Interplay between Plant Functional Traits and Soil Carbon Sequestration under Ambient and Elevated CO2 Levels
Abstract
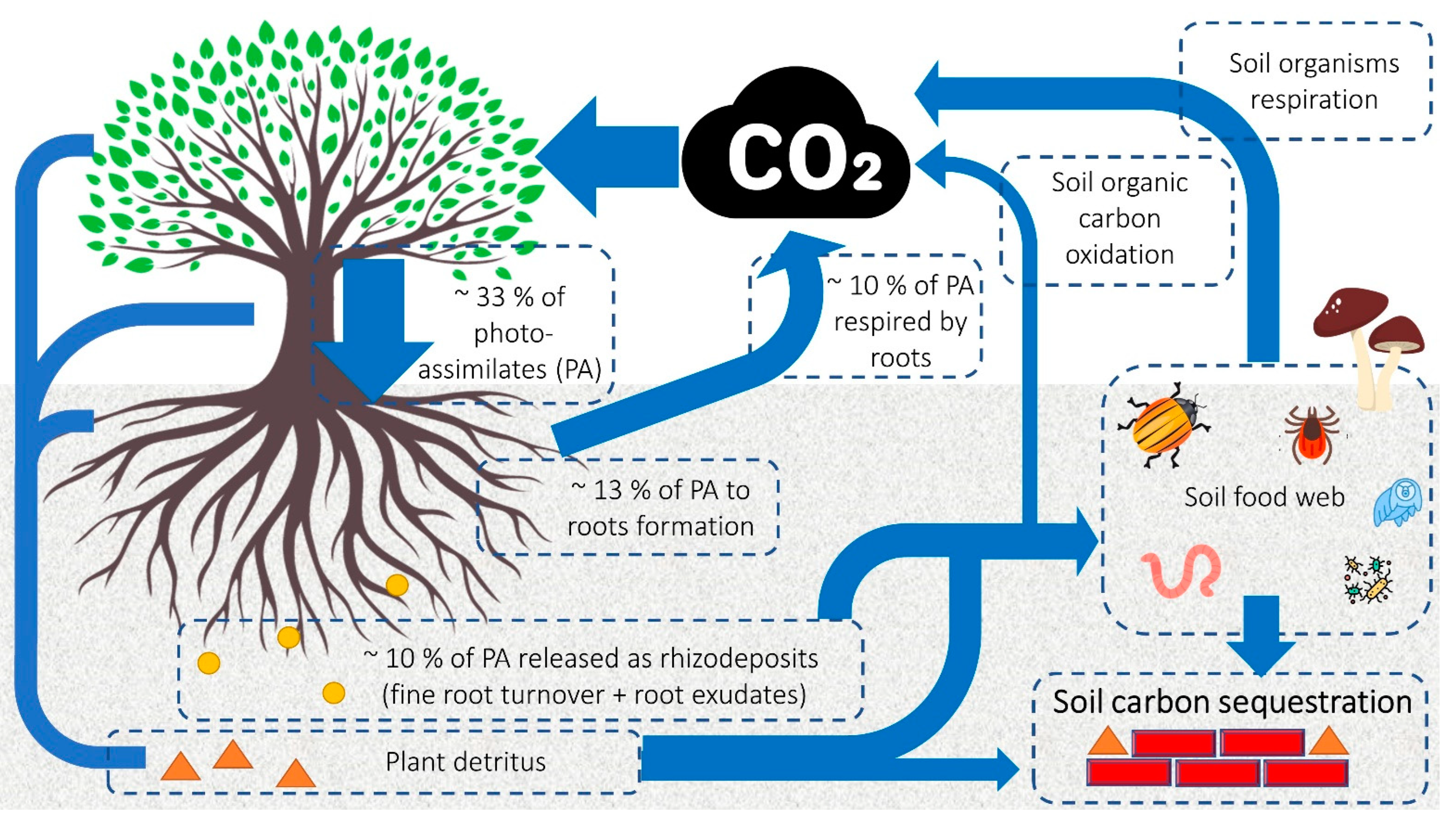
1. Introduction
- How do plant functional traits (morpho-physio-anatomical features) affect C storage and partitioning under eCO2 in different plant parts?
- How do plant functional traits influence C transfer to the soil and rhizosphere services?
2. Impacts of Morphological Characteristics on Carbon Accumulation in Biomass and Soil
3. Impacts of Physiological Traits on Carbon Retention
4. Impacts of Plant Anatomical Traits on Carbon Storage in Plant Biomass and Soil
4.1. Aboveground Plant Anatomical Features and Carbon Sequestration
4.2. In-Depth Overview of Anatomical Features of Belowground Plant Parts in Soil Carbon Sequestration
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Soil management for carbon sequestration. S. Afr. J. Plant Soil 2021, 38, 231–237. [Google Scholar] [CrossRef]
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide; NOAA Climate: Washington, DC, USA, 2022. [Google Scholar]
- Van De Wal, R.S.W.; De Boer, B.; Lourens, L.J.; Köhler, P.; Bintanja, R. Reconstruction of a continuous high-resolution CO2 record over the past 20 million years. Clim. Past 2011, 7, 1459–1469. [Google Scholar] [CrossRef]
- Gayathri, R.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Vodovnik, M.; Vijayalakshmi, S. A review on biological carbon sequestration: A sustainable solution for a cleaner air environment, less pollution and lower health risks. J. King Saud Univ.—Sci. 2021, 33, 101282. [Google Scholar] [CrossRef]
- The Core Writing Team IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2015; ISBN 9789291691432. [Google Scholar]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Lal, R. World cropland soils as a source or sink for atmospheric carbon. Adv. Agron. 2001, 71, 145–191. [Google Scholar]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Gilfillan, D.; Marland, G. CDIAC-FF: Global and National CO2 Emissions from Fossil Fuel Combustion and Cement Manufacture: 1751–2017. Earth Syst. Sci. Data 2021, 13, 1667–1680. [Google Scholar] [CrossRef]
- Ward, D.S.; Mahowald, N.M. Contributions of developed and developing countries to global climate forcing and surface temperature change. Environ. Res. Lett. 2014, 9, 74008. [Google Scholar] [CrossRef]
- Wei, D.; Qi, Y.; Ma, Y.; Wang, X.; Ma, W.; Gao, T.; Huang, L.; Zhao, H.; Zhang, J.; Wang, X. Plant uptake of CO2 outpaces losses from permafrost and plant respiration on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2021, 118, 2015283118. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Shi, G. An updated estimation of radiative forcing due to CO2 and its effect on global surface temperature change. Adv. Atmos. Sci. 2013, 30, 1017–1024. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Leite, F.F.G.D.; Adeyemi, M.A.; Sarker, A.J.; Cambareri, G.S.; Faverin, C.; Tieri, M.P.; Castillo-Zacarías, C.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; et al. A paradigm shift to CO2 sequestration to manage global warming—With the emphasis on developing countries. Sci. Total Environ. 2021, 790, 148169. [Google Scholar] [CrossRef]
- Honegger, M.; Michaelowa, A.; Roy, J. Potential implications of carbon dioxide removal for the sustainable development goals. Clim. Policy 2021, 21, 678–698. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon sequestration in soil. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar] [CrossRef]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Zomer, R.J.; Bossio, D.A.; Sommer, R.; Verchot, L.V. Global Sequestration Potential of Increased Organic Carbon in Cropland Soils. Sci. Rep. 2017, 7, 15554. [Google Scholar] [CrossRef]
- Janzen, H.H. Beyond carbon sequestration: Soil as conduit of solar energy. Eur. J. Soil Sci. 2015, 66, 19–32. [Google Scholar] [CrossRef]
- Powlson, D.S.; Whitmore, A.P.; Goulding, K.W.T. Soil carbon sequestration to mitigate climate change: A critical re-examination to identify the true and the false. Eur. J. Soil Sci. 2011, 62, 42–55. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Faucon, M.P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Colman, B.; Espie, G.S. CO2 uptake and transport in leaf mesophyll cells. Plant. Cell Environ. 1985, 8, 449–457. [Google Scholar] [CrossRef]
- Emerson, R.; Arnold, W. The photochemical reaction in photosynthesis. J. Gen. Physiol. 1932, 16, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Harrison, E.L.; Arce Cubas, L.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Liu, X.; Mathesius, U.; Wang, G.; Tang, C.; Wu, J.; Liu, J.; Zhang, S.; Jin, J. Elevated CO2 increases Nitrogen fixation at the reproductive phase contributing to various yield responses of soybean cultivars. Front. Plant Sci. 2017, 8, 1546. [Google Scholar] [CrossRef]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Interactive impacts of temperature and elevated CO2 on basil (Ocimum basilicum L.) root and shoot morphology and growth. Horticulturae 2021, 7, 112. [Google Scholar] [CrossRef]
- Wang, N.; Gao, G.; Wang, Y.; Wang, D.; Wang, Z.; Gu, J. Coordinated responses of leaf and absorptive root traits under elevated CO2 concentration in temperate woody and herbaceous species. Environ. Exp. Bot. 2020, 179, 104199. [Google Scholar] [CrossRef]
