Dynamic Mechanical Performance of Sulfate-Bearing Soils Stabilized by Magnesia-Ground Granulated Blast Furnace Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Sample Preparation
2.3. Test Method
3. Results and Analysis
3.1. Vertical Expansion
3.2. Dynamic Cyclic Loading Test
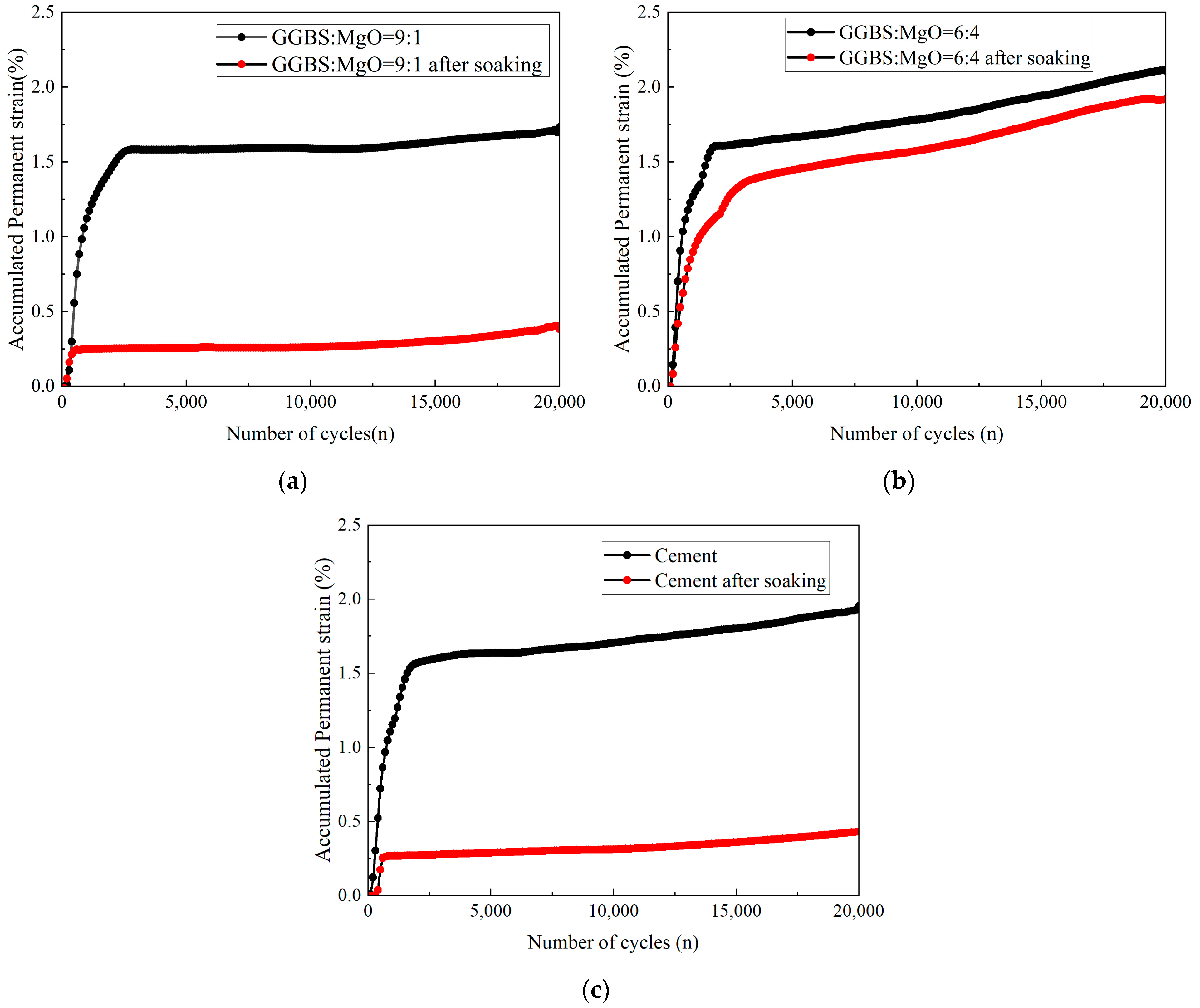
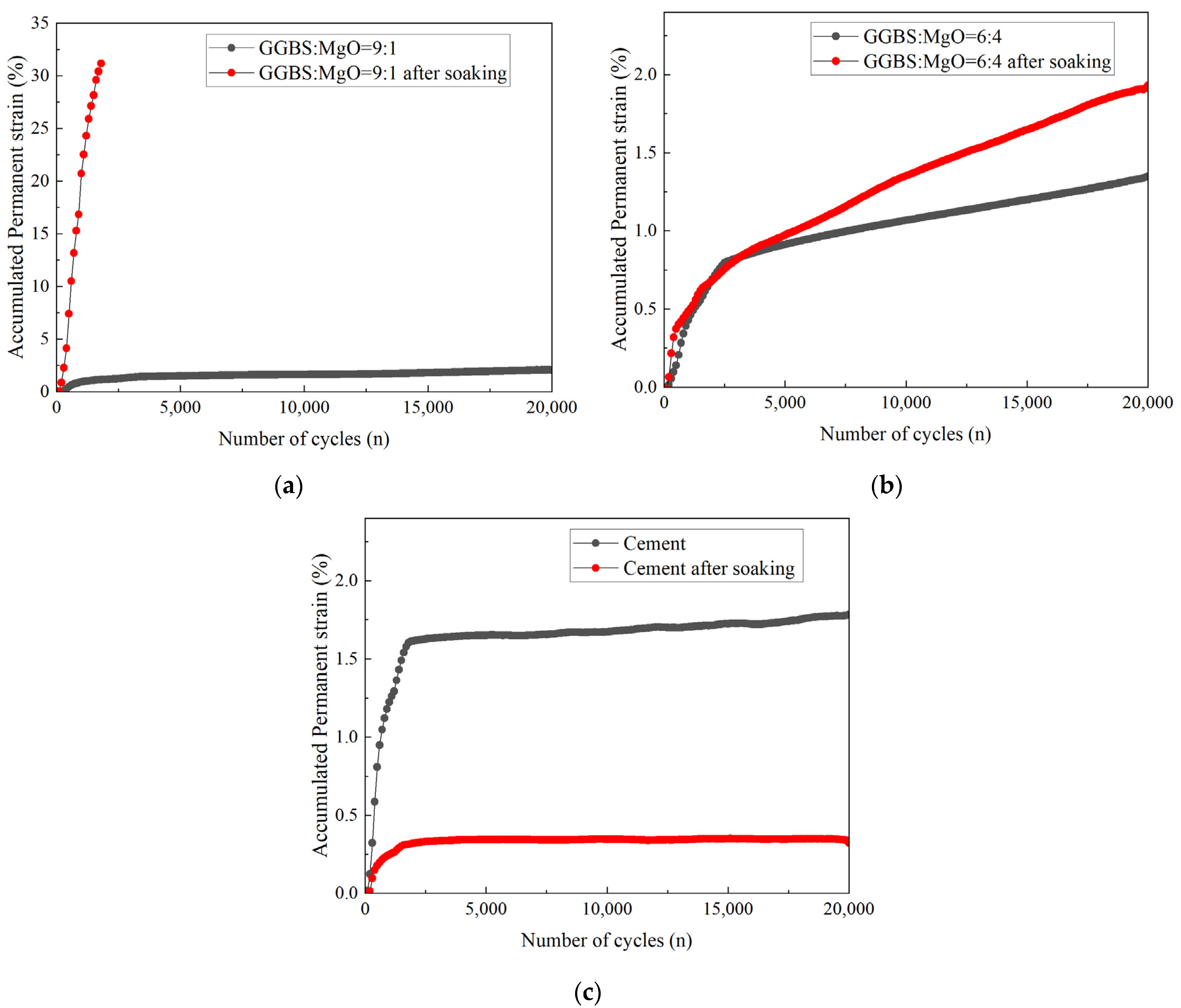
3.2.1. Accumulated Permanent Strain
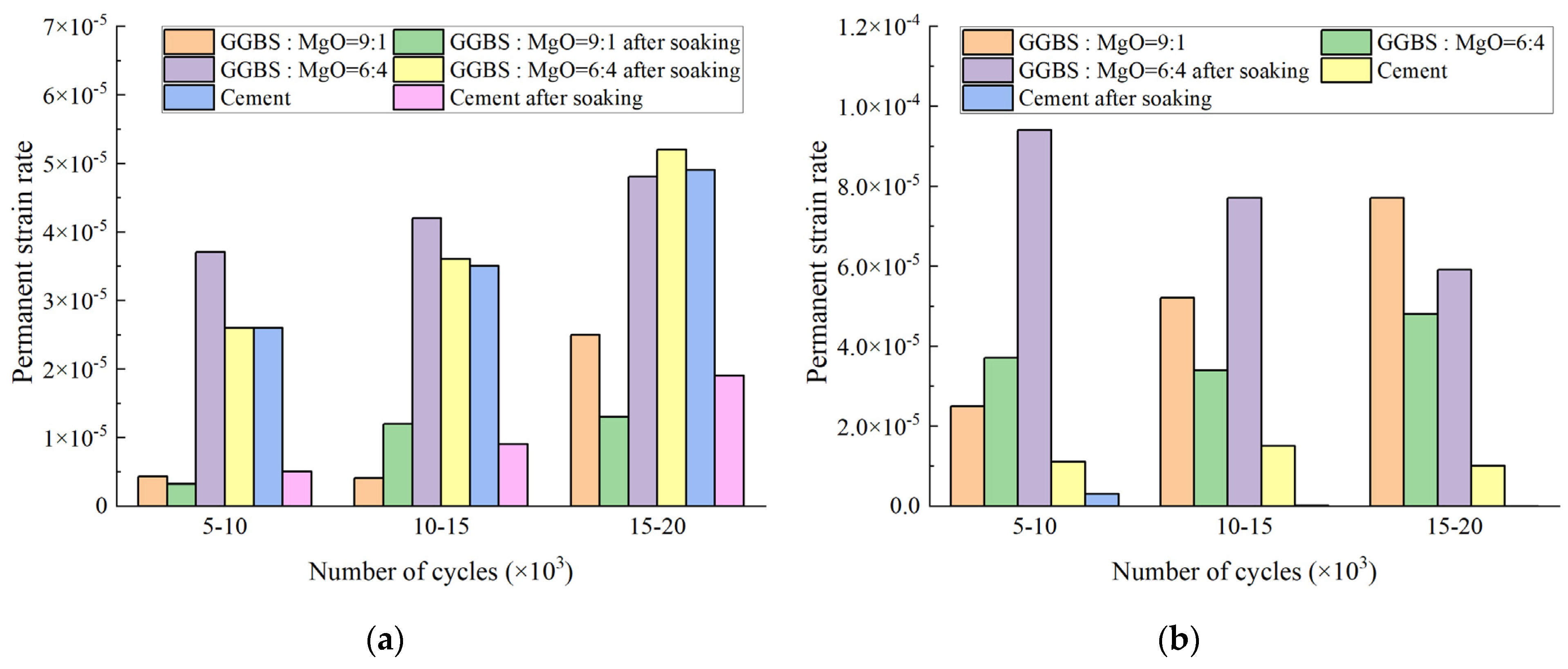
3.2.2. Permanent Strain Rate
3.3. Dynamic Stress–Strain Hysteresis Curves
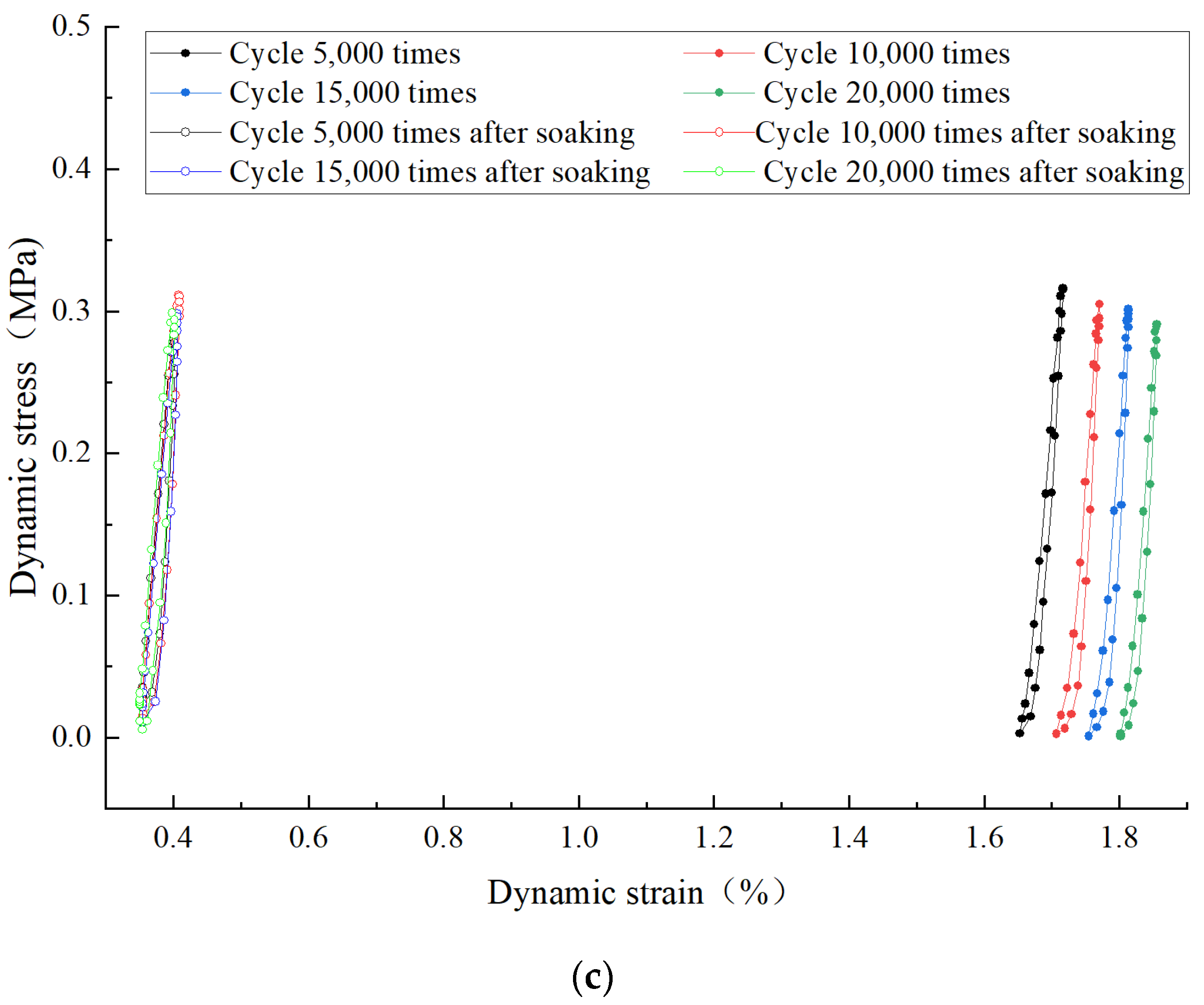
3.3.1. Strain Hysteresis Curve
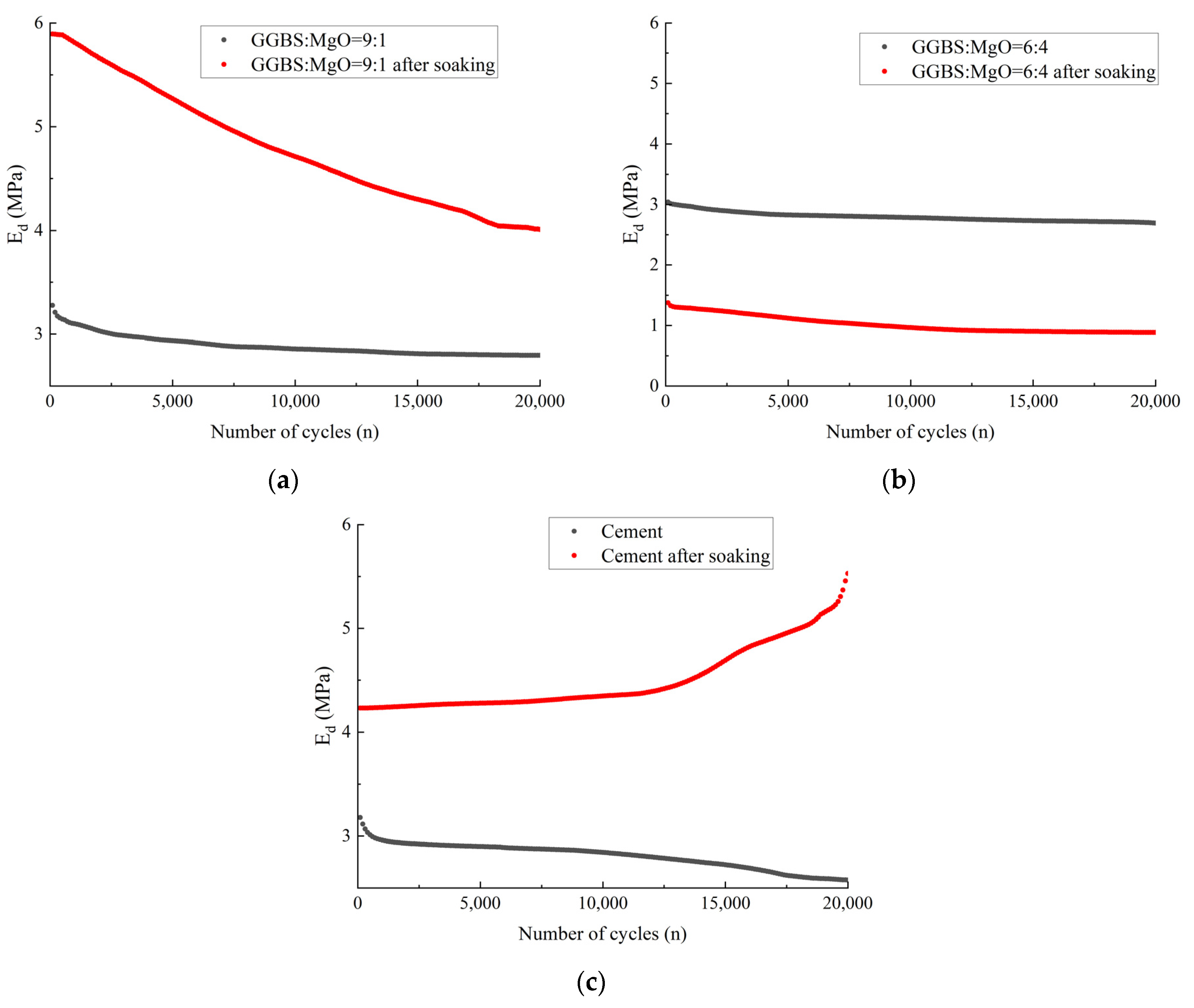
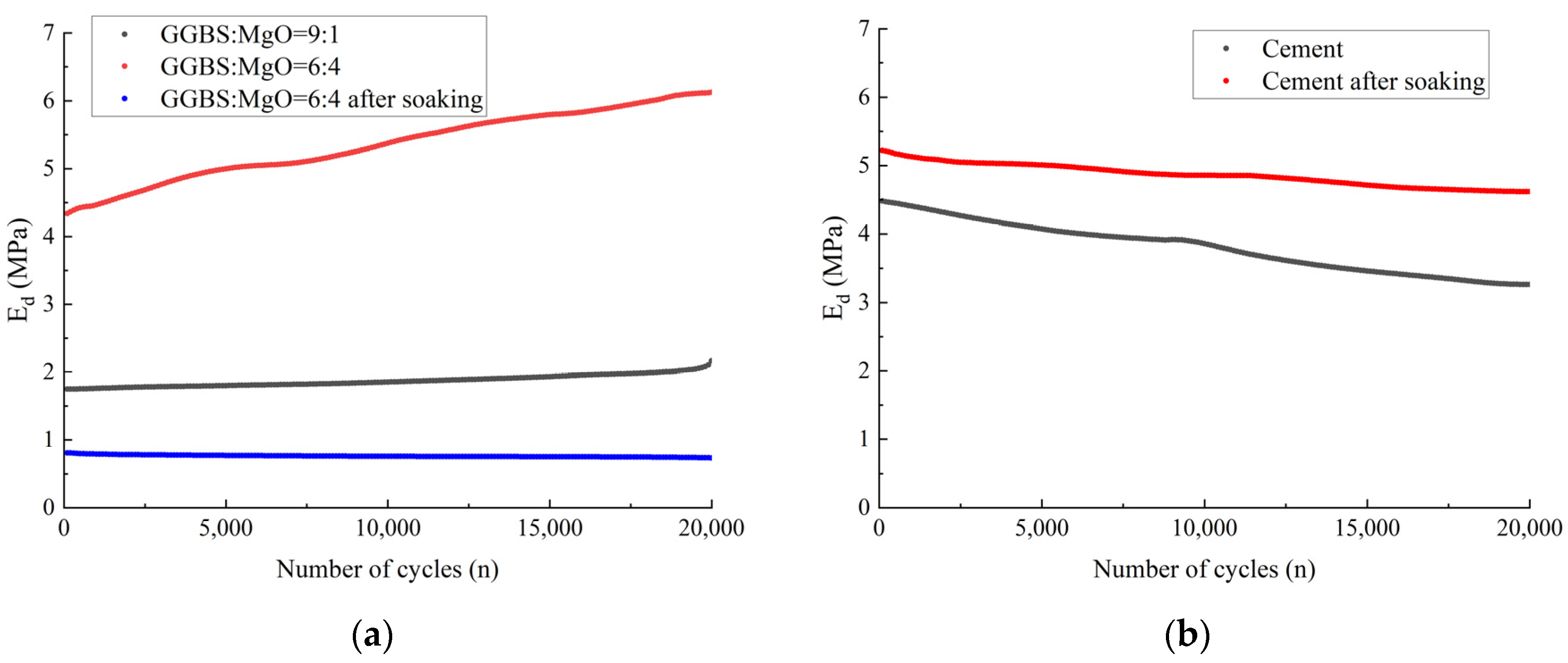
3.3.2. Dynamic Elastic Modulus
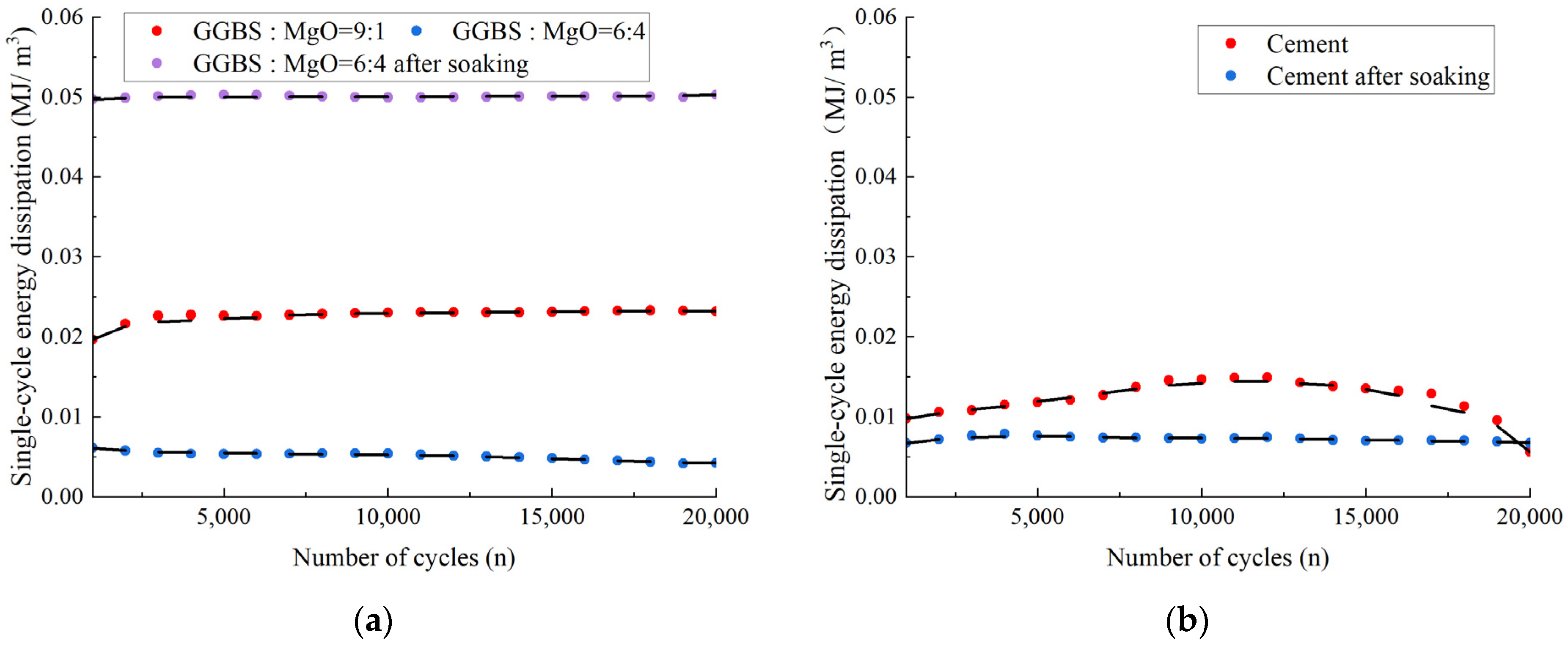
3.3.3. Single-Cycle Energy Dissipation under Fatigue Loading
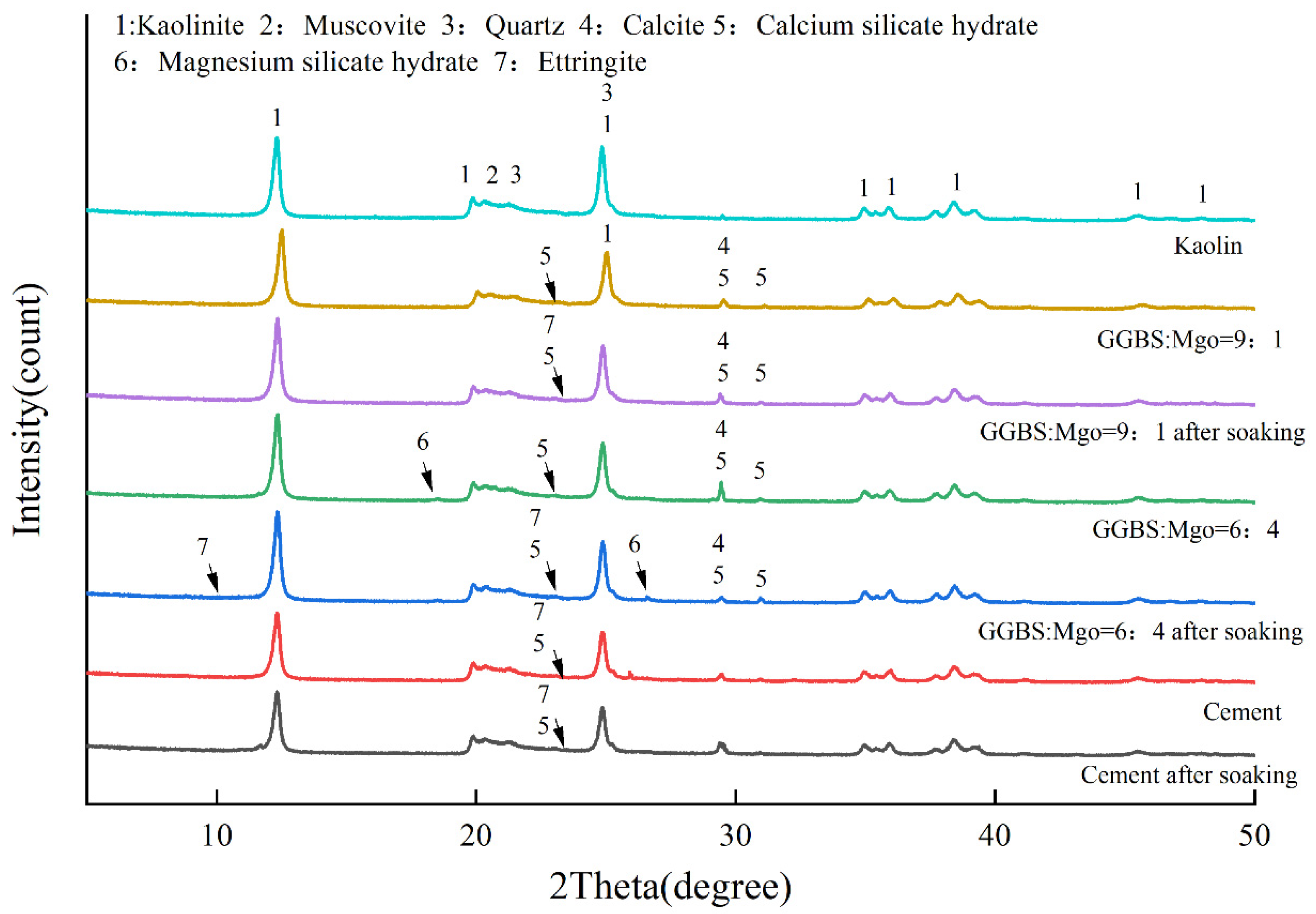
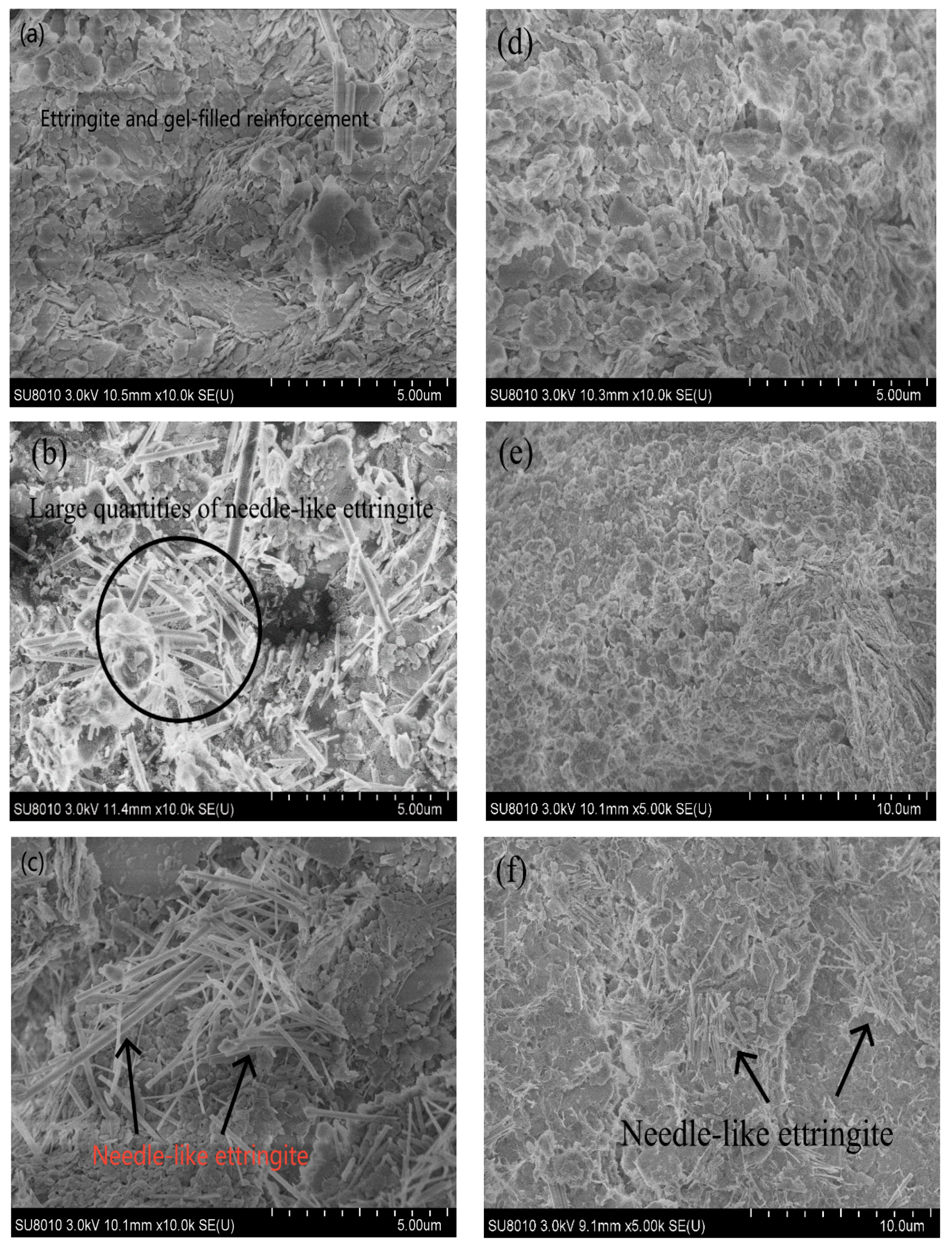
3.4. XRD and SEM
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoshida, N. Sulfate attack on residential concrete foundations in Japan. J. Sustain. Cem.-Based Mater. 2019, 8, 327–336. [Google Scholar] [CrossRef]
- Kampala, A.; Jitsangiam, P.; Pimraksa, K.; Chindaprasirt, P. An investigation of sulfate effects on compaction characteristics and strength development of cement-treated sulfate bearing clay subgrade. Road Mater. Pavement Des. 2021, 22, 2396–2409. [Google Scholar] [CrossRef]
- Wan, X.; You, Z.; Wen, H.; Crossley, W. An experimental study of salt expansion in sodium saline soils under transient conditions. J. Arid Land 2017, 9, 865–878. [Google Scholar] [CrossRef]
- Vo, T.B.T.; Wassmann, R.; Tirol-Padre, A.; Cao, V.P.; MacDonald, B.; Espaldon, M.V.O.; Sander, B.O. Methane emission from rice cultivation in different agro-ecological zones of the Mekong river delta: Seasonal patterns and emission factors for baseline water management. Soil Sci. Plant Nutr. 2018, 64, 47–58. [Google Scholar] [CrossRef]
