Biosorption of Cd(II), Co(II), and Cu(II) onto Microalgae under Acidic and Neutral Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Microalgae
2.2. Recovery and Processing of Biomass
2.3. FT-IR Characterisation of Biomass
2.4. Preparation of Stock Solution
2.5. Biosorption Experiments
2.6. Analytic Methods
2.7. Biosorption Isotherm Models
2.8. Determination of the Point of Zero Charge (pHpzc)
2.9. Determination of DOC
3. Results and Discussion
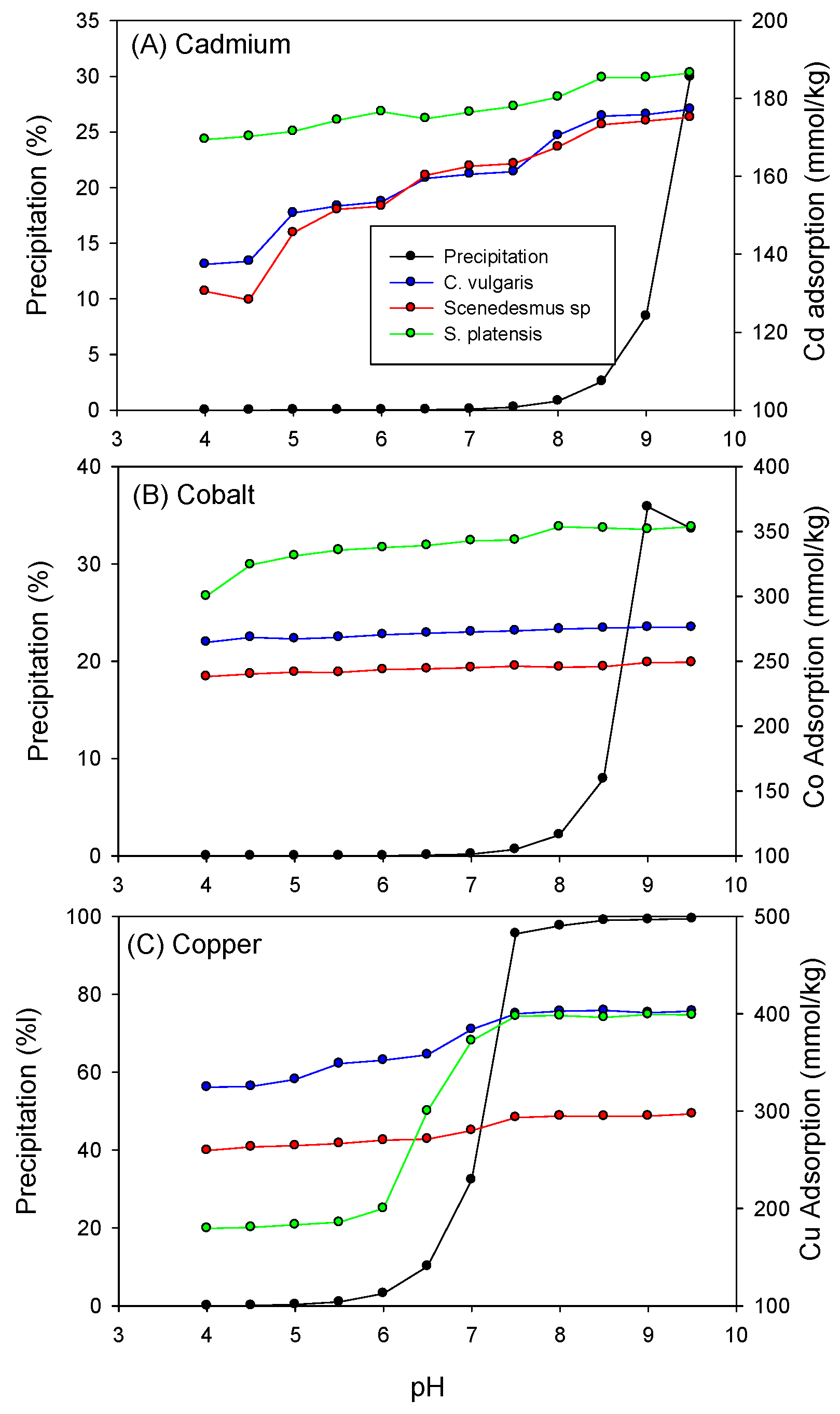
3.1. Effect of pH on the Adsorption of Heavy Metals by Biomass
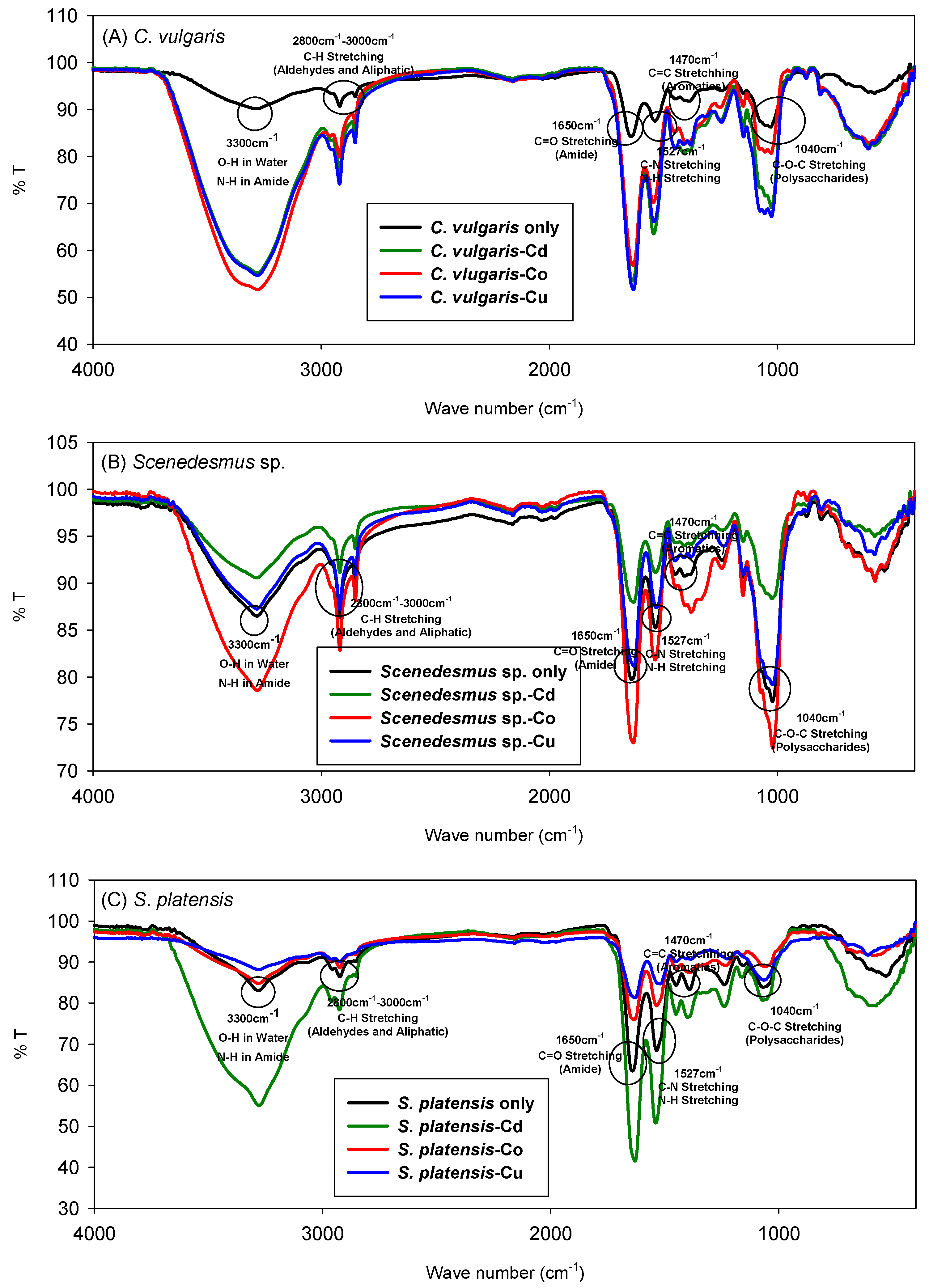
3.2. FTIR Spectra
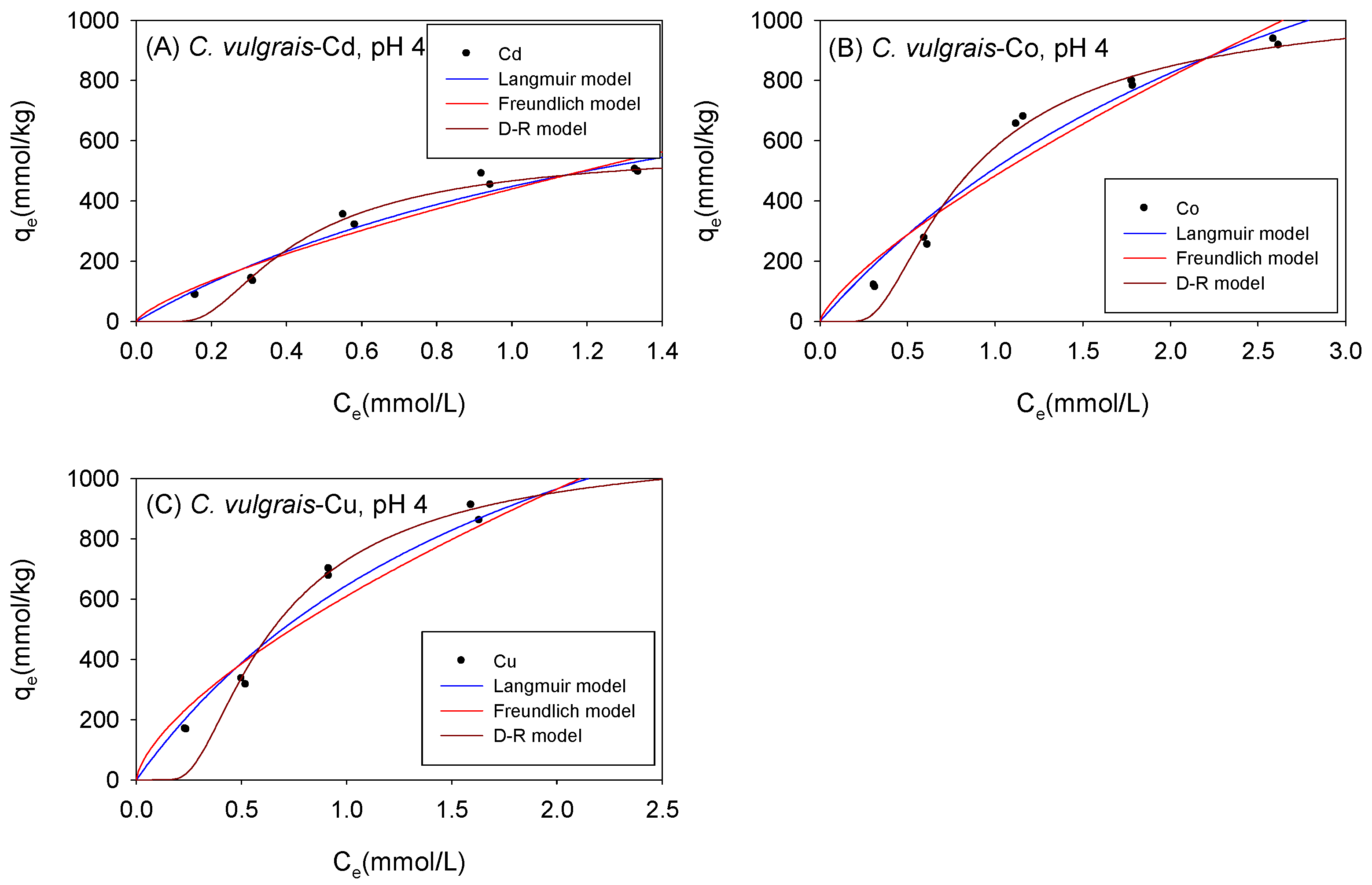
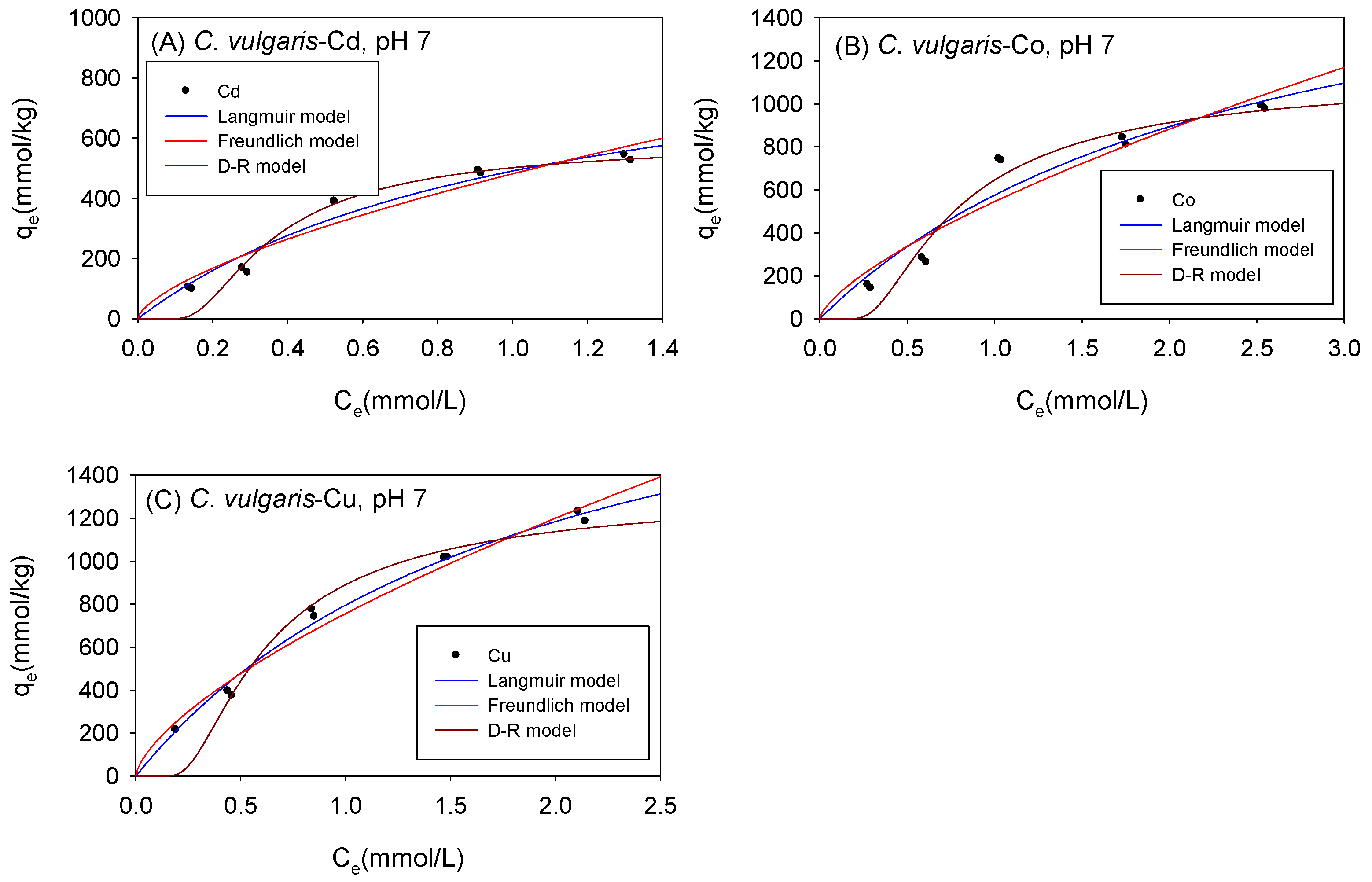
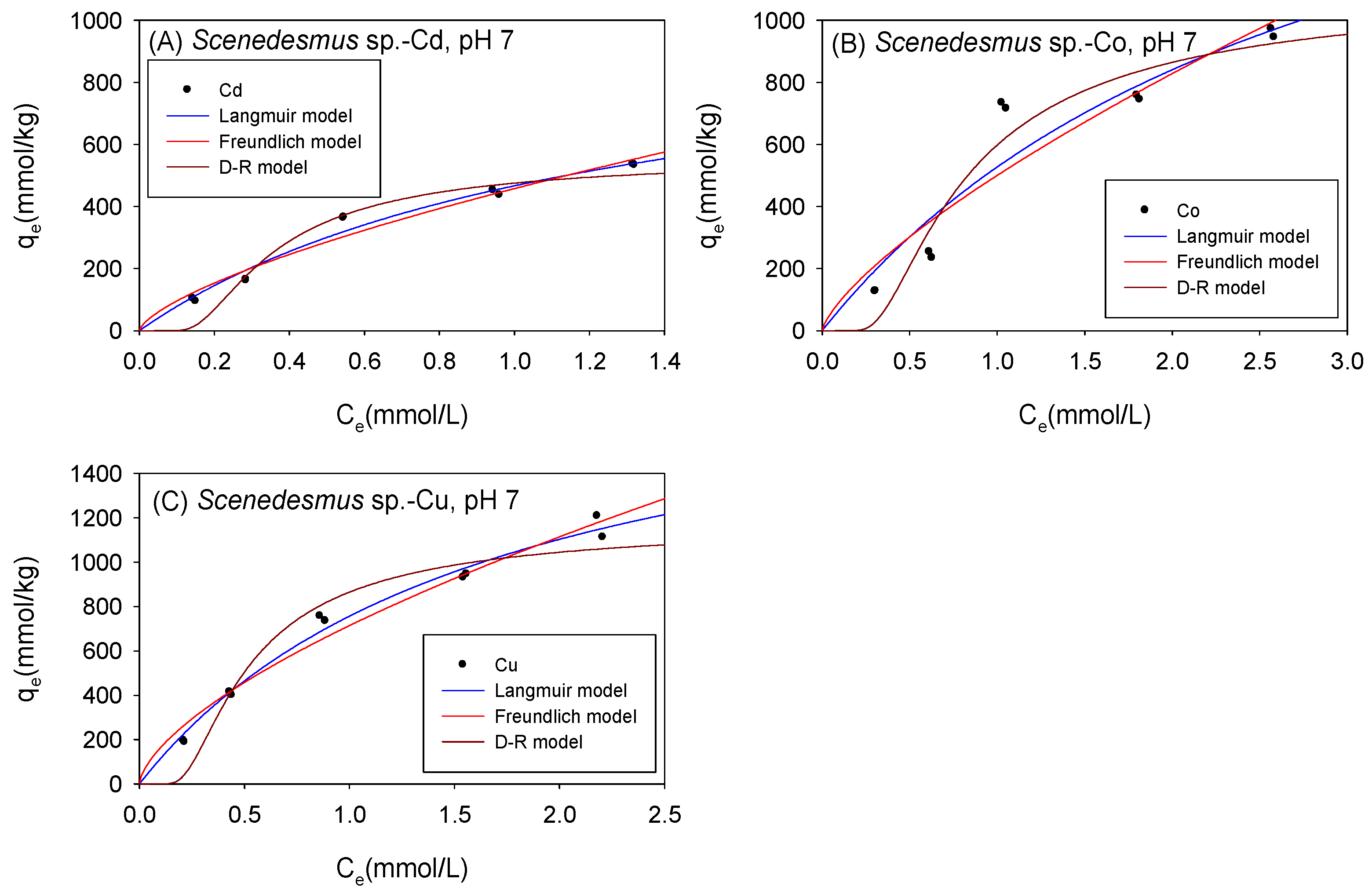
3.3. Biosorption under Acidic Conditions
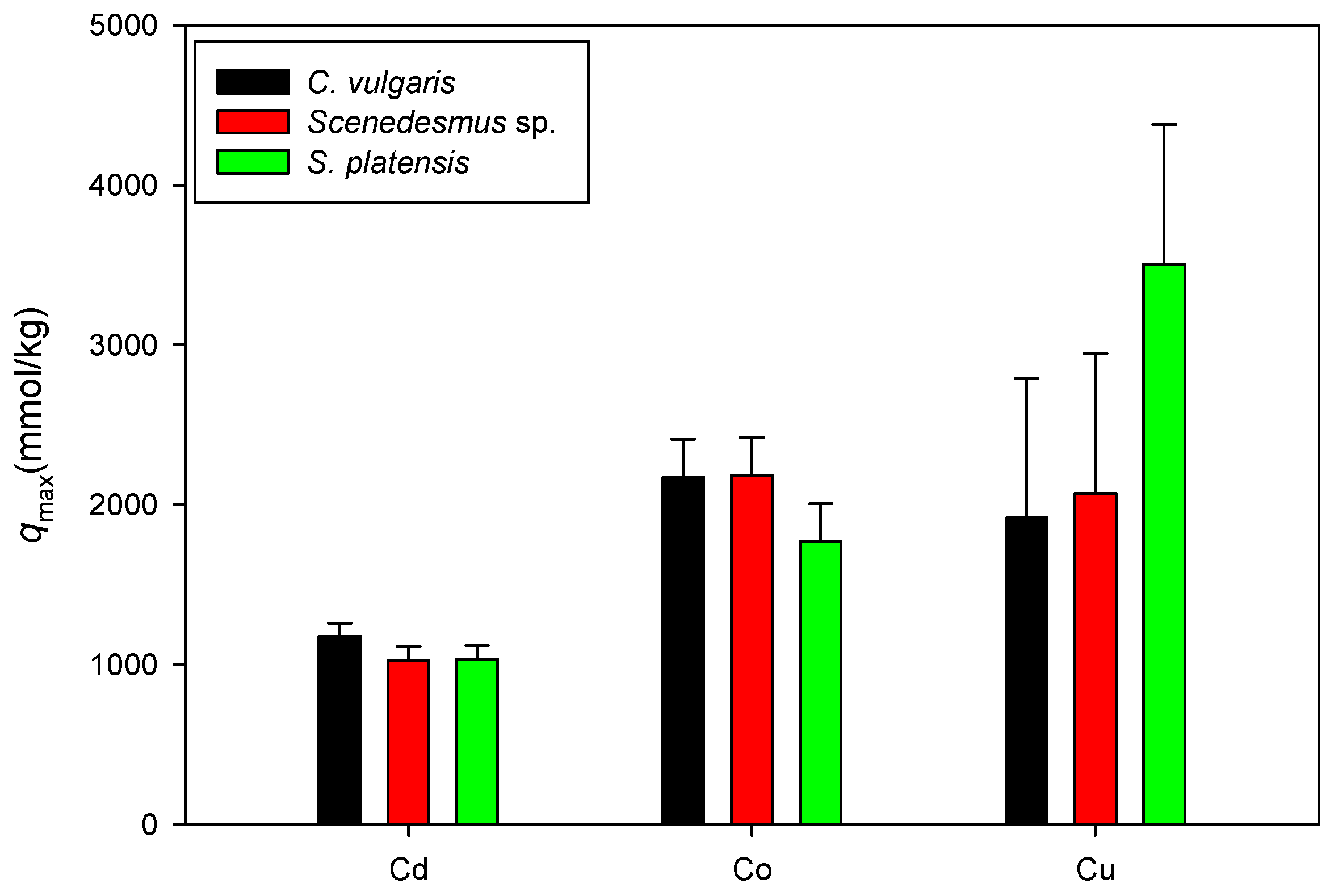
3.4. Comparison of the Maximum Adsorption Capacities of the Cations onto Biomass under Acidic Condition
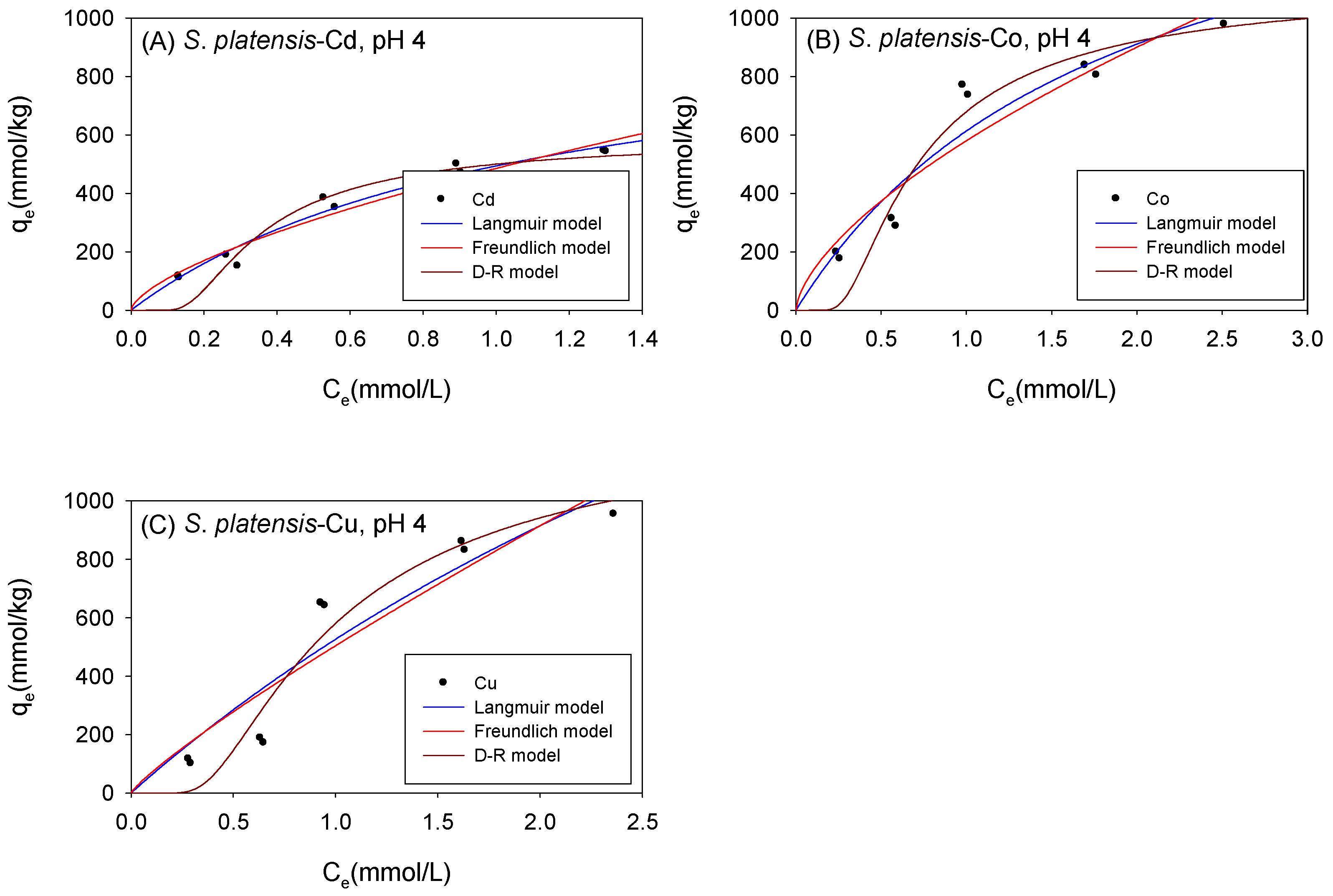
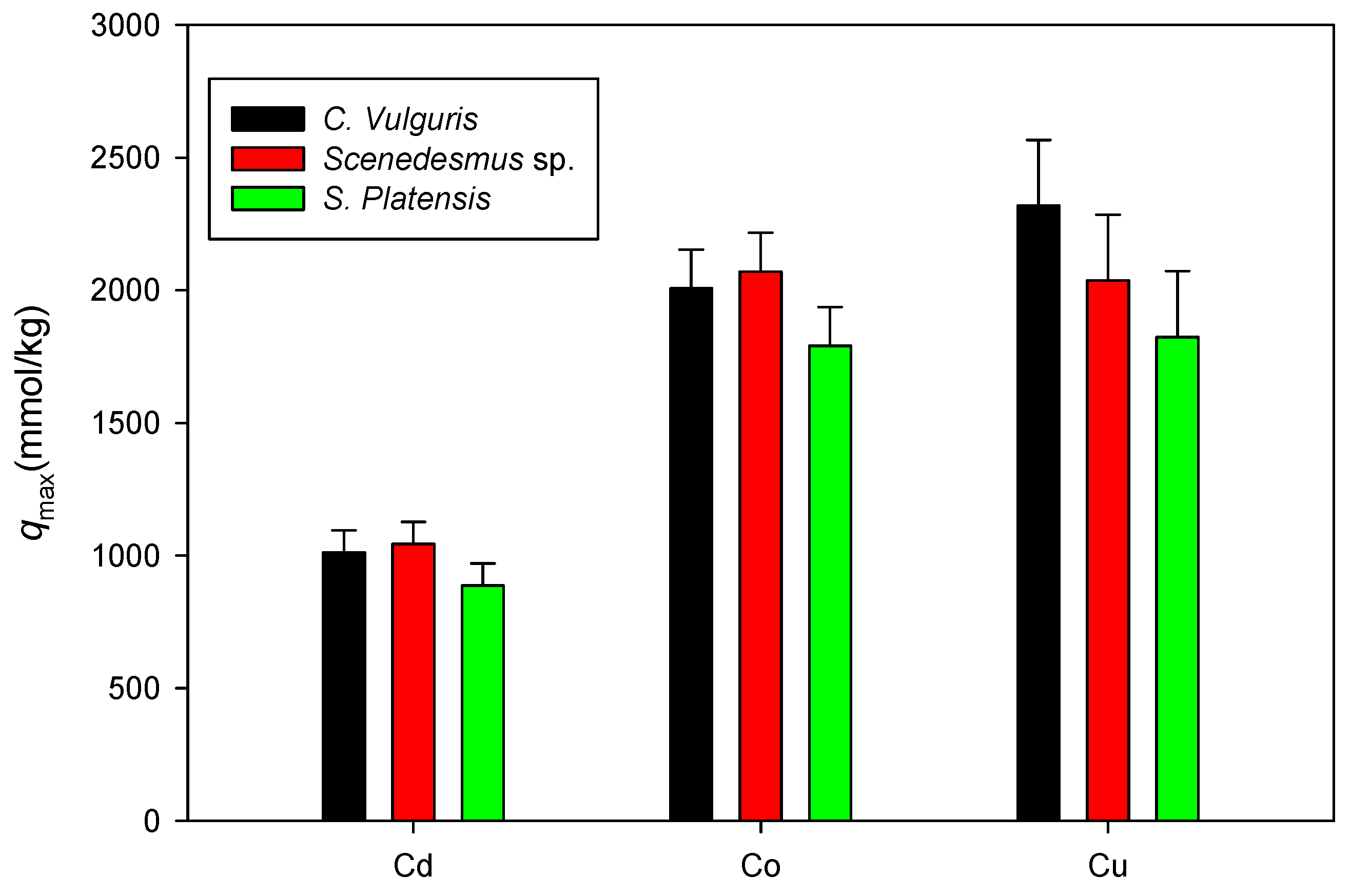
3.5. Biosorption under Neutral Conditions
3.6. Comparison of the Maximum Adsorption Capacities of the Cations onto Biomass under Neutral Conditions
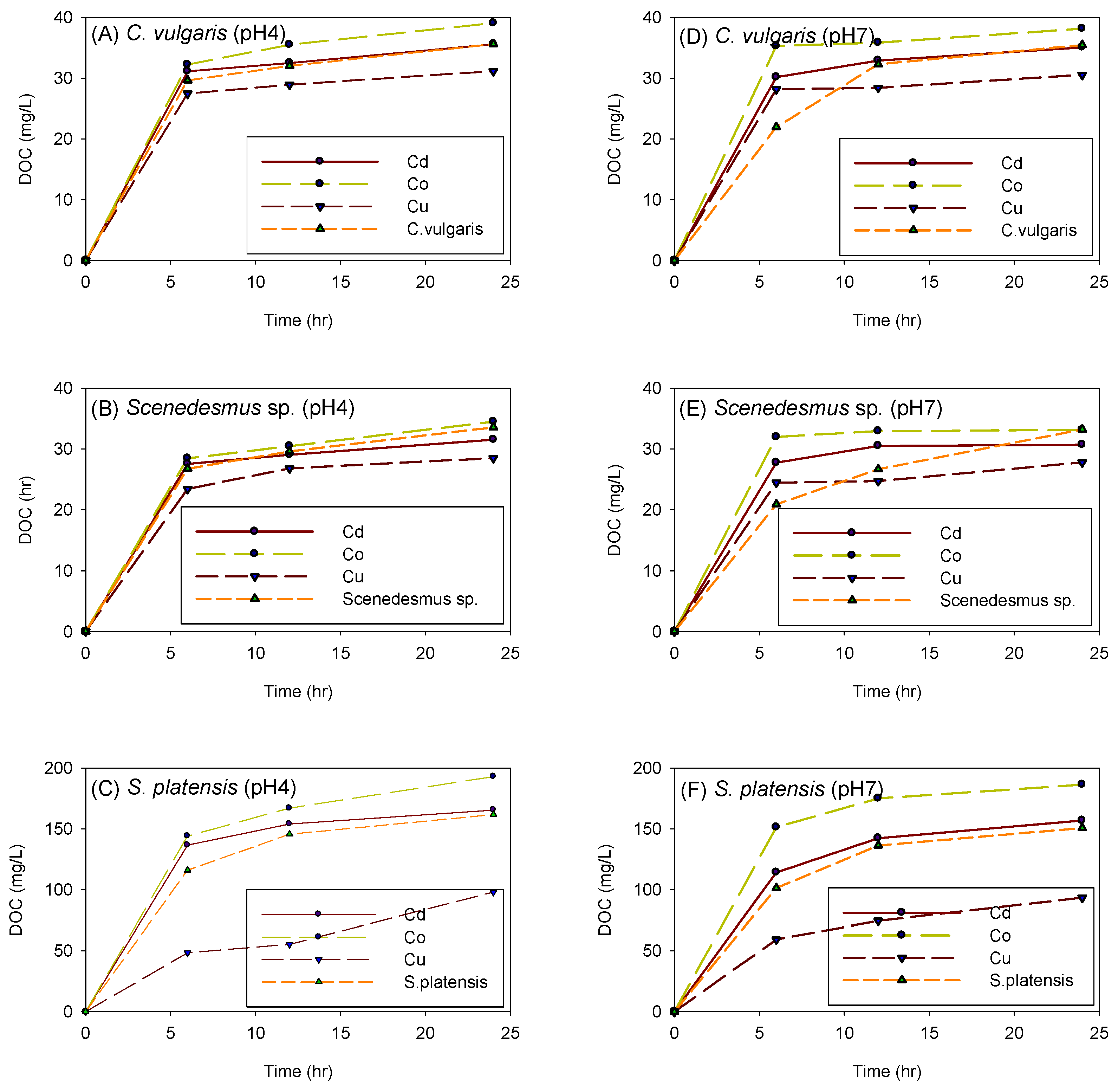
3.7. The Effect of Sorption on the Concentration of Dissolved Carbon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, X.; Zhu, Y.; Zhuo, R. Recent Advances in Phyto-Combined Remediation of Heavy Metal Pollution in Soil. Biotechnol. Adv. 2024, 72, 108337. [Google Scholar] [CrossRef]
- Sharpe, S.; Lenton, T.M. Are We on the Brink of an Electric Vehicle Boom? Only with More Action. Clim. Policy 2021, 21, 421–433. [Google Scholar] [CrossRef]
