Ultrasound Application in Potato Cultivation: Potential for Enhanced Yield and Sustainable Agriculture
Abstract
:1. Introduction
- –
- Cellular Permeability: Ultrasound can increase the permeability of plant cell membranes, facilitating the absorption of nutrients and water [2].
- –
- –
- Pathogen Control: Ultrasound has been explored for its antimicrobial effects, potentially helping to control pathogens and pests in crops [17].
- –
- –
- –
- Research on ultrasonic treatment for increasing plant yield and improving quality has been conducted on various crops, including but not limited to:
- –
- Rice: Studies have explored the application of ultrasound in rice cultivation to enhance seed germination and grain yield [1].
- –
- Wheat: Ultrasound has been investigated for its effects on wheat seed germination and plant growth [1].
- –
- Soybeans: Research has examined the impact of ultrasound on soybean seed germination and plant development [2].
- –
- Subjecting potato tubers to ultrasound treatment prior to planting is anticipated to elevate the yield per unit area and produce a yield structure with a favorable proportion of marketable fractions.
- Physical treatments on potato tubers before planting will significantly influence the structure, overall yield, and commercial yield of the tubers of the studied cultivars, in contrast to the null hypothesis, which assumes that physical treatments will not have a significant impact on these characteristics.
2. Material and Methods
2.1. Field Research
2.2. Characteristics of Cultivars
- ‘Denar’: Very early, light-yellow skin, slightly yellow flesh, round-oval tubers, versatile for salads, taste rating: 7/9.
- ‘Lord’: Very early, light-yellow skin, slightly yellow flesh, round-oval tubers, versatile for salads, taste rating: 7/9.
- ‘Owacja’: Early, Light-yellow skin, slightly yellow flesh, round-oval tubers, versatile (B-BC), suitable for general consumption, taste rating: 7/9.
- ‘Vineta’: Early, light-yellow skin, yellow flesh, round tubers, suitable for general consumption, taste rating: 7/9.
