Culturable Bioaerosols Assessment in a Waste-Sorting Plant and UV-C Decontamination
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Methods
2.3. Techniques for Bioaerosols Assessment
- Mesophilic bacteria from environmental sources: 25 °C for 5 days;
- Bacteria from human sources: 37 °C for 2 days;
- Fungi: 25 °C for 5 days.
2.4. Quality Control
2.5. Statistical Analysis
3. Results and Discussion
3.1. Outdoor Air Quality
3.2. Indoor Air Quality in Waste-Sorting Unit 1 (Campaign 1)
3.2.1. Overall Indoor Air Quality Parameters
3.2.2. Seasonal Effect
3.2.3. Workday Time Effect
3.3. Indoor Air Quality in Administrative Offices and Canteen (Campaign 2)
3.4. Physicochemical and Microbiological Parameter Correlations
3.5. UV Disinfection Effect on Indoor Air Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Directive 2008/98/EC. European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008L0098-20180705 (accessed on 21 February 2023).
- Jedynska, A.; Kuijpers, E.; van den Berg, C.; Kruizinga, A.; Meima, M.; Spaan, S. Biological Agents and Work-Related Diseases: Results of a Literature Review, Expert Survey and Analysis of Monitoring Systems; European Agency for Safety and Health at Work: Bilbao, Spain, 2019. [Google Scholar] [CrossRef]
- Park, D.-U.; Ryu, S.-H.; Kim, S.-B.; Yoon, C.-S. An Assessment of Dust, Endotoxin, and Microorganism Exposure during Waste Collection and Sorting. J. Air Waste Manag. Assoc. 2011, 61, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ryu, S.; Kim, S.; Byun, H.; Yoon, C.; Lee, K. Airborne Bacteria and Fungi Associated with Waste-Handling Work. Int. J. Occup. Environ. Health 2013, 19, 311–318. [Google Scholar] [CrossRef]
- Lavoie, J.; Guertin, S. Evaluation of Health and Safety Risks in Municipal Solid Waste Recycling Plants. J. Air Waste Manag. Assoc. 2001, 51, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Kozajda, A.; Jeżak, K.; Cyprowski, M.; Szadkowska-Stańczyk, I. Inhalable Dust, Endotoxins and (1–3)-β-d-Glucans as Indicators of Exposure in Waste Sorting Plant Environment. Aerobiologia 2017, 33, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Hebisch, R.; Linsel, G. Workers’ Exposure to Hazardous Substances and Biological Agents in Recycling Enterprises. Gefahrstoffe Reinhalt. Luft 2012, 72, 163–169. [Google Scholar]
- Cyprowski, M.; Ławniczek-Wałczyk, A.; Stobnicka-Kupiec, A.; Górny, R.L. Occupational Exposure to Anaerobic Bacteria in a Waste Sorting Plant. J. Air Waste Manag. Assoc. 2021, 71, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Černá, K.; Wittlingerová, Z.; Zimová, M.; Janovský, Z. Exposure to Airborne Fungi during Sorting of Recyclable Plastics in Waste Treatment Facilities. Med. Pr. 2017, 68, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breum, N.O.; Nielsen, B.H.; Nielsen, E.M.; Midtgaard, U.; Poulsen, O.M. Dustiness of Compostable Waste: A Methodological Approach to Quantify the Potential of Waste to Generate Airborne Micro-Organisms and Endotoxin. Waste Manag. Res. 1997, 15, 169–187. [Google Scholar] [CrossRef]
- Nair, A.T. Bioaerosols in the Landfill Environment: An Overview of Microbial Diversity and Potential Health Hazards. Aerobiologia 2021, 37, 185–203. [Google Scholar] [CrossRef]
- Kiviranta, H.; Tuomainen, A.; Reiman, M.; Laitinen, S.; Nevalainen, A.; Liesivuori, J. Exposure to Airborne Microorganisms and Volatile Compounds in Different Types of Waste Handling. Ann. Agric. Environ. Med. 1999, 6, 39–44. [Google Scholar]
- Wikuats, C.F.H.; da Silva, I.; Prates, K.V.M.C.; da Silva, J.C.R.; Duarte, E.H.; Silva, D.d.M.C.e.; Ribeiro, M.; Simão, A.N.C.; Martins, L.D. Health Symptoms and Inflammatory Blood Biomarkers from Exposure of Recyclable Waste Workers to Particulate Matter and Bioaerosols. Atmos. Pollut. Res. 2022, 13, 101323. [Google Scholar] [CrossRef]
- Malta-Vacas, J.; Viegas, S.; Sabino, R.; Viegas, C. Fungal and Microbial Volatile Organic Compounds Exposure Assessment in a Waste Sorting Plant. J. Toxicol. Environ. Health Part A 2012, 75, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.; Afanou, A.K.; Madsen, A.M.; Straumfors, A.; Graff, P. An Assessment of Occupational Exposure to Bioaerosols in Automated versus Manual Waste Sorting Plants. Environ. Res. 2023, 218, 115040. [Google Scholar] [CrossRef] [PubMed]
- Degois, J.; Clerc, F.; Simon, X.; Bontemps, C.; Leblond, P.; Duquenne, P. First Metagenomic Survey of the Microbial Diversity in Bioaerosols Emitted in Waste Sorting Plants. Ann. Work Expo. Health 2017, 61, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Brągoszewska, E. Exposure to Bacterial and Fungal Aerosols: Microorganism Indices in a Waste-Sorting Plant in Poland. Int. J. Environ. Res. Public Health 2019, 16, 3308. [Google Scholar] [CrossRef] [PubMed]
- Baghani, A.N.; Sorooshian, A.; Delikhoon, M.; Nabizadeh, R.; Nazmara, S.; Bakhtiari, R. Pollution Characteristics and Noncarcinogenic Risk Assessment of Fungal Bioaerosol in Different Processing Units of Waste Paper and Cardboard Recycling Factory. Toxin Rev. 2020, 40, 752–763. [Google Scholar] [CrossRef]
- Baghani, A.N.; Golbaz, S.; Ebrahimzadeh, G.; Guzman, M.I.; Delikhoon, M.; Rastani, M.J.; Barkhordari, A.; Nabizadeh, R. Characteristics and Assessing Biological Risks of Airborne Bacteria in Waste Sorting Plant. Ecotoxicol. Environ. Saf. 2022, 232, 113272. [Google Scholar] [CrossRef] [PubMed]
- Breum, N.O.; Würtz, H.; Midtgaard, U.; Ebbehøj, N. Dustiness and Bio-Aerosol Exposure in Sorting Recyclable Paper. Waste Manag. Res. 1999, 17, 100–108. [Google Scholar] [CrossRef]
- Directive 2000/54/EC. European Parliament and of the Council of 18 September 2000 on the Protection of Workers from Risks Related to Exposure to Biological Agents at Work. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32000L0054 (accessed on 21 February 2023).
