Pots to Plots: Microshock Weed Control Is an Effective and Energy Efficient Option in the Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment and Materials
2.2. Environmental Data
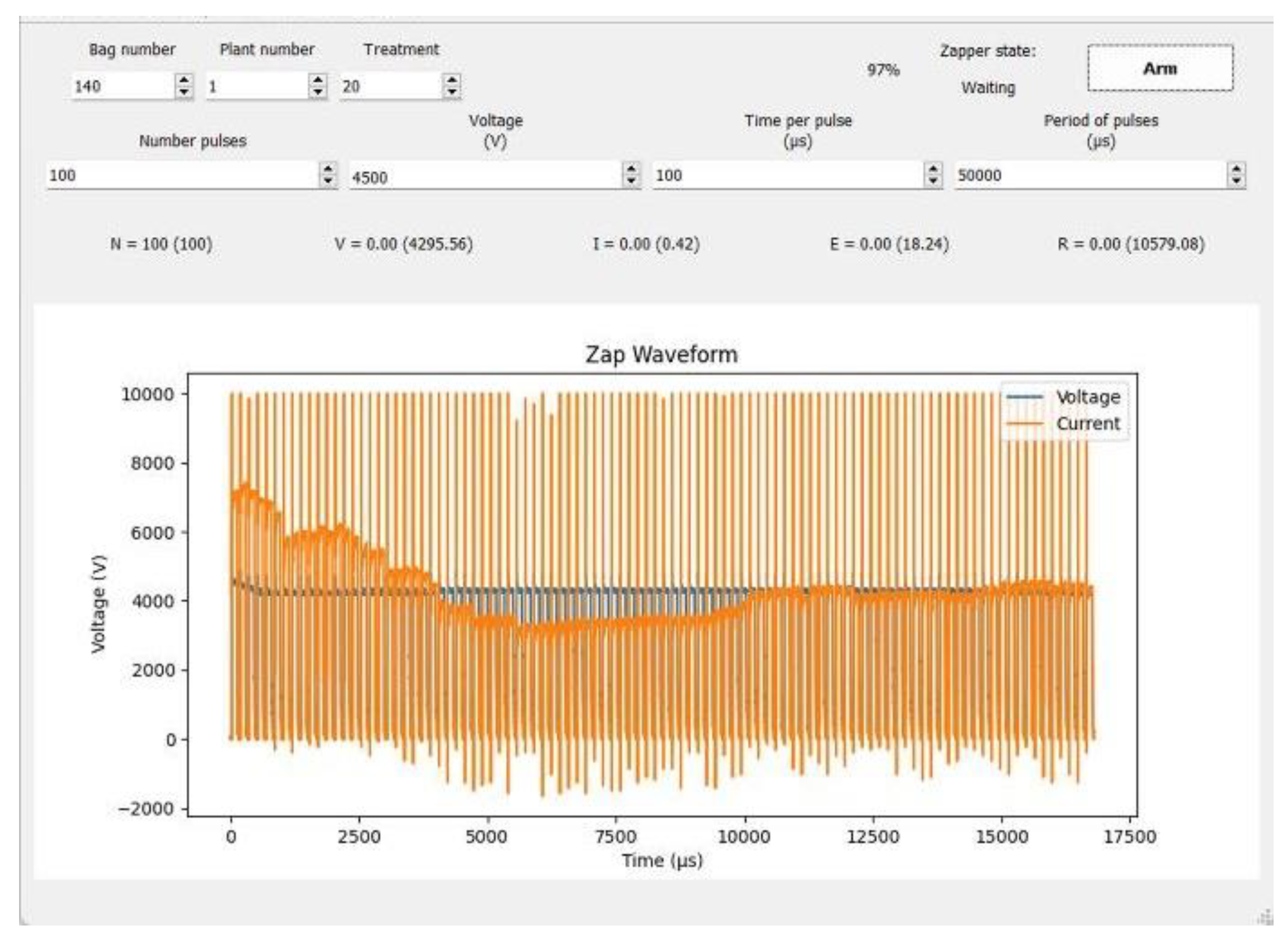
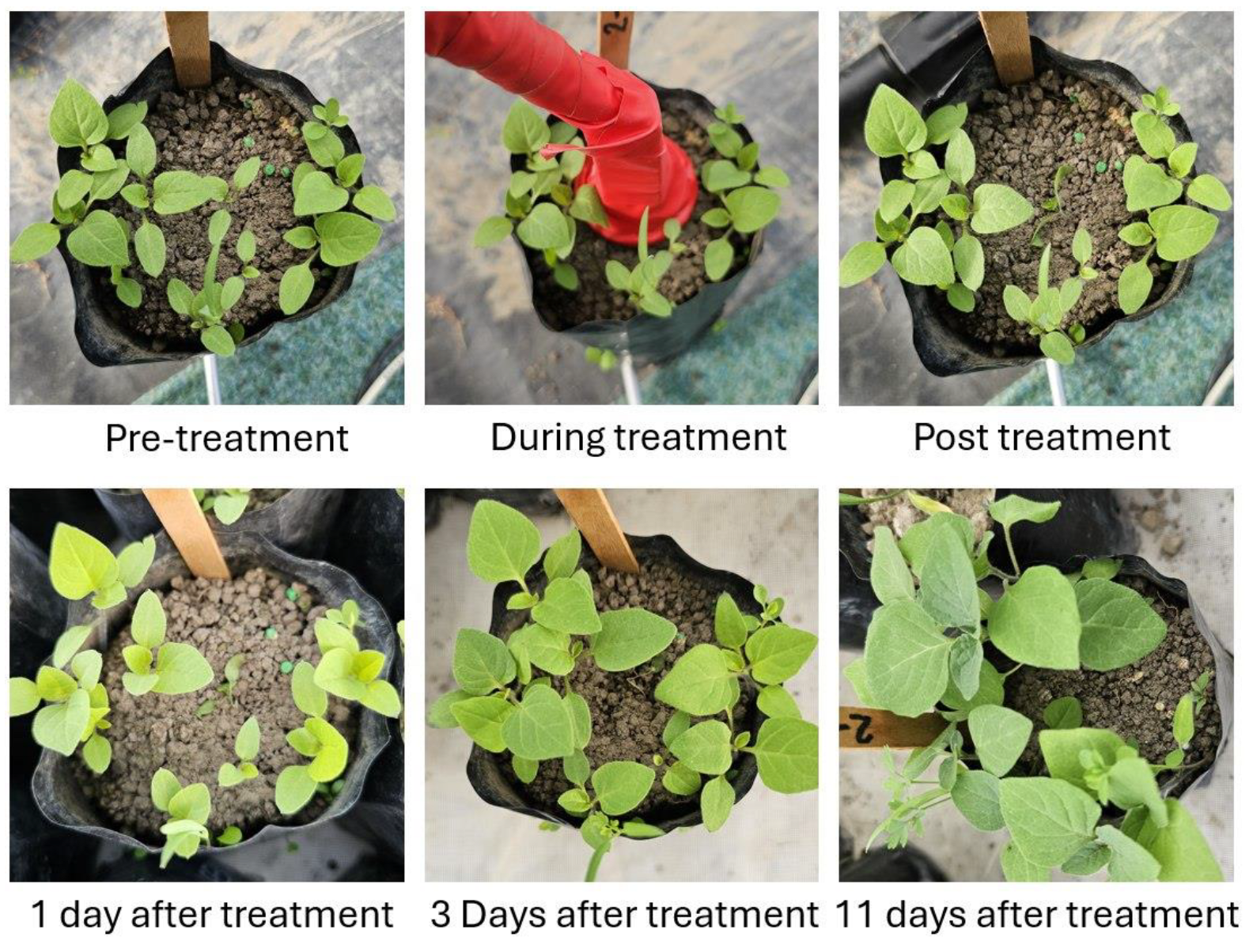
2.3. Methods
2.4. Treatments
2.5. Statistical Analysis
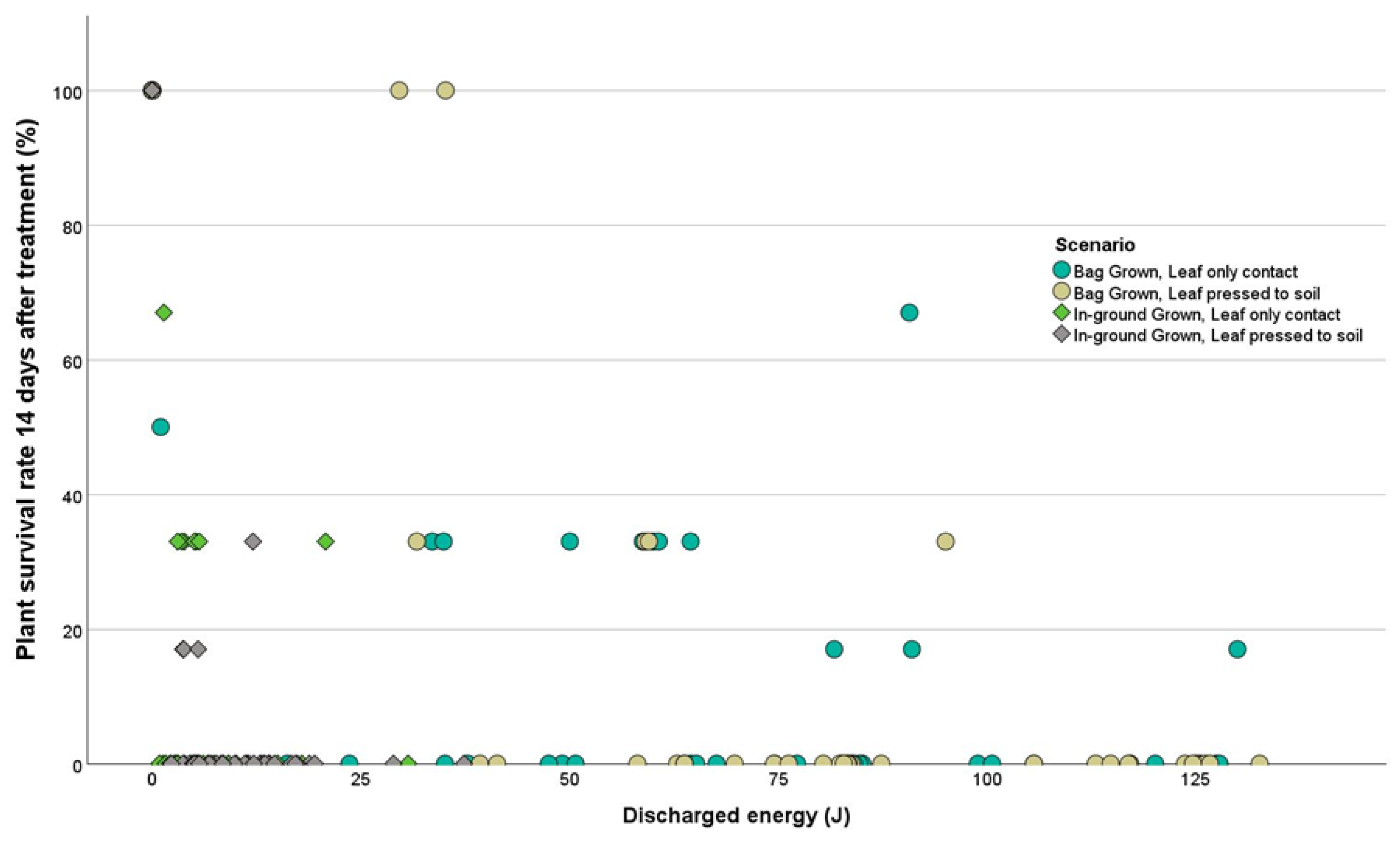
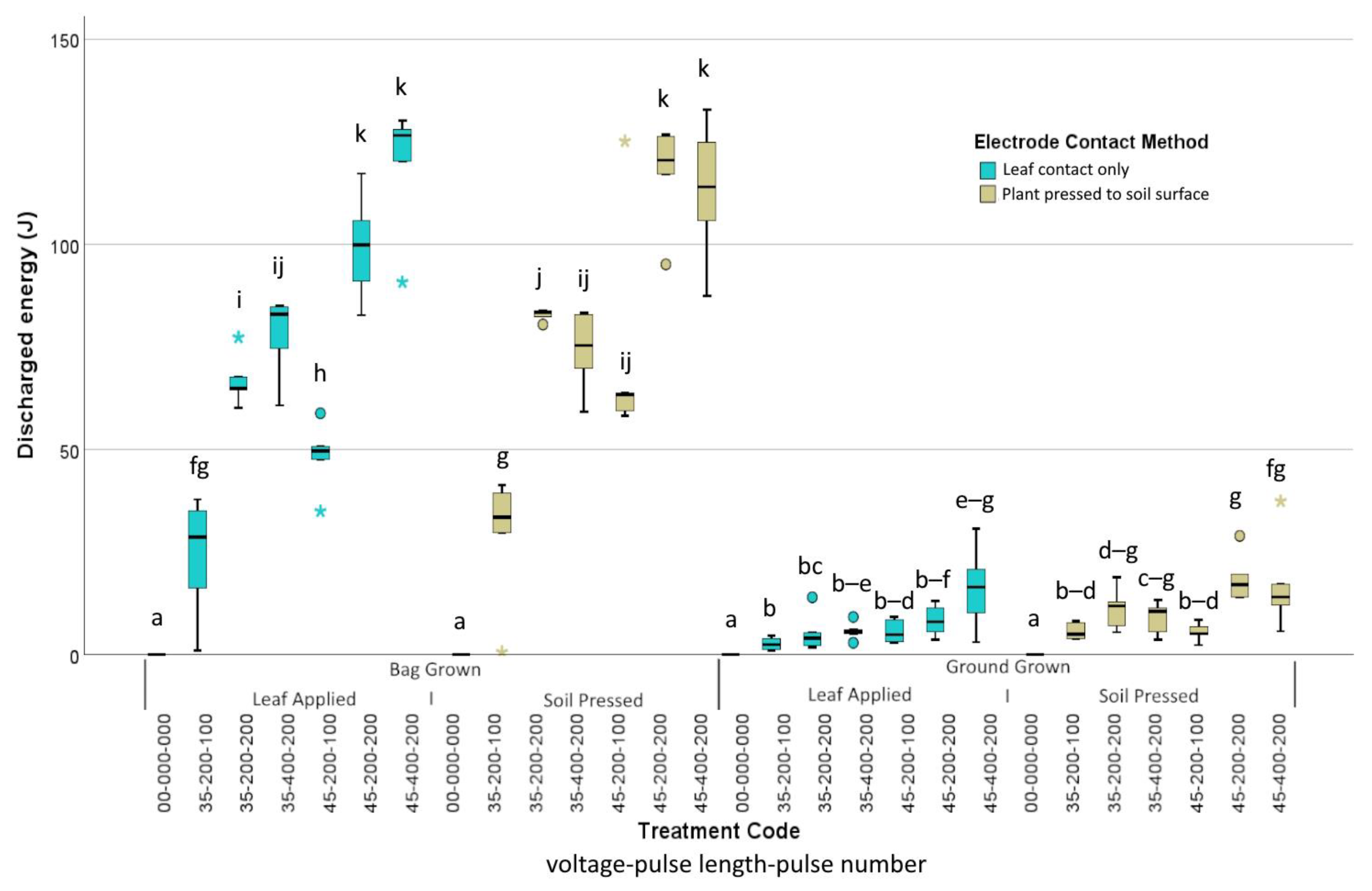
3. Results
3.1. Trial 1 Lepidium didymum
3.2. Trial 2 Amaranthus powellii
3.3. Trial 3 Lolium multiflorum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Donoghue, T.; Minasny, B.; McBratney, A. Regenerative Agriculture and Its Potential to Improve Farmscape Function. Sustainability 2022, 14, 5815. [Google Scholar] [CrossRef]
- Rempelos, L.; Kabourakis, E.; Leifert, C. Innovative Organic and Regenerative Agricultural Production. Agronomy 2023, 13, 1344. [Google Scholar] [CrossRef]
- Grelet, G.; Lang, S. Regenerative agriculture in Aotearoa New Zealand: Research Pathways to Build Science-Based Evidence and National Narratives; Manaaki Whenua-Landcare Research: Lincoln, NZ, USA, 2021; p. 59. [Google Scholar]
- Burns, E.A. Thinking sociologically about regenerative agriculture. N. Z. Sociol. 2020, 35, 189–213. [Google Scholar]
- LaCanne, C.E.; Lundgren, J.G. Regenerative agriculture: Merging farming and natural resource conservation profitably. PeerJ 2018, 6, e4428. [Google Scholar] [CrossRef] [PubMed]
- Hageman, K.J.; Aebig, C.H.F.; Luong, K.H.; Kaserzon, S.L.; Wong, C.S.; Reeks, T.; Greenwood, M.; Macaulay, S.; Matthaei, C.D. Current-use pesticides in New Zealand streams: Comparing results from grab samples and three types of passive samplers. Environ. Pollut. 2019, 254, 112973. [Google Scholar] [CrossRef]
- Jayasinghe, S.L.; Thomas, D.T.; Anderson, J.P.; Chen, C.; Macdonald, B.C.T. Global Application of Regenerative Agriculture: A Review of Definitions and Assessment Approaches. Sustainability 2023, 15, 15941. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Weed science in sustainable agriculture. Am. J. Altern. Agric. 1995, 10, 138–142. [Google Scholar] [CrossRef]
