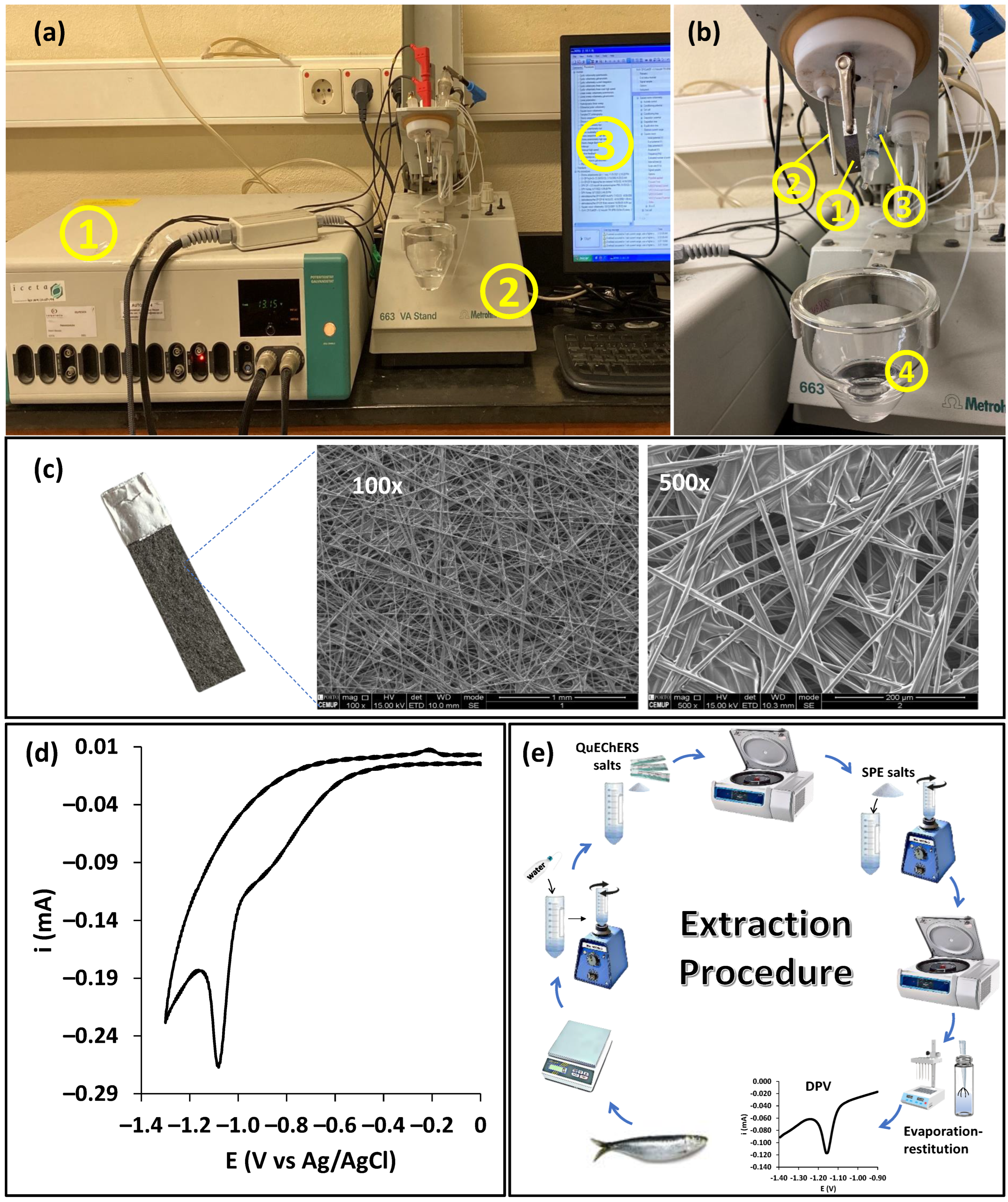
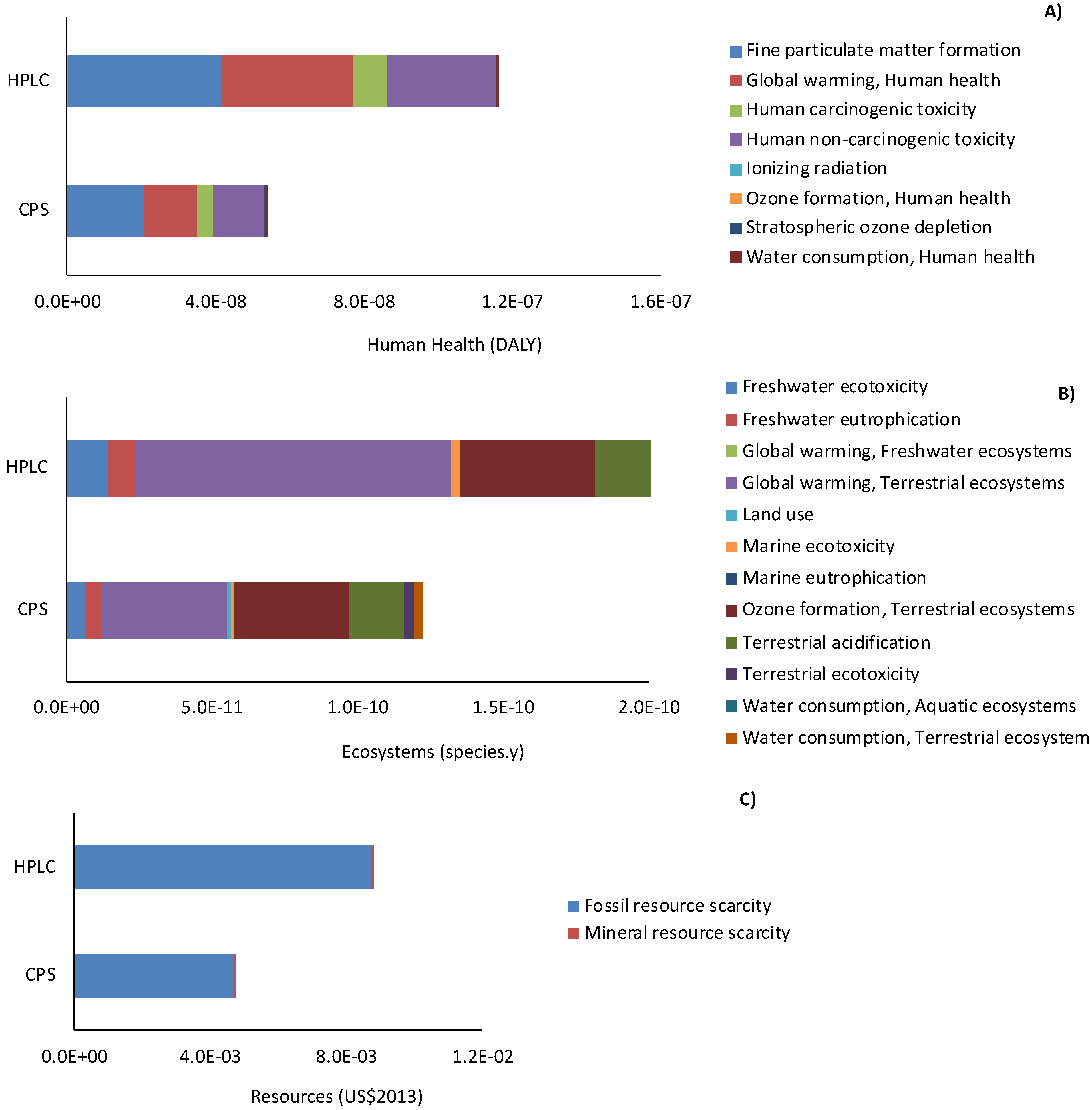3.1. Life Cycle Assessment
Table 8 shows a comparison of the environmental burdens associated with one ketoprofen analysis performed on fish extract samples using the novel CPS and an analysis conducted using the traditional chromatographic method (HPLC). The results were calculated at midpoint and endpoint levels, and they showed that the novel CPS had lower environmental impacts in almost all the categories when compared to HPLC, with percentages of decrease ranging, on average, from 15% (“ozone formation”) to 62% (“stratospheric ozone depletion”). These results suggest that the use of CPS for the determination of ketoprofen in fish sample extract is environmentally more advantageous than traditional HPLC methodology.
