The Role of Surface Functional Groups of Iron Oxide, Organic Matter, and Clay Mineral Complexes in Sediments on the Adsorption of Copper Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Basic Physical and Chemical Properties
2.3. Preparation of the Complex
2.4. Fitting of Adsorption Isotherms
2.5. Characterization
2.5.1. X-ray Diffraction (XRD)
2.5.2. Fourier Infrared Spectroscopy (FTIR)
2.6. Data Processing
3. Results
3.1. Three Complex Adsorption Isothermal Curves
3.2. Adsorption Studies
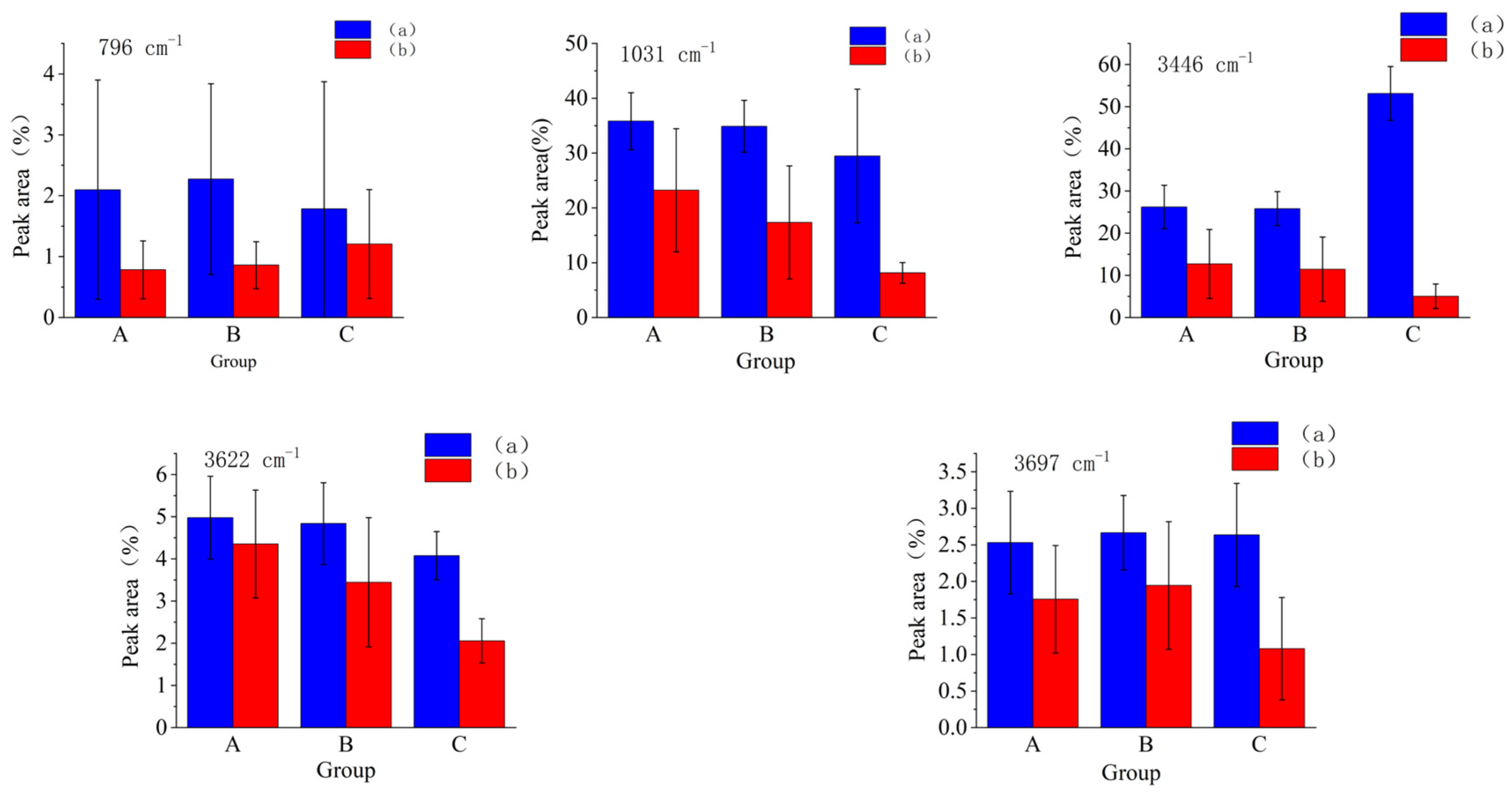
3.2.1. Fourier Infrared Spectroscopy Analysis of Three Groups of Complexes
3.2.2. Influence of Clay Mineral Composition and Intrinsic Structure on Copper Adsorption by Sediment Complex
4. Discussion and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Y.; Liu, L.; Xiang, H.; Wang, Y.; Sun, X. Biomass-based carbon microspheres for removing heavy metals from the environment: A review. Mater. Today Sustain. 2022, 18, 100136. [Google Scholar] [CrossRef]
- Peng, J.-F.; Song, Y.-H.; Yuan, P.; Cui, X.-Y.; Qiu, G.-L. The remediation of heavy metals contaminated sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-s.; Liu, L.; Fang, Y.-c.; Sun, X.-l. The adsorption characteristics of Cu(II) and Zn(II) on the sediments at the mouth of a typical urban polluted river in Dianchi Lake: Taking Xinhe as an example. Sci. Rep. 2021, 11, 17067. [Google Scholar] [CrossRef]
- Bi, S.; Yang, Y.; Xu, C.; Zhang, Y.; Zhang, X.; Zhang, X. Distribution of heavy metals and environmental assessment of surface sediment of typical estuaries in eastern China. Mar. Pollut. Bull. 2017, 121, 357–366. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Carbohydrate biopolymers, lignin based adsorbents for removal of heavy metals (Cd2+, Pb2+, Zn2+) from wastewater, regeneration and reuse for spent adsorbents including latent fingerprint detection: A review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef]
- Bruemmer, G.W.; Gerth, J.; Herms, U. Heavy metal species, mobility and availability in soils. Z. Pflanz. Bodenkd. 1986, 149, 382–398. [Google Scholar] [CrossRef]
