Bioremediation of Hydrocarbon Pollutants: Recent Promising Sustainable Approaches, Scope, and Challenges
Abstract
1. Introduction
2. An overview of Bioremediation of Petroleum Pollutants
2.1. Role of Microorganisms in Hydrocarbon Biodegradation
2.2. Optimization of Bioremediation Conditions
3. Metabolic Pathways for Hydrocarbon Degradation
3.1. Major Intermediates and Biproducts
3.2. Mechanisms Used by Microorganisms to Enhance Degradation of Hydrocarbons
4. Mechanistic Insights of Biodegradation of Phenolic Compounds PAH Pollutants
4.1. Biodegradation of Phenolic Compounds
4.2. Biodegradation of Naphthalene
4.3. Biodegradation of Phenanthrene
4.4. Biodegradation of Anthracene
4.5. Biodegradation of Pyrene
4.6. Biodegradation of Benzopyrene
5. Challenges in Bioremediation Process and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Kang, S.H.; Mulchandani, A.; Chen, W. Bioremediation: Environmental clean-up through pathway engineering. Curr. Opin. Biotechnol. 2008, 19, 437–444. [Google Scholar] [CrossRef]
- Joutey, N.T.; Bahafid, W.; Sayel, H.; El Ghachtouli, N. Biodegradation: Involved microorganisms and genetically engineered microorganisms. Biodegrad. Sci. 2013, 1, 289–320. [Google Scholar]
- Liu, S.; Sun, S.; Cui, P.; Ding, Y. Molecular modification of fluoroquinolone-biodegrading enzymes based on molecular docking and homology modelling. Int. J. Environ. Res. Public Health 2019, 16, 3407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, D.-F.; Hao, J.; Zhao, Z.-H.; Zhang, Y.-J. The crucial role of bacterial laccases in the bioremediation of petroleum hydrocarbons. World J. Microbiol. Biotechnol. 2020, 36, 116. [Google Scholar] [CrossRef] [PubMed]
- Shakerian, F.; Zhao, J.; Li, S.-P. Recent development in the application of immobilized oxidative enzymes for bioremediation of hazardous micropollutants–A review. Chemosphere 2020, 239, 124716. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Parhamfar, M.; Abtahi, H.; Godini, K.; Saeedi, R.; Sartaj, M.; Villaseñor, J.; Coulon, F.; Kumar, V.; Soltanighias, T.; Ghaznavi-Rad, E. Biodegradation of heavy oily sludge by a two-step inoculation composting process using synergistic effect of indigenous isolated bacteria. Process Biochem. 2020, 91, 223–230. [Google Scholar] [CrossRef]
- Poorsoleiman, M.S.; Hosseini, S.A.; Etminan, A.; Abtahi, H.; Koolivand, A. Bioremediation of Petroleum Hydrocarbons by using a two-step inoculation composting process scaled-up from a mineral-based medium: Effect of biostimulation of an indigenous bacterial strain. Waste Biomass Valoriz. 2021, 12, 2089–2096. [Google Scholar] [CrossRef]
- Poorsoleiman, M.S.; Hosseini, S.A.; Etminan, A.; Abtahi, H.; Koolivand, A. Effect of two-step bioaugmentation of an indigenous bacterial strain isolated from oily waste sludge on petroleum hydrocarbons biodegradation: Scaling-up from a liquid mineral medium to a two-stage composting process. Environ. Technol. Innov. 2020, 17, 100558. [Google Scholar] [CrossRef]
- Tormoehlen, L.M.; Tekulve, K.J.; Nañagas, K.A. Hydrocarbon toxicity: A review. Clin. Toxicol. 2014, 52, 479–489. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Sáez, J.A.; Moral, R.; Moreno, J. Bioremediation of Olive Mill Wastewater sediments in evaporation ponds through in situ composting assisted by bioaugmentation. Sci. Total Environ. 2020, 703, 135537. [Google Scholar] [CrossRef] [PubMed]
