Prediction of the Potentially Suitable Areas of Actinidia latifolia in China Based on Climate Change Using the Optimized MaxEnt Model
Abstract
1. Introduction
2. Materials and Methods
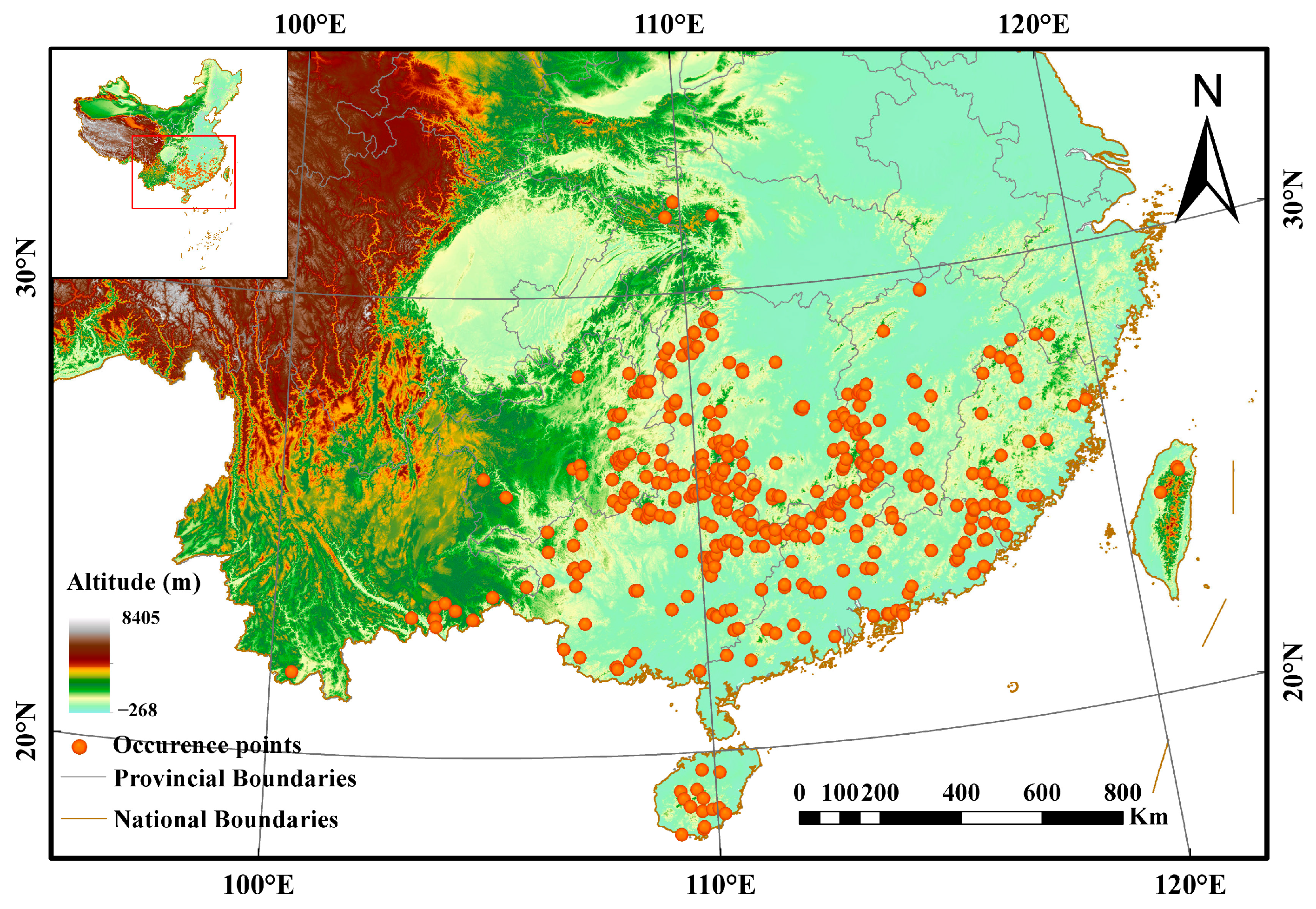
2.1. Occurrence Data
2.2. Environment Variables
2.3. Optimization and Evaluation of Model
2.4. Potential Distribution Ranges and Centroid Shifts for A. latifolia
3. Results
3.1. Model Optimization and Environmental Variables
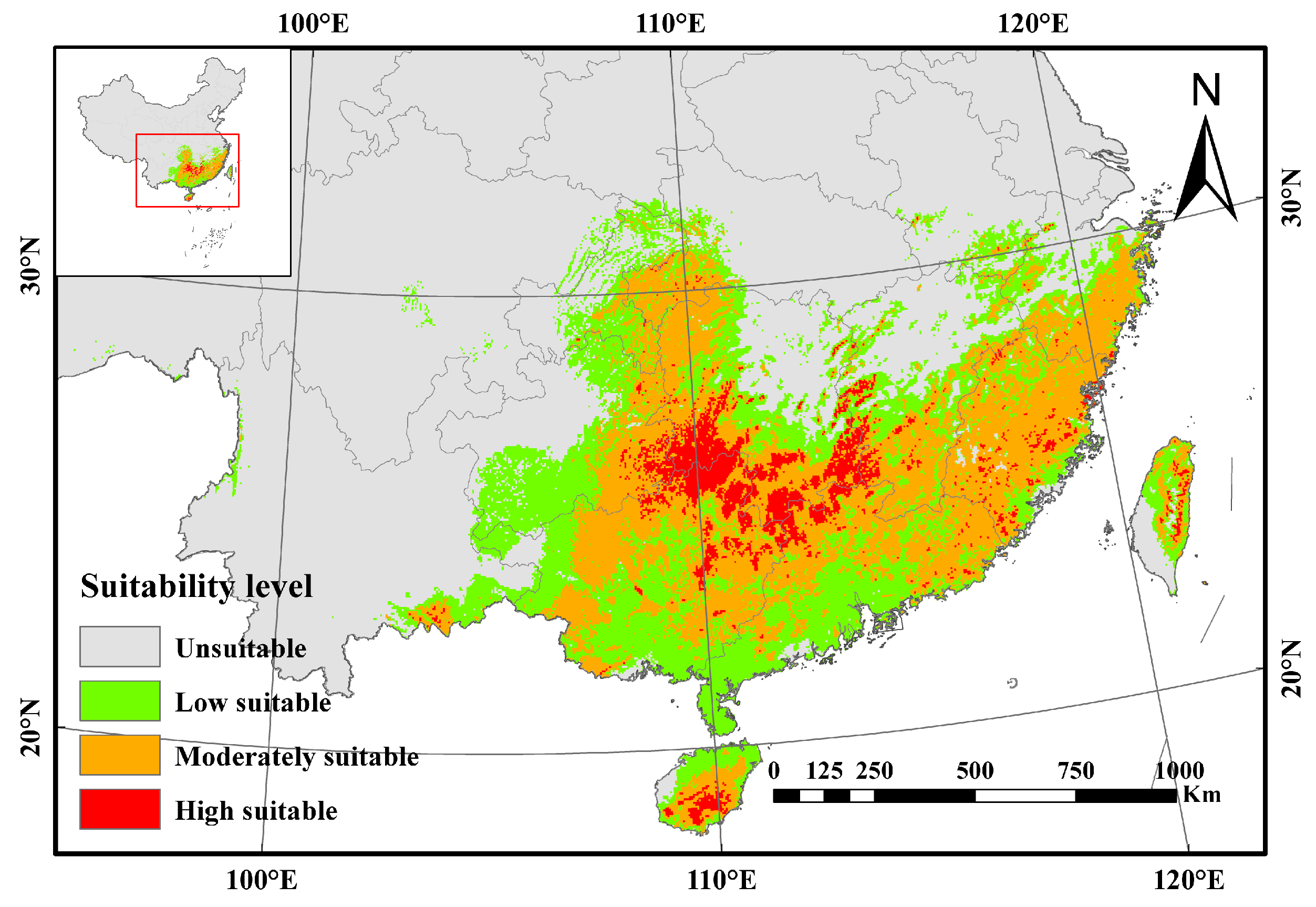
3.2. Current Potentially Suitable Areas of A. latifolia in China
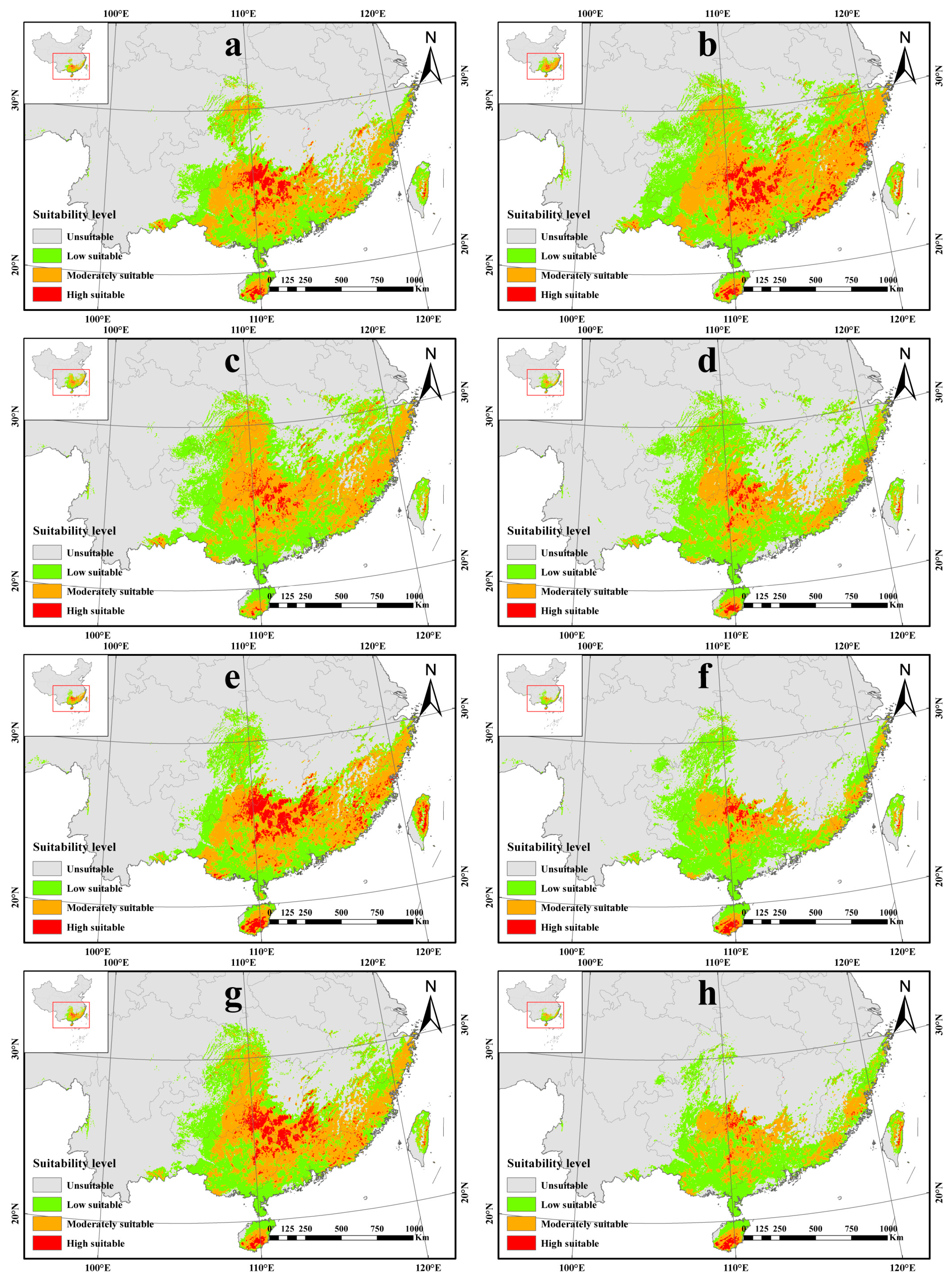
3.3. Future Potentially Suitable Areas of A. latifolia in China
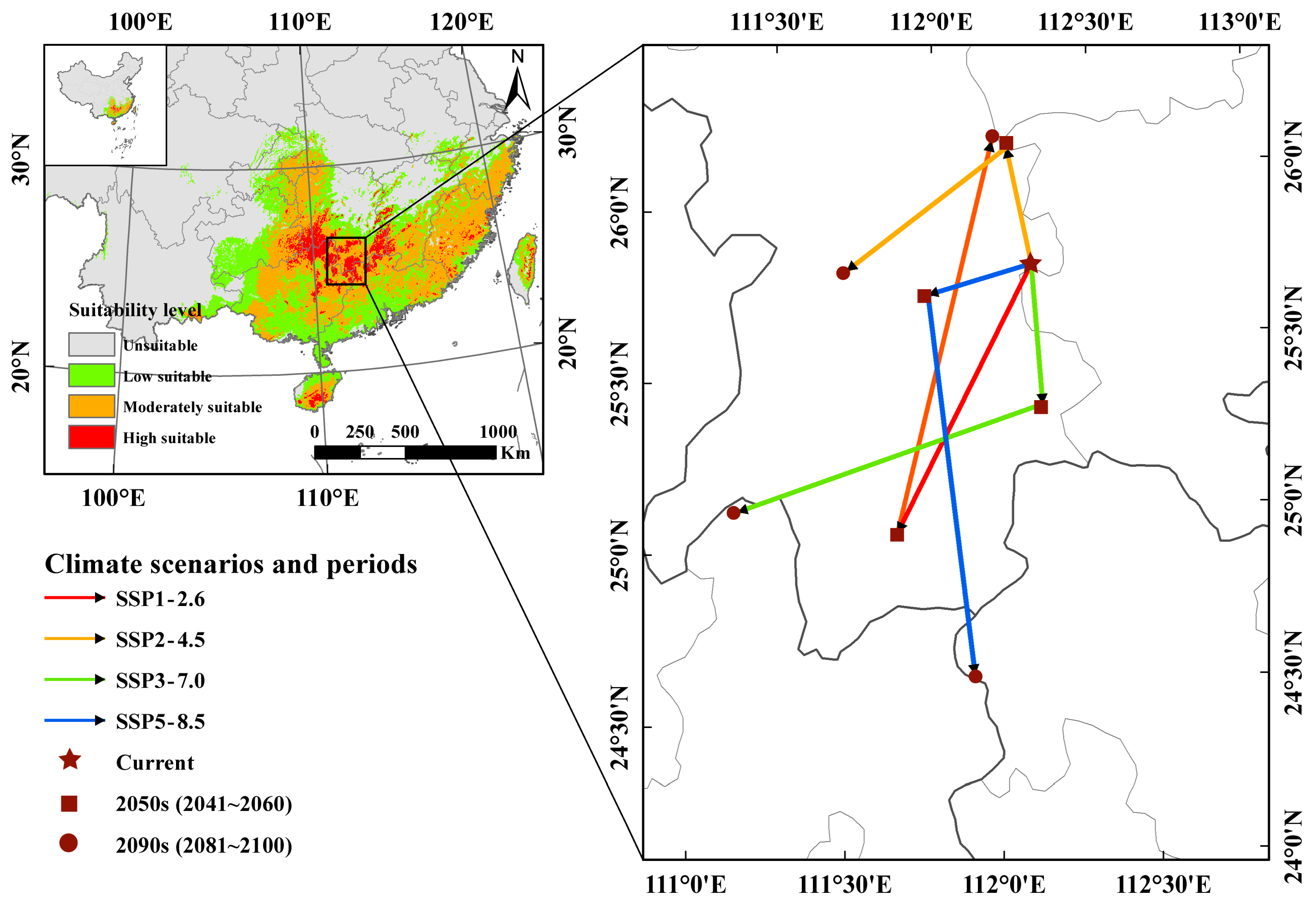
3.4. The Core Distribution Shifts
4. Discussion
4.1. Model Prediction Accuracy
4.2. Effects of the Main Environmental Factors on the Distribution of A. latifolia
4.3. Effects of Climate Change on the Distribution of A. latifolia
4.4. Conservation and Utilization of Wild Kiwifruit Relatives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond Predictions: Biodiversity Conservation in a Changing Climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- Zhou, T. New Physical Science behind Climate Change: What Does IPCC AR6 Tell Us? Innovation 2021, 2, 100173. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Shen, X.-L.; Tong, L.; Lei, F.-W.; Mu, X.-Y.; Zhang, Z.-X. Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications. Forests 2021, 12, 125. [Google Scholar] [CrossRef]
- Ostad-Ali-Askar, K.; Su, R.; Liu, L. Water Resources and Climate Change. J. Water Clim. Change 2018, 9, 239. [Google Scholar] [CrossRef]
- Jing-Song, S.; Guang-Sheng, Z.; Xing-Hua, S. Climatic Suitability of the Distribution of the Winter Wheat Cultivation Zone in China. Eur. J. Agron. 2012, 43, 77–86. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Li, K.; Wang, C.; Guna, A.; Zhang, J. Prediction of Potential Geographical Distribution Patterns of Actinidia Arguta under Different Climate Scenarios. Sustainability 2021, 13, 3526. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, P.; Shang, Y. The Potential Global Distribution and Dynamics of Wheat under Multiple Climate Change Scenarios. Sci. Total Environ. 2019, 688, 1308–1318. [Google Scholar] [CrossRef]