- Dutkiewicz, J. Bacteria and Fungi in Organic Dust as Potential Health Hazard. Ann. Agric. Environ. Med. Lublin 1997, 4, 11–16. [Google Scholar]
- Ivens, U.I.; Ebbehøj, N.; Poulsen, O.M.; Skov, T. Gastrointestinal Symptoms among Waste Recycling Workers. Ann. Agric. Environ. Med. 1997, 4, 153–157. [Google Scholar]
- Heldal, K.K.; Halstensen, A.S.; Thorn, J.; Diupesland, P.; Wouters, I.; Eduard, W.; Halstensen, T.S. Upper Airway Inflammation in Waste Handlers Exposed to Bioaerosols. Occup. Environ. Med. 2003, 60, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Pepper, I.L.; Gerba, C.P. Aeromicrobiology. In Environmental Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 89–110. [Google Scholar] [CrossRef]
- Madsen, A.M.; Raulf, M.; Duquenne, P.; Graff, P.; Cyprowski, M.; Beswick, A.; Laitinen, S.; Rasmussen, P.U.; Hinker, M.; Kolk, A.; et al. Review of Biological Risks Associated with the Collection of Municipal Wastes. Sci. Total Environ. 2021, 791, 148287. [Google Scholar] [CrossRef] [PubMed]
- Brągoszewska, E.; Biedroń, I.; Hryb, W. Microbiological Air Quality and Drug Resistance in Airborne Bacteria Isolated from a Waste Sorting Plant Located in Poland―A Case Study. Microorganisms 2020, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, A.; Santos, J.; Ramos, C.; Vasconcelos, M.; Fernandes, P. Ambient Air Waste Sorting Facilities Could Be a Source of Antibiotic Resistant Bacteria. Microbiol. Biotechnol. Lett. 2021, 49, 367–373. [Google Scholar] [CrossRef]
- Chen, J.; Hoek, G. Long-Term Exposure to PM and All-Cause and Cause-Specific Mortality: A Systematic Review and Meta-Analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Chen, T.; Han, Z.; Ji, W.; Bai, Y.; Zheng, Z.; Su, Y.; Jin, L.; Xie, B.; Wu, D. From Air to Airway: Dynamics and Risk of Inhalable Bacteria in Municipal Solid Waste Treatment Systems. J. Hazard. Mater. 2023, 460, 132407. [Google Scholar] [CrossRef]
- Viegas, C.; Gomes, A.Q.; Abegão, J.; Sabino, R.; Graça, T.; Viegas, S. Assessment of Fungal Contamination in Waste Sorting and Incineration—Case Study in Portugal. J. Toxicol. Environ. Health Part A 2014, 77, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Górny, R.; Cyprowski, M.; Ławniczek-Wałczyk, A.; Gołofit-Szymczak, M.; Zapór, L. Biohazards in the Indoor Environment—A Role for Threshold Limit Values in Exposure Assessment. In Management of Indoor Air Quality; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–20. [Google Scholar] [CrossRef]
- European Agency for Safety and Health at Work. Occupational Safety and Health Encyclopedia and Home of the Safety Science Monitor Archives. Biological Agents. Available online: https://oshwiki.osha.europa.eu/en/themes/biological-agents (accessed on 25 February 2024).
- German Federal Institute for Occupational Safety and Health. Technical Rules for Biological Agents (TRBA). Available online: https://www.baua.de/DE/Angebote/Regelwerk/TRBA/TRBA-214.html (accessed on 21 February 2024).
- Decree-Law no 102-A/2020 of December 9th. Presidência Do Conselho de Ministros (Presidency of the Council of Ministers). Diário da República, 1a Série, Portugal. 2020, Volume 238, pp. 36(2)–36(50). Available online: https://files.dre.pt/1s/2020/12/23801/0000200050.pdf (accessed on 21 February 2024).
- Ordinance no 138-G/2021 of July 1st. Saúde e Ambiente e Ação Climática (Health and Environment and Climate Action). Diário da República, 1a Série, Portugal. 2021, Volume 126, pp. 128(2)–128(6). Available online: https://diariodarepublica.pt/dr/detalhe/portaria/138-g-2021-166296490 (accessed on 21 February 2024).