- Fenster, T.; LaCanne, C.; Pecenka, J.; Schmid, R.; Bredeson, M.; Busenitz, K.; Michels, A.; Welch, K.; Lundgren, J. Defining and validating regenerative farm systems using a composite of ranked agricultural practices [version 1; peer review: 2 approved]. F1000Research 2021, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Elrick, W.; Luke, H.; Stimpson, K. Exploring opportunities and constraints of a certification scheme for regenerative agricultural practice. Agroecol. Sustain. Food Syst. 2022, 46, 1527–1549. [Google Scholar] [CrossRef]
- McCain Foods. McCain’s Regenerative Agriculture Framework. 2023. Available online: https://www.mccain.com/media/4594/mccain_regenag_framework_2024.pdf (accessed on 6 May 2024).
- Cornell University College of Agriculture and Life Sciences. Environmental Impact Quotient (EIQ) Explained. Available online: https://turf.cals.cornell.edu/pests-and-weeds/environmental-impact-quotient-eiq-explained/ (accessed on 5 March 2024).
- Bloomer, D.J.; Harrington, K.C.; Ghanizadeh, H.; James, T.K. Robots and shocks: Emerging non-herbicide weed control options for vegetable and arable cropping. N. Z. J. Agric. Res. 2024, 67, 81–103. [Google Scholar] [CrossRef]
- Crinnion, W.J. Organic foods contain higher levels of certain nutrients, lower levels of pesticides, and may provide health benefits for the consumer. Environ. Med. 2010, 15, 9. [Google Scholar] [PubMed]
- Forbes, S.L.; Cohen, D.A.; Cullen, R.; Wratten, S.D.; Fountain, J. Consumer attitudes regarding environmentally sustainable wine: An exploratory study of the New Zealand marketplace. J. Clean. Prod. 2009, 17, 1195–1199. [Google Scholar] [CrossRef]
- Galati, A.; Schifani, G.; Crescimanno, M.; Migliore, G. “Natural wine” consumers and interest in label information: An analysis of willingness to pay in a new Italian wine market segment. J. Clean. Prod. 2019, 227, 405–413. [Google Scholar] [CrossRef]
