Evaluation of Life Cycle Assessment of Jatropha Biodiesel Processed by Esterification of Thai Domestic Rare Earth Oxide Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. LCA Goal, Scope, and Functional Unit
2.2. Data Sources and Assumptions
- This study applied the energy allocation method, which allocates the environmental impacts of all products and co-products based on the energy value of the Jatropha plantation (the fruit and the co-products), the Jatropha oil production (Jatropha oil and seed cake) [12,38], and the hydrolysis of the Jatropha oil (oleic acid and glycerol) [28,33].
- Energy consumption and CO2 emissions from manual labor are not included in this analysis.
3. Results and Discussion
3.1. Life Cycle Inventory (LCI) Analysis
3.1.1. Jatropha curcas Linnaeus (Jatropha) Plantation
3.1.2. Jatropha Oil Extraction
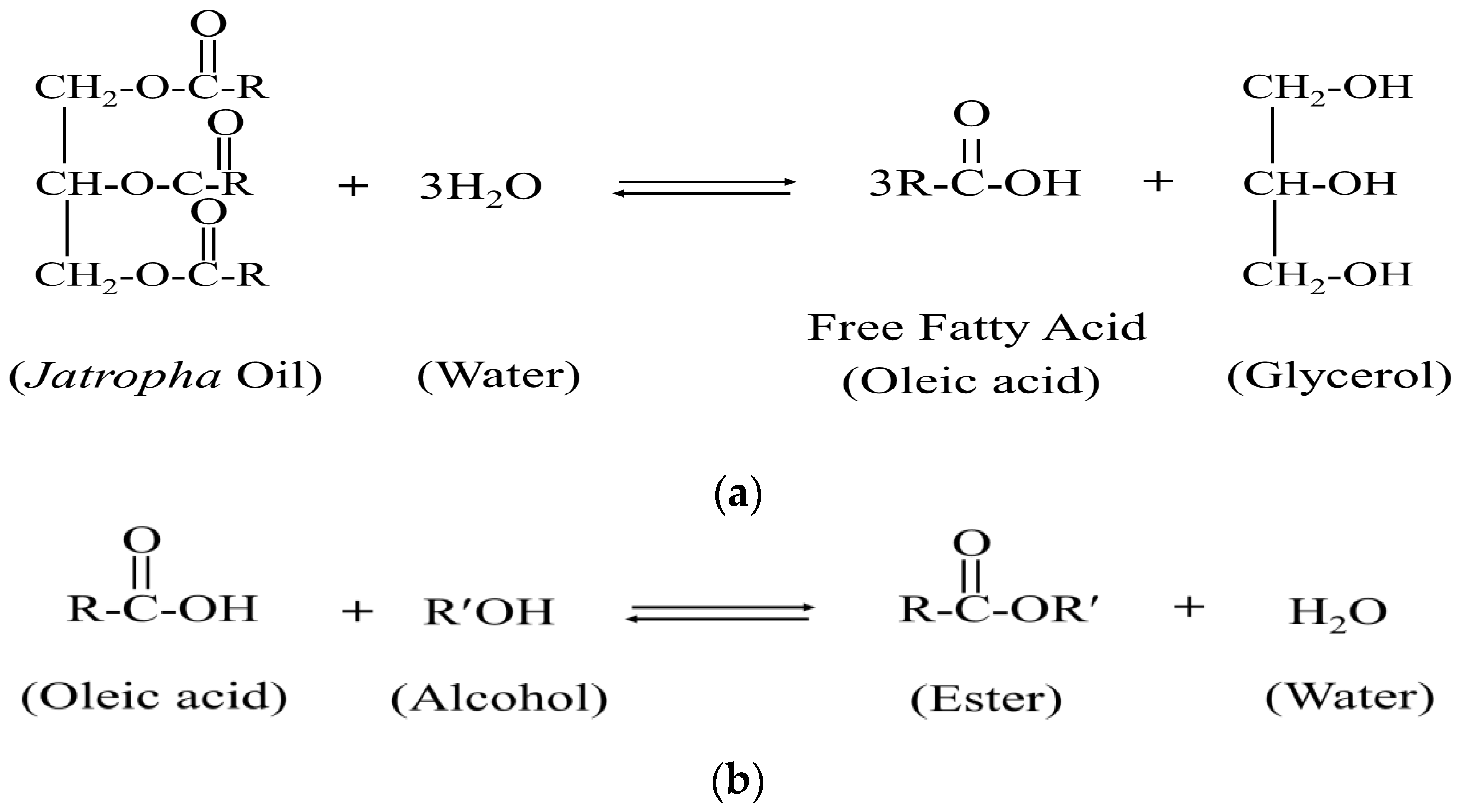
3.1.3. Hydrolysis of Jatropha Oil Triglycerides
3.1.4. Esterification of Fatty Acid
3.1.5. Transportation
3.1.6. Use in Vehicle Engines
