From Waste to Energy: Enhancing Fuel and Hydrogen Production through Pyrolysis and In-Line Reforming of Plastic Wastes
Abstract
1. Introduction
Sustainability of Pyrolysis Process
2. Plastic Pyrolysis
2.1. Plastic
2.2. Pyrolysis
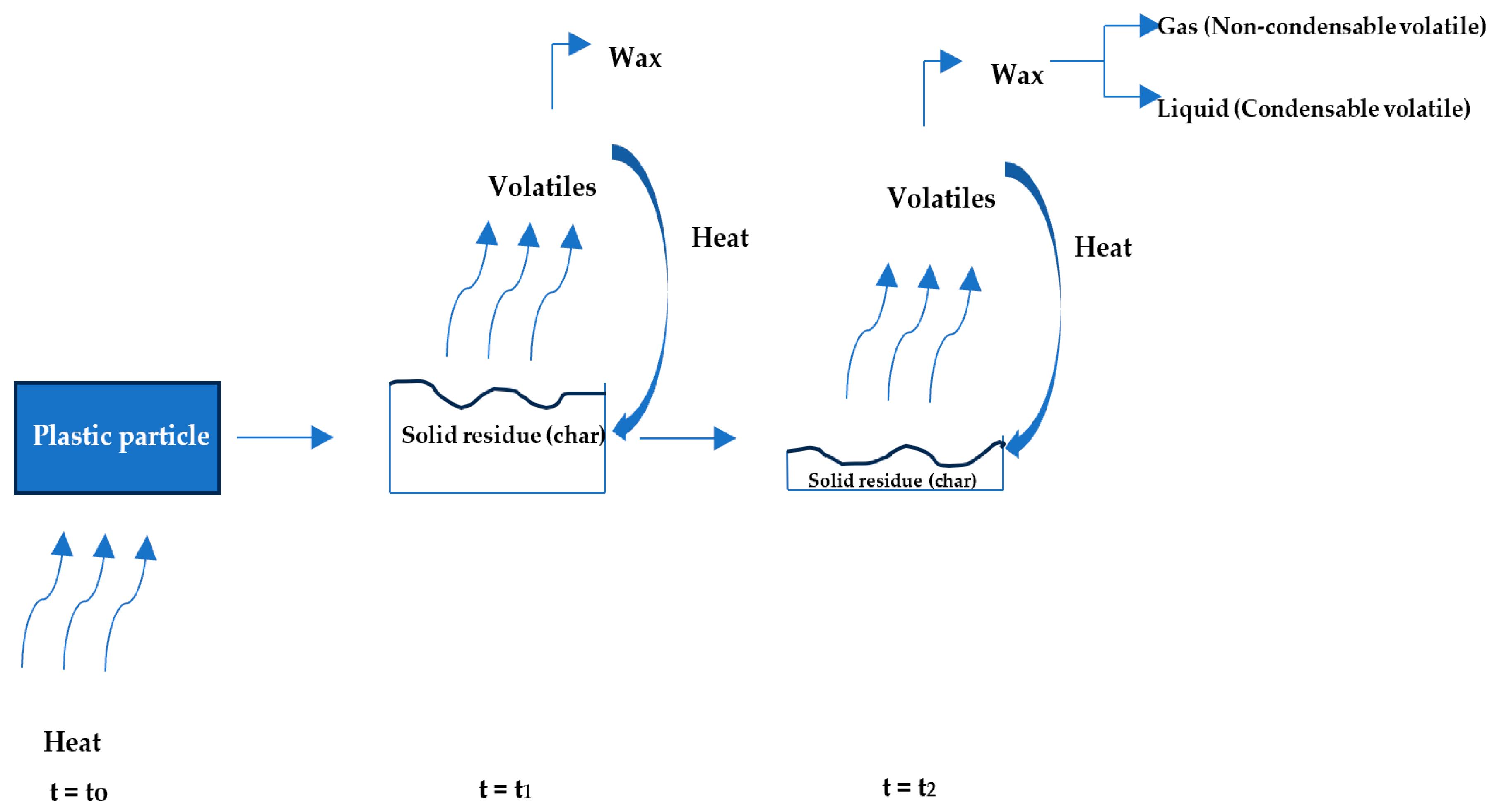
2.3. Mechanism of Plastic Pyrolysis
2.4. Factors Affecting Plastic Pyrolysis
2.4.1. Temperature
2.4.2. Heating Rate and Residence Time
2.4.3. Pressure
2.4.4. Catalyst
2.4.5. Feedstock Composition
2.4.6. Type of Reactors
Batch/Semi-Batch Reactors
Continuous Flow Reactors
- Fixed bed reactor: This type of reactor can be used for catalytic pyrolysis and is predominantly used for laboratory-scale studies due to its ease of design. However, it has certain limitations including a restricted available surface area for the catalyst and challenges relating to the irregular shape and particle size of the plastics used as feedstock, which can pose difficulties during feeding [18]. The feedstock is introduced into the reactor, usually made of stainless steel and heated externally with an electric furnace. Typically, the heating rate of a fixed bed reactor is usually low [17]. Moreover, since there is no movement of feedstock during the process, achieving the uniform heating of a significant amount of municipal solid waste (MSW) on an industrial scale can be challenging, unless technologies such as heat pipes are employed to improve the heat transfer [17,69]. Consequently, this type of reactor is mostly employed for gathering experimental data on the pyrolysis parameters and its products [21] or utilised as secondary reactors [54].
- Fluidised bed reactor (FBR): Unlike the fixed bed reactor, the fluidised bed reactor has better heat and mass transfer efficiencies [10]. This reactor is usually employed to examine the behaviour of fast pyrolysis due to its ability to effectively blend feedstock and achieve high heating rates. Fluidised bed reactors offer a significant advantage in their ability to control operational parameters like temperature, providing direct flexibility to achieve the desired product distribution. Furthermore, fluidised bed reactors are renowned for their excellent heat and mass transfer capabilities, facilitated by a heated fluidising medium within the reactor [21]. The heat transfer characteristics are improved due to the remarkable mixing within the bed induced by the motion of fluidised particles. When compared to fixed bed reactors, fluidised bed reactors reduce the time needed for degradation. The favourable heat transfer rate of this reactor is notable, especially considering the low thermal conductivity of plastics and their melt [10]. Additionally, this reactor type is well-suited for catalytic pyrolysis. Unlike in fixed bed reactors where the catalyst is packed into a static bed, in fluidised bed reactors, the catalyst rests on a distributor plate through which the fluidising gas passes [21]. The industrial application of this reactor presents challenges when handling non-uniform particle sizes, as it necessitates different velocities for fluidising particles of varying shapes and sizes [10].
- Conical spouted bed reactor (CSBR): This reactor is a type of fluidising bed reactor that utilises spouting characteristic behaviours. It has a small inlet connected to a fixed-diameter column containing static spouting media via a conical section. In contrast to a fluidised bed reactor, this reactor can effectively handle particles with a wide size distribution, sticky solids, irregular textures, and varying densities. Sand is employed in this reactor as a heat transfer medium due to its crystalline structure, which improves heat transfer to plastic melts surrounding the particle, thereby decreasing de-fluidisation problems [10]. This reactor offers attractive conditions for the pyrolysis of plastic wastes due to lower attrition, low bed segregation, and drops in pressure in comparison to the fluidised bed reactors, as observed by Elordi et al. [70]. Waste plastic melt, as they are fed into this type of reactor, and because of their cyclic movement, coat the sand particles uniformly. It provides a high heat transfer between phases and minimal de-fluidisation issues when handling sticky solids. The action of the spout and the solid flow pattern contribute to a decrease in the formation of agglomerates. Additionally, it is very versatile in terms of gas flow, enabling operations with short gas residence times. This reactor is appropriate for flash pyrolysis because of its excellent solid movement, resulting in rapid heat transfer rates between phases. Notably, the CSBR is capable of continuous operation, a crucial feature for implementing pyrolysis on a larger scale [17,21]. The spouted bed design proved effective in wax generation for pyrolysis at low temperatures. However, some of the technical limitations during the reactor’s operation include catalyst feeding, catalyst entrainment, and the collection of products (solid and liquid) [18,71]. Also, the high running costs associated with its complex design, which necessitates the use of numerous pumps in the system to provide sufficient pressure for spouting behaviours, makes it less favourable [5,21].
- Rotary/screw kiln reactor: A rotary kiln reactor is recommended for the industrial pyrolysis of plastics, given its capability in handling irregular particles with a wide range of heat capacity. It is beneficial in terms of the heat input to the feedstock material. By varying the screw speed which influences the product distribution, the heat input and residence time can be regulated. To optimise the product distribution, rotary kiln reactors set at an angle can help control the degree of mixing [10]. Rotary kilns are less complicated to operate than fluidised bed reactors and offer better heat transfer to the feedstock than fixed bed reactors. A crucial parameter in the pyrolysis process of rotary kilns is the residence time of the feedstock in the reactor since it affects how much energy the charge receives at a specific heating rate. As reported by Fantozzi et al. [72], the residence time in rotary kilns is often a function of the mean volumetric flow and kiln rotation speed [17]. Good mixing is enabled by the slow rotation of the inclined kiln in order to yield more homogenous pyrolytic products [17,73]. The heating is uniform; however, because only the reactor wall transports heat, the heating is relatively slow. These reactors are widely used for conventional pyrolysis (slow pyrolysis), usually carried out at 500 °C for a residence time of 1 h [73]. Similar to the CSBR, these reactors are highly flexible for treating mixed waste plastic with a wide variety of shape and size distributions, but with a simpler design and mode of operation [5]. More benefits of using this reactor type over other reactors are low capital costs, the flexible modification of residence times and temperature control, good mixing, and an ease of maintenance [73].
- e.