- Lange, I.; Kotiukov, P.; Lebedeva, Y. Analyzing Physical-Mechanical and Hydrophysical Properties of Sandy Soils Exposed to Long-Term Hydrocarbon Contamination. Sustainability 2023, 15, 3599. [Google Scholar] [CrossRef]
- Blenis, N.; Hue, N.; Maaz, T.M.; Kantar, M. Biochar Production, Modification, and Its Uses in Soil Remediation: A Review. Sustainability 2023, 15, 3442. [Google Scholar] [CrossRef]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, biostimulation and bioaugmention: A review. Int. J. Environ. Bioremediat. Biodegrad. 2015, 3, 28–39. [Google Scholar]
- Sayara, T.; Sánchez, A. Bioremediation of PAH-contaminated soils: Process enhancement through composting/compost. Appl. Sci. 2020, 10, 3684. [Google Scholar] [CrossRef]
- Mahiudddin, M.; Fakhruddin, A.N.M. Degradation of phenol via meta cleavage pathway by Pseudomonas fluorescens PU1. Int. Sch. Res. Not. 2012, 2012, 741820. [Google Scholar]
- Xi, B.; Dang, Q.; Wei, Y.; Li, X.; Zheng, Y.; Zhao, X. Biogas slurry as an activator for the remediation of petroleum contaminated soils through composting mediated by humic acid. Sci. Total Environ. 2020, 730, 139117. [Google Scholar] [CrossRef]
- Gaur, V.K.; Gautam, K.; Sharma, P.; Gupta, P.; Dwivedi, S.; Srivastava, J.K.; Varjani, S.; Ngo, H.H.; Kim, S.-H.; Chang, J.-S. Sustainable strategies for combating hydrocarbon pollution: Special emphasis on mobil oil bioremediation. Sci. Total Environ. 2022, 832, 155083. [Google Scholar] [CrossRef]
- Sharma, S.; Pandey, L.M. Biodegradation kinetics of binary mixture of Hexadecane and Phenanthrene by the bacterial microconsortium. Bioresour. Technol. 2022, 358, 127408. [Google Scholar] [CrossRef]
- Haripriyan, U.; Gopinath, K.P.; Arun, J.; Govarthanan, M. Bioremediation of organic pollutants: A mini review on current and critical strategies for wastewater treatment. Arch. Microbiol. 2022, 204, 286. [Google Scholar] [CrossRef]
- Osei-Twumasi, D.; Fei-Baffoe, B.; Anning, A.K.; Danquah, K.O. Synergistic effects of compost, cow bile and bacterial culture on bioremediation of hydrocarbon-contaminated drill mud waste. Environ. Pollut. 2020, 266, 115202. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, H.; Parhamfar, M.; Saeedi, R.; Villasenor, J.; Sartaj, M.; Kumar, V.; Coulon, F.; Parhamfar, M.; Didehdar, M.; Koolivand, A. Effect of competition between petroleum-degrading bacteria and indigenous compost microorganisms on the efficiency of petroleum sludge bioremediation: Field application of mineral-based culture in the composting process. J. Environ. Manag. 2020, 258, 110013. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, M.; Natarajan, N. Towards achieving sustainable bioplastics production and nutrient recovery from wastewater—A comprehensive overview on polyhydroxybutyrate. Biomass Convers. Biorefin. 2022, 1–20. [Google Scholar] [CrossRef]
- Leech, C.; Tighe, M.K.; Pereg, L.; Winter, G.; McMillan, M.; Esmaeili, A.; Wilson, S.C. Bioaccessibility constrains the co-composting bioremediation of field aged PAH contaminated soils. Int. Biodeterior. Biodegrad. 2020, 149, 104922. [Google Scholar] [CrossRef]
- Mahyudin, R.P.; Firmansyah, M.; Purwanti, M.A.; Najmina, D. Bioremediation of iron on diamond post mining soil using compost made from cow manure and traditional market organic waste. J. Ecol. Eng. 2020, 21, 221–228. [Google Scholar] [CrossRef]