For the categories of impact of “land use” and “ionizing radiation ”, the CPS showed worse environmental performance than HPLC, represented by increases of 183% and 120%, respectively (
Table 8). This is attributed, in the case of “ionizing radiation”, to the higher environmental credits accounted by the ReCiPe2016 method based on the Ecoinvent 3.7 database [
20] for the electric energy mix associated with the production of acetonitrile and formic acid in the global market, with benefits of renewable biomass sources and avoidance of non-renewable sources of energy.
Applying the “weighting” function, which expresses all the impact results in a single scoring unit (points), it is possible to rank, group, and assign importance to the different impact categories based on stakeholder concerns, established policies, scientific targets, and economic aspects. The environmental impacts calculated as weighted values [
29] (highlighted in gray in
Table S4 of Supplementary Material) show that the categories of impact with the highest environmental relevance in the three areas of protection are “fine particulate matter formation”, “global warming, human health”, “human non-carcinogenic and carcinogenic toxicity”, and “fossil resource scarcity”.
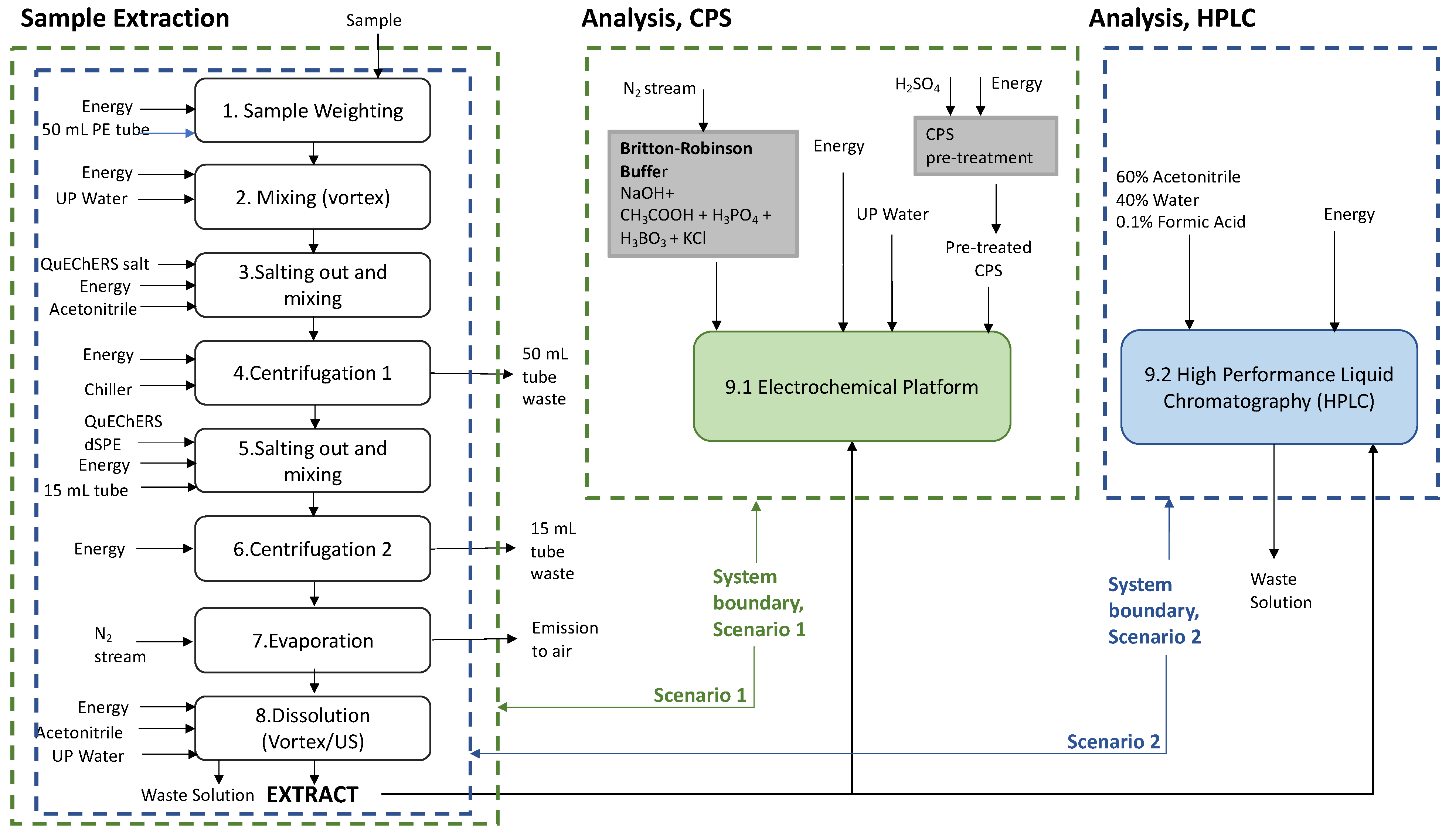
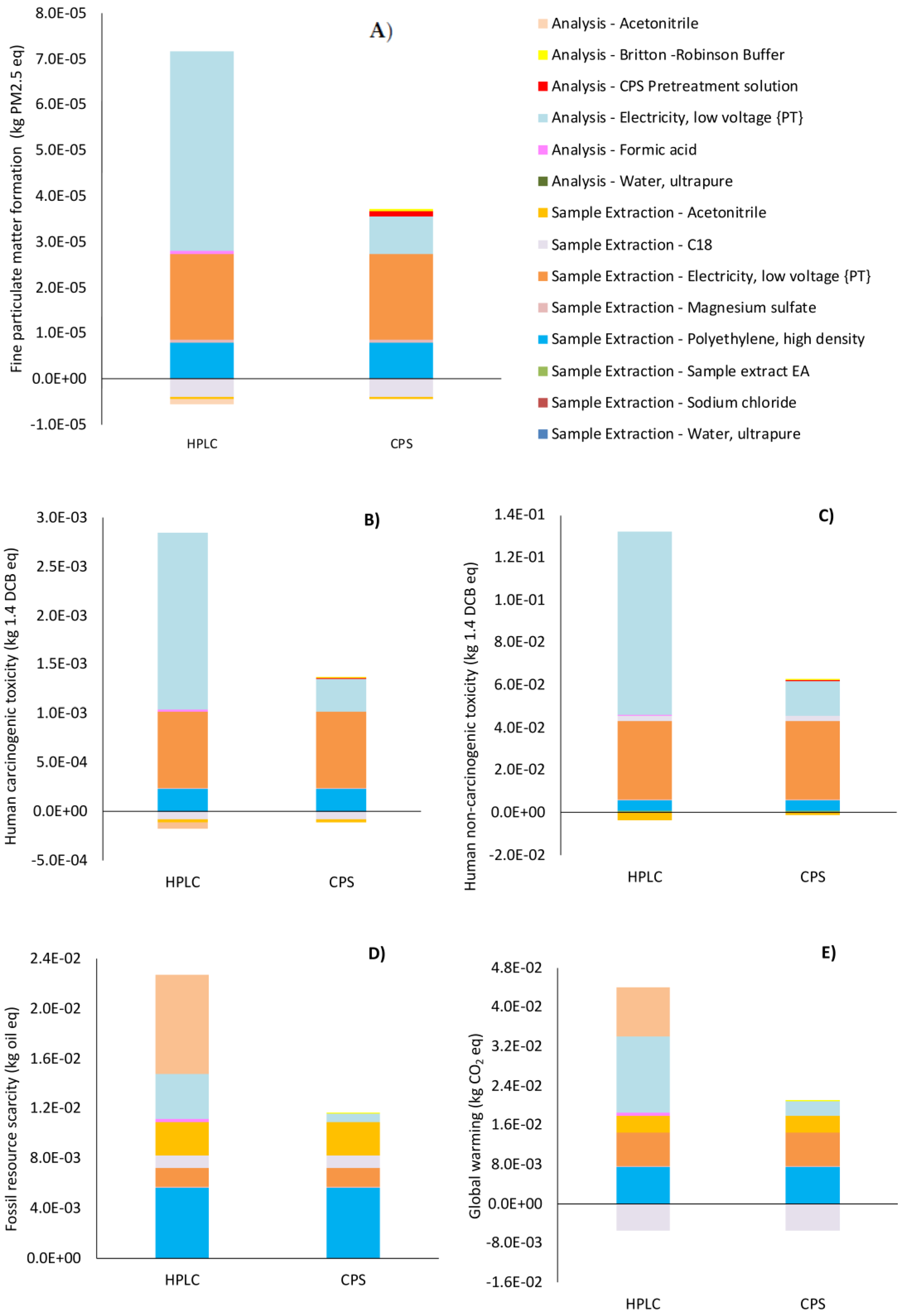
Figure 3A–E show the process contributions to the final values of the main environmental categories of impact (Midpoint). The results, as reported with a specific indication if the subprocesses contributed to the “sample extraction” or to the final “analysis” step (
