, 14, 1228084. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought stress memory at the plant cycle level: A review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, Y.; Chen, Y.; Gao, M.; Zhao, Y.; Wu, L. Effects of drought stress and rehydration on physiological and biochemical properties of four oak species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Bo, W.; Fu, B.; Qin, G.; Xing, G.; Wang, Y. Evaluation of drought resistance in Iris germanica L. based on subordination function and principal component analysis. Emir. J. Food Agric. 2017, 29, 770–778. [Google Scholar] [CrossRef]
- Füzy, A.; Kovács, R.; Cseresnyés, I.; Parádi, I.; Szili-Kovács, T.; Kelemen, B. Selection of plant physiological parameters to detect stress effects in potexperiments using principal component analysis. Acta Physiol. Plant 2019, 41, 56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).