- de Roo, L.; Lauriks, F.; Salomón, R.L.; Oleksyn, J.; Steppe, K. Woody tissue photosynthesis increases radial stem growth of young poplar trees under ambient atmospheric CO2 but its contribution ceases under elevated CO2. Tree Physiol. 2020, 40, 1572–1582. [Google Scholar] [CrossRef]
- Liu, J.; Kang, S.; Davies, W.J.; Ding, R. Elevated [CO2] alleviates the impacts of water deficit on xylem anatomy and hydraulic properties of maize stems. Plant Cell Environ. 2020, 43, 563–578. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, 1356534. [Google Scholar] [CrossRef]
- Arndal, M.F.; Tolver, A.; Larsen, K.S.; Beier, C.; Schmidt, I.K. Fine Root Growth and Vertical Distribution in Response to Elevated CO2, Warming and Drought in a Mixed Heathland–Grassland. Ecosystems 2018, 21, 15–30. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Liu, W.; Zha, L.; Shao, M.; Li, B. Light quality affected the growth and root organic carbon and autotoxin secretions of hydroponic lettuce. Plants 2020, 9, 1542. [Google Scholar] [CrossRef]
- Cohen, I.; Halpern, M.; Yermiyahu, U.; Bar-Tal, A.; Gendler, T.; Rachmilevitch, S. CO2 and nitrogen interaction alters root anatomy, morphology, nitrogen partitioning and photosynthetic acclimation of tomato plants. Planta 2019, 250, 1423–1432. [Google Scholar] [CrossRef]
- Srinivasan, V.; Kumar, P.; Long, S.P. Decreasing, not increasing, leaf area will raise crop yields under global atmospheric change. Glob. Chang. Biol. 2017, 23, 1626–1635. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R.; Chen, H.Y.H.; Chang, S.X.; Zhao, Y.T.; Yang, X.D.; Xu, M.S. Stand structural diversity rather than species diversity enhances aboveground carbon storage in secondary subtropical forests in Eastern China. Biogeosciences 2016, 13, 4627–4635. [Google Scholar] [CrossRef]
- Druart, N.; Rodriguez-Buey, M.; Barron-Gafford, G.; Sjödin, A.; Bhalerao, R.; Hurry, V. Molecular targets of elevated [CO2] in leaves and stems of Populus deltoides: Implications for future tree growth and carbon sequestration. Funct. Plant Biol. 2006, 33, 121–131. [Google Scholar] [CrossRef]
- Benlloch-Gonzalez, M.; Bochicchio, R.; Berger, J.; Bramley, H.; Palta, J.A. High temperature reduces the positive effect of elevated CO2 on wheat root system growth. Field Crop. Res. 2014, 165, 71–79. [Google Scholar] [CrossRef]
- Bu, W.; Huang, J.; Xu, H.; Zang, R.; Ding, Y.; Li, Y.; Lin, M.; Wang, J.; Zhang, C. Plant functional traits are the mediators in regulating effects of abiotic site conditions on aboveground carbon stock-evidence from a 30 ha tropical forest plot. Front. Plant Sci. 2019, 9, 1958. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A Perspective on root sugar sensing and hormonal crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C. Carbon sequestration and foliar dust retention by woody plants in the greenbelts along two major Taiwan highways. Ann. Appl. Biol. 2011, 159, 244–251. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Han, H.; Shi, Z.; Yang, X. Biomass accumulation and carbon sequestration in an age-sequence of Mongolian pine plantations in Horqin sandy land, China. Forests 2019, 10, 197. [Google Scholar] [CrossRef]
- Panahi, P.; Pourhashemi, M.; Nejad, M.H. Estimation of leaf biomass and leaf carbon sequestration of Pistacia atlantica in National Botanical Garden of Iran. Iran. J. For. 2011, 3, 1–12. [Google Scholar]
- Birouste, M.; Kazakou, E.; Blanchard, A.; Roumet, C. Plant traits and decomposition: Are the relationships for roots comparable to those for leaves? Ann. Bot. 2012, 109, 463–472. [Google Scholar] [CrossRef]
- Igbari, A.D.; Nodza, G.I.; Adeusi, A.D.; Ogundipe, O.T. Morphological characterization of mango (Mangifera indica L.) cultivars from south-west Nigeria. Ife J. Sci. 2019, 21, 155. [Google Scholar] [CrossRef]
- Kaplan, D.R. The science of plant morphology: Definition, history, and role in modern biology. Am. J. Bot. 2001, 88, 1711–1741. [Google Scholar] [CrossRef]
- Van De Wouw, M.; Mohammed, J.; Jorge, M.A.; Hanson, J. Agro-morphological characterisation of a collection of Cynodon. Trop. Grassl. 2009, 43, 151–161. [Google Scholar]
- Gratani, L.; Catoni, R.; Varone, L. Quercus ilex L. carbon sequestration capability related to shrub size. Environ. Monit. Assess. 2011, 178, 383–392. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Rogers, H.H.; Prior, S.A.; Peterson, C.M. Elevated CO2 and plant structure: A review. Glob. Chang. Biol. 1999, 5, 807–837. [Google Scholar] [CrossRef]
- Diaz, S. Elevated CO2 Responsiveness, Interactions at the Community Level and Plant Functional Types. J. Biogeogr. 1995, 22, 289. [Google Scholar] [CrossRef]
- Taub, D. Effects of rising atmospheric concentrations of carbon dioxide on plants. Nat. Educ. Knowl. 2010, 3, 21. [Google Scholar]
- Poorter, H.; Navas, M.L. Plant growth and competition at elevated CO2: On winners, losers and functional groups. New Phytol. 2003, 157, 175–198. [Google Scholar] [CrossRef]
- Cha, S.; Chae, H.M.; Lee, S.H.; Shim, J.K. Effect of elevated atmospheric CO2 concentration on growth and leaf litter decomposition of Quercus acutissima and Fraxinus rhynchophylla. PLoS ONE 2017, 12, e0171197. [Google Scholar] [CrossRef]
- Thinh, N.C.; Kumagai, E.; Shimono, H.; Kawasaki, M. Effects of elevated atmospheric CO2 concentration on morphology of leaf blades in Chinese yam. Plant Prod. Sci. 2018, 21, 311–321. [Google Scholar] [CrossRef]
- Nie, M.; Lu, M.; Bell, J.; Raut, S.; Pendall, E. Altered root traits due to elevated CO2: A meta-analysis. Glob. Ecol. Biogeogr. 2013, 22, 1095–1105. [Google Scholar] [CrossRef]