Figure 1), confirmed the clear environmental advantages of CPS in comparison with HPLC in all the main categories of impact analyzed in this study.
Higher final values of impact were obtained in the HPLC scenario compared with the CPS scenario due to the higher contribution provided by the “analysis” step, which accounts for 52% (fossil resource scarcity) to 68% (global warming) of the total values of the main categories of impact. This is a direct consequence of the higher electric energy consumption associated with the HPLC “analysis” step, which is more than fivefold higher for HPLC than for the CPS scenario (
Table 3 and
Table 4). This result is in full agreement with the literature, wherein electricity consumption is considered the main contributor to the environmental impacts associated with chromatographic analysis [
6,
12,
30]. In fact, Schneider et al. [
6] observed that electricity consumption, rather than solvents, was the major contributor to the environmental burden associated with the use of the HPLC system.
Figure 3A shows that in both the HPLC and CPS scenarios the “fine particulate matter formation” (FPM) category of impact is mainly affected by the indirect loads associated with the electric energy production in the Portuguese energy mix, with the “sample extraction” step contributing 1.87 × 10
−5 kg PM2.5 eq in both the HPLC and CPS scenarios. The “analysis” step in the CPS scenario provides 8.20 × 10
−6 kg PM2.5 eq, which is 81% lower than the corresponding values generated by HPLC (4.35 × 10
