Distribution Patterns and Driving Factors of the Phytoplankton Community in the Middle Reaches of the Yarlung Zangbo River
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey of Study Area and Layout of Sampling Points
2.2. Sample Collection and Processing
2.3. Data Processing and Analysis
3. Results
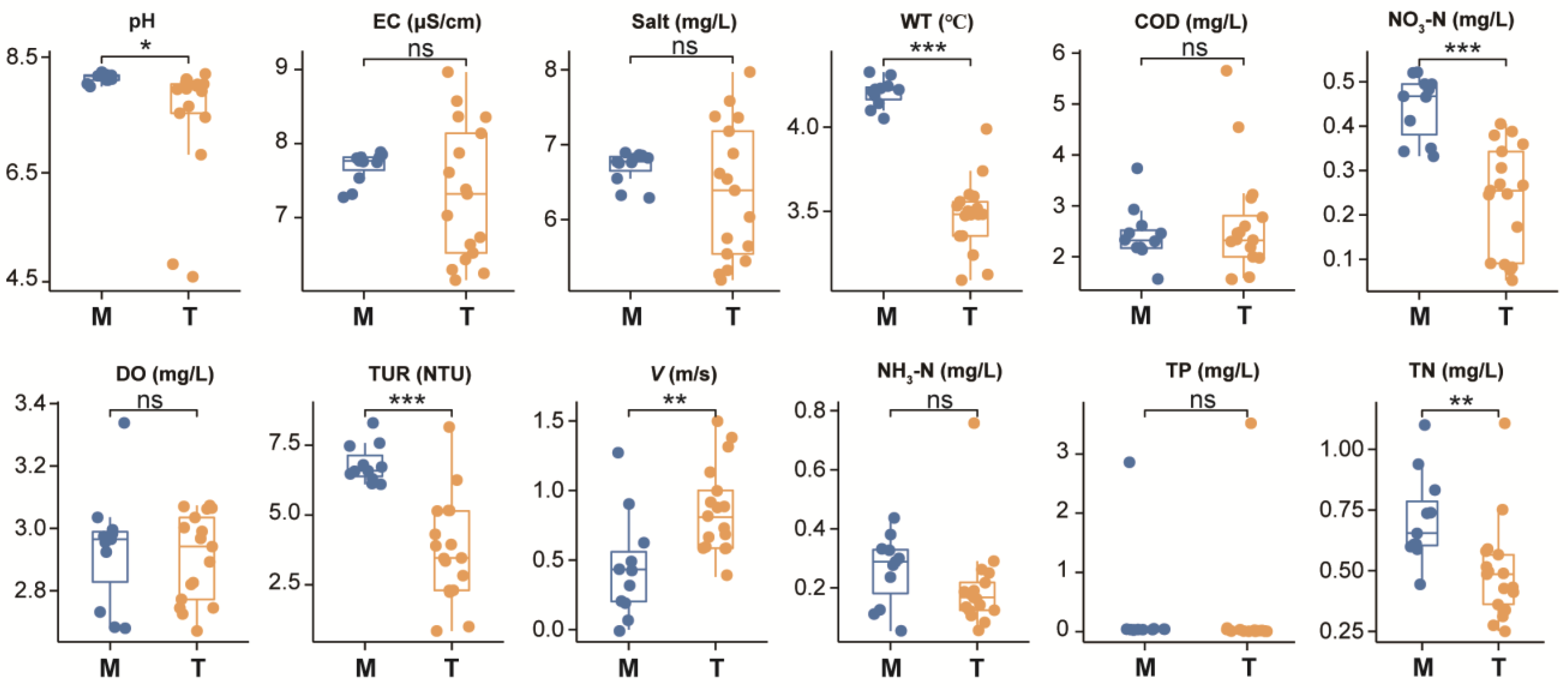
3.1. Difference Analysis of Water Environmental Factors in Different River Reaches
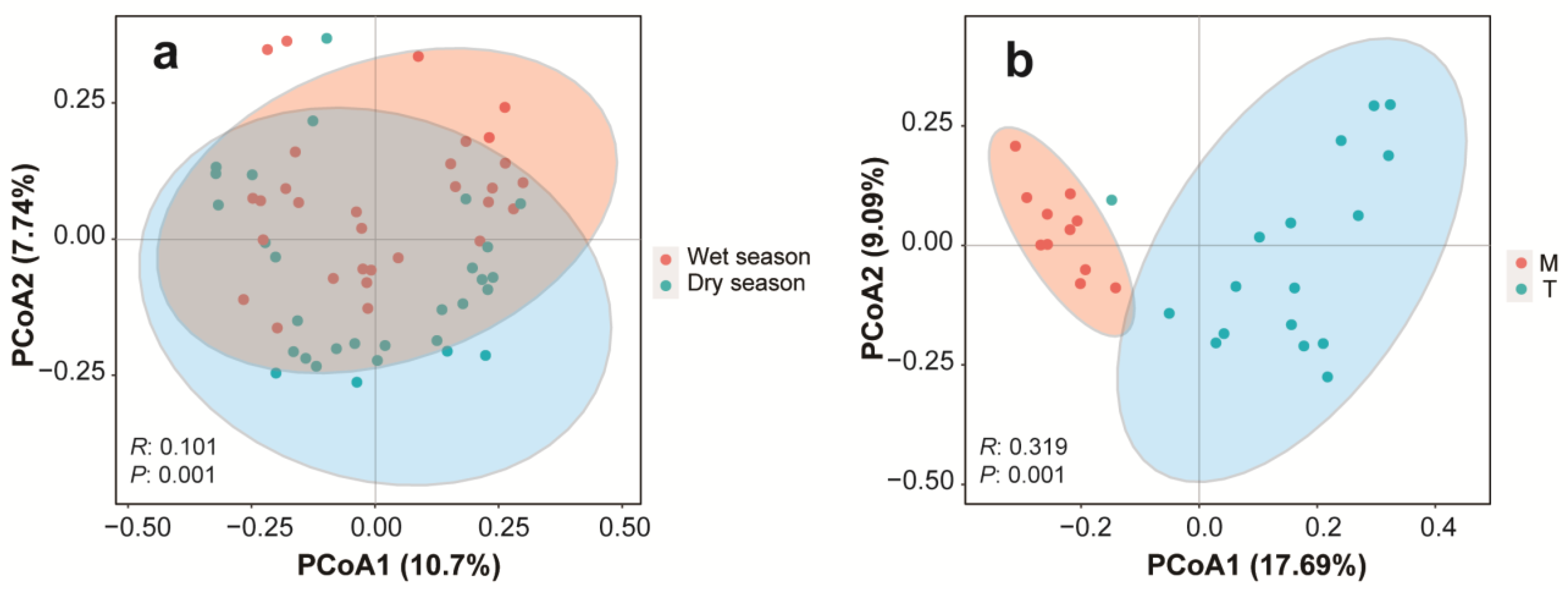
3.2. Spatio-Temporal Distribution Patterns of Phytoplankton Communities
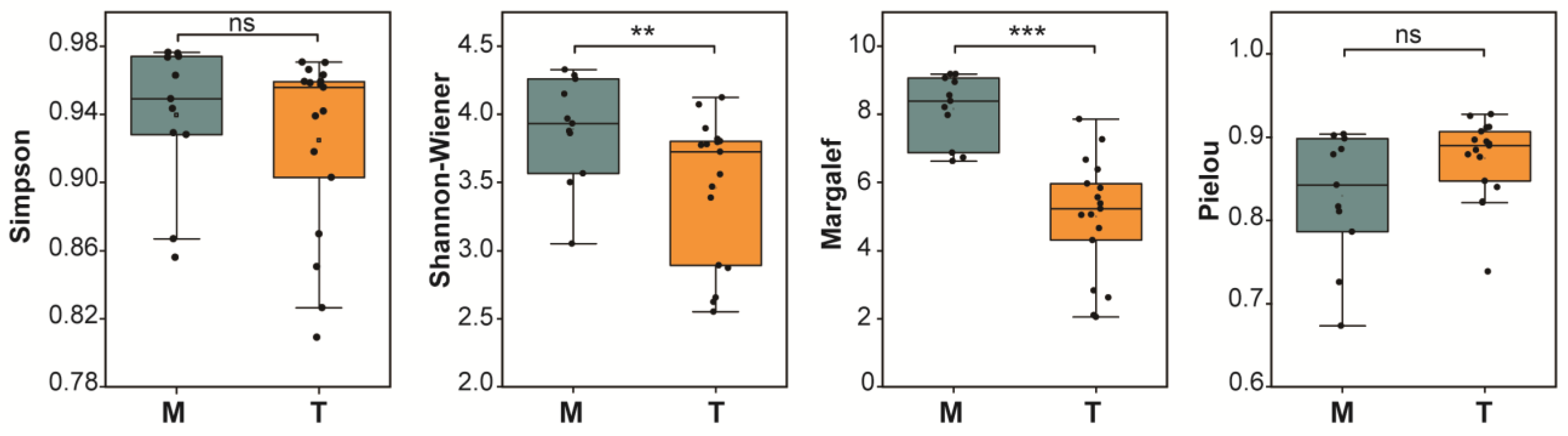
3.3. Differences in Alpha Diversity of Phytoplankton Communities
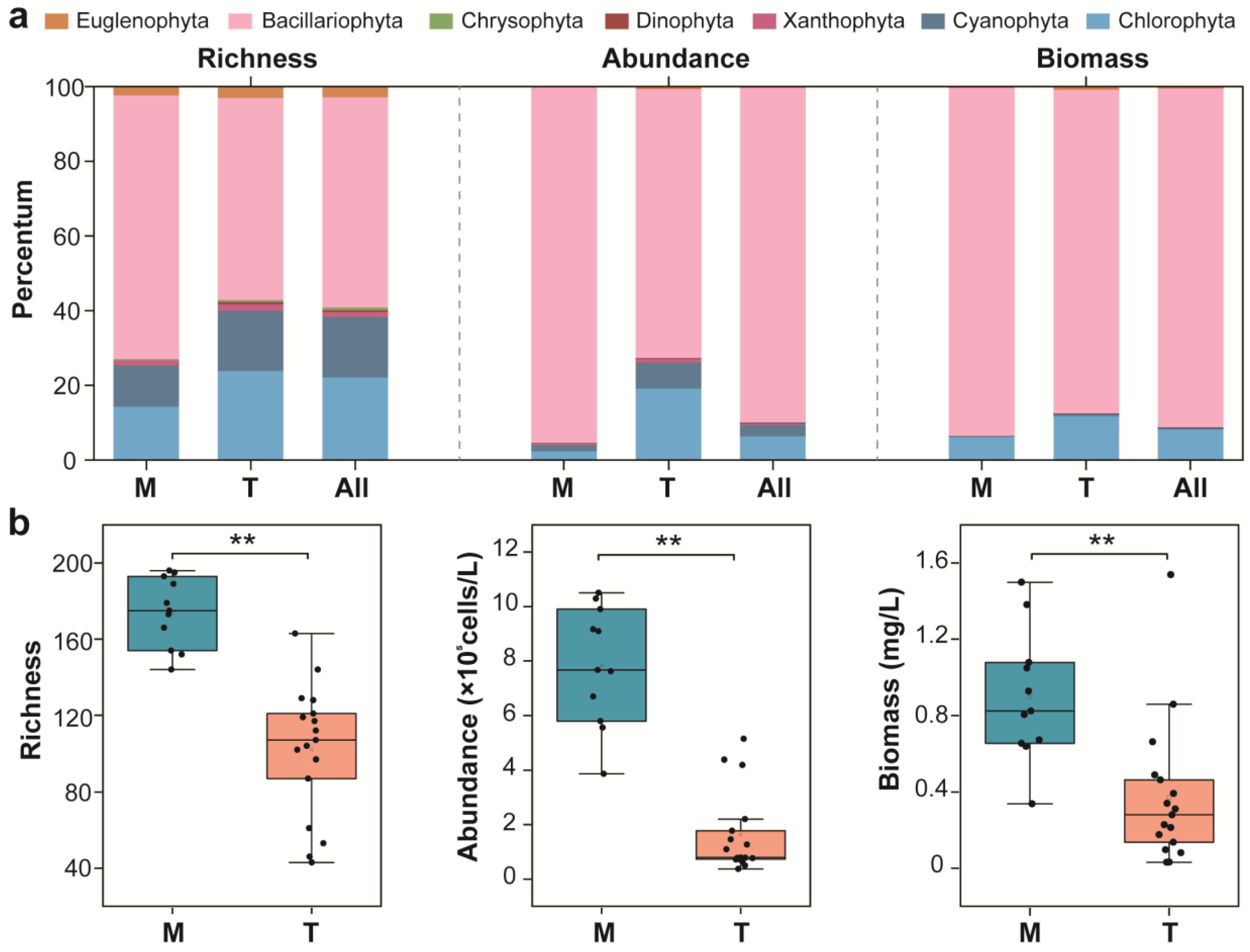
3.4. Characteristics of the Phytoplankton Community’s Structure
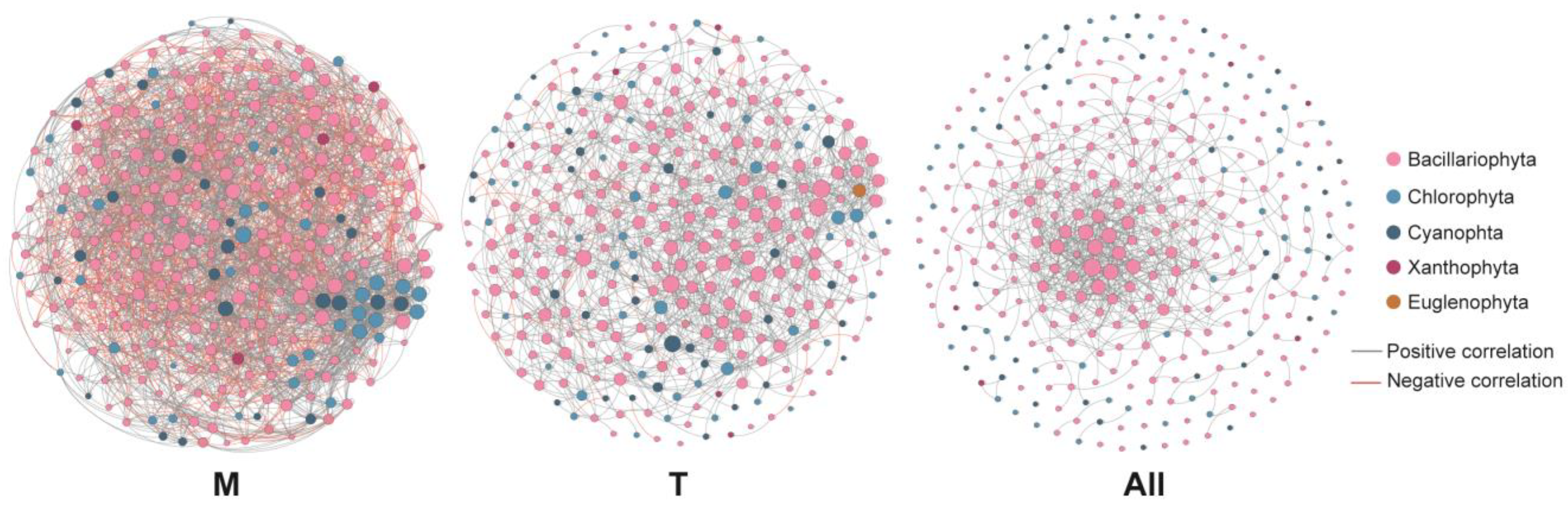
3.5. Co-Occurrence Network of the Phytoplankton Community
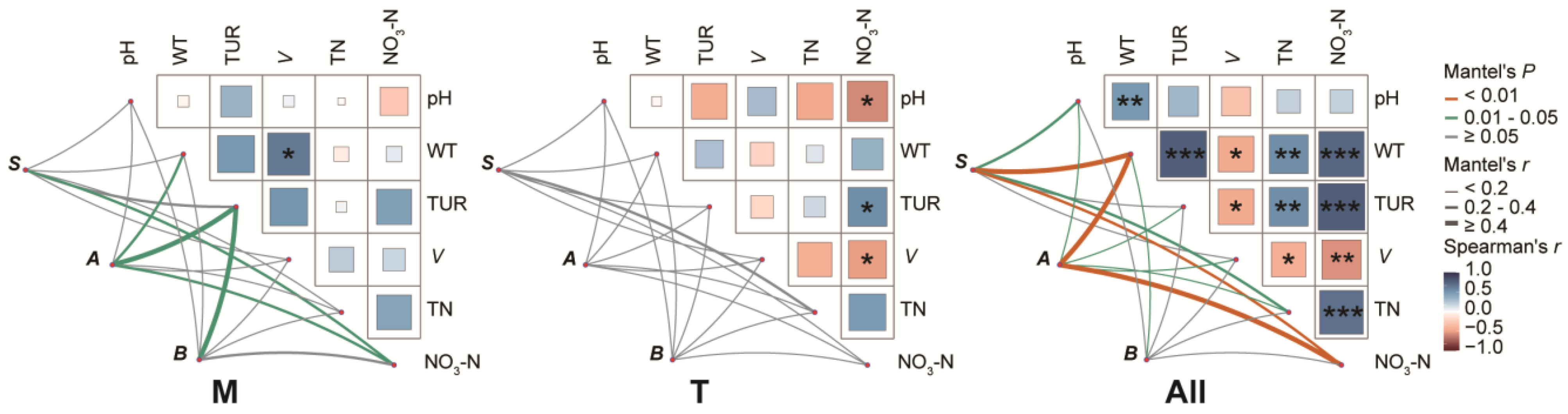
3.6. Environmental Factors Drive Diversity and Composition of the Phytoplankton Community
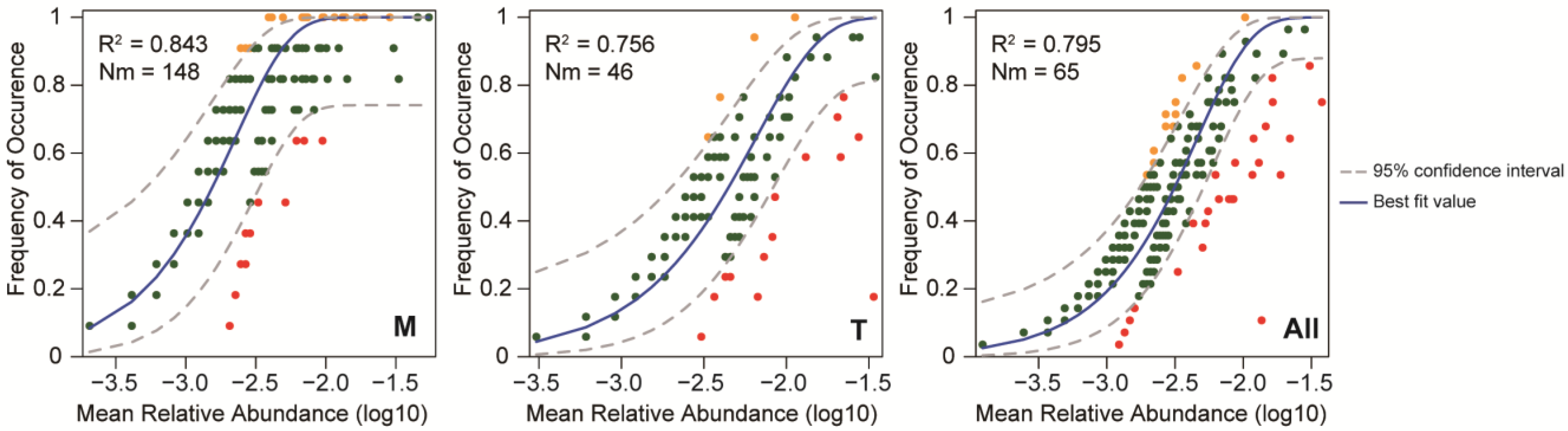
3.7. The Dispersal Limitation Effect on the Phytoplankton Community’s Assembly Processes
4. Discussion
4.1. Phytoplankton Community Diversity and Its Driving Factors
4.2. The Characteristics and Driving Factors of the Phytoplankton Community’s Structure
4.3. Co-Occurrence Patterns and Driving Factors in Phytoplankton Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salmaso, N.; Naselli, F.L.; Padisák, J. Impairing the largest and most productive forest on our planet: How do human activities impact phytoplankton. Hydrobiologia 2012, 698, 375–384. [Google Scholar] [CrossRef]
- Ishida, K.N. Seasonal distribution of photosynthetically active phytoplankton using pulse amplitude modulated fluorometry in the large monomictic Lake Biwa, Japan. J. Plankton Res. 2008, 30, 1169–1177. [Google Scholar]