3.2. Life Cycle Impact Assessment (LCIA) Analysis
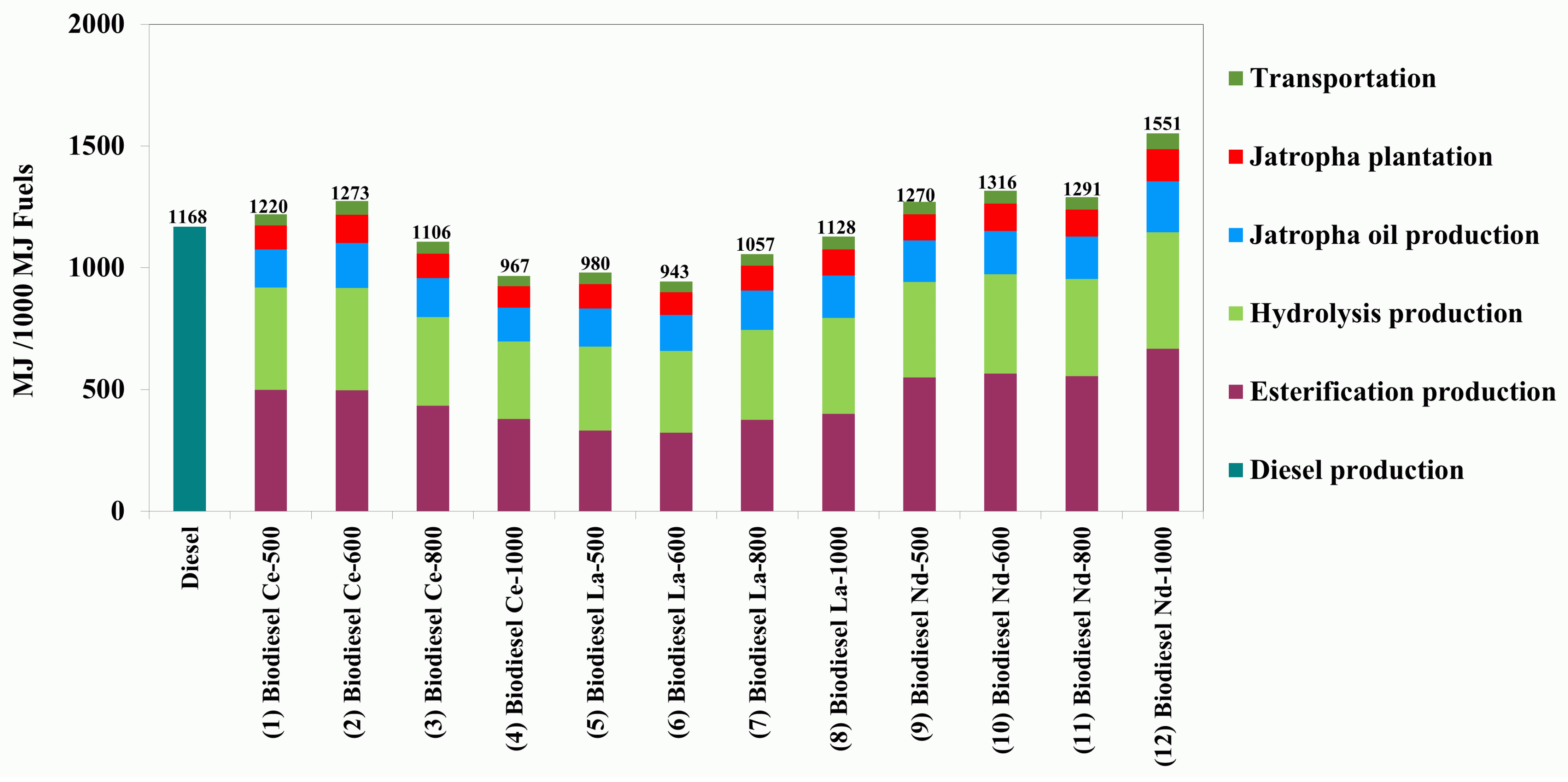
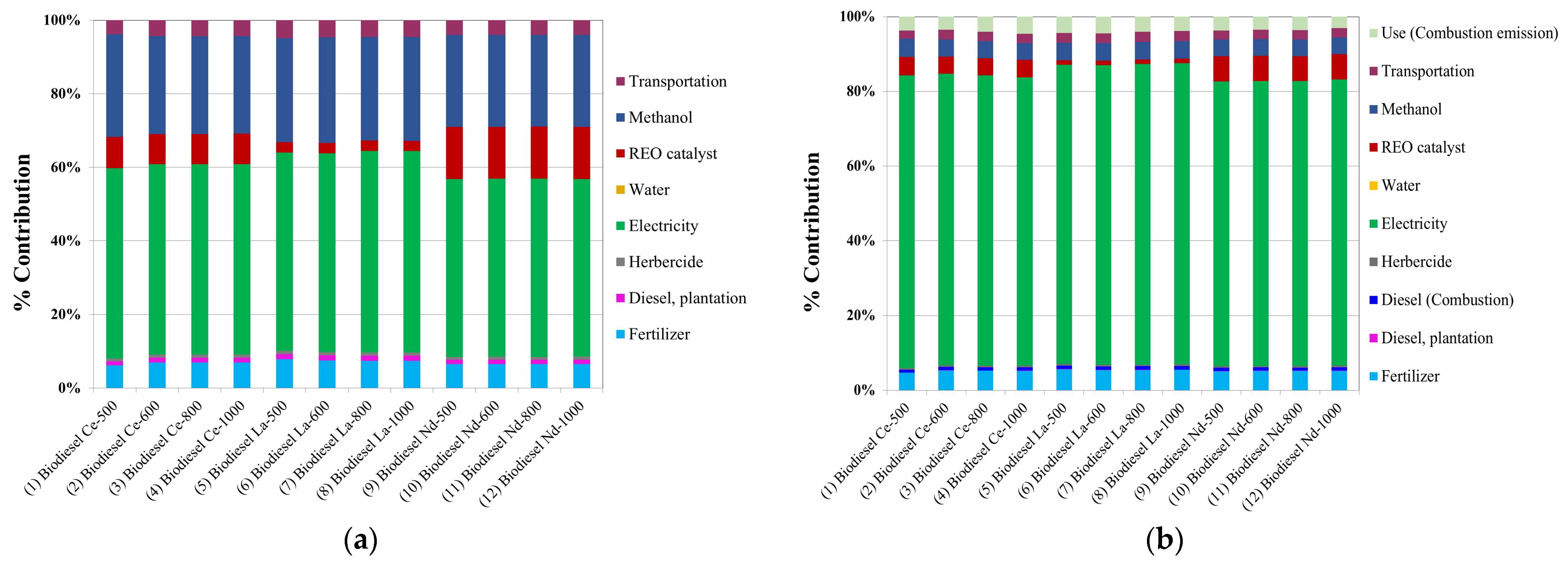
3.2.1. Energy Efficiency
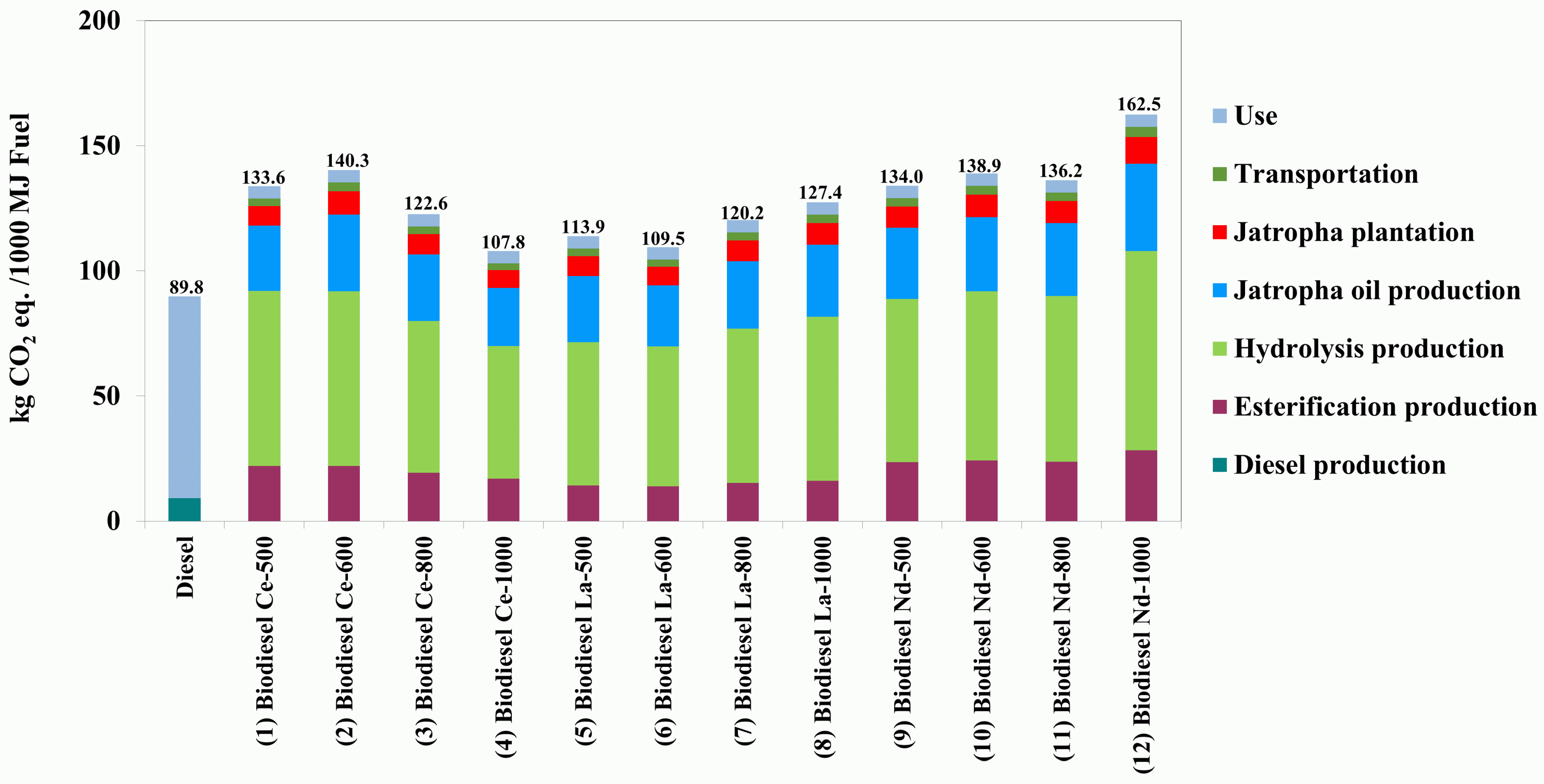
3.2.2. Global Warming Impact Assessment
3.2.3. Process Analysis: Improvement of Waste Heat Recovery
3.2.4. Impacts on Land Use Change
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Economic Forum. Analysis: Global CO2 Emissions from Fossil Fuels Hits Record High in 2022. 2023. Available online: https://www.weforum.org/agenda/2022/11/global-co2-emissions-fossil-fuels-hit-record-2022/ (accessed on 11 November 2022).
- Intergovernmental Panel on Climate Change (IPCC). AR5 Climate Change 2014: Mitigation of Climate Change. 2014. Available online: https://www.ipcc.ch/report/ar5/wg3/ (accessed on 11 November 2022).
- Economic Research Institute for Asean and East Asia (ERIA). Study of Renewable Energy Potential and Its Effective Usage in East Asia Summit Countries. 2017. Available online: https://www.eria.org/uploads/media/ERIA-RPR-2017_09.pdf (accessed on 1 July 2022).
- Department of Alternative Energy Development and Efficiency (DEDE). The Alternative Energy Development Plan: AEDP2015. 2015. Available online: www.eppo.go.th/images/POLICY/ENG/AEDP2015ENG.pdf (accessed on 1 July 2022).
- Rattanaphra, D.; Kingkam, W.; Nuchdang, S.; Suwanmane, U. Characterization of rare earths obtained from monazite concentrate processing and their application in esterification for biodiesel production. Energy Rep. 2022, 8, 6914–6928. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. Bioenergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T. Chapter 15—Production of biodiesel using palm oil. In Biofuels: Alternative Feedstocks and Conversion Processes; Elsevier: Amsterdam, The Netherlands, 2011; pp. 353–374. [Google Scholar]
- Romero, R.; Martínez, S.L.; Natividad, R. Biodiesel Production by Using Heterogeneous Catalysts. 2011. Available online: https://www.intechopen.com/chapters/17583 (accessed on 11 November 2022).