- Hoek, A.C.; Pearson, D.; James, S.W.; Lawrence, M.A.; Friel, S. Shrinking the food-print: A qualitative study into consumer perceptions, experiences and attitudes towards healthy and environmentally friendly food behaviours. Appetite 2017, 108, 117–131. [Google Scholar] [CrossRef]
- Koch, S.; Epp, A.; Lohmann, M.; Bol, G.F. Pesticide residues in food: Attitudes, beliefs, and misconceptions among conventional and organic consumers. J. Food Prot. 2017, 80, 2083–2089. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Mahajan, G.; Chauhan, B.S. Nonconventional weed management strategies for modern agriculture. Weed Sci. 2015, 63, 723–747. [Google Scholar] [CrossRef]
- Jun, I.; Feng, Z.; Avanasi, R.; Brain, R.A.; Prosperi, M.; Bian, J. Evaluating the perceptions of pesticide use, safety, and regulation and identifying common pesticide-related topics on Twitter. Integr. Environ. Assess. Manag. 2023, 19, 1581–1599. [Google Scholar] [CrossRef]
- Ministry for Primary Industries. Regenerating Aotearoa: Investigating the Impacts of Regenerative Farming Practices; Ministry for Primary Industries: Wellington, New Zealand, 2022; ISBN 978-1-99-105209-4.
- Buddenhagen, C.E.; James, T.K.; Ngow, Z.; Hackell, D.L.; Rolston, M.P.; Chynoweth, R.J.; Gunnarsson, M.; Li, F.; Harrington, K.C.; Ghanizadeh, H. Resistance to post-emergent herbicides is becoming common for grass weeds on New Zealand wheat and barley farms. PLoS ONE 2021, 16, e0258685. [Google Scholar] [CrossRef]
- Doole, G.J.; James, T.K. Profitable management of a finite herbicide resource. Crop Prot. 2023, 172, 106314. [Google Scholar] [CrossRef]
- Espig, M.; Henwood, R.J.T. The social foundations for re-solving herbicide resistance in Canterbury, New Zealand. PLoS ONE 2023, 18, e0286515. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Online 2020. Available online: www.weedscience.com (accessed on 14 April 2024).