- Reed, N.G. The History of Ultraviolet Germicidal Irradiation for Air Disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef]
- Lee, B.U. Life Comes from the Air: A Short Review on Bioaerosol Control. Aerosol Air Qual. Res. 2011, 11, 921–927. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, C.S. Control Effectiveness of Ultraviolet Germicidal Irradiation on Bioaerosols. Aerosol Sci. Technol. 2002, 36, 474–478. [Google Scholar] [CrossRef]
- Wang, C.; Lu, S.; Zhang, Z. Inactivation of Airborne Bacteria Using Different UV Sources: Performance Modeling, Energy Utilization, and Endotoxin Degradation. Sci. Total Environ. 2019, 655, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh, M.; Adeli, B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.H.; Bhardwaj, N. UVC Radiation for Food Safety: An Emerging Technology for the Microbial Disinfection of Food Products. Chem. Eng. J. 2021, 417, 128084. [Google Scholar] [CrossRef]
- Ramos, C.C.R.; Roque, J.L.A.; Sarmiento, D.B.; Suarez, L.E.G.; Sunio, J.T.P.; Tabungar, K.I.B.; Tengco, G.S.C.; Rio, P.C.; Hilario, A.L. Use of Ultraviolet-C in Environmental Sterilization in Hospitals: A Systematic Review on Efficacy and Safety. Int. J. Health Sci. 2020, 14, 52–65. [Google Scholar]
- Paidalwar, A.A.; Khedikar, I. Overview of Water Disinfection by UV Technology. Int. J. Sci. Technol. Eng. 2016, 2, 213–219. [Google Scholar] [CrossRef]
- Manda, V.; Balachandran, S.; Mahesh, D.; Houji, R.; Raj, B.S.S.; Kumar, J.A.; Govindu, G.S.; Vilasagaram, R.K. UV Sterilizing Dustbin. Int. J. Mod. Trends Sci. Technol. 2021, 7, 71–75. [Google Scholar]
- Bergman, R.; Brenner, D.; Buonanno, M.; Eadie, E.; Forbes, P.D.; Jensen, P.; Nardell, E.A.; Sliney, D.; Vincent, R.; Welch, D.; et al. Air Disinfection with Germicidal Ultraviolet: For This Pandemic and the Next. Photochem. Photobiol. 2021, 97, 464–465. [Google Scholar] [CrossRef]
- Marchand, G.; Lavoie, J.; Lazure, L. Evaluation of Bioaerosols in a Municipal Solid Waste Recycling and Composting Plant. J. Air Waste Manag. Assoc. 1995, 45, 778–781. [Google Scholar] [CrossRef]
- ISO 16000-1:2004E; Indoor Air—Part 1: General Aspects of Sampling Strategy. ISO: Geneva, Switzerland, 2004.
- Jensen, P.A.; Schafer, M.P. Sampling and Characterization of Bioaerosol. In Manual of Analytical Methods; Center for Disease Control and Prevention: Atlanta, GA, USA, 2014; pp. 84–100. [Google Scholar]
- EN13098:2019E; European Standard “Workplace Exposure-Measurement of Airborne Microorganisms and Microbial Compounds–General Requirements”. The European Committee for Standardization: Brussels, Belgium, 2019; CEN/TC 137.
- ADENE. Technical Note NT-SCE-02 Metodologia Para Auditorias Periódicas de QAI Em Edifícios de Serviços Existentes No Âmbito Do RSECE (Methodology for Periodical Auditing of IAQ in Service Buildings in RCESE); Portuguese Agency Energy: Porto, Portugal, 2009. [Google Scholar]
- VWR®. Microbiological Air Sampler SAS Super ISO USB Manual. 2021. Version 2, UK. Available online: https://uk.vwr.com/assetsvc/asset/en_GB/id/35782738/contents/manual-vwr-microbiological-air-sampler-sas-super-iso-usb.pdf (accessed on 21 February 2024).
- Rastmanesh, A.; Boruah, J.S.; Lee, M.-S.; Park, S. On-Site Bioaerosol Sampling and Airborne Microorganism Detection Technologies. Biosensors 2024, 14, 122. [Google Scholar] [CrossRef]
- Neto, F.A.; Siqueira, L.F.G. Guidelines for Indoor Air Quality in Offices in Brazil. In Proceedings of the Sixth International Conference on Healthy Buildings, Espoo, Finland, 6–10 August 2000; Volume 4, p. 549. [Google Scholar]
- Stryjakowska-Sekulska, M.; Piotraszewska-Pajak, A.; Szyszka, A.; Nowicki, M.; Filipiak, M. Microbiological Qualtiy of Indoor Air in University Rooms. Polish J. Environ. Stud. 2007, 16, 623–632. [Google Scholar]
- Chegini, F.M.; Baghani, A.N.; Hassanvand, M.S.; Sorooshian, A.; Golbaz, S.; Bakhtiari, R.; Ashouri, A.; Joubani, M.N.; Alimohammadi, M. Indoor and Outdoor Airborne Bacterial and Fungal Air Quality in Kindergartens: Seasonal Distribution, Genera, Levels, and Factors Influencing Their Concentration. Build. Environ. 2020, 175, 106690. [Google Scholar] [CrossRef]
- Bragoszewska, E.; Mainka, A.; Pastuszka, J.S. Concentration and Size Distribution of Culturable Bacteria in Ambient Air during Spring and Winter in Gliwice: A Typical Urban Area. Atmosphere 2017, 8, 239. [Google Scholar] [CrossRef]
- Zuraimi, M.S.; Fang, L.; Tan, T.K.; Chew, F.T.; Tham, K.W. Airborne Fungi in Low and High Allergic Prevalence Child Care Centers. Atmos. Environ. 2009, 43, 2391–2400. [Google Scholar] [CrossRef]
- Fang, Z.; Ouyang, Z.; Zheng, H.; Wang, X.; Hu, L. Culturable Airborne Bacteria in Outdoor Environments in Beijing, China. Microb. Ecol. 2007, 54, 487–496. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; dos Santos, M.; Carolino, E.; Gomes, A.Q.; Sabino, R.; Viegas, S. Fungal Burden in Waste Industry: An Occupational Risk to Be Solved. Environ. Monit. Assess. 2015, 187, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, J.; Tolvanen, O.; Nivukoski, U.; Veijanen, A.; Hänninen, K. Occupational Hygiene in Terms of Volatile Organic Compounds (VOCs) and Bioaerosols at Two Solid Waste Management Plants in Finland. Waste Manag. 2013, 33, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Malmros, P.; Sigsgaard, T.; Bach, B. Occupational Health Problems Due to Garbage Sorting. Waste Manag. Res. 1992, 10, 227–234. [Google Scholar] [CrossRef]
- Sigsgaard, T.; Malmros, P.; Nersting, L.; Petersen, C. Respiratory Disorders and Atopy in Danish Refuse Workers. Am. J. Respir. Crit. Care Med. 1994, 149, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.; Graff, P.; Pedersen, I.; Straumfors, A.; Afanou, A.K. Bioaerosol Exposure and in Vitro Activation of Toll-like Receptors in a Norwegian Waste Sorting Plant. Saf. Health Work 2022, 13, 9–16. [Google Scholar] [CrossRef]
- Wouters, I.M.; Hilhorst, S.K.M.; Kleppe, P.; Doekes, G.; Douwes, J.; Peretz, C.; Heederik, D. Upper Airway Inflammation and Respiratory Symptoms in Domestic Waste Collectors. Occup. Environ. Med. 2002, 59, 106–112. [Google Scholar] [CrossRef]
- Szulc, J.; Okrasa, M.; Majchrzycka, K.; Sulyok, M.; Nowak, A.; Szponar, B.; Górczyńska, A.; Ryngajłło, M.; Gutarowska, B. Microbiological and Toxicological Hazard Assessment in a Waste Sorting Plant and Proper Respiratory Protection. J. Environ. Manag. 2022, 303, 114257–114271. [Google Scholar] [CrossRef]
- Decree-Law no 243/86 of August 20th. Regulamento Geral de Higiene e Segurança Do Trabalho Nos Estabelecimentos Comerciais, de Escritório e Serviços (General Occupational Hygiene and Safety Regulations in Commercial, Office and Service Establishments). Diário da República, Portugal. 1986, pp. 2099–2106. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/243-1986-219080 (accessed on 21 February 2024).
