Activated Carbons for Removing Ammonia from Piggery Vent Air: A Promising Tool for Mitigating the Environmental Impact of Large-Scale Pig Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbonization
2.3. Steam and CO2 Activation
2.4. Chemical (KOH) Activation
2.5. Proximate Analysis
2.6. Ultimate Analysis
2.7. Heat of Combustion
2.8. Thermogravimetric Analysis
2.9. Porous Texture Determination
2.10. Ammonia Sorption Measurements
2.11. Methodology of Optimization the Activation Method
2.12. Economic Analysis of Activated Carbon Production
3. Results and Discussion
3.1. Raw Materials Characterization
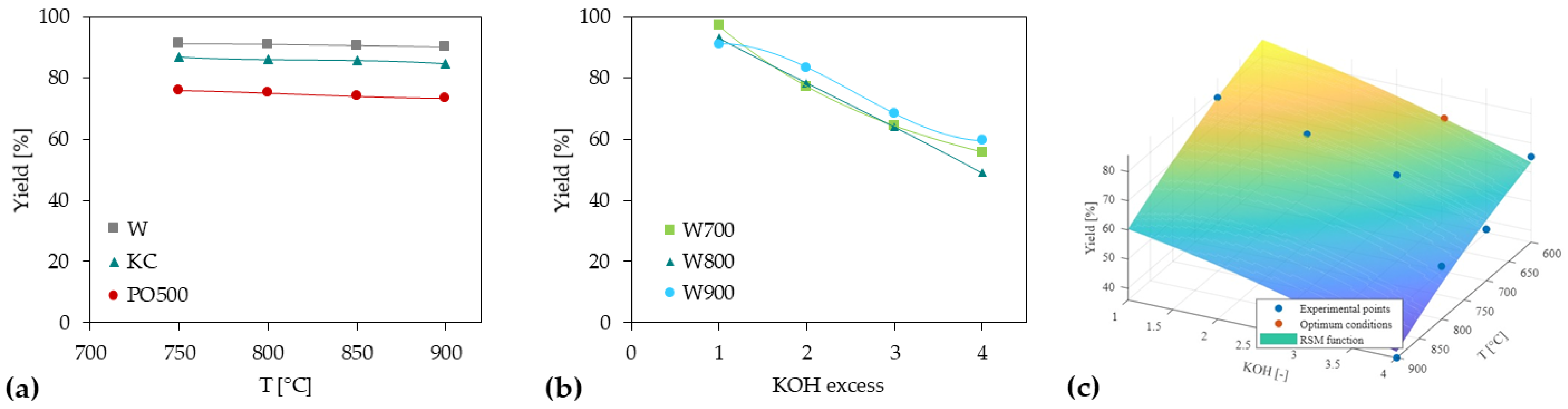
3.2. Activated Carbon Preparation
3.3. Porous Structure of Chars and Activated Carbons
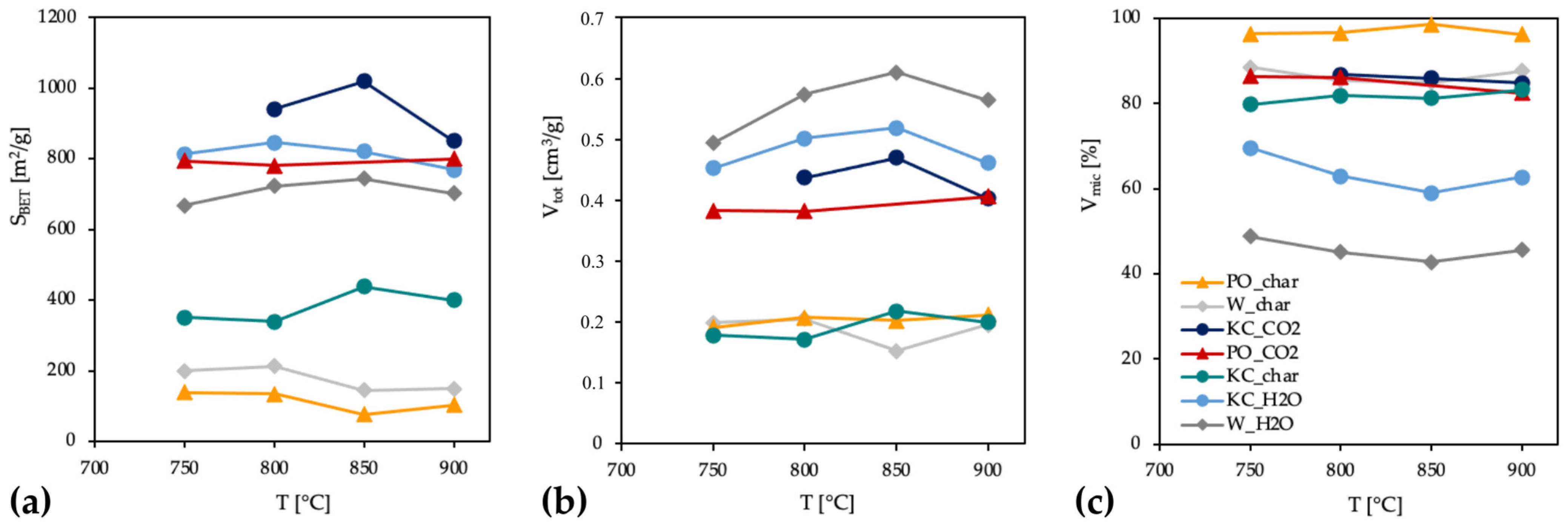
3.3.1. Chars and ACs from Physical Activation
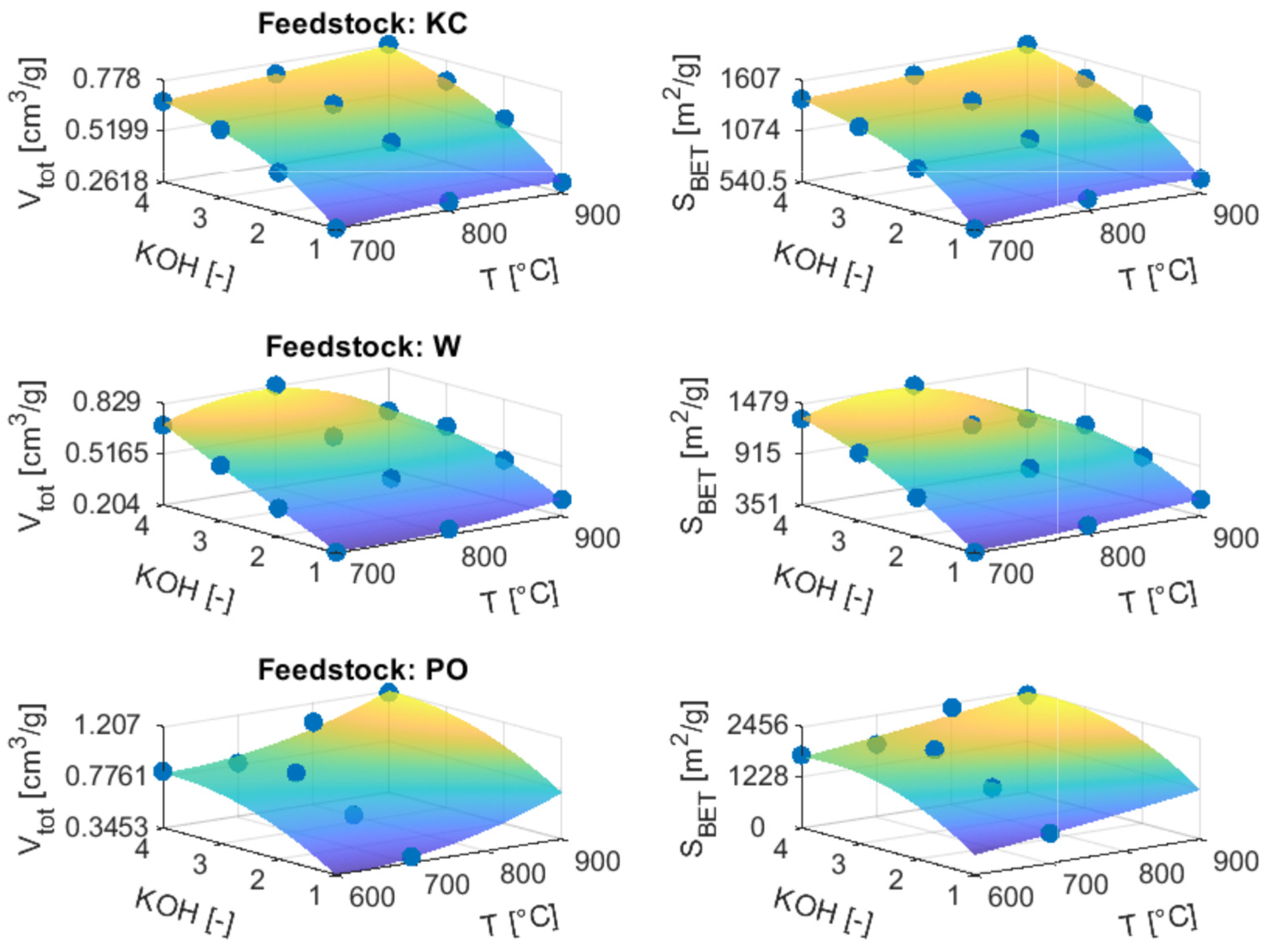
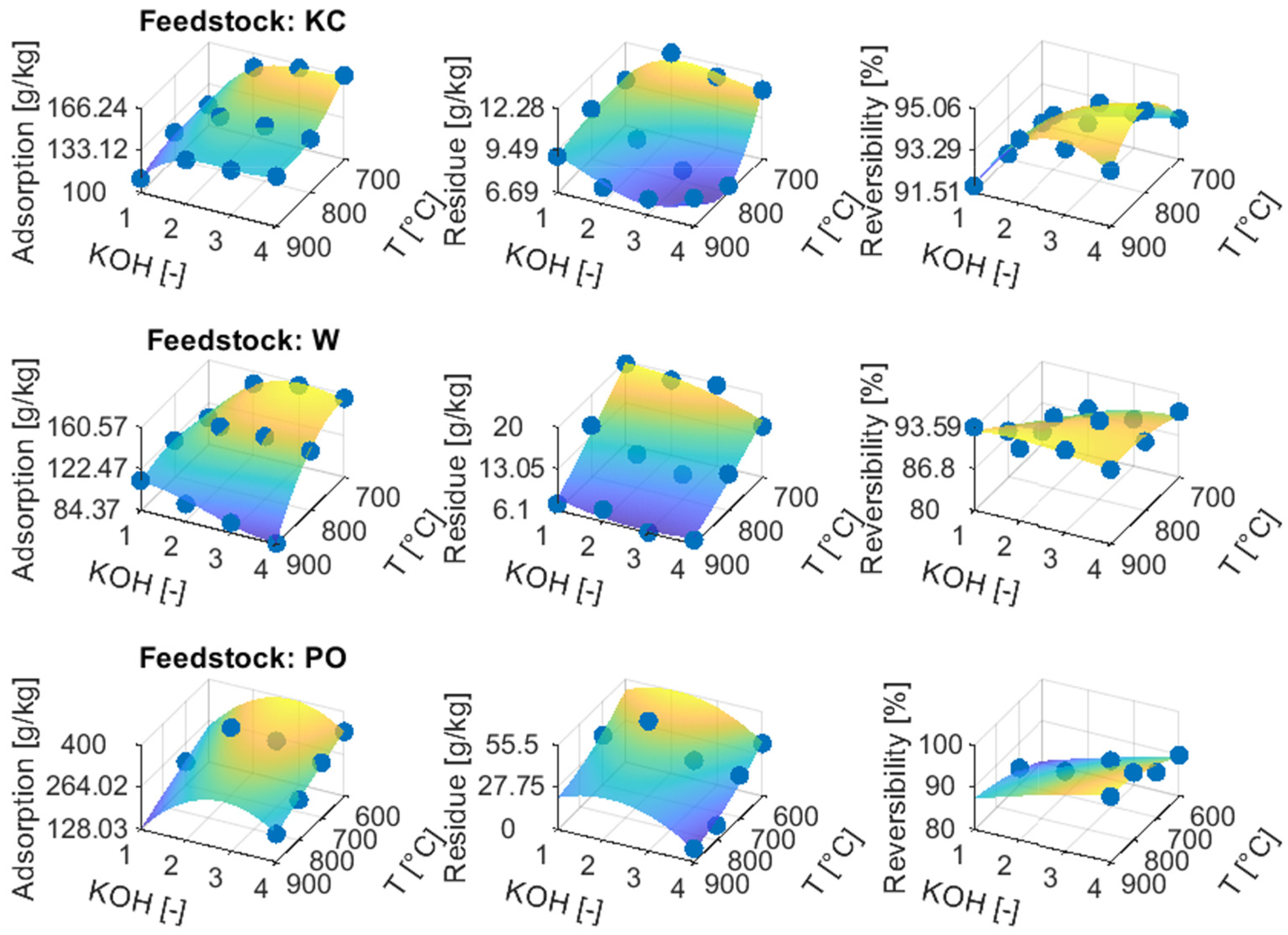
3.3.2. ACs from Chemical Activation
3.4. Ammonia Sorption
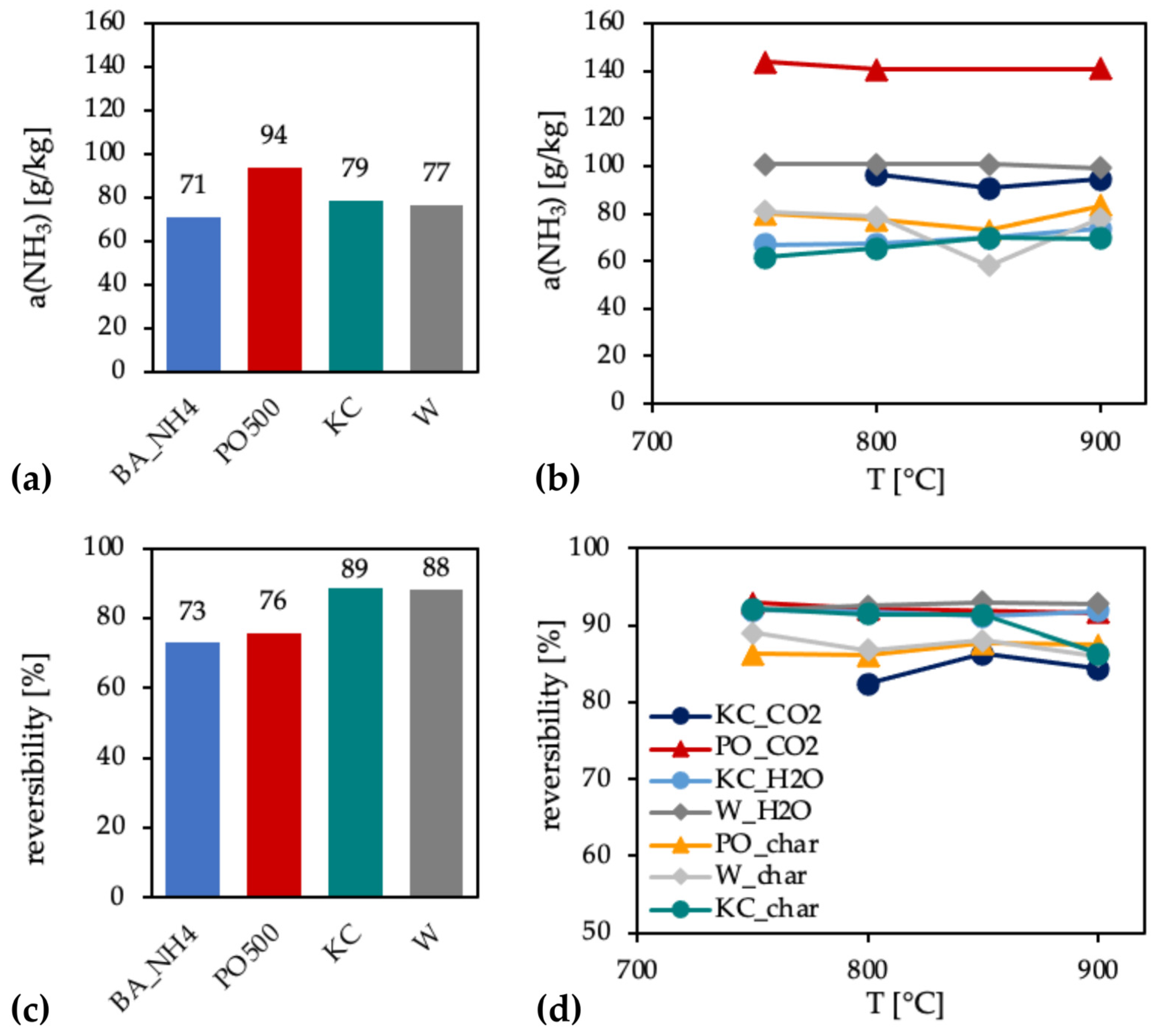
3.4.1. Chars and ACs from Physical Activation
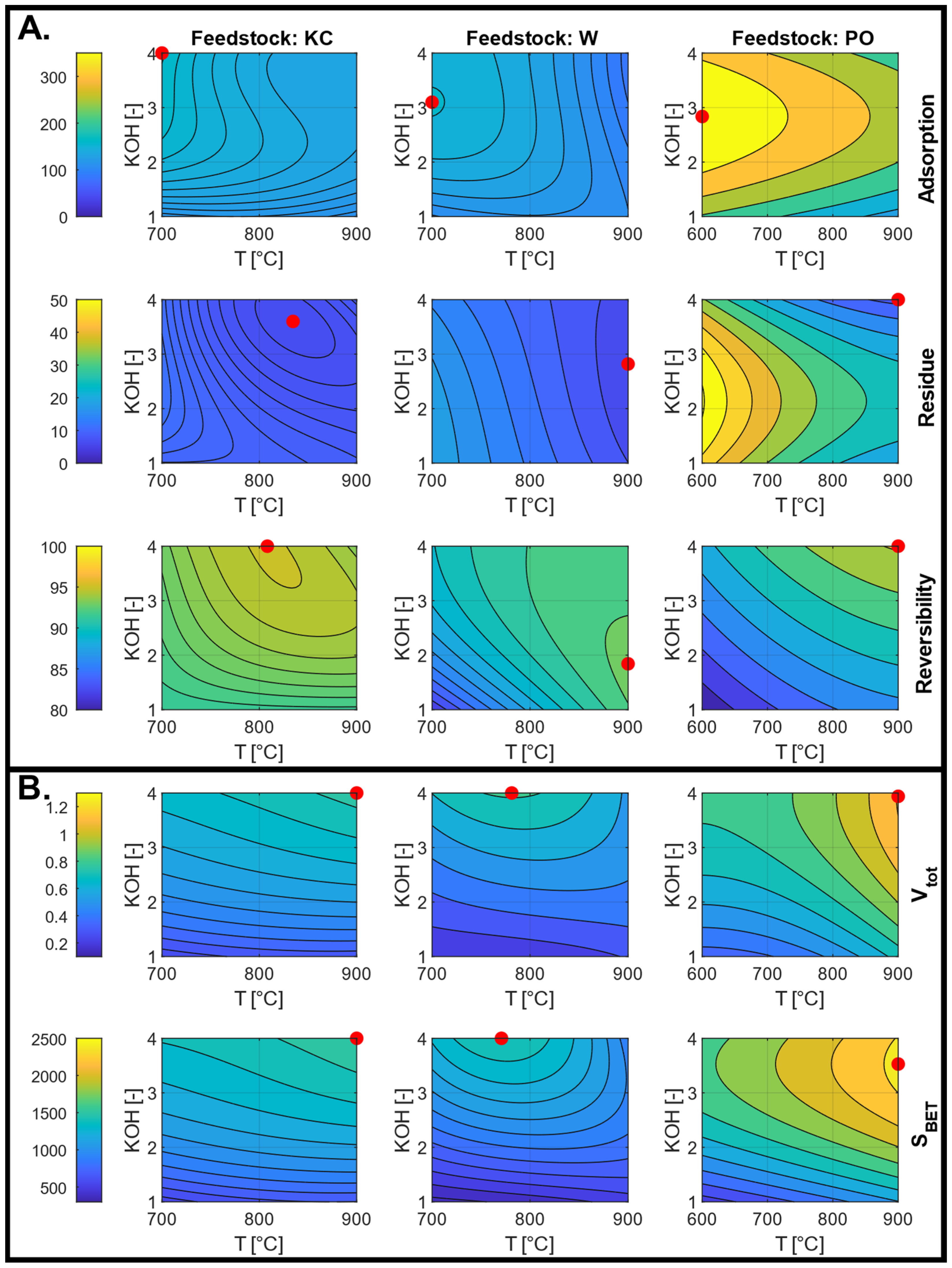
3.4.2. ACs from Chemical Activation
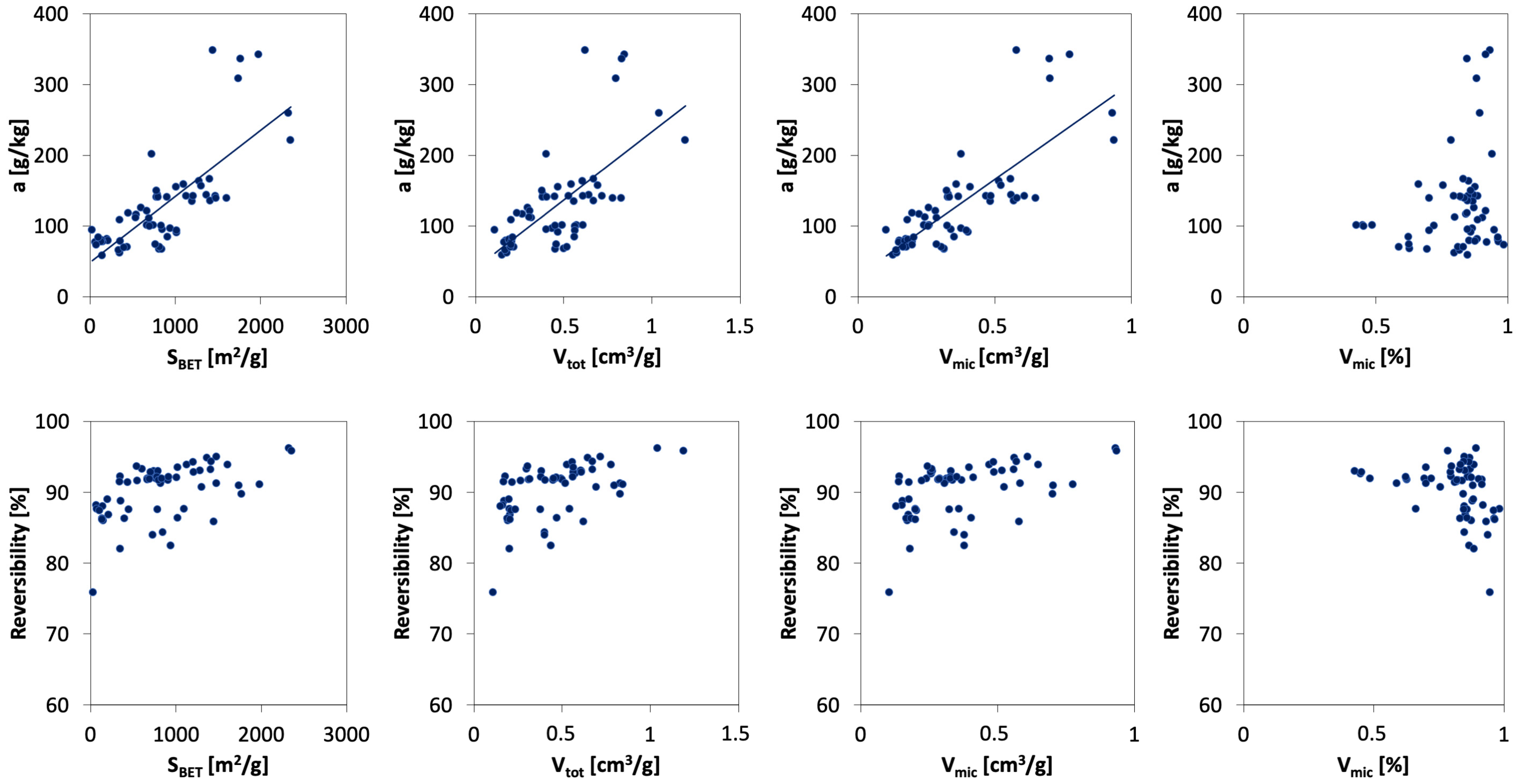
3.5. Effect of Porous Texture Parameters on Ammonia Sorption
3.6. Economic Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.W.; Gormley, A.; Jang, K.B.; Duarte, M.E. Invited Review—Current Status of Global Pig Production: An Overview and Research Trends. Anim. Biosci. 2024, 37, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Colmenero, M.; Costantini, M.; Bàllega, A.; Zoli, M.; Andón, M.; Cerrillo, M.; Fàbrega, E.; Bonmatí, A.; Guarino, M.; Bacenetti, J. Air Treatment Technologies in Pig Farms. Life Cycle Assessment of Dry and Wet Scrubbers in Northern Italy and Northeastern Spain. Sci. Total Environ. 2024, 922, 171197. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, M.; Eglin, T.; Cellier, P.; Colomb, V.; Cohan, J.-P.; Decuq, C.; Delabuis, M.; Edouard, N. Measuring Emissions from Livestock Farming: Greenhouse Gases, Ammonia and Nitrogen Oxides; INRA-ADEME: Paris, France, 2016. [Google Scholar]
- Guthrie, S.; Giles, S.; Dunkerley, F.; Tabaqchali, H.; Harshfield, A.; Ioppolo, B.; Manville, C. The Impact of Ammonia Emissions from Agriculture on Biodiversity An Evidence Synthesis; RAND Corporation and The Royal Society: Cambridge, UK, 2018. [Google Scholar]
- Ma, R.; Li, K.; Guo, Y.; Zhang, B.; Zhao, X.; Linder, S.; Guan, C.H.; Chen, G.; Gan, Y.; Meng, J. Mitigation Potential of Global Ammonia Emissions and Related Health Impacts in the Trade Network. Nat. Commun. 2021, 12, 6308. [Google Scholar] [CrossRef] [PubMed]