- Tanaka, T. Regulation of plant type and carbon assimilation of rice. Japan Agric. Res. Q. 1976, 10, 161–167. [Google Scholar]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Sarlikioti, V.; De Visser, P.H.B.; Buck-Sorlin, G.H.; Marcelis, L.F.M. How plant architecture affects light absorption and photosynthesis in tomato: Towards an ideotype for plant architecture using a functional structural plant model. Ann. Bot. 2011, 108, 1065–1073. [Google Scholar] [CrossRef]
- Strauss, S.; Lempe, J.; Prusinkiewicz, P.; Tsiantis, M.; Smith, R.S. Phyllotaxis: Is the golden angle optimal for light capture? New Phytol. 2020, 225, 499–510. [Google Scholar] [CrossRef]
- Takenaka, A. Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot. Ecol. Res. 1994, 9, 109–114. [Google Scholar] [CrossRef]
- Righetti, T.L.; Vasconcelos, C.; Sandrock, D.R.; Ortega-Farias, S.; Moreno, Y.; Meza, F.J. Assessments of CO2 assimilation on a per-leaf-area basis are related to total leaf area. J. Am. Soc. Hortic. Sci. 2007, 132, 230–238. [Google Scholar] [CrossRef]
- Kuronuma, T.; Watanabe, H. Relevance of Carbon Sequestration to the Physiological and Morphological Traits of Several Green Roof Plants during the First Year after Construction. Am. J. Plant Sci. 2017, 8, 14–27. [Google Scholar] [CrossRef]
- Peng, S. Single-leaf and canopy photosynthesis of rice. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 2000; Volume 7, pp. 213–228. [Google Scholar]
- Grigulis, K.; Lavorel, S.; Krainer, U.; Legay, N.; Baxendale, C.; Dumont, M.; Kastl, E.; Arnoldi, C.; Bardgett, R.D.; Poly, F.; et al. Relative contributions of plant traits and soil microbial properties to mountain grassland ecosystem services. J. Ecol. 2013, 101, 47–57. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Masters, M.D.; Black, C.K.; Zeri, M.; Hussain, M.Z.; Bernacchi, C.J.; DeLucia, E.H. Altered Belowground Carbon Cycling Following Land-Use Change to Perennial Bioenergy Crops. Ecosystems 2013, 16, 508–520. [Google Scholar] [CrossRef]
- Hudiburg, T.W.; Davis, S.C.; Parton, W.; Delucia, E.H. Bioenergy crop greenhouse gas mitigation potential under a range of management practices. GCB Bioenergy 2015, 7, 366–374. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Mutema, M.; Chaplot, V. What crop type for atmospheric carbon sequestration: Results from a global data analysis. Agric. Ecosyst. Environ. 2017, 243, 34–46. [Google Scholar] [CrossRef]
- Singh, M.; Guleria, N.; Prakasa Rao, E.V.S.; Goswami, P. Efficient C sequestration and benefits of medicinal vetiver cropping in tropical regions. Agron. Sustain. Dev. 2014, 34, 603–607. [Google Scholar] [CrossRef]
- Stewart, C.E.; Roosendaal, D.; Denef, K.; Pruessner, E.; Comas, L.H.; Sarath, G.; Jin, V.L.; Schmer, M.R.; Soundararajan, M. Seasonal switchgrass ecotype contributions to soil organic carbon, deep soil microbial community composition and rhizodeposit uptake during an extreme drought. Soil Biol. Biochem. 2017, 112, 191–203. [Google Scholar] [CrossRef]
- Nath, A.J.; Lal, R.; Das, A.K. Managing woody bamboos for carbon farming and carbon trading. Glob. Ecol. Conserv. 2015, 3, 654–663. [Google Scholar] [CrossRef]
- Nigatu, A.; Wondie, M.; Alemu, A.; Gebeyehu, D.; Workagegnehu, H. Productivity of highland bamboo (Yushania alpina) across different plantation niches in West Amhara, Ethiopia. For. Sci. Technol. 2020, 16, 116–122. [Google Scholar] [CrossRef]
- Yen, T.M. Culm height development, biomass accumulation and carbon storage in an initial growth stage for a fast-growing moso bamboo (Phyllostachy pubescens). Bot. Stud. 2016, 57, 10. [Google Scholar] [CrossRef]
- Devi, A.S.; Singh, K.S. Carbon storage and sequestration potential in aboveground biomass of bamboos in North East India. Sci. Rep. 2021, 11, 837. [Google Scholar] [CrossRef]
- Berhongaray, G.; Cotrufo, F.M.; Janssens, I.A.; Ceulemans, R. Below-ground carbon inputs contribute more than above-ground inputs to soil carbon accrual in a bioenergy poplar plantation. Plant Soil 2019, 434, 363–378. [Google Scholar] [CrossRef]
- Balesdent, J.; Balabane, M. Major contribution of roots to soil carbon storage inferred from maize cultivated soils. Soil Biol. Biochem. 1996, 28, 1261–1263. [Google Scholar] [CrossRef]
- Broadbent, F.E.; Nakashima, T. Mineralization of Carbon and Nitrogen in Soil Amended with Carbon-13 and Nitrogen-15 Labeled Plant Material. Soil Sci. Soc. Am. J. 1974, 38, 313–315. [Google Scholar] [CrossRef]
- Chimento, C.; Amaducci, S. Characterization of fine root system and potential contribution to soil organic carbon of six perennial bioenergy crops. Biomass Bioenergy 2015, 83, 116–122. [Google Scholar] [CrossRef]
- Kell, D.B. Large-scale sequestration of atmospheric carbon via plant roots in natural and agricultural ecosystems: Why and how. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1589–1597. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Finér, L.; Helmisaari, H.S.; Lõhmus, K.; Majdi, H.; Brunner, I.; Børja, I.; Eldhuset, T.; Godbold, D.; Grebenc, T.; Konôpka, B.; et al. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosyst. 2007, 141, 394–405. [Google Scholar] [CrossRef]
