Application of Flotation for Removing Barium(II) Ions Using Ionized Acyclic Polyethers in the Context of Sustainable Waste Management
Abstract
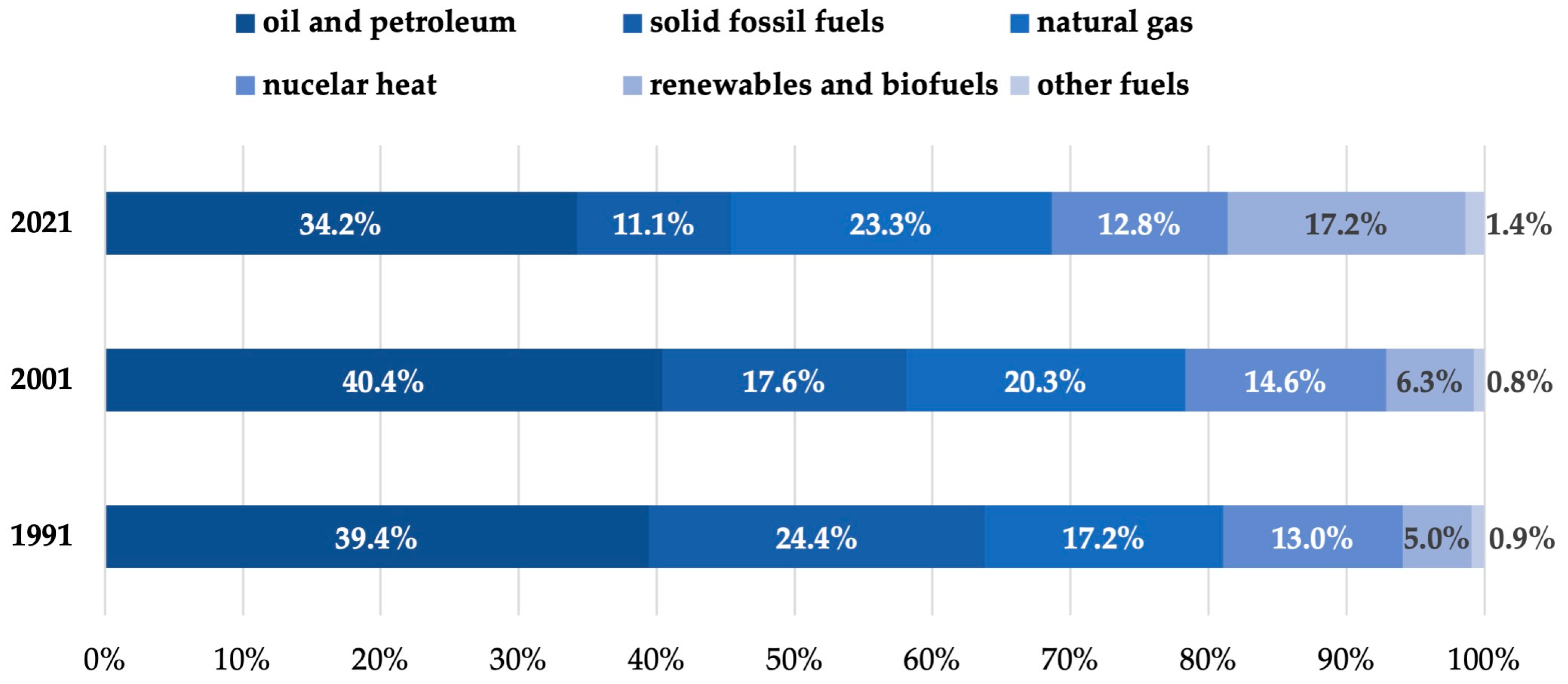
1. Introduction
2. Materials and Methods
2.1. Reagents
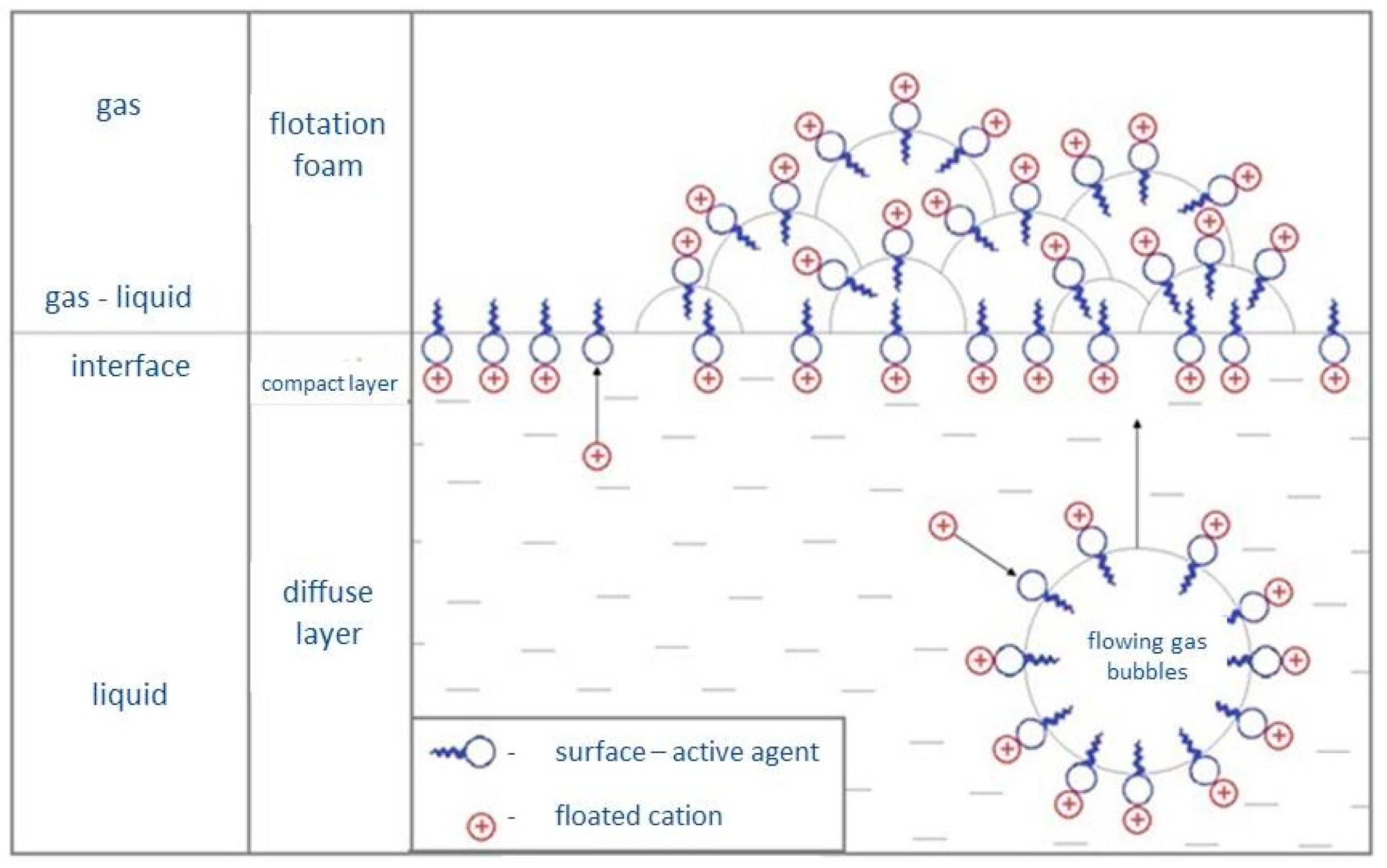