- Ngow, Z. Herbicide Resistant Weeds in Maize in New Zealand: A Survey for Herbicide Resistant Weeds in the Bay of Plenty and Waikato; Waikato: Hamilton, NZ, USA, 2022. [Google Scholar]
- Alluvione, F.; Moretti, B.; Sacco, D.; Grignani, C. EUE (energy use efficiency) of cropping systems for a sustainable agriculture. Energy 2011, 36, 4468–4481. [Google Scholar] [CrossRef]
- Bloomer, D.J.; Harrington, K.C.; Ghanizadeh, H.; James, T.K. Micro electric shocks control broadleaved and grass weeds. Agronomy 2022, 12, 2039. [Google Scholar] [CrossRef]
- Coleman, G.Y.; Stead, A.; Rigter, M.; Xu, Z.; Johnson, D.W.; Brooker, G.; Sukkarieh, S.; Walsh, M.J. Using energy requirements to compare the suitability of alternative methods for broadcast and site-specific weed control. Weed Technol. 2019, 33, 633. [Google Scholar] [CrossRef]
- Kaufman, K.R.; Schaffner, L.W. Energy and economics of electrical weed control. Trans. ASAE 1982, 25, 297–0300. [Google Scholar] [CrossRef]
- Vigneault, C.; Benoit, D.L.; McLaughlin, N.B. Energy aspects of weed electrocution. Rev. Weed Sci. 1990, 5, 15–26. [Google Scholar]
- Bloomer, D.J.; Harrington, K.C.; Ghanizadeh, H.; James, T.K. The electric spatula: Killing weeds with pulsed microshocks from a flat-plate electrode. Agronomy 2023, 13, 2694. [Google Scholar] [CrossRef]
- Andreasen, C.; Saberi, M.; Rakhmatulin, I. Weed control with laser beams using autonomous vehicles: Pros and cons. In Proceedings of the FIRA 2021, Online, 7–9 December 2021; ACM: New York, NY, USA, 2021. [Google Scholar]
- Coleman, G.Y.; Betters, C.; Squires, C.; Leon-Saval, S.; Walsh, M.J. Low energy laser treatments control annual ryegrass (Lolium rigidum). Front. Agron. 2021, 2, 601542. [Google Scholar] [CrossRef]
- De Cauwer, B.; Bogaert, S.; Claerhout, S.; Bulcke, R.; Reheul, D.; Kim, D.-S. Efficacy and reduced fuel use for hot water weed control on pavements. Weed Res. 2015, 55, 195–205. [Google Scholar] [CrossRef]
- Denmukhammadiev, A.; Matchanov, O.; Sobirov, E.; Azamov, S. Energy-efficient environmentally friendly electric device for the destruction of weeds by heating them. IOP Conf. Ser. Earth Environ. Sci. 2022, 1076, 012036. [Google Scholar] [CrossRef]
- Fergedal, S. Weed control by freezing with liquid nitrogen and carbon dioxide snow. A comparison between flaming and freezing. In Proceedings of the Maitrise Des Adventices Par Voie Non Chimique, Communications de la Quatrieme Conference Internationale I.F.O.A.M., Dijon, France, 5–9 July 1993; pp. 163–166. [Google Scholar]
- Gay, P.; Piccarolo, P.; Ricauda Aimonino, D.; Tortia, C. A high efficiency steam soil disinfestation system, part I: Physical background and steam supply optimisation. Biosyst. Eng. 2010, 107, 74–85. [Google Scholar] [CrossRef]
- Goel, A.; Foshee, W.; Kirkici, H.; Ieee, I. Pulsed Electric Field Studies of Bio-Dielectrics; IEEE: Piscataway, NJ, USA, 2003; pp. 56–59. [Google Scholar]
- Gonzalez-de-Soto, M.; Emmi, L.; Garcia, I.; Gonzalez-de-Santos, P. Reducing fuel consumption in weed and pest control using robotic tractors. Comput. Electron. Agric. 2015, 114, 96–113. [Google Scholar] [CrossRef]
- Helsel, Z.R.; Pimentel, D. Energy in pesticide production and use. Encycl. Pest Manag. 2007, 2, 157–160. [Google Scholar]
- Kaufman, K.R.; Schaffner, L.W. Energy Requirements and Economic Analysis of Electrical Weed Control; The Sugarbeet Research and Education Board of Minnesota and North Dakota: Fargo, ND, USA, 1980; pp. 72–85. [Google Scholar]
- Mwitta, C.; Rains, G.C.; Prostko, E. Evaluation of Diode Laser Treatments to Manage Weeds in Row Crops. Agronomy 2022, 12, 2681. [Google Scholar] [CrossRef]
- Lati, R.N.; Rosenfeld, L.; David, I.B.; Bechar, A. Power on! Low-energy electrophysical treatment is an effective new weed control approach. Pest Manag. Sci. 2021, 77, 4138–4147. [Google Scholar] [CrossRef]
- Diprose, M.F.; Hackam, R.; Benson, F.A. Weed control by high voltage electric shocks. In Proceedings of the 1978 British Crop Protection Conference-Weeds, Brighton, UK, 20–23 November 1978; pp. 443–450. [Google Scholar]
- Chandler, J.M. Crop and weed response to an electrical discharge. In Proceedings of the 31st Annual Meeting of the Southern Weed Science Society, Charleston, South Carolina, 20–25 January 1978; Volume 63. [Google Scholar]
- Mizuno, A.; Tenma, T.; Yamano, N. Destruction of weeds by pulsed high voltage discharges. In Proceedings of the Record of the 1990 IEEE Industry Applications Society Annual Meeting, Seattle, WA, USA, 7–12 October 1990; pp. 720–727. [Google Scholar]
- Blasco, J.; Aleixos, N.; Roger, J.M.; Rabatel, G.; Moltó, E. Automation and emerging technologies. Robotic weed control using machine vision. Biosyst. Eng. 2002, 83, 149–157. [Google Scholar] [CrossRef]
- Cao, Y.; Repo, T.; Silvennoinen, R.; Lehto, T.; Pelkonen, P. An appraisal of the electrical resistance method for assessing root surface area. J. Exp. Bot. 2010, 61, 2491–2497. [Google Scholar] [CrossRef]
- Scheible, A. Apparatus for Exterminating Vegetation. US Patent 546,682, 24 September 1895. [Google Scholar]
- Sharp, A.A. Vegetation Exterminator. US Patent 492,635, 28 February 1893. [Google Scholar]
- Diprose, M.F.; Benson, F.A. Electrical methods of killing plants. J. Agric. Eng. Res. 1984, 30, 197–209. [Google Scholar] [CrossRef]
- Diprose, M.F.; Fletcher, R.; Longden, P.C.; Champion, M.J. Use of electricity to control bolters in sugar beet (Beta vulgaris L.): A comparison of the electrothermal with chemical and mechanical cutting methods. Weed Res. 1985, 25, 53. [Google Scholar] [CrossRef]
- Vigoureux, A. Results of trials carried out in Belgium in 1980 about killing weed beets by electric discharge. Meded. Van De Fac. Landbouwwet. Rijksuniv. Gent 1981, 46, 163–172. [Google Scholar]
- Mizuno, A.; Nagura, A.; Miyamoto, T.; Chakrabarti, A.; Sato, T.; Kimura, K.; Kimura, T.; Kobayashi, M. A portable weed control device using high frequency AC voltage. In Proceedings of the Record of the 1993 IEEE Industry Applications Conference, Toronto, ON, USA, 2–8 October 1993; pp. 2000–2003. [Google Scholar]
- Manaaki Whenua-Landcare Research. The New Zealand Soils Portal. 2024, Version: 5.0.154. Available online: https://soils.landcareresearch.co.nz/ (accessed on 3 January 2024).