- Guo, D.; Li, H.; Mitchell, R.J.; Han, W.; Hendricks, J.J.; Fahey, T.J.; Hendrick, R.L. Fine root heterogeneity by branch order: Exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol. 2008, 177, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Mitchell, R.J.; Withington, J.M.; Fan, P.P.; Hendricks, J.J. Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: Root branch order predominates. J. Ecol. 2008, 96, 737–745. [Google Scholar] [CrossRef]
- Rytter, R.M. The potential of willow and poplar plantations as carbon sinks in Sweden. Biomass Bioenergy 2012, 36, 86–95. [Google Scholar] [CrossRef]
- Tierney, G.L.; Fahey, T.J. Fine root turnover in a northern hardwood forest: A direct comparison of the radiocarbon and minirhizotron methods. Can. J. For. Res. 2002, 32, 1692–1697. [Google Scholar] [CrossRef]
- Wells, C.E.; Eissenstat, D.M. Marked differences in survivorship among apple roots of different diameters. Ecology 2001, 82, 882–892. [Google Scholar] [CrossRef]
- Nelson, L.; Blumenthal, D.M.; Williams, D.G.; Pendall, E. Digging into the roots of belowground carbon cycling following seven years of Prairie Heating and CO2 Enrichment (PHACE), Wyoming USA. Soil Biol. Biochem. 2017, 115, 169–177. [Google Scholar] [CrossRef]
- Czarnota, M.A.; Paul, R.N.; Weston, L.A.; Duke, S.O. Anatomy of sorgoleone-secreting root hairs of Sorghum species. Int. J. Plant Sci. 2003, 164, 861–866. [Google Scholar] [CrossRef]
- Groleau-Renaud, V.; Plantureux, S.; Guckert, A. Influence of plant morphology on root exudation of maize subjected to mechanical impedance in hydroponic conditions. Plant Soil 1998, 201, 231–239. [Google Scholar] [CrossRef]
- Darwent, M.J.; Paterson, E.; McDonald, A.J.S.; Tomos, A.D. Biosensor reporting of root exudation from Hordeum vulgare in relation to shoot nitrate concentration. J. Exp. Bot. 2003, 54, 325–334. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie 2003, 23, 375–396. [Google Scholar] [CrossRef]
- Piñeros, M.A.; Magalhaes, J.V.; Carvalho Alves, V.M.; Kochian, L.V. The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize. Plant Physiol. 2002, 129, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Tückmantel, T.; Leuschner, C.; Preusser, S.; Kandeler, E.; Angst, G.; Mueller, C.W.; Meier, I.C. Root exudation patterns in a beech forest: Dependence on soil depth, root morphology, and environment. Soil Biol. Biochem. 2017, 107, 188–197. [Google Scholar] [CrossRef]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; Jackson, R.B. Root dynamics and global change: Seeking an ecosystem perspective. New Phytol. 2000, 147, 3–12. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Amézketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Angers, D.A.; Prévost, D.; Vézina, L.-P.; Chalifour, F.-P. Soil Aggregation and Fungal and Bacterial Biomass under Annual and Perennial Cropping Systems. Soil Sci. Soc. Am. J. 1997, 61, 262–267. [Google Scholar] [CrossRef]
- Helfrich, M.; Ludwig, B.; Potthoff, M.; Flessa, H. Effect of litter quality and soil fungi on macroaggregate dynamics and associated partitioning of litter carbon and nitrogen. Soil Biol. Biochem. 2008, 40, 1823–1835. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Demenois, J.; Rey, F.; Ibanez, T.; Stokes, A.; Carriconde, F. Linkages between root traits, soil fungi and aggregate stability in tropical plant communities along a successional vegetation gradient. Plant Soil 2018, 424, 319–334. [Google Scholar] [CrossRef]
- Angers, D.A.; Caron, J. Plant-induced changes in soil structure: Processes and feedbacks. Biogeochemistry 1998, 42, 55–72. [Google Scholar] [CrossRef]
- Pérès, G.; Cluzeau, D.; Menasseri, S.; Soussana, J.F.; Bessler, H.; Engels, C.; Habekost, M.; Gleixner, G.; Weigelt, A.; Weisser, W.W.; et al. Mechanisms linking plant community properties to soil aggregate stability in an experimental grassland plant diversity gradient. Plant Soil 2013, 373, 285–299. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable Plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Kennedy, P.G.E. Moving beyond the Black-box: Fungal Traits, Community Structure, and Carbon Sequestration in Forest Soils. New Phytol. 2015, 205, 1378–1380. [Google Scholar] [CrossRef]
- Erktan, A.; Cécillon, L.; Graf, F.; Roumet, C.; Legout, C.; Rey, F. Increase in soil aggregate stability along a Mediterranean successional gradient in severely eroded gully bed ecosystems: Combined effects of soil, root traits and plant community characteristics. Plant Soil 2016, 398, 121–137. [Google Scholar] [CrossRef]
- Poirier, V.; Roumet, C.; Munson, A.D. The root of the matter: Linking root traits and soil organic matter stabilization processes. Soil Biol. Biochem. 2018, 120, 246–259. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Miller, R.M.; Lussenhop, J. Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol. Biochem. 1998, 30, 905–916. [Google Scholar] [CrossRef]
- Miller, R.; Jastrow, J. The application of VA mycorrhizae to ecosystem restoration and reclamation. In Mycorrhizal Functioning—An Integrative Plant-Fungal Process; Allen, M., Ed.; Chapman and Hall: Boca Raton, FL, USA, 1992; pp. 438–467. [Google Scholar]
- Ontl, T.A.; Cambardella, C.A.; Schulte, L.A.; Kolka, R.K. Factors influencing soil aggregation and particulate organic matter responses to bioenergy crops across a topographic gradient. Geoderma 2015, 255–256, 1–11. [Google Scholar] [CrossRef]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224–225, 121–131. [Google Scholar] [CrossRef]
- Teskey, R.O.; McGuire, M.A. Carbon dioxide transport in xylem causes errors in estimation of rates of respiration in stems and branches of trees. Plant Cell Environ. 2002, 25, 1571–1577. [Google Scholar] [CrossRef]