- Microwave-assisted technology: This technology has not received as much research attention as other reactor setups. It is an attractive technique in providing volumetric heating at improved heating efficiencies [73]. In this type of reactor, the plastic feedstock is mixed with a microwave absorbent material, such as particulate carbon [74]. This material provides the required heating for feedstock conversion into the desired product through transforming microwave radiation energy into thermal energy [8,21]. In the electromagnetic spectrum, microwave frequencies fall between infrared and radio frequencies [17]. Microwaves have wavelengths between 1 mm and 1 m, and their corresponding frequencies range from 300 GHz to 300 MHz, respectively. The most popular microwave frequencies are 2.45 GHz and 916 MHz [17,75]. Suitable materials for converting microwave radiation into heat at a frequency of 2.45 or 0.972 GHz are metals or carbon. Moreover, the reducing nature of carbon prevents undesired oxygenated compounds from forming if the plastic waste contains oxygen-bearing contaminants. Unlike conventional reactors, microwave energy is supplied directly to the material via molecular interactions with the electromagnetic field. This leads to a shorter time being required for heating the surrounding area, resulting in a reduced heating time and lower operational costs. Plastics have low dielectric constants [8]; therefore, a microwave absorbent with a high dielectric constant is required to be mixed with the plastics in order to absorb the microwave energy and attain the required temperature [30]. The efficiency of microwave heating is significantly dependent on the dielectric properties of the material [76]. A pyrolytic temperature of 1000 °C can be attained quickly using microwave heating because of the strength and amount of microwave absorbent materials used [8]. Therefore, this technique offers many benefits over conventional heating methods, including rapid and uniform internal heating rates, operational flexibility (prompt response for quick start-up and shutdown), economical and high-product selectivity, and consistent volumetric heating. Unfortunately, research on microwave-assisted reactors is still in its early stages, and there are still many challenges to its usage [8,21]. For instance, Lam and Chase [74] employed microwave pyrolysis in waste-to-energy processes, accurately characterising the process, but concluded that the development of industrial microwave heating applications was constrained by an apparent lack of technical knowledge in terms of designing commercial equipment for this pyrolysis and a lack of understanding of microwave systems [17]. Using microwave absorbents on an industrial scale may be very challenging given that the heating efficiency may vary greatly for different absorbents [8]. The varying composition of plastic wastes and the lack of knowledge regarding the dielectric characteristics of various materials further compound this limitation. In addition to the universal parameters for all reactors, other factors influencing the efficiency of microwave pyrolysis include the microwave output power, microwave type (single-mode or multimode), reactor design/type, and microwave receptor properties (size, type, and amount/concentration) [5]. The microwave heating in the pyrolysis of polyolefin wastes (PP and HDPE) was investigated by Undri et al. [77] using two types of microwave absorbers, namely carbon and tyres. According to the results, HDPE had the highest liquid yield at 83.9 wt%, whereas PP had a yield of 74.7 wt%. Microwave powers ranging from 3 to 6 kW were employed. The results indicated that, due to the reduction in the residence time caused by the high power, more polymers were converted into liquid rather than gases. Additionally, utilising tyre as the microwave absorber increased the solid residue (33 wt%) due to the other tyre components that could not be pyrolyzed. However, when carbon was used as the microwave absorber, the solid residue was far less (0.4 wt%) [21,77]. Therefore, carbon material was a better microwave absorbent with a high capacity to absorb and transform microwave energy into heat [77]. In addition, to maximise the liquid yield, the microwave power and absorber type must be prioritised [21]. A pyrowave is a recently developed microwave pyrolysis unit with the aim to recover styrene from polystyrene waste [78].