- Konkol, D.; Popiela, E.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Moustakas, K.; Opaliński, S.; Korczyński, M.; Witek-Krowiak, A.; Chojnacka, K. Recent Innovations in Various Methods of Harmful Gases Conversion and Its Mechanism in Poultry Farms. Environ. Res. 2022, 214, 113825. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Strader, R.; Davidson, C. Airborne Reduced Nitrogen: Ammonia Emissions from Agriculture and Other Sources. Environ. Int. 2003, 29, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Philippe, F.X.; Cabaraux, J.F.; Nicks, B. Ammonia Emissions from Pig Houses: Influencing Factors and Mitigation Techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Vogel, G.J.; Kayser, W.C.; Savides, M.C.; Edmonds, M.; Van Engen, N.; Edwards, T.M.; Allen, J.B.; Rincker, P.J.; Lawrence, T.E.; McKeith, F.K.; et al. Effects of Lubabegron Removal on Tissue Concentration, Calculated Ammonia Gas Emissions, Live Performance, and Carcass Characteristics of Beef Steers. Appl. Anim. Sci. 2023, 39, 484–493. [Google Scholar] [CrossRef]
- Ossowski, M.; Wlazło, Ł.; Bis-Wencel, H.; Krzaczek, P.; Nowakowicz-Dębek, B. The Use of Natural Sorbents in the Diet of Pigs as a Method for Reducing Gaseous Pollutants and Manure Nutrients from Livestock Housing. Przem. Chem. 2022, 1, 39–45. [Google Scholar] [CrossRef]
- Le Dinh, P.; van der Peet-Schwering, C.M.C.; Ogink, N.W.M.; Aarnink, A.J.A. Effect of Diet Composition on Excreta Composition and Ammonia Emissions from Growing-Finishing Pigs. Animals 2022, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Rong, L. Effect of Partial Pit Exhaust Ventilation System on Ammonia Removal Ratio and Mass Transfer Coefficients from Different Emission Sources in Pig Houses. Energy Built Environ. 2020, 1, 343–350. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Pilot Plant for the Capture of Ammonia from the Atmosphere of Pig and Poultry Farms Using Gas-Permeable Membrane Technology. Membranes 2021, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Cao, F.; Bai, L.; Chang, F.; Zhou, B.; Duan, Y.; Zeng, S.; Dong, H.; Li, Z.; Zhang, X. Process Simulation and Optimization of Ammonia-Containing Gas Separation and Ammonia Recovery with Ionic Liquids. ACS Sustain. Chem. Eng. 2021, 9, 312–325. [Google Scholar] [CrossRef]
- Chmielarz, L.; Jabłońska, M. Advances in Selective Catalytic Oxidation of Ammonia to Dinitrogen: A Review. RSC Adv. 2015, 5, 43408–43431. [Google Scholar] [CrossRef]