- Marco, A.; Napoletano, P.; Panico, S.C.; Memoli, V.; Santorufo, L.; Ruggiero, A.R.; Colombo, C.; Barile, R.; Maisto, G. Combined Effect of Black Locust Invasion and Fire on Soils of Mediterranean Shrublands and Pine Forests. Catena 2023, 220, 106656. [Google Scholar] [CrossRef]
- Heid, L.; Calvaruso, C.; Andrianantenaina, A.; Granier, A.; Conil, S.; Rathgeber, C.B.K.; Turpault, M.P.; Longdoz, B. Seasonal time-course of the above ground biomass production efficiency in beech trees (Fagus sylvatica L.). Ann. For. Sci. 2018, 75, 31. [Google Scholar] [CrossRef]
- Teskey, R.O.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, fate and significance of CO2 in tree stems. New Phytol. 2008, 177, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Jackson, R.B.; Mooney, H.A. Stomatal responses to increased CO2: Implications from the plant to the global scale. Plant. Cell Environ. 1995, 18, 1214–1225. [Google Scholar] [CrossRef]
- Rogers, A.; Humphries, S.W. A mechanistic evaluation of photosynthetic acclimation at elevated CO2. Glob. Chang. Biol. 2000, 6, 1005–1011. [Google Scholar] [CrossRef]
- Byeon, S.; Song, W.; Park, M.; Kim, S.; Kim, S.H.; Lee, H.; Jeon, J.; Kim, K.; Lee, M.; Lim, H.; et al. Canopy Height Affects the Allocation of Photosynthetic Carbon and Nitrogen in Two Deciduous Tree Species under Elevated CO2. J. Plant Physiol. 2021, 268, 153584. [Google Scholar] [CrossRef]
- Smith, M.G.; Miller, R.E.; Arndt, S.K.; Kasel, S.; Bennett, L.T. Whole-tree distribution and temporal variation of non-structural carbohydrates in broadleaf evergreen trees. Tree Physiol. 2018, 38, 570–581. [Google Scholar] [CrossRef]
- Shen, Y.; Gilbert, G.S.; Li, W.; Fang, M.; Lu, H.; Yu, S. Linking Aboveground Traits to Root Traits and Local Environment: Implications of the Plant Economics Spectrum. Front. Plant Sci. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Lugassi, N.; Egbaria, A.; Granot, D.; Yermiyahu, U. The role of nitrogen in photosynthetic acclimation to elevated [CO2] in tomatoes. Plant Soil 2019, 434, 397–411. [Google Scholar] [CrossRef]
- Smith, N.G. Plant Respiration Responses to Elevated CO2: An Overview from Cellular Processes to Global Impacts. In Plant Respiration: Metabolic Fluxes and Carbon Balance. Advances in Photosynthesis and Respiration (Including Bioenergy and Related Processes; Tcherkez, G., Ghashghaie, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 43, pp. 69–87. [Google Scholar]
- Gimeno, T.E.; Crous, K.Y.; Cooke, J.; O’Grady, A.P.; Ósvaldsson, A.; Medlyn, B.E.; Ellsworth, D.S. Conserved stomatal behaviour under elevated CO2 and varying water availability in a mature woodland. Funct. Ecol. 2016, 30, 700–709. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; Wang, J.; Ding, Y.; Song, X. Effects of Elevated CO2 and Drought on Plant Physiology, Soil Carbon and Soil Enzyme Activities. Pedosphere 2017, 27, 846–855. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Pokharel, S.S.; Li, C.; Parajulee, M.N.; Chen, F.; Fang, W. Effects of elevated CO2 on foliar soluble nutrients and functional components of tea, and population dynamics of tea aphid, Toxoptera aurantii. Plant Physiol. Biochem. 2019, 145, 84–94. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrera-Bosquet, L.; Morcuende, R.; Avice, J.C.; Nogués, S.; Araus, J.L.; Martínez-Carrasco, R.; Pérez, P. Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J. Exp. Bot. 2011, 62, 3957–3969. [Google Scholar] [CrossRef]
- Quick, W.P.; Schurr, U.; Fichtner, K.; Schulze, E.-D.; Rodermel, S.R.; Bogorad, L.; Stitt, M. The impact of decreased Rubisco on photosynthesis, growth, allocation and storage in tobacco plants which have been transformed with antisense rbcS. Plant J. 1991, 1, 51–58. [Google Scholar] [CrossRef]
- Soh, W.K.; Yiotis, C.; Murray, M.A.; Parnell, A.C.; Wright, I.M.R.; Spicer, R.A.; Lawson, T.; Caballero, R.; McElwain, J.C. Rising CO2 Drives Divergence in Water Use Efficiency of Evergreen and Deciduous Plants. Sci. Adv. 2019, 5, eaax7906. [Google Scholar] [CrossRef]
- Peñuelas, J.; Fernández-Martínez, M.; Vallicrosa, H.; Maspons, J.; Zuccarini, P.; Carnicer, J.; Sanders, T.G.M.; Krüger, I.; Obersteiner, M.; Janssens, I.A.; et al. Increasing Atmospheric CO2 Concentrations Correlate with Declining Nutritional Status of European Forests. Commun. Biol. 2020, 3, 125. [Google Scholar] [CrossRef]
- Skinner, C.B.; Poulsen, C.J.; Mankin, J.S. Amplification of heat extremes by plant CO2 physiological forcing. Nat. Commun. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Purcell, C.; Batke, S.P.; Yiotis, C.; Caballero, R.; Soh, W.K.; Murray, M.; McElwain, J.C. Increasing stomatal conductance in response to rising atmospheric CO2. Ann. Bot. 2018, 121, 1137–1149. [Google Scholar] [CrossRef]