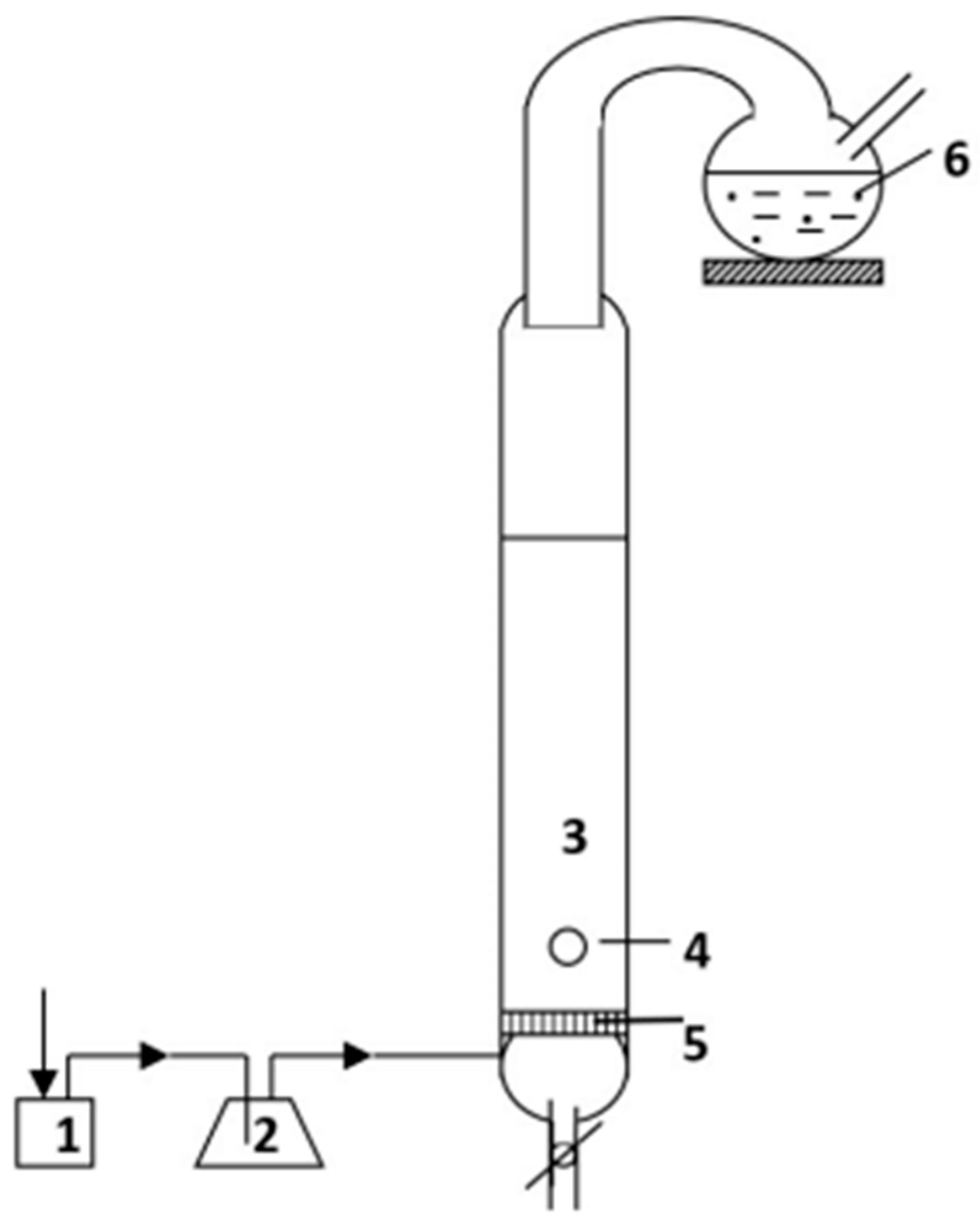
2.2. Ion Flotation Procedure
3. Results and Discussion
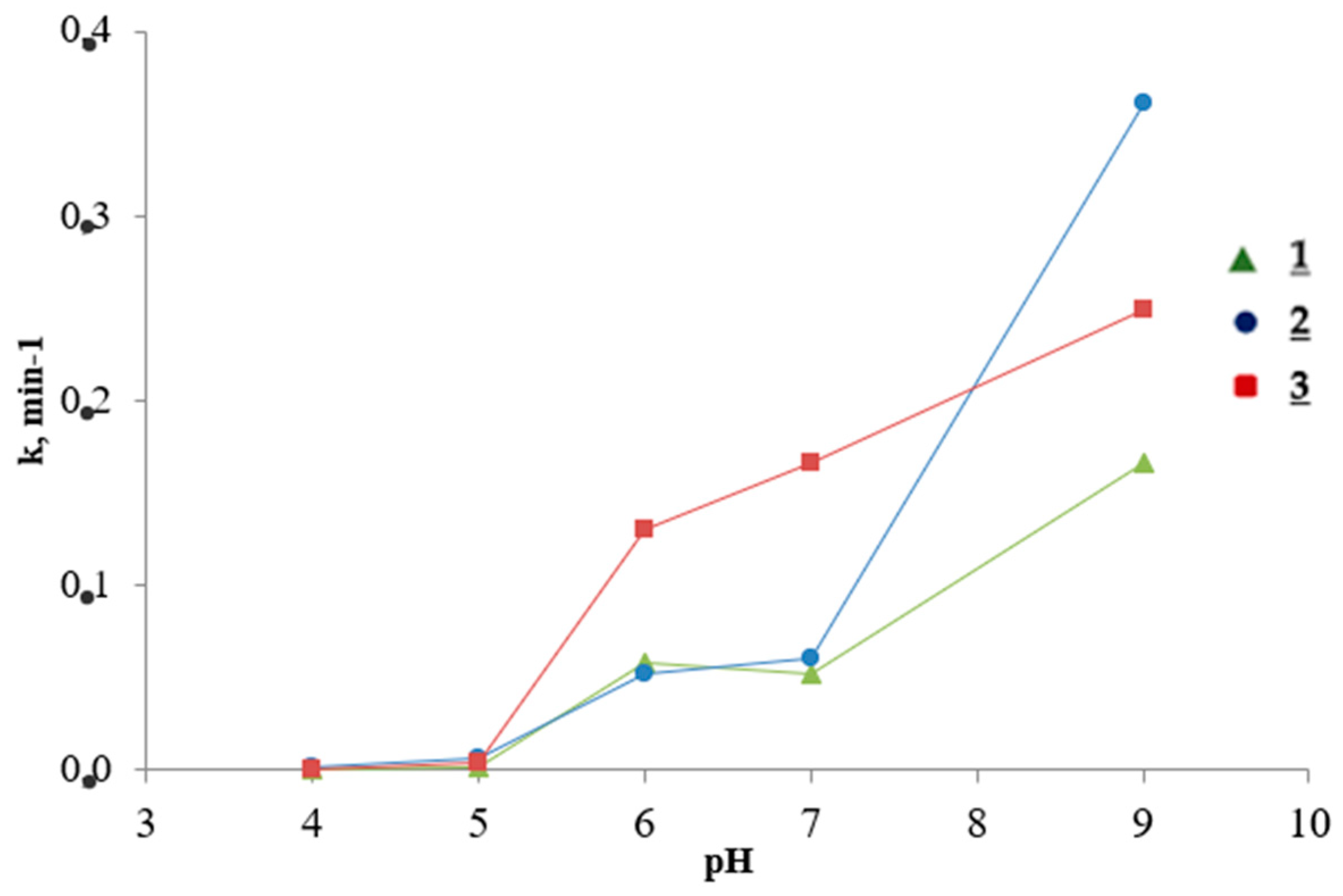