- Atagana, H.I. Compost bioremediation of hydrocarbon-contaminated soil inoculated with organic manure. Afr. J. Biotechnol. 2008, 7, 1516–1525. [Google Scholar]
- Chang, B.-V.; Chang, I.T.; Yuan, S.Y. Anaerobic degradation of phenanthrene and pyrene in mangrove sediment. Bull. Environ. Contam. Toxicol. 2008, 80, 145–149. [Google Scholar] [CrossRef]
- Saha, J.K.; Selladurai, R.; Coumar, M.V.; Dotaniya, M.L.; Kundu, S.; Patra, A.K. Soil Pollution—An Emerging Threat to Agriculture; Springer: Singapore, 2017; ISBN 9811042748. [Google Scholar]
- Vasudevan, M.; Nambi, I.M.; Kumar, G.S. Scenario-based modelling of mass transfer mechanisms at a petroleum contaminated field site-numerical implications. J. Environ. Manag. 2016, 175, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, M.; Suresh Kumar, G.; Nambi, I.M. Numerical modelling on rate-limited dissolution mass transfer of entrapped petroleum hydrocarbons in a saturated sub-surface system. ISH J. Hydraul. Eng. 2016, 22, 3–15. [Google Scholar] [CrossRef]
- Koolivand, A.; Abtahi, H.; Villaseñor, J.; Saeedi, R.; Godini, K.; Parhamfar, M. Effective scale-up of oily sludge bioremediation from a culture-based medium to a two-phase composting system using an isolated hydrocarbon-degrading bacterium: Effect of two-step bioaugmentation. J. Mater. Cycles Waste Manag. 2020, 22, 1475–1483. [Google Scholar] [CrossRef]
- Bhanse, P.; Kumar, M.; Singh, L.; Awasthi, M.K.; Qureshi, A. Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: Opportunities, challenges, and prospects. Chemosphere 2022, 303, 134954. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tang, S.; Gong, J.; Zeng, G.; Tang, W.; Song, B.; Zhang, P.; Yang, Z.; Luo, Y. Responses of enzymatic activity and microbial communities to biochar/compost amendment in sulfamethoxazole polluted wetland soil. J. Hazard. Mater. 2020, 385, 121533. [Google Scholar] [CrossRef]
- Sáez, J.A.; Pérez-Murcia, M.D.; Vico, A.; Martínez-Gallardo, M.R.; Andreu-Rodríguez, F.J.; López, M.J.; Bustamante, M.A.; Sanchez-Hernandez, J.C.; Moreno, J.; Moral, R. Olive mill wastewater-evaporation ponds long term stored: Integrated assessment of in situ bioremediation strategies based on composting and vermicomposting. J. Hazard. Mater. 2021, 402, 123481. [Google Scholar] [CrossRef] [PubMed]
- Waigi, M.G.; Kang, F.; Goikavi, C.; Ling, W.; Gao, Y. Phenanthrene biodegradation by sphingomonads and its application in the contaminated soils and sediments: A review. Int. Biodeterior. Biodegrad. 2015, 104, 333–349. [Google Scholar] [CrossRef]
- Grover, R.; Burse, S.A.; Shankrit, S.; Aggarwal, A.; Kirty, K.; Narta, K.; Srivastav, R.; Ray, A.K.; Malik, G.; Vats, A. Myg1 exonuclease couples the nuclear and mitochondrial translational programs through RNA processing. Nucleic Acids Res. 2019, 47, 5852–5866. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Hussain, I.; Khan, A.H.A.; Muhammad, Y.S.; Iqbal, M.; Soja, G.; Reichenauer, T.G.; Yousaf, S. Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ. Exp. Bot. 2018, 153, 80–88. [Google Scholar] [CrossRef]
- Nzila, A. Current status of the degradation of aliphatic and aromatic petroleum hydrocarbons by thermophilic microbes and future perspectives. Int. J. Environ. Res. Public Health 2018, 15, 2782. [Google Scholar] [CrossRef]