- Ghildiyal, M.C.; Sharma-Natu, P. Photosynthetic acclimation to rising atmospheric carbon dioxide concentration. Indian J. Exp. Biol. 2000, 38, 961–966. [Google Scholar] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising Atmospheric Carbon Dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Maroco, J.P.; Edwards, G.E.; Ku, M.S.B. Photosynthetic acclimation of maize to growth under elevated levels of carbon dioxide. Planta 1999, 210, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Dingkuhn, M.; Luquet, D.; Fabre, D.; Muller, B.; Yin, X.; Paul, M.J. The case for improving crop carbon sink strength or plasticity for a CO2-rich future. Curr. Opin. Plant Biol. 2020, 56, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sáez, Á.; Erice, G.; Aranjuelo, I.; Nogués, S.; Irigoyen, J.J.; Sánchez-Díaz, M. Photosynthetic down-regulation under elevated CO2 exposure can be prevented by nitrogen supply in nodulated alfalfa. J. Plant Physiol. 2010, 167, 1558–1565. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Wujeska-Klause, A.; Crous, K.Y.; Ghannoum, O.; Ellsworth, D.S. Lower photorespiration in elevated CO2 reduces leaf N concentrations in mature Eucalyptus trees in the field. Glob. Chang. Biol. 2019, 25, 1282–1295. [Google Scholar] [CrossRef]
- Asensio, J.S.R.; Rachmilevitch, S.; Bloom, A.J. Responses of arabidopsis and wheat to rising CO2 depend on nitrogen source and nighttime CO2 levels. Plant Physiol. 2015, 168, 156–163. [Google Scholar] [CrossRef]
- Bravdo, B.-A.; Canvin, D. Effect of Carbon Dioxide on Photorespiration. Plant Physiol. 1979, 63, 399–401. [Google Scholar] [CrossRef]
- Bunce, J.A. Three new methods indicate that CO2 concentration affects plant respiration in the range relevant to global change. AoB Plants 2021, 13, plab004. [Google Scholar] [CrossRef]
- Fry, E.L.; De Long, J.R.; Bardgett, R.D. Plant communities as modulators of soil carbon storage. In Soil Carbon Storage: Modulators, Mechanisms and Modeling; Singh, B., Ed.; Academic Press: London, UK, 2018; pp. 29–71. ISBN 9780128127667. [Google Scholar]
- Kim, K.; Labbé, N.; Warren, J.M.; Elder, T.; Rials, T.G. Chemical and anatomical changes in Liquidambar styraciflua L. xylem after long term exposure to elevated CO2. Environ. Pollut. 2015, 198, 179–185. [Google Scholar] [CrossRef]
- Milhinhos, A.; Miguel, C.M. Hormone interactions in xylem development: A matter of signals. Plant Cell Rep. 2013, 32, 867–883. [Google Scholar] [CrossRef]
- Savage, J.A.; Clearwater, M.J.; Haines, D.F.; Klein, T.; Mencuccini, M.; Sevanto, S.; Turgeon, R.; Zhang, C. Allocation, stress tolerance and carbon transport in plants: How does phloem physiology affect plant ecology? Plant Cell Environ. 2016, 39, 709–725. [Google Scholar] [CrossRef]
- Liesche, J.; Schulz, A. Phloem transport in gymnosperms: A question of pressure and resistance. Curr. Opin. Plant Biol. 2018, 43, 36–42. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S. Plant Physiological Ecology; Springer: New York, NY, USA, 2019; ISBN 9783030296391. [Google Scholar]
- Tarvainen, L.; Wallin, G.; Linder, S.; Näsholm, T.; Oren, R.; Ottosson Löfvenius, M.; Räntfors, M.; Tor-Ngern, P.; Marshall, J.D. Limited vertical CO2 transport in stems of mature boreal Pinus sylvestris trees. Tree Physiol. 2021, 41, 63–75. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Valverde-Barrantes, O.J.; Kardol, P. A framework to assess the carbon supply–consumption balance in plant roots. New Phytol. 2021, 229, 659–664. [Google Scholar] [CrossRef]
- Weiss, D.; Shomer-Ilan, A.; Vainstein, A.; Halevy, A.H. Photosynthetic carbon fixation in the corollas of Petunia hybrida. Physiol. Plant. 1990, 78, 345–350. [Google Scholar] [CrossRef]
- Niinemets, Ü. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef]
- Woodward, F.I.; Lake, J.A.; Quick, W.P. Stomatal development and CO2: Ecological consequences. New Phytol. 2002, 153, 477–484. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Seo, Y.O.; Lumbres, R.I.C.; Lee, Y.J. Partitioning of above and belowground biomass and allometry in the two stand age classes of Pinus rigida in South Korea. Life Sci. J. 2012, 9, 3553–3559. [Google Scholar]
- Nabais, C.; Hansen, J.K.; David-Schwartz, R.; Klisz, M.; López, R.; Rozenberg, P. The effect of climate on wood density: What provenance trials tell us? For. Ecol. Manage. 2018, 408, 148–156. [Google Scholar] [CrossRef]
- Lundqvist, S.O.; Seifert, S.; Grahn, T.; Olsson, L.; García-Gil, M.R.; Karlsson, B.; Seifert, T. Age and weather effects on between and within ring variations of number, width and coarseness of tracheids and radial growth of young Norway spruce. Eur. J. For. Res. 2018, 137, 719–743. [Google Scholar] [CrossRef]
- Furze, M.E.; Trumbore, S.; Hartmann, H. Detours on the phloem sugar highway: Stem carbon storage and remobilization. Curr. Opin. Plant Biol. 2018, 43, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cernusak, L.A.; Marshall, J.D. Photosynthetic refixation in branches of Western White Pine. Funct. Ecol. 2000, 14, 300–311. [Google Scholar] [CrossRef]
- Ma, S.H.; Eziz, A.; Tian, D.; Yan, Z.B.; Cai, Q.; Jiang, M.W.; Ji, C.J.; Fang, J.Y.; Sun, O.J. Size- and age-dependent increases in tree stem carbon concentration: Implications for forest carbon stock estimations. J. Plant Ecol. 2020, 13, 233–240. [Google Scholar] [CrossRef]
- Farrar, J.F.; Jones, D.L. The control of carbon acquisition by roots. In New Phytologist; Kroon, H.D., Visser, E.J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 147, pp. 43–53. [Google Scholar]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of Internal and External Factors on Root Growth and Development. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 331–346. ISBN 9780123849052. [Google Scholar]
- Hennion, N.; Durand, M.; Vriet, C.; Doidy, J.; Maurousset, L.; Lemoine, R.; Pourtau, N. Sugars en route to the roots. Transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere. Physiol. Plant. 2019, 165, 44–57. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef]