- Jiang, Y.; Xu, H.L.; Zhu, M.Z. Use of planktonic protists for assessing water quality in Jiaozhou Bay, northern China. Protistology 2012, 7, 34–41. [Google Scholar]
- Filker, S.; Sommaruga, R.; Vila, I.; Stoeck, T. Microbial eukaryote plankton communities of high-mountain lakes from three continents exhibit strong biogeographic patterns. Mol. Ecol. 2016, 25, 2286–2301. [Google Scholar] [CrossRef]
- Jia, H.Y.; Xu, J.F.; Lei, J.S. Relationship of community structure of phytoplankton and environmental factors in Danjiangkou Reservoir bay. Yangtze River 2019, 50, 52–58. [Google Scholar]
- Torremorell, A.; Llames, M.E.; Pérez, G.L.; Escaray, R.; Bustingorry, J.; Zagarese, H. Annual patterns of phytoplankton density and primary production in a large, shallow lake: The central role of light. Freshw. Biol. 2009, 54, 437–449. [Google Scholar] [CrossRef]
- Shen, W.F. Planktonic Algal Science; Science Press: Beijing, China, 1999. [Google Scholar]
- Guo, K.; Wu, N.C.; Wang, C.; Yang, D.G.; He, Y.F.; Luo, J.B.; Chai, Y.; Duan, M.; Huang, X.F.; Riis, T. Trait dependent roles of environmental factors, spatial processes and grazing pressure on lake phytoplankton metacommunity. Ecol. Indic. 2019, 103, 312–320. [Google Scholar] [CrossRef]
- Cheng, R.; Chen, H.X.; Qi, C.; Hou, F.; Cao, X.X.; Zheng, X.; Zeng, F.G.; Li, Z.H. Characteristics of plankton community structure in Shahe Reservoir of the North Canal. Acta Sci. Circumstantiae 2021, 41, 239–246. [Google Scholar]
- Yang, M.; Zhang, S.; Liu, S.R. Phytoplankton community structure and water quality assessment in Jialing River after the impoundment of Caojie Reservoir. Environ. Sci. 2015, 36, 2480–2486. [Google Scholar]
- Xu, Z.; Chen, X.H.; Shen, G.X.; Zhu, Y.; Qian, X.Y.; Zhang, X.L.; Zhang, W.; Hu, S.Q.; Bai, Y.J. Spatial and Temporal Variation of Phytoplankton Community Structure and Its Influencing Factors in Shanghai River Channels. Environ. Sci. 2020, 41, 3621–3628. [Google Scholar]