- ‘Satina’: Medium-early, light-yellow skin, yellow flesh, round-oval tubers, suitable for general consumption, taste rating: 7.5/9.
- ‘Tajfun’: Medium-early, light-yellow skin, yellow flesh, oval tubers, slightly floury, versatile for culinary uses, taste rating: 7/9.
- ‘Syrena’: Medium-late, light-yellow skin, yellow flesh, oval tubers, ideal for various culinary uses, taste rating: 7/9.
- ‘Zagłoba’: Medium late, light-yellow skin, yellow flesh, round-oval tubers, suitable for general consumption, taste rating: 6.5/9.
2.3. Cultivation Practices
- –
- before planting, the potato tubers were sonicated in an aquatic environment at 18 °C for the time specified in the pilot studies (10 min),
- –
- control object in which the tubers were soaked in distilled water for 10 min before planting.
2.4. Growing Conditions
2.5. Soil Sampling
2.6. Soil Minerals Analysis
2.7. Meteorological Conditions
2.8. Statistical Calculations
3. Results
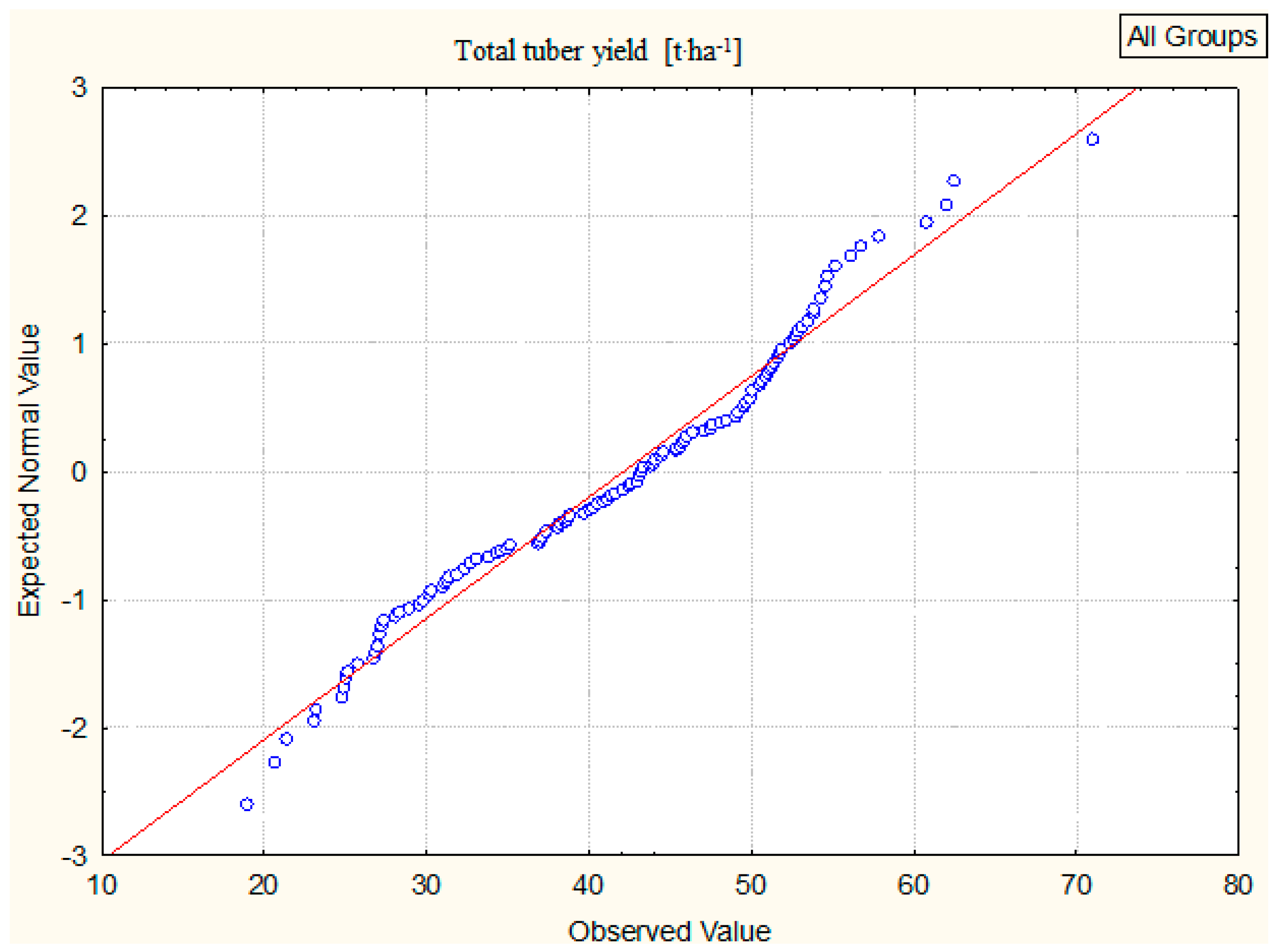
3.1. Total Tuber Yield
3.2. Tuber Mass Structure
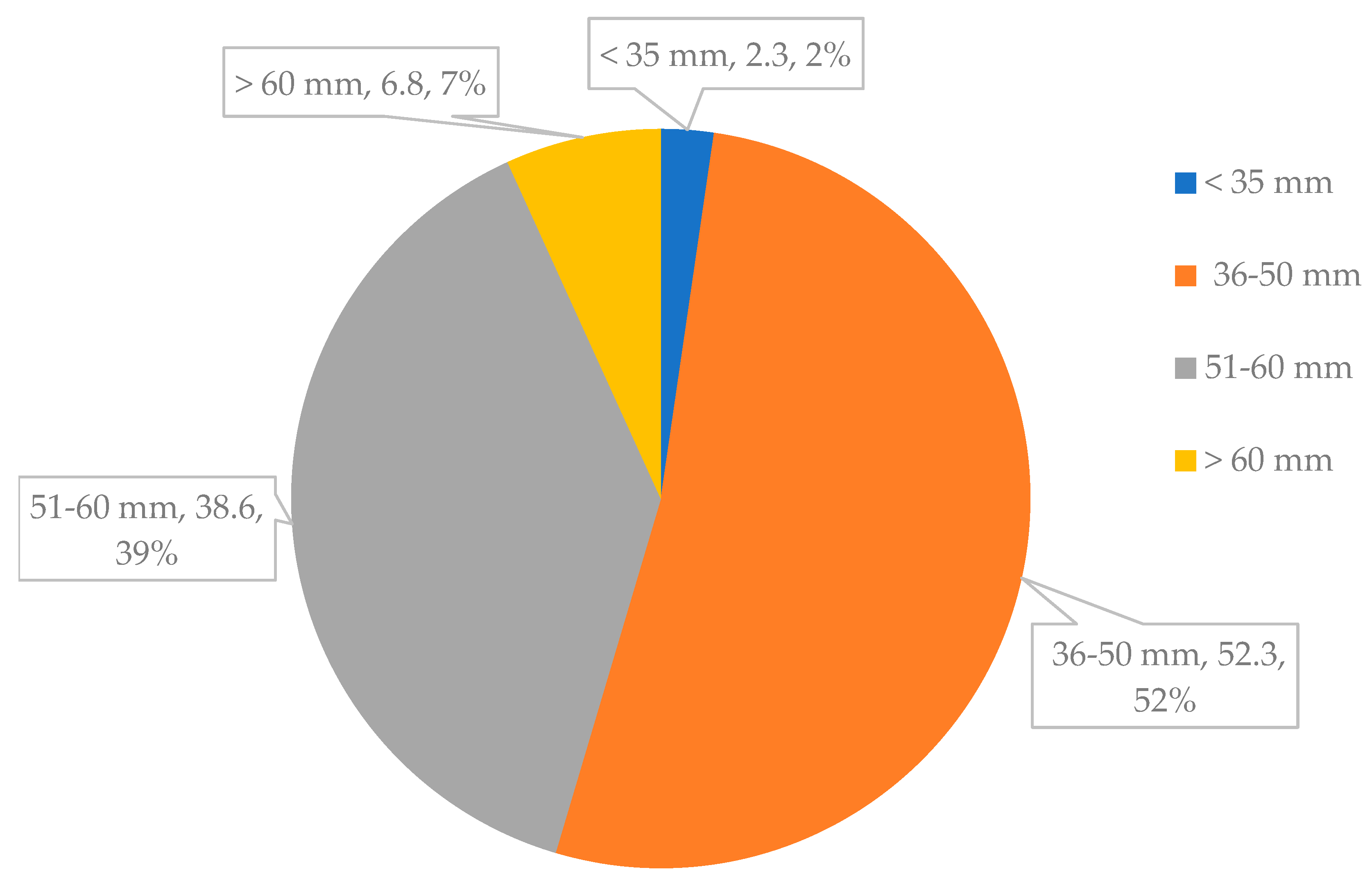
3.3. Percentage of Marketable Yield
3.4. Marketable Tuber Yield
3.5. Descriptive Statistics of Dependent and Independent Variables
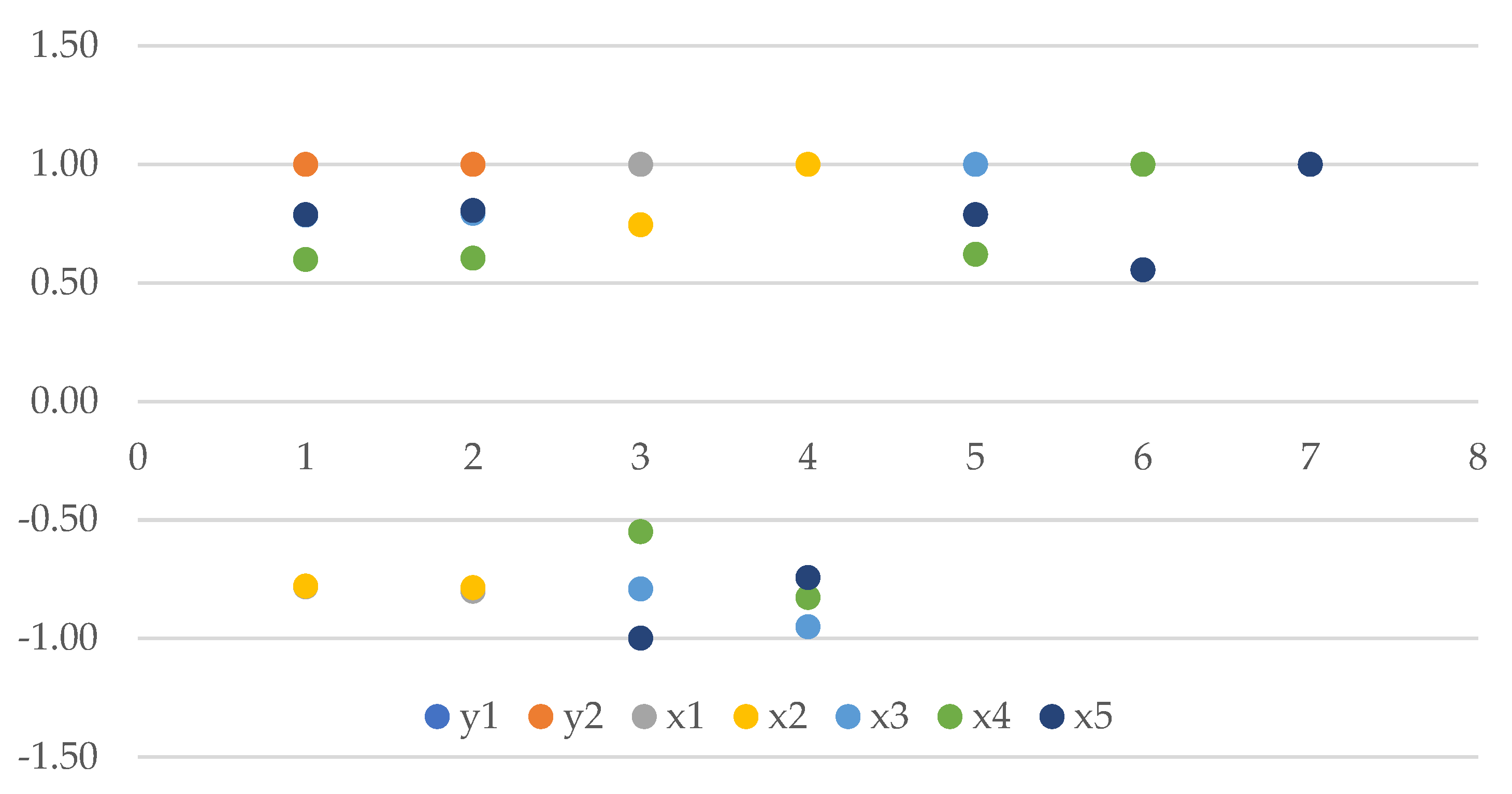
3.6. Dependence of Total and Marketable Yield on Its Structure
- –
- y1 (total yield) and y2 (commercial yield) have a perfect positive correlation of 1.00. This means that they move together in the same direction, indicating that as total yield increases, so does commercial yield, and vice versa;
- –
- The variable x1 (percentage of tuber mass with a diameter <36 mm) exhibits a robust negative correlation of r = −0.78 with y1 (total yield). An increase in the percentage of small tubers is associated with a decrease in total yield;
- –
- x2 (percentage of the mass of tubers with a diameter 36–50 mm) and y1 (total yield) also have a strong negative correlation of r = −0.78. This suggests that an increase in the percentage of tubers in the 36–50 mm range is associated with a decrease in total yield;