- Kourim, A.; Malouki, M.A.; Ziouche, A.; Boulahbal, M.; Mokhtari, M. Tamanrasset’s Clay Characterization and Use as Low Cost, Ecofriendly and Sustainable Material for Water Treatment: Progress and Challenge in Copper Cu (II). Defect Diffus. Forum 2021, 406, 457–472. [Google Scholar] [CrossRef]
- Shu, Q.; Qiu, W.; Luo, M.; Xiao, L. Morphology-controlled hydrothermal synthesis of copper selenides with orange juice for highly efficient cationic dyes adsorption. Mater. Today Sustain. 2022, 17, 100094. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Teng, H.; Yang, H.; Liu, A.; Li, M.; Niu, X. Historical Evolution of Sources and Pollution Levels of Heavy Metals in the Sediment of the Shuanglong Reservoir, China. Water 2020, 12, 1855. [Google Scholar] [CrossRef]
- Wei, M.-p.; Chai, H.; Cao, Y.-l.; Jia, D.-z. Sulfonated graphene oxide as an adsorbent for removal of Pb2+ and methylene blue. J. Colloid Interface Sci. 2018, 524, 297–305. [Google Scholar] [CrossRef]
- Herrero, J.; Perez-Coveta, O. Soil salinity changes over 24 years in a Mediterranean irrigated district. Geoderma 2005, 125, 287–308. [Google Scholar] [CrossRef]
- Jankauskas, B.; Slepetiene, A.; Jankauskiene, G.; Fullen, M.A.; Booth, C.A. A comparative study of analytical methodologies to determine the soil organic matter content of Lithuanian Eutric Albeluvisols. Geoderma 2006, 136, 763–773. [Google Scholar] [CrossRef]
- Dohrmann, R.; Kaufhold, S. Three new, quick cec methods for determining the amounts of exchangeable calcium cations in calcareous clays. Clays Clay Miner. 2009, 57, 338–352. [Google Scholar] [CrossRef]
- Syu, C.-H.; Jiang, P.-Y.; Huang, H.-H.; Chen, W.-T.; Lin, T.-H.; Lee, D.-Y. Arsenic sequestration in iron plaque and its effect on As uptake by rice plants grown in paddy soils with high contents of As, iron oxides, and organic matter. Soil Sci. Plant Nutr. 2013, 59, 463–471. [Google Scholar] [CrossRef]
- Silva-Yumi, J.; Escudey, M.; Gacitua, M.; Pizarro, C. Kinetics, adsorption and desorption of Cd(II) and Cu(II) on natural allophane: Effect of iron oxide coating. Geoderma 2018, 319, 70–79. [Google Scholar] [CrossRef]