- Ostrem Loss, E.M.; Lee, M.-K.; Wu, M.-Y.; Martien, J.; Chen, W.; Amador-Noguez, D.; Jefcoate, C.; Remucal, C.; Jung, S.; Kim, S.-C. Cytochrome P450 monooxygenase-mediated metabolic utilization of benzo [a] pyrene by Aspergillus species. MBio 2019, 10, e00558-19. [Google Scholar] [CrossRef]
- Li, J.; Yang, H. Polycyclic Aromatic Hydrocarbon Improves the Anaerobic Biodegradation of Benz [α] Anthracene in Sludge Via Boosting the Microbial Activity and Bioavailability. Pak. J. Zool. 2021, 53, 2445–2450. [Google Scholar] [CrossRef]
- Soleimani, M.; Farhoudi, M.; Christensen, J.H. Chemometric assessment of enhanced bioremediation of oil contaminated soils. J. Hazard. Mater. 2013, 254, 372–381. [Google Scholar] [CrossRef]
- Pandya, D.K.; Kumar, M.A. Chemo-metric engineering designs for deciphering the biodegradation of polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2021, 411, 125154. [Google Scholar] [CrossRef] [PubMed]
- Dudhagara, D.R.; Rajpara, R.K.; Bhatt, J.K.; Gosai, H.B.; Dave, B.P. Bioengineering for polycyclic aromatic hydrocarbon degradation by Mycobacterium litorale: Statistical and artificial neural network (ANN) approach. Chemom. Intell. Lab. Syst. 2016, 159, 155–163. [Google Scholar] [CrossRef]
- Oliveira, L.G.; Araújo, K.C.; Barreto, M.C.; Bastos, M.E.P.; Lemos, S.G.; Fragoso, W.D. Applications of chemometrics in oil spill studies. Microchem. J. 2021, 166, 106216. [Google Scholar] [CrossRef]
- Tavares, T.S.; da Rocha, E.P.; Esteves Nogueira, F.G.; Torres, J.A.; Silva, M.C.; Kuca, K.; Ramalho, T.C. Δ-FeOOH as support for immobilization peroxidase: Optimization via a chemometric approach. Molecules 2020, 25, 259. [Google Scholar] [CrossRef] [PubMed]
- de Castro, A.A.; Soares, F.V.; Pereira, A.F.; Silva, T.C.; Silva, D.R.; Mancini, D.T.; Caetano, M.S.; da Cunha, E.F.F.; Ramalho, T.C. Asymmetric biodegradation of the nerve agents Sarin and VX by human dUTPase: Chemometrics, molecular docking and hybrid QM/MM calculations. J. Biomol. Struct. Dyn. 2019, 37, 2154–2164. [Google Scholar] [CrossRef]
- Shavandi, M.; Mohebali, G.; Haddadi, A.; Shakarami, H.; Nuhi, A. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloids Surf. B Biointerfaces 2011, 82, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Bezza, F.A.; Chirwa, E.M. Production and applications of lipopeptide biosurfactant for bioremediation and oil recovery by Bacillus subtilis CN2. Biochem. Eng. J. 2015, 101, 168–178. [Google Scholar] [CrossRef]
- Chandankere, R.; Yao, J.; Cai, M.; Masakorala, K.; Jain, A.K.; Choi, M.M. Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel 2014, 122, 140–148. [Google Scholar] [CrossRef]
- Chirwa, E.M.; Mampholo, T.; Fayemiwo, O. Biosurfactants as demulsifying agents for oil recovery from oily sludge--performance evaluation. Water Sci Technol. 2013, 67, 2875–2881. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M. Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil. Chemosphere 2016, 144, 635–644. [Google Scholar] [CrossRef]
- Bengtsson, G.; Törneman, N.; Yang, X. Spatial uncoupling of biodegradation, soil respiration, and PAH concentration in a creosote contaminated soil. Environ. Pollut. 2010, 158, 2865–2871. [Google Scholar] [CrossRef]
- Shin, K.H.; Kim, K.W.; Ahn, Y. Use of biosurfactant to remediate phenanthrenecontaminated soil by the combined solubilizationebiodegradation process. J. Hazard. Mater. 2006, 137, 1831–1837. [Google Scholar] [CrossRef]