- Hussain, Z.; Khattak, R.; Irshad, M.; Eneji, A. Ameliorative effect of potassium sulphate on the growth and chemical composition of wheat (Triticum aestivum L.) in salt-affected soils. J. Soil Sci. Plant Nutr. 2013, 13, 401–415. [Google Scholar] [CrossRef]
- Basit, A.; Hussain, S.; Abid, M.; Zafar-ul-Hye, M.; Ahmed, N. Zinc and potassium priming of maize (Zea mays L.) seeds for salt-affected soils. J. Plant Nutr. 2020, 44, 130–141. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Zhang, J.; Liu, N. Variations of soil salinity and cotton growth under six-years mulched drip irrigation. Agronomy 2021, 11, 1127. [Google Scholar] [CrossRef]
- Zhang, J.; Weng, X.; Qu, B.; Liu, J.; Yang, B.; Li, Y. Failure modes and mechanisms of pavements in saline foundations. Proc. Inst. Civ. Eng. Transp. 2018, 171, 174–182. [Google Scholar] [CrossRef]
- You, Z.; Lai, Y.; Zhang, M.; Liu, E. Quantitative analysis for the effect of microstructure on the mechanical strength of frozen silty clay with different contents of sodium sulfate. Environ. Earth Sci. 2017, 76, 143. [Google Scholar] [CrossRef]
- Abdi, M.R.; Askarian, A.; Safdari Seh Gonbad, M. Effects of sodium and calcium sulphates on volume stability and strength of lime-stabilized kaolinite. Bull. Eng. Geol. Environ. 2020, 79, 941–957. [Google Scholar] [CrossRef]
- Wild, S.; Kinuthia, J.; Robinson, R.; Humphreys, I. Effects of ground granulated blast furnace slag (GGBS) on the strength and swelling properties of lime-stabilized kaolinite in the presence of sulphates. Clay Miner. 1996, 31, 423–433. [Google Scholar] [CrossRef]
- Adeleke, B.; Kinuthia, J.; Oti, J. Strength and Swell Performance of High-Sulphate Kaolinite Clay Soil. Sustainability 2020, 12, 10164. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Chen, Y.; Li, R.; Xiao, H. Treating sulfate-bearing soil by using sodium silicate and NaOH-activated ground granulated blast-furnace slag. Acta Geotech. 2023, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Li, Z.; Shi, J.; Liu, Z.; Wu, M.; Liu, C.; Chen, Y.; Liu, G.; Yang, Y. Degradation of mortar fully buried in saline soil containing sodium sulfate or magnesium sulfate. Constr. Build. Mater. 2023, 369, 130620. [Google Scholar] [CrossRef]