- Sun, Y.; Pang, H.; Li, Z.; Kang, H.; Zhang, S. Polyphenols induced in situ organic-inorganic crosslinking/mineralization strategy for constructing eco-friendly soy adhesive with high waterproof bonding strength. Compos. Part B Eng. 2022, 242, 110027. [Google Scholar] [CrossRef]
- Li, Z.; Huang, B.; Huang, J.; Chen, G.; Zhang, C.; Nie, X.; Luo, N.; Yao, H.; Ma, W.; Zeng, G. Influence of removal of organic matter and iron and manganese oxides on cadmium adsorption by red paddy soil aggregates. RSC Adv. 2015, 5, 90588–90595. [Google Scholar] [CrossRef]
- Mehra, O.P.; Jackson, M.L. Iron Oxide Removal from Soils and Clays by a Dithionite-Citrate System Buffered with Sodium Bicarbonate. Clays Clay Miner. 1958, 7, 317–327. [Google Scholar] [CrossRef]
- Edwards, A.; Bremner, J. Dispersion of soil particles by sonic Vibration. J. Soil Sci. 2006, 18, 47–63. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Soulages, O.E.; Acebal, S.G.; Rueda, E.H.; Torres Sanchez, R.M. Sorption of Zn(II) and Cu(II) by four Argentinean soils as affected by pH, oxides, organic matter and clay content. Environ. Earth Sci. 2015, 74, 4201–4214. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Zhang, J.; Jiao, C.; Ding, L.; Yang, S. Synthesis and Adsorption Properties of Novel Bacterial Cellulose/Graphene Oxide/Attapulgite Materials for Cu and Pb Ions in Aqueous Solutions. Materials 2020, 13, 3703. [Google Scholar] [CrossRef]
- Alexander, J.A.; Zaini, M.A.A.; Abdulsalam, S.; El-Nafaty, U.A.; Aroke, U.O. Isotherm studies of lead(II), manganese(II), and cadmium(II) adsorption by Nigerian bentonite clay in single and multimetal solutions. Part. Sci. Technol. 2019, 37, 399–409. [Google Scholar] [CrossRef]
- Luo, M.; Yang, S.; Shen, S.; Li, Y. Adsorption Characteristics of Oxytetracycline by Different Fractions of the Organic Matter from Humus Soil: Insight from Internal Structure and Composition. Int. J. Environ. Res. Public Health 2020, 17, 914. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Iskrenova-Tchoukova, E.; Ahn, W.-Y.; Clark, M.M.; Kirkpatrick, R.J. Effects of Ca2+ on supramolecular aggregation of natural organic matter in aqueous solutions: A comparison of molecular modeling approaches. Geoderma 2011, 169, 27–32. [Google Scholar] [CrossRef]