- Zhong, X.H.; Liu, S.Z.; Wang, X.D.; Zhu, W.Z.; Li, X.M.; Yang, L. A Research on the Protection and Construction of the State Ecological Safe Shelter Zone on the Tibet Plateau. J. Mt. Sci. 2006, 24, 129–136. [Google Scholar]
- Jun, S.; Wang, D.B.; Zhou, J.H.; Bai, X.Y.; Bai, K. Community structures of phytoplankton and its relationship with environmental factors in the Lhasa River. Acta Ecol. Sin. 2019, 39, 787–798. [Google Scholar]
- Ba, S.; Yang, X.L.; Huang, X.; Gui, Q. Phytoplankton community characteristics and water quality evaluation in the lower reaches of Lhasa River in spring and summer. Res. Plateau Sci. 2017, 1, 25–38. [Google Scholar]
- Liu, H.P.; Ye, S.W.; Yang, X.F.; Zhang, L.S.; Zhong, G.H.; Li, Z.J. Spatial-temporal dynamics of aquatic organism community and their relationships to environment in Niyang River, Tibet: 1. Phytoplankton. J. Lake Sci. 2013, 25, 695–706. [Google Scholar]
- Pei, G.F.; Cao, J.X.; Liu, G.X. Diversity of phytoplankton community in different reaches of Niyang River. Resour. Environ. Yangtze Basin 2012, 21, 24–29. [Google Scholar]
- Zhang, R.; Xu, Z.X.; Liu, X.W.; Liu, J.T.; Bai, J.R. Spatiotemporal Characteristics of Land Use/Cover Change for the Yarlung Zangbo River Basin from 1980 to 2015. China Rural. Water Hydropower 2019, 3, 106–111. [Google Scholar]
- Guan, Z.H.; Chen, C.Y. Tibetan Rivers and Lakes; Science Press: Beijing, China, 1984. [Google Scholar]
- Wang, S.; Wang, S.S.; Fan, F.L. Change patterns of NDVl (1985–2018) in the Yarlung Zangbo River Basin of China based on time series segmentation algorithm. Acta Ecol. Sin. 2020, 40, 6863–6871. [Google Scholar]
- Liu, H.; Li, X.Y.; Yao, Z.Y. Rainfall erosivity in Yarlung Zangbo River Basin during 1961–2015. J. Desert Res. 2019, 39, 166–176. [Google Scholar]
- Zhang, Z.S.; Huang, X.F. Research Methods of Freshwater Plankton; Science Press: Beijing, China, 1991. [Google Scholar]