- Su, P.; Zhang, A.; Wang, R.; Wang, J.; Gao, Y.; Liu, F. Prediction of Future Natural Suitable Areas for Rice under Representative Concentration Pathways (RCPs). Sustainability 2021, 13, 1580. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Shi, X.; Bo, X.; Li, S.; Shang, M.; Chen, F.; Chu, Q. Modeling Climatically Suitable Areas for Soybean and Their Shifts across China. Agric. Syst. 2021, 192, 103205. [Google Scholar] [CrossRef]
- Ureta, C.; Martínez-Meyer, E.; Perales, H.R.; Álvarez-Buylla, E.R. Projecting the Effects of Climate Change on the Distribution of Maize Races and Their Wild Relatives in Mexico. Glob. Chang. Biol. 2012, 18, 1073–1082. [Google Scholar] [CrossRef]
- Suwardi, A.B.; Mukhtar, E. Potential Geographic Distribution of Durio Oxleyanus (Malvaceae): A Threatened Wild Fruit Plant Species in Sumatra, Indonesia. Pol. J. Environ. Stud. 2023, 32, 2845. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the Potentially Suitable Areas of Litsea cubeba in China Based on Future Climate Change Using the Optimized MaxEnt Model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- He, Y.; Ma, J.; Chen, G. Potential Geographical Distribution and Its Multi-Factor Analysis of Pinus Massoniana in China Based on the Maxent Model. Ecol. Indic. 2023, 154, 110790. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Gong, Z.N.; Su, S.; Du, B.; Guan, H.; Zhang, Q. Habitat Selection and Dispersal of Red-Crowned Cranes during Breeding Period in Zhalong Wetland National Nature Reserve. J. Nat. Resour. 2021, 36, 1964–1975. [Google Scholar] [CrossRef]
- Soilhi, Z.; Sayari, N.; Benalouache, N.; Mekki, M. Predicting Current and Future Distributions of Mentha Pulegium L. in Tunisia under Climate Change Conditions, Using the MaxEnt Model. Ecol. Inform. 2022, 68, 101533. [Google Scholar] [CrossRef]
- Huang, H. The Genus Actinidia, a World Monograph; Science Press: Beijing, China, 2014. [Google Scholar]
- Li, X.W.; Li, X.; Li, J.Q.; Soejarto, D.D. Progress in the Phylogeny and Taxonomy of Actinidia during the Past Decade. In Proceedings of the VII International Symposium on Kiwifruit 913, Faenza, Italy, 12–17 September 2010; pp. 71–76. [Google Scholar]