- Mattos, F.C.G.; de Souza, J.A.S.; Cotrim, A.B.A.; Macedo, J.L.; Dias, J.A.; Dias, S.C.L.; Ghesti, G.F. Lewis acid/surfactant rare earth trisdodecylsulfate catalysts for biodiesel production from waste cooking oil. Appl. Catal. A Gen. 2012, 423–424, 1–6. [Google Scholar] [CrossRef]
- Ratthanaphra, D.; Suwanmanee, U. Uncertainty analysis of environmental sustainability of biodiesel production using Thai domestic rare earth oxide solid catalysts. Sustain. Prod. Consump. 2019, 18, 237–249. [Google Scholar] [CrossRef]
- Gnanaserkhar, S.; Asikin-Mijan, N.; AbdulKareem-Alsultan, G.; Seenivasagam, S.; Izham, S.M.; Taufiq-Yap, Y.H. Biodiesel production via simultaneous esterification and transesterification of chicken fat oil by mesoporous sulfated Ce supported activated carbon. Biomass Bioenergy 2020, 141, 105714. [Google Scholar]
- Prueksakorn, K.; Gheewala, S.H.; Malakul, P.; Bonnet, S. Energy analysis of Jatropha plantation systems for biodiesel production in Thailand. Energy Sustain. Dev. 2010, 14, 1–5. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pragya, N.; Sahoo, P.K. Life cycle assessment of small scale high input Jatropha biodiesel production in India. Appl. Energy 2011, 88, 4831–4839. [Google Scholar] [CrossRef]
- Basili, M.; Fontini, F. Biofuel from Jatropha curcas: Environmental sustainability and option value. Ecol. Econ. 2012, 78, 1–8. [Google Scholar] [CrossRef]
- Kumar, S.; Chaube, A.; Jain, S.K. Sustainability issues for promotion of Jatropha biodiesel in Indian scenario: A review. Renew. Sustain. Energy Rev. 2012, 16, 1089–1098. [Google Scholar] [CrossRef]
- Ajayebi, A.; Gnansounou, E.; Raman, J.K. Comparative life cycle assessment of biodiesel from algae and Jatropha: A case study of India. Bioresour. Technol. 2013, 150, 429–437. [Google Scholar] [CrossRef]
- Obligado, A.B.; Demafelis, R.X.; Matanguihan, A.E.D.; Villancio, V.T.; Magadia, R.V.; Manaig, L.M.A., Jr. Carbon emission inventory of a commercial scale Jatropha (Jatropha curcas L.) biodiesel processing plant. J. Environ. Sci. Manag. 2017, 20–32. [Google Scholar] [CrossRef]
- Giraldi-Diaz, M.R.; Medina-Salas, L.D.; Castillo-Gonzalez, E.; Cruz-Benavides, M.D. Environmental impact associated with the supply chain and production of biodiesel from Jatropha curcas L. through life cycle analysis. Sustainability 2018, 10, 1451. [Google Scholar] [CrossRef]
- ISO 14040:2020/And 14044:2020; Environmental Management—Life Cycle Assessment—Requirements and Guide-Lines—Amendment 2. International Organization for Standardization: Geneva, Switzerland, 2020.
- Guinee, J.B.; Heijungs, R.; Huppes, G.; Zamagni, A.; Masoni, P.; Buonamici, R.; Ekvall, T.; Rydberg, T. Life cycle assessment past, present, and future. Environ. Sci. Technol. 2011, 45, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Algren, M.; Fisher, W.; Landis, A.E. Chapter 8—Machine learning in life cycle assessment. In Data Science Applied to Sustainability Analysis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 167–190. [Google Scholar]
- Xia, X.; Li, P. A review of the life cycle assessment of electric vehicles: Considering the influence of batteries. Sci. Total Environ. 2022, 814, 152870. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, P.; Xia, Z.; Wu, R.; Cheng, Y. Life cycle carbon footprint of electric vehicles in different countries: A review. Sep. Purif. Technol. 2022, 301, 122063. [Google Scholar] [CrossRef]
- Permpool, N.; Gheewala, S.H. Environmental and energy assessment of alternative fuels for diesel in Thailand. J. Clean. Prod. 2017, 142, 1176–1182. [Google Scholar] [CrossRef]
- Suwanmanee, U.; Bangjang, T.; Kaewchada, A.; Jaree, A.A. Greenhouse gas emissions and energy assessment of modified diesohol using cashew nut shell liquid and biodiesel as additives. Sustain. Prod. Consum. 2020, 24, 232–253. [Google Scholar] [CrossRef]
- Ecoinvent. Swiss Center for Life Cycle Inventories. 2006. Available online: https://ecoinvent.org/ (accessed on 11 November 2022).
- Tobin, J. Life Cycle Assessment of the Production of Biodiesel from Jatropha. Master’s Dissertation, School of Construction Management and Engineering, The University of Reading, Whiteknights, UK, 2005. [Google Scholar]
- Silalertruksa, T.; Bonnet, S.; Gheewala, H.S. Life cycle costing and externalities of palm oil biodiesel in Thailand. J. Clean. Prod. 2012, 28, 225–232. [Google Scholar] [CrossRef]
- Thailand Environment Institute (TEI). Final Report for the Project on Life Cycle Assessment for Asian Countries–Phases III; Thailand Environment Institute (TEI): Pak Kret, Thailand, 2003. [Google Scholar]
- Kittiyopas, D.; Ladawan, N.A. Development and Extension Prospect: Emphasison Jatropha curcas Linn; Department of Agricultural Extension: Bangkok, Thailand, 2006. [Google Scholar]
- Opeshaw, K. A reiew of Jatropha curcus: An oil plant of unfulfilled promise. Biomass Energy 2000, 19, 1–15. [Google Scholar] [CrossRef]
- Fassinou, W.F.; Sako, A.; Fofana, A.; Koua, K.B.; Toure, S. Fatty acids composition as a means to estimate the high heating value (HHV). Energy 2010, 35, 4949–4954. [Google Scholar] [CrossRef]
- Demirbas, A. Calculation of higher heating values of fatty acids. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2693–2697. [Google Scholar] [CrossRef]
- Becker, K.; Francis, G. Bio-Diesel from Jatropha Plantations on Degraded Land, Department of Aquaculture System and Animal Nutrition; University of Hohenheim: Stuttgart, Germany, 2000. [Google Scholar]
- Thailand Greenhouse Gas Management Organization Public Organization (TGO). Emission Factor for Carbon Footprint of Product. 2021. Available online: http://thaicarbonlabel.tgo.or.th/admin/uploadfiles/emission/ts_b934985782.pdf (accessed on 1 July 2022).
- Thailand Greenhouse Gas Management Organization Public Organization (TGO). Emission Factor for Carbon Footprint of Organization. 2022. Available online: http://thaicarbonlabel.tgo.or.th/admin/uploadfiles/emission/ts_578cd2cb78.pdf (accessed on 1 July 2022).