- Chi, N.W. Atlas of Bacillariophyta in Tibet; Tibet People’s Publishing House: Lhasa, China, 1990. [Google Scholar]
- Zhu, H.Z.; Chen, J.Y. Diatoms of Tibet, China; Science Press: Beijing, China, 2000. [Google Scholar]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China—Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Sun, J.; Liu, D.Y. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Kremer, C.T.; Gillette, J.P.; Rudstam, L.G.; Brettum, P.; Ptacnik, R. A compendium of cell and natural unit biovolumes for >1200 freshwater phytoplankton species. Ecology 2014, 95, 2984. [Google Scholar] [CrossRef]
- Margalef, R. Perspectives in ecological theory. Oikos 1969, 20, 571. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W.; Wiener, N. The mathematical theory of communication. Phys. Today 1950, 3, 31–32. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Li, Y.R.; Ma, J.; Qiu, X.C. Species Diversity of Phytoplankton Community in the Lakes in Ningxia Taiyangshan National Wetland Park. Wetl. Sci. 2021, 19, 375–383. [Google Scholar]
- Tang, Y.; Zheng, Y.H.; Liu, J.H.; Fu, M.; Wu, R.H.; Feng, X.W. Analysis on community structure and diversity of phytoplankton in the middle and lower reaches of the Hengjiang River in spring. Freshw. Fish. 2016, 46, 51–58. [Google Scholar]
- Qiu, Y.L.; Lin, Y.Q.; Liu, J.J.; Tang, L.; Guan, T.S.; Chen, Q.W.; Chen, K.; Wang, L. The biodiversity assessment of phytoplankton community in summer within main stream and tributary of Huaihe River. Acta Sci. Circumstantiae 2018, 38, 1665–1672. [Google Scholar]
- Yan, G.H.; Yin, X.Y.; Wang, X.; Wang, L.Q.; Li, Y.J.; Li, H.; Chen, W. Effects of environmental factors on phytoplankton community composition in the three mouths of the Yangtze River and west Dongting Lake. China Environ. Sci. 2019, 39, 2532–2540. [Google Scholar]