- Djayaprabha, H.S.; Chang, T.-P.; Shih, J.-Y.; Nguyen, H.-A. Mechanical Properties of Eco-Friendly Self-consolidating Concrete Containing Ground Granulated Blast Furnace Slag and Calcined Dolomite. In Proceedings of the International Conference on Sustainable Civil Engineering Structures and Construction Materials; Springer Nature: Singapore, 2020; pp. 285–296. [Google Scholar]
- Venkatesan, R.P.; Pazhani, K. Strength and durability properties of geopolymer concrete made with ground granulated blast furnace slag and black rice husk ash. KSCE J. Civ. Eng. 2016, 20, 2384–2391. [Google Scholar] [CrossRef]
- Muthalvan, R.S.; Selvaraj, L.; Avudaiappan, S.; Liseitsev, Y. Performance evaluation of super absorbent polymer modified cement mortar with nano-silica/GGBS. Case Stud. Constr. Mater. 2023, 19, e02359. [Google Scholar] [CrossRef]
- Wild, S.; Kinuthia, J.; Jones, G.; Higgins, D. Suppression of swelling associated with ettringite formation in lime stabilized sulphate bearing clay soils by partial substitution of lime with ground granulated blastfurnace slag (GGBS). Eng. Geol. 1999, 51, 257–277. [Google Scholar] [CrossRef]
- Celik, E.; Nalbantoglu, Z. Effects of ground granulated blastfurnace slag (GGBS) on the swelling properties of lime-stabilized sulfate-bearing soils. Eng. Geol. 2013, 163, 20–25. [Google Scholar] [CrossRef]
- Aldaood, A.; Bouasker, M.; Al-Mukhtar, M. Impact of wetting–drying cycles on the microstructure and mechanical properties of lime-stabilized gypseous soils. Eng. Geol. 2014, 174, 11–21. [Google Scholar] [CrossRef]
- Khadka, S.D.; Jayawickrama, P.W.; Senadheera, S.; Segvic, B. Stabilization of highly expansive soils containing sulfate using metakaolin and fly ash based geopolymer modified with lime and gypsum. Transp. Geotech. 2020, 23, 100327. [Google Scholar] [CrossRef]