- Täubel, M.; Rintala, H.; Pitkäranta, M.; Paulin, L.; Laitinen, S.; Pekkanen, J.; Hyvärinen, A.; Nevalainen, A. The Occupant as a Source of House Dust Bacteria. J. Allergy Clin. Immunol. 2009, 124, 834–840.e47. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.K.; Schlünssen, V.; Broberg, K.; Østergaard, K.; Frederiksen, M.W.; Madsen, A.M.; Kolstad, H.A. Exposure Levels of Dust, Endotoxin, and Microorganisms in the Danish Recycling Industry. Ann. Work Expo. Health 2023, 67, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.U.; Frederiksen, M.W.; Carøe, T.K.; Madsen, A.M. Health Symptoms, Inflammation, and Bioaerosol Exposure in Workers at Biowaste Pretreatment Plants. Waste Manag. 2023, 167, 173–182. [Google Scholar] [CrossRef]
- Guo, K.; Qian, H.; Ye, J.; Sun, F.; Zhuge, Y.; Wang, S.; Liu, C.; Cao, G.; Zheng, X. Assessment of Airborne Bacteria and Fungi in Different-Type Buildings in Nanjing, a Hot Summer and Cold Winter Moist Chinese City. Build. Environ. 2021, 205, 108258. [Google Scholar] [CrossRef]
- Pestana, M.H.; Gageiro, J.N. Análise de Dados Para Ciências Sociais—A Complementaridade Do SPSS (Data Analysis for Social Sciences—A SPSS Complementarity), 6th ed.; Robalo, M., Ed.; Silabo Editions, Lda: Lisboa, Portugal, 2014. [Google Scholar]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Dorizas, P.V.; Kapsanaki-Gotsi, E.; Assimakopoulos, M.N.; Santamouris, M. Correlation of Particulate Matter with Airborne Fungi in Schools in Greece. Int. J. Vent. 2013, 12, 1–15. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, V.; Thakur, M.; Singh, G.; Bhargava, B. A Systematic Review on Mitigation of Common Indoor Air Pollutants Using Plant-Based Methods: A Phytoremediation Approach. Air Qual. Atmos. Health 2023, 16, 1501–1527. [Google Scholar] [CrossRef]
