A Comprehensive Review on Recent Advancements in Absorption-Based Post Combustion Carbon Capture Technologies to Obtain a Sustainable Energy Sector with Clean Environment
Abstract
1. Introduction
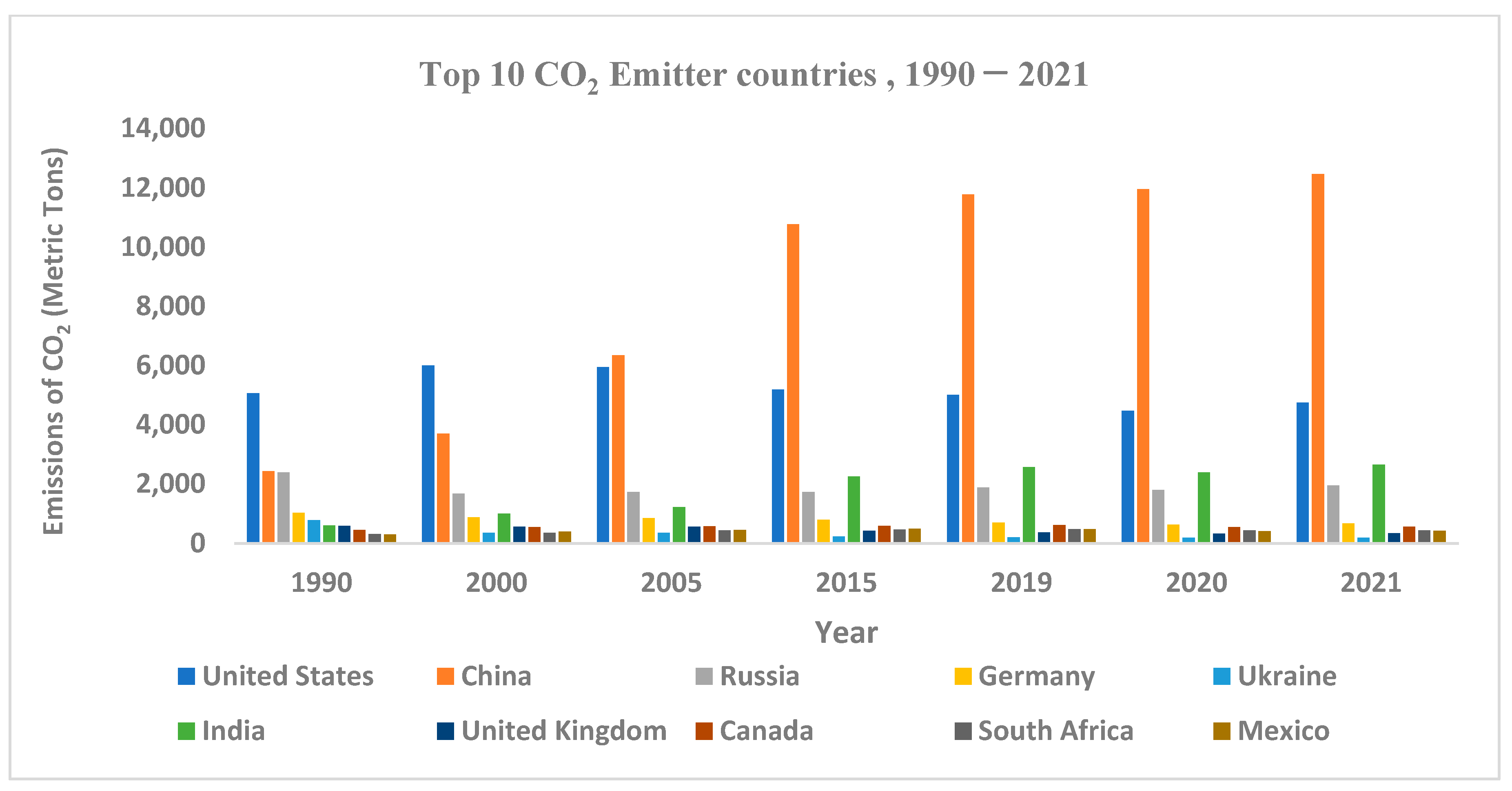
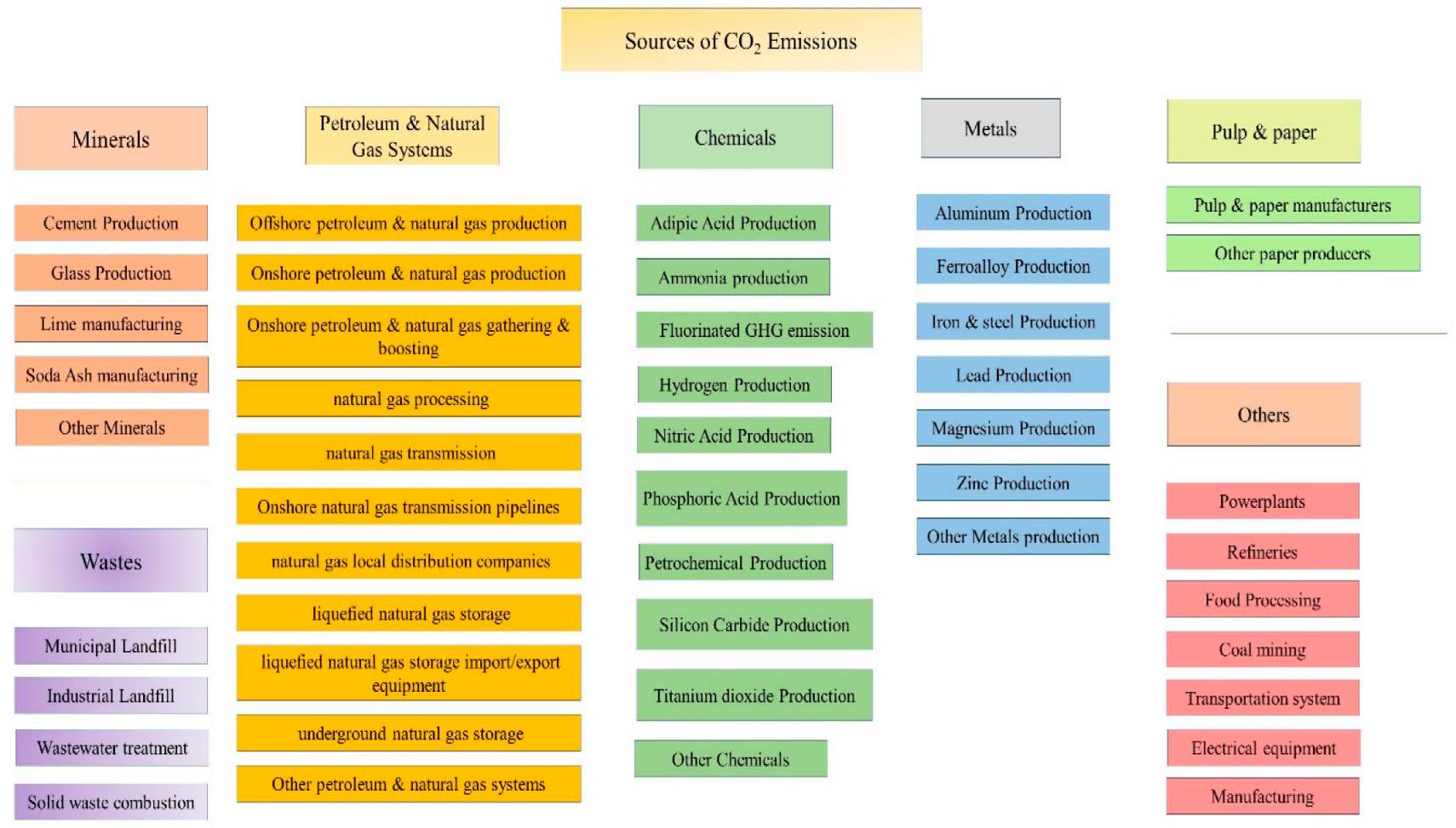
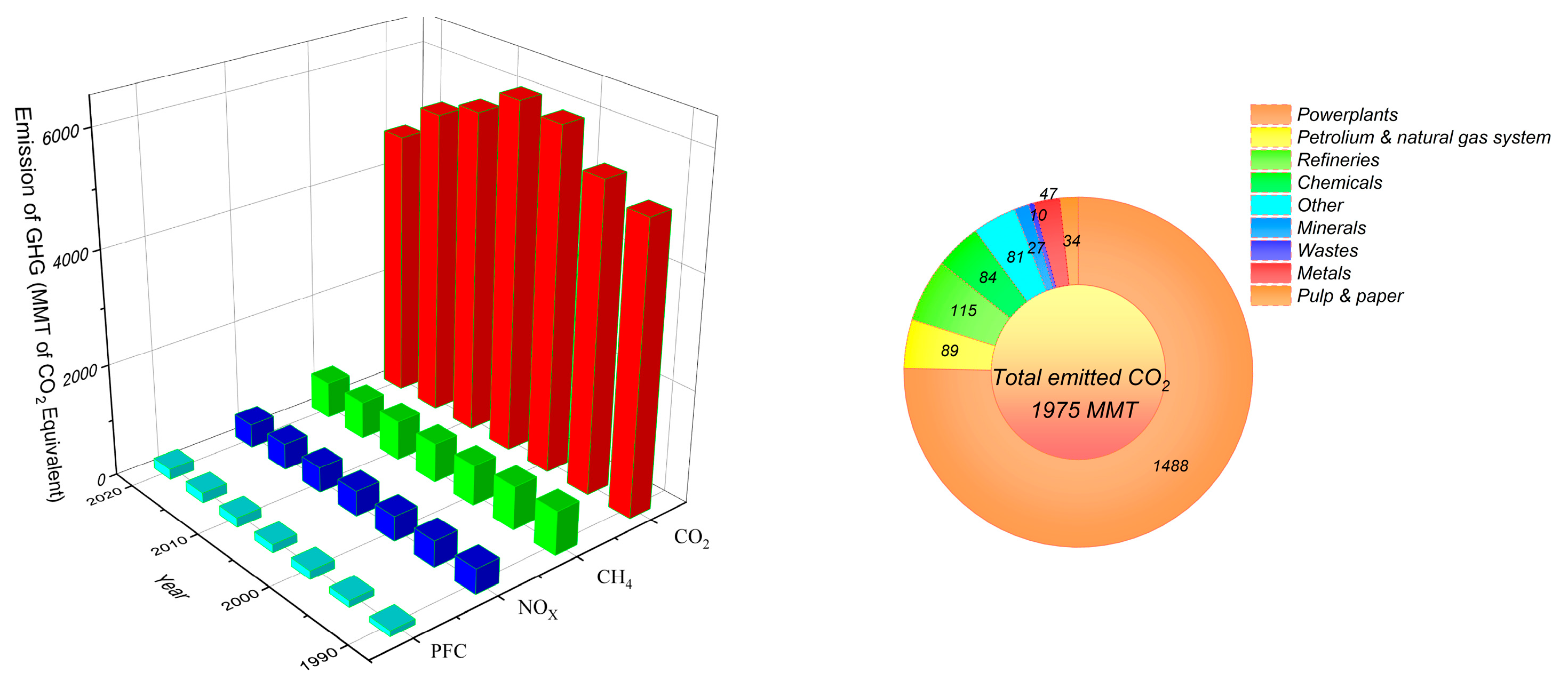
Status of Global CO2 Emissions
2. Absorption-Based Carbon Capture
2.1. Physical Absorption
2.2. Chemical Absorption
3. Developed Chemical Absorption Processes
3.1. Amine-Based Absorption