- Ehwailat, K.I.A.; Ismail, M.A.M.; Ezreig, A.M.A. Novel approach for suppression of ettringite formation in sulfate-bearing soil using blends of nano-magnesium oxide, ground granulated blast-furnace slag and rice husk ash. Appl. Sci. 2021, 11, 6618. [Google Scholar] [CrossRef]
- Sindhu, A.; Minukrishna, P.; Abraham, B. Experimental study on the impact of type of sulphate in lime stabilised clays. In Proceedings of the International Web Conference in Civil Engineering for a Sustainable Planet, Virtual, 5–6 March 2021; pp. 109–118. [Google Scholar] [CrossRef]
- Estabragh, A.; Jahani, A.; Javadi, A.; Babalar, M. Assessment of different agents for stabilisation of a clay soil. Int. J. Pavement Eng. 2022, 23, 160–170. [Google Scholar] [CrossRef]
- Yi, Y.; Zheng, X.; Liu, S.; Al-Tabbaa, A. Comparison of reactive magnesia-and carbide slag-activated ground granulated blastfurnace slag and Portland cement for stabilisation of a natural soil. Appl. Clay Sci. 2015, 111, 21–26. [Google Scholar] [CrossRef]
- Pu, H.; Mastoi, A.K.; Chen, X.; Song, D.; Qiu, J.; Yang, P. An integrated method for the rapid dewatering and solidification/stabilization of dredged contaminated sediment with a high water content. Front. Environ. Sci. Eng. 2021, 15, 67. [Google Scholar] [CrossRef]
- Ehwailat, K.I.A.; Mohamad Ismail, M.A.; Ezreig, A.M.A. Novel approach to the treatment of gypseous soil-induced ettringite using blends of non-calcium-based stabilizer, ground granulated blast-furnace slag, and metakaolin. Materials 2021, 14, 5198. [Google Scholar] [CrossRef]
- Seco, A.; Miqueleiz, L.; Prieto, E.; Marcelino, S.; García, B.; Urmeneta, P. Sulfate soils stabilization with magnesium-based binders. Appl. Clay Sci. 2017, 135, 457–464. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S.; Jin, F. Magnesium sulfate attack on clays stabilised by carbide slag-and magnesia-ground granulated blast furnace slag. Géotechnique Lett. 2015, 5, 306–312. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y.; Puppala, A.J. Utilization of carbide slag-activated ground granulated blastfurnace slag to treat gypseous soil. Soils Found. 2019, 59, 1496–1507. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y.; Puppala, A.J. Suppressing ettringite-induced swelling of gypseous soil by using magnesia-activated ground granulated blast-furnace slag. J. Geotech. Geoenvironmental Eng. 2020, 146, 06020008. [Google Scholar] [CrossRef]
- ASTM D698-12e2; Standard Test Methods for Laboratory Compaction Characeristics of Soil Using Standard Effort (12,400 ft-lbf/ft3 (600 kN-m/m3)). ASTM: West Conshohocken, PA, USA, 2012.
- AASHTO T307-99; Standard Method of Test for Determining the Resilient Modulus of Soils and Aggregates. AASHTO: West Conshohocken, PA, USA, 2003.
- Werkmeister, S. Permanent Deformation Behaviour of Unbound Granular Materials in Pavement Constructions. Ph.D. Thesis, Technischen Universität Dresden, Dresden, Germany, 2004. [Google Scholar]
- ASTM D1633; Standard Method for Compressive Strength of Molded Soil-Cement Cylinders. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM D1883; Standard Test Method for CBR (California Bearing Ratio) of Laboratory–Compacted Soils. ASTM (American Society for Testing and Materials): West Conshohocken, PA, USA, 1999.
- Arnold, G.K. Rutting of Granular Pavements. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2004. [Google Scholar]
- Zhuang, X.; Zhao, J. Experimental Study of the Dynamic Characteristics and Microscopic Mechanism of Lightweight Soil Modified with Expanded Polystyrene and Sisal Fibre. Appl. Sci. 2023, 13, 11502. [Google Scholar] [CrossRef]
- Xie, H.; Li, X.; Shan, C.; Xia, Z.; Yu, L. Study on the damage mechanism and energy evolution characteristics of water-bearing coal samples under cyclic loading. Rock Mech. Rock Eng. 2023, 56, 1367–1385. [Google Scholar] [CrossRef]
- Song, Z.; Frühwirt, T.; Konietzky, H. Characteristics of dissipated energy of concrete subjected to cyclic loading. Constr. Build. Mater. 2018, 168, 47–60. [Google Scholar] [CrossRef]