- Drummond, L. The Composition and Nutritional Value of Kiwifruit. Adv. Food Nutr. Res. 2013, 68, 33–57. [Google Scholar] [PubMed]
- Liang, D.; Deng, H.; Deng, Q.; Lin, L.; Lv, X.; Wang, J.; Wang, Z.; Xiong, B.; Zhao, X.; Xia, H. Dynamic Changes of Phenolic Compounds and Their Associated Gene Expression Profiles Occurring during Fruit Development and Ripening of the Donghong Kiwifruit. J. Agric. Food Chem. 2020, 68, 11421–11433. [Google Scholar] [CrossRef]
- Wu, H.; Ma, T.; Kang, M.; Ai, F.; Zhang, J.; Dong, G.; Liu, J. A High-Quality Actinidia chinensis (Kiwifruit) Genome. Hortic. Res. 2019, 6, 117. [Google Scholar] [CrossRef]
- Jian-Qiang, L.; Huang, H.; Sheng-Mei, W.; Zi-Can, H.; Zhong-Hui, Z.; Jun-Jie, G. Genetic Diversity in the Genus Actinidia. Biodivers. Sci. 2000, 8, 1–12. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Zhang, Q.; Ma, N.; Liu, X.; Tao, W.; Lou, Z.; Zhong, C.; Deng, X.W.; Li, D. Two Haplotype-Resolved, Gap-Free Genome Assemblies for Actinidia Latifolia and Actinidia chinensis Shed Light on the Regulatory Mechanisms of Vitamin C and Sucrose Metabolism in Kiwifruit. Mol. Plant 2023, 16, 452–470. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xia, H.; Guo, Y.; Liu, X.; Lin, L.; Wang, J.; Xu, K.; Lv, X.; Hu, R.; Liang, D. Dynamic Changes in Ascorbic Acid Content during Fruit Development and Ripening of Actinidia latifolia (an Ascorbate-Rich Fruit Crop) and the Associated Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 5808. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, X.; Zhong, C.; Li, D. Comparative Transcriptome Analysis Revealed the Key Genes Regulating Ascorbic Acid Synthesis in Actinidia. Int. J. Mol. Sci. 2021, 22, 12894. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Qi, X.; Xie, X.; Zhong, C.; Li, D. The Complete Chloroplast Genome of Actinidia latifolia, a Species with High Vitamin C Content in Fruit. Mitochondrial DNA Part B 2020, 5, 3425–3426. [Google Scholar] [CrossRef]