- Hashmi, M.Z.; Kumar, V.; Varma, A. Xenobiotics in the Soil Environment: Monitoring, Toxicity and Management; Springer: Cham, Switzerland, 2017; Volume 49, ISBN 3319477447. [Google Scholar]
- Srivastav, R.; Suneja, G. Recent advances in microbial genome sequencing. In Microbial Genomics in Sustainable Agroecosystems; Springer: Singapore, 2019; pp. 131–144. [Google Scholar]
- Suneja, G.; Srivastav, R. Impact of Microbial Genome Sequencing Advancements in Understanding Extremophiles. In Extreme Environments; CRC Press: Boca Raton, FL, USA, 2021; pp. 330–342. ISBN 0429343450. [Google Scholar]
- Wang, S.; Li, X.; Liu, W.; Li, P.; Kong, L.; Ren, W.; Wu, H.; Tu, Y. Degradation of pyrene by immobilized microorganisms in saline-alkaline soil. J. Environ. Sci. 2012, 24, 1662–1669. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sust. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Lignocellulosic biomass: Biosynthesis, degradation, and industrial utilization. Eng. Life Sci. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Li, L.O.; Klett, E.L.; Coleman, R.A. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochem. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 246–251. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Gupta, R.; Beg, Q.; Lorenz, P. Bacterial alkaline proteases: Molecular approaches and industrial applications. Appl. Microb. Biotechnol. 2002, 59, 15–32. [Google Scholar]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, S.P.; Iqbal, H.M.N.; Tong, Y.W. Omics approaches in bioremediation of environmental contaminants: An integrated approach for environmental safety and sustainability. Environ. Res. 2022, 211, 113102. [Google Scholar] [CrossRef]
- Kapardar, R.K.; Ranjan, R.; Grover, A.; Puri, M.; Sharma, R. Identification and characterization of genes conferring salt tolerance to Escherichia coli from pond water metagenome. Bioresour Technol. 2010, 101, 3917–3924. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F. Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J. Environ. Chem. Eng. 2019, 7, 102805. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, M. Bioremediation of crude oil-contaminated soil: Comparison of different biostimulation and bioaugmentation treatments. J. Hazard. Mater. 2010, 183, 395–401. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef] [PubMed]
- Christova, N.; Kabaivanova, L.; Nacheva, L.; Petrov, P.; Stoineva, I. Biodegradation of crude oil hydrocarbons by a newly isolated biosurfactant producing strain. Biotechnol. Biotechnol. Equip. 2019, 33, 863–872. [Google Scholar] [CrossRef]
- Dutta, S.; Singh, P. Chemotaxis of biofilm producing Pseudomonas spp. towards refined petroleum oil. J. Sci. Res. 2016, 8, 199–207. [Google Scholar] [CrossRef]
- Antoniou, E.; Fodelianakis, S.; Korkakaki, E.; Kalogerakis, N. Biosurfactant production from marine hydrocarbon-degrading consortia and pure bacterial strains using crude oil as carbon source. Front. Microbiol. 2015, 6, 274. [Google Scholar] [CrossRef]
- Yaohua, G.; Ping, X.; Feng, J.; Keren, S. Co-immobilization of laccase and ABTS onto novel dual-functionalized cellulose beads for highly improved biodegradation of indole. J. Hazard. Mater. 2018, 365, 118–124. [Google Scholar] [CrossRef]