- Chojer, H.; Branco, P.T.B.S.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Sousa, S.I.V. Source Identification and Mitigation of Indoor Air Pollution Using Monitoring Data–Current Trends. Environ. Technol. Innov. 2024, 33, 103534. [Google Scholar] [CrossRef]
- Lindblad, M.; Tano, E.; Lindahl, C.; Huss, F. Ultraviolet-C Decontamination of a Hospital Room: Amount of UV Light Needed. Burns 2020, 46, 842–849. [Google Scholar] [CrossRef]
- Li, P.; Koziel, J.A.; Paris, R.V.; Macedo, N.; Zimmerman, J.J.; Wrzesinski, D.; Sobotka, E.; Balderas, M.; Walz, W.B.; Liu, D.; et al. Indoor Air Quality Improvement with Filtration and UV-C on Mitigation of Particulate Matter and Airborne Bacteria: Monitoring and Modeling. J. Environ. Manag. 2024, 351, 119764. [Google Scholar] [CrossRef] [PubMed]
- Nerandzic, M.M.; Fisher, C.W.; Donskey, C.J. Sorting through the Wealth of Options: Comparative Evaluation of Two Ultraviolet Disinfection Systems. PLoS ONE 2014, 9, e107444. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, M.; Ponnaiya, B.; Welch, D.; Stanislauskas, M.; Randers-Pehrson, G.; Smilenov, L.; Lowy, F.D.; Owens, D.M.; Brennera, D.J. Germicidal Efficacy and Mammalian Skin Safety of 222-Nm UV Light. Radiat. Res. 2017, 187, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Graeffe, F.; Luo, Y.; Guo, Y.; Ehn, M. Unwanted Indoor Air Quality Effects from Using Ultraviolet C Lamps for Disinfection. Environ. Sci. Technol. Lett. 2023, 10, 172–178. [Google Scholar] [CrossRef]
- Claus, H. Ozone Generation by Ultraviolet Lamps. Photochem. Photobiol. 2021, 97, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Q. Culture-Based Analytical Methods for Investigation of Indoor Fungi. In Sampling and Analysis of Indoor Microorganisms; Yang, C.S., Heinsohn, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 105–122. [Google Scholar]
- Harkawy, A.; Górny, R.L.; Ogierman, L.; Wlazło, A.; Ławniczek-Wałczyk, A.; Niesler, A. Bioaerosol Assessment in Naturally Ventilated Historical Library Building with Restricted Personnel Access. Ann. Agric. Environ. Med. 2011, 18, 323–329. [Google Scholar]