- Mohtasham, J. Review Article—Renewable Energies. Energy Procedia 2015, 74, 1289–1297. [Google Scholar] [CrossRef]
- Solangi, K.H.; Islam, M.R.; Saidur, R.; Rahim, N.A.; Fayaz, H. A Review on Global Solar Energy Policy. Renew. Sustain. Energy Rev. 2011, 15, 2149–2163. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Jain, A.; Khurana, P.; Kumar, D.; Thatai, S. Ni-Cd Batteries. In Rechargeable Batteries: History, Progress, and Applications; Scrivener Publishing LLC: Austin, TX, USA, 2020; pp. 177–194. [Google Scholar] [CrossRef]
- Stanley, A.G. Cadmium Sulfide Solar Cells. Appl. Solid State Sci. 1975, 5, 251–366. [Google Scholar] [CrossRef]
- DeCarlo, S.; Matthews, D. More than a Pretty Color: The Renaissance of the Cobalt Industry. J. Int. Commer. Econ. 2019, 2019, 1–23. [Google Scholar]
- Iglesias-Émbil, M.; Valero, A.; Ortego, A.; Villacampa, M.; Vilaró, J.; Villalba, G. Raw Material Use in a Battery Electric Car—A Thermodynamic Rarity Assessment. Resour. Conserv. Recycl. 2020, 158, 104820. [Google Scholar] [CrossRef]
- Machura, P.; Li, Q. A Critical Review on Wireless Charging for Electric Vehicles. Renew. Sustain. Energy Rev. 2019, 104, 209–234. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Zhang, M.; Zhu, Y.; Zhuo, R. Removal of Heavy-Metal Pollutants by White Rot Fungi: Mechanisms, Achievements, and Perspectives. J. Clean. Prod. 2022, 354, 131681. [Google Scholar] [CrossRef]
- Kogel, J.E. Sustainable Development and the Minerals Industry. In Engineering Solutions for Sustainability: Materials and Resources II; Springer: Cham, Switzerland, 2016; pp. 25–34. [Google Scholar] [CrossRef]