- Ren, W.; Wang, L.; Feng, G.; Tao, C.; Liu, Y.; Yang, J. High-Quality Assembly and Comparative Analysis of Actinidia latifolia and A. valvata Mitogenomes. Genes 2023, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Bao, H.; Ling, H.; Li, P.; Zhang, Y.; He, Y.; Hu, X.; Ling, C.; Liu, Y.; Tang, W. Comparative Transcriptomic Analysis Revealed the Suppression and Alternative Splicing of Kiwifruit (Actinidia latifolia) NAP1 Gene Mediating Trichome Development. Int. J. Mol. Sci. 2023, 24, 4481. [Google Scholar] [CrossRef]
- Liu, X.; Wu, R.; Bulley, S.M.; Zhong, C.; Li, D. Kiwifruit MYBS1-like and GBF3 Transcription Factors Influence L-ascorbic Acid Biosynthesis by Activating Transcription of GDP-L-galactose Phosphorylase 3. N. Phytol. 2022, 234, 1782–1800. [Google Scholar] [CrossRef]
- Wang, R.L.; Jiang, C.; Huang, T.; Zhang, Z.; Wang, M.; Shen, Z.; Wang, Y.; Li, Q. A simulation study of the geographical distribution of Actinidia arguta in China. Pol. J. Environ. Stud. 2020, 29, 1889. [Google Scholar] [CrossRef]
- Gao, B.; Yuan, S.; Guo, Y.; Zhao, Z. Potential geographical distribution of Actinidia spp. and its predominant indices under climate change. Ecol. Inform. 2022, 72, 101865. [Google Scholar] [CrossRef]
- Ma, Y.; Guga, S.; Xu, J.; Zhang, J.; Tong, Z.; Liu, X. Comprehensive risk assessment of high temperature disaster to kiwifruit in Shaanxi province, China. Int. J. Environ. Res. Public Health 2021, 18, 10437. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L. SDM Toolbox: A Python-based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The Next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O. The Shared Socioeconomic Pathways and Their Energy, Land Use, and Greenhouse Gas Emissions Implications: An Overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, Z.; Li, L. Biases and Improvements in Three Dynamical Downscaling Climate Simulations over China. Clim. Dyn. 2016, 47, 3235–3251. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Dai, Y.; Hu, G. Climate Sensitivity and Feedback of BCC-CSM to Idealized CO2 Forcing from CMIP5 to CMIP6. J. Meteorol. Res. 2020, 34, 865–878. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; Van Ruijven, B.J.; Van Vuuren, D.P.; Birkmann, J.; Kok, K. The Roads Ahead: Narratives for Shared Socioeconomic Pathways Describing World Futures in the 21st Century. Glob. Environ. Chang. 2017, 42, 169–180. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Xiao-Long, C.; Xiao-Ge, X.I.N. Short Commentary on CMIP6 Scenario Model Intercomparison Project (ScenarioMIP). Adv. Clim. Chang. Res. 2019, 15, 519. [Google Scholar]
- Ostad-Ali-Askari, K.; Ghorbanizadeh Kharazi, H.; Shayannejad, M.; Zareian, M.J. Effect of Climate Change on Precipitation Patterns in an Arid Region Using GCM Models: Case Study of Isfahan-Borkhar Plain. Nat. Hazards Rev. 2020, 21, 04020006. [Google Scholar] [CrossRef]
- Liu, F.; Wu, H.; Zhao, Y.; Li, D.; Yang, J.-L.; Song, X.; Shi, Z.; Zhu, A.-X.; Zhang, G.-L. Mapping High-Resolution National Soil Information Grids of China. Sci. Bull. 2022, 67, 328–340. [Google Scholar] [CrossRef]
- Yin, Y.; He, Q.; Pan, X.; Liu, Q.; Wu, Y.; Li, X. Predicting Current Potential Distribution and the Range Dynamics of Pomacea Canaliculata in China under Global Climate Change. Biology 2022, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM Eval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for MAXENT Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists: Statistical Explanation of MaxEnt. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting Thresholds for the Prediction of Species Occurrence with Presence-only Data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Freeman, E.A.; Moisen, G.G. A Comparison of the Performance of Threshold Criteria for Binary Classification in Terms of Predicted Prevalence and Kappa. Ecol. Model. 2008, 217, 48–58. [Google Scholar] [CrossRef]
- He, Q.; Zhou, G.; Lü, X.; Zhou, M. Climatic Suitability and Spatial Distribution for Summer Maize Cultivation in China at 1.5 and 2.0 C Global Warming. Sci. Bull. 2019, 64, 690–697. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Warren, D.L.; Wright, A.N.; Seifert, S.N.; Shaffer, H.B. Incorporating Model Complexity and Spatial Sampling Bias into Ecological Niche Models of Climate Change Risks Faced by 90 C Alifornia Vertebrate Species of Concern. Divers. Distrib. 2014, 20, 334–343. [Google Scholar] [CrossRef]