- Li, J.L.; Zheng, B.H.; Liu, L.S.; Tang, J.L. Phytoplankton Community Structure in the Yangtze River Estuary and Its Relation to Environmental Factors. Res. Environ. Sci. 2013, 26, 403–409. [Google Scholar]
- Mi, W.M.; Shi, J.Q.; Yang, Y.J.; Yang, S.Q.; He, S.H.; Wu, Z.X. Changes in epilithic algae community and its relationship with environmental factors in the Meixi River, a tributary of the Three Gorges Reservoir. Environ. Sci. 2020, 41, 1636–1647. [Google Scholar]
- Li, Y.M.; Zhao, Q.; Feng, G.P.; Liu, H.M.; Wang, Y.F. The diatom assemblages and their response to different environments of Baiyangdian Lake, China. Acta Ecol. Sin. 2010, 30, 4559–4570. [Google Scholar]
- Wei, J.W.; Li, H.R.; Wang, X.Z.; Qi, W.H.; Wang, Y.; Zhao, B.J.; Tan, X.; Zhang, Q.F. Structure Characteristics and Driving Variables of Epilithic Algae Community in Lhasa River Basin of Qinghai-Tibet Plateau. Environ. Sci. 2021, 42, 1879–1888. [Google Scholar]
- Myrstener, M.; Rocher-Ros, G.; Burrows, R.M.; Bergström, A.-K.; Giesler, R.; Sponseller, R.A. Persistent nitrogen limitation of stream biofilm communities along climate gradients in the Arctic. Glob. Chang. Biol. 2018, 24, 3680–3691. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.S.; Li, L.; Zhou, J.; Wang, Y.; Xia, F.; Xia, Y. Correlation analysis of algae composition and environmental factors in Jingpo Lake. China Environ. Sci. 2015, 35, 3403–3413. [Google Scholar]
- Schneider, S.C.; Kahlert, M.; Kelly, M.G. Interactions between pH and nutrients on benthic algae in streams and consequences for ecological status assessment and species richness patterns. Sci. Total Environ. 2013, 444, 73–84. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Shah, R.D.T.; Shah, D.N.; Hoppeler, F.; Jähnig, S.C.; Pauls, S.U. Metacommunity structuring in Himalayan streams over large elevational gradients: The role of dispersal routes and niche characteristics. J. Biogeogr. 2017, 44, 62–74. [Google Scholar] [CrossRef]