- Lei, D.; Zhang, P.; He, J.; Bai, P.; Zhu, F. Fatigue life prediction method of concrete based on energy dissipation. Constr. Build. Mater. 2017, 145, 419–425. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Al-Tabbaa, A. Properties and microstructure of GGBS–magnesia pastes. Adv. Cem. Res. 2014, 26, 114–122. [Google Scholar] [CrossRef]
- Hekal, E.E.; Kishar, E.; Mostafa, H. Magnesium sulfate attack on hardened blended cement pastes under different circumstances. Cem. Concr. Res. 2002, 32, 1421–1427. [Google Scholar] [CrossRef]
- Park, H.; Jeong, Y.; Jun, Y.; Jeong, J.-H.; Oh, J.E. Strength enhancement and pore-size refinement in clinker-free CaO-activated GGBFS systems through substitution with gypsum. Cem. Concr. Compos. 2016, 68, 57–65. [Google Scholar] [CrossRef]
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.E. Impacts of MgO waste: GGBS formulations on the performance of a stabilised natural high sulphate bearing soil. Constr. Build. Mater. 2022, 315, 125745. [Google Scholar] [CrossRef]
- Saranya, P.; Nagarajan, P.; Shashikala, A.; Salam, A.P. Flexural behaviour of GGBS-dolomite geopolymer concrete beams under cyclic loading. In Proceedings of the Materials Science Forum, Singapore, 19–21 October 2019; pp. 291–296. [Google Scholar]
- You, L.; Jiang, L.; Chu, H. Influence of carbonation on fatigue of concrete with high volume of ground granulated blast-furnace slag. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2015, 30, 361–368. [Google Scholar] [CrossRef]
- Reddy, P.S.; Indhu, B.; Gangha, G.; Padmarekha, A. Determination of fatigue behaviour of concrete using GGBS and robosand. Int. J. Civ. Eng. Technol. 2017, 8, 244–251. [Google Scholar]
- Lang, L.; Chen, B.; Chen, B. Strength evolutions of varying water content-dredged sludge stabilized with alkali-activated ground granulated blast-furnace slag. Constr. Build. Mater. 2021, 275, 122111. [Google Scholar] [CrossRef]