- Ang, E.L.; Zhao, H.; Obbard, J.P. Recent advances in the bioremediation of persistent organic pollutants via biomolecular engineering. Enzyme Microb. Technol. 2005, 37, 487–496. [Google Scholar] [CrossRef]
- Li, Q.-S.; Ogawa, J.; Schmid, R.D.; Shimizu, S. Engineering cytochrome P450 BM-3 for oxidation of polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 2001, 67, 5735–5739. [Google Scholar] [CrossRef]
- Staats, M.; Braster, M.; Röling, W.F.M. Molecular diversity and distribution of aromatic hydrocarbon-degrading anaerobes across a landfill leachate plume. Environ. Microbiol. 2011, 13, 1216–1227. [Google Scholar] [CrossRef]
- Somu, P.; Narayanasamy, S.; Gomez, L.A.; Rajendran, S.; Lee, Y.R.; Balakrishnan, D. Immobilization of enzymes for bioremediation: A future remedial and mitigating strategy. Environ. Res. 2022, 212, 113411. [Google Scholar] [CrossRef]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of microbes for bioremediation of crude oil polluted environments: A mini review. Open Microbiol. J. 2015, 9, 48. [Google Scholar] [PubMed]
- Botton, S.; Van Harmelen, M.; Braster, M.; Parsons, J.R.; Röling, W.F.M. Dominance of Geobacteraceae in BTX-degrading enrichments from an iron-reducing aquifer. FEMS Microbiol. Ecol. 2007, 62, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Winderl, C.; Schaefer, S.; Lueders, T. Detection of anaerobic toluene and hydrocarbon degraders in contaminated aquifers using benzylsuccinate synthase (bssA) genes as a functional marker. Environ. Microbiol. 2007, 9, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N.; Shah, S.Z.H.; Hu, H.; Wang, W.; Zhang, X. Horseradish peroxidase-assisted approach to decolorize and detoxify dye pollutants in a packed bed bioreactor. J. Environ. Manag. 2016, 183, 836–842. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Hu, H.; Zhang, X. Kinetic characterization, thermo-stability and Reactive Red 195A dye detoxifying properties of manganese peroxidase-coupled gelatin hydrogel. Water Sci. Technol. 2016, 74, 1809–1820. [Google Scholar] [CrossRef]
- Vasudevan, M.; Kumar, G.S.; Nambi, I.M. Numerical studies on kinetics of sorption and dissolution and their interactions for estimating mass removal of toluene from entrapped soil pores. Arab. J. Geosci. 2015, 8, 6895–6910. [Google Scholar] [CrossRef]
- Kuntze, K.; Shinoda, Y.; Moutakki, H.; McInerney, M.J.; Vogt, C.; Richnow, H.; Boll, M. 6-Oxocyclohex-1-ene-1-carbonyl-coenzyme A hydrolases from obligately anaerobic bacteria: Characterization and identification of its gene as a functional marker for aromatic compounds degrading anaerobes. Environ. Microbiol. 2008, 10, 1547–1556. [Google Scholar] [CrossRef]
- Srivastav, R.; Sharma, R.; Tandon, S.; Tandon, C. Role of DHH superfamily proteins in nucleic acids metabolism and stress tolerance in prokaryotes and eukaryotes. Int. J. Biol. Macromol. 2019, 127, 66–75. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Naidu, R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo [a] pyrene. Int. Biodeterior. Biodegrad. 2000, 45, 57–88. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, S.N. Biodegradation of benzo (a) pyrene mediated by catabolic enzymes of bacteria. Int. J. Environ. Sci. Technol. 2014, 11, 1571–1580. [Google Scholar] [CrossRef]
- Ostrem Loss, E.M.; Yu, J. Bioremediation and microbial metabolism of benzo (a) pyrene. Mol. Microbiol. 2018, 109, 433–444. [Google Scholar] [CrossRef]