- Liu, L.M.; Chen, H.H.; Liu, M.; Yang, J.R.; Xiao, P.; Wilkinson, D.M.; Yang, J. Response of the eukaryotic plankton community to the cyanobacterial biomass cycle over 6 years in two subtropical reservoirs. ISME J. 2019, 13, 2196–2208. [Google Scholar] [CrossRef]
- Morris, L.A.; Voolstra, C.R.; Quigley, K.M.; Bourne, D.G.; Bay, L.K. Nutrient availability and metabolism affect the stability of coral-Symbiodiniaceae symbioses. Trends Microbiol. 2019, 27, 678–689. [Google Scholar] [CrossRef]
- Proulx, S.R.; Promislow, D.E.L.; Phillips, P.C. Network thinking in ecology and evolution. Trends Ecol. Evol. 2005, 20, 345–353. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.F.; He, Z.L.; Luo, F.; Zhou, J.Z. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Deng, Y.; Luo, F.; He, Z.L.; Tu, Q.C.; Zhi, X.Y. Functional molecular ecological networks. mBio 2010, 1, e00169-10. [Google Scholar] [CrossRef]
- Zhang, B.G.; Zhang, J.; Liu, Y.; Shi, P.; Wei, G.H. Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biol. Biochem. 2018, 118, 178–186. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, P.; An, R.Z.; Qiao, N.Q.; Da, Z.; Sang, B. Spatial and temporal distribution patterns and driving mechanisms of ciliate communities in the midstream and downstream reaches of the Lhasa River. Biodivers. Sci. 2022, 30, 136–150. [Google Scholar] [CrossRef]
- Selbmann, L.; Egidi, E.; Isola, D.; Onofri, S.; Zucconi, L.; de Hoog, G.S.; Chinaglia, S.; Testa, L.; Tosi, S.; Balestrazzi, A.; et al. Biodiversity, evolution and adaptation of fungi in extreme environments. Plant Biosyst. 2013, 147, 237–246. [Google Scholar] [CrossRef]
- Chen, W.D.; Ren, K.X.; Isabwe, A.; Chen, H.H.; Liu, M.; Yang, J. Correction to: Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 148. [Google Scholar] [CrossRef]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
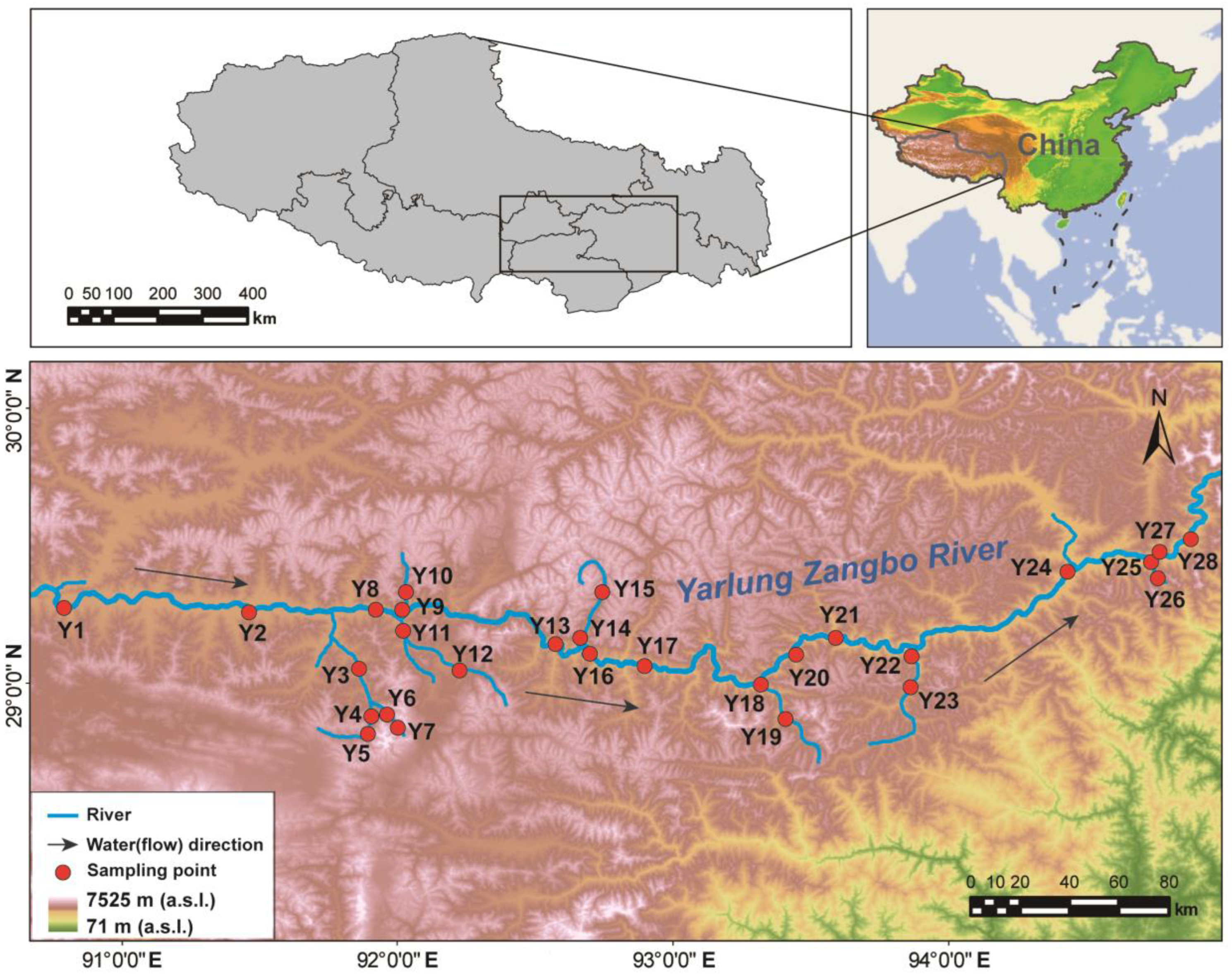