- Lambers, H.; Atkin, O.; Millenaar, F. Respiratory Patterns in Roots in Relation to Their Functioning. In Plant Roots; Waisel, Y., Eshel, A., Kafkaki, U., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 521–552. [Google Scholar]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Hoang, N.V.; Park, C.; Kamran, M.; Lee, J.Y. Gene Regulatory Network Guided Investigations and Engineering of Storage Root Development in Root Crops. Front. Plant Sci. 2020, 11, 762. [Google Scholar] [CrossRef]
- Le Deunff, E.; Lecourt, J.; Malagoli, P. Fine-tuning of root elongation by ethylene: A tool to study dynamic structure-function relationships between root architecture and nitrate absorption. Ann. Bot. 2016, 118, 607–620. [Google Scholar] [CrossRef]
- Iversen, C.M. Digging deeper: Fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol. 2010, 186, 346–357. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Prieto, I.; Marañón, T.; Pérez-Ramos, I.M.; Olmo, M.; Villar, R. Root economics spectrum and construction costs in Mediterranean woody plants: The role of symbiotic associations and the environment. J. Ecol. 2021, 109, 1873–1885. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Kurze, S.; Engelbrecht, B.M.J.; Bilton, M.C.; Tielbörger, K.; Álvarez-Cansino, L. Rethinking the Plant Economics Spectrum for Annuals: A Multi-Species Study. Front. Plant Sci. 2021, 12, 640862. [Google Scholar] [CrossRef]
- Hishi, T.; Tateno, R.; Fukushima, K.; Fujimaki, R.; Itoh, M.; Tokuchi, N. Changes in the anatomy, morphology and mycorrhizal infection of fine root systems of Cryptomeria japonica in relation to stand ageing. Tree Physiol. 2017, 37, 61–70. [Google Scholar] [CrossRef]
- Funayama-Noguchi, S.; Shibata, M.; Noguchi, K.; Terashima, I. Effects of root morphology, respiration and carboxylate exudation on carbon economy in two non-mycorrhizal lupines under phosphorus deficiency. Plant Cell Environ. 2021, 44, 598–612. [Google Scholar] [CrossRef]
- Nielsen, K.L.; Eshel, A.; Lynch, J.P. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J. Exp. Bot. 2001, 52, 329–339. [Google Scholar] [CrossRef]
- Ding, W.; Cong, W.F.; Lambers, H. Plant phosphorus-acquisition and -use strategies affect soil carbon cycling. Trends Ecol. Evol. 2021, 36, 899–906. [Google Scholar] [CrossRef]
- Escandón, A.B.; Rojas, R.; Morales, L.V.; Corcuera, L.J.; Coopman, R.E.; Paula, S. Physiological differences between root suckers and saplings enlarge the regeneration niche in Eucryphia cordifolia Cav. Tree Physiol. 2018, 38, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Strock, C.F.; Lynch, J.P. Root secondary growth: An unexplored component of soil resource acquisition. Ann. Bot. 2020, 126, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Germon, A.; Laclau, J.P.; Robin, A.; Jourdan, C. Tamm Review: Deep fine roots in forest ecosystems: Why dig deeper? For. Ecol. Manage. 2020, 466, 118135. [Google Scholar] [CrossRef]
- Wu, X.J.; Sun, S.; Xing, G.M.; Wang, G.L.; Wang, F.; Xu, Z.S.; Tian, Y.S.; Hou, X.L.; Xiong, A.S. Elevated carbon dioxide altered morphological and anatomical characteristics, ascorbic acid accumulation, and related gene expression during taproot development in carrots. Front. Plant Sci. 2017, 7, 2026. [Google Scholar] [CrossRef]
- Gray, S.B.; Rodriguez-Medina, J.; Rusoff, S.; Toal, T.W.; Kajala, K.; Runcie, D.E.; Brady, S.M. Translational regulation contributes to the elevated CO2 response in two Solanum species. Plant J. 2020, 102, 383–397. [Google Scholar] [CrossRef]
- Cohen, I.; Rapaport, T.; Berger, R.T.; Rachmilevitch, S. The effects of elevated CO2 and nitrogen nutrition on root dynamics. Plant Sci. 2018, 272, 294–300. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Rhizosphere Chemistry in Relation to Plant Nutrition. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 347–368. ISBN 9780123849052. [Google Scholar]
- Koo, B.J.; Adriano, D.C.; Bolan, N.S.; Barton, C.D. Root Exudates and Microorganisms. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2004; Volume 4, pp. 421–428. ISBN 9780080547954. [Google Scholar]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Makinen, H.; Prislan, P.; Rossi, S.; Del Castillo, E.M.; Campelo, F.; Vavrčík, H.; et al. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 2015, 1, 15160. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, S.; Ciais, P.; Manzoni, S.; Fang, J.; Yu, G.; Tang, X.; Zhou, P.; Wang, W.; Yan, J.; et al. Climate and litter C/N ratio constrain soil organic carbon accumulation. Natl. Sci. Rev. 2019, 6, 746–757. [Google Scholar] [CrossRef]
- Kicklighter, D.W.; Melillo, J.M.; Monier, E.; Sokolov, A.P.; Zhuang, Q. Future nitrogen availability and its effect on carbon sequestration in Northern Eurasia. Nat. Commun. 2019, 10, 3024. [Google Scholar] [CrossRef]
- Cao, Y.; He, Z.; Zhu, T.; Zhao, F. Organic-C quality as a key driver of microbial nitrogen immobilization in soil: A meta-analysis. Geoderma 2021, 383, 114784. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Hueso, R.; Hughes, J.; Delgado-Baquerizo, M.; Drake, J.E.; Tjoelker, M.G.; Piñeiro, J.; Power, S.A. Rhizosphere-driven increase in nitrogen and phosphorus availability under elevated atmospheric CO2 in a mature Eucalyptus woodland. Plant Soil 2017, 416, 283–295. [Google Scholar] [CrossRef]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Ostle, N.; Smith, P.; Fisher, R.; Woodward, F.I.; Fisher, J.B.; Smith, J.; Galbraith, D.W.; Levy, P.M.; Meir, P.; McNamara, N.P.; et al. Integrating Plant-Soil Interactions into Global Carbon Cycle Models. J. Ecol. 2009, 97, 851–863. [Google Scholar] [CrossRef]