3.1. Effect of pH
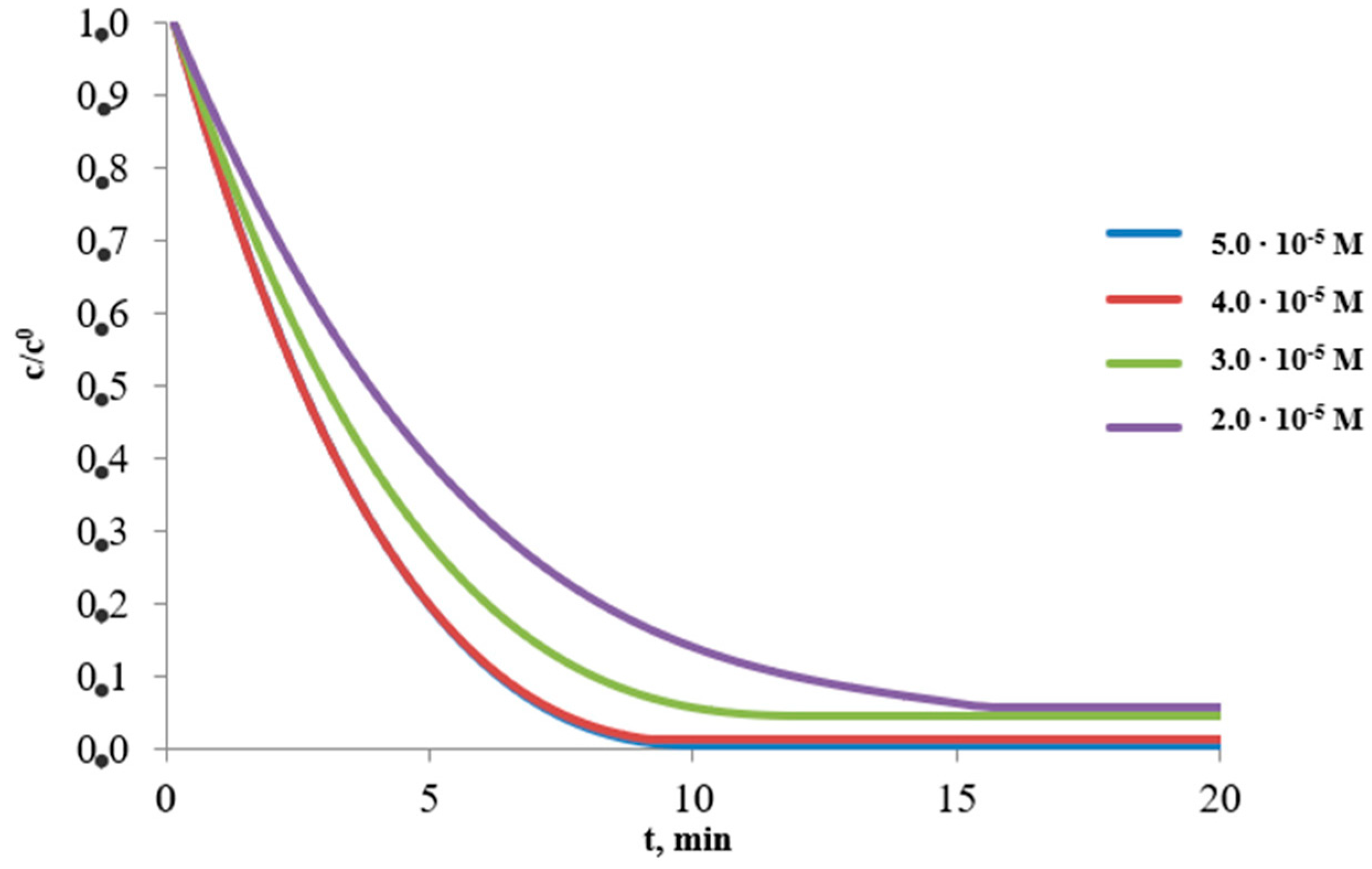
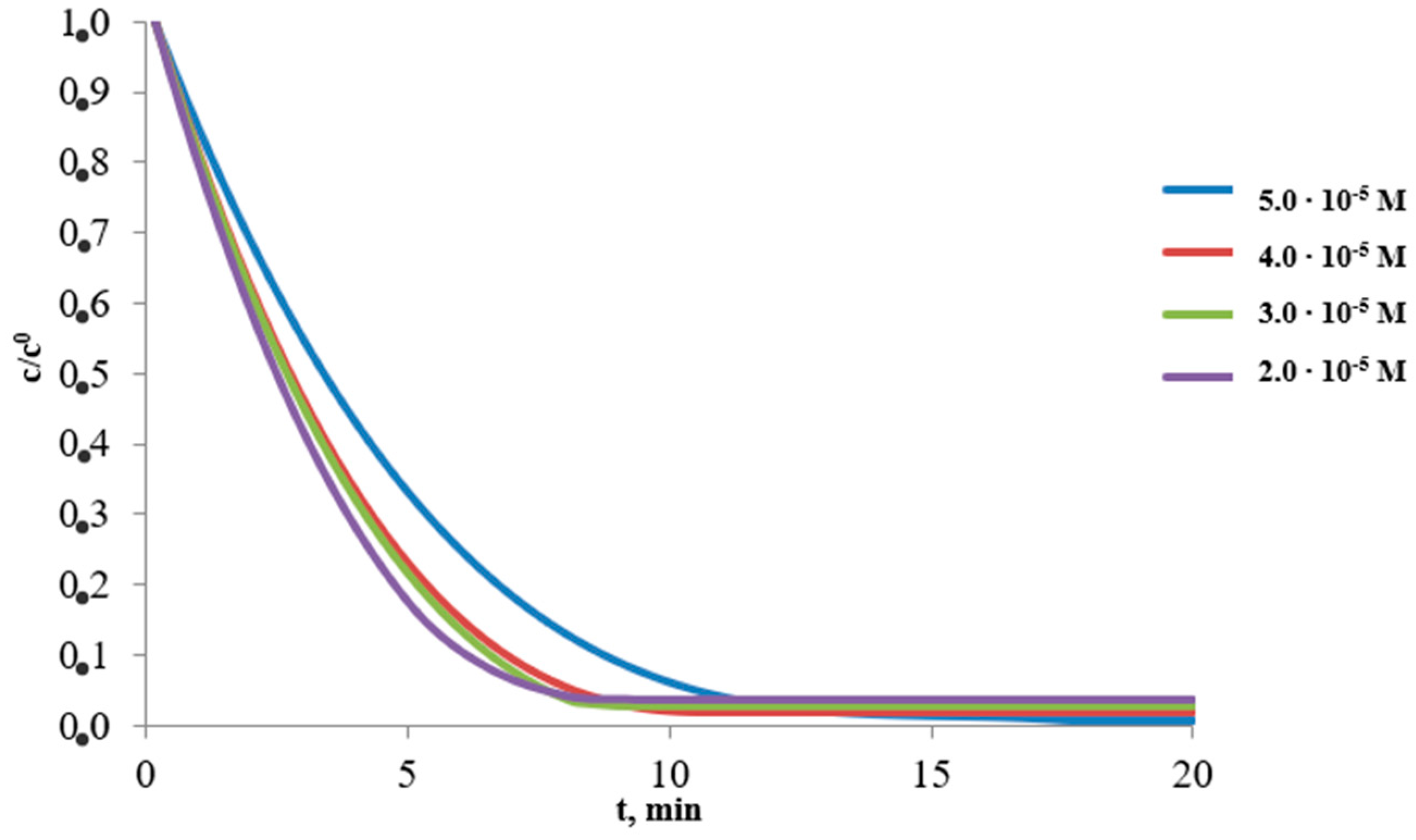
3.2. Effect of Collector Concentration