- Huang, Z.; Lv, J.; Caoa, D.; Zhang, S. Iron plays an important role in molecular fractionation of dissolved organic matter at soil-water interface. Sci. Total Environ. 2019, 670, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.-H.; Li, H.-Y.; Dong, X.-Y.; Zang, S.-Q. Robust multifunctional Zr-based metal-organic polyhedra for high proton conductivity and selective CO2 capture. J. Mater. Chem. A 2018, 6, 7724–7730. [Google Scholar] [CrossRef]
- Ndiaye, D.; Coufourier, S.; Mbaye, M.D.; Gaillard, S.; Renaud, J.-L. Cyclopentadienone Iron Tricarbonyl Complexes-Catalyzed Hydrogen Transfer in Water. Molecules 2020, 25, 421. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.L.A.; Alleoni, L.R.F.; Camargo, O.A.; Casagrande, J.C. Copper adsorption in oxidic soils after removal of organic matter and iron oxides. Commun. Soil Sci. Plant Anal. 2002, 33, 3581–3592. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.-l.; Ding, T.; Song, Y.-J.; Li, H.-C.; Li, D.-q.; Chen, S.; Xu, F. The role of surface functional groups of pectin and pectin-based materials on the adsorption of heavy metal ions and dyes. Carbohydr. Polym. 2022, 276, 118789. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Kukkadapu, R.; Cliff, J.B.; Smallwood, C.R.; Kovarik, L.; Wirth, M.G.; Engelhard, M.H.; Varga, T.; Dohnalkova, A.; Perea, D.E.; et al. Calcareous organic matter coatings sequester siderophores in alkaline soils. Sci. Total Environ. 2020, 724, 138250. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Ziola-Frankowska, A.; Nowicki, P.; Frankowski, M.; Wolski, R.; Pietrzak, R. Removal of Heavy Metal Ions from One- and Two-Component Solutions via Adsorption on N-Doped Activated Carbon. Materials 2021, 14, 7045. [Google Scholar] [CrossRef] [PubMed]