- f.
- Plasma reactors: This is also a relatively new pyrolysis technology. It has been gaining increased attention because of its manageability, rapid heating, and ability to function effectively at a relatively low power consumption [17,79]. Plasma, characterised as an ionised gas, is often considered as the fourth state of matter following solid, liquid, and gas. Typically, it is considered as a gaseous mixture of positively charged ions and negatively charged electrons, produced either through the intense heating of a gas or the exposure of the gas to a strong electromagnetic field. The three categories of plasmas are high-temperature plasma (also known as Equilibrium plasma), thermal plasmas (quasi-equilibrium plasma), and non-equilibrium plasma (also cold plasma) [80]. Thermal plasma can be generated by employing direct current, alternating current, microwave discharge, or radio frequency induction. Plasma can also be produced using a 2.45 GHz magnetron from a commercial microwave oven [17]. When waste-derived carbonaceous particles are introduced into plasma, the rapid heating by the plasma induces the release and cracking of volatile materials. This process generates hydrogen and light hydrocarbons such as acetylene and methane [17,81]. Huang and Tang [81] conducted a review on the thermal plasma pyrolysis of organic waste, noting the production of the following two main streams: a solid residue and a combustible gas. The gas yield, composed of H2, CH4, CO, C2H2, and C2H4, ranged from 50 to 98 wt%, with the heating value varying from 4 to 9 MJ/Nm3. Therefore, it can be utilised directly as fuel in many energy applications, including direct firing in boilers, gas engines, and gas turbines [17].
2.5. Pyrolysis of Single-Type Plastics
2.5.1. Polyethylene (PE)
High-Density Polyethylene (HDPE)
Low-Density Polyethylene (LDPE)
2.5.2. Polypropylene (PP)
2.5.3. Polystyrene (PS)
2.5.4. Polyethylene Terephthalate (PET)
2.5.5. Polyvinyl Chloride (PVC)
2.6. Pyrolysis of Mixed Plastics
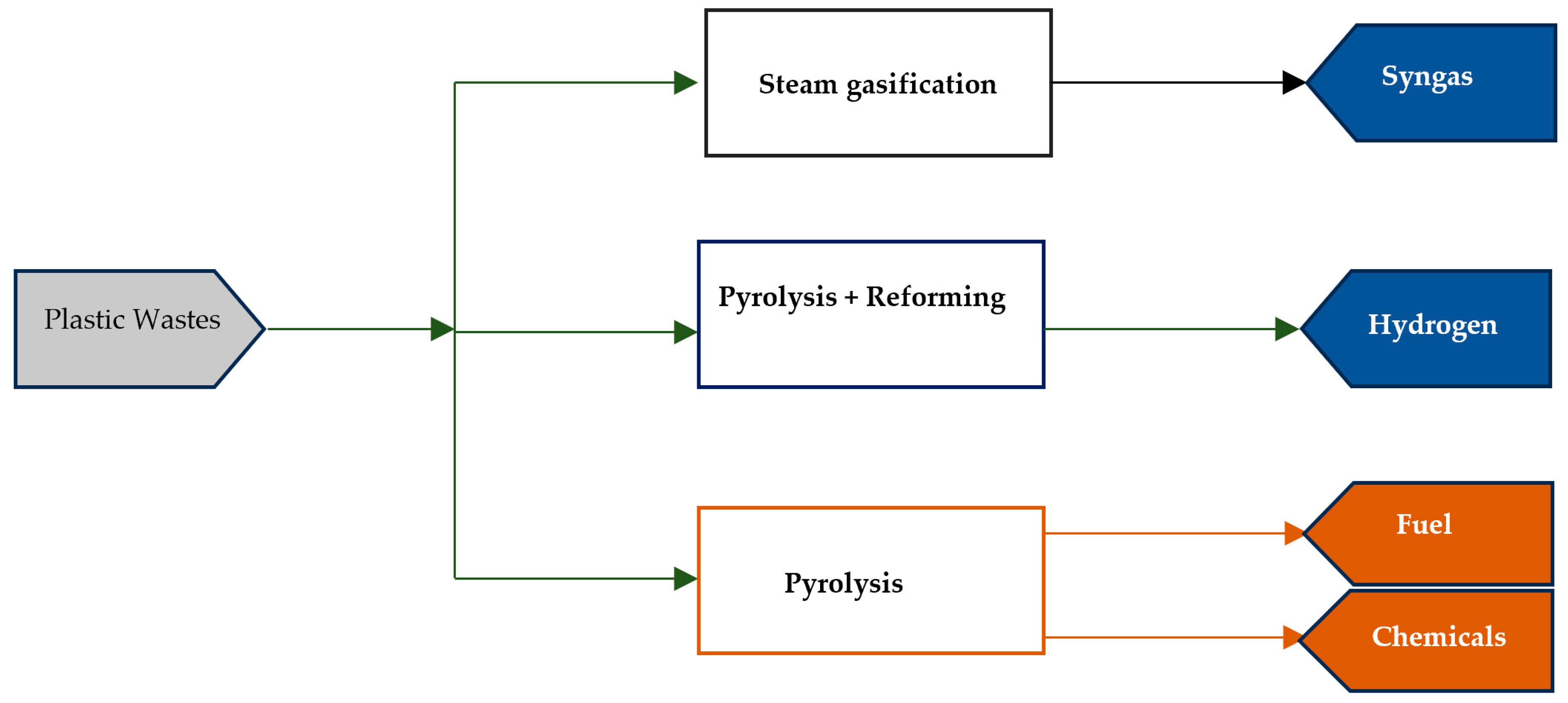
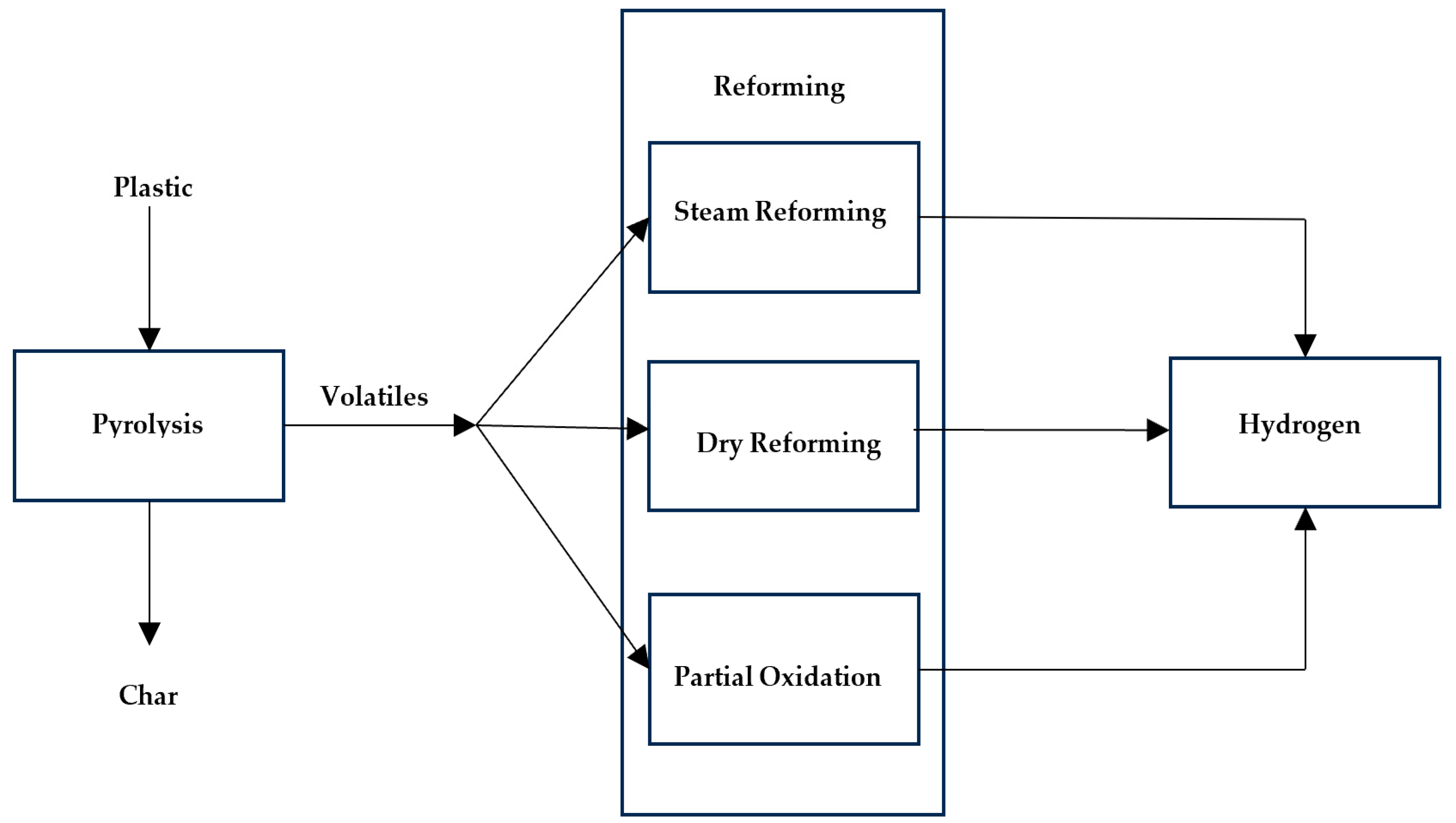
2.7. Hydrogen Production from Pyrolysis
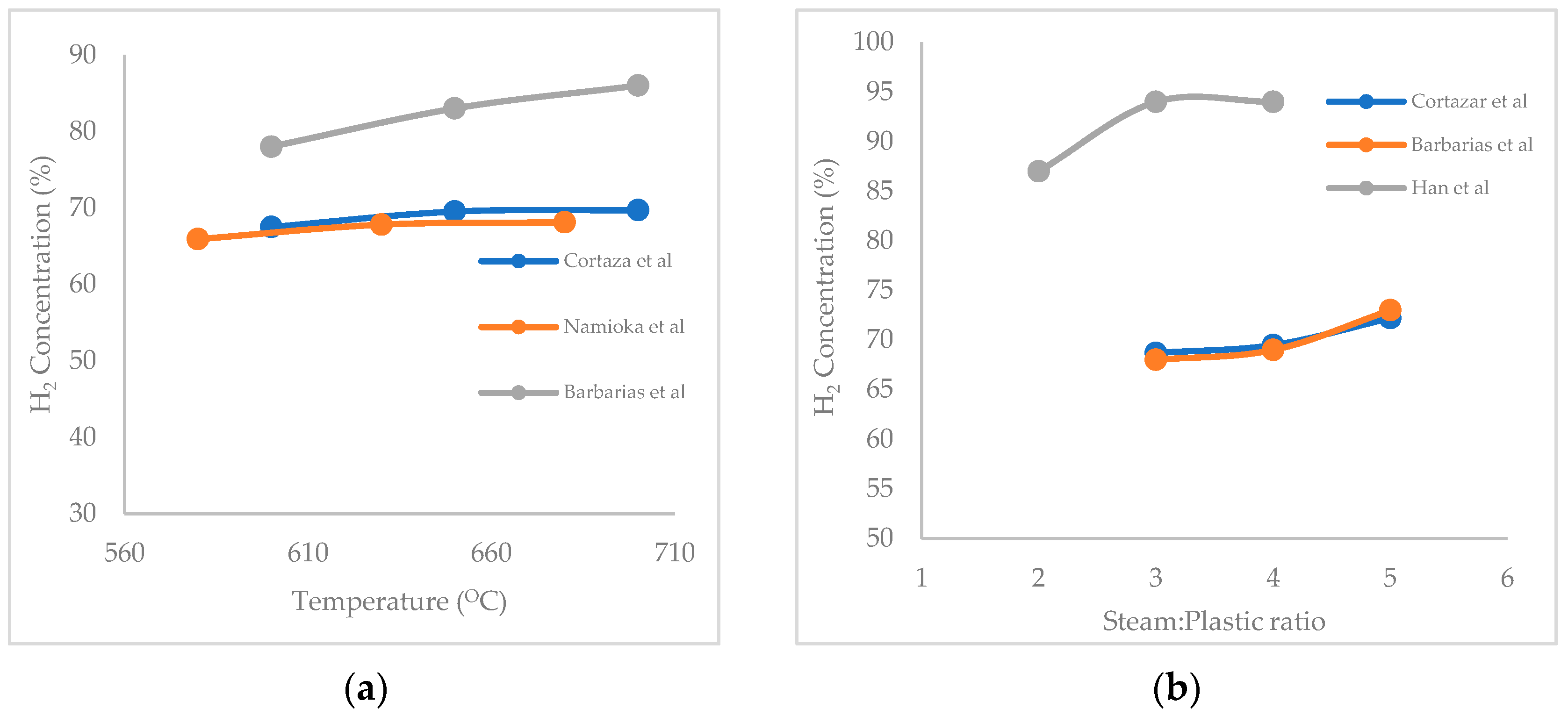
2.7.1. Steam Reforming
2.7.2. Dry Reforming (DR)
2.7.3. Partial Oxidation
2.8. Environmental Impact and Energy Efficiency of Pyrolysis
3. Conclusions
4. Future Recommendation
Funding
Acknowledgments
Conflicts of Interest
References
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Al-Garadi, N.Y.; Osman, A.I.; Al-Mubaddel, F.S.; Ibrahim, A.A.; Khan, W.U.; Alanazi, Y.M.; Alrashed, M.M.; Alothman, O.Y. From plastic waste pyrolysis to Fuel: Impact of process parameters and material selection on hydrogen production. Fuel 2023, 344, 128107. [Google Scholar] [CrossRef]
- PlasticsEurope. Annual Production of Plastics Worldwide from 1950 to 2022 (in Million Metric Tons) [Graph]. Statista. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 11 February 2024).
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Mirkarimi, S.M.R.; Bensaid, S.; Chiaramonti, D. Conversion of mixed waste plastic into fuel for diesel engines through pyrolysis process: A review. Appl. Energy 2022, 327, 120040. [Google Scholar] [CrossRef]
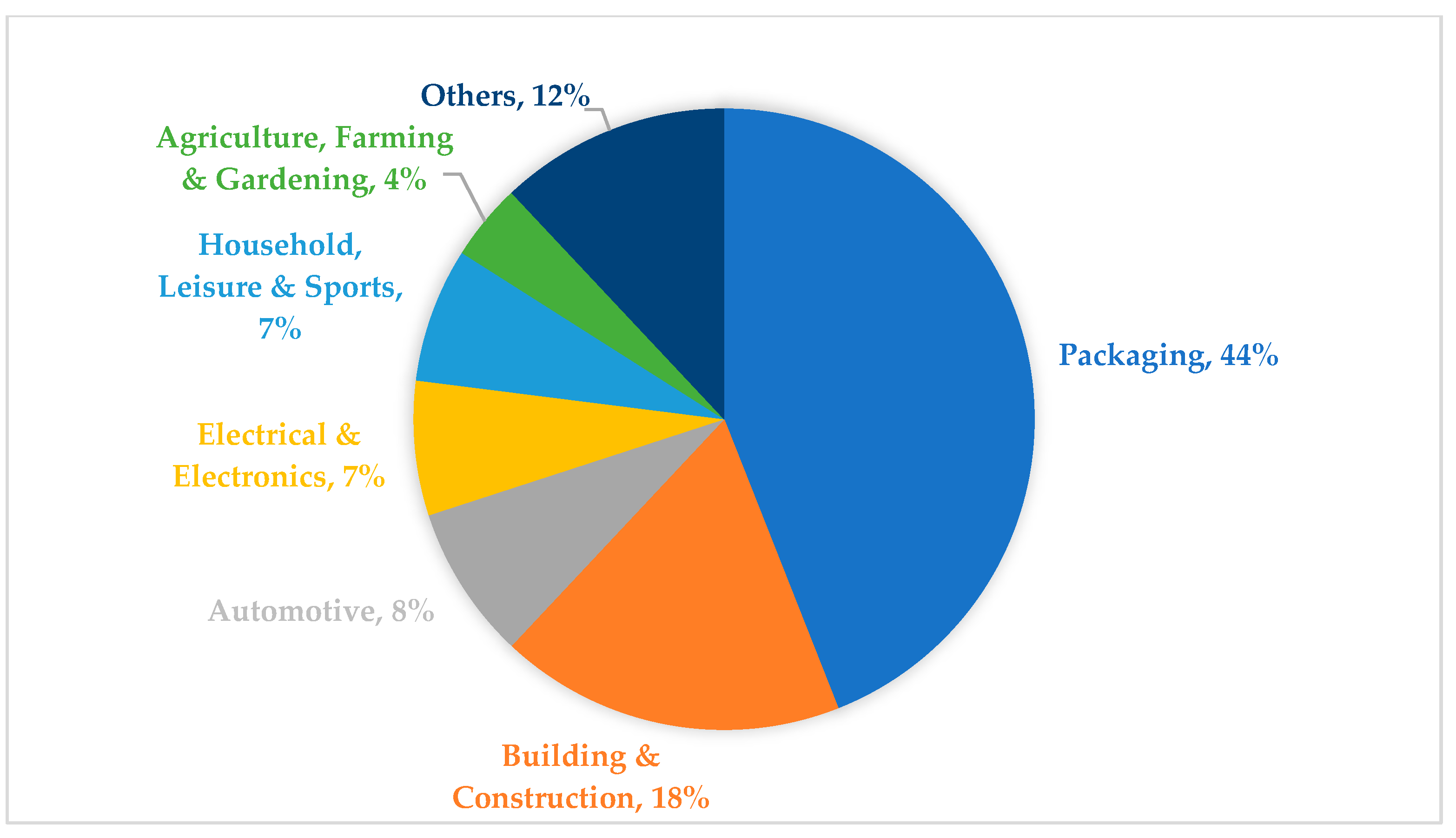
- PlasticsEurope AISBL and European Association of Plastics Recycling & Recovery Organisations. Plastics—The Facts 2022. Belgium. October 2022. Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 15 May 2024).
- Davidson, M.G.; Furlong, R.A.; McManus, M.C. Developments in the life cycle assessment of chemical recycling of plastic waste—A review. J. Clean. Prod. 2021, 293, 126163. [Google Scholar] [CrossRef]
- Xayachak, T.; Haque, N.; Parthasarathy, R.; King, S.; Emami, N.; Lau, D.; Pramanik, B.K. Pyrolysis for plastic waste management: An engineering perspective. J. Environ. Chem. Eng. 2022, 10, 108865. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/en/resources/publications/1804-plastics-facts-2019 (accessed on 15 April 2024).