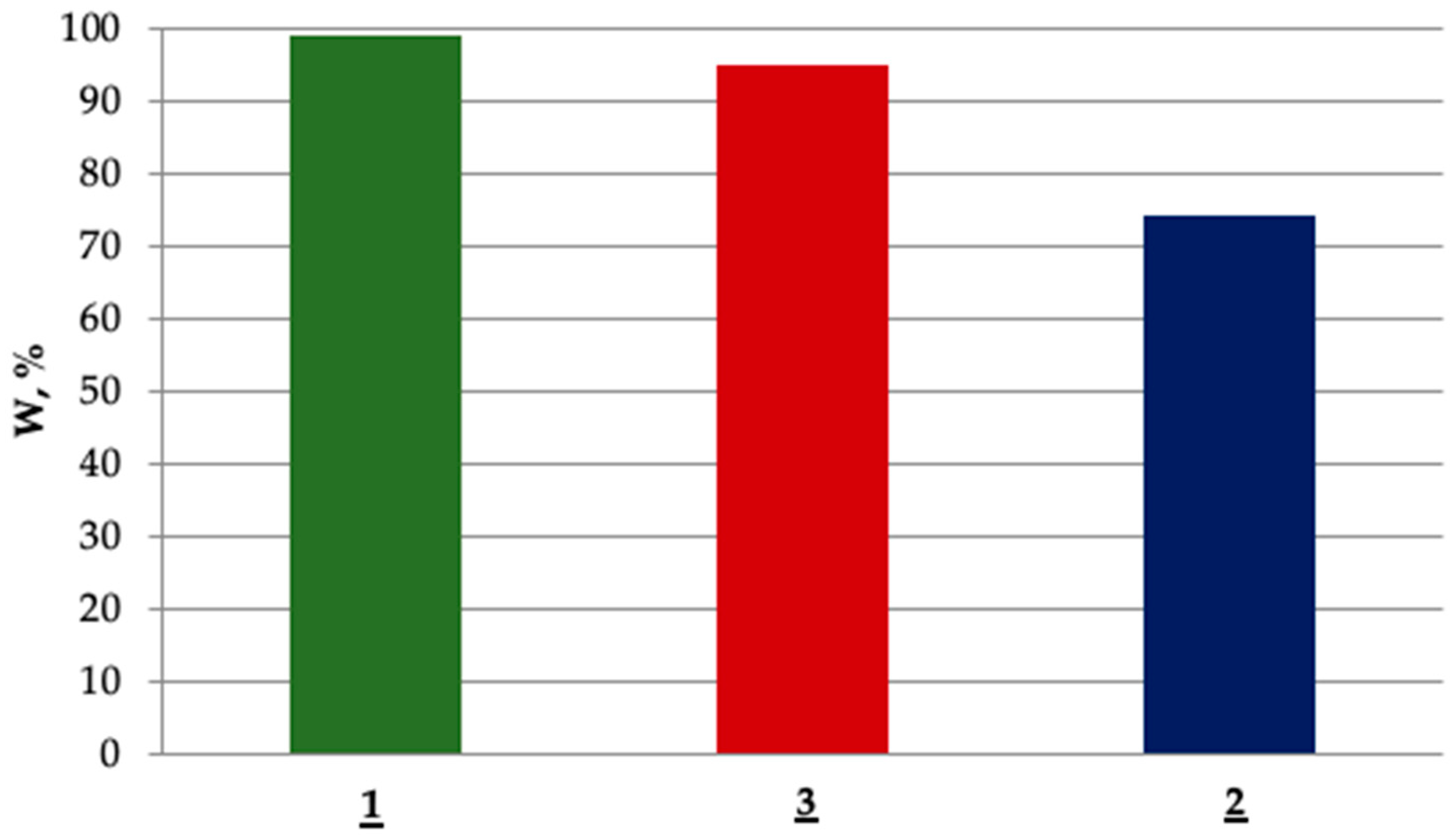
3.3. Effect of End-Group
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 3 October 2021).