−5 kg PM2.5eq). This evidence reflects the low electric energy demand required by CPS. The use of polyethylene tubes in the “sample extraction” step accounts for 24% and 12% of the final value of the FPM category of impact in the CPS and HPLC scenarios, respectively. It must be noted that FPM category of impact provides some environmental benefits, which are connected to acetonitrile and C18 salt, the production processes of which are able to reduce the final value of this category of impact by −1.20%, −2.37% (acetonitrile) and −12.3%, −6.1% (C18) in CPS and HPLC scenarios, respectively. These credits are derived from the market of hexane used for the synthesis of C18 QuEChERS salt and from the market of acetonitrile, the production processes of which, as modeled by the Ecoinvent 3.7 database, are based on hexane produced by molecular sieve separation of naphtha and methanol, both of which are supported by the process of industrial or district production of products other than natural gas. This last process is based on the global biomass market (wood pellets use) or other renewable fuels able to provide environmental credits to the system. The use of reagents marginally contribute to the final value of the FPM category of impact both in the “sample extraction” and “analysis” steps. For instance, magnesium sulfate accounts for 2.45% of the final FPM value, whereas sodium chloride accounts for 0.2% and 0.4% during the “sample extraction” step in the CPS and HPLC scenarios, respectively. The use of formic acid contributes 0.97% in the HPLC scenario, and CPS pretreatment reagents and Britton–Robinson buffer contribute 3.1% and 1.78% of the total value of FPM, respectively.