- Lang, L.; Chen, B.; Li, N. Utilization of lime/carbide slag-activated ground granulated blast-furnace slag for dredged sludge stabilization. Mar. Georesources Geotechnol. 2021, 39, 659–669. [Google Scholar] [CrossRef]
- Sarkkinen, M.; Kujala, K.; Gehör, S. Efficiency of MgO activated GGBFS and OPC in the stabilization of highly sulfidic mine tailings. J. Sustain. Min. 2019, 18, 115–126. [Google Scholar] [CrossRef]
- Fasihnikoutalab, M.H.; Pourakbar, S.; Ball, R.J.; Unluer, C.; Cristelo, N. Sustainable soil stabilisation with ground granulated blast-furnace slag activated by olivine and sodium hydroxide. Acta Geotech. 2020, 15, 1981–1991. [Google Scholar] [CrossRef]
- Dung, N.; Hooper, T.; Unluer, C. Enhancing the performance of MgO-activated slag-fly ash mixes by accelerated carbonation. J. CO2 Util. 2020, 42, 101356. [Google Scholar] [CrossRef]
- Liu, T.; Li, S.; Chen, Y.; Brouwers, H.; Yu, Q. Insitu formation of layered double hydroxides in MgO–NaAlO2-activated GGBS/MSWI BA: Impact of Mg2+ on reaction mechanism and leaching behavior. Cem. Concr. Compos. 2023, 140, 105114. [Google Scholar] [CrossRef]
- Zhong, Y.; Cai, G.; Wang, S.; Qin, H.; Zhang, C.; Li, J. Influence of Organic Content on the Mechanical Properties of Organic-Rich Soils Stabilized with CaO-GGBS Binder and PC. Water 2022, 14, 3053. [Google Scholar] [CrossRef]
- Korde, C.; Cruickshank, M.; West, R.P. Activation of slag: A comparative study of cement, lime, calcium sulfate, GGBS fineness and temperature. Mag. Concr. Res. 2021, 73, 15–31. [Google Scholar] [CrossRef]
- Adeleke, B.; Kinuthia, J.; Oti, J. Optimization of MgO-GGBS Cementitious Systems Using Thermo-Chemical Approaches. Sustainability 2021, 13, 9378. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Bai, W.; Hu, T.; He, J.; Song, X. Effect of reactive MgO on hydration and properties of alkali-activated slag pastes with different activators. Constr. Build. Mater. 2021, 271, 121608. [Google Scholar] [CrossRef]