- –
- x3 (percentage of the mass of tubers with a diameter 51–60 mm) and y1 (total yield) have a strong positive correlation of r = 0.79. This means that an increase in the percentage of tubers in the 51–60 mm range is associated with an increase in total yield;
- –
- x4 (percentage of the mass of tubers with a diameter >60 mm) and y1 (total yield) have a moderate positive correlation of r = 0.60. This indicates that as the percentage of large tubers (>60 mm) increases, the total yield tends to increase as well;
- –
- x5 (percentage of commercial yield) and y2 (commercial yield) have a perfect positive correlation of r = 1.00. This shows that they are directly proportional, with increases in one corresponding to increases in the other (Figure 5).
4. Discussion
- –
- Resource Efficiency: Research on ultrasound allows for the efficient use of resources such as water and fertilizers. By increasing the permeability of plant cell membranes, ultrasound helps with the better absorption of nutrients, potentially reducing the amount of fertilizers used and minimizing excessive water consumption in the cultivation process [2,4].
- –
- Reduction in Chemical Usage: The application of ultrasound in combating pathogens and pests can contribute to reducing the need for chemical plant protection products. The reduced use of pesticides (only three fungicide treatments were applied against Phytophthora infestans, providing full protection against this pathogen, and the Colorado potato beetle was controlled 2–3 times in the L2–L3 stage of the pest) promotes biodiversity conservation and minimizes negative impacts on the environment [20,21].
- –
- –
- –
- Optimization of Manufacturing Processes: In ultrasound research, there is potential for optimizing food manufacturing processes. These processes may include storage, packaging, or food processing, impacting sustainable food production by reducing losses and eliminating unhealthy substances [15,16,17].