- Sustainable Development Goals, Goal 13—Climate Acdtion: Take Urgent Action to Combat Climate Change and Its Impacts. Available online: https://www.un.org/sustainabledevelopment/climate-change/ (accessed on 3 October 2021).
- Sustainable Development Goals, Goal 7—Affordable and Clean Energy: Ensure Access to Affordable, Reliable, Sustainable and Modern Energy. Available online: https://www.un.org/sustainabledevelopment/energy/ (accessed on 3 October 2021).
- Keleş, S. Fossil Energy Sources, Climate Change, and Alternative Solutions. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1184–1195. [Google Scholar] [CrossRef]
- Krane, J. Climate change and fossil fuel: An examination of risks for the energy industry and producer states. MRS Energy Sustain. 2017, 4, E2. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Güneş, H.; Simba, H.M.A.; Karadağ, H.; Şit, M. Global Energy Transformation and the Impacts of Systematic Energy Change Policy on Climate Change Mitigation. Sustainability 2023, 15, 14298. [Google Scholar] [CrossRef]
- Jałowiec, T.; Wojtaszek, H.; Miciuła, I. Analysis of the Potential Management of the Low-Carbon Energy Transformation by 2050. Energies 2022, 15, 2351. [Google Scholar] [CrossRef]
- Soeder, D.J. Fossil Fuels and Climate Change. In Fracking and the Environment; Springer: Cham, Switzerland, 2020; pp. 155–185. [Google Scholar] [CrossRef]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Eurostat. Energy Consumption in Households. Statistical Article. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Energy_consumption_in_households (accessed on 3 October 2021).
- Eurostat. Energy Statistics—An Overview. Statistical Article. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Energy_statistics_-_an_overview#Gross_available_energy (accessed on 3 October 2021).
- Eurostat. Nuclear Energy Statistics. Statistical Article. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Nuclear_energy_statistics (accessed on 3 October 2021).
- Eurostat. Drop in Nuclear Power Production in 2022. News Artic. 2024. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20240112-1 (accessed on 3 October 2021).
- Siqueira, D.S.; de Almeida Meystre, J.; Hilário, M.Q.; Rocha, D.H.D.; Menon, G.J.; da Silva, R.J. Current perspectives on nuclear energy as a global climate change mitigation option. Mitig. Adapt. Strateg. Glob. Chang. 2019, 24, 749–777. [Google Scholar] [CrossRef]
- Muellner, N.; Arnold, N.; Gufler, K.; Kromp, W.; Renneberg, W.; Liebert, W. Nuclear energy—The solution to climate change? Energy Policy 2021, 155, 112363. [Google Scholar] [CrossRef]
- Krūmiņš, J.; Kļaviņš, M. Investigating the Potential of Nuclear Energy in Achieving a Carbon-Free Energy Future. Energies 2023, 16, 3612. [Google Scholar] [CrossRef]
- Imran, M.; Zaman, K.; Nassani, A.A.; Dincă, G.; Khan, H.U.R.; Haffar, M. Does nuclear energy reduce carbon emissions despite using fuels and chemicals? Transition to clean energy and finance for green solutions. Geosci. Front. 2023, 101608. [Google Scholar] [CrossRef]
- World Nuclear Association, Emerging Nuclear Energy Countries. Available online: https://world-nuclear.org/information-library/country-profiles/others/emerging-nuclear-energy-countries.aspx (accessed on 3 October 2021).
- Aszódi, A.; Biró, B.; Adorján, L.; Dobos, A.C.; Illés, G.; Tóth, N.K.; Zagyi, D.; Zsiborás, Z.T. The effect of the future of nuclear energy on the decarbonization pathways and continuous supply of electricity in the European Union. Nucl. Eng. Des. 2023, 415, 112688. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Oremek, G.M.; Brüggmann, D.; Groneberg, D.A. Global research on nuclear energy in the context of health and environmental risks, considering economic interests. WIREs Energy Environ. 2024, 13, e497. [Google Scholar] [CrossRef]
- Rehm, T.E. Advanced nuclear energy: The safest and most renewable clean energy. Curr. Opin. Chem. Eng. 2023, 39, 100878. [Google Scholar] [CrossRef]
- Santana, L.P.; Cordeiro, T.C. Management of radioactive waste: A review. Proc. Int. Acad. Ecol. Environ. Sci. 2016, 6, 38–43. Available online: http://www.iaees.org/publications/journals/piaees/articles/2016-6(2)/management-of-radioactive-waste.pdf (accessed on 3 October 2021).