- Manuel, C.M.; Nunes, O.C.; Melo, L.F. Unsteady State Flow and Stagnation in Distribution Systems Affect the Biological Stability of Drinking Water. Biofouling 2010, 26, 129–139. [Google Scholar] [CrossRef]
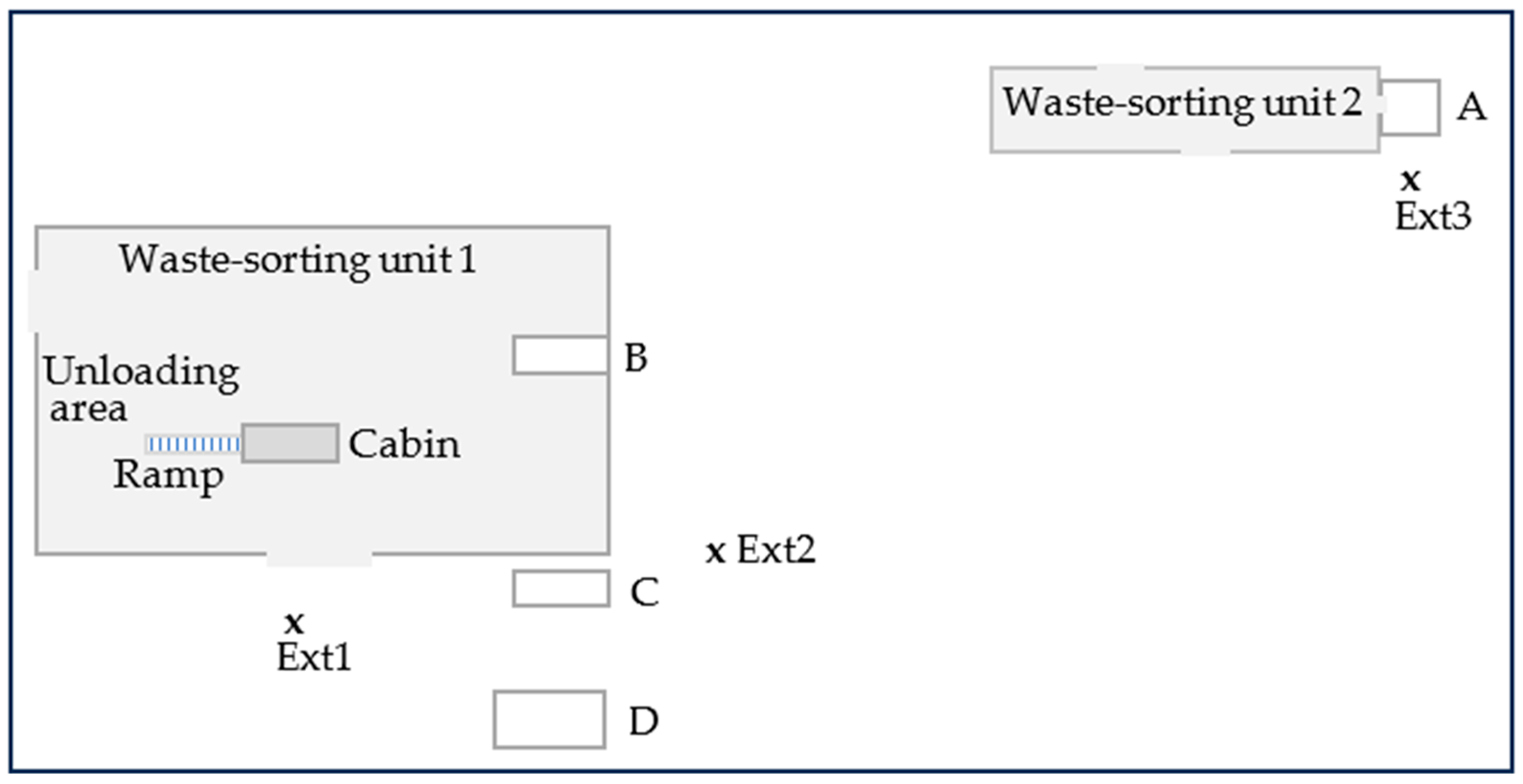

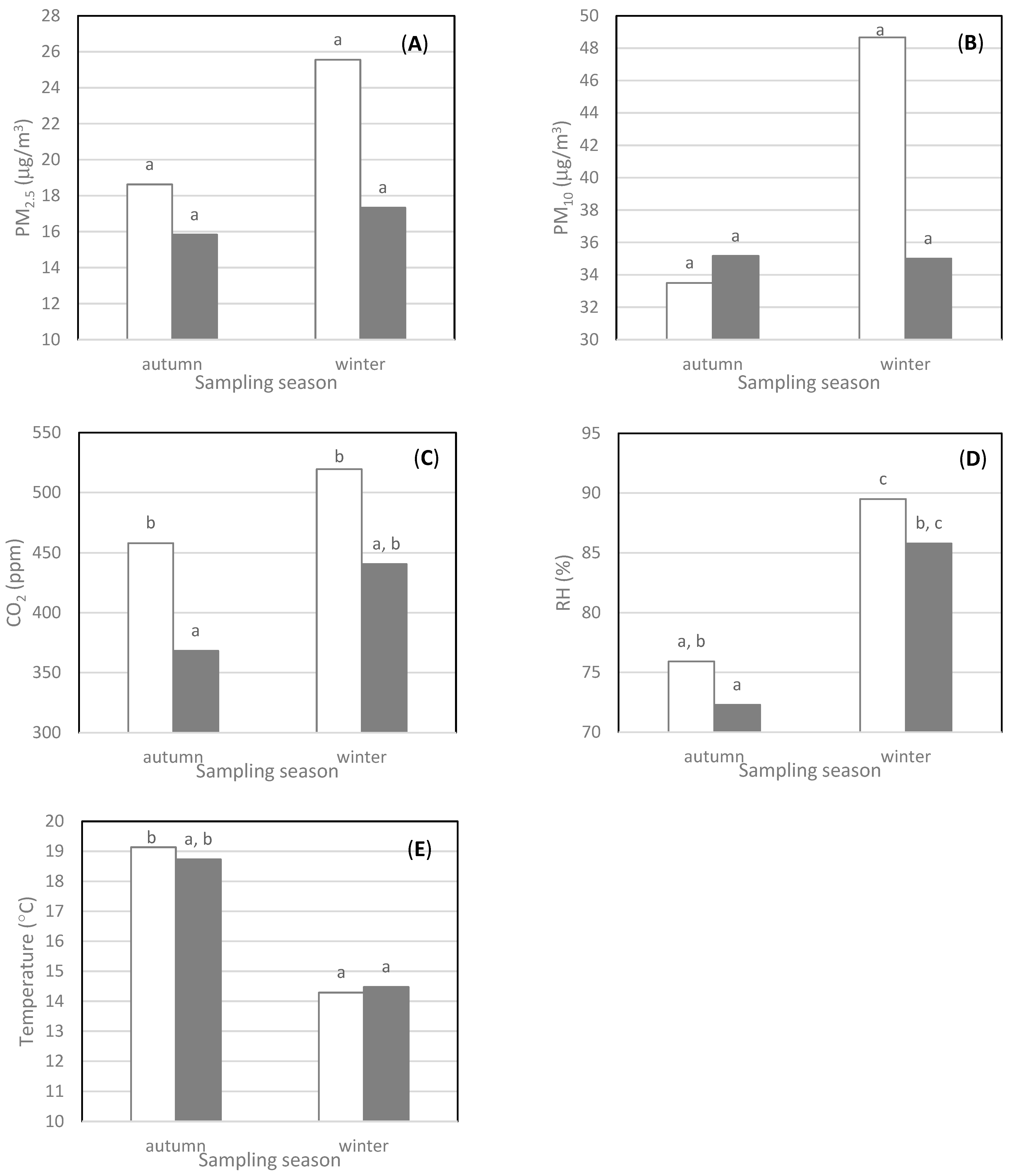
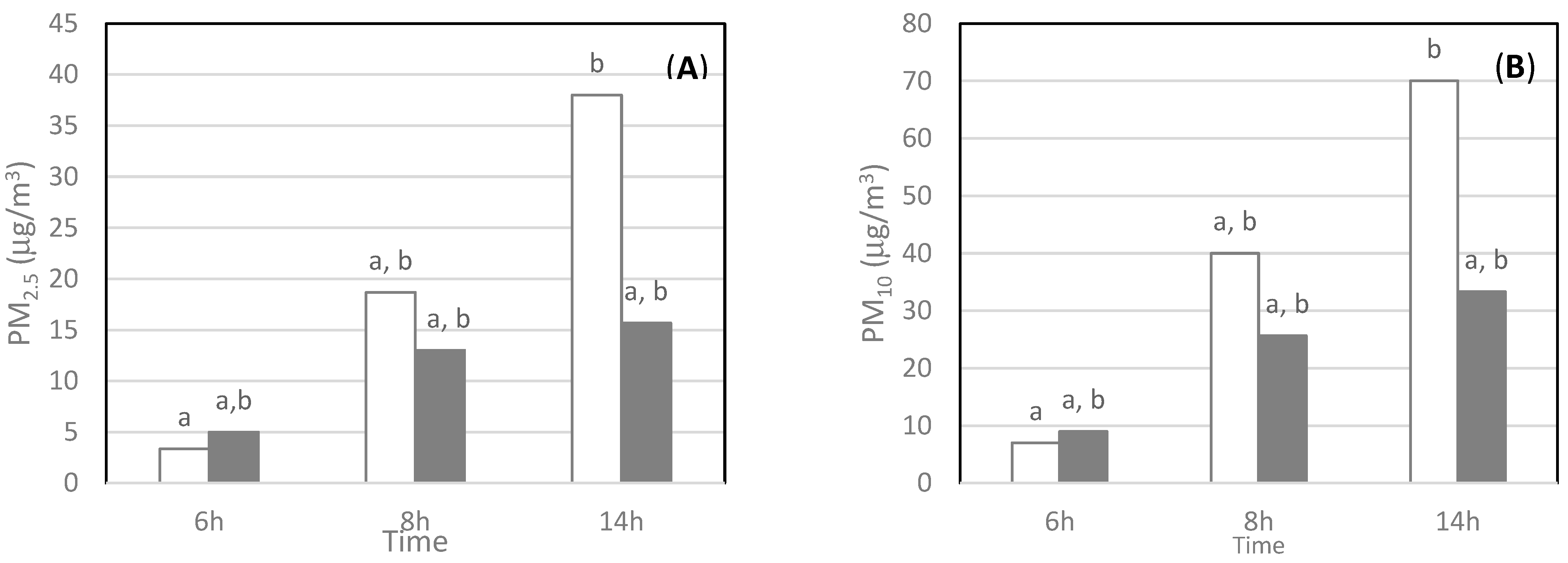
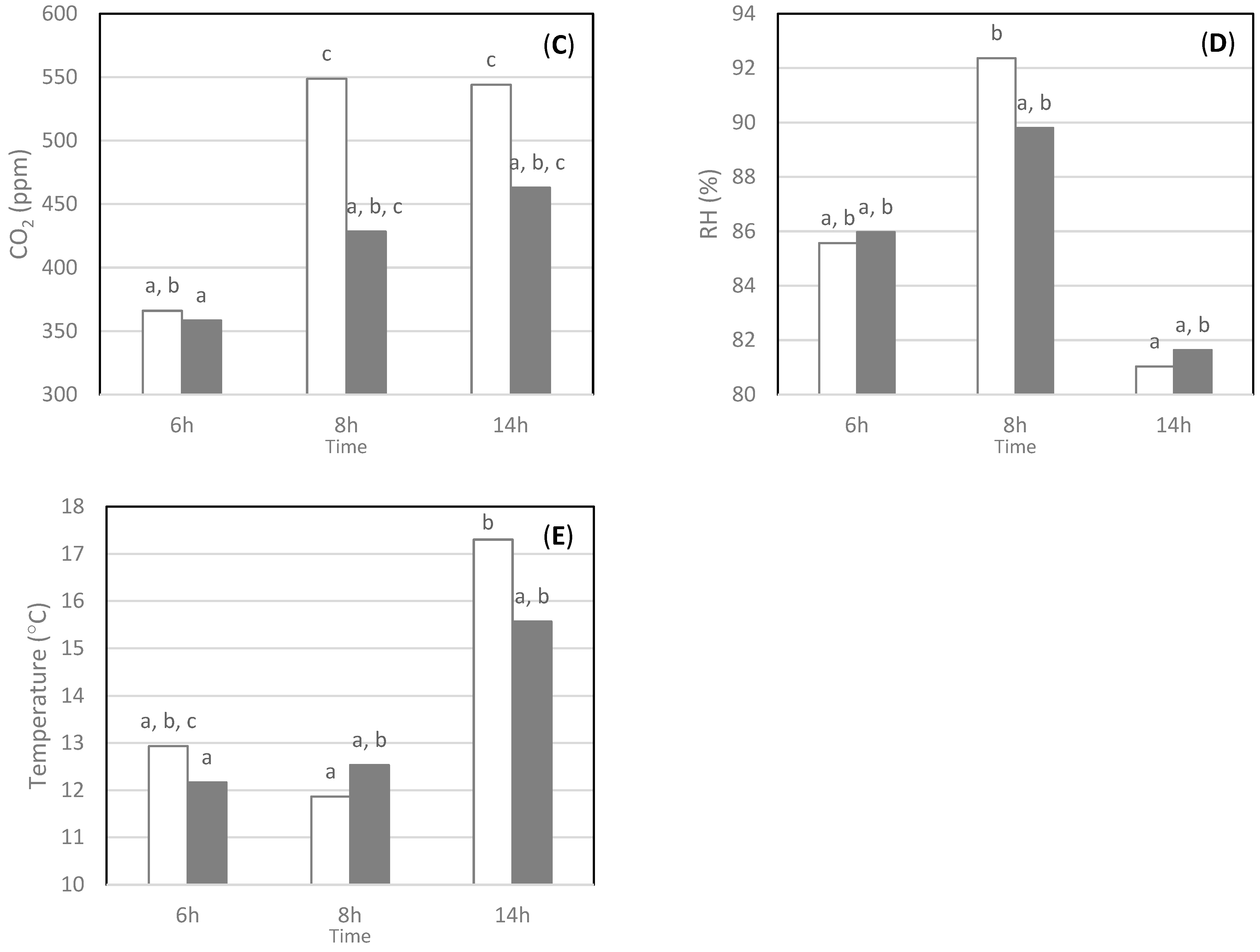
| Sites | Volume (m3) | Average Occupancy | Maximum Occupancy | Electrical Equipment and Ventilation |
|---|---|---|---|---|
| A—Administrative office | 71 | 1 | 1 | 1 computer, 1 air conditioner |
| B—Administrative office | 98 | 1–2 | 5 | 5 computers, 1 air conditioner with HEPA filters |
| C—Administrative office | 173 | 5–8 | 22 | 22 computers, 4 air conditioners |
| D—Canteen | 149 | 4–30 | 54 | 14 kitchen appliances, 6 air conditioners |
| PM2.5 (μg/m3) | PM10 (μg/m3) | CO2 (ppm) | T (°C) | RH (%) | |
|---|---|---|---|---|---|
| Mean | 80 | 112 | 368 | 17.9 | 79.2 |
| SD | 180 | 240 | 55 | 5.5 | 14.4 |
| Median | 7 | 13 | 357 | 15.6 | 79.1 |
| Min | 1 | 2 | 296 | 11.0 | 52.5 |
| Max | 686 | 913 | 669 | 26.2 | 99.9 |
| Bacteria at 25 °C (CFU/m3) | Bacteria at 37 °C (CFU/m3) | Fungi (CFU/m3) | |
|---|---|---|---|
| Mean | 1654 | 422 | 1044 |
| SD | 1150 | 347 | 497 |
| Median | 1568 | 338 | 1028 |
| Min | 333 | 48 | 425 |
| Max | 3208 | 1 150 | 1872 |
| PM2.5 (μg/m3) | PM10 (μg/m3) | CO2 (ppm) | T (°C) | RH (%) | |
|---|---|---|---|---|---|
| Cabin | 22 | 42 | 491 | 16.6 | 83.1 |
| Ramp | 17 | 35 | 416 | 15.9 | 81.3 |
| I/O Cabin | 0.131 | 0.177 | 1.41 | 0.959 | 1.08 |
| I/O Ramp | 0.0990 | 0.149 | 1.19 | 0.920 | 1.05 |
| Bacteria at 25 °C (CFU/m3) | Bacteria at 37 °C (CFU/m3) | Fungi (CFU/m3) | |
|---|---|---|---|
| Cabin | >21,470 | 2565 | 1488 |
| Ramp | >14,684 | >1397 | 2412 |
| I/O Cabin | >14.56 * | 3.96 * | 1.44 |