| Number | Type | Longitude | Latitude | Altitude (m) | Depth of Water (cm) | Velocity of Flow (m/s) |
|---|---|---|---|---|---|---|
| Y1 | main stream | 90°47′26.88″ | 29°16′35.76″ | 3537.0 | 58.0 | 0.1 |
| Y2 | main stream | 91°27′47.16″ | 29°15′46.08″ | 3527.0 | 70.0 | 0.4 |
| Y3 | tributary | 91°51′42.48″ | 29°3′25.56″ | 3714.0 | 69.0 | 0.7 |
| Y4 | tributary | 91°54′29.52″ | 28°52′57.36″ | 4113.0 | 41.0 | 0.6 |
| Y5 | tributary | 91°53′37.68″ | 28°49′14.16″ | 4505.0 | 55.0 | 1.1 |
| Y6 | tributary | 91°57′5.04″ | 28°53′30.12″ | 4221.0 | 37.0 | 1.7 |
| Y7 | tributary | 92°0′12.96″ | 28°50′33.36″ | 4740.0 | 39.0 | 0.4 |
| Y8 | main stream | 91°55′21″ | 29°16′15.96″ | 3510.0 | 30.0 | 0.2 |
| Y9 | tributary | 92°1′25.68″ | 29°11′38.04″ | 3571.0 | 45.0 | 0.6 |
| Y10 | tributary | 92°13′41.88″ | 29°3′4.32″ | 3880.0 | 36.0 | 1.3 |
| Y11 | tributary | 92°1′13.8″ | 29°15′26.64″ | 3513.0 | 25.0 | 0.4 |
| Y12 | tributary | 92°2′37.32″ | 29°20′11.04″ | 3752.0 | 34.0 | 0.7 |
| Y13 | main stream | 92°34′29.28″ | 29°8′52.08″ | 3166.0 | 47.0 | 1.9 |
| Y14 | tributary | 92°40′0.84″ | 29°10′2.64″ | 3369.0 | 60.0 | 1.9 |
| Y15 | tributary | 92°44′47.76″ | 29°20′4.2″ | 4137.0 | 70.0 | 0.5 |
| Y16 | main stream | 92°42′7.92″ | 29°6′36.36″ | 3116.0 | 50.0 | 0.2 |
| Y17 | main stream | 92°53′52.44″ | 29°3′57.24″ | 3076.0 | 70.0 | 0.6 |
| Y18 | tributary | 93°19′15.96″ | 28°59′57.12″ | 3012.0 | 41.0 | 1.6 |
| Y19 | tributary | 93°24′36″ | 28°52′33.24″ | 3479.0 | 37.0 | 1.0 |
| Y20 | main stream | 93°26′54.96″ | 29°6′23.76″ | 2945.0 | 50.0 | 0.7 |
| Y21 | main stream | 93°35′40.92″ | 29°10′5.16″ | 2934.0 | 50.0 | 0.1 |
| Y22 | tributary | 93°52′10.92″ | 29°6′3.6″ | 2956.0 | 40.0 | 1.0 |
| Y23 | tributary | 93°51′54.72″ | 28°59′18.24″ | 3203.0 | 50.0 | 3.1 |
| Y24 | main stream | 94°26′4.56″ | 29°24′33.48″ | 2896.0 | 50.0 | 0.1 |
| Y25 | tributary | 94°43′32.52″ | 29°26′36.96″ | 2914.0 | 45.0 | 1.2 |