- Intergovernmental Panel on Climate Change (IPCC). IPCC Guidelines for National Greenhouse Gas Inventories: Agriculture, Forestry and Other Land Use; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006. [Google Scholar]
- Prueksakorn, K.; Gheewala, S.H. Full chain energy analysis of biodiesel from Jatropha curcas L. in Thailand. Environ. Sci. Technol. 2008, 42, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Phuenduang, S.; Chatsirisook, P.; Chatsirisook, P.; Simasatitkul, L.; Arpornwichanop, A. Heat-integrated reactive distillation for biodiesel production from Jatropha oil. Comput. Aided Chem. Eng. 2012, 31, 250–254. [Google Scholar]
- US Department of Energy. Thermoelectric Conversion of Waste Heat to Electricity in an IC Engine Powered Vehicle. 2011. Available online: https://www.osti.gov/servlets/purl/1045212 (accessed on 1 July 2022).
- Chen, W.; Huang, Z.; Chua, K.J. Sustainable energy recovery from thermal processes: A review. Energy Sustain. Soc. 2022, 12, 1–25. [Google Scholar] [CrossRef]
- Electricity Generating Authority of Thailand (EGAT). Laboratory Measurements Conducted by EGA. Bangkok, Thailand. 2006. Available online: https://www.egat.co.th/ (accessed on 11 November 2022).
- EN 14103:2020; Fat and Oil Derivatives-Fatty Acid Methyl Esters (FAME). Determination of Ester and Linolenic Acid Methyl Ester Contents: Brussels, Belgium, 2020.
- Sebos, I. Fossil fraction of CO2 emissions of biofuels. Carbon Manag. 2022, 13, 154–163. [Google Scholar] [CrossRef]
- USDA. Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus. 1998. Available online: https://www.nrel.gov/docs/legosti/fy98/24089.pdf (accessed on 31 March 2020).
- Varadharajan, A.; Venkateswaran, W.S.; Banerjee, R. Energy Analysis of Biodiesel from Jatropha; World Renewable Energy Congress (Energy WREC): Brighton, UK, 2008; pp. 147–152. [Google Scholar]
- Garcia, C.A.; Fuentes, A.; Hennecke, A.; Riegelhaupt, E.; Manzini, F.; Masera, O. Life-cycle greenhouse gas emissions and energy balance of sugarcane ethanol production in Mexico. Appl. Energy 2011, 88, 2088–2097. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Carvalho, J.L.N.; Cerri, C.E.P.; Nogueira, L.A.H.; Souza, G.M.; Cantarella, H. Land use and management effects on sustainable sugarcane-derived bioenergy. Land 2021, 10, 72. [Google Scholar] [CrossRef]
- Bailis, R.E.; Baka, J.F. Greenhouse gas emissions and land use change from Jatropha curcas-based jet fuel in Brazil. Environ. Sci. Technol. 2010, 44, 8684–8691. [Google Scholar] [CrossRef]