- Kartik, S.; Balsora, H.K.; Sharma, M.; Saptoro, A.; Jain, R.K.; Joshi, J.B.; Sharma, A. Valorization of plastic wastes for production of fuels and value-added chemicals through pyrolysis—A review. Therm. Sci. Eng. Prog. 2022, 32, 101316. [Google Scholar] [CrossRef]
- Balu, R.; Dutta, N.K.; Choudhury, N.R. Plastic Waste Upcycling: A Sustainable Solution for Waste Management, Product Development, and Circular Economy. Polymers 2022, 14, 4788. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, H.; Krüger, C.; Russ, M.; Horlacher, M.; Antony, F.; Hann, S.; Azapagic, A. Life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste in comparison with mechanical recycling and energy recovery. Sci. Total Environ. 2021, 769, 144483. [Google Scholar] [CrossRef]
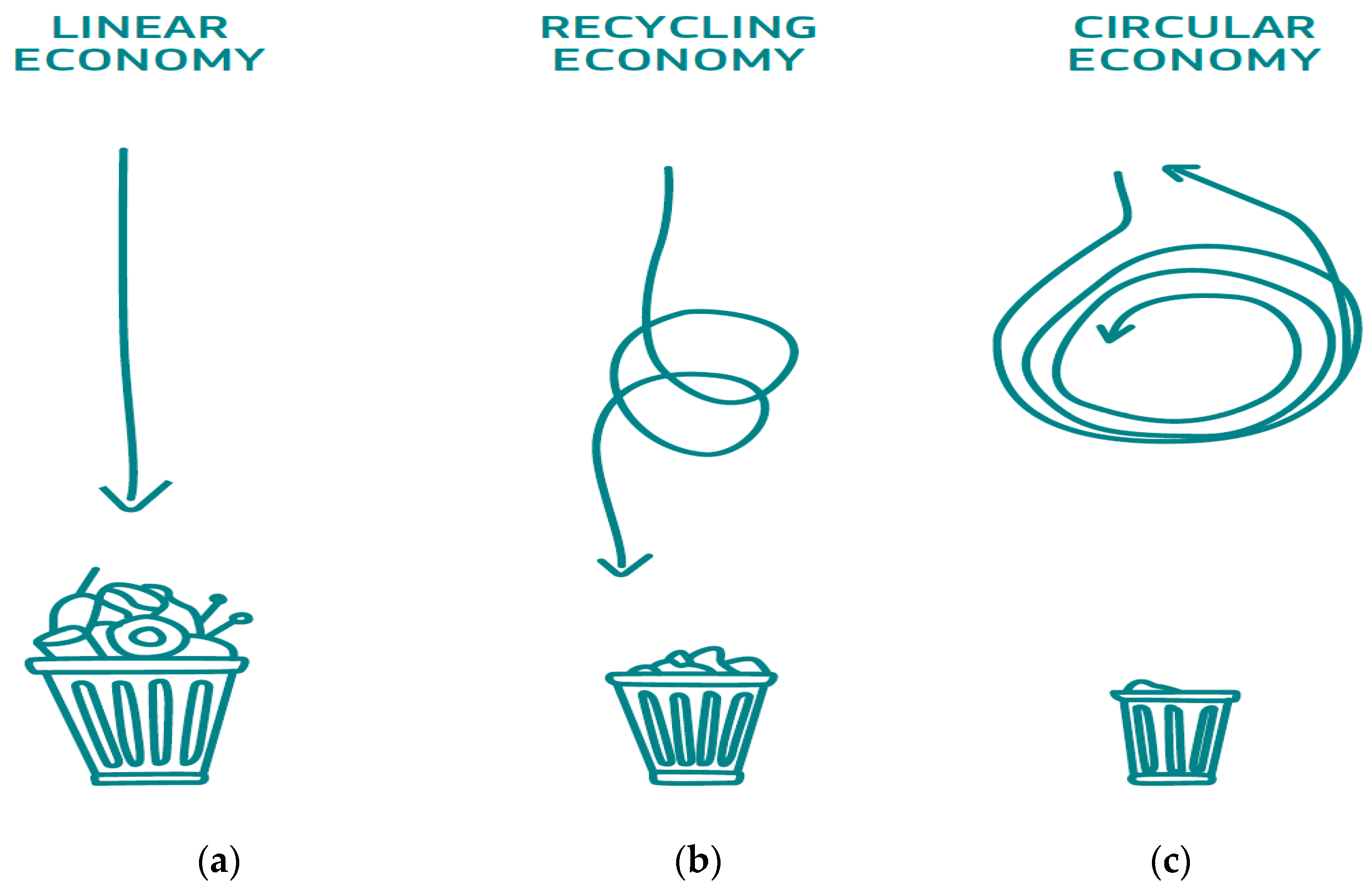
- The World Economic Forum; Stuchtey, M.R.; MacArthur, D.E.; Waughray, D. The New Plastics Economy: Rethinking the Future of Plastics. January 2016. Available online: https://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf (accessed on 15 April 2024).
- Getor, R.Y.; Mishra, N.; Ramudhin, A. The role of technological innovation in plastic production within a circular economy framework. Resour. Conserv. Recycl. 2020, 163, 105094. [Google Scholar] [CrossRef]
- Chang, S.H. Plastic waste as pyrolysis feedstock for plastic oil production: A review. Sci. Total. Environ. 2023, 877, 162719. [Google Scholar] [CrossRef] [PubMed]
- Paradela, F.; Pinto, F.; Gulyurtlu, I.; Cabrita, I.; Lapa, N. Study of the co-pyrolysis of biomass and plastic wastes. Clean Technol. Environ. Policy 2009, 11, 115–122. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Shah, H.H.; Amin, M.; Iqbal, A.; Nadeem, I.; Kalin, M.; Soomar, A.M.; Galal, A.M. A review on gasification and pyrolysis of waste plastics. Front. Chem. 2023, 10, 960894. [Google Scholar] [CrossRef] [PubMed]
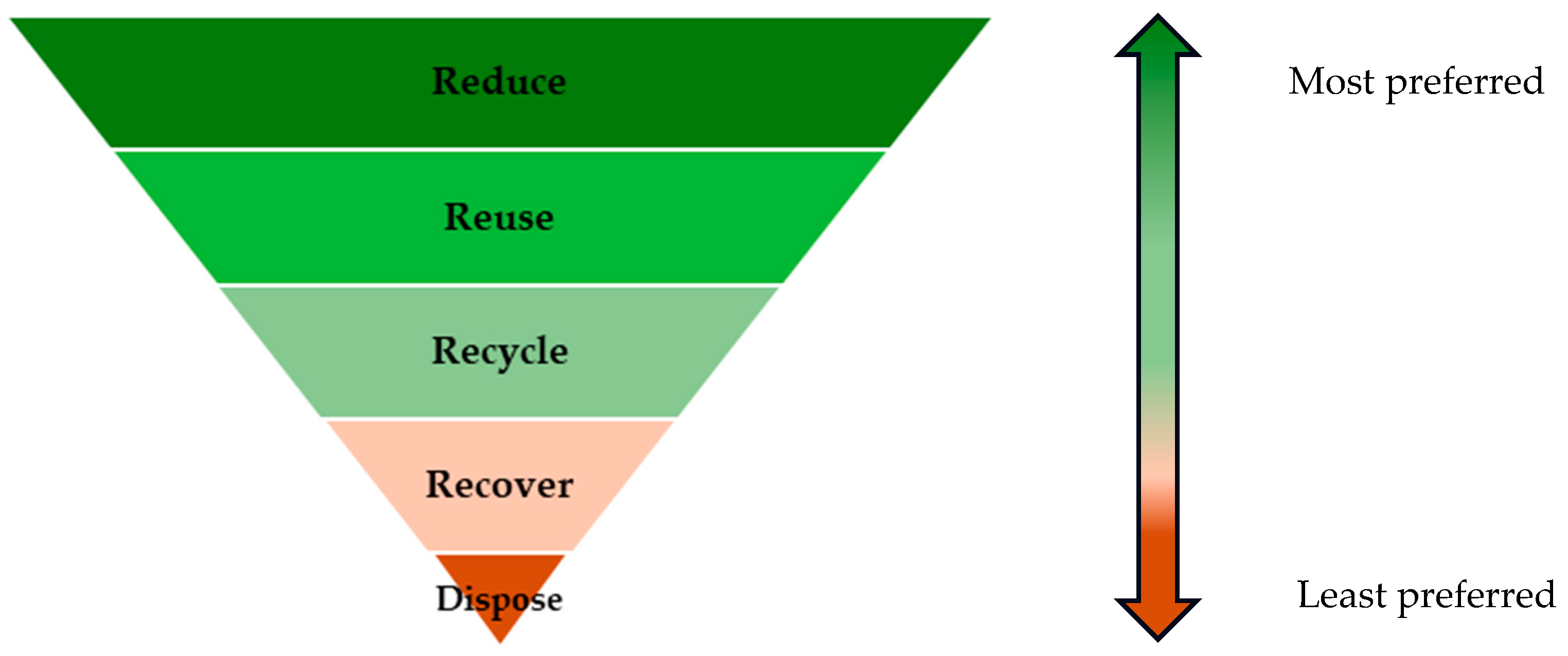
- CIRIA. The Waste Hierarchy. Ciriasoil. Available online: https://www.ciria.org/SOILCOP/SOILCOP/Knowledgebase/Waste-Hierarchy.aspx (accessed on 18 April 2024).
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Thermochemical conversion of plastic waste to fuels: A review. Environ. Chem. Lett. 2021, 19, 123–148. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics’s Contribution to the Circular Economy. Available online: https://www.plasticseurope.org/en/focus-areas/circular-economy (accessed on 21 April 2024).
- Moraga, G.; Huysveld, S.; Mathieux, F.; Blengini, G.A.; Alaerts, L.; Van Acker, K.; de Meester, S.; Dewulf, J. Circular economy indicators: What do they measure? Resour. Conserv. Recycl. 2019, 146, 452–461. [Google Scholar] [CrossRef]
- Crippa, M.; De Wilde, B.; Koopmans, R.; Leyssens, J.; Linder, M.; Muncke, J.; Ritschkoff, A.C.; Van Doorsselaer, K.; Velis, C.; Wagner, M. A Circular Economy for Plastics—Insights from Research and Innovation to Inform Policy and Funding Decisions; Publications Office of the European Union: Brussels, Belgium, 2019; Available online: https://data.europa.eu/doi/10.2777/269031 (accessed on 19 April 2024).
- The Society Plastic Industry: The Plastic Industry Trade Association. SPI Resin Identification Code—Guide to Correct Use. Available online: https://web.archive.org/web/20160126213345/http://www.plasticsindustry.org/AboutPlastics/content.cfm?ItemNumber=823&navItemNumber=1125 (accessed on 15 April 2024).