- World Nuclear Association, Radioactive Waste Management. Available online: https://world-nuclear.org/information-library/nuclear-fuel-cycle/nuclear-wastes/radioactive-waste-management.aspx (accessed on 3 October 2021).
- Zhang, X.; Gu, P.; Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 2019, 215, 543–553. [Google Scholar] [CrossRef]
- IAEA Nuclear Energy Series. Status and Trends in Spent Fuel and Radioactive Waste Management; International Atomic Energy Agency: Vienna, Austria, 2022; Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/PUB1963_web.pdf (accessed on 3 October 2021).
- Darda, S.A.; Gabbar, H.A.; Damideh, V.; Hassen, I. A comprehensive review on radioactive waste cycle from generation to disposal. J. Radioanal. Nucl. Chem. 2021, 329, 15–31. [Google Scholar] [CrossRef]
- Reissig, F.; Kopka, K.; Mamat, C. The impact of barium isotopes in radiopharmacy and nuclear medicine—From past to presence. Nucl. Med. Biol. 2021, 98–99, 59–68. [Google Scholar] [CrossRef]
- Gad, H.M.H.; Lasheen, Y.F.; El-Zakla, T.S. Efficiency of Locally Prepared Activated Carbon in the Preconcentration of Barium- 133 and Radium-226 Radionuclides in Single and Binary Systems. Radiochemistry 2013, 55, 589–595. [Google Scholar] [CrossRef]
- Celebi, O.; Kilikli, A.; Erten, H.N. Sorption of cesium and barium ions onto solid humic acid. J. Hazard. Mater. 2009, 168, 695–703. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Youssef, M.A.; Aboterika, A.H.A.; Awwad, N.S. Removal of barium and strontium from wastewater and radioactive wastes using a green bioadsorbent, Salvadora persica (Miswak). Desalination Water Treat. 2020, 192, 306–314. [Google Scholar] [CrossRef]
- Kaveeshwar, A.R.; Kumar, P.S.; Revellame, E.D.; Gang, D.D.; Zappi, M.E.; Subramaniam, R. Adsorption properties and mecha nism of barium (II) and strontium (II) removal from fracking wastewater using pecan shell based activated carbon. J. Clean. Prod. 2018, 193, 1–13. [Google Scholar] [CrossRef]
- Ghaemi, A.; Torab-Mostaedi, M.; Ghannadi-Maragheh, M. Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J. Hazard. Mater. 2011, 190, 916–921. [Google Scholar] [CrossRef]
- Seliman, A.F.; Lasheen, Y.F.; Youssief, M.A.E.; Abo-Aly, M.M.; Shehata, F.A. Removal of some radionuclides from contaminated solution using natural clay: Bentonite. J. Radioanal. Nucl. Chem. 2014, 300, 969–979. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Liu, D.; Zhong, C. Radioactive Barium Ion Trap Based on Metal–Organic Framework for Efficient and Irreversible Removal of Barium from Nuclear Wastewater. ACS Appl. Mater. Interfaces 2016, 8, 8527–8535. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.-K. Selective adsorption of Ba2+ using chemically modified alginate beads with enhanced Ba2+ affinity and its application to 131Cs production. Nucl. Eng. Technol. 2022, 54, 3017–3026. [Google Scholar] [CrossRef]
- Azam, A.; Rafig, M.; Shafique, M.; Zhang, H.; Yuan, J. Analyzing the effect of natural gas, nuclear energy and renewable energy on GDP and carbon emissions: A multi-variate panel data analysis. Energy 2019, 219, 119592. [Google Scholar] [CrossRef]
- Wang, H.; Mu, S.; Li, G.; Yang, Z.; Yang, J.; Matquez, F.P.G.M.; Zhou, X.; Ma, Y.; Chen, Z. Modeling and operation strategy of nuclear power plant with electric heat storage in the ancillary service market. Nucl. Eng. Des. 2023, 415, 112686. [Google Scholar] [CrossRef]
- Horsley, D.M.C.; Hallington, P.J. Nuclear Power and the Management of the Radioactive Waste Legacy. Chem. Eng. Res. Des. 2005, 83, 773–776. [Google Scholar] [CrossRef]
- Hassas, B.V.; Kouachi, S.; Eskanlou, A.; Bouhenguel, M.; Celik, M.S.; Miller, J.D. The significance of positive and negative inertial forces in Particle-Bubble interaction and their role in the general flotation kinetics model. Miner. Eng. 2021, 170, 107006. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Grudniewska, K.; Maciejewski, P.; Gawlik-Kobylańska, M. Removal of Zn(II) and Mn(II) by Ion Flotation from Aqueous Solutions Derived from Zn-C and Zn-Mn(II) Batteries Leaching. Energies 2021, 14, 1335. [Google Scholar] [CrossRef]