- Shang, B.; Zhou, T.; Tao, X.; Chen, Y. Greenhouse Gas Emissions From Biofilters for Composting Exhaust Ammonia Removal. Front. Bioeng. Biotechnol. 2022, 10, 918365. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Baig, Z.T.; Yeung, M.; Soomro, A.F.; Lu, L.; Xi, J. Low-Concentration Organics Mitigate the Inhibition of Free Nitrous Acid on Nitrification in Biofilters for Gaseous Ammonia Removal. Chem. Eng. J. 2023, 476, 146757. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. Volatile Organic Compounds, Ammonia and Hydrogen Sulphide Removal Using a Two-Stage Membrane Biofiltration Process. Chem. Eng. Res. Des. 2021, 165, 69–80. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, D.; Chen, X.; Wang, X.; Dong, B.; Dai, X. Vacuum Ammonia Stripping from Liquid Digestate: Effects of PH, Alkalinity, Temperature, Negative Pressure and Process Optimization. J. Environ. Sci. 2025, 149, 638–650. [Google Scholar] [CrossRef]
- Jeong, M.; Zhao, S.; Ji, Y.; Khan, S.A.; Saqlain, S.; Kim, Y.D. Efficacious Removal of Benzene and Ammonia Gases over Visible Light Irradiated Activated Carbon. Appl. Surf. Sci. 2023, 613, 156012. [Google Scholar] [CrossRef]
- Johnson, K.I.; Borges, W.; Sharma, P.R.; Sharma, S.K.; Chang, H.Y.; Abou-Krisha, M.M.; Alhamzani, A.G.; Hsiao, B.S. Cellulose Sulfate Nanofibers for Enhanced Ammonium Removal. Nanomaterials 2024, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, F.; Jeyavelan, M.; Hussain, T.; Hudson, M.S.L.; Nagarajan, S. Ammonia Gas Adsorption Study on Graphene Oxide Based Sensing Device under Different Humidity Conditions. Mater. Chem. Phys. 2020, 242, 122485. [Google Scholar] [CrossRef]
- Sha, Y.; Liu, J.; Yu, J.; Xu, S.; Yan, W.; Li, Z.; Shahbaz, M. Effect of Graphene Oxide on the Ammonia Removal and Bacterial Community in a Simulated Wastewater Treatment Process. J. Environ. Eng. 2020, 146, 04020097. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.; Fang, D.; Zhang, P.; Li, F.; Qiu, X.; Mo, X.; Li, K. Polyaniline-Derived Mesoporous Carbon Electrode for Selective and Efficient Ammonium Removal with in a Flow-Electrode Capacitive Deionization System. J. Environ. Chem. Eng. 2023, 11, 110857. [Google Scholar] [CrossRef]
- Li, J.F.; Li, Z.M.; Xu, J.B.; Guo, C.Y.; Fang, G.W.; Zhou, Y.; Sun, M.S.; Tao, D.J. Demethylation of Waste Alkali Lignin for Rapid and Efficient Ammonia Adsorption. Ind. Eng. Chem. Res. 2024, 63, 3282–3289. [Google Scholar] [CrossRef]