- Ben Ali, M.; Wang, F.; Boukherroub, R.; Lei, W.; Xia, M. Phytic acid-doped polyaniline nanofibers-clay mineral for efficient adsorption of copper (II) ions. J. Colloid Interface Sci. 2019, 553, 688–698. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Ni, F.; Liu, Q.; Yang, Y.; Wang, M.; Ao, T.; Chen, W. Synthesis of Magnesium Modified Biochar for Removing Copper, Lead and Cadmium in Single and Binary Systems from Aqueous Solutions: Adsorption Mechanism. Water 2021, 13, 599. [Google Scholar] [CrossRef]
- Tatsumi, T.; Tahara, Y.; Matsumoto, M. Adsorption of Metallic Ions on Amidoxime-Chitosan/Cellulose Hydrogels. Separations 2021, 8, 202. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Li, G.; Jiang, T. Effects of metal cations on the fulvic acid (FA) adsorption onto natural iron oxide in iron ore pelletizing process. Powder Technol. 2016, 302, 90–99. [Google Scholar] [CrossRef]
- Yang, R.; Li, Z.; Huang, M.; Luo, N.; Wen, J.; Zeng, G. Characteristics of fulvic acid during coprecipitation and adsorption to iron oxides-copper aqueous system. J. Mol. Liq. 2019, 274, 664–672. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Han, X.; Tang, Z.; Song, F.; Zhang, S.; Zhu, Y.; Guo, W.; He, Z.; Guo, Q.; et al. Colloidal stability of Fe3O4 magnetic nanoparticles differentially impacted by dissolved organic matter and cations in synthetic and naturally-occurred environmental waters. Environ. Pollut. 2018, 241, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, B.; Tang, B.; Luan, L.; Xu, W.; Zhang, B.; Niu, Y. Adsorption of aqueous Cu(II) and Ag(I) by silica anchored Schiffbase decorated polyamidoamine dendrimers: Behavior and mechanism. Chin. Chem. Lett. 2022, 33, 2721–2725. [Google Scholar] [CrossRef]
- Jiang, L.; Guan, J.; Zhao, L.; Li, J.; Yang, W. pH-dependent aggregation of citrate-capped Au nanoparticles induced by Cu2+ ions: The competition effect of hydroxyl groups with the carboxyl groups. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 216–220. [Google Scholar] [CrossRef]