- Kotterman, M.J.J.; Vis, E.H.; Field, J.A. Successive mineralization and detoxification of benzo [a] pyrene by the white rot fungus Bjerkandera sp. strain BOS55 and indigenous microflora. Appl. Environ. Microbiol. 1998, 64, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, Y.; Sun, G. Synergistic degradation of pyrene and volatilization of arsenic by cocultures of bacteria and a fungus. Front. Environ. Sci. Eng. 2013, 7, 191–199. [Google Scholar] [CrossRef]
- Luo, S.; Chen, B.; Lin, L.; Wang, X.; Tam, N.F.-Y.; Luan, T. Pyrene degradation accelerated by constructed consortium of bacterium and microalga: Effects of degradation products on the microalgal growth. Environ. Sci. Technol. 2014, 48, 13917–13924. [Google Scholar] [CrossRef]


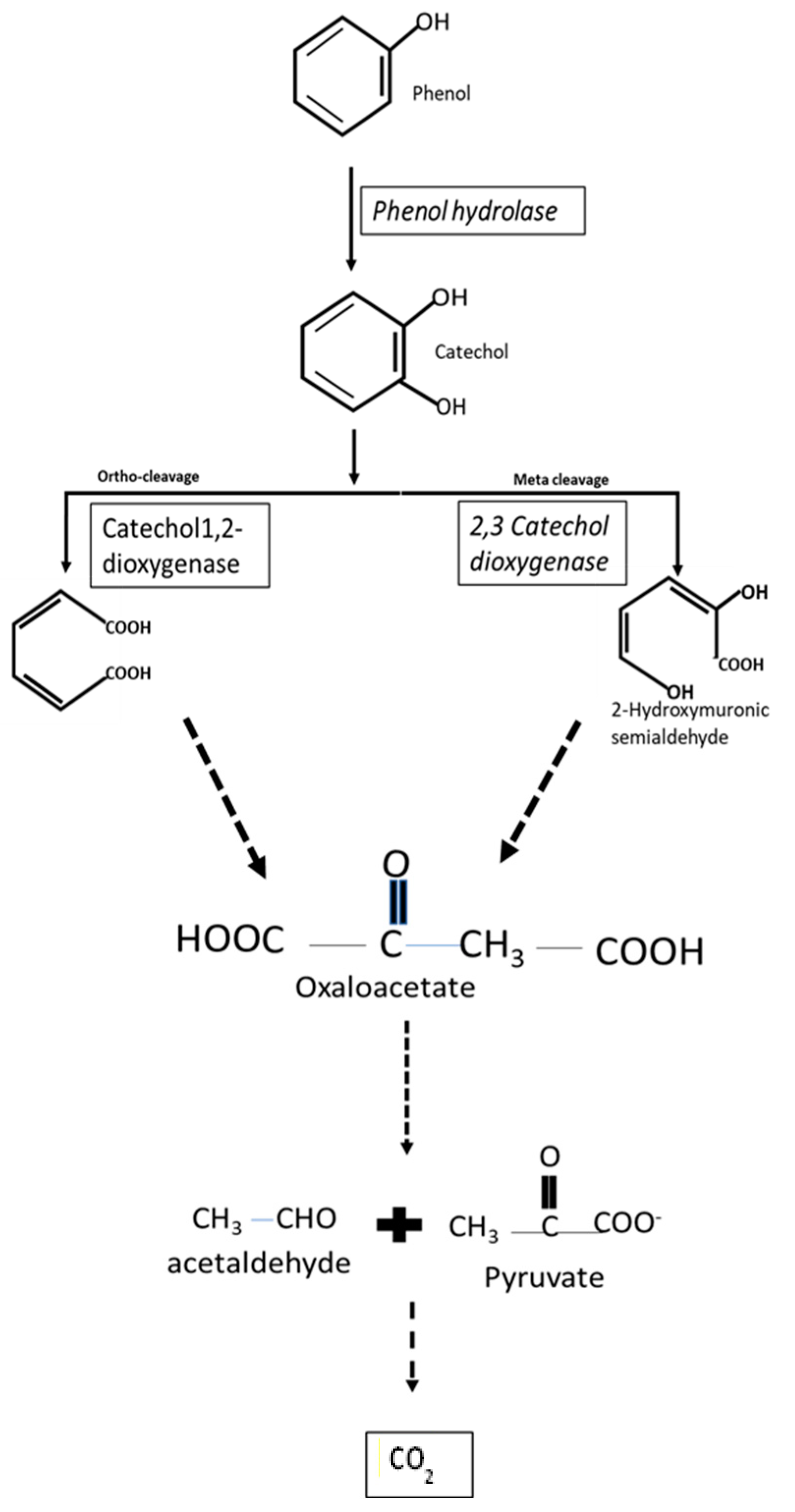
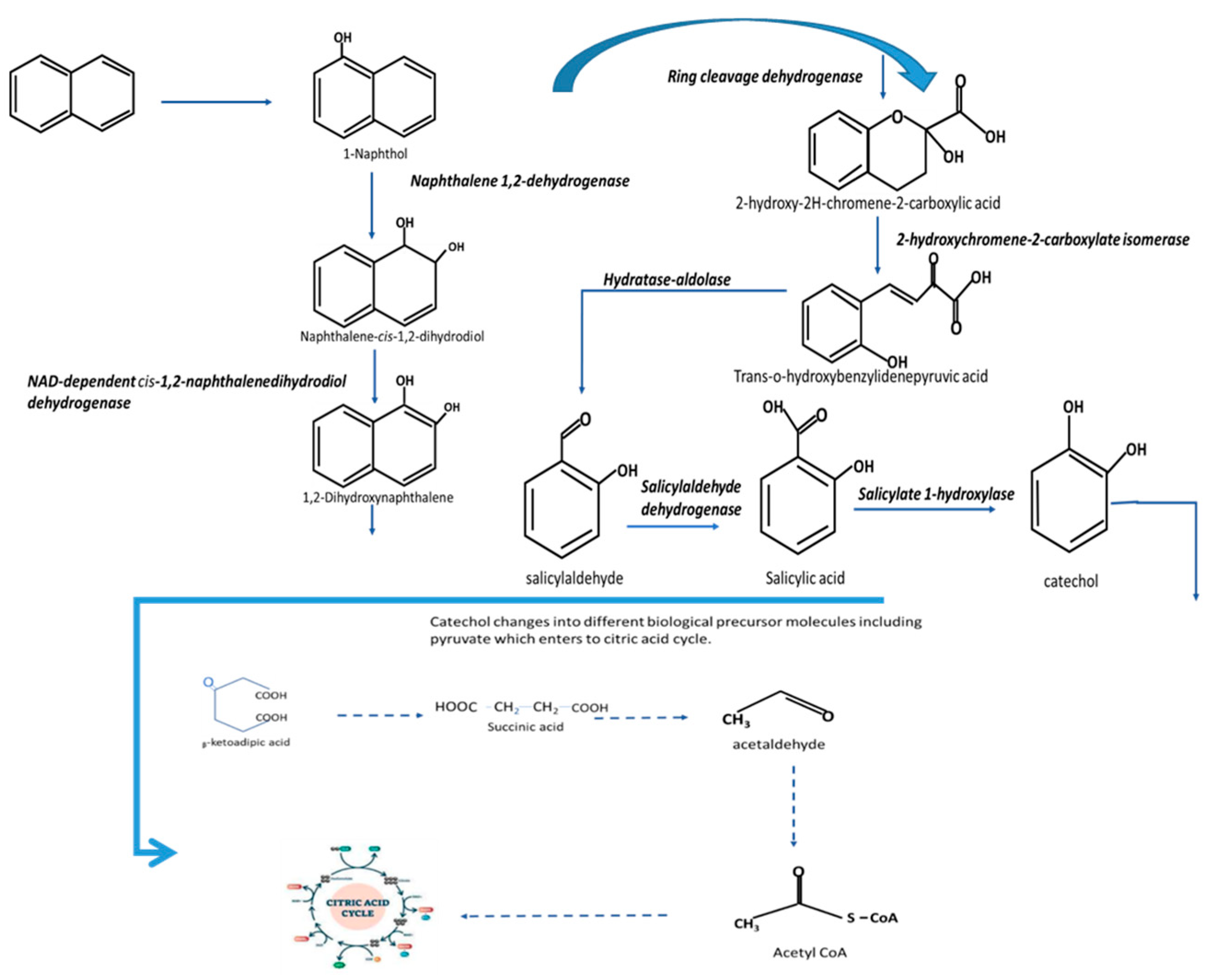
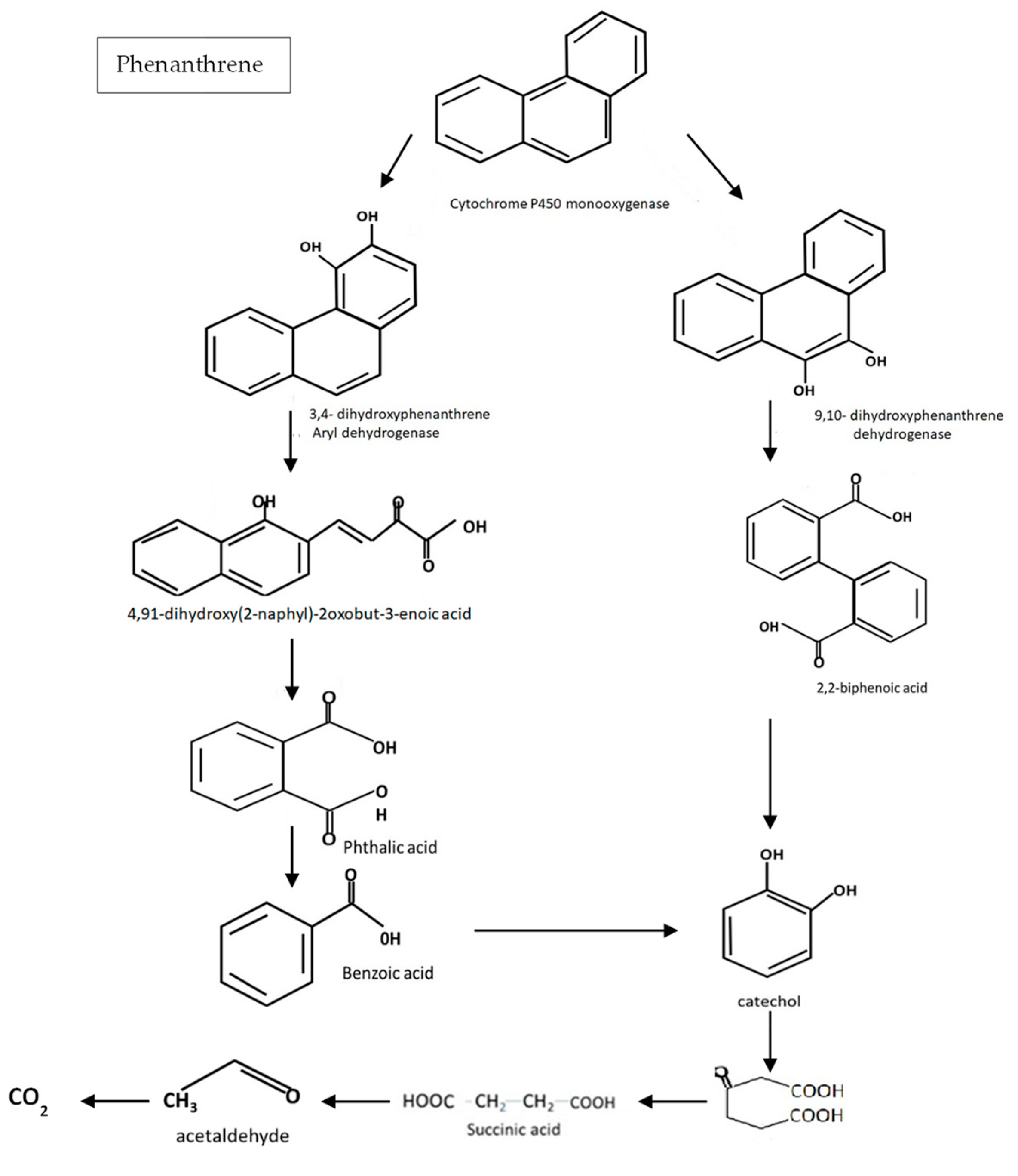


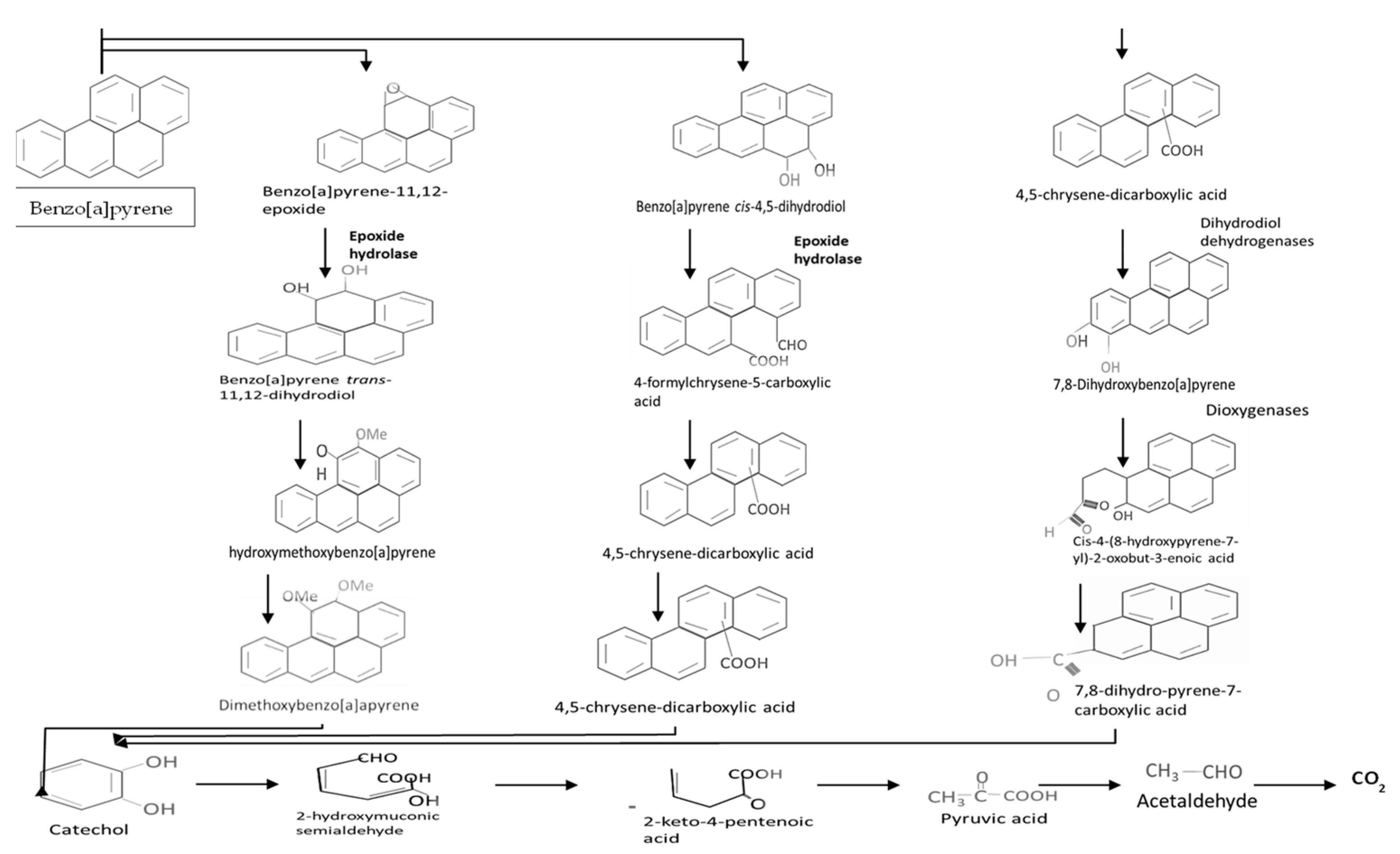
| Microorganisms Degrading Xenobiotics | Isolation Sites | References |
|---|---|---|
| Micrococcus and Pseudomonas | Soil samples contaminated with spent engine oil; from a workshop in Ado-Ekiti | [29] |
| Proteus vulgaris SR1 | Freshly killed fish samples close to the point of oil spill in the Niger Delta, Nigeria | [30] |
| Pseudomonas sp., Achromobacter sp., Bacillus sp. and Flavobacterium sp. | Soil sample; obtained from a diesel spill region in north-central Alberta, British Columbia | [31] |
| Flavobacterium sp., and Acinetobacterium calcoaceticum | Soil sample; collected from Amanzimtoti, South Africa | [32] |
| Bacillus coagulans CR31, Klebsiella pneumonia CR23, Klebsiella aerogenes CR21 and Pseudomonas putrefacience CR33 | Rhizosphere soil contaminated with spent engine oil in Sokoto, Nigeria | [33] |
| Pseudomonas sp., Acinetobacter sp., Bacillus sp., Corynebacterium sp. and Flavobacterium sp. | Soil samples from auto-mechanic workshops at Mgbukankpor, Nigeria | [34] |
| Pseudomonas putida, (Strain G1) and Pseudomonas aeruginosa (Strain K1) | Soil samples from abandoned coal power plant (PHC) at Ijora-Olapa, Lagos | [25] |
| Bacillus sp. S6 and S35 | Soil samples from storage centre of oil products in Tehran refinery and Siri Island | [35] |
| Substrate | Microbes | Duration | Removal Efficiency (%) | References |
|---|---|---|---|---|
| Oily sludge | Acinetobacter radioresistens KA2 | Two stage (8 + 8 weeks) | 90 | [9] |
| Petroleum waste sludge | Acinetobacter radioresistens KA5, Enterobacter hormaechei KA6 | Two stage composting, 12 weeks | 84 | [20] |
| Olive mill wastewater | - | Two stage composting | 84 | [29] |
| Petroleum sludge | Acinetobacter radioresistens KA2 | In vessel reactor, two phase composting (8 + 8 weeks) | 88 | [9] |
| Oily sludge | Enterobacter hormaechei KA6 | In vessel experiment, 16 weeks | 81 | [26] |
| Contaminated soil | - | Soil inoculated sewage sludge, wood chips and incubated for 19 months | 99 | [23] |
| Heavy oily sludge | Staphylococcus equorum KA4, Enterobacter hormaechei KA3 | Composting bioreactor (2 phase composting process 8 + 8 weeks) | 89 | [19] |
| Hydrocarbon contaminated drill mud waste | Brevibacterium casei, Bacillus sp. | Composting bioreactor, 6 weeks process | 99 | [7] |
| Substrate (s) | Medium | Conditions | References |
|---|---|---|---|