- Dai, L.; Zhou, N.; Lv, Y.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. Pyrolysis technology for plastic waste recycling: A state-of-the-art review. Prog. Energy Combust. Sci. 2022, 93, 101021. [Google Scholar] [CrossRef]
- Ryan, J. Polymer from Pyrolysis Liquid: Pyrolymer. Ph.D. Thesis, The University of Nottingham, Nottingham, UK, 2021. [Google Scholar]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic conversion of plastic waste to value-added products and fuels: A review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Mohamed, A.; Abdelnaby, M.A. Pyrolysis Kinetic Behavior and Thermodynamic Analysis of PET Nonwoven Fabric. Materials 2023, 16, 6079. [Google Scholar] [CrossRef] [PubMed]
- Habtewold, A.T.; Ambie, D.A.; Eremed, W.B. Solar assisted pyrolysis system for High-Density polyethylene plastic waste to fuel conversion. AIMS Energy 2020, 8, 455–473. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Zhao, P.; Wang, Z.; Cui, X.; Gao, L.; Guo, Q.; Tian, H. Synergistic Effect and Chlorine-Release Behaviors During Co-pyrolysis of LLDPE, PP, and PVC. ACS Omega 2020, 5, 11291–11298. [Google Scholar] [CrossRef] [PubMed]
- Adoga, S.O.; Igbum, O.G.; Okopi, G.; Ikyenge, B.A.; Ochi, O.I.; Ode, P. Catalytic pyrolysis of low density polyethylene and polypropylene wastes to fuel oils by N-clay. Ovidius Univ. Ann. Chem. 2022, 33, 50–55. [Google Scholar] [CrossRef]
- Heikkinen, J.M.; Hordijk, J.C.; de Jong, W.; Spliethoff, H. Thermogravimetry as a tool to classify waste components to be used for energy generation. J. Anal. Appl. Pyrolysis 2004, 71, 883–900. [Google Scholar] [CrossRef]
- Chai, Y. Pyrolysis and Gasification of Biomass and Waste Plastics under Novel Catalyst for H2 Production in the Context of Carbon Capture and Utilisation. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2021. [Google Scholar]
- Diaz Silvarrey, L.S. Advanced Pyrolysis of Plastic Waste for Chemicals, Fuel and Materials. Ph.D. Thesis, Newcastle University, Newcastle, UK, 2019. [Google Scholar]
- Okoro, F.E. Plastic Wastes to Energy: Pyrolysis Simulation by Thermogravimetry; IST Institute Superior Tecnico: Lisbon, Portugal, 2019. [Google Scholar]
- Stamouli, K. Fuel Production and Optimisation from Mixed Plastic Waste. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2017. [Google Scholar]
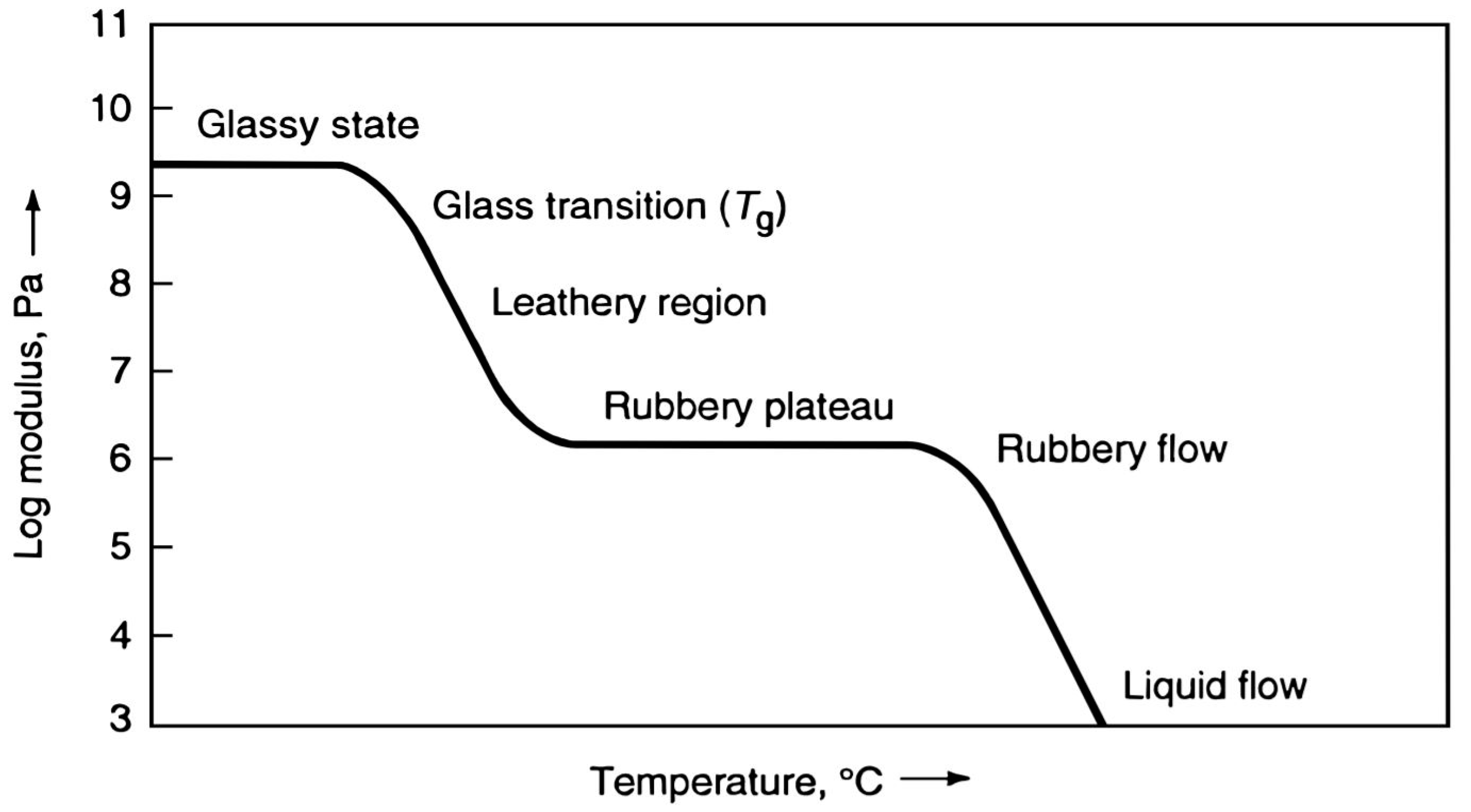
- The Welding Institute. Thermoset vs. Thermoplastic (What Is the Difference?). TWI Ltd. Available online: https://www.twi-global.com/technical-knowledge/faqs/thermoset-vs-thermoplastic#:~:text=Thermoplastics%20are%20resins%20that%20are,glass%20transition%20temperature%20(Tg) (accessed on 21 April 2024).
- Baker, A.-M.M.; Mead, J. Thermoplastics. In Modern Plastics Handbook, 1st ed.; Harper, C.A., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2000; p. 1. [Google Scholar]
- Babu, B.V.; Chaurasia, A. Modeling, simulation and estimation of optimum parameters in pyrolysis of biomass. Energy Convers. Manag. 2003, 44, 2135–2158. [Google Scholar] [CrossRef]
- Hernández, M.d.R.; Gómez, A.; García, N.; Agulló, J.; Marcilla, A. Effect of the temperature in the nature and extension of the primary and secondary reactions in the thermal and HZSM-5 catalytic pyrolysis of HDPE. Appl. Catal. A Gen. 2007, 317, 183–194. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Braithwaite, M.J.; Hassur, S.M.; Papenfuhs, T. Feedstock Recycling of Plastic Wastes; The Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.; Laresgoiti, M.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Thermal degradation of waste plastics under non-sweeping atmosphere: Part 1: Effect of temperature, product optimization, and degradation mechanism. J. Environ. Manag. 2019, 239, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Hervier, A.; Sablier, M. Study on the pyrolysis of polyethylene in the presence of iron and copper chlorides. Chemosphere 2006, 65, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W.; Kim, J.-S. Pyrolysis of mixed plastics into aromatics. J. Anal. Appl. Pyrolysis 1999, 51, 127–134. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Williams, E.A.; Williams, P.T. Analysis of products derived from the fast pyrolysis of plastic waste. J. Anal. Appl. Pyrolysis 1997, 40–41, 347–363. [Google Scholar] [CrossRef]
- Gracida-Alvarez, U.R.; Mitchell, M.K.; Sacramento-Rivero, J.C.; Shonnard, D.R. Effect of Temperature and Vapor Residence Time on the Micropyrolysis Products of Waste High Density Polyethylene. Ind. Eng. Chem. Res. 2018, 57, 1912–1923. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Wang, D.; Yan, M.; Zhang, J.; Zhang, P.; Ding, T.; Chen, L.; Chen, C. Current technologies for plastic waste treatment: A review. J. Clean. Prod. 2021, 282, 124523. [Google Scholar] [CrossRef]
- Yansaneh, O.Y.; Zein, S.H. Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview. Processes 2022, 10, 332. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 11524. [Google Scholar] [CrossRef]
- Mastral, F.J.; Esperanza, E.; Berrueco, C.; Juste, M.; Ceamanos, J. Fluidized bed thermal degradation products of HDPE in an inert atmosphere and in air–nitrogen mixtures. J. Anal. Appl. Pyrolysis 2003, 70, 1–17. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J. Pyrolysis of synthetic polymers and plastic wastes. Kinetic study. Fuel Process. Technol. 2008, 89, 678–686. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Martins-Vieira, J.C.; Missau, J.; Anshu, K.; Siakpebru, O.K.; Thengane, S.K.; Morais, A.R.C.; Tanabe, E.H.; Bertuol, D.A. Review on Biomass Pyrolysis with a Focus on Bio-Oil Upgrading Techniques. Analytica 2023, 4, 182–205. [Google Scholar] [CrossRef]
- Al-Haj Ibrahim, H. Introductory Chapter: Pyrolysis. Recent Adv. Pyrolysis 2020, 1, 90366. [Google Scholar] [CrossRef]
- Lopez, G.; Amutio, M.; Elordi, G.; Artetxe, M.; Altzibar, H.; Olazar, M. A Conical Spouted Bed Reactor for The Valorisation of Waste Tires. In Proceedings of the 13th International Conference on Fluidization—New Paradigm in Fluidization Engineering, Gyeongju, Republic of Korea, 16–21 May 2010; Available online: http://dc.engconfintl.org/fluidizationxiii/87 (accessed on 25 May 2024).