- Krounbi, L.; Enders, A.; Anderton, C.R.; Engelhard, M.H.; Hestrin, R.; Torres-Rojas, D.; Dynes, J.J.; Lehmann, J. Sequential Ammonia and Carbon Dioxide Adsorption on Pyrolyzed Biomass to Recover Waste Stream Nutrients. ACS Sustain. Chem. Eng. 2020, 8, 7121–7131. [Google Scholar] [CrossRef]
- Tokushige, M.; Ryu, J. Adsorption and Desorption Behaviors of Ammonia on Zeolites at 473 K by the Pressure-Swing Method. ACS Omega 2023, 8, 32536–32543. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.T.; Kim, J.; Kim, N.Y.; Lee, J.K.; Joo, J.B. Effect of Pore Texture Property of Mesoporous Alumina on Adsorption Performance of Ammonia Gas. J. Ind. Eng. Chem. 2020, 91, 129–138. [Google Scholar] [CrossRef]
- Rezaei, E.; Schlageter, B.; Nemati, M.; Predicala, B. Evaluation of Metal Oxide Nanoparticles for Adsorption of Gas Phase Ammonia. J. Environ. Chem. Eng. 2017, 5, 422–431. [Google Scholar] [CrossRef]
- Zong, K.; Li, K.; Zhou, Z.; Gong, L.; Deng, D. Highly Efficient and Reversible Adsorption of Ammonia by Incorporation of Deep Eutectic Solvents into Silica Gel and Al2O3. New J. Chem. 2022, 46, 15959–15966. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, J.; Li, P.; Dong, H.; Wang, H.; Zhang, X.; Zhang, X. Efficient Adsorption of Ammonia by Incorporation of Metal Ionic Liquids into Silica Gels as Mesoporous Composites. Chem. Eng. J. 2019, 370, 81–88. [Google Scholar] [CrossRef]
- Jung, D.; Chen, Z.; Alayoglu, S.; Mian, M.R.; Goetjen, T.A.; Idrees, K.B.; Kirlikovali, K.O.; Islamoglu, T.; Farha, O.K. Postsynthetically Modified Polymers of Intrinsic Microporosity (PIMs) for Capturing Toxic Gases. ACS Appl. Mater. Interfaces 2021, 13, 10409–10415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Wang, H.; Zhao, Y.; Xia, Q.; Qiu, J.; Wang, J. Regulating the Pore Microenvironment of Microporous Metal-Organic Frameworks for Efficient Adsorption of Low-Concentration Ammonia. ACS Sustain. Chem. Eng. 2022, 10, 10945–10954. [Google Scholar] [CrossRef]
- Takahashi, A.; Tanaka, H.; Parajuli, D.; Nakamura, T.; Minami, K.; Sugiyama, Y.; Hakuta, Y.; Ohkoshi, S.I.; Kawamoto, T. Historical Pigment Exhibiting Ammonia Gas Capture beyond Standard Adsorbents with Adsorption Sites of Two Kinds. J. Am. Chem. Soc. 2016, 138, 6376–6379. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H.; Watanabe, T.; Ishikawa, T.; Nakashima, N.; Noda, K.; Takahashi, A.; Kawamoto, T.; Minami, K. Ammonia Concentrator for Repeatable Adsorption/Desorption Using Nickel Hexacyanoferrate as Adsorbent and Production of Solid Ammonium Bicarbonate. ACS Sustain. Chem. Eng. 2024, 12, 2183–2190. [Google Scholar] [CrossRef]