- Huang, C.-H.; Shan-Yi, S.; Cheng-Di, D.; Mohanraj, K.; Jih-HsingTI, C. Removal Mechanism Effective Current of Electrocoagulation for Treating Wastewater Containing Ni, Cu, Cr. Water 2020, 12, 2614. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, K.; Kim, B.K.; Yang, J.W. Humic substance-enhanced ultrafiltration for removal of cobalt. J. Hazard. Mater. 2005, 122, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Z.; Yuan, Y.; Liu, F.; Zhu, C.; Ling, C.; Li, A. Insight into Cu(II) Adsorption on Polyamine Resin in the Presence of HEDP by Tracking the Evolution of Amino Groups and Cu(II)-HEDP Complexes. Acs Sustain. Chem. Eng. 2019, 7, 5256–5263. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, C.; Zhou, S.; Li, G.; Lu, T.; Tian, Y.; He, P. Low-Temperature Co-hydroxylated Cu/SiO2 Hybrid Bonding Strategy for a Memory-Centric Chip Architecture. ACS Appl. Mater. Interfaces 2021, 13, 38866–38876. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Hsu, L.-C.; Chan, Y.-T.; Cho, Y.-L.; Tsao, F.-Y.; Tzou, Y.-M.; Hsieh, Y.-C.; Liu, Y.-T. Phosphate Removal in Relation to Structural Development of Humic Acid-Iron Coprecipitates. Sci. Rep. 2018, 8, 11584. [Google Scholar] [CrossRef]
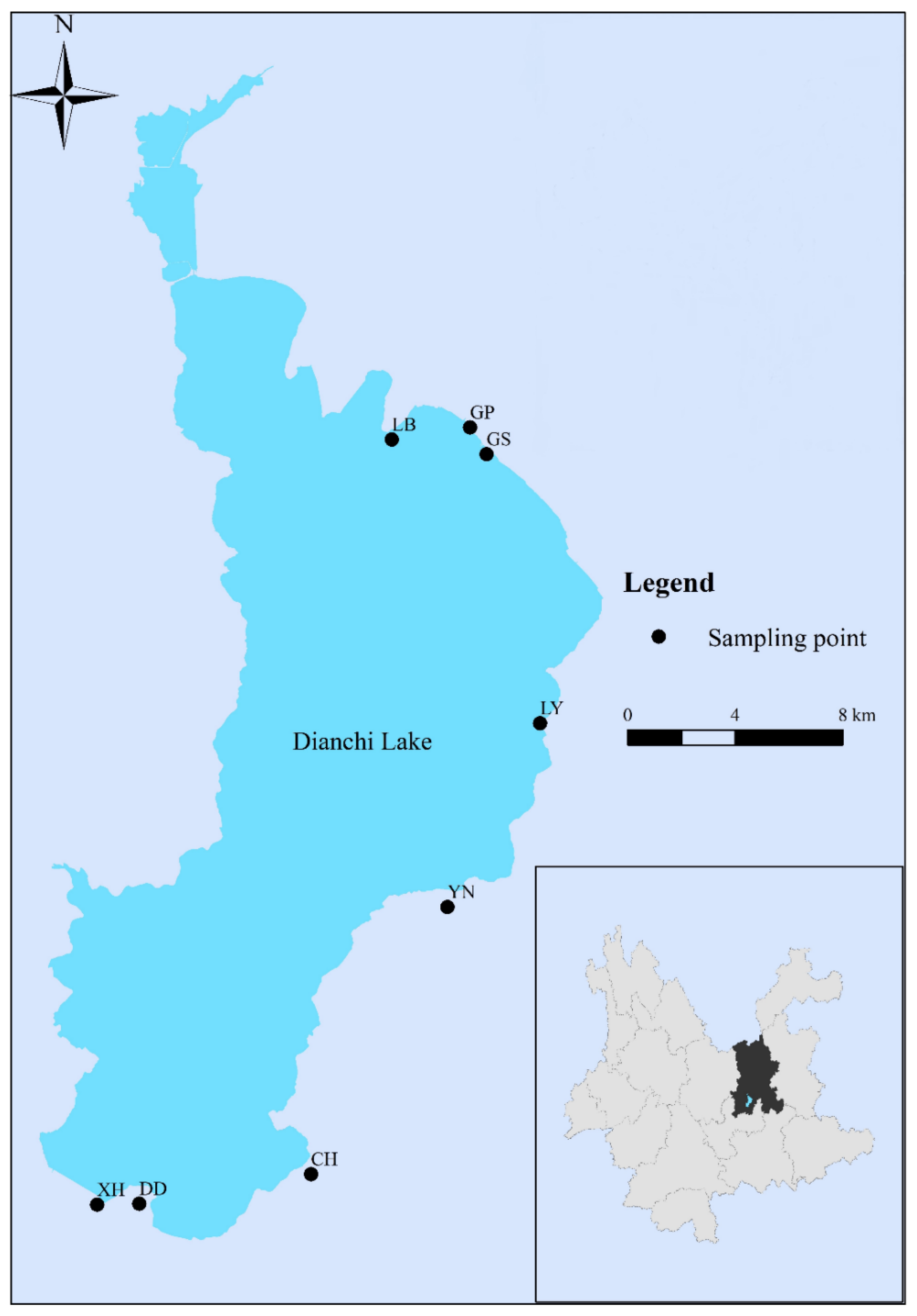

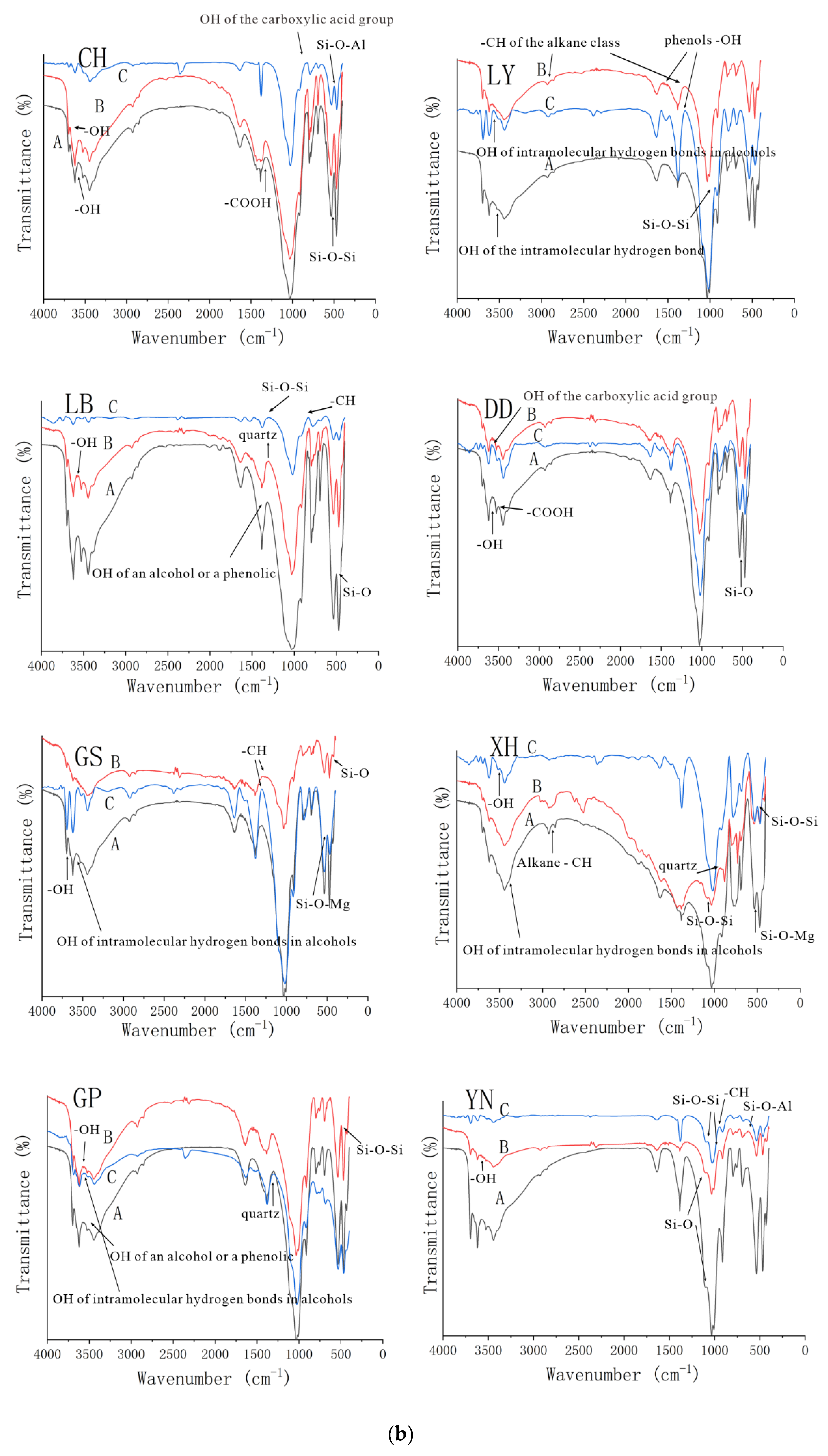
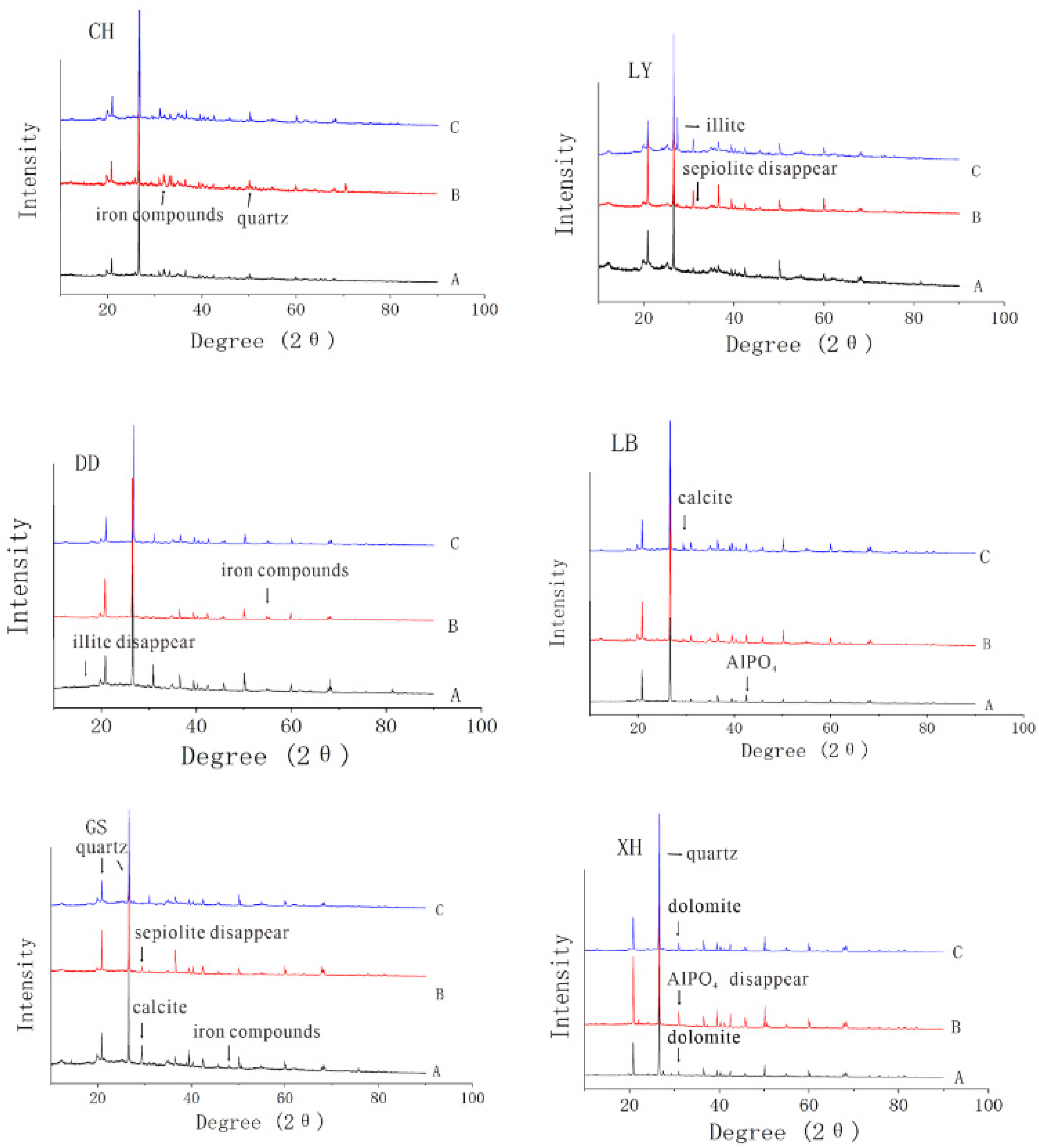
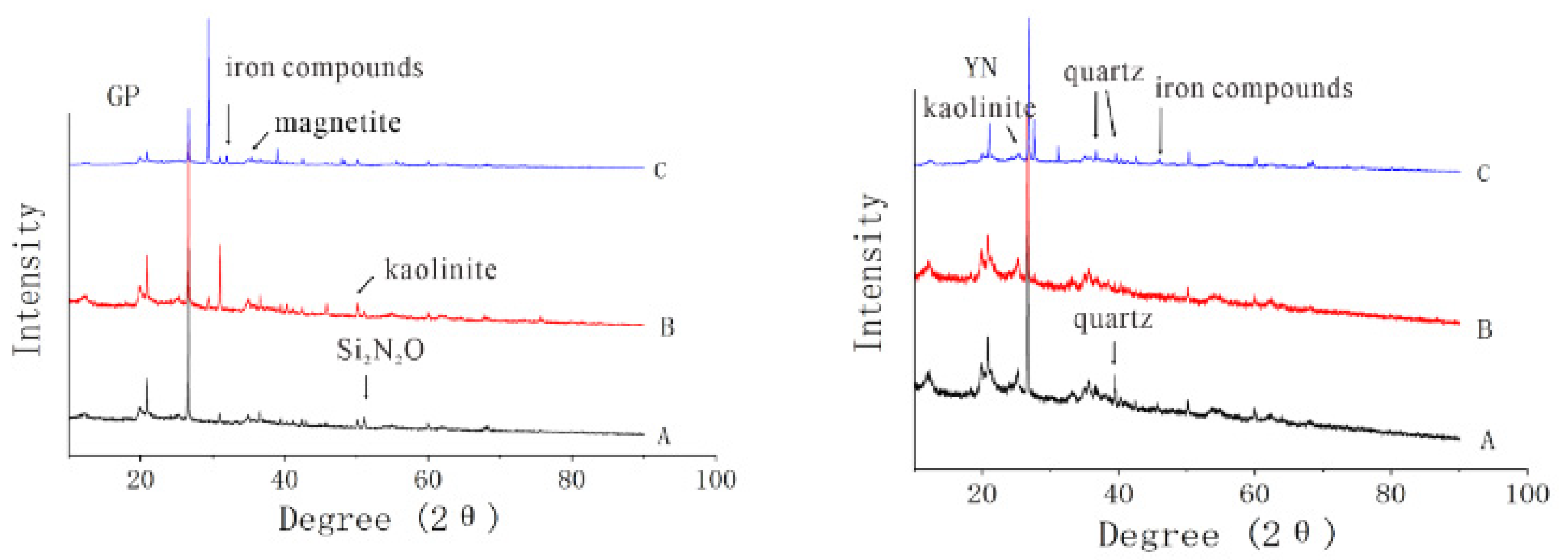
| Location | Group | CEC (coml/kg) | SOM (mg/g) | Free Iron Oxide (mg/g) | Aluminum Oxide (mg/g) | pH |
|---|---|---|---|---|---|---|
| CH | A | 1.09 | 32.57 | 62.13 | 11.72 | 8.18 |
| CH | B | 9.73 | 44.69 | 7.83 | 1.22 | 9.15 |
| CH | C | 31.27 | 34.39 | 35.51 | 6.06 | 8.49 |
| DD | A | 0.80 | 20.72 | 41.60 | 4.27 | 7.25 |
| DD | B | 9.63 | 35.89 | 3.37 | 0.81 | 9.50 |
| DD | C | 32.25 | 46.58 | 26.55 | 4.92 | 7.97 |
| GS | A | 1.48 | 33.66 | 45.71 | 3.42 | 8.14 |
| GS | B | 14.45 | 44.54 | 4.50 | 0.91 | 8.81 |
| GS | C | 45.36 | 49.12 | 30.73 | 4.68 | 8.25 |
| GP | A | 2.15 | 31.08 | 52.60 | 4.94 | 8.06 |
| GP | B | 26.01 | 66.98 | 5.85 | 1.18 | 8.69 |
| GP | C | 56.87 | 48.81 | 35.23 | 5.33 | 8.02 |
| LY | A | 1.80 | 21.44 | 76.04 | 8.16 | 7.95 |
| LY | B | 20.92 | 46.90 | 5.79 | 0.81 | 9.18 |