- –
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB-US | Air-based Ultrasound |
| PE-US | Piezoelectric Ultrasound |
| US | Ultrasonic |
| SFW | Shoot Fresh Weight |
| DEG | Differentially Expressed Gene |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| WRKY | Transcription Factor |
| RFT | Reactive Oxygen Species |
| MYB | Proto-Oncogene, Transcription Factor |
| DREBP | Dehydration Responsive Element Binding Protein |
| G × E | Genotype × Environment Interaction |
References
- FAO. Food Security and Nutrition. In Statistical Yearbook. World Food and Agriculture; FAO: Rome, Italy, 2020; Chapter 3; Available online: https://www.fao.org/3/cb1329en/online/cb1329en.html (accessed on 29 October 2023).
- Śliwiński, A. Ultrasound and Their Applications; WNT: Warszawa, Poland, 2001; p. 426. [Google Scholar]
- Wu, M.; Wu, J. In-vitro investigations on ultrasonic control of water chestnut. J. Aquat. Plant Manag. 2007, 45, 76–83. [Google Scholar]
- Kaczmarski, Ł.K.; Lewicki, P.P. Application of Ultrasound in Food Processing; SIGMA-NOT: Ronkonkoma, NY, USA, 2005; Volume 59, pp. 34–36. [Google Scholar]
- Miłowska, K. Ultrasound—Mechanisms of action and application in sonodynamic therapy. Postępy Hig. Med. Dośw. 2007, 61, 338–349. [Google Scholar]
- Dobrowolska, D.; Kolasa, J. Acoustics, Ultrasounds, Mechanical Vibrations, Field Guide; GUM: Warszawa, Poland, 2020; pp. 1–76. Available online: https://gum.gov.pl/ftp/pdf (accessed on 29 October 2023).
- Gagliano, M.; Mancuso, S.; Robert, D. Towards understanding plant bioacoustics. Trends Plant Sci. 2012, 17, 323–325. [Google Scholar] [CrossRef]
- Gagliano, M. Green symphonies: A call for studies on acoustic communication in plants. Behav. Ecol. 2013, 24, 789–796. [Google Scholar] [CrossRef]
- Dobránszki, J.; Hidvégi, N.; Gulyás, A.; Tóth, B.; Teixeira da Silva, J.A. Abiotic stress elements in in vitro potato (Solanum tuberosum L.) exposed to air-based and liquid-based ultrasound: A comparative transcriptomic assessment. Prog. Biophys. Mol. Biol. 2020, 158, 47–56. [Google Scholar] [CrossRef]
- Chowdhury, E.K.; Lim, H.-S.; Bae, H. Update on the effects of sound wave on plants. Research in Plant 2014. Disease 2014, 20, 1–7. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J. Sonication, and ultrasound: Impact on plant growth and development. Plant Cell Tissue Organ Cult 2014, 117, 131–143. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.-K.; Kim, J.Y.; Jeong, M.J.; Ryu, C.M. Beyond chemical triggers: Evidence for sound361 evoked physiological reactions in plants. Front. Plant Sci. 2018, 9, 25. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Hidvégi, N.; Gulyás, A.; Tóth, B.; Dobránszki, J. Transcriptomic Response of In vitro Potato (Solanum tuberosum L.) to Piezoelectric Ultrasound. Plant Mol. Biol. Rep. 2020, 38, 404–418. [Google Scholar] [CrossRef]
- Dolatowski, Z.J.; Stadnik, J.; Stasiak, D. Application of ultrasound in food technology. Acta Sci. Pol. Technol. Aliment. 2007, 6, 89–99. [Google Scholar]
- Witrowa-Rajchert, D. Ultrasound in designed food production. Przemysł Spożywczy 2012, 66, 41–43. [Google Scholar]
- Kentish, S.; Feng, H. Applications of Power Ultrasound in Food Processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Konopacka, D.; Plocharski, W.; Siucinska, K. The possibilities of ultrasound application in the fruit and vegetable processing industry. Przemysł Ferment. I Owocowo-Warzywny 2015, 59, 16–21. [Google Scholar]
- Fijałkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The influence of ultrasound pre-treatment on drying kinetics and the color and betaines content in beetroot. Zesz. Probl. Postępów Nauk Rol. 2015, 581, 11–20. [Google Scholar]
- Maksymiec, M.; Frąckiewicz, A.; Stasiak, D.M. Ultrasonic assisted production of food. In Review of Selected Issues in the Field of the Food Industry; Szala, M., Kropiwiec, K., Eds.; Wydawnictwo Naukowe TYGIEL: Lublin, Poland, 2016; pp. 199–213. [Google Scholar]
- Sawicka, B.; Noaema, A.H.; Barbaś, P.; Skiba, D.; Bienia, B. Environmentally friendly methods used to improve the quality seed potatoes. In Contemporary Research on the State of the Environment; Chwil, M., Skoczylas, M.M., Eds.; Wydawnictwo Uniwersytetu Przyrodniczego w Lublinie: Lublin, Poland, 2019; pp. 109–123. [Google Scholar] [CrossRef]
- Sawicka, B.; Pszczółkowski, P.; DanilČenko, H.; Jarienė, E. Impact of ultrasounds on physicochemical characteristics of potato tubers. Agron. Sci. 2020, 75, 85–104. [Google Scholar] [CrossRef]
- Sawicka, B. Physical method of stimulation of seed—A new technology in the production of potatoes. Pol. Ziemn. 2013, 1, 13–18. [Google Scholar]
- Safari, M.; Ghanati, F.; Behmanesh, M.; Hajnorouzi, A.; Nahidian, B.; Mina, G. Enhancement of antioxidant enzymes activity and expression of CAT and PAL genes in hazel (Corylus avellana L.) cells in response to low-intensity ultrasound. Acta Physiol Plant 2013, 35, 2847–2855. [Google Scholar] [CrossRef]