| I/O Ramp | >9.96 * | >2.16 * | 2.34 * |
| I/O Ratio | ||||||
|---|---|---|---|---|---|---|
| PM2.5 | PM10 | CO2 | T | RH | ||
| Cabin | autumn | 0.0578 | 0.0764 | 1.36 | 0.90 | 1.16 |
| winter | 1.45 | 1.52 | 1.44 | 1.08 | 1.01 | |
| Ramp | autumn | 0.0491 | 0.0802 | 1.09 | 0.880 | 1.11 |
| winter | 0.981 | 1.09 | 1.22 | 1.09 | 0.964 | |
| Bacteria at 25 °C (CFU/m3) | Bacteria at 37 °C (CFU/m3) | Fungi (CFU/m3) | ||||
|---|---|---|---|---|---|---|
| Autumn | Winter | Autumn | Winter | Autumn | Winter | |
| Cabin | 1475 | 26,469 | 408 | 3021 | 675 | 2300 |
| Ramp | 5567 | 16,964 | >2650 | 1084 | 2025 | 2800 |
| I/O Cabin | 0.681 | 20.3 * | 0.584 | 4.61 * | 1.51 | 1.73 |
| I/O Ramp | 2.57 * | 13.0 * | >3.80 * | 1.65 | 4.54 * | 2.10 * |
| Bacteria at 25 °C (CFU/m3) | Bacteria at 37 °C (CFU/m3) | |||||
|---|---|---|---|---|---|---|
| 6 h | 8 h | 14 h | 6 h | 8 h | 14 h | |
| Cabin | 1540 | >53,000 | 24,000 | 108 | 3567 | 1740 |
| Ramp | 3395 | >53,000 | 10,700 | 47 | 2533 | 698 |
| I/O Cabin | 0.604 | >16.52 * | 24.74 * | 0.806 | 7.46 * | 5.16 * |
| I/O Ramp | 1.33 | >16.52 * | 11.03 * | 0.351 | 5.30 * | 2.07 * |
| PM2.5 (μg/m3) | PM10 (μg/m3) | CO2 (ppm) | T (°C) | RH (%) | |
|---|---|---|---|---|---|
| Site A | 90 | 118 | 561 | 19.4 | 81.5 |
| Site B | 4 | 8 | 1194 | 19.3 | 81.8 |
| Site C | 6 | 11 | 1338 | 19.3 | 82.1 |
| Site D | 16 | 34 | 826 | 21.8 | 78.4 |
| I/O site A | 17.18 * | 12.4 * | 1.38 | 1.03 | 0.954 |
| I/O site B | 0.731 | 0.753 | 3.22 * | 0.915 | 1.13 |
| I/O site C | 0.917 | 1.08 | 3.61 * | 0.92 | 1.13 |
| I/O site D | 2.74 * | 3.20 * | 2.23 * | 1.04 | 1.08 |
| Bacteria at 37 °C (CFU/m3) | Fungi (CFU/m3) | |
|---|---|---|
| Site A | 295 | 373 |
| Site B | 464 | 723 |
| Site C | 424 | 851 |
| Site D | 370 | 1604 |
| I/O site A | 2.33 * | 0.307 |
| I/O site B | 1.21 | 0.848 |
| I/O site C | 1.10 | 1.00 |
| I/O site D | 0.964 | 1.88 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. PM2.5 | -- | ||||||