- Cheroennet, N.; Pongpinyopap, S.; Leejarkpai, T.; Suwanmanee, U. A tradeoff between carbon and water impacts in bio-based box production chains in Thailand: A case study on PS, PLAS, PLAS/starch, and PBS. J. Clean. Prod. 2017, 167, 987–1001. [Google Scholar] [CrossRef]


| Options | Abbreviations | Explanation |
|---|---|---|
| 1 | Biodiesel Ce-500 | Jatropha biodiesl-CeO2 at calcination temperature of 500 °C |
| 2 | Biodiesel Ce-600 | Jatropha biodiesl-CeO2 at calcination temperature of 600 °C |
| 3 | Biodiesel Ce-800 | Jatropha biodiesl-CeO2 at calcination temperature of 800 °C |
| 4 | Biodiesel Ce-1000 | Jatropha biodiesl-CeO2 at calcination temperature of 1000 °C |
| 5 | Biodiesel La-500 | Jatropha biodiesl-La2O3 at calcination temperature of 500 °C |
| 6 | Biodiesel La-600 | Jatropha biodiesl-La2O3 at calcination temperature of 600 °C |
| 7 | Biodiesel La-800 | Jatropha biodiesl-La2O3 at calcination temperature of 800 °C |
| 8 | Biodiesel La-1000 | Jatropha biodiesl-La2O3 at calcination temperature of 1000 °C |
| 9 | Biodiesel Nd-500 | Jatropha biodiesl-Nd2O3 at calcination temperature of 500 °C |
| 10 | Biodiesel Nd-600 | Jatropha biodiesl-Nd2O3 at calcination temperature of 600 °C |
| 11 | Biodiesel Nd-800 | Jatropha biodiesl-Nd2O3 at calcination temperature of 800 °C |
| 12 | Biodiesel Nd-1000 | Jatropha biodiesl-Nd2O3 at calcination temperature of 1000 °C |
| Subjects | Unit | Energy Factors |
|---|---|---|
| Fertilizer production | ||
| Fertilizer N | (MJ/kg) | 65.0 a |
| Fertilizer P | (MJ/kg) | 29.9 a |
| Fertilizer K | (MJ/kg) | 21.5 a |
| Herbicides production | ||
| Glyphosate | (MJ/kg) | 151.0 a |
| Paraquat | (MJ/kg) | 202.0 a |
| Utilities/Fuels | ||
| Water | (MJ/kg) | 0.006 a |
| Methanol | (MJ/kg) | 38.08 b |
| Diesel | (MJ/L) | 36.42 c |
| Electricity | (MJ/kWh) | 3.60 d |
| Products/Co-products | ||
| Wood and leaves (dry) | (MJ/kg) | 16.54–16.8 d,e |
| Fruit (dry coat) | (MJ/kg) | 11.10–13.07 f |
| Fruit (seed) | (MJ/kg) | 18.81–25.10 f |
| Seed cake (as fuel) | (MJ/kg) | 18.81–25.10 f |
| Jatropha oil (triolein) | (MJ/kg) | 39.03 g |
| Oleic acid | (MJ/kg) | 38.84 h |
| Glycerol | (MJ/kg) | 25.60 c |
| Jatropha biodiesel | (MJ/kg) | 37.30 i |
| Subjects | Unit | Emission Factors |
|---|---|---|
| Fertilizer production | ||
| Fertilizer N | (kg CO2 eq./kg N) | 6.700 j |
| Fertilizer P | (kg CO2 eq./kg P) | 1.800 j |
| Fertilizer K | (kg CO2 eq./kg K) | 1.290 j |
| Herbicides production | ||
| Glyphosate (g.l.) | (kg CO2 eq./kg g.l.) | 10.200 j |
| Paraquat (p.a.) | (kg CO2 eq./kg p.a.) | 7.600 j |
| Utilities/Fuels | ||
| Water | (kg CO2 eq./m3) | 0.795 k |