- Cai, G.-H.; Liu, S.-Y.; Zheng, X. Influence of drying-wetting cycles on engineering properties of carbonated silt admixed with reactive MgO. Constr. Build. Mater. 2019, 204, 84–93. [Google Scholar] [CrossRef]
- Seo, J.; Choi, S.; Kim, J.; Lee, H. Potential for the liquid carbonation curing of Portland cement using sodium bicarbonate solutions. Constr. Build. Mater. 2024, 427, 136220. [Google Scholar] [CrossRef]










| Composition | CaO | Si2O | Al2O3 | MgO | Fe2O3 | SO3 | TiO2 | K2O | Others |
|---|---|---|---|---|---|---|---|---|---|
| Kaolin | ND | 53.90 | 43.24 | ND | 0.89 | 0.08 | 1.36 | 0.19 | 0.34 |
| Cement | 59.39 | 20.66 | 5.60 | 3.87 | 3.23 | 4.99 | ND | 0.10 | 2.16 |
| CaSO4·2H2O | 58.39 | 8.32 | 2.61 | 5.21 | 0.72 | 23.70 | ND | 0.55 | 0.50 |
| MgSO4·7H2O | 1.21 | 14.64 | 7.73 | 20.09 | 0.22 | 55.11 | 0.07 | 0.30 | 0.63 |
| GGBS | 42.22 | 31.33 | 14.82 | 6.83 | ND | 2.31 | 0.79 | ND | 1.70 |
| MgO | 5.62 | 10.23 | 6.34 | 76.72 | ND | 0.55 | ND | ND | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Yang, K.; Cheng, Y.; Huang, K.; Hu, Y.; Liu, L.; Li, X. Dynamic Mechanical Performance of Sulfate-Bearing Soils Stabilized by Magnesia-Ground Granulated Blast Furnace Slag. Sustainability 2024, 16, 4313. https://doi.org/10.3390/su16104313
Li W, Yang K, Cheng Y, Huang K, Hu Y, Liu L, Li X. Dynamic Mechanical Performance of Sulfate-Bearing Soils Stabilized by Magnesia-Ground Granulated Blast Furnace Slag. Sustainability. 2024; 16(10):4313. https://doi.org/10.3390/su16104313
Chicago/Turabian StyleLi, Wentao, Kang Yang, Yang Cheng, Ke Huang, Yan Hu, Le Liu, and Xing Li. 2024. "Dynamic Mechanical Performance of Sulfate-Bearing Soils Stabilized by Magnesia-Ground Granulated Blast Furnace Slag" Sustainability 16, no. 10: 4313. https://doi.org/10.3390/su16104313
APA StyleLi, W., Yang, K., Cheng, Y., Huang, K., Hu, Y., Liu, L., & Li, X. (2024). Dynamic Mechanical Performance of Sulfate-Bearing Soils Stabilized by Magnesia-Ground Granulated Blast Furnace Slag. Sustainability, 16(10), 4313. https://doi.org/10.3390/su16104313