- Field, A.P. Discovering Statistics Using IBM SPSS Statistics: And Sex and Drugs and Rock ‘n’ Roll, 4th ed.; Sage: London, UK, 2013. [Google Scholar]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236. [Google Scholar] [CrossRef]
- Baev, I.V.; Yudaev, V.I. The determination of the most effective current type for electrical damage of plants. Indian J. Sci. Technol. 2017, 10, 1. [Google Scholar] [CrossRef][Green Version]
- Gusbeth, C.; Frey, W.; Bluhm, H. Evidences for membrane electroporation during application of nanoseconds electrical pulses. In IEEE Conference Record-Abstracts; IEEE: Piscataway, NJ, USA, 2004; Volume 195. [Google Scholar] [CrossRef]
- Yudaev, I.V. Analysis of Variation in Circuit Parameters for Substitution of Weed Plant Tissue under Electric Impulse Action. Surf. Eng. Appl. Electrochem. 2019, 55, 219–224. [Google Scholar] [CrossRef]
- Slaven, M.J.; Koch, M.; Borger, C.P.D. Exploring the potential of electric weed control: A review. Weed Sci. 2023, 1–19. [Google Scholar] [CrossRef]
- McNeill, J. Electrical Conductivity of Soil and Rocks; Geonics Ltd.: Mississauga, ON, Canada, 1980. [Google Scholar]
- Ahmad, J.; Muhammad, K.; Ahmad, I.; Ahmad, W.; Smith, M.L.; Smith, L.N.; Jain, D.K.; Wang, H.; Mehmood, I. Visual features based boosted classification of weeds for real-time selective herbicide sprayer systems. Comput. Ind. 2018, 98, 23–33. [Google Scholar] [CrossRef]
- Anken, T.; Latsch, A. Detection rate and spraying accuracy of Ecorobotix ARA. In 42nd GIL Annual Conference, Artificial Intelligence in the Agricultural and Food Sector; Gandorfer, M., Hoffmann, C., El Benni, N., Cockburn, M., Anken, T., Floto, H., Eds.; Society for Informatics: Bonn, Germany, 2022; pp. 45–50. [Google Scholar]
- Coleman, G.; Bender, A.; Hu, K.; Sharpe, S.; Schumann, A.; Wang, Z.; Bagavathiannan, M.; Boyd, N.; Walsh, M. Weed detection to weed recognition: Reviewing 50 years of research to identify constraints and opportunities for large-scale cropping systems. Weed Technol. 2022, 36, 741–757. [Google Scholar] [CrossRef]
- Coleman, G.; Salter, W. More eyes on the prize: Open-source data, software and hardware for advancing plant science through collaboration. AoB Plants 2023, 15, plad010. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Zhang, C.; Guo, H.; Sun, Z.; Wu, X. Real-Time Crop Recognition in Transplanted Fields With Prominent Weed Growth: A Visual-Attention-Based Approach. IEEE Access 2019, 7, 185310–185321. [Google Scholar] [CrossRef]
- Ziwen, C.; Chunlong, Z.; Nan, L.; Zhe, S.; Wei, L.; Bin, Z. Study review and analysis of high performance intra-row weeding robot. Trans. Chin. Soc. Agric. Eng. 2015, 31, 5. [Google Scholar]