- Custodio, M.; Cuadrado, W.; Peñaloza, R.; Montalvo, R.; Ochoa, S.; Quispe, J. Human Risk from Exposure to Heavy Metals and Arsenic in Water from Rivers with Mining Influence in the Central Andes of Peru. Water 2020, 12, 1946. [Google Scholar] [CrossRef]
- Delgado, A.; Fernandez, A.; Chirinos, B.; Barboza, G.; Huamaní, E.L. Impact of the Mining Activity on the Water Quality in Peru Applying the Fuzzy Logic with the Grey Clustering Method. IJACSA Int. J. Adv. Comput. Sci. Appl. 2021, 12, 348–357. [Google Scholar] [CrossRef]
- Muma, D.; Besa, B.; Manchisi, J.; Banda, W. Effects of Mining Operations on Air and Water Quality in Mufulira District of Zambia: A Case Study of Kankoyo Township. J. S. Afr. Inst. Min. Metall. 2020, 120, 287–298. [Google Scholar] [CrossRef]
- Atibu, E.K.; Devarajan, N.; Laffite, A.; Giuliani, G.; Salumu, J.A.; Muteb, R.C.; Mulaji, C.K.; Otamonga, J.P.; Elongo, V.; Mpiana, P.T.; et al. Assessment of Trace Metal and Rare Earth Elements Contamination in Rivers around Abandoned and Active Mine Areas. The Case of Lubumbashi River and Tshamilemba Canal, Katanga, Democratic Republic of the Congo. Geochemistry 2016, 76, 353–362. [Google Scholar] [CrossRef]
- Atibu, E.K.; Lacroix, P.; Sivalingam, P.; Ray, N.; Giuliani, G.; Mulaji, C.K.; Otamonga, J.P.; Mpiana, P.T.; Slaveykova, V.I.; Poté, J. High Contamination in the Areas Surrounding Abandoned Mines and Mining Activities: An Impact Assessment of the Dilala, Luilu and Mpingiri Rivers, Democratic Republic of the Congo. Chemosphere 2018, 191, 1008–1020. [Google Scholar] [CrossRef]