- Wang, M.; Xie, R.; Chen, Y.; Pu, X.; Jiang, W.; Yao, L. A Novel Mesoporous Zeolite-Activated Carbon Composite as an Effective Adsorbent for Removal of Ammonia-Nitrogen and Methylene Blue from Aqueous Solution. Bioresour. Technol. 2018, 268, 726–732. [Google Scholar] [CrossRef] [PubMed]
- McHugh, L.N.; Terracina, A.; Wheatley, P.S.; Buscarino, G.; Smith, M.W.; Morris, R.E. Metal–Organic Framework-Activated Carbon Composite Materials for the Removal of Ammonia from Contaminated Airstreams. Angew. Chemie—Int. Ed. 2019, 58, 11747–11751. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, E.; Azar, R.; Nemati, M.; Predicala, B. Gas Phase Adsorption of Ammonia Using Nano TiO2-Activated Carbon Composites—Effect of TiO2 Loading and Composite Characterization. J. Environ. Chem. Eng. 2017, 5, 5902–5911. [Google Scholar] [CrossRef]
- Nazir, M.A.; Javed, M.S.; Islam, M.; Assiri, M.A.; Hassan, A.M.; Jamshaid, M.; Najam, T.; Shah, S.S.A.; ur Rehman, A. MOF@graphene Nanocomposites for Energy and Environment Applications. Compos. Commun. 2024, 45, 101783. [Google Scholar] [CrossRef]
- Mousavi, S.V.; Ahranjani, P.J.; Saei, S.F.; Mehrdadi, N.; Bidhendi, G.N.; Jume, B.H.; Rezania, S.; Mojiri, A. Ammonia Removal from Industrial Effluent Using Zirconium Oxide and Graphene-Oxide Nanocomposites. Chemosphere 2022, 297, 134008. [Google Scholar] [CrossRef] [PubMed]
- Eljamal, O.; Eljamal, R.; Maamoun, I.; Khalil, A.M.E.; Shubair, T.; Falyouna, O.; Sugihara, Y. Efficient Treatment of Ammonia-Nitrogen Contaminated Waters by Nano Zero-Valent Iron/Zeolite Composite. Chemosphere 2022, 287, 131990. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A. A Review on the Technologies for Converting Biomass into Carbon-Based Materials: Sustainability and Economy. Bioresour. Technol. Rep. 2024, 25, 101771. [Google Scholar] [CrossRef]
- Fan, Q.; Song, C.; Fu, P. Advances in the Improvement of the Quality and Efficiency of Biomass-Derived Porous Carbon: A Comprehensive Review on Synthesis Strategies and Heteroatom Doping Effects. J. Clean. Prod. 2024, 452, 142169. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Ye, G.; Fan, J.; Yao, F.; Wang, Y.; Jiao, Y.; Zhu, W.; Huang, H.; Ye, D. Key Factors and Primary Modification Methods of Activated Carbon and Their Application in Adsorption of Carbon-Based Gases: A Review. Chemosphere 2022, 287, 131995. [Google Scholar] [CrossRef] [PubMed]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Gopalan, J.; Buthiyappan, A.; Raman, A.A.A. Insight into Metal-Impregnated Biomass Based Activated Carbon for Enhanced Carbon Dioxide Adsorption: A Review. J. Ind. Eng. Chem. 2022, 113, 72–95. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.; Yang, X. Organic Soil Additives for the Remediation of Cadmium Contaminated Soils and Their Impact on the Soil-Plant System: A Review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, J.; Han, L.; Tan, J.; Ge, X.; Nan, Q. Biochar Addition Reduces Salinity in Salt-Affected Soils with No Impact on Soil PH: A Meta-Analysis. Geoderma 2024, 443, 116845. [Google Scholar] [CrossRef]
- Lo, S.L.Y.; How, B.S.; Leong, W.D.; Teng, S.Y.; Rhamdhani, M.A.; Sunarso, J. Techno-Economic Analysis for Biomass Supply Chain: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2021, 135, 110164. [Google Scholar] [CrossRef]
- Ng, C.; Marshall, W.E.; Rao, R.M.; Bansode, R.R.; Losso, J.N. Activated Carbon from Pecan Shell: Process Description and Economic Analysis. Ind. Crops Prod. 2003, 17, 209–217. [Google Scholar] [CrossRef]
- Wlazło, Ł.; Kwiecień, M.; Bis-Wencel, H.; Łopuszyński, W.; Buszewicz, G.; Karpińska, K.; Rodzyń, I.; Kasela, M.; Sobczak, P.; Nowakowicz-Dębek, B. Assessment of Health Safety of Pigs Taking Natural Sorbents with Feed. BMC Vet. Res. 2023, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Postawa, K.; Szczygieł, J.; Kułażyński, M. Heuristic Methods in Optimization of Selected Parameters of Two-Phase Anaerobic Digestion (TPAD) Model. Fuel 2020, 281, 118257. [Google Scholar] [CrossRef]
- Postawa, K.; Szczygieł, J.; Wrzesińska-Jędrusiak, E.; Klimek, K.; Kułażyński, M. The Pump-Mixed Anaerobic Digestion of Pig Slurry: New Technology and Mathematical Modeling. Waste Manag. 2021, 123, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Pstrowska, K.; Kaczmarczyk, J.; Czapor-Irzabek, H.; Kułażyński, M. Characterization of Activated Beech Wood Char—Methane Storage Application. Stud. Univ. Babeș-Bolyai Chem. 2018, 63, 21–39. [Google Scholar] [CrossRef]
- Fałtynowicz, H.; Kaczmarczyk, J.; Kułażyński, M. Preparation and Characterization of Activated Carbons from Biomass Material—Giant Knotweed (Reynoutria Sachalinensis). Open Chem. 2015, 13, 1150–1156. [Google Scholar] [CrossRef]
- PN-ISO 1171:2002; Paliwa Stałe—Oznaczanie Popiołu. Polish Committee for Standardization: Warsaw, Poland, 2002.
- PN-G-04511:1980; Paliwa Stałe—Oznaczanie Zawartości Wilgoci. Polish Committee for Standardization: Warsaw, Poland, 1980.
- PN-G-04516:1988; Paliwa Stałe—Oznaczanie Zawartości Części Lotnych Metodą Wagową. Polish Committee for Standardization: Warsaw, Poland, 1998.