- Walkowiak, W.; Walus, K.; Charewicz, W.A.; Bartsch, R.; Elshani, S.; Węgiel, M. Competitive Solvent Extraction of Ra(II) and Ba(II) from Aqueous Solutions with Lipophilic, Acyclic, Di-ionizable Polyethers. Sep. Sci. Technol. 2005, 40, 1673–1683. [Google Scholar] [CrossRef]
- Sobianowska, K. Flotation of Cs+, Sr2+, Ba2+ and Co2+ Ions from Dilute Aqueous Solutions Using Macrocyclic Compounds (In Polish). Ph.D. Dissertation, Wrocław University of Science and Technoloy, Wrocław, Poland, 2012. [Google Scholar]
- Lis, T.; Nowacki, K.; Małysa, T. Utilization of Metallurgical Waste in Non-Metallurgical Industry. Solid State Phenom. 2013, 212, 195–200. [Google Scholar] [CrossRef]
- Maciejewski, P.; Robak, W.; Ulewicz, M.; Sobianowska, K. The use of macrocyclic compounds to remove toxic metal cations from aqueous solutions in the ion flotation process (in polish). Zesz. Nauk. WSOWL 2008, 1, 147. [Google Scholar]
- Nazari, S.; Vakylabad, A.B.; Asgari, K.; Li, J.; Khoshdast, H.; He, Y.; Hassanzadeh, A. Bubbles to batteries: A review of froth flotation for sustainably recycling spent lithium-ion batteries. J. Energy Storage 2024, 84, 110702. [Google Scholar] [CrossRef]


| Number of the Chemical | Structural Formula | -R | -Y | -X |
|---|---|---|---|---|
| 1 | 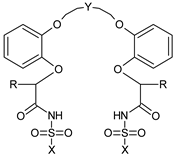 | -C8H17 | -(CH2CH2)2O | -CH3 |
| 2 | -CF3 | |||
| 3 | -C6H4-pNO2 |
| pH | Ba2+ | |||||
| Maximal Percent Removal, W, %/Rate Constant, k, min−1 | ||||||
| 1 | 2 | 3 | ||||
| 4.0 | - | - | 2.18 | 0.0019 | - | - |
| 5.0 | 6.27 | 0.0012 | 14.9 | 0.0064 | 14.1 | 0.0039 |
| 6.0 | 81.4 | 0.0581 | 60.8 | 0.0517 | 95.2 | 0.1304 |
| 7.0 | 81.7 | 0.0527 | 72.6 | 0.0612 | 97.5 | 0.1665 |
| 9.0 | 86.8 | 0.1667 | 99.4 | 0.3616 | 99.3 | 0.2498 |
| Number of the Chemical | Structural Formula | -R | -Y | -X |
|---|---|---|---|---|
| 4 | 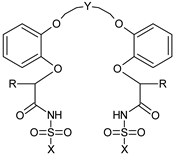 | -C8H17 | -(CH2)2 | -CH3 |
| 5 | -CF3 | |||
| 6 | -C6H5 | |||
| 7 | -C6H4-pNO2 | |||
| 8 | -(CH2)3 | -CH3 | ||
| 9 | -CF3 | |||
| 10 | -C6H5 | |||
| 11 | -C6H4-pNO2 | |||
| 1 | -(CH2CH2)2O | -CH3 | ||
| 2 | -CF3 | |||
| 12 | -C6H5 | |||
| 3 | -C6H4-pNO2 | |||
| 13 | -C14H27 | -CF3 |
| Collector Concentration [M] | Ba2+ | |||||
|---|---|---|---|---|---|---|
| Maximal Percent Removal, W, %/Rate Constant, k, min−1 | ||||||
| 1 | 2 | 3 | ||||
| 5.0 × 10−5 | 86.8 | 0.1667 | 99.4 | 0.3616 | 99.3 | 0.2498 |
| 4.0 × 10−5 | 98.5 | 0.2459 | 98.6 | 0.3273 | 98.2 | 0.3007 |
| 3.0 × 10−5 | 99.8 | 0.2053 | 95.3 | 0.2383 | 97.3 | 0.2963 |
| 2.0 × 10−5 | 99.7 | 0.1583 | 94.2 | 0.1817 | 96.3 | 0.2939 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobianowska-Turek, A.; Grudniewska, K.; Fornalczyk, A.; Willner, J.; Bialik, W.; Urbańska, W.; Janda, A. Application of Flotation for Removing Barium(II) Ions Using Ionized Acyclic Polyethers in the Context of Sustainable Waste Management. Sustainability 2024, 16, 4665. https://doi.org/10.3390/su16114665
Sobianowska-Turek A, Grudniewska K, Fornalczyk A, Willner J, Bialik W, Urbańska W, Janda A. Application of Flotation for Removing Barium(II) Ions Using Ionized Acyclic Polyethers in the Context of Sustainable Waste Management. Sustainability. 2024; 16(11):4665. https://doi.org/10.3390/su16114665
Chicago/Turabian StyleSobianowska-Turek, Agnieszka, Katarzyna Grudniewska, Agnieszka Fornalczyk, Joanna Willner, Wojciech Bialik, Weronika Urbańska, and Anna Janda. 2024. "Application of Flotation for Removing Barium(II) Ions Using Ionized Acyclic Polyethers in the Context of Sustainable Waste Management" Sustainability 16, no. 11: 4665. https://doi.org/10.3390/su16114665
APA StyleSobianowska-Turek, A., Grudniewska, K., Fornalczyk, A., Willner, J., Bialik, W., Urbańska, W., & Janda, A. (2024). Application of Flotation for Removing Barium(II) Ions Using Ionized Acyclic Polyethers in the Context of Sustainable Waste Management. Sustainability, 16(11), 4665. https://doi.org/10.3390/su16114665