- Dobránszki, J.; Hidvégi, N.; Gulyás, A.; Teixeira da Silva, J.A. mRNA transcription profile of potato (Solanum tuberosum L.) exposed to ultrasound during different stages of in vitro plantlet development. Plant Mol Biol 2019, 100, 511–525. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Dobránszki, J.; Asboth, G.; Homoki, D.; Bíró-Molnár, P.; Teixeira da Silva, J.A.; Remenyik, J. Ultrasonication of in vitro potato single node explants: Activation and recovery of antioxidant defence system and growth responses. Plant Physiol Biochem 2017, 121, 153–160. [Google Scholar] [CrossRef]
- Hidvégi, N.; Gulyás, A.; Dobránszki, J.; Teixeira da Silva, J.A. Mining sequences with similarity to XTH genes in the Solanum tuberosum L. transcriptome: Introductory step for identifying homologous XTH genes. Plant Signal Behav. 2020, 15, 1797294. [Google Scholar] [CrossRef]
- Laschimke, R.; Burger, M.; Vallen, H. Acoustic emission analysis and experiments with physical model systems reveal a peculiar nature of the xylem tension. J. Plant Physiol. 2006, 163, 996–1007. [Google Scholar] [CrossRef]
- Perel’man, M.E.; Rubinstein, G.M. Ultrasound vibrations of plant cells membranes: Water lift in trees, electrical phenomena. arXiv 2006, arXiv:physics/0611133. [Google Scholar]
- Wu, J.Y.; Ge, X.C. Oxidative burst, jasmonic acid biosynthesis, and Taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnol. Bioeng. 2004, 85, 714–721. [Google Scholar] [CrossRef]
- Sawicka, B.; Dolatowski, Z. The variability of tuber flesh darkening of new potato cultivars under the action of ultrasound. Zesz. Probl. Postępów Nauk Rol. 2007, 517, 639–649. [Google Scholar]
- Natural Resources Conservation Service Soils. Soil Texture Calculator. United States Department of Agriculture. Soil Classification. 2021. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/?cid=nrcs142p2_054167 (accessed on 29 October 2023).
- Lenartowicz, T. Descriptive List of Agricultural Cultivars; COBORU Publishing House: Słupia Wielka, Poland, 2017; p. 38. ISSN 1641-7003. (In Polish) [Google Scholar]
- Polish Standard PN-IEC 60038; Standard Voltages IEC. Polish Committee for Standardization: Warsaw, Poland, 1999.
- Duer, I.; Fotyma, M.; Madej, A. Code of Good Agricultural Practice; Ministry of Agriculture and Rural Development: Warsaw, Poland, 2004; p. 93.
- Roztropowicz, S.; Czerko, Z.; Głuska, A.; Goliszewski, W.; Gruczek, T.; Lis, B.; Lutomirska, B.; Nowacki, W.; Rykaczewska, K.; Sowa-Niedziałkowska, G.; et al. Methodic of Observation, Measurements and Sample Take in Agricultural Experiments with Potato; Plant Breeding Acclimatization Institute, Section: Jadwisin, Poland, 1999; p. 50. [Google Scholar]
- Bleinholder, H.L.; Weber, E.; Buhr, L.; Feller, C.; Hess, M.; Wicke, H.; Meier, U.; Van Den Boom, T.; Lancashire, P.; Buhr, D.L.; et al. Compendium of growth stage identification keys for mono- and dicotyledonous plants. In BBCH Monograph, 2nd ed.; Federal Center for Agricultural and Forest Biological Research Uwe Meier, Ed.; EPPO: Luxembourg, 2001. [Google Scholar]
- Agriculture and Food Economy in Poland. Collective Work Edited by the Institute of Agricultural and Food Economics; Ministry of Agriculture and Rural Development: Warsaw, Poland, 2015; p. 155.
- Lenartowicz, T. Potato. Methodology of Economic Value Analysis of Cultivars (WGO); COBORU: Słupia Wielka, Poland, 2013; p. 34.
- Polish Standard PN–R–04031:1997P; Chemical and Agricultural Analysis of Soil. Sampling. Polish Committee for Standardization: Warsaw, Poland, 1997.
- Polish Standard PN-ISO 10390:1997P; Soil Quality—Determination of pH. Polish Committee for Standardization: Warsaw, Poland, 1997; p. 15.
- Barančíková, G.; Makovníková, J. Comparison of two methods for determining organic carbon in soil. Pol. J. Soil Sci. 2015, 48, 47–56. [Google Scholar] [CrossRef]
- Mocek, A. Soil Science; State Scientific Publisher: Warsaw, Poland, 2015; p. 571. [Google Scholar]
- Polish Standard PN-R-04023: 1996P; Chemical and Agricultural Analysis of Soil. Determination of Available Phosphorus Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 1996.
- Polish Standard PN-R-04022: 1996 +Az1: 2002P; Chemical and Agricultural Analysis of Soil. Determination of Available Potassium Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Polish Standard PN-R-04020 1994 + Az1: 2004P; Chemical and Agricultural Analysis of Soil. Polish Committee for Standardization: Warsaw, Poland, 2004.
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1971−2010). Fragm. Agron. 2014, 31, 74–87. [Google Scholar]
- SAS Institute Inc. SAS/STAT®9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- StatSoft. Electronic Textbook of Statistics PL, Krakow. 2006. Available online: http://www.statsoft.pl/textbook/stathome.html (accessed on 29 October 2023).