| Methanol (m.e.) | (kg CO2 eq./kg m.e.) | 0.721 k |
| Diesel | (kg CO2 eq./L fuel) | 0.296 k |
| Diesel (Combustion) | (kg CO2 eq./L fuel) | 2.741 l |
| Electricity | (kg CO2 eq./kWh) | 0.599 k |
| Catalysts | ||
| CeO2 | (kg CO2 eq./kg CeO2) | 4.860 m |
| La2O3 | (kg CO2 eq./kg La2O3) | 1.240 m |
| Nd2O3 | (kg CO2 eq./kg Nd2O3) | 7.120 m |
| Transportation | ||
| Pickup (4-wheel truck) | (kg CO2 eq./tkm) | 0.519 j |
| 10-wheel truck | (kg CO2 eq./tkm) | 0.216 j |
| Use phase | ||
| Biodiesel (Combustion) | (kg CO2 eq./L fuel) | 0.160 n |
| Diesel (Combustion) | (kg CO2 eq./L fuel) | 2.741 l |
| Parameters | Jatropha Biodiesl-CeO2 | Jatropha Biodiesl-La2O3 | Jatropha Biodiesl-Nd2O3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Options | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) |
| Materials/Chemicals | ||||||||||||
| Jatropha, kg | 58.611 | 69.269 | 60.112 | 52.459 | 59.567 | 55.348 | 61.093 | 64.945 | 63.968 | 67.004 | 65.607 | 78.899 |
| Jatropha oil, kg | 38.139 | 45.073 | 39.115 | 34.135 | 38.761 | 36.015 | 39.753 | 42.260 | 41.625 | 43.600 | 42.691 | 51.340 |
| Oleic acid, kg | 45.195 | 45.073 | 39.115 | 34.135 | 36.938 | 36.015 | 39.753 | 42.260 | 42.154 | 43.600 | 42.691 | 51.340 |
| Methanol, kg | 8.927 | 8.903 | 7.726 | 6.743 | 7.296 | 7.114 | 7.853 | 8.348 | 8.327 | 8.612 | 8.433 | 10.141 |
| Solid catalyst, kg | 1.356 | 1.352 | 1.173 | 1.024 | 1.108 | 1.080 | 1.193 | 1.268 | 1.265 | 1.308 | 1.281 | 1.540 |
| Utilities/Fuels, kWh | ||||||||||||
| n Electricity (Hydrolysis) | 43.338 | 51.218 | 44.448 | 38.788 | 44.045 | 40.925 | 45.173 | 48.021 | 47.299 | 49.544 | 48.510 | 58.339 |
| O Electricity, (Calcination) | 12.642 | 15.216 | 17.733 | 19.427 | 10.332 | 12.158 | 18.022 | 24.051 | 11.791 | 14.719 | 19.354 | 29.219 |
| p Electricity, (Esterification) | 10.528 | 10.500 | 9.112 | 7.952 | 8.605 | 8.390 | 9.261 | 9.844 | 9.820 | 10.157 | 9.945 | 11.964 |
| Q Electricity (Purification) | 4.487 | 4.481 | 4.291 | 3.999 | 4.123 | 4.082 | 4.247 | 4.357 | 4.352 | 4.416 | 4.376 | 4.757 |
| Products | ||||||||||||
| Biodiesel yield | 0.593 | 0.595 | 0.685 | 0.785 | 0.725 | 0.744 | 0.678 | 0.634 | 0.636 | 0.615 | 0.628 | 0.522 |
| Energy efficiency | ||||||||||||
| Summation of energy input (MJ) | 1220 | 1273 | 1106 | 967 | 980 | 943 | 1056 | 1128 | 1270 | 1316 | 1290 | 1551 |
| NER | 0.819 | 0.785 | 0.904 | 1.034 | 1.020 | 1.060 | 0.946 | 0.886 | 0.787 | 0.759 | 0.775 | 0.645 |
| Parameters | Jatropha Biodiesl-CeO2 | Jatropha Biodiesl-La2O3 | Jatropha Biodiesl-Nd2O3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Options | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) |