- Zayoud, A.; Thi, H.D.; Kusenberg, M.; Eschenbacher, A.; Kresovic, U.; Alderweireldt, N.; Djokic, M.; Van Geem, K.M. Pyrolysis of end-of-life polystyrene in a pilot-scale reactor: Maximizing styrene production. Waste Manag. 2022, 139, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Parku, G.K.; Collard, F.-X.; Görgens, J.F. Pyrolysis of waste polypropylene plastics for energy recovery: Influence of heating rate and vacuum conditions on composition of fuel product. Fuel Process. Technol. 2020, 209, 106522. [Google Scholar] [CrossRef]
- Murata, K.; Sato, K.; Sakata, Y. Effect of pressure on thermal degradation of polyethylene. J. Anal. Appl. Pyrolysis 2004, 71, 569–589. [Google Scholar] [CrossRef]
- Newalkar, G.; Iisa, K.; D’amico, A.D.; Sievers, C.; Agrawal, P. Effect of Temperature, Pressure, and Residence Time on Pyrolysis of Pine in an Entrained Flow Reactor. Energy Fuels 2014, 28, 5144–5157. [Google Scholar] [CrossRef]
- Rehan, M.; Miandad, R.; Barakat, M.; Ismail, I.; Almeelbi, T.; Gardy, J.; Hassanpour, A.; Khan, M.; Demirbas, A.; Nizami, A. Effect of zeolite catalysts on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 162–175. [Google Scholar] [CrossRef]
- Pandey, U.; Stormyr, J.A.; Hassani, A.; Jaiswal, R.; Haugen, H.H.; Moldestad, B.M.E. Pyrolysis of plastic waste to environmentally friendly products. WIT Trans. Ecol. Environ. 2020, 246, 61–74. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.; Mishra, D. Thermolysis of waste plastics to liquid fuelA suitable method for plastic waste management and manufacture of value added products—A world prospective. Renew. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Jouhara, H.; Nannou, T.; Anguilano, L.; Ghazal, H.; Spencer, N. Heat pipe based municipal waste treatment unit for home energy recovery. Energy 2017, 139, 1210–1230. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Amutio, M.; Artetxe, M.; Aguado, R.; Bilbao, J. Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2009, 85, 345–351. [Google Scholar] [CrossRef]
- Lopez, G.; Olazar, M.; Amutio, M.; Aguado, R.; Bilbao, J. Influence of Tire Formulation on the Products of Continuous Pyrolysis in a Conical Spouted Bed Reactor. Energy Fuels 2009, 23, 5423–5431. [Google Scholar] [CrossRef]
- Fantozzi, F.; Colantoni, S.; Bartocci, P.; Desideri, U. Rotary Kiln Slow Pyrolysis for Syngas and Char Production from Biomass and Waste—Part I: Working Envelope of the Reactor. J. Eng. Gas Turbines Power 2007, 129, 901–907. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Chase, H.A. A Review on Waste to Energy Processes Using Microwave Pyrolysis. Energies 2012, 5, 4209–4232. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Quadri, M.W.; Dohare, D. A Study to Optimise Plastic to Fuel Technology—A Review. Int. J. Eng. Res. Technol. 2020, 9, 192–222. [Google Scholar] [CrossRef]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Efficient disposal of waste polyolefins through microwave assisted pyrolysis. Fuel 2014, 116, 662–671. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Khongkrapan, P.; Thanompongchart, P.; Tippayawong, N.; Kiatsiriroat, T. Fuel gas and char from pyrolysis of waste paper in a microwave plasma reactor. Int. J. Energy Environ. 2013, 4, 969–974. [Google Scholar]
- Dave, P.N.; Joshi, A.K. Plasma pyrolysis and gasification of plastics waste—A review. J. Sci. Ind. Res. 2010, 69, 177–179. [Google Scholar]
- Huang, H.; Tang, L. Treatment of organic waste using thermal plasma pyrolysis technology. Energy Convers. Manag. 2007, 48, 1331–1337. [Google Scholar] [CrossRef]
- Mortezaeikia, V.; Tavakoli, O.; Khodaparasti, M.S. A review on kinetic study approach for pyrolysis of plastic wastes using thermogravimetric analysis. J. Anal. Appl. Pyrolysis 2021, 160, 105340. [Google Scholar] [CrossRef]
- Polychem USA. Plastic Coding System. Polychem USA: Raw Materials for the Plastics Industry. Available online: https://polychem-usa.com/plastic-coding-system/ (accessed on 16 April 2024).
- Piringer, O.G.; Baner, A.L. Plastic Packaging: Interactions with Food and Pharmaceuticals, 2nd ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kalargaris, I. Pyrolysis of Waste Plastics and Utilisation of the Produced Oils in Diesel Engines; University of Surrey: Surrey, UK, 2018. [Google Scholar]
- Eltayef, A. Handbook of Plastic Films; Rapra: Shawbury, UK, 2003. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. 2017. Available online: https://www.science.org (accessed on 25 May 2024).
- Mariappan, M.; Panithasan, M.S.; Venkadesan, G. Pyrolysis plastic oil production and optimisation followed by maximum possible replacement of diesel with bio-oil/methanol blends in a CRDI engine. J. Clean. Prod. 2021, 312, 127687. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Thermal pyrolysis of high density polyethylene (HDPE) in a novel fixed bed reactor system for the production of high value gasoline range hydrocarbons (HC). Process. Saf. Environ. Prot. 2019, 127, 171–179. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K. Recovery of hydrocarbon liquid from waste high density polyethylene by thermal pyrolysis. Braz. J. Chem. Eng. 2011, 28, 659–667. [Google Scholar] [CrossRef]
- Mastral, F.; Esperanza, E.; García, P.; Juste, M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J. Anal. Appl. Pyrolysis 2002, 63, 1–15. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Sebastian, J. Pyrolysis process to produce fuel from different types of plastic—A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 396, 012062. [Google Scholar] [CrossRef]
- Bagri, R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- FakhrHoseini, S.M.; Dastanian, M. Predicting Pyrolysis Products of PE, PP, and PET Using NRTL Activity Coefficient Model. J. Chem. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. The effect of slow pyrolysis on the conversion of packaging waste plastics (PE and PP) into fuel. Waste Manag. 2018, 79, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, Y.; Abdelouahed, L.; El Hage, R.; El Samrani, A.; Taouk, B. Pyrolysis of common plastics and their mixtures to produce valuable petroleum-like products. Polym. Degrad. Stab. 2022, 195, 109770. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; El Bouadili, A. Non-isothermal kinetic studies on co-processing of olive residue and polypropylene. Energy Convers. Manag. 2008, 49, 3666–3671. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- Sakata, Y.; Uddin, M.A.; Muto, A. Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. J. Anal. Appl. Pyrolysis 1999, 51, 135–155. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Park, K.-B.; Jeong, Y.-S.; Guzelciftci, B.; Kim, J.-S. Two-stage pyrolysis of polystyrene: Pyrolysis oil as a source of fuels or benzene, toluene, ethylbenzene, and xylenes. Appl. Energy 2020, 259, 114240. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, J.; Wang, J. Pyrolysis of polystyrene waste in a fluidized-bed reactor to obtain styrene monomer and gasoline fraction. Fuel Process. Technol. 2000, 63, 45–55. [Google Scholar] [CrossRef]
- Al-Salem, S.; Khan, A. On the degradation kinetics of poly(ethylene terephthalate) (PET)/poly(methyl methacrylate) (PMMA) blends in dynamic thermogravimetry. Polym. Degrad. Stab. 2014, 104, 28–32. [Google Scholar] [CrossRef]
- Ko, K.-H.; Rawal, A.; Sahajwalla, V. Analysis of thermal degradation kinetics and carbon structure changes of co-pyrolysis between macadamia nut shell and PET using thermogravimetric analysis and 13C solid state nuclear magnetic resonance. Energy Convers. Manag. 2014, 86, 154–164. [Google Scholar] [CrossRef]
- Çepelioğullar, Ö.; Pütün, A.E. Thermal and kinetic behaviors of biomass and plastic wastes in co-pyrolysis. Energy Convers. Manag. 2013, 75, 263–270. [Google Scholar] [CrossRef]
- King, S.; Locock, K.E. A circular economy framework for plastics: A semi-systematic review. J. Clean. Prod. 2022, 364, 132503. [Google Scholar] [CrossRef]
- Kim, N.-K.; Lee, S.-H.; Park, H.-D. Current biotechnologies on depolymerization of polyethylene terephthalate (PET) and repolymerization of reclaimed monomers from PET for bio-upcycling: A critical review. Bioresour. Technol. 2022, 363, 127931. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, C. Pyrolysis-Catalysis of Plastic Wastes for Production of Liquid Fuels and Chemicals. Ph.D. Thesis, University of Leeds, Leeds, UK, 2015. [Google Scholar]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; De Meester, S.; Van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, D.; Yin, L.; Wang, Z.; Zhao, L.; Wang, J. High efficiency chlorine removal from polyvinyl chloride (PVC) pyrolysis with a gas–liquid fluidized bed reactor. Waste Manag. 2014, 34, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Yang, J.; Roy, C.; Vasile, C. Vacuum pyrolysis of PVC I. Kinetic study. Polym. Degrad. Stab. 1999, 64, 127–144. [Google Scholar] [CrossRef]
- Cepeliogullar, O.P.A. Utilization of two different types of plastic wastes from daily and industrial life. J. Selcuk. Univ. Nat. Appl. Sci. 2013, 2, 694–706. [Google Scholar]
- Shan, T.; Bian, H.; Wang, K.; Li, Z.; Qiu, J.; Zhu, D.; Wang, C.; Tian, X. Study on pyrolysis characteristics and kinetics of mixed waste plastics under different atmospheres. Thermochim. Acta 2023, 722, 179467. [Google Scholar] [CrossRef]
- Briceno, J.; Lemos, M.A.; Lemos, F. Kinetic analysis of the degradation of HDPE+PP polymer mixtures. Int. J. Chem. Kinet. 2021, 53, 660–674. [Google Scholar] [CrossRef]
- Tuffi, R.; D’Abramo, S.; Cafiero, L.M.; Trinca, E.; Ciprioti, S.V. Thermal behavior and pyrolytic degradation kinetics of polymeric mixtures from waste packaging plastics. Express Polym. Lett. 2018, 12, 82–99. [Google Scholar] [CrossRef]
- Hujuri, U.; Ghoshal, A.K.; Gumma, S. Modeling pyrolysis kinetics of plastic mixtures. Polym. Degrad. Stab. 2008, 93, 1832–1837. [Google Scholar] [CrossRef]
- Anene, A.F.; Fredriksen, S.B.; Sætre, K.A.; Tokheim, L.-A. Experimental Study of Thermal and Catalytic Pyrolysis of Plastic Waste Components. Sustainability 2018, 10, 3979. [Google Scholar] [CrossRef]
- Kruse, T.M.; Levine, S.E.; Wong, H.-W.; Duoss, E.; Lebovitz, A.H.; Torkelson, J.M.; Broadbelt, L.J. Binary mixture pyrolysis of polypropylene and polystyrene: A modeling and experimental study. J. Anal. Appl. Pyrolysis 2005, 73, 342–354. [Google Scholar] [CrossRef]
- Marongiu, A.; Faravelli, T.; Ranzi, E. Detailed kinetic modeling of the thermal degradation of vinyl polymers. J. Anal. Appl. Pyrolysis 2007, 78, 343–362. [Google Scholar] [CrossRef]
- Ziad, M.; Khan, S.; Gohar, A. Thermal Pyrolysis of Individual and Mixed Plastic Waste of Polypropylene, Polyethylene and Polystyrene. 2022. Available online: https://ssrn.com/abstract=4091209 (accessed on 25 May 2024).