- Mishra, D.; Shekhar, S.; Singh, D.; Chakraborty, S.; Chakraborty, N. Heat shock proteins and abiotic stress tolerance in plants. In Regulation of Heat Shock Protein Responses, Heat; Asea, A., Kaur, P., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 41–69. [Google Scholar] [CrossRef]
- Wang, B.C.; Zhou, J.; Wang, Y.C.; Zhu, L.C.; Teixeira da Silva, J.A. Physical stress, and plant growth. In Floriculture, Ornamental, and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J., Ed.; Global Science Books, Ltd.: London, UK, 2006; Volume II, Chapter 7; pp. 68–85. [Google Scholar]
- Rokhina, E.V.; Lens, P.; Virkutyte, J. Low-frequency ultrasound in biotechnology: State of the art. Trends Biotechnol. 2009, 27, 298–306. [Google Scholar] [CrossRef]
- Nowacki, W. Polish potato sector during the COVID-19 pandemic. Pol. Potato 2020, 2. Available online: http://ziemniak-bonin.pl/ziemniak-polski-02-2020/ (accessed on 29 October 2023).
- Nowacki, W. Opportunities and threats of potato market in Poland: Association of Agricultural Economists. Sci. Ann. 2018, 17, 169–175. [Google Scholar]
- Arvanitoyannis, I.S.; Mavromatis, A.G.; Vaitsi, O.; Korkovelos, A.; Golia, E. Effect of genotype and geographical origin on potato properties (physical and sensory) for authenticity purposes. J. Agric. Sci. 2012, 4, 63–74. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. Phenotypic variability of the yield and structure of mid-early potato cultivars. Acta Sci. Pol. Agric. 2017, 16, 147–161. [Google Scholar]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Degradation during application of ultrasound in food processing A review. Food Control 2013, 31, 593–606. [Google Scholar] [CrossRef]


| 2015 | 2016 | 2017 |
|---|---|---|
| Fungicides | ||
| Infinito 687.5 SC (propamocarb hydrochloride + fluopicolide) (625 + 62.5)—1.6 dm3·ha−1 Ridomil Gold MZ 67.8 (mancozeb + metalaxyl) (640 + 38.3)—2 kg·ha−1 Infinito 687.5 SC (propamocarb hydrochloride + fluopicolide) (625 + 62.5)—1.6 dm3·ha−1 | Acrobat MZ 69 WG (dimethomorph + mancozeb) (90 +600)—2.0 kg·ha−1 Infinito 687.5 SC (propamocarb hydrochloride + fluopicolide) (625+62.5)—1.6 dm3·ha−1 Acrobat MZ 69 WG (dimethomorph + mancozeb) (90 + 600)—2.0 kg·ha−1 | Acrobat MZ 69 WG (dimethomorph + mancozeb) (90 + 600)—2.0 kg·ha−1 Infinito 687.5 SC (propamocarb hydrochloride + fluopicolide) (625 + 62.5)—1.6 dm3·ha−1 Acrobat MZ 69 WG (dimethomorph + mancozeb) (90 + 600)—2.0 kg·ha−1 |
| Insecticides | ||
| Apacz 50 WG (clothianidin 500)—0.04 kg·ha−1 Proteus OD 110 (thiacloprid + deltamethrin) (100 + 10)—0.4 dm3·ha−1 | Actara 25 WG (thiamethoxam 250)—0.08 kg·ha−1 Nuprid 200 SC (imidacloprid 200)—0.15 dm3·ha−1 Apacz 50 WG (clothianidin 500)—0.04 kg·ha−1 | Actara 25 WG (thiamethoxam 250)—0.08 kg·ha−1 Apacz 50 WG (clothianidin 500)—0.04 kg·ha−1 Proteus OD 110 (thiacloprid + deltamethrin) (100 + 10)—0.4 dm3·ha−1 |
| Year | Composition of Granulometric Fractions [%] | Soil Classification | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Loam | ||||||||
| mm | ||||||||||
| 2.0–1.0 | 1.0–0.5 | 0.5–0.25 | 0.25–0.10 | 0.10–0.05 | 0.05–0.02 | 0.02–0.005 | 0.005–0.002 | <0.002 | ||
| 2015 | 0.10 | 16.58 | 29.56 | 12.05 | 8.61 | 16.02 | 11.17 | 3.30 | 2.61 | Sandy loam |
| 2016 | 0.98 | 17.86 | 28.27 | 11.75 | 8.33 | 15.40 | 11.16 | 3.56 | 2.69 | Sandy loam |
| 2017 | 0.71 | 15.09 | 25.39 | 13.59 | 12.05 | 18.48 | 10.27 | 2.37 | 2.05 | Sandy loam |
| Mean | 0.60 | 16.51 | 27.74 | 12.50 | 9.66 | 16.63 | 10.87 | 3.08 | 2.45 | |
| Year of Research | Macronutrients Content [mg·kg−1 Soil] | Humus Content [g·kg−1] | pH [KCL] | Micronutrients Content [mg·kg−1 Soil] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Mg | Cu | Mn | Zn | Fe | B | |||