| 2. PM10 | 0.980 * | ||||||
| 3. CO2 | −0.091 | −0.125 | |||||
| 4. RH | 0.199 | 0.161 | 0.429 | ||||
| 5. T | 0.309 | 0.350 | 0.007 | −0.675 * | |||
| 6. Bact. at 25 °C | 0.017 | 0.033 | 0.683 ** | −0.033 | 0.383 | ||
| 7. Bact. at 37 °C | 0.232 | 0.200 | 0.236 | 0.355 | −0.036 | 0.633 *** | |
| 8. Fungi | 0.204 | 0.096 | 0.359 | 0.731 ** | −0.587 | 0.314 | 0.299 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. PM2.5 | -- | ||||||
| 2. PM10 | 0.986 * | ||||||
| 3. CO2 | 0.112 | 0.165 | |||||
| 4. RH | −0.114 | −0.157 | 0.213 | ||||
| 5. T | 0.052 | 0.126 | 0.058 | −0.399 *** | |||
| 6. Bacteria at 37 °C | 0.190 | 0.240 | 0.361 *** | −0.252 | 0.495 ** | ||
| 7. Fungi | 0.482 ** | 0.510 ** | 0.446 ** | 0.150 | 0.292 | 0.491 ** | |
| 8. Workers number | 0.185 | 0.192 | 0.696 * | 0.292 | 0.065 | 0.305 | 0.512 ** |
| Concentration (CFU/m3) | Disinfection Efficiency (%) | ||
|---|---|---|---|
| BEFORE | AFTER | ||
| Bacteria at 25 °C | 11,875 | Surface: 421 | 96.5 |
| Bacteria at 37 °C | 464 | Surface: 58 | 87.6 |
| Air: 47 | 89.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manuel, C.D.; Samardjieva, K. Culturable Bioaerosols Assessment in a Waste-Sorting Plant and UV-C Decontamination. Sustainability 2024, 16, 4299. https://doi.org/10.3390/su16104299
Manuel CD, Samardjieva K. Culturable Bioaerosols Assessment in a Waste-Sorting Plant and UV-C Decontamination. Sustainability. 2024; 16(10):4299. https://doi.org/10.3390/su16104299
Chicago/Turabian StyleManuel, Candida Duarte, and Kalina Samardjieva. 2024. "Culturable Bioaerosols Assessment in a Waste-Sorting Plant and UV-C Decontamination" Sustainability 16, no. 10: 4299. https://doi.org/10.3390/su16104299
APA StyleManuel, C. D., & Samardjieva, K. (2024). Culturable Bioaerosols Assessment in a Waste-Sorting Plant and UV-C Decontamination. Sustainability, 16(10), 4299. https://doi.org/10.3390/su16104299