- Järup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Boonamnuayvitaya, V.; Chaiya, C.; Tanthapanichakoon, W.; Jarudilokkul, S. Removal of Heavy Metals by Adsorbent Prepared from Pyrolyzed Coffee Residues and Clay. Sep. Purif. Technol. 2004, 35, 11–22. [Google Scholar] [CrossRef]
- Shamsollahi, Z.; Partovinia, A. Recent Advances on Pollutants Removal by Rice Husk as a Bio-Based Adsorbent: A Critical Review. J. Environ. Manag. 2019, 246, 314–323. [Google Scholar] [CrossRef]
- Pak, H.; Phiri, J.; We, J.; Jung, K.; Oh, S. Adsorptive Removal of Arsenic and Lead by Stone Powder/Chitosan/Maghemite Composite Beads. Int. J. Environ. Res. Public Health 2021, 18, 8808. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Zhao, D.; Lin, J.; Gao, P.; Yao, L. Adsorption of Pb2+ onto Freeze-Dried Microalgae and Environmental Risk Assessment. J. Environ. Manag. 2020, 265, 110472. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, M.; Guo, X.; Yang, B.; Zhuo, R. Coupling of Fenton Reaction and White Rot Fungi for the Degradation of Organic Pollutants. Ecotoxicol. Environ. Saf. 2023, 254, 114697. [Google Scholar] [CrossRef]
- Gao, X.; Wei, M.; Zhang, X.; Xun, Y.; Duan, M.; Yang, Z.; Zhu, M.; Zhu, Y.; Zhuo, R. Copper Removal from Aqueous Solutions by White Rot Fungus Pleurotus Ostreatus GEMB-PO1 and Its Potential in Co-Remediation of Copper and Organic Pollutants. Bioresour. Technol. 2024, 395, 130337. [Google Scholar] [CrossRef]