- PN-C-04375-2:2013–07; Badanie Paliw Stałych i Ciekłych—Oznaczanie Ciepła Spalania w Bombie Kalorymetrycznej i Obliczanie Wartości Opałowej—Część 2: Metoda z Zastosowaniem Kalorymetru Izoperibolicznego lub Kalorymetru z Płaszczem Statycznym. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Veza, I.; Spraggon, M.; Fattah, I.M.R.; Idris, M. Response Surface Methodology (RSM) for Optimizing Engine Performance and Emissions Fueled with Biofuel: Review of RSM for Sustainability Energy Transition. Results Eng. 2023, 18, 101213. [Google Scholar] [CrossRef]
- Pereira, L.M.S.; Milan, T.M.; Tapia-Blácido, D.R. Using Response Surface Methodology (RSM) to Optimize 2G Bioethanol Production: A Review. Biomass Bioenergy 2021, 151, 106166. [Google Scholar] [CrossRef]
- Aziz, S.H. Polynomial Interpolation in Matlab. J. Eng. Sci. Res. 2018, 2, 12–19. [Google Scholar] [CrossRef]
- Goldberg, D.E. Genetic Algorithms in Search, Optimization and Machine Learning, 1st ed.; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1989; ISBN 978-0-201-15767-3. [Google Scholar]
- Katoch, S.; Chauhan, S.S.; Kumar, V. A Review on Genetic Algorithm: Past, Present, and Future. Multimed. Tools Appl. 2021, 80, 8091–8126. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.F.U.; Ali, H.O.; Aziz, S.; Kouotou, P.M.; Waqas, M.; Naqvi, S.M.A.; Athar, M.H.; Ammar, M.; Shah, I.; Jung, D.W. Optimization and Techno-Economic Analysis of Catalytic Gasification of Wheat Straw Biomass Using ASPEN PLUS Model. Arab. J. Chem. 2024, 17, 105821. [Google Scholar] [CrossRef]
- Liu, L.; Qian, H.; Mu, L.; Wu, J.; Feng, X.; Lu, X.; Zhu, J. Techno-Economic Analysis of Biomass Processing with Dual Outputs of Energy and Activated Carbon. Bioresour. Technol. 2021, 319, 124108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Khalil, R.A.; Bakken, J.; Skreiberg, Ø. Production and Characterization of Biocarbon from Woody Biomasses Produced at 1000 °C. Fuel 2024, 370, 131667. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, G. Physical Characterisation and Chemical Composition of Densified Biomass Fuels with Regard to Their Combustion Behaviour. Biomass Bioenergy 2004, 27, 653–669. [Google Scholar] [CrossRef]
- Ferraro, G.; Pecori, G.; Rosi, L.; Bettucci, L.; Fratini, E.; Casini, D.; Rizzo, A.M.; Chiaramonti, D. Biochar from Lab-Scale Pyrolysis: Influence of Feedstock and Operational Temperature. Biomass Convers. Biorefinery 2024, 14, 5901–5911. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Chen, S.; Li, Q.; Zhang, Y. A Novel Method for Kinetics Analysis of Pyrolysis of Hemicellulose, Cellulose, and Lignin in TGA and Macro-TGA. RSC Adv. 2015, 5, 26509–26516. [Google Scholar] [CrossRef]
- Postawa, K.; Fałtynowicz, H.; Szczygieł, J.; Beran, E.; Kułażyński, M. Analyzing the Kinetics of Waste Plant Biomass Pyrolysis via Thermogravimetry Modeling and Semi-Statistical Methods. Bioresour. Technol. 2022, 344, 126181. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.H.; Kirkham, M.B.; et al. Multifunctional Applications of Biochar beyond Carbon Storage. Int. Mater. Rev. 2022, 67, 150–200. [Google Scholar] [CrossRef]
- Cardenas, C.; Sigot, L.; Vallières, C.; Marsteau, S.; Marchal, M.; Latifi, A.M. Ammonia Capture by Adsorption on Doped and Undoped Activated Carbon: Isotherm and Breakthrough Curve Measurements. Sep. Purif. Technol. 2023, 313, 123454. [Google Scholar] [CrossRef]
- Corre, Y.; Seredych, M.; Bandosz, T.J. Analysis of the Chemical and Physical Factors Affecting Reactive Adsorption of Ammonia on Graphene/Nanoporous Carbon Composites. Carbon 2013, 55, 176–184. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; González, M.T.; Rodriguez-Reinoso, F.; Sepúlveda-Escribano, A. Effect of Steam and Carbon Dioxide Activation in the Micropore Size Distribution of Activated Carbon. Carbon 1996, 34, 505–509. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kubota, M.; Matsuda, H.; D’Elia Camacho, L.F. Adsorption Behaviors of Ammonia and Hydrogen Sulfide on Activated Carbon Prepared from Petroleum Coke by KOH Chemical Activation. Fuel Process. Technol. 2016, 144, 164–169. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Petit, C. On the Reactive Adsorption of Ammonia on Activated Carbons Modified by Impregnation with Inorganic Compounds. J. Colloid Interface Sci. 2009, 338, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, G.G.; Zabaniotou, A.A. Minimizing Activated Carbons Production Cost. Fuel Process. Technol. 2009, 90, 952–957. [Google Scholar] [CrossRef]
- León, M.; Silva, J.; Carrasco, S.; Barrientos, N. Design, Cost Estimation and Sensitivity Analysis for a Production Process of Activated Carbon from Waste Nutshells by Physical Activation. Processes 2020, 8, 945. [Google Scholar] [CrossRef]
- Qajar, A.; Peer, M.; Andalibi, M.R.; Rajagopalan, R.; Foley, H.C. Enhanced Ammonia Adsorption on Functionalized Nanoporous Carbons. Microporous Mesoporous Mater. 2015, 218, 15–23. [Google Scholar] [CrossRef]


| Parameter | Unit | BA_NH4 |
|---|---|---|
| Iodine number * | mg/g | 1000 |
| Acid concentration | % | ~10 |
| pH | - | 1.5 |