3.2. KMALC
3.3. Fluor EFG + Process
3.4. KM-CDR Process
3.5. Chilled Ammonia Process (CAP)
3.6. Aqueous Ammonia Scrubbing
3.7. Amino Acid Absorption
3.8. Dual Alkali Absorption (DAA)
3.9. Alkaline Solvent Absorption
4. Different Types of Absorbents
4.1. Polymeric Solvent
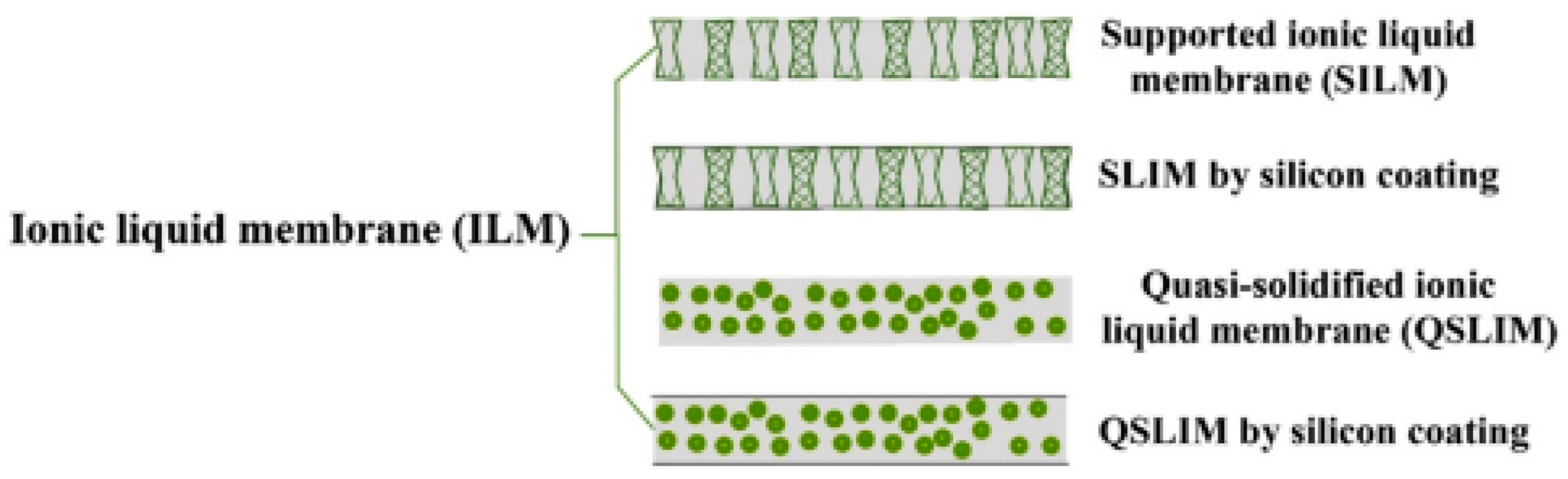
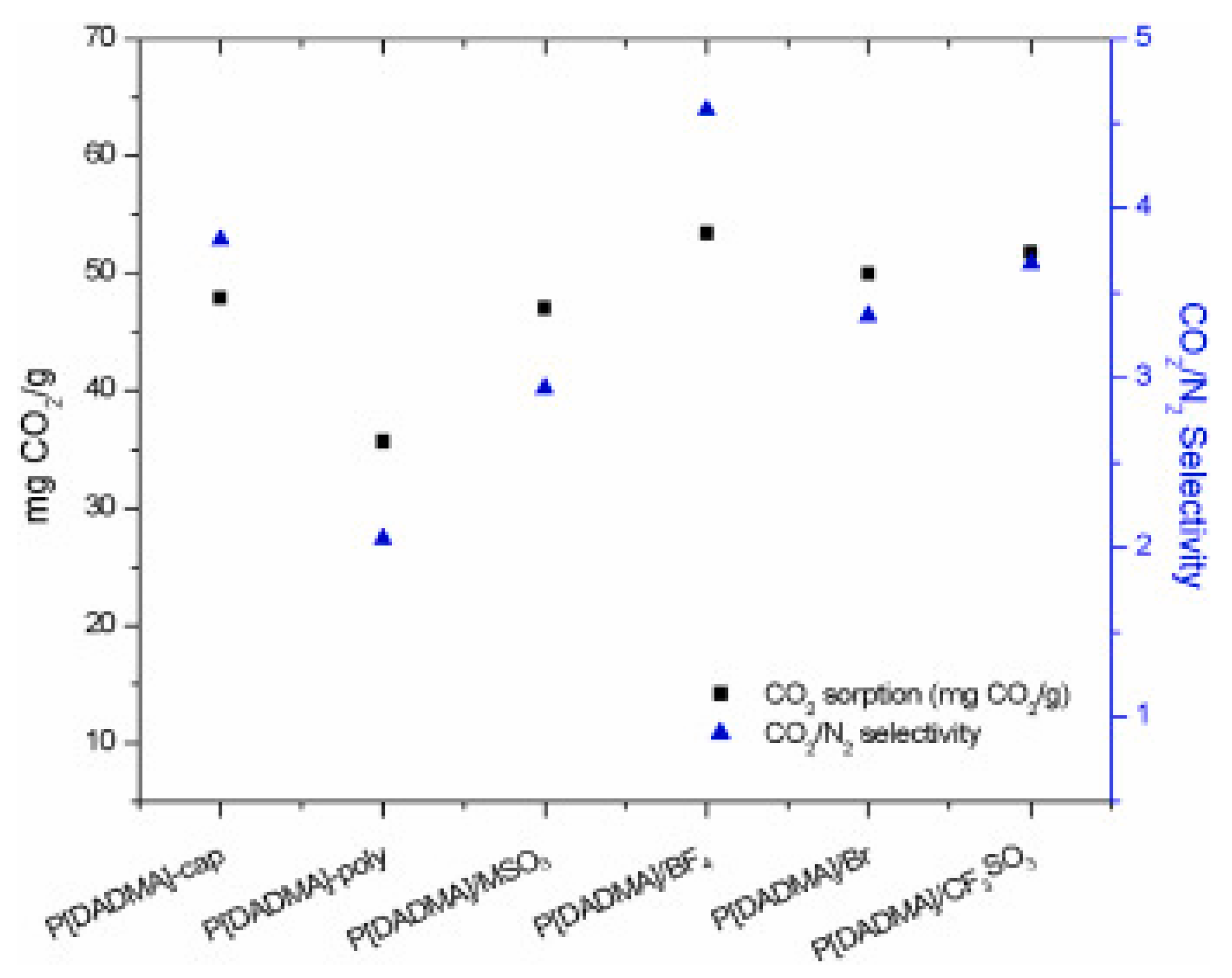
4.2. Poly-Ionic Liquid
4.3. Nano Sorbent
4.3.1. Nanofluids for CO2 Absorption
4.3.2. Nano-Emulsions for CO2 Capture
4.4. Amino Acid Solution