- Grause, G.; Matsumoto, S.; Kameda, T.; Yoshioka, T. Pyrolysis of Mixed Plastics in a Fluidized Bed of Hard Burnt Lime. Ind. Eng. Chem. Res. 2011, 50, 5459–5466. [Google Scholar] [CrossRef]
- Ciliz, N.K.; Ekinci, E.; Snape, C.E. Pyrolysis of virgin and waste polypropylene and its mixtures with waste polyethylene and polystyrene. Waste Manag. 2004, 24, 173–181. [Google Scholar] [CrossRef]
- Aminu, I.; Nahil, M.A.; Williams, P.T. High-yield hydrogen from thermal processing of waste plastics. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2022, 175, 3–13. [Google Scholar] [CrossRef]
- Cortazar, M.; Gao, N.; Quan, C.; Suarez, M.A.; Lopez, G.; Orozco, S.; Santamaria, L.; Amutio, M.; Olazar, M. Analysis of hydrogen production potential from waste plastics by pyrolysis and in line oxidative steam reforming. Fuel Process. Technol. 2022, 225, 107044. [Google Scholar] [CrossRef]
- Sudalaimuthu, P.; Sathyamurthy, R. The clean energy aspect of plastic waste—Hydrogen gas production, CO2 reforming, and plastic waste management coincide with catalytic pyrolysis—An extensive review. Environ. Sci. Pollut. Res. 2023, 30, 66559–66584. [Google Scholar] [CrossRef] [PubMed]
- Arman, A.; Hagos, F.Y.; Abdullah, A.A.; Mamat, R.; Aziz, A.R.A.; Cheng, C.K. Syngas production through steam and CO2 reforming of methane over Ni-based catalyst-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 042032. [Google Scholar] [CrossRef]
- Han, D.; Shin, S.; Jung, H.; Cho, W.; Baek, Y. Hydrogen Production by Steam Reforming of Pyrolysis Oil from Waste Plastic over 3 wt.% Ni/Ce-Zr-Mg/Al2O3 Catalyst. Energies 2023, 16, 2656. [Google Scholar] [CrossRef]
- Li, Y.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic steam reforming of waste plastics for enhanced hydrogen/syngas yield using sacrificial tire pyrolysis char catalyst. Chem. Eng. J. 2023, 467, 143427. [Google Scholar] [CrossRef]
- Alshareef, R.; Nahil, M.A.; Williams, P.T. Hydrogen Production by Three-Stage (i) Pyrolysis, (ii) Catalytic Steam Reforming, and (iii) Water Gas Shift Processing of Waste Plastic. Energy Fuels 2023, 37, 3894–3907. [Google Scholar] [CrossRef] [PubMed]
- Fakeeha, A.; Ibrahim, A.A.; Aljuraywi, H.; Alqahtani, Y.; Alkhodair, A.; Alswaidan, S.; Abasaeed, A.E.; Kasim, S.O.; Mahmud, S.; Al-Fatesh, A.S. Hydrogen production by partial oxidation reforming of methane over ni catalysts supported on high and low surface area Alumina and Zirconia. Processes 2020, 8, 499. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Wysocka, I.; Gębicki, J. Pyrolysis Combined with the Dry Reforming of Waste Plastics as a Potential Method for Resource Recovery—A Review of Process Parameters and Catalysts. Catalysts 2022, 12, 362. [Google Scholar] [CrossRef]
- Williams, P.T. Hydrogen and Carbon Nanotubes from Pyrolysis-Catalysis of Waste Plastics: A Review. Waste Biomass Valorization 2021, 12, 1–28. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A short review on Ni based catalysts and related engineering issues for methane steam reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Le, P.-A.; Trung, V.D.; Nguyen, P.L.; Phung, T.V.B.; Natsuki, J.; Natsuki, T. The current status of hydrogen energy: An overview. RSC Adv. 2023, 13, 28262–28287. [Google Scholar] [CrossRef] [PubMed]
- Tanios, C.; Labaki, M. Catalytic reforming: A sustainable technology for hydrogen production. In Recent Advances in Renewable Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 199–247. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Ghouri, M.M.; Sengupta, D.; El-Halwagi, M.M.; Elbashir, N.O. A Process Integration Approach to the Optimization of CO2 Utilization via Tri-Reforming of Methane. Comput. Aided Chem. Eng. 2017, 40, 1993–1998. [Google Scholar] [CrossRef]
- Wang, X.; Economides, M. Gas-To-Liquids (GTL). In Advanced Natural Gas Engineering; Elsevier: Amsterdam, The Netherlands, 2009; pp. 243–287. [Google Scholar] [CrossRef]
- Boscherini, M.; Storione, A.; Minelli, M.; Miccio, F.; Doghieri, F. New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas. Energies 2023, 16, 6375. [Google Scholar] [CrossRef]
- Department of Energy. Hydrogen Production: Natural Gas Reforming. Hydrogen and Fuel Cell Technologies Office. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming (accessed on 9 February 2024).
- Subramani, A.; Basile, A.; Veziroğlu, T.N. (Eds.) Compendium of Hydrogen Energy: Hydrogen Production and Purification; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1. [Google Scholar]
- Namioka, T.; Saito, A.; Inoue, Y.; Park, Y.; Min, T.-J.; Roh, S.-A.; Yoshikawa, K. Hydrogen-rich gas production from waste plastics by pyrolysis and low-temperature steam reforming over a ruthenium catalyst. Appl. Energy 2011, 88, 2019–2026. [Google Scholar] [CrossRef]
- Aouad, S.; Labaki, M.; Ojala, S.; Seelam, P.; Turpeinen, E.; Gennequin, C.; Estephane, J.; Abi Aad, E. A review on the dry reforming processes for hydrogen production: Catalytic materials and technologies. Catal. Mater. Hydrog. Prod. Electro Oxid. React. Front. Ceram. Sci. 2018, 2, 60–128. [Google Scholar]
- Manfro, R.L.; Souza, M.M.V.M. Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming. Catalysts 2023, 13, 1296. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Liland, S.E.; Regli, S.K.; Yang, J.; Rout, K.R.; Luo, J.; Rønning, M.; Ran, J.; Chen, D. Unraveling enhanced activity, selectivity, and coke resistance of Pt–Ni bimetallic clusters in dry reforming. ACS Catal. 2021, 11, 2398–2411. [Google Scholar] [CrossRef]
- Cho, M.-H.; Mun, T.-Y.; Kim, J.-S. Air gasification of mixed plastic wastes using calcined dolomite and activated carbon in a two-stage gasifier to reduce tar. Energy 2013, 53, 299–305. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Effects of Gasification Temperature and Catalyst Ratio on Hydrogen Production from Catalytic Steam Pyrolysis-Gasification of Polypropylene. Energy Fuels 2008, 22, 4125–4132. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Hydrogen Production from the Pyrolysis–Gasification of Polypropylene: Influence of Steam Flow Rate, Carrier Gas Flow Rate and Gasification Temperature. Energy Fuels 2009, 23, 5055–5061. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Barbarias, I.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. HDPE pyrolysis-steam reforming in a tandem spouted bed-fixed bed reactor for H-2 production. J. Anal. Appl. Pyrolysis 2015, 116, 34–41. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Alvarez, J.; Artetxe, M.; Arregi, A.; Bilbao, J.; Olazar, M. A sequential process for hydrogen production based on continuous HDPE fast pyrolysis and in-line steam reforming. Chem. Eng. J. 2016, 296, 191–198. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Amutio, M.; Artetxe, M.; Alvarez, J.; Arregi, A.; Bilbao, J.; Olazar, M. Steam reforming of plastic pyrolysis model hydrocarbons and catalyst deactivation. Appl. Catal. A Gen. 2016, 527, 152–160. [Google Scholar] [CrossRef]
- Isicheli, P.; Oji, A.; Okwonna, O.; Muwarure, P.; Ibrahim, A.D.; Erazele, A. Analysing the effects of process parameters on HDPE pyrolysis and in-line reforming. Glob. J. Eng. Technol. Adv. 2023, 17, 139–149. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Manipulating the H2/CO ratio from dry reforming of simulated mixed waste plastics by the addition of steam. Fuel Process. Technol. 2017, 156, 331–338. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Artetxe, M.; Arregi, A.; Bilbao, J.; Olazar, M. Valorisation of different waste plastics by pyrolysis and in-line catalytic steam reforming for hydrogen production. Energy Convers. Manag. 2018, 156, 575–584. [Google Scholar] [CrossRef]
- Czernik, S.; French, R.J. Production of Hydrogen from Plastics by Pyrolysis and Catalytic Steam Reform. Energy Fuels 2006, 20, 754–758. [Google Scholar] [CrossRef]
- Martínez-Lera, S.; Torrico, J.; Pallarés, J.; Gil, A. Thermal valorization of post-consumer film waste in a bubbling bed gasifier. Waste Manag. 2013, 33, 1640–1647. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Syngas from steam gasification of polyethylene in a conical spouted bed reactor. Fuel 2013, 109, 461–469. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Hu, Z.; Liu, S.; Guo, X.; Luo, S. Syngas production from catalytic gasification of waste polyethylene: Influence of temperature on gas yield and composition. Int. J. Hydrog. Energy 2009, 34, 1342–1348. [Google Scholar] [CrossRef]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic waste recycling via pyrolysis: A bibliometric survey and literature review. J. Anal. Appl. Pyrolysis 2021, 158, 105265. [Google Scholar] [CrossRef]
- Azam, M.U.; Vete, A.; Afzal, W. Process Simulation and Life Cycle Assessment of Waste Plastics: A Comparison of Pyrolysis and Hydrocracking. Molecules 2022, 27, 8084. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Qureshi, M.S. Catalyzed pyrolysis of plastics: A thermogravimetric study. Afr. J. Pure Appl. Chem. 2011, 5, 284–292. Available online: https://academicjournals.org/article/article1379428874_Ali%20and%20Qureshi.pdf (accessed on 21 April 2024).