| Bulk density | g/L | 530 ± 30 |
| Particle size | mm | 4 |
| Moisture (Ma) | wt.% | max. 10 |
| Sample | M a (wt.%) | A a (wt.%) | A d (wt.%) | V a (wt.%) | V daf (wt.%) | Fc a (wt.%) | Qc (MJ/kg) |
|---|---|---|---|---|---|---|---|
| PO | 6.43 | 0.22 | 0.23 | 79.09 | 84.72 | 14.26 | 20.04 |
| PO500 | 2.37 | 0.83 | 0.85 | 25.32 | 26.16 | 71.49 | - |
| KC | 9.87 | 9.87 | 10.96 | 3.66 | 4.57 | 76.60 | 27.45 |
| W | 3.23 | 7.74 | 8.00 | 4.10 | 4.61 | 84.93 | 29.52 |
| Sample | C daf (wt.%) | H daf (wt.%) | N daf (wt.%) | S daf (wt.%) | O daf (wt.%) * | H/C | O/C |
|---|---|---|---|---|---|---|---|
| PO | 51.12 | 5.97 | 0.03 | 0.06 | 42.82 | 0.117 | 0.838 |
| PO500 | 84.78 | 3.7 | 0.13 | 0 | 11.39 | 0.044 | 0.134 |
| W | 95.95 | 0.41 | 0.62 | 0 | 3.02 | 0.004 | 0.032 |
| Sample | SBET (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Vmic (%) | Vmes (cm3/g) | d (nm) |
|---|---|---|---|---|---|---|
| PO | 7 | 0.041 | 0.027 | 65.9 | 0.014 | 7.00 |
| PO500 | 29 | 0.110 | 0.104 | 94.8 | 0.006 | 3.71 |
| KC | 356 | 0.174 | 0.153 | 87.9 | 0.021 | 4.39 |
| W | 66 | 0.164 | 0.151 | 92.1 | 0.013 | 4.73 |
| Steam | ||||
|---|---|---|---|---|
| W | KC | |||
| Temperature (°C) | t (min) | R daf (g/(g·h)) | t (min) | R daf (g/(g·h)) |
| 750 | 195 | 0.154 | 560 | 0.054 |
| 800 | 77 | 0.390 | 200 | 0.150 |
| 850 | 35 | 0.857 | 90 | 0.333 |
| 900 | 25 | 1.200 | 47 | 0.638 |
| CO2 | ||||
| PO500 | KC | |||
| 750 | 410 | 0.073 | - * | - * |
| 800 | 133 | 0.226 | 885 | 0.034 |
| 850 | 47 | 0.638 | 402 | 0.075 |
| 900 | 36 | 0.833 | 98 | 0.306 |
| Adsorption | Desorption | Reversibility of Sorption | Vtot | SBET | |
|---|---|---|---|---|---|
| KC | 0.993 | 0.908 | 0.942 | 0.995 | 0.996 |
| W | 0.985 | 0.820 | 0.743 | 0.978 | 0.986 |
| PO | 0.894 | 0.608 | 0.824 | 0.930 | 0.875 |
| KC | W | PO | ||||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value * | p-Value * | |
| Adsorption (X1) | 8.99 | 0.0157 | 8.05 | 0.0200 | 77.02 | 0.0835 |
| Adsorption (X2) | 15.99 | 0.0029 | 0.74 | 0.5644 | 132.44 | 0.0638 |
| Desorption (X1) | 7.71 | 0.0220 | 41.01 | 0.0003 | 14.75 | 0.1886 |
| Desorption (X2) | 0.32 | 0.8100 | 1.28 | 0.3621 | 8.98 | 0.2394 |
| Reversibility (X1) | 6.52 | 0.0313 | 6.74 | 0.0292 | 19.57 | 0.1643 |
| Reversibility (X2) | 11.18 | 0.0072 | 1.68 | 0.2687 | 38.96 | 0.1171 |
| Vtot (X1) | 32.41 | 0.0006 | 1.15 | 0.3785 | 66.81 | 0.0896 |
| Vtot (X2) | 534.93 | 0.0000 | 19.66 | 0.0017 | 93.77 | 0.0757 |
| SBET (X1) | 42.89 | 0.0003 | 1.52 | 0.2926 | 24.90 | 0.1460 |
| SBET (X2) | 652.74 | 0.0000 | 15.45 | 0.0032 | 86.59 | 0.0788 |
| T (°C) | KOH (-) | Vtot (cm3/g) | SBET (m2/g) | |
|---|---|---|---|---|
| KC | 900 | 4.00 | 0.774 | 1598 |
| W | 774 | 4.00 | 0.814 | 1478 |
| PO | 900 | 3.62 | 1.201 | 2454 |
| T (°C) | KOH (-) | Adsorption (g/kg) | Desorption residue (g/kg) | |
| KC | 700 | 4.00 | 166.2 | 11.3 |
| W | 725 | 3.40 | 158.4 | 15.6 |
| PO | 600 | 3.03 | 396.9 | 50.9 |
| Economic Quantity | Price | Unit |
|---|---|---|
| Energy from NG * combustion | 0.057 | EUR/kWh |
| Water | 1.92 | EUR/m3 |
| Carbon dioxide | 0.70 | EUR/kg |
| Sawdust pellet (PO) | 0.04 | EUR/kg |
| Activated carbon (BA_NH4) | 4.18 | EUR/kg |
| Temperature (°C) | 750 | 800 | 850 | 900 | ||||
|---|---|---|---|---|---|---|---|---|
| Cost (EUR/kg) | ETE (kW/kg) | Cost (EUR/kg) | ETE (kW/kg) | Cost (EUR/kg) | ETE (kW/kg) | Cost (EUR/kg) | ETE (kW/kg) | |
| H2O | ||||||||
| W | 0.22 | 3.92 | 0.24 | 4.09 | 0.25 | 3.96 | 0.26 | 3.86 |
| PO | 0.26 | 2.84 | 0.27 | 2.64 | 0.28 | 2.46 | 0.29 | 2.29 |
| CO2 | ||||||||
| W | 2.17 | 2.36 | 2.52 | 3.58 | 2.72 | 4.19 | 2.73 | 4.11 |
| PO | 2.43 | 3.10 | 2.44 | 2.91 | 2.45 | 2.73 | 2.46 | 2.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fałtynowicz, H.; Kaczmarczyk, J.; Łużny, R.; Jaroszewska, K.; Pstrowska, K.; Hull, S.; Kułażyński, M.; Postawa, K. Activated Carbons for Removing Ammonia from Piggery Vent Air: A Promising Tool for Mitigating the Environmental Impact of Large-Scale Pig Breeding. Sustainability 2024, 16, 6122. https://doi.org/10.3390/su16146122
Fałtynowicz H, Kaczmarczyk J, Łużny R, Jaroszewska K, Pstrowska K, Hull S, Kułażyński M, Postawa K. Activated Carbons for Removing Ammonia from Piggery Vent Air: A Promising Tool for Mitigating the Environmental Impact of Large-Scale Pig Breeding. Sustainability. 2024; 16(14):6122. https://doi.org/10.3390/su16146122
Chicago/Turabian StyleFałtynowicz, Hanna, Jan Kaczmarczyk, Rafał Łużny, Karolina Jaroszewska, Katarzyna Pstrowska, Sylwia Hull, Marek Kułażyński, and Karol Postawa. 2024. "Activated Carbons for Removing Ammonia from Piggery Vent Air: A Promising Tool for Mitigating the Environmental Impact of Large-Scale Pig Breeding" Sustainability 16, no. 14: 6122. https://doi.org/10.3390/su16146122
APA StyleFałtynowicz, H., Kaczmarczyk, J., Łużny, R., Jaroszewska, K., Pstrowska, K., Hull, S., Kułażyński, M., & Postawa, K. (2024). Activated Carbons for Removing Ammonia from Piggery Vent Air: A Promising Tool for Mitigating the Environmental Impact of Large-Scale Pig Breeding. Sustainability, 16(14), 6122. https://doi.org/10.3390/su16146122