- Michaels, A.; Haug, S.; Albert, A. Vision-based high-speed manipulation for robotic ultra-precise weed control. In Proceedings of the International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–3 October 2015; pp. 5498–5505. [Google Scholar]
- Nguyen, V.-T.; Tung, T. Development of a prototype of weeding robot. Eng. Res. Express 2024, 6, 015411. [Google Scholar] [CrossRef]
- Malewar, A. New agricultural Robots Kill Individual Weeds with Electricity. Available online: https://www.inceptivemind.com/small-robot-company-tom-dick-kill-individual-weeds-electricity/19481/ (accessed on 3 August 2022).
- Lysakov, A.A.; Masyutina, G.V.; Rostova, A.T.; Eliseeva, A.A.; Lubentsov, V.F. Development of a weeding robot with tubular linear electric motors. IOP Conf. Ser. Earth Environ. Sci. 2021, 852, 012063. [Google Scholar] [CrossRef]
- Vaqar, A. New Weed Killer Robot Scours the Fields and Takes Out Weeds with a Single Chemical Jet Blast. Wonderful Engeneering. 2018. Available online: https://wonderfulengineering.com/new-weed-killer-robot-scours-the-fields-and-takes-out-weeds-with-a-single-chemical-jet-blast/ (accessed on 2 May 2024).
- Abdelghafour, F.; Rançon, F.; Liu, S.; Champ, J.; De Rudnicki, V.; Guizard, C.; Goëau, H.; Doussan, C.; Joly, A.; Bonnet, P. 83. WeedElec: A robotic research platform for individual weed detection and selective electrical weeding. In Precision Agriculture’21; Wageningen Academic: Wageningen, The Netherlands, 2021; pp. 695–702. [Google Scholar]




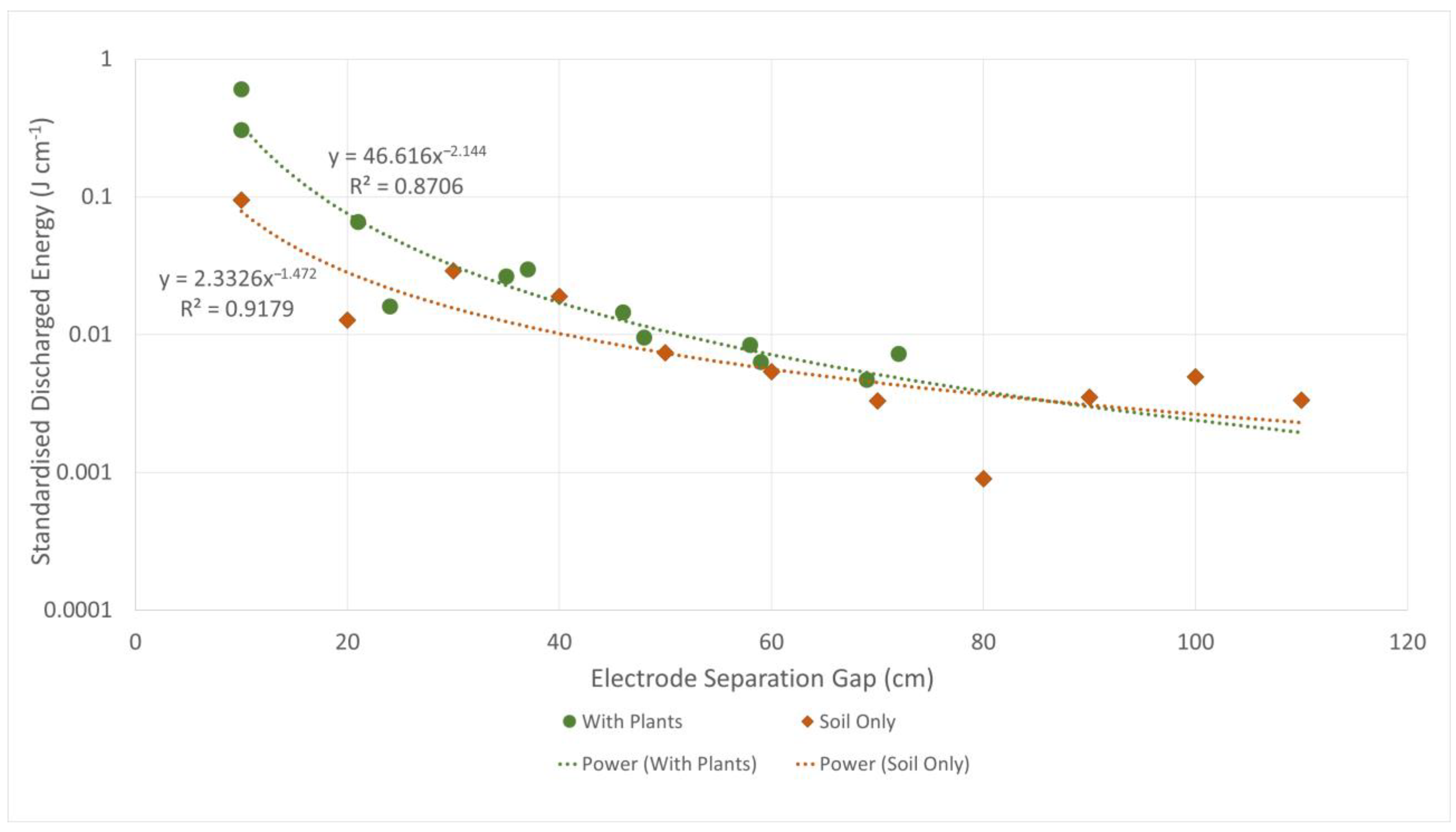
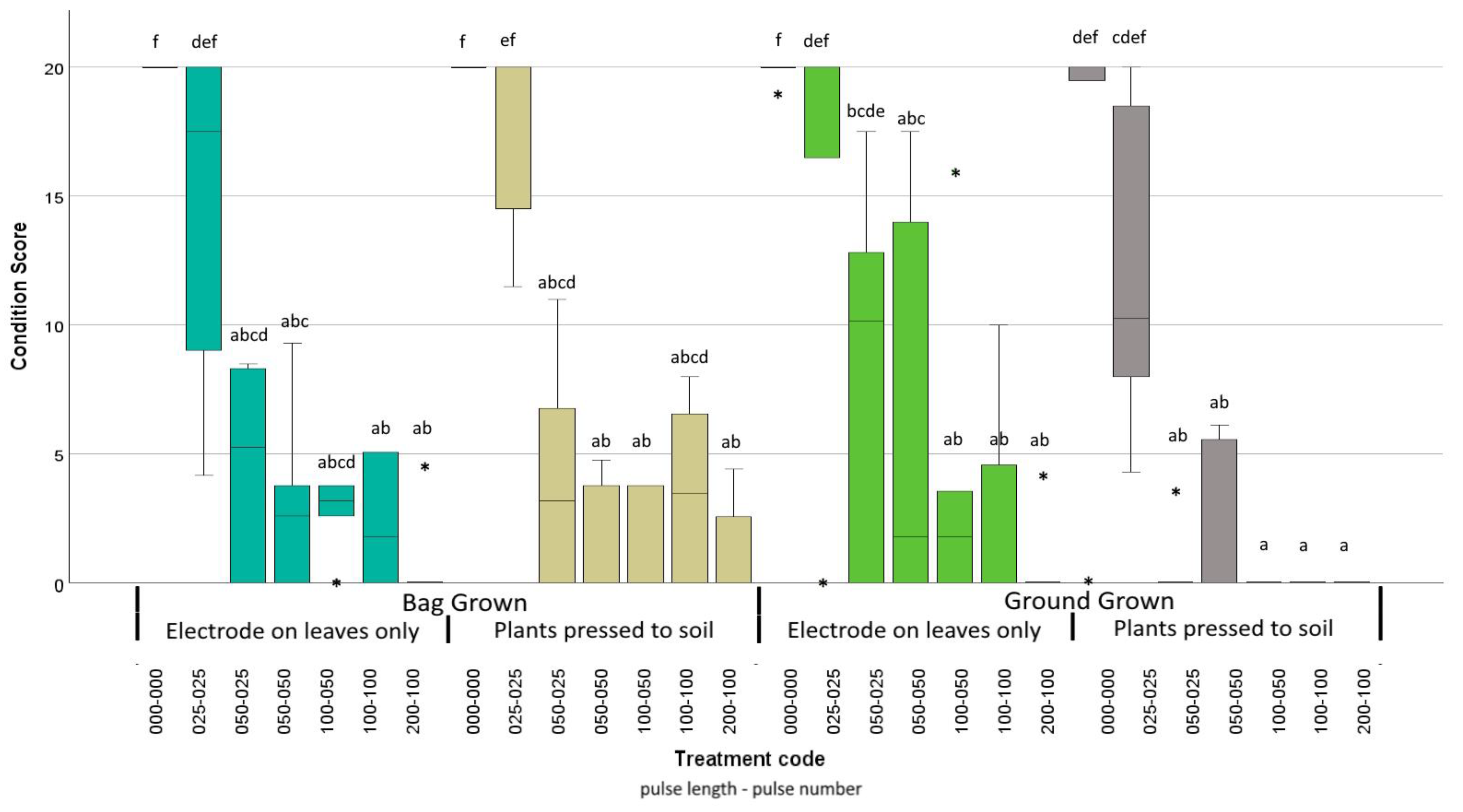

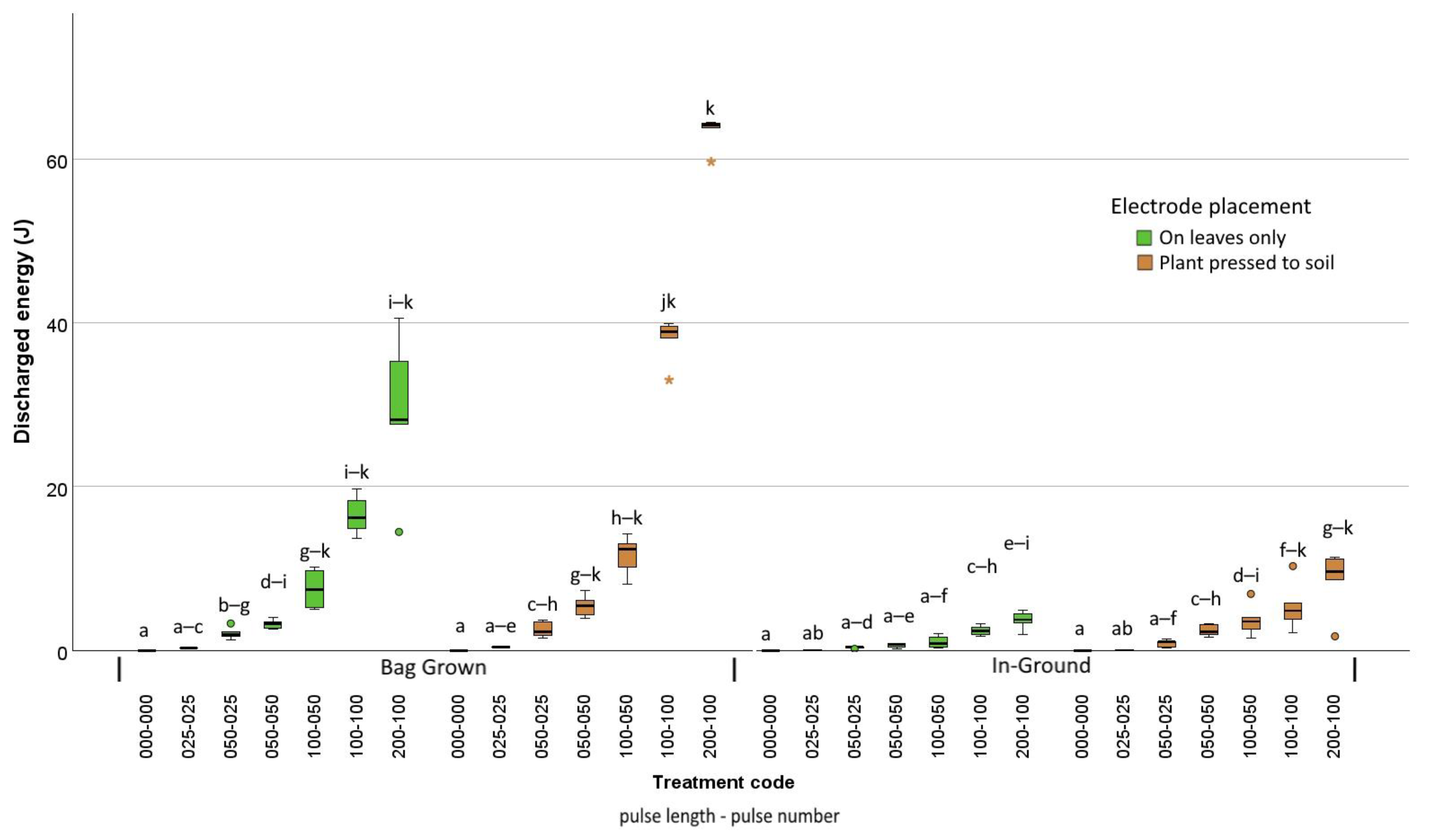
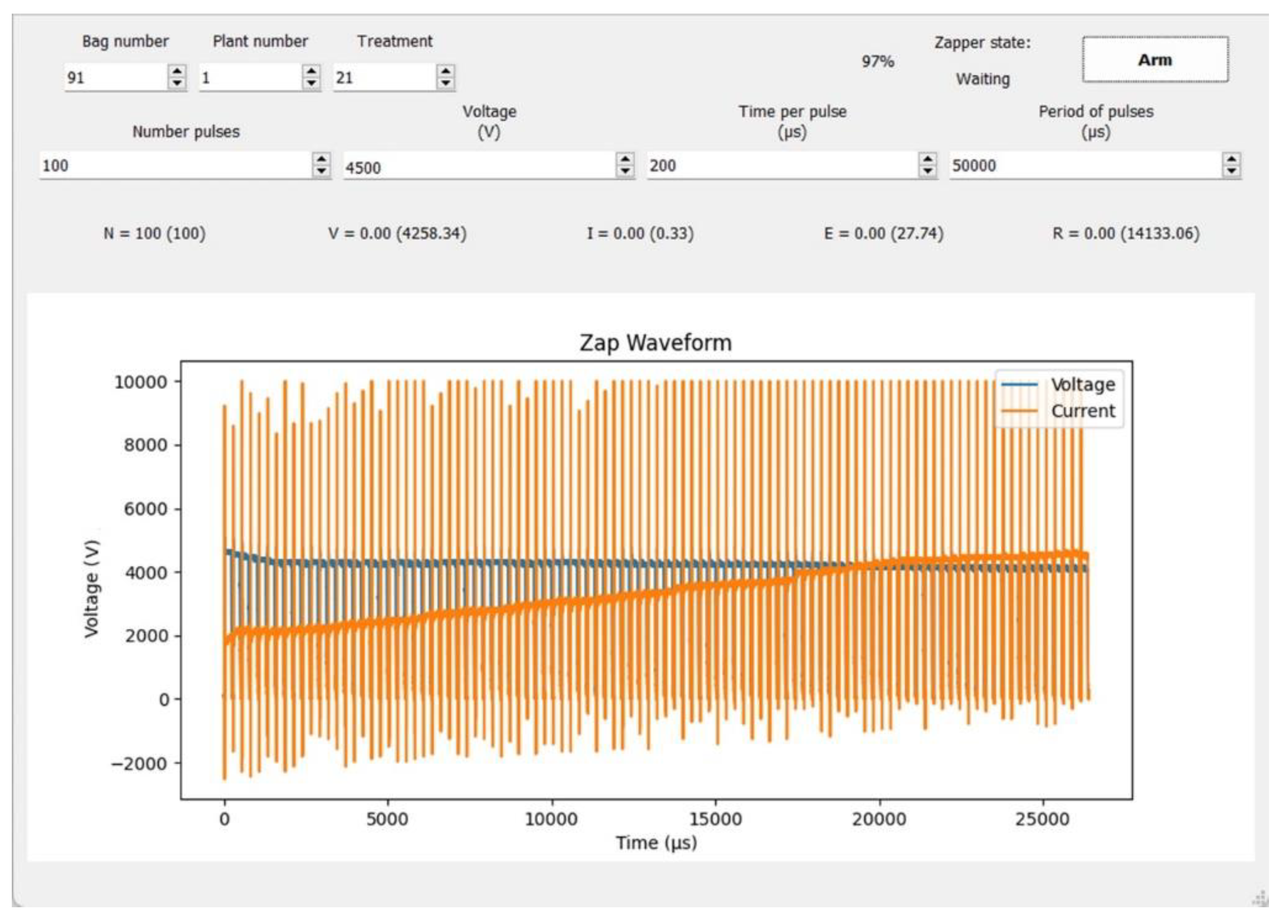

| Trial | Species | Mean Size | Treatments |
|---|---|---|---|
| 1 | L. didymum | Stem length 64.0 mm (SD = 13.9 mm) Stem basal diameter 1.9 mm (SD = 0.5 mm) | Plants grown: in bags vs. in ground Application: to leaves pressed to dry soil surface Dose applied: no treatment vs. 25, 50 or 100 × 100 µs pulse lengths at 4.5 kV with electrode disc pressing plant to soil. Extra treatments: inserted rod vs. surface-pressed disc earthing electrode applying 100 × 100 µs pulses at 4.5 kV with different electrode separation distances. |
| 2 | A. powellii | Stem length 72.9 mm (SD = 12.3 mm) Stem basal diameter 2.1 mm (SD = 0.3 mm) | Plants grown: in bags vs. in ground Application: to leaf canopy only vs. leaves pressed to dry soil surface Dose applied: no treatment vs. 25 × 25 µs, 50 × 50 µs, 50 × 100 µs, 100 × 100 µs, or 100 × 200 µs pulses at 4.5 kV. |
| 3 | L. multiflorum | Tiller No. 1.2 (SD = 0.3) Leaf No. 2.9 (SD = 0.5) Longest leaf length 157.6 mm (SD = 17.1 mm) | Plants grown: in bags vs. in ground Application: to leaf canopy only vs. leaves pressed to dry soil surface Dose applied: no treatment vs. 100 × 200 µs pulses, 200 × 200 µs pulses and 200 × 400 µs pulses at 3.5 kV or 4.5 kV. |