- Jia, X.; Wang, C.; Jin, H.; Zhao, Y.; Liu, L.; Chen, Q.; Li, B.; Xiao, Y.; Yin, H. Assessing the Suitable Distribution Area of Pinus Koraiensis Based on an Optimized MaxEnt Model. Chin. J. Ecol. 2019, 38, 2570. [Google Scholar]
- Guo, J.; Liu, X.P.; Zhang, Q.; Zhang, D.F.; Xie, C.X.; Liu, X. Prediction for the Potential Distribution Area of Codonopsis Pilosula at Global Scale Based on Maxent Model. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2017, 28, 992–1000. [Google Scholar]
- Huang, H. Kiwifruit: The Genus Actinidia; Academic Press: London, UK, 2016. [Google Scholar]
- Khan, A.M.; Li, Q.; Saqib, Z.; Khan, N.; Habib, T.; Khalid, N.; Majeed, M.; Tariq, A. MaxEnt Modelling and Impact of Climate Change on Habitat Suitability Variations of Economically Important Chilgoza Pine (Pinus gerardiana Wall.) in South Asia. Forests 2022, 13, 715. [Google Scholar] [CrossRef]
- Kim, C.; Kim, W.; Song, W.; Cho, J.; Choi, J. Prediction of Native Seed Habitat Distribution According to SSP Scenario and Seed Transfer Zones: A Focus on Acer pictum Subsp. Mono and Quercus acuta. Forests 2023, 14, 87. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, L.; Wang, H.; Zhong, J.; Li, Y.; Liu, J.; Ou, Z.; Liang, X.; Li, Y.; Huang, H.; et al. Geographic distribution and impacts of climate change on the suitable habitats of Zingiber species in China. Ind. Crops Prod. 2019, 138, 111429. [Google Scholar] [CrossRef]
- Bradie, J.; Leung, B. A Quantitative Synthesis of the Importance of Variables Used in MaxEnt Species Distribution Models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Punyasena, S.W.; Eshel, G.; McElwain, J.C. The influence of climate on the spatial patterning of Neotropical plant families. J. Biogeogr. 2008, 35, 117–130. [Google Scholar] [CrossRef]
- Gengping, Z.; Guoqing, L.; Wenjun, B.; Yubao, G. Ecological Niche Modeling and Its Applications in Biodiversity Conservation: Ecological Niche Modeling and Its Applications in Biodiversity Conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar] [CrossRef]