- Kowalchuk, G.A. Bad news for soil carbon sequestration? Science 2012, 337, 1049–1050. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Meena, V.S.; Gupta, V.K.; Rana, K.; Choudhary, M.; Tiwari, G.; Mishra, P.K.; Pattanayak, A.; Bisht, J.K.; et al. The potential of arbuscular mycorrhizal fungi in C cycling: A review. Arch. Microbiol. 2020, 202, 1581–1596. [Google Scholar] [CrossRef]


| Species | Treatments | RD (mm) | RTD (mg mm−3) | SRL (mm mg−1) | SRSA (mm2 mg−1) |
|---|---|---|---|---|---|
| Bouteloua gracilis | AC + AT | 0.26 (0.01) | 0.554 (0.039) | 35 (3) | 28 (2) |
| AC + ET | 0.29 (0.01) | 0.523 (0.030) | 30 (3) | 27 (2) | |
| EC + AT | 0.26 (0.01) | 0.552 (0.024) | 34 (2) | 28 (1) | |
| EC + ET | 0.28 (0.01) | 0.520 (0.015) | 32 (2) | 28 (1) | |
| Carex eleocharis | AC + AT | 0.23 (0.02) | 0.476 (0.032) | 56 (8) | 39 (4) |
| AC + ET | 0.22 (0.01) | 0.466 (0.013) | 57 (6) | 39 (2) | |
| EC + AT | 0.24 (0.02) | 0.520 (0.019) | 44 (6) | 32 (3) | |
| EC + ET | 0.22 (0.01) | 0.502 (0.032) | 56 (5) | 37 (3) | |
| Pascopyrum smithii | AC + AT | 0.41 (0.04) | 0.540 (0.032) | 16 (4) | 19 (3) |
| AC + ET | 0.41 (0.04) | 0.472 (0.021) | 17 (3) | 21 (2) | |
| EC + AT | 0.35 (0.03) | 0.467 (0.047) | 25 (5) | 26 (2) | |
| EC + ET | 0.32 (0.05) | 0.431 (0.026) | 35 (9) | 31 (4) | |
| Community | AC + AT | 0.23 (0.004) | 0.495 (0.015) | 50 (3) | 36 (2) |
| AC + ET | 0.25 (0.02) | 0.475 (0.022) | 44 (4) | 34 (1) | |
| EC + AT | 0.22 (0.01) | 0.494 (0.024) | 55 (4) | 37 (2) | |
| EC + ET | 0.20 (0.01) | 0.507 (0.048) | 65 (7) | 40 (3) |
| Explored Morphological Traits | Definition | Relationship with Plant CS | Relationship with Soil CS | Deduced Stage of C Accumulation That Is Affected | Refs. |
|---|---|---|---|---|---|
| Leaf angle | Angle between stem and leaves | +/− | n.d | Photoassimilation of atmospheric C | [49,116,117] |
| Leaf curvature | To the degree leaves are curved | − | n.d | Photoassimilation of atmospheric C | [117] |
| Leaf shape | Structural outline and appearance of leaf | + (Length), − (width) | n.d | Photoassimilation of atmospheric C | [118] |
| Internode length | Distance between leaf nodes | +/− | n.d | Photoassimilation of atmospheric C | [118,119] |
| Petiole length | Length of leaf petiole | + | n.d | Photoassimilation of atmospheric C | [119] |
| Leaf area | |||||
| Leaf area ratio per whole plant C | Leaf area to whole plant C ratio | − | n.d | Photoassimilation of atmospheric C and C storage in plant body | [93] |
| Specific leaf area | Leaf area to leaf drymass ratio and indicates leaf thickness | − | n.d | Photoassimilation of atmospheric C and C storage in plant body | [62] |
| Crown diameter | Diameter of the span of tree crown | n.k | n.d | Photoassimilation of atmospheric C | [120] |
| Culm diameter | Diameter of culm (modified stem) | + | n.d | C Storage in plant body | [40] |
| Root diameter | Diameter of roots | n.d | − | SOC sequestration | [39,52] |
| Root length and area | [34,35,63,85,121,122,123,124] | ||||
| Root length | The depth to which root extends | n.d | + | SOC sequestration | |
| Specific root length | Root length to root drymass ratio | n.d | + | SOC sequestration | |
| Root surface area | Total surface area of root mass | n.d | + | SOC sequestration | |
| Root length density | Total length of roots per unit soil volume | n.d | + | SOC sequestration | [7,39,125,126] |
| Species/Crops | Net Photosynthesis Rate | Leaf Transpiration | Stomatal Conductance | Intercellular CO2 Concentration | Reference(s) |
|---|---|---|---|---|---|
| Soybean (Glycine max) | +++ | −−− | −−− | +++ | [45] |
| Basil (Ocimum basilicum L.) | +++ | n.d | −−− | n.d | [115] |
| Peppermint (Mentha piperita L.) | +++ | n.d | −−− | n.d | [115] |
| Tea (Longjing changye) | +++ | −−− | −−− | +++ | [129] |
| Winter wheat (Triticum aestivum L. cv. MV 16) | +++ | −−− | 000 | 000 | [96] |
| Rhizosphere | AG | BG | CBH | XYL |
|---|---|---|---|---|
| Fine-root biomass | 0.333 | −0.373 | 0.561 | 0.496 |
| Rhizosphere soil mass | 0.273 | −0.097 | 0.33 | 0.69 |
| Coarse-root biomass | 0.646 | −0.329 | 0.099 | 0.389 |
| Total root biomass | 0.677 | −0.373 | 0.175 | 0.449 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharyya, S.S.; Mondaca, P.; Shushupti, O.; Ashfaq, S. Interplay between Plant Functional Traits and Soil Carbon Sequestration under Ambient and Elevated CO2 Levels. Sustainability 2023, 15, 7584. https://doi.org/10.3390/su15097584
Bhattacharyya SS, Mondaca P, Shushupti O, Ashfaq S. Interplay between Plant Functional Traits and Soil Carbon Sequestration under Ambient and Elevated CO2 Levels. Sustainability. 2023; 15(9):7584. https://doi.org/10.3390/su15097584
Chicago/Turabian StyleBhattacharyya, Siddhartha Shankar, Pedro Mondaca, Oloka Shushupti, and Sharjeel Ashfaq. 2023. "Interplay between Plant Functional Traits and Soil Carbon Sequestration under Ambient and Elevated CO2 Levels" Sustainability 15, no. 9: 7584. https://doi.org/10.3390/su15097584
APA StyleBhattacharyya, S. S., Mondaca, P., Shushupti, O., & Ashfaq, S. (2023). Interplay between Plant Functional Traits and Soil Carbon Sequestration under Ambient and Elevated CO2 Levels. Sustainability, 15(9), 7584. https://doi.org/10.3390/su15097584