| Planting | Earthing | Voltage | Pulse Length (µs) | Number of Pulses | Mean Energy Discharge (J) |
|---|---|---|---|---|---|
| Bagged | Probe | 4500 | 100 | 25 | 4.6 b |
| Bagged | Probe | 4500 | 100 | 50 | 8.4 c |
| Bagged | Probe | 4500 | 100 | 100 | 23.0 d |
| Bagged | Disc | 4500 | 100 | 100 | 4.5 b |
| In-ground | Disc | 4500 | 100 | 100 | 2.2 a |
| Mean Energy Discharge (kJ s−1) | ||||
|---|---|---|---|---|
| Treatment | Bag-Grown | Ground-Grown | ||
| Dose Applied | Leaves Only | Pressed to Soil | Leaves Only | Pressed to Soil |
| 45-025-025 | 0.518 | 0.667 | 0.053 | 0.050 |
| 45-050-025 | 1.674 | 2.022 | 0.303 | 0.724 |
| 45-050-050 | 1.302 | 2.178 | 0.252 | 0.976 |
| 45-100-050 | 1.505 | 2.345 | 0.207 | 0.740 |
| 45-100-100 | 1.649 | 3.807 | 0.241 | 0.529 |
| 45-100-100 | 1.452 | 3.170 | 0.1851 | 0.459 |
| Bag-Grown Plants | In-Ground-Grown Plants | ||||||
|---|---|---|---|---|---|---|---|
| Dose Discharge Duration (ms) | >0 | 0.625 | >0.625 | >0 | 0.625 | >0.625 | |
| Average of all plants | Mean death rate (%) | 86.1 | 25.0 | 98.3 | 88.7 | 58.3 | 94.9 |
| Mean energy discharge/plant (J) | 12.9 | 0.37 | 17.9 | 2.01 | 0.032 | 2.82 | |
| Mean energy discharge rate (kJ s−1) | 1.86 | 0.59 | 2.11 | 0.391 | 0.052 | 0.459 | |
| Electrode contacting leaf canopy only | Mean death rate (%) | 88.9 | 33.3 | 100 | 80.6 | 33.3 | 90.0 |
| Mean energy discharge/plant (J) | 8.39 | 0.32 | 11.7 | 1.17 | 0.033 | 1.63 | |
| Mean energy discharge rate (kJ s−1) | 1.35 | 0.51 | 1.52 | 0.207 | 0.053 | 0.238 | |
| Electrode pressing whole plant to soil | Mean death rate (%) | 83.3 | 16.7 | 96.7 | 97.1 | 83.3 | 100 |
| Mean energy discharge/plant (J) | 17.4 | 0.41 | 24.2 | 2.87 | 0.031 | 4.21 | |
| Mean energy discharge rate (kJ s−1) | 2.36 | 0.66 | 2.70 | 0.576 | 0.050 | 0.681 | |
| Variables in the Equation | 95% C.I. for EXP(B) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | Lower | Upper | ||
| Step 1 | Electrode Contact | 1.116 | 0.784 | 2.028 | 1 | 0.154 | 3.054 | 0.657 | 14.193 |
| Soil Moisture (%) | 0.259 | 0.143 | 3.303 | 1 | 0.069 | 1.296 | 0.980 | 1.715 | |
| Stem Length (mm) | −0.030 | 0.034 | 0.799 | 1 | 0.371 | 0.970 | 0.908 | 1.037 | |
| Stem Diameter (mm) | −0.133 | 1.258 | 0.011 | 1 | 0.916 | 0.875 | 0.074 | 10.300 | |
| Mean Voltage (V) | 0.001 | 0.000 | 7.965 | 1 | 0.005 | 1.001 | 1.000 | 1.001 | |
| Mean Current (I) | −8.397 | 3.765 | 4.974 | 1 | 0.026 | 0.000 | 0.000 | 0.362 | |
| Discharged energy (J) | 3.048 | 1.076 | 8.023 | 1 | 0.005 | 21.080 | 2.557 | 173.768 | |
| Constant | −8.436 | 4.435 | 3.618 | 1 | 0.057 | 0.000 | |||
| Voltage (kV) | Bag-Grown Plants | In-Ground Plants | |||||
|---|---|---|---|---|---|---|---|
| 3.5 | 4.5 | All | 3.5 | 4.5 | All | ||