| LY | C | 52.33 | 46.65 | 49.59 | 5.85 | 8.02 |
| LB | A | 0.81 | 22.66 | 37.03 | 5.89 | 7.39 |
| LB | B | 6.90 | 29.24 | 3.52 | 0.84 | 8.51 |
| LB | C | 20.77 | 37.47 | 28.27 | 4.78 | 7.91 |
| XH | A | 0.44 | 21.52 | 26.52 | 4.71 | 8.39 |
| XH | B | 2.42 | 19.50 | 1.10 | 0.30 | 9.46 |
| XH | C | 18.71 | 31.96 | 8.98 | 1.07 | 8.35 |
| YN | A | 2.08 | 18.38 | 131.02 | 15.06 | 7.51 |
| YN | B | 19.15 | 24.50 | 17.30 | 2.11 | 9.18 |
| YN | C | 57.92 | 24.83 | 85.05 | 9.59 | 8.06 |
| Site | Group Number | Freundlich Model | Langmuir Model | |||
|---|---|---|---|---|---|---|
| Kf (mg/g) [23] | 1/n | R2 | Qmax (mg/kg) | R2 | ||
| CH | A | 0.35 ± 0.06 | 1.28 ± 0.51 | 0.9956 | 4.85 ± 4.43 | 0.92616 |
| B | 4.01 ± 0.64 | 0.64 ± 0.08 | 0.99698 | 26.38 ± 4.00 | 0.99831 | |
| C | 1.79 ± 0.52 | 0.77 ± 0.12 | 0.99375 | 55.11 ± 53.37 | 0.99084 | |
| DD | A | 0.42 ± 0.10 | 0.87 ± 0.07 | 0.9995 | 43.69 ± 15.02 | 0.99967 |
| B | 0.75 ± 0.36 | 0.23 ± 0.16 | 0.99928 | 2.06 ± 0.53 | 0.99961 | |
| C | 1.86 ± 1.05 | 0.43 ± 0.18 | 0.99332 | 7.53 ± 1.76 | 0.99088 | |
| GS | A | 1.71 ± 0.22 | 0.75 ± 0.05 | 0.99851 | 33.33 ± 4.39 | 0.99892 |
| B | 3.57 ± 0.77 | 0.82 ± 0.14 | 0.99635 | 52.51 ± 0.36 | 0.99721 | |
| C | 3.52 ± 0.20 | 0.27 ± 0.02 | 0.99993 | 10.66 ± 0.94 | 0.99904 | |
| GP | A | 0.63 ± 0.21 | 1.03 ± 0.12 | 0.99453 | 219.97 ± 743.21 | 0.99457 |
| B | 2.84 ± 0.17 | 0.94 ± 0.04 | 0.99975 | 153.14 ± 1.19 | 0.99979 | |
| C | 4.82 ± 0.42 | 0.54 ± 0.05 | 0.99894 | 21.82 ± 0.84 | 0.99978 | |
| LY | A | 0.51 ± 0.28 | 0.68 ± 0.14 | 0.9993 | 46.25 ± 90.69 | 0.99898 |
| B | 2.56 ± 0.64 | 0.65 ± 0.11 | 0.99751 | 19.28 ± 1.38 | 0.99882 | |
| C | 5.29 ± 0.43 | 0.13 ± 0.02 | 0.99987 | 9.22 ± 0.28 | 0.9999 | |
| LB | A | 0.53 ± 0.29 | 0.74 ± 0.15 | 0.99815 | 23.51 ± 12.12 | 0.99827 |
| B | 1.42 ± 0.27 | 0.85 ± 0.08 | 0.99683 | 46.30 ± 14.51 | 0.99808 | |
| C | 2.83 ± 0.40 | 0.46 ± 0.05 | 0.99869 | 17.38 ± 2.83 | 0.99507 | |
| XH | A | 0.39 ± 0.08 | 0.88 ± 0.06 | 0.9997 | 45.60 ± 14.48 | 0.9998 |
| B | 1.01 ± 0.50 | 0.52 ± 0.13 | 0.99799 | 10.39 ± 1.72 | 0.999 | |