| Utilities/Fuels, kWh | ||||||||||||
| p Electricity, (Esterification) | 3.969 | 3.959 | 3.436 | 2.998 | 3.244 | 3.163 | 3.492 | 3.712 | 3.702 | 3.829 | 3.749 | 4.511 |
| Energy efficiency | ||||||||||||
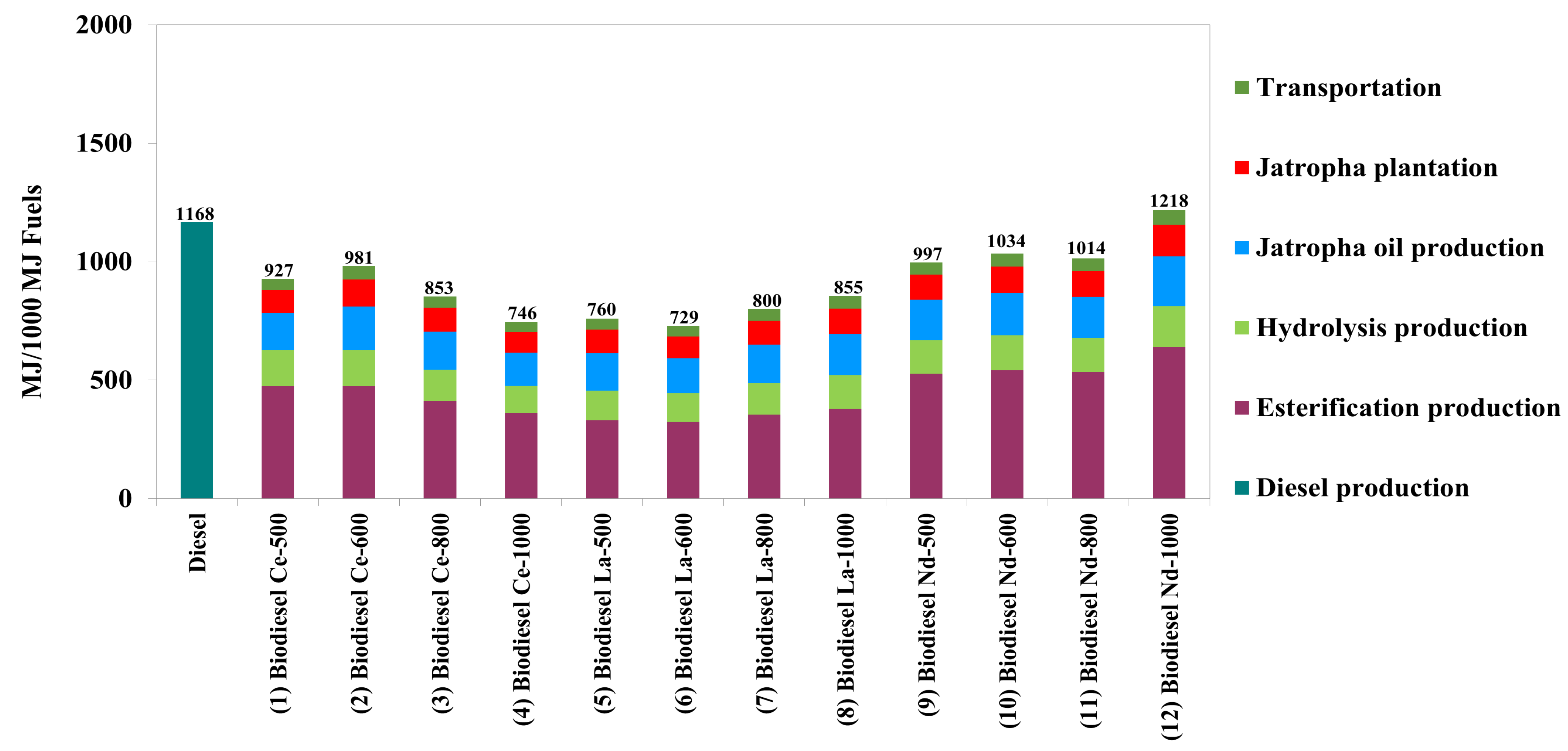
| Summation of energy input (MJ) | 928 | 981 | 853 | 746 | 760 | 729 | 801 | 855 | 997 | 1034 | 1014 | 1218 |
| NER | 1.078 | 1.019 | 1.172 | 1.341 | 1.315 | 1.372 | 1.249 | 1.170 | 1.003 | 0.967 | 0.986 | 0.821 |
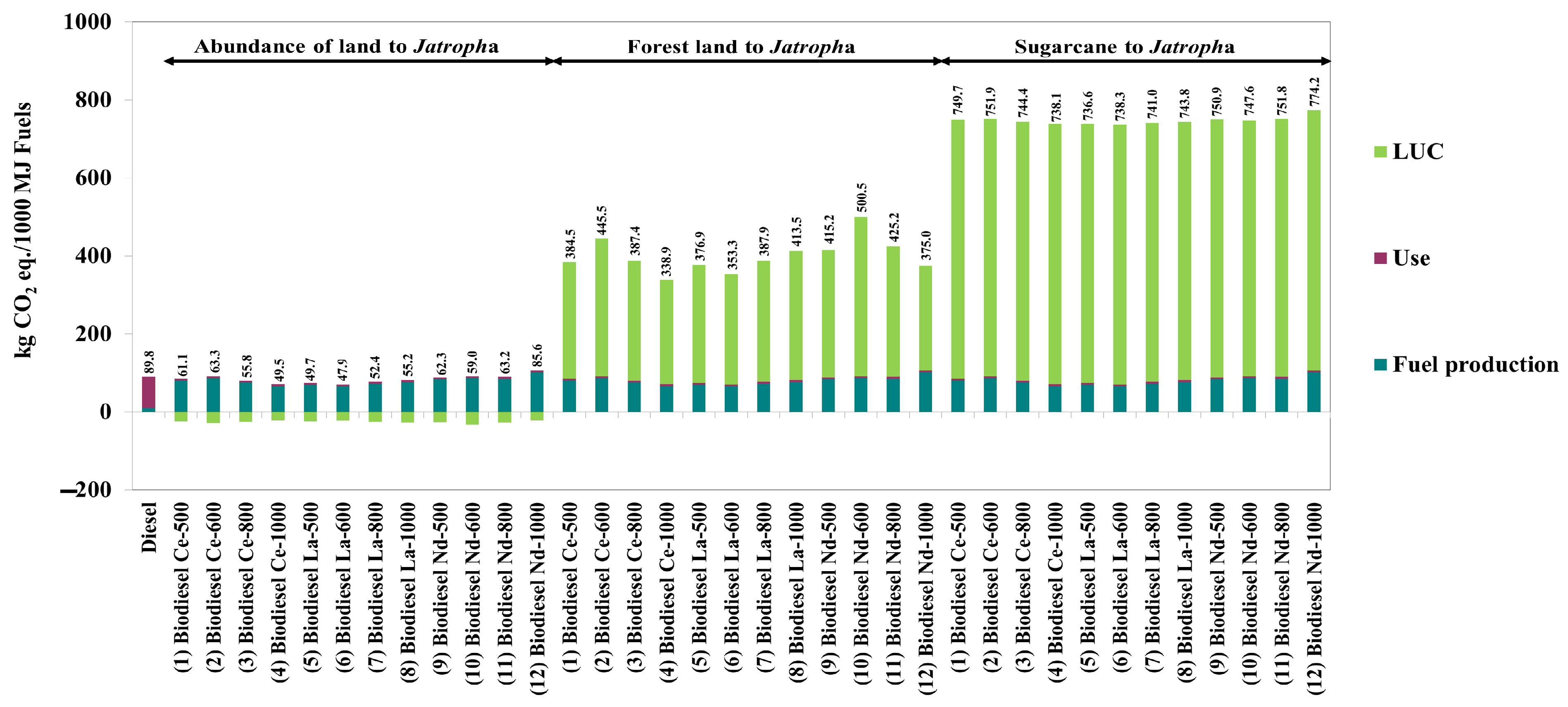
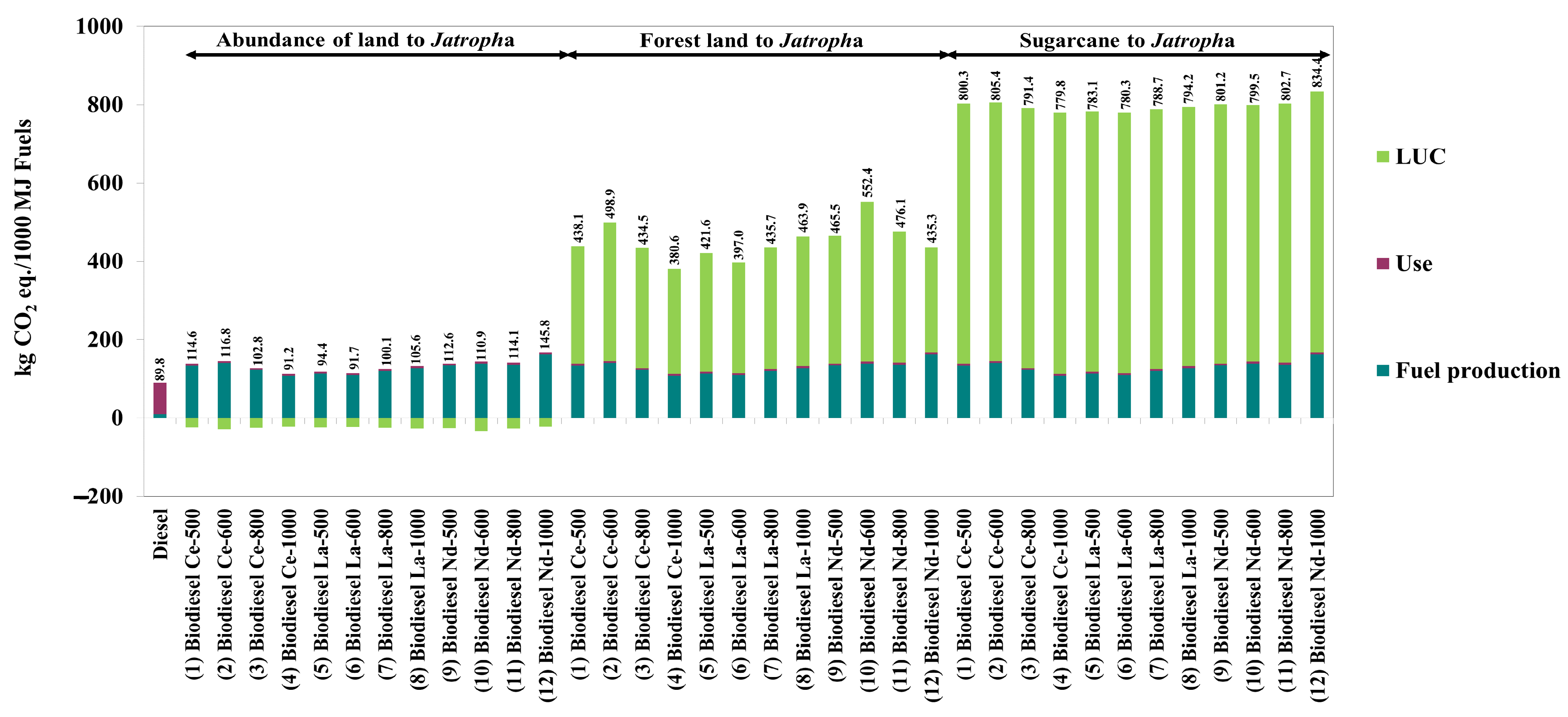
| Case Descriptions | Units | Abundance of Land to Sugarcane | Abundance of Land to Jatropha | Forest Land to Jatropha | Sugarcane to Jatropha |
|---|---|---|---|---|---|
| Soil carbon | kg CO2 equivalent/hectare | −1210 | −1210 | 6197 | 0 |
| Soil carbon stock | kg CO2 equivalent/hectare | −6270 | −917 | 18,316 | 5353 |
| Non-CO2 gases from crop burning | kg CO2 equivalent/hectare | 0 r | 0 r | 5240 s | 0 r |
| Emissions from soil management | kg CO2 equivalent/hectare | −527.70 | 0.31 | −3331 | 528.01 |
| Total LUC | kg CO2 equivalent/hectare | −6952 | −2126 | 26,421 | 5881 |
| kg CO2 equivalent/kg crop | −0.102 | −0.425 | 5.284 | _ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattanaphra, D.; Tawkaew, S.; Chuichulcherm, S.; Kingkam, W.; Nuchdang, S.; Kitpakornsanti, K.; Suwanmanee, U. Evaluation of Life Cycle Assessment of Jatropha Biodiesel Processed by Esterification of Thai Domestic Rare Earth Oxide Catalysts. Sustainability 2024, 16, 100. https://doi.org/10.3390/su16010100
Rattanaphra D, Tawkaew S, Chuichulcherm S, Kingkam W, Nuchdang S, Kitpakornsanti K, Suwanmanee U. Evaluation of Life Cycle Assessment of Jatropha Biodiesel Processed by Esterification of Thai Domestic Rare Earth Oxide Catalysts. Sustainability. 2024; 16(1):100. https://doi.org/10.3390/su16010100
Chicago/Turabian StyleRattanaphra, Dussadee, Sittinun Tawkaew, Sinsupha Chuichulcherm, Wilasinee Kingkam, Sasikarn Nuchdang, Kittiwan Kitpakornsanti, and Unchalee Suwanmanee. 2024. "Evaluation of Life Cycle Assessment of Jatropha Biodiesel Processed by Esterification of Thai Domestic Rare Earth Oxide Catalysts" Sustainability 16, no. 1: 100. https://doi.org/10.3390/su16010100
APA StyleRattanaphra, D., Tawkaew, S., Chuichulcherm, S., Kingkam, W., Nuchdang, S., Kitpakornsanti, K., & Suwanmanee, U. (2024). Evaluation of Life Cycle Assessment of Jatropha Biodiesel Processed by Esterification of Thai Domestic Rare Earth Oxide Catalysts. Sustainability, 16(1), 100. https://doi.org/10.3390/su16010100