- Ye, X.; Zhao, G.; Zhang, M.; Cui, X.; Fan, H.; Liu, B. Distribution Pattern of Endangered Plant Semiliquidambar cathayensis (Hamamelidaceae) in Response to Climate Change after the Last Interglacial Period. Forests 2020, 11, 434. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Zhou, X.; Chen, R.; Zhao, G.; Zhang, F. Prediction of the Potentially Suitable Areas of Leonurus japonicus in China Based on Future Climate Change Using the Optimized MaxEnt Model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B. Niche Properties and Geographical Extent as Predictors of Species Sensitivity to Climate Change. Glob. Ecol. Biogeogr. 2005, 14, 347–357. [Google Scholar] [CrossRef]
- Diamond, S.E.; Frame, A.M.; Martin, R.A.; Buckley, L.B. Species’ Traits Predict Phenological Responses to Climate Change in Butterflies. Ecology 2011, 92, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhang, Y.-H.; Zhang, H.-J.; Landis, J.B.; Zhang, X.; Wang, H.-C.; Yao, X.-H. Molecular Phylogeography and Species Distribution Modelling Evidence of ‘Oceanic’ Adaptation for Actinidia eriantha with a Refugium along the Oceanic–Continental Gradient in a Biodiversity Hotspot. BMC Plant Biol. 2022, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, G.; Li, Z.; Zhong, C.; Yao, X. Characterizing Tetraploid Populations of Actinidia chinensis for Kiwifruit Genetic Improvement. Plants 2022, 11, 1154. [Google Scholar] [CrossRef]
- Pardini, R.; Nichols, E.; Püttker, T. Biodiversity Response to Habitat Loss and Fragmentation. Encycl. Anthr. 2017, 3, 229–239. [Google Scholar]



| Code | Description | Percent Contribution | Permutation Importance |
|---|---|---|---|
| Bio12 | Annual precipitation | 55.71 | 9.98 |
| Bio7 | Temperature annual range (bio5-bio6) | 20.73 | 35.70 |
| Bio2 | Mean diurnal range (mean of monthly (max.temp.-min.temp.) | 10.07 | 8.10 |
| Bio17 | Precipitation of the driest quarter | 7.30 | 14.19 |
| pH | Topsoil pH (H2O) | 2.07 | 6.46 |
| Bio14 | Precipitation of the driest month | 0.84 | 5.11 |
| TN | Total nitrogen | 0.83 | 4.84 |
| Elev | Elevations | 0.76 | 2.08 |
| TK | Total potassium | 0.65 | 4.92 |
| Bio18 | Precipitation of the warmest quarter | 0.32 | 5.57 |
| Bio3 | Isothermality (bio2/bio7) (×100) | 0.28 | 1.52 |