| 2015 | 89.0 | 109.0 | 78.0 | 0.94 | 5.9 | 7.51 | 318 | 40.1 | 3760 | 7.24 |
| 2016 | 83.0 | 91.0 | 70.0 | 1.06 | 5.8 | 4.92 | 337 | 56.7 | 3925 | 5.28 |
| 2017 | 106.0 | 98.0 | 63.0 | 1.03 | 6.6 | 8.99 | 166 | 41.1 | 3600 | 6.04 |
| Mean | 93.0 | 99.0 | 70.0 | 1.02 | - | 7.02 | 274 | 46.0 | 3762 | 6.17 |
| Year | Month | Sum of Rainfall [mm] | Air Temperature [°C] | Hydrothermal Coefficient of Sielianinov * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Decade of Month | Month | Decade of Month | Mean | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | |||||
| 2015 | April | 14.6 | 5.9 | 41.3 | 61.8 | 5.4 | 8.6 | 12.4 | 8.8 | 2.3 |
| May | 23.4 | 13.9 | 83.0 | 120.3 | 12.6 | 12.0 | 13.7 | 12.8 | 3.0 | |
| June | 5.4 | 16.5 | 24.8 | 46.7 | 17.7 | 16.3 | 16.1 | 16.7 | 0.9 | |
| July | 10.5 | 21.6 | 13.1 | 45.2 | 19.6 | 18.7 | 19.9 | 19.4 | 0.8 | |
| August | 0.4 | 0 | 5.7 | 6.1 | 23.4 | 20.6 | 20.3 | 21.4 | 0.1 | |
| September | 32.4 | 32.6 | 65.2 | 130.2 | 16.0 | 17.7 | 12.8 | 15.5 | 2.8 | |
| Total | 410.3 | |||||||||
| 2016 | April | 11.5 | 22.2 | 13.4 | 47.1 | 10.9 | 10.1 | 9.0 | 10.0 | 1.6 |
| May | 4.9 | 2.8 | 38.6 | 46.3 | 14.4 | 17.8 | 12.9 | 15.3 | 1.0 | |
| June | 10.1 | 43.2 | 34.0 | 87.3 | 16.6 | 17.5 | 23.0 | 19.1 | 1.5 | |
| July | 22.4 | 30.8 | 60.9 | 114.1 | 19.5 | 20.1 | 21.9 | 20.5 | 1.8 | |
| August | 22.8 | 17.7 | 0.5 | 41.0 | 20.7 | 17.1 | 20.4 | 19.5 | 0.7 | |
| September | 7.6 | 0.1 | 4.1 | 11.8 | 19.5 | 15.5 | 11.5 | 15.5 | 0.3 | |
| Total | 347.6 | |||||||||
| 2017 | April | 6.4 | 7.2 | 38.2 | 51.8 | 10.6 | 6.8 | 6.9 | 8.1 | 2.1 |
| May | 45.1 | 1.3 | 19.1 | 65.5 | 10.5 | 13.0 | 17.4 | 13.7 | 1.5 | |
| June | 1.9 | 9.2 | 12.0 | 23.1 | 16.6 | 17.7 | 20.7 | 18.3 | 0.4 | |
| July | 10.1 | 80.9 | 41.0 | 132.0 | 17.9 | 19.0 | 21.0 | 19.4 | 2.2 | |
| August | 0.4 | 24.4 | 2.2 | 27.0 | 22.8 | 21.3 | 17.1 | 20.3 | 0.4 | |
| September | 38.7 | 35.9 | 8.7 | 83.3 | 16.3 | 15.3 | 12.8 | 14.8 | 1.9 | |
| Total | 382.7 | |||||||||
| Cultivars | Managements’ Practices Cultivation | Years | Mean | |||
|---|---|---|---|---|---|---|
| Control Object | Ultrasounds | 2015 | 2016 | 2017 | ||
| ‘Denar’ | 43.69 a * | 43.47a | 28.59 a | 54.20 bc | 47.96 c | 43.58 ab |
| ‘Lord’ | 39.30 a | 43.11a | 29.57 a | 49.94 a | 44.11 b | 41.21 a |
| ‘Owacja’ | 37.27a | 40.14 a | 31.56 ab | 47.32 a | 37.24 a | 38.71 a |
| ‘Vineta’ | 37.53 a | 40.50 a | 27.43 a | 48.04 a | 41.56 b | 39.01 a |
| ‘Satina’ | 40.80 a | 45.51 a | 36.78 b | 48.09 a | 44.60 b | 43.16 ab |
| ‘Tajfun’ | 39.10 a | 39.82 a | 28.82 a | 50.36 b | 39.20 a | 39.46 a |
| ‘Syrena’ | 43.33 a | 45.57a | 36.67 b | 51.71 b | 44.98 b | 44.45 c |
| ‘Zagłoba’ | 46.30 a | 47.96 a | 34.07 ab | 60.14 c | 47.18 c | 47.13 c |
| LSDp0.05 | ns ** | 11.9 | 5.7 | |||
| Mean | 40.91 a | 43.26 b | 31.69 a | 51.23 c | 43.35 b | 42.09 |
| LSDp0.05 | 1.8 | 2.7 | ||||
| Factors of the Experiment | Tuber Fraction Share [%] | ||||
|---|---|---|---|---|---|
| <35 mm | 36–50 mm | 51–60 mm | >60 mm | ||
| Management practices in cultivation | Control object | 2.3 a * | 51.7 a | 38.8 a | 7.2 a |
| Ultrasounds | 2.2 a | 53.0 a | 38.3 a | 6.5 a | |
| LSDp0.05 | ns ** | ns | ns | ns | |
| Cultivars | ‘Denar’ | 2.3 b | 52.3 bc | 40.3 bc | 4.8 ba |
| ‘Lord’ | 2.2 b | 51.3 bc | 39.8 bc | 6.9 bc | |
| ‘Owacja’ | 3.1 d | 55.5 c | 35.8 b | 5.4 bca | |
| ‘Vineta’ | 2.1b | 47.6 b | 42.3 c | 8.0 c | |
| ‘Satina’ | 2.2 b | 54.0 c | 38.7 bc | 5.2 bc | |
| ‘Tajfun’ | 2.5 cd | 64.8 d | 30.1 a | 2.6 a | |
| ‘Syrena’ | 1.7 a | 54.2 c | 38.2 bc | 6.0 bc | |