- Vale, M.A.; Ferreira, A.; Pires, J.C.M.; Gonçalves, G.A.L. CO2 Capture Using Microalgae. In Advances in Carbon Capture: Methods, Technologies and Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 381–405. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Çelekli, A.; Bozkurt, H. Bio-Sorption of Cadmium and Nickel Ions Using Spirulina Platensis: Kinetic and Equilibrium Studies. Desalination 2011, 275, 141–147. [Google Scholar] [CrossRef]
- Aksu, Z.; Dönmez, G. Binary Biosorption of Cadmium(II) and Nickel(II) onto Dried Chlorella Vulgaris: Co-Ion Effect on Mono-Component Isotherm Parameters. Process Biochem. 2006, 41, 860–868. [Google Scholar] [CrossRef]
- Tien, C.J. Biosorption of Metal Ions by Freshwater Algae with Different Surface Characteristics. Process Biochem. 2002, 38, 605–613. [Google Scholar] [CrossRef]
- Tüzün, I.; Bayramoǧlu, G.; Yalçin, E.; Başaran, G.; Çelik, G.; Arica, M.Y. Equilibrium and Kinetic Studies on Biosorption of Hg(II), Cd(II) and Pb(II) Ions onto Microalgae Chlamydomonas Reinhardtii. J. Environ. Manag. 2005, 77, 85–92. [Google Scholar] [CrossRef]
- Fraile, A.; Penche, S.; González, F.; Blázquez, M.L.; Muñoz, J.A.; Ballester, A. Biosorption of Copper, Zinc, Cadmium and Nickel by Chlorella Vulgaris. Chem. Ecol. 2005, 21, 61–75. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Removal of Ni and Cu from Single and Binary Metal Solutions by Free and Immobilized Chlorella Vulgaris. Eur. J. Protistol. 2001, 37, 261–271. [Google Scholar] [CrossRef]
- Plöhn, M.; Escudero-Oñate, C.; Funk, C. Biosorption of Cd(II) by Nordic Microalgae: Tolerance, Kinetics and Equilibrium Studies. Algal Res. 2021, 59, 102471. [Google Scholar] [CrossRef]