| Oil sludge | Bushnell-Haas, 1% Kerosene | 150 rpm shaking, 1 week at 35 °C | [26] |
| Heavy oil sludge | Bushnell-Haas, 1% Crude Oil | 160 rpm shaking, 1 week at 30 °C | [7] |
| Oily waste sludge | Bushnell-Haas, 1% Crude Oil | 160 rpm shaking, 1 week at 30 °C | [7] |
| Petroleum sludge | Bushnell-Haas, 1% Crude Oil | 120 rpm shaking, 12 days at 30 °C | [9] |
| Petroleum sludge | Bushnell-Haas, 1% Crude Oil | 120 rpm shaking, 12 days at 30 °C | [20] |
| Olive mill sludge | Remazol brilliant blue R (RBBR) plate count agar-tannic acid or potato dextrose agar-tannic acid | Incubation at 30 °C for 48 h (bacteria) and 96 h fungi | [11] |
| Product Name | Function | Source | Significance of Microbes | Cost of Remediation ($ per acre) | Reference |
|---|---|---|---|---|---|
| BioSpill | Biodegradation of hydrocarbons | Bacillus sp. | Bacillus sp. can degrade a wide range of hydrocarbons, including crude oil, gasoline, and diesel. | 100–500 | [67] |
| Bio-Solve Pink | Biodegradation of petroleum hydrocarbons | Pseudomonas sp. and other bacterial strains | Pseudomonas sp. is known to degrade petroleum hydrocarbons efficiently and has been widely used in bioremediation. | 200–1000 | [68] |
| Petrox | Biodegradation of hydrocarbons and other pollutants | Mixed culture of microorganisms | The mixed culture of microorganisms can degrade a wide range of pollutants, including crude oil, gasoline, and diesel. | 1000–5000 | [69] |
| Nualgi | Biostimulation of indigenous microbes | Diatomaceous earth and micronutrients | Nualgi provides micronutrients to the indigenous microbial population to enhance their hydrocarbon-degrading abilities. | 100–500 | [70] |
| Oilgone | Biodegradation of hydrocarbons | Bacillus sp. and other bacterial strains | Bacillus sp. is known to degrade hydrocarbons efficiently and has been widely used in bioremediation. Oilgone contains a blend of bacterial strains. | 500–2000 | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radhakrishnan, A.; Balaganesh, P.; Vasudevan, M.; Natarajan, N.; Chauhan, A.; Arora, J.; Ranjan, A.; Rajput, V.D.; Sushkova, S.; Minkina, T.; et al. Bioremediation of Hydrocarbon Pollutants: Recent Promising Sustainable Approaches, Scope, and Challenges. Sustainability 2023, 15, 5847. https://doi.org/10.3390/su15075847
Radhakrishnan A, Balaganesh P, Vasudevan M, Natarajan N, Chauhan A, Arora J, Ranjan A, Rajput VD, Sushkova S, Minkina T, et al. Bioremediation of Hydrocarbon Pollutants: Recent Promising Sustainable Approaches, Scope, and Challenges. Sustainability. 2023; 15(7):5847. https://doi.org/10.3390/su15075847
Chicago/Turabian StyleRadhakrishnan, Arathi, Pandiyan Balaganesh, Mangottiri Vasudevan, Narayanan Natarajan, Abhishek Chauhan, Jayati Arora, Anuj Ranjan, Vishnu D. Rajput, Svetlana Sushkova, Tatiana Minkina, and et al. 2023. "Bioremediation of Hydrocarbon Pollutants: Recent Promising Sustainable Approaches, Scope, and Challenges" Sustainability 15, no. 7: 5847. https://doi.org/10.3390/su15075847
APA StyleRadhakrishnan, A., Balaganesh, P., Vasudevan, M., Natarajan, N., Chauhan, A., Arora, J., Ranjan, A., Rajput, V. D., Sushkova, S., Minkina, T., Basniwal, R. K., Kapardar, R., & Srivastav, R. (2023). Bioremediation of Hydrocarbon Pollutants: Recent Promising Sustainable Approaches, Scope, and Challenges. Sustainability, 15(7), 5847. https://doi.org/10.3390/su15075847