- Bockhorn, H.; Hornung, A.; Hornung, U. Stepwise pyrolysis for raw material recovery from plastic waste. J. Anal. Appl. Pyrolysis 1998, 46, 1–13. [Google Scholar] [CrossRef]
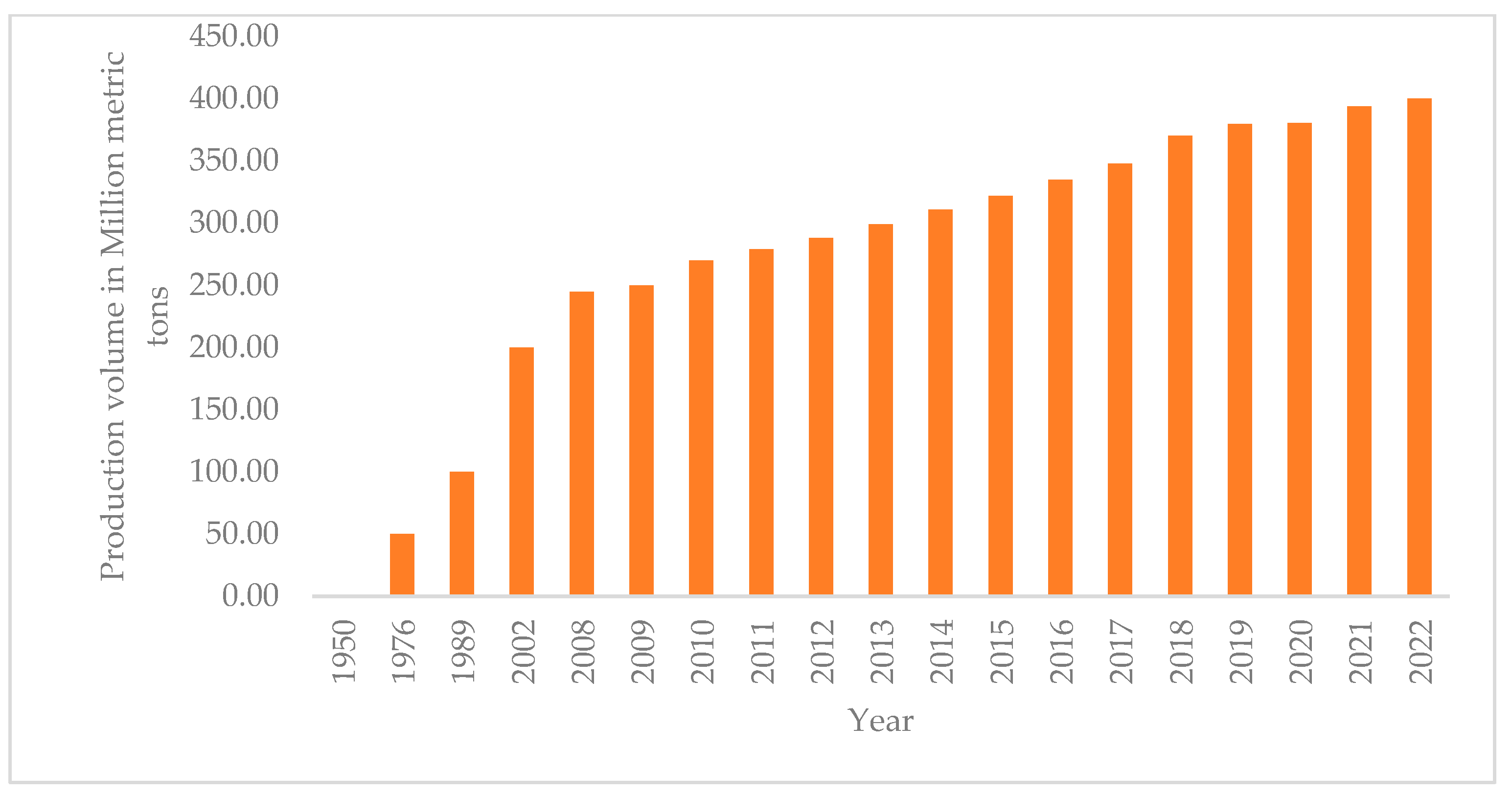
| Proximate Analysis | PET [32] | HDPE [33] | PVC [34] | LDPE [35] | PP [31] | PS [33] |
|---|---|---|---|---|---|---|
| Moisture content (%) | 0.27 | 0.15 | 0.01 | 0.76 | 0.20 | 0.79 |
| Fixed carbon (%) | 17.89 | 4.08 | 3.47 | 2.82 | 1.20 | 0.80 |
| Volatile matter (%) | 79.92 | 92.04 | 96.52 | 95.59 | 97.80 | 98.31 |
| Ash (%) | 1.92 | 3.73 | 0.00 | 0.83 | 1.90 | 0.10 |
| Ultimate analysis | [36] | [33] | [34] | [35] | [34] | [33] |
| Carbon (%) | 62.51 | 81.69 | 37.78 | 85.68 | 85.71 | 93.32 |
| Hydrogen (%) | 4.19 | 13.72 | 4.83 | 14.20 | 14.18 | 6.14 |
| Oxygen (%) | 33.30 | 0.26 | 0.36 | 0.02 | 0.04 | 0.50 |
| Nitrogen (%) | 0.00 | 3.75 | 0.14 | 0.05 | 0.03 | 0.04 |
| Sulphur (%) | 0.00 | 0.58 | 0.16 | 0.00 | 0.04 | 0.00 |
| Chlorine (%) | 0.00 | 0.00 | 56.73 | 0.05 | 0.00 | 0.00 |
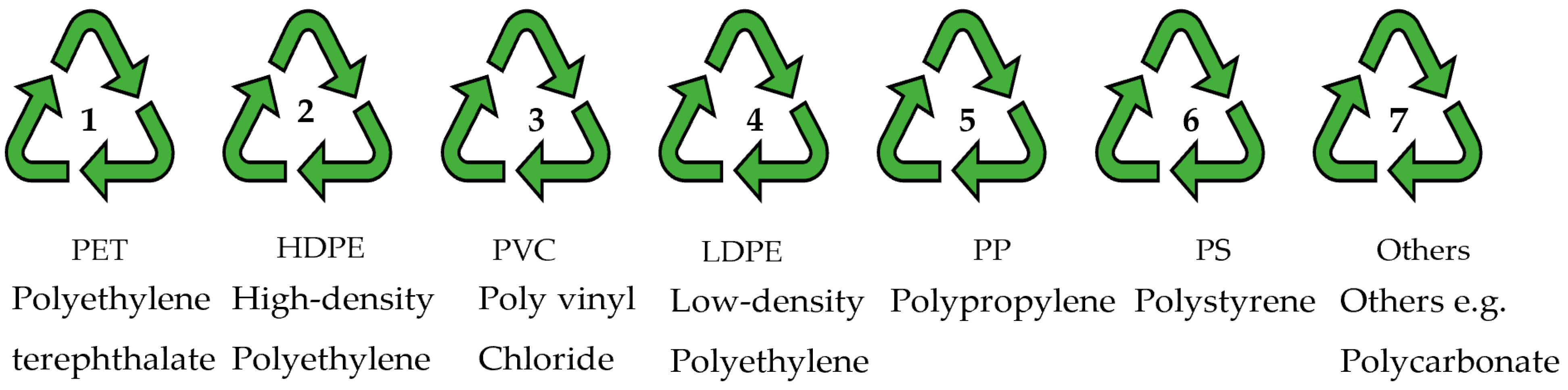
| Plastic Resin Code | Chemical Structure [8] | Applications [5,8,82,83] |
|---|---|---|
 Polyethylene terephthalate |  | Soft drink and mineral water plastic bottles, food trays, textiles, and fibres |
 High-density Polyethylene | 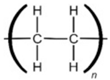 | Detergent and bleach containers and milk and shampoo bottles |
 Polyvinyl Chloride | 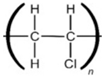 | Plumbing pipes, wall and floor coverings, and wiring and cable insulation |
 Low-density Polyethylene | 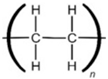 | Cling wraps, Ziploc, and grocery and trash bags |
 Polypropylene | 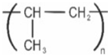 | Microwave-safe containers, bottle caps, and reusable food containers |
 Polystyrene | 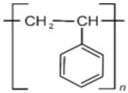 | Plastic utensils, Styrofoam, and disposable cups |
 | Baby bottle, 5-gallon water bottle, fibreglass, and acrylic nylon |
| Process | Advantages [127,135,144] | Disadvantages [140,144] |
|---|---|---|
| Dry reforming (DR) | Reforms two of the most abundant greenhouse gases (CO2 and CH4) Less CO2 product compared to steam reforming | High energy requirements (more endothermic than steam reforming) Requires constant supply of pure CO2 Formation and deposition of coke occurs more rapidly in contrast to steam reforming |
| Partial oxidation | Commercially viable External heat not required Low overall cost Compactness | Less efficient than steam reforming Pure O2 required which incurs extra costs |
| Steam reforming (SR) | Commercially viable High efficiency High H2/CO ratio | High energy requirements Large reformer required |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medaiyese, F.J.; Nasriani, H.R.; Khajenoori, L.; Khan, K.; Badiei, A. From Waste to Energy: Enhancing Fuel and Hydrogen Production through Pyrolysis and In-Line Reforming of Plastic Wastes. Sustainability 2024, 16, 4973. https://doi.org/10.3390/su16124973
Medaiyese FJ, Nasriani HR, Khajenoori L, Khan K, Badiei A. From Waste to Energy: Enhancing Fuel and Hydrogen Production through Pyrolysis and In-Line Reforming of Plastic Wastes. Sustainability. 2024; 16(12):4973. https://doi.org/10.3390/su16124973
Chicago/Turabian StyleMedaiyese, Fiyinfoluwa Joan, Hamid Reza Nasriani, Leila Khajenoori, Khalid Khan, and Ali Badiei. 2024. "From Waste to Energy: Enhancing Fuel and Hydrogen Production through Pyrolysis and In-Line Reforming of Plastic Wastes" Sustainability 16, no. 12: 4973. https://doi.org/10.3390/su16124973
APA StyleMedaiyese, F. J., Nasriani, H. R., Khajenoori, L., Khan, K., & Badiei, A. (2024). From Waste to Energy: Enhancing Fuel and Hydrogen Production through Pyrolysis and In-Line Reforming of Plastic Wastes. Sustainability, 16(12), 4973. https://doi.org/10.3390/su16124973