The human non-carcinogenic toxicity (HT-nc) category of impact is affected by the production of electric energy required for CPS and HPLC operations, accounting for 60.4% and 29% in the “sample extraction” step and by 26.5% and 67% in the “analysis” step, respectively, demonstrating, again, that electric energy production is the main contributor to HT-nc (
Figure 3B). The use of polyethylene tubes in the “sample extraction” step accounts for 3.7% and 7.8% of the total value of HT-nc in the HPLC and CPS scenarios, respectively (
Figure 3B). C18 preparation generates 2.15
10
−3 kg 1.4 DCB eq of human non-cancerogenic toxicity, corresponding to 1.7% and 3.5% of the final HT-nc value in the HPLC and CPS scenarios, respectively. This is mainly due to the contributions of the activated silica and polymethyl siloxane processes.
Similar relative percentages of contribution are observed for human carcinogenic toxicity (HT-c), with absolute values two orders of magnitude lower than for HT-nc (
Figure 3C). The use of polyethylene tubes is responsible for 17.4% and 8.3% of the total value of HT-c, with a contribution of 2.21
10
−4 kg 1.4 DCB eq. It must be noted that for both HT-nc and HT-c, the use of reagents such as magnesium chloride, sulfuric acid used for CPS pretreatment, formic acid, and Britton–Robinson buffer accounts for less than 1% to the final value of HT-nc and HT-c both in the CPS and HPLC scenarios, suggesting the low indirect toxic effect on human health derived by the processing of most of the reagents used in these processes. Environmental credits from acetonitrile in HT-nc and HT-c (>1.4%), as well as from C18 in HT-c (>3%), are also observed (
Figure 3C). This is due, as previously mentioned for the FPM category of impact, to the use of renewable resource for the production process in the global market (Ecoinvent 3.7) of acetonitrile and C18 constituents [
20].
The fossil resource scarcity (FRS) category of impact is affected in the “sample extraction” step by the production of polyethylene (5.6
10
−3 kg oil eq), acetonitrile (2.7
10
−3 kg oil eq), electric energy 1.5
10
−3 kg oil eq, and C18 (9.8
10
−4 kg oil eq) (
Figure 3D). In the CPS scenario, the “sample extraction” step contributes 94% to the final value of the FRS category of impact, with 48% due to polyethylene tube production, 23% due to acetonitrile, 13% to electric energy, and 8% due to C18 synthesis, whereas the remaining 6% is due to the electric energy required for the “analysis” step (
Figure 2D). In the HPLC scenario, approximately equal contributions are provided by the “sample extraction” (48%) and “analysis” (52%) steps to the final value of FRS, with the “analysis” step mainly affected by acetonitrile (7.9
10
−3 kg oil eq) and electric energy production (3.6
10
−3 kg oil eq), accounting for 35% and 16% of total FRS value, respectively. In the FRS category of impact, the main contributors are represented by the reagents rather than energy generation, and the use of CPS shows a decrease in FRS by 49% when compared with HPLC, which, on an annual basis, allows for savings of 43.6 kg oil eq. This result highlights the importance of the process contribution for each category of impact, since it provides information for further environmental improvements.