- Hockaday, J.; Harvey, A.; Velasquez-Orta, S. A Comparative Analysis of the Adsorption Kinetics of Cu2+ and Cd2+ by the Microalgae Chlorella Vulgaris and Scenedesmus Obliquus. Algal Res. 2022, 64, 102710. [Google Scholar] [CrossRef]
- Bordoloi, N.; Goswami, R.; Kumar, M.; Kataki, R. Biosorption of Co (II) from Aqueous Solution Using Algal Biochar: Kinetics and Isotherm Studies. Bioresour. Technol. 2017, 244, 1465–1469. [Google Scholar] [CrossRef]
- Skousen, J.G.; Sexstone, A.; Ziemkiewicz, P.F. Acid Mine Drainage Control and Treatment. In Reclamation of Drastically Disturbed Lands; American Society of Agronomy: Madison, WI, USA, 2015; pp. 131–168. [Google Scholar] [CrossRef]
- Phiri, J.T. Biosorption of Cd2+, Co2+, and Cu2+ onto Chlorella Vulgaris, Scenedesmus sp., and Spirulina Platensis under Acidic and Neutral Conditions. Master’s Thesis, School of Architectural, Civil, Environmental and Energy Engineering, Major in Civil Engineering The Graduate School, Kyungpook National University, Daegu, Republic of Korea, 2022. [Google Scholar]
- Oh, S.; Kwak, M.Y.; Shin, W.S. Competitive Sorption of Lead and Cadmium onto Sediments. Chem. Eng. J. 2009, 152, 376–388. [Google Scholar] [CrossRef]
- Dubinin, M.M. The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Equilibrium and Kinetic Modelling of Cadmium(II) Biosorption by Nonliving Algal Biomass Oedogonium Sp. from Aqueous Phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Sikandar, M. Study of Sorption and Desorption of Cd (II) from Aqueous Solution Using Isolated Green Algae Chlorella Vulgaris. Appl. Water Sci. 2018, 8, 225. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Biosorption of Heavy Metal Ions by Green Alga Neochloris oleoabundans: Effects of Metal Ion Properties and Cell Wall Structure. J. Hazard Mater. 2021, 418, 126336. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Macena, M.; Esteves, B.; Guiné, R.P.F. Ideal PH for the Adsorption of Metal Ions Cr6+, Ni2+, Pb2+ in Aqueous Solution with Different Adsorbent Materials. Open Agric. 2021, 6, 115–123. [Google Scholar] [CrossRef]
- Driver, T.; Bajhaiya, A.K.; Allwood, J.W.; Goodacre, R.; Pittman, J.K.; Dean, A.P. Metabolic Responses of Eukaryotic Microalgae to Environmental Stress Limit the Ability of FT-IR Spectroscopy for Species Identification. Algal Res. 2015, 11, 148–155. [Google Scholar] [CrossRef]
- Mecozzi, M.; Pietroletti, M.; Tornambé, A. Molecular and Structural Characteristics in Toxic Algae Cultures of Ostreopsis ovata and Ostreopsis spp. Evidenced by FTIR and FTNIR Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1572–1580. [Google Scholar] [CrossRef]
- Eren, E.; Afsin, B.; Onal, Y. Removal of Lead Ions by Acid Activated and Manganese Oxide-Coated Bentonite. J. Hazard. Mater. 2009, 161, 677–685. [Google Scholar] [CrossRef]
- Chang, H.H.; Tung, H.H.; Chao, C.C.; Wang, G.S. Occurrence of Haloacetic Acids (HAAs) and Trihalomethanes (THMs) in Drinking Water of Taiwan. Environ. Monit. Assess. 2010, 162, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Dissolved Organic Carbon I DOC Analyzers & Sensors I Real Tech Water. Available online: https://realtechwater.com/parameters/dissolved-organic-carbon/ (accessed on 29 January 2023).