| C | 3.07 ± 4.50 | 0.37 ± 0.50 | 0.94052 | 10.05 ± 4.21 | 0.94166 | |
| YN | A | 2.52 ± 0.39 | 0.72 ± 0.06 | 0.99237 | 28.60 ± 6.55 | 0.99581 |
| B | 5.02 ± 2.30 | 0.34 ± 0.15 | 0.9735 | 19.40 ± 3.35 | 0.9844 | |
| C | 1.24 ± 0.22 | 0.65 ± 0.06 | 0.99916 | 24.64 ± 8.09 | 0.99748 | |
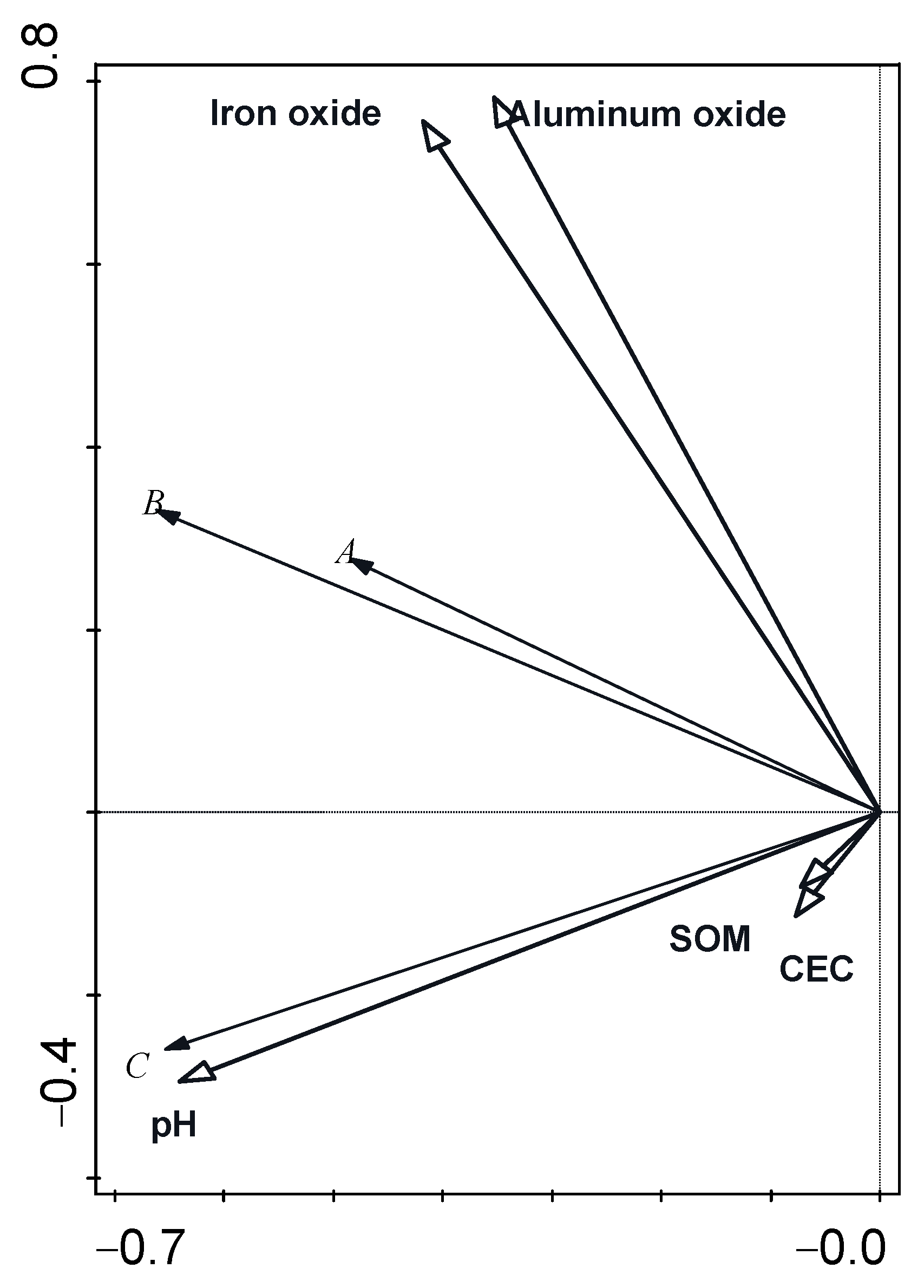
| Name | Interpretation % | Contribution % | Pseudo-F | p |
|---|---|---|---|---|
| pH | 17 | 34.2 | 4.5 | 0.028 |
| iron oxide | 11.7 | 23.6 | 3.5 | 0.042 |
| aluminum oxide | 11.5 | 23.2 | 3.8 | 0.026 |
| SOM | 2.7 | 5.5 | 0.9 | 0.384 |
| CEC | 6.7 | 13.5 | 2.4 | 0.116 |
| Group | Free Iron Oxide | Free Aluminum Oxide | pH | SOM | CEC |
|---|---|---|---|---|---|
| A | 0.044 | 0.426 | 0.253 | 0.551 | 0.511 |
| B | 0.905 ** | 0.919 ** | 0.393 | 0.161 | 0.773 * |
| C | 0.081 | −0.045 | 0.429 | −0.296 | 0.234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.-L.; Wang, Y.; Xiong, H.-Q.; Wu, F.; Lv, T.-X.; Fang, Y.-C.; Xiang, H. The Role of Surface Functional Groups of Iron Oxide, Organic Matter, and Clay Mineral Complexes in Sediments on the Adsorption of Copper Ions. Sustainability 2023, 15, 6711. https://doi.org/10.3390/su15086711
Sun X-L, Wang Y, Xiong H-Q, Wu F, Lv T-X, Fang Y-C, Xiang H. The Role of Surface Functional Groups of Iron Oxide, Organic Matter, and Clay Mineral Complexes in Sediments on the Adsorption of Copper Ions. Sustainability. 2023; 15(8):6711. https://doi.org/10.3390/su15086711
Chicago/Turabian StyleSun, Xiao-Long, Yuan Wang, Hao-Qin Xiong, Fan Wu, Tian-Xin Lv, Yi-Chuan Fang, and Hong Xiang. 2023. "The Role of Surface Functional Groups of Iron Oxide, Organic Matter, and Clay Mineral Complexes in Sediments on the Adsorption of Copper Ions" Sustainability 15, no. 8: 6711. https://doi.org/10.3390/su15086711
APA StyleSun, X.-L., Wang, Y., Xiong, H.-Q., Wu, F., Lv, T.-X., Fang, Y.-C., & Xiang, H. (2023). The Role of Surface Functional Groups of Iron Oxide, Organic Matter, and Clay Mineral Complexes in Sediments on the Adsorption of Copper Ions. Sustainability, 15(8), 6711. https://doi.org/10.3390/su15086711