| ‘Zagłoba’ | 1.9 ab | 38.9 a | 43.3 c | 15.6 d | |
| LSDp0.05 | 0.6 | 6.2 | 5.3 | 3.2 | |
| Years | 2015 | 3.5 c | 69.4 c | 25.4 a | 1.8 a |
| 2016 | 0.9 a | 37.3 a | 50.6 c | 11.2 c | |
| 2017 | 2.4 b | 50.3 b | 39.7 b | 7.5 b | |
| Mean | 2.3 | 52.3 | 38.6 | 6.8 | |
| LSDp0.05 | 0.3 | 2.9 | 2.5 | 1.5 | |
| Cultivars | Managements Cultivation Practices | Years | Mean | |||
|---|---|---|---|---|---|---|
| Control Object | Ultrasounds | 2015 | 2016 | 2017 | ||
| ‘Denar’ | 97.5 b * | 97.8 a | 96.1 ab | 99.0 a | 98.0 bc | 97.7 b |
| ‘Lord’ | 97.7 b | 97.8 a | 96.8 b | 99.1 ab | 97.4 ab | 97.8 bc |
| ‘Owacja’ | 96.6 a | 97.3 a | 95.2 a | 98.9 a | 96.7 a | 96.9 a |
| ‘Vineta’ | 97.9 b | 97.9 b | 96.4 ab | 99.4 c | 97.9 b | 97.9 bc |
| ‘Satina’ | 97.7 b | 98.0 b | 96.8 b | 99.0 a | 97.8 b | 97.9 bc |
| ‘Tajfun’ | 97.7 b | 97.2 a | 96.4 ab | 98.9 a | 97.1 ab | 97.5 bc |
| ‘Syrena’ | 98.3 c | 98.4 c | 97.4 c | 99.3 ab | 98.3 c | 98.3 c |
| ‘Zagłoba’ | 98.0 b | 98.1 b | 96.9 b | 99.4 c | 97.9 b | 98.1 b |
| LSDp0.05 | 0.9 | 1.2 | 0.6 | |||
| Mean | 97.7 a | 97.8 a | 96.5 a | 99.1 c | 97.6 b | 97.8 |
| LSDp0.05 | 0.2 | 0.3 | ||||
| Cultivars | Managements Cultivation Practices | Years | Mean | |||
|---|---|---|---|---|---|---|
| Control Object | Ultrasounds | 2015 | 2016 | 2017 | ||
| ‘Denar’ | 42.76 bc * | 42.66 abc | 27.47a | 53.66 b | 47.00 bc | 42.7 b |
| ‘Lord’ | 38.49 ab | 42.24 ab | 28.62 a | 49.49 a | 42.99 b | 40.37 ab |
| ‘Owacja’ | 36.14 a | 39.11 a | 30.06 ab | 46.80 a | 36.00 a | 37.62 a |
| ‘Vineta’ | 36.84 a | 39.77a | 26.46 a | 47.72 a | 40.71 ab | 38.30 a |
| ‘Satina’ | 39.29 ab | 44.63 bc | 35.59 bc | 47.62 a | 43.61 b | 42.28 b |
| ‘Tajfun’ | 38.31 a | 38.79 a | 27.79 ab | 49.82 ab | 38.05 a | 38.55 a |
| ‘Syrena’ | 42.65 bc | 44.86 bc | 35.70 bc | 51.37 ab | 44.20 b | 43.76 bc |
| ‘Zagłoba’ | 45.46 c | 47.20 c | 33.02 b | 59.79 bc | 46.17bc | 46.33 c |
| LSDp0.05 | 4.2 | 6.3 | 2.1 | |||
| Mean | 40.07 a | 42.41 b | 30.59 a | 50.79 c | 42.34 b | 41.24 |
| Specification | y1 | y2 | x1 | x2 | x3 | x4 | x5 |
|---|---|---|---|---|---|---|---|
| Mean | 42.32 | 41.48 | 2.24 | 52.33 | 38.56 | 6.83 | 97.75 |
| Standard Error | 0.87 | 0.89 | 0.11 | 1.38 | 1.05 | 0.55 | 0.11 |
| Median | 43.53 | 42.49 | 2.15 | 51.83 | 40.56 | 5.89 | 97.80 |
| Standard deviations | 10.49 | 10.67 | 1.29 | 16.56 | 12.64 | 6.58 | 1.29 |
| Kurtosis | −0.63 | −0.66 | −0.28 | −1.07 | −0.94 | 1.23 | −0.31 |
| Skewness | −0.19 | −0.16 | 0.50 | 0.05 | −0.30 | 1.21 | −0.50 |
| Range | 52.07 | 52.60 | 5.95 | 63.29 | 50.22 | 30.26 | 5.90 |
| Minimum | 18.87 | 17.90 | 0.27 | 22.73 | 10.75 | 0.00 | 93.80 |
| Maximum | 70.93 | 70.51 | 6.22 | 86.02 | 60.97 | 30.26 | 99.70 |
| CV (%) | 24.78 | 25.71 | 57.39 | 31.64 | 32.79 | 96.23 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pszczółkowski, P.; Sawicka, B. Ultrasound Application in Potato Cultivation: Potential for Enhanced Yield and Sustainable Agriculture. Sustainability 2024, 16, 108. https://doi.org/10.3390/su16010108
Pszczółkowski P, Sawicka B. Ultrasound Application in Potato Cultivation: Potential for Enhanced Yield and Sustainable Agriculture. Sustainability. 2024; 16(1):108. https://doi.org/10.3390/su16010108
Chicago/Turabian StylePszczółkowski, Piotr, and Barbara Sawicka. 2024. "Ultrasound Application in Potato Cultivation: Potential for Enhanced Yield and Sustainable Agriculture" Sustainability 16, no. 1: 108. https://doi.org/10.3390/su16010108
APA StylePszczółkowski, P., & Sawicka, B. (2024). Ultrasound Application in Potato Cultivation: Potential for Enhanced Yield and Sustainable Agriculture. Sustainability, 16(1), 108. https://doi.org/10.3390/su16010108