| Model | Parameter | Cd | Co | Cu |
|---|---|---|---|---|
| Langmuir | qmL (mmol/kg) | 1175 | 2172 | 1917 |
| bL (L/mmol) | 0.617 | 0.306 | 0.507 | |
| R2 | 0.947 | 0.95 | 0.97 | |
| SSE | 2434 | 14,430 | 5475 | |
| RL | 0.474 | 0.487 | 0.377 | |
| Freundlich | KF (mmol1-NLN/kg) | 439.7 | 483.7 | 609.8 |
| N(-) | 1.363 | 1.338 | 1.51 | |
| R2 | 0.923 | 0.925 | 0.944 | |
| SSE | 1375 | 7322 | 4111 | |
| D-R | qmD (mmol/kg) | 647.7 | 1383 | 1347 |
| Kdr (mol2/J2) | 1.380 × 10−8 | 3.669 × 10−8 | 2.584 × 10−8 | |
| R2 | 0.945 | 0.978 | 0.953 | |
| SSE | 18,700 | 16,274 | 62,132 | |
| E (kJ/mol) | 6.019 | 3.691 | 4.4 |
| Model | Parameter | Cd | Co | Cu |
|---|---|---|---|---|
| Langmuir | qmL (mmol/kg) | 1027 | 2182 | 2070 |
| bL (L/mmol) | 0.682 | 0.292 | 0.415 | |
| R2 | 0.946 | 0.899 | 0.92 | |
| SSE | 1773 | 25,008 | 13,751 | |
| RL | 0.449 | 0.498 | 0.426 | |
| Freundlich | KF (mmol1-NLN/kg) | 407.7 | 466.4 | 575.3 |
| N(-) | 1.407 | 1.31 | 1.421 | |
| R2 | 0.923 | 0.873 | 0.889 | |
| SSE | 1026 | 1111 | 7448 | |
| D-R | qmD (mmol/kg) | 592.8 | 1398 | 1474 |
| Kdr (mol2/J2) | 1.310 × 10−8 | 3.839 × 10−8 | 3.290 × 10−8 | |
| R2 | 0.945 | 0.97 | 0.948 | |
| SSE | 14,720 | 1920 | 46,868 | |
| E (kJ/mol) | 6.178 | 3.609 | 3.894 |
| Model | Parameter | Cd | Co | Cu |
|---|---|---|---|---|
| Langmuir | qmL (mmol/kg) | 1034 | 1768 | 3503 |
| bL (L/mmol) | 0.913 | 0.531 | 0.177 | |
| R2 | 0.969 | 0.926 | 0.895 | |
| SSE | 291.1 | 3,449 | 22,557 | |
| RL | 0.378 | 0.351 | 0.635 | |
| Freundlich | KF (mmol1-NLN/kg) | 485.3 | 579.6 | 503.7 |
| N(-) | 1.532 | 1.576 | 1.163 | |
| R2 | 0.95 | 0.9 | 0.882 | |
| SSE | 537.7 | 3028 | 1001 | |
| D-R | qmD (mmol/kg) | 638.6 | 1359 | 1754 |
| Kdr (mol2/J2) | 1.033 × 10−8 | 2.923 × 10−8 | 4.662 × 10−8 | |
| R2 | 0.901 | 0.905 | 0.943 | |
| SSE | 39,385 | 80,097 | 21,410 | |
| E (kJ/mol) | 6.957 | 4.136 | 3.275 |
| Model | Parameter | Cd | Co | Cu |
|---|---|---|---|---|
| Langmuir | qmL (mmol/kg) | 1012 | 2007 | 2318 |
| bL (L/mmol) | 0.941 | 0.402 | 0.522 | |
| R2 | 0.951 | 0.921 | 0.99 | |
| SSE | 1867 | 11,272 | 526.1 | |
| RL | 0.371 | 0.419 | 0.371 | |
| Freundlich | KF (mmol1-NLN/kg) | 481.5 | 544.6 | 755.7 |
| N(-) | 1.531 | 1.438 | 1.501 | |
| R2 | 0.92 | 0.893 | 0.977 | |
| SSE | 1259 | 6159 | 2057 | |
| D-R | qmD (mmol/kg) | 642.6 | 1424 | 1558 |
| Kdr (mol2/J2) | 1.041 × 10−8 | 3.334 × 10−8 | 2.359 × 10−8 | |
| R2 | 0.939 | 0.942 | 0.926 | |
| SSE | 22,382 | 34,713 | 168,086 | |
| E (kJ/mol) | 6.93 | 3.873 | 4.604 |
| Model | Parameter | Cd | Co | Cu |
|---|---|---|---|---|
| Langmuir | qmL (mmol/kg) | 1044 | 2070 | 2037 |
| bL (L/mmol) | 0.808 | 0.342 | 0.591 | |
| R2 | 0.975 | 0.894 | 0.986 | |
| SSE | 724 | 12,638 | 1486 | |
| RL | 0.408 | 0.459 | 0.343 | |
| Freundlich | KF (mmol1-NLN/kg) | 457.7 | 500.1 | 713.868 |
| N(-) | 1.471 | 1.374 | 1.556 | |
| R2 | 0.958 | 0.872 | 0.972 | |
| SSE | 637.9 | 6280 | 2376.231 | |
| D-R | qmD (mmol/kg) | 605.8 | 1389 | 1332 |
| Kdr (mol2/J2) | 1.021 × 10−8 | 3.550 × 10−8 | 1.819 × 10−8 | |
| R2 | 0.934 | 0.927 | 0.929 | |
| SSE | 18,822 | 17,851 | 62,185 | |
| E (kJ/mol) | 6.998 | 3.753 | 5.243 |
| Model | Parameter | Cd | Co | Cu |
|---|---|---|---|---|
| Langmuir | qmL (mmol/kg) | 886.7 | 1790 | 1824 |
| bL (L/mmol) | 1.267 | 0.621 | 0.646 | |
| R2 | 0.942 | 0.925 | 0.969 | |
| SSE | 677.5 | 5081 | 357.2 | |
| RL | 0.305 | 0.319 | 0.323 | |
| Freundlich | KF (mmol1-NLN/kg) | 488.6 | 643.8 | 675.4 |
| N(-) | 1.718 | 1.636 | 1.616 | |
| R2 | 0.943 | 0.897 | 0.956 | |
| SSE | 12.82 | 3782 | 1405 | |
| D-R | qmD (mmol/kg) | 624.6 | 1349 | 1318 |
| Kdr (mol2/J2) | 9.397 × 10−9 | 2.344 × 10−8 | 2.104 × 10−8 | |
| R2 | 0.812 | 0.911 | 0.902 | |
| SSE | 76,511 | 58,372 | 117,988 | |
| E (kJ/mol) | 7.294 | 4.619 | 4.875 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phiri, J.T.; Oh, S. Biosorption of Cd(II), Co(II), and Cu(II) onto Microalgae under Acidic and Neutral Conditions. Sustainability 2024, 16, 6342. https://doi.org/10.3390/su16156342
Phiri JT, Oh S. Biosorption of Cd(II), Co(II), and Cu(II) onto Microalgae under Acidic and Neutral Conditions. Sustainability. 2024; 16(15):6342. https://doi.org/10.3390/su16156342
Chicago/Turabian StylePhiri, Jesse T., and Sanghwa Oh. 2024. "Biosorption of Cd(II), Co(II), and Cu(II) onto Microalgae under Acidic and Neutral Conditions" Sustainability 16, no. 15: 6342. https://doi.org/10.3390/su16156342
APA StylePhiri, J. T., & Oh, S. (2024). Biosorption of Cd(II), Co(II), and Cu(II) onto Microalgae under Acidic and Neutral Conditions. Sustainability, 16(15), 6342. https://doi.org/10.3390/su16156342