| TP | Total phosphorus | 0.20 | 0.26 |
| CF | Coarse fragment content | 0.16 | 0.36 |
| Bio5 | Max temperature of the warmest month | 0.09 | 0.90 |
| Type | RM | FC | Delta.AICc | AUC | Mean.diff.AUC | Mean.OR10 |
|---|---|---|---|---|---|---|
| default | 1 | LQHP | 87.661 | 0.961 | 0.032 | 0.129 |
| optimized | 1.5 | LQHPT | 0.000 | 0.951 | 0.031 | 0.146 |
| Scenario | Current | SSP1-2.6 | SSP2-4.5 | SSP3-7.0 | SSP5-8.5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Period | 2050s | 2090s | 2050s | 2090s | 2050s | 2090s | 2050s | 2090s | |
| Unsuitable habitats | 87.34 | 90.61 | 85.35 | 86.71 | 89.49 | 89.44 | 91.71 | 88.53 | 92.51 |
| Low suitable habitats | 5.59 | 4.59 | 6.54 | 7.12 | 7.00 | 4.64 | 5.83 | 5.74 | 4.86 |
| Moderately suitable habitats | 6.02 | 4.19 | 7.18 | 5.82 | 3.28 | 4.82 | 2.21 | 4.91 | 2.44 |
| Highly suitable habitats | 1.05 | 0.60 | 0.93 | 0.34 | 0.22 | 1.10 | 0.25 | 0.82 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Luo, M.; Ye, L.; Peng, J.; Luo, X.; Gao, L.; Huang, Q.; Chen, Q.; Zhang, L. Prediction of the Potentially Suitable Areas of Actinidia latifolia in China Based on Climate Change Using the Optimized MaxEnt Model. Sustainability 2024, 16, 5975. https://doi.org/10.3390/su16145975
Wang Z, Luo M, Ye L, Peng J, Luo X, Gao L, Huang Q, Chen Q, Zhang L. Prediction of the Potentially Suitable Areas of Actinidia latifolia in China Based on Climate Change Using the Optimized MaxEnt Model. Sustainability. 2024; 16(14):5975. https://doi.org/10.3390/su16145975
Chicago/Turabian StyleWang, Zhi, Minmin Luo, Lixia Ye, Jue Peng, Xuan Luo, Lei Gao, Qiong Huang, Qinghong Chen, and Lei Zhang. 2024. "Prediction of the Potentially Suitable Areas of Actinidia latifolia in China Based on Climate Change Using the Optimized MaxEnt Model" Sustainability 16, no. 14: 5975. https://doi.org/10.3390/su16145975
APA StyleWang, Z., Luo, M., Ye, L., Peng, J., Luo, X., Gao, L., Huang, Q., Chen, Q., & Zhang, L. (2024). Prediction of the Potentially Suitable Areas of Actinidia latifolia in China Based on Climate Change Using the Optimized MaxEnt Model. Sustainability, 16(14), 5975. https://doi.org/10.3390/su16145975