| All plants | Death Rate (%) | 83.3 | 91.7 | 87.5 | 91.7 | 96.3 | 94.0 |
| Energy Discharge (J) | 59.4 | 95.2 | 77.3 | 6.58 | 11.8 | 9.18 | |
| Energy ha−1 (MJ ha−1) * | 2.97 | 4.76 | 3.86 | 0.329 | 0.589 | 0.507 | |
| Leaf canopy only contacted | Death Rate (%) | 87.0 | 87.0 | 87.0 | 88.9 | 92.6 | 90.7 |
| Energy Discharge (J) | 56.5 | 89.4 | 73.0 | 4.49 | 10.0 | 7.26 | |
| Energy ha−1 (MJ ha−1) * | 2.82 | 4.47 | 3.65 | 0.224 | 0.502 | 0.363 | |
| Leaves pressed to soil | Death Rate (%) | 79.6 | 96.3 | 88.0 | 94.4 | 100 | 92.7 |
| Energy Discharge (J) | 62.2 | 101 | 81.6 | 8.68 | 13.5 | 11.1 | |
| Energy ha−1 (MJ ha−1) * | 3.11 | 5.05 | 4.10 | 0.434 | 0.675 | 0.555 | |
| Variables in the Equation | 95% C.I. for EXP(B) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | Lower | Upper | ||
| Step 1 | Electrode Contact | 0.792 | 0.349 | 5.150 | 1 | 0.023 | 2.208 | 1.114 | 4.375 |
| Leaf number | −0.506 | 0.244 | 4.290 | 1 | 0.038 | 0.603 | 0.374 | 0.973 | |
| Longest leaf (mm) | 0.014 | 0.007 | 4.509 | 1 | 0.034 | 1.014 | 1.001 | 1.028 | |
| Soil moisture (%) | −0.025 | 0.028 | 0.834 | 1 | 0.361 | 0.975 | 0.924 | 1.029 | |
| Mean Voltage (V) | 0.001 | 0.000 | 58.849 | 1 | <0.001 | 1.001 | 1.001 | 1.001 | |
| Mean Current (I) | −0.690 | 1.049 | 0.433 | 1 | 0.510 | 0.501 | 0.064 | 3.916 | |
| Discharged energy (J) | 0.026 | 0.009 | 9.370 | 1 | 0.002 | 1.027 | 1.010 | 1.044 | |
| Constant | −2.334 | 1.311 | 3.172 | 1 | 0.075 | 0.097 | |||
| Trial | Tiller No. | Leaf No. | Longest Leaf Length |
|---|---|---|---|
| Current | 1.2 | 2.9 | 158 mm |
| Previous [28] 1 * | 1.0 | 2.0 | 109.mm |
| Previous [28] 2 ^ | 1.0 | 2.0 | 149 mm |
| Previous [32] 1 ^ | 1.6 | 3.7 | 141 mm |
| Previous [32] 2 ^ | 1.9 | 4.0 | 197 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloomer, D.J.; Harrington, K.C.; Ghanizadeh, H.; James, T.K. Pots to Plots: Microshock Weed Control Is an Effective and Energy Efficient Option in the Field. Sustainability 2024, 16, 4324. https://doi.org/10.3390/su16114324
Bloomer DJ, Harrington KC, Ghanizadeh H, James TK. Pots to Plots: Microshock Weed Control Is an Effective and Energy Efficient Option in the Field. Sustainability. 2024; 16(11):4324. https://doi.org/10.3390/su16114324
Chicago/Turabian StyleBloomer, Daniel J., Kerry C. Harrington, Hossein Ghanizadeh, and Trevor K. James. 2024. "Pots to Plots: Microshock Weed Control Is an Effective and Energy Efficient Option in the Field" Sustainability 16, no. 11: 4324. https://doi.org/10.3390/su16114324
APA StyleBloomer, D. J., Harrington, K. C., Ghanizadeh, H., & James, T. K. (2024). Pots to Plots: Microshock Weed Control Is an Effective and Energy Efficient Option in the Field. Sustainability, 16(11), 4324. https://doi.org/10.3390/su16114324