| Y26 | tributary | 94°44′24″ | 29°25′13.08″ | 2986.0 | 40.0 | 0.7 |
| Y27 | main stream | 94°44′41.28″ | 29°27′19.8″ | 2886.0 | 40.0 | 0.2 |
| Y28 | main stream | 94°52′53.76″ | 29°31′32.88″ | 2874.0 | 43.0 | 0.1 |
| Parameter | Main Stream | Tributary | All |
|---|---|---|---|
| Number of nodes | 323 | 324 | 345 |
| The number of connections | 3129 | 1541 | 987 |
| Average degree | 19.375 | 9.512 | 5.722 |
| Average weighting degree | 6.548 | 8.204 | 6.383 |
| Proportion of positive correlation (%) | 69.93 | 97.66 | 99.7 |
| Proportion of negative correlation (%) | 30.07 | 2.34 | 0.30 |
| The density of figure | 0.06 | 0.029 | 0.017 |
| Coefficient of modularity | 1.145 | 0.661 | 0.493 |
| Average clustering coefficient | 0.377 | 0.527 | 0.658 |
| Average path length | 2.588 | 3.869 | 4.648 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, P.; Yang, Q.; Liu, H.; Chao, X.; Yang, S.; Ba, S. Distribution Patterns and Driving Factors of the Phytoplankton Community in the Middle Reaches of the Yarlung Zangbo River. Sustainability 2023, 15, 7162. https://doi.org/10.3390/su15097162
Li X, Zhang P, Yang Q, Liu H, Chao X, Yang S, Ba S. Distribution Patterns and Driving Factors of the Phytoplankton Community in the Middle Reaches of the Yarlung Zangbo River. Sustainability. 2023; 15(9):7162. https://doi.org/10.3390/su15097162
Chicago/Turabian StyleLi, Xiaodong, Peng Zhang, Qing Yang, Huiqiu Liu, Xin Chao, Shengxian Yang, and Sang Ba. 2023. "Distribution Patterns and Driving Factors of the Phytoplankton Community in the Middle Reaches of the Yarlung Zangbo River" Sustainability 15, no. 9: 7162. https://doi.org/10.3390/su15097162
APA StyleLi, X., Zhang, P., Yang, Q., Liu, H., Chao, X., Yang, S., & Ba, S. (2023). Distribution Patterns and Driving Factors of the Phytoplankton Community in the Middle Reaches of the Yarlung Zangbo River. Sustainability, 15(9), 7162. https://doi.org/10.3390/su15097162