Finally,
Figure 2E represents a comparison of the impacts associated with the global warming (GW) category of impact for the CPS and HPLC scenarios. HPLC shows a final value of impact (3.85 × 10
−2 kg CO
2 eq) 150% higher than that for the CPS scenario (1.54 × 10
−2 kg CO
2 eq), which, on an annual basis, corresponds to a savings of 91.4 kg CO
2 eq. The main contributions associated with the “sample extraction” step, which are equal for both the HPLC and CPS scenarios, include polyethylene (high density, granulate for tube production) (7.4
10
−3 kg CO
2 eq), acetonitrile (3.4
10
−3 kg CO
2 eq), electric energy production (1.34
10
−2 kg CO
2 eq) and rely on significant environmental credits provided by C18 synthesis (−5.6
10
−3 kg CO
2 eq). The “analysis” step is mainly affected by electric energy consumption, the contribution of which in the HPLC scenario is 430% higher than in the CPS scenario and represents 41% of total HPLC GW value, whereas acetonitrile represents 26% (1.0
10
−2 kg CO
2 eq). This evidence is confirmed by available literature data [
6,
12,
30]. In the CPS scenario, electric energy consumption accounts for 19% of the final GW value, and reagents such as formic acid, Britton–Robinson buffer, and sulfuric acid used for CPS pretreatment contribute individually, with percentages always lower than 1.7%, representing minor contributions.
Figure 4A–C show the relative contributions of the categories of impact to the areas of protection of human health (DALY), ecosystems (species.y), and resources (USD 2013) calculated at the endpoint level. In all cases, CPS has fewer associated damages than HPLC, with 54%, 47.5%, and 46% lower damages in the three areas of protection, respectively. These results are in agreement with those found in a previous study [
12], although the method used for calculation was different (ReCiPe2016 vs. IMPac 2000+).
Regarding human health (
Figure 4A), “fine matter formation”, “global warming, human health”, and “human non-carcinogenic toxicity” are the main categories responsible for human health damage, whereas for the ecosystem area of protection (
Figure 4B) “global warming, terrestrial ecosystems”, “ozone formation”, and “terrestrial acidification” account for, on average, 41%, 26%, and 16% of the final value, respectively.
Finally, as shown in
Figure 3C, “mineral resource scarcity” marginally contributes to resource depletion, with damage associated with “fossil resource scarcity” accounting for 99% of the total damage in the area of protection of resources in both the CPS and HPLC scenarios.
3.3. Discussion
Analysis of the categories of impact with the highest environmental relevance according to ReCiPe2016(H) weighting factors shows that the worse environmental performance provided by the HPLC scenario is mainly caused by the higher indirect impact associated with electric energy consumption during the “analysis” step for the FPM, HTR-nc, and HT-c categories of impact. For FRS and GW, beyond electric energy consumption, the use of acetonitrile as major component of the mobile phase contributes to the final impact values always being higher than 25%.
It must be noted that the use of reagents such as magnesium chloride, sulfuric acid solution for CPS pretreatment, formic acid, and Britton–Robinson buffer provides individually contributions always lower than 3.1% to the final value of the FPM, HT-nc, HT-c, and GWP categories of impact both in the CPS and HPLC scenarios; therefore, they can be considered minor environmental contributors.
Quantification of the advantages of the CPS on an annual basis shows an annual savings of 333 kg 1.4 DB eq of non-carcinogenic toxicity and 6.9 kg 1.4 DB eq of carcinogenic toxicity for human health, 43.6 kg oil eq in terms of fossil resources, and 91.4 kg CO2 eq of greenhouse gases emissions in comparison with HPLC.
In the “sample extraction” step, the use of polyethylene tubes to contain QuEChERS salts represents a significant contribution to the final values of the FPM, HTR-nc, HT-c, FRS, and GW categories of impact, accounting for 24 %, 17.4%, 7.8%, 48%, and 48% in the CPS scenario and 12 %, 8.3%, 3.7%, 25%, and 19% in the HPLC scenario, respectively.
The critical environmental aspects associated with the operation of the novel portable CPS device are related to energy supply and the use of fossil-derived polyethylene tubes. The systematization of the use of recycled polyethylene tubes, as well as the design of a CPS device energetically supplied by solar batteries or by 100% renewable sources, may drastically improve its environmental performance, making the novel device close to carbon-neutral, since processes that currently have higher impacts provide credits to the system.