4.5. Hydroxide Absorbent
4.5.1. Potassium Hydroxide
4.5.2. Sodium Hydroxide-Based Hybrid Absorbents
4.6. Carbonate Absorbent
4.6.1. Potassium Carbonate
4.6.2. Sodium Carbonate
4.6.3. Calcium Carbonate
4.7. Amine-Based Absorbents
4.7.1. Primary Amines
4.7.2. Secondary Amines
4.7.3. Tertiary Amines
| Year | Alkanolamine | Temperature, K | The Partial Pressure of CO2, kPa | Amine Concentration, % | CO2 Loading, α | Ref. |
|---|---|---|---|---|---|---|
| 1972 | DEA | 323 | 7–3370 | 19.2 | 0.45–1.13 | [89] |
| 1976 | DEA | 338.5–366.9 | 32–767 | 25 | 0.4–0.79 | [90] |
| 1977 | DIPA | 313–373 | 2.7–5888 | 33.63 | 0.07–1.11 | [91] |
| 1978 | DGA | 323–373 | 1.58–4720 | 60 | 0.13–0.62 | [92] |
| 1988 | AMP | 313.2 | 1.25–144 | 28 | 0.4–0.9 | [93] |
| 1990 | AMP | 313, 343 | 0.16–5279 | 18.8 | 0.03–1.65 | [94] |
| 1991 | AMP | 293–353 | 1.59–94 | 18.76, 28.14 | 0.13–0.94 | [95] |
| MDEA | 313 | 0.18–92.8 | 22.9 | 0.04–0.84 | [96] | |
| 1992 | MDEA+MEA | 313.15–373.15 | 1.12–2080 | MDEA: 12–24/MEA: 6–18 | 0.188–1.015 | [97] |
| 1996 | AMP | 313–353 | 3.94–336.6 | 30 | 0.28–0.9 | [98] |
| DEA | 313–353 | 4.85–357.3 | 30 | 0.4–0.73 | [98] | |
| 1998 | DEA+AMP | 313.15–373.15 | 22–2838 | DEA: 20–25/AMP: 5–10 | 0.337–1.2 | [99] |
| DEA+MDEA | 313.15–393.15 | 0.4–2833.6 | DEA: 10–32.5/MDEA: 10–35 | 0.038–1.119 | [99] | |
| 2000 | MDEA | 297.7 | 0.02–1.64 | 23.63 | 0.02–0.26 | [100] |
| PZ | 313–343 | 0.03–40 | 4.7 | 0.16–0.96 | [101] | |
| 313–343 | 29–40,200 | 4.7 | 0.6–0.96 | [101] | ||
| 2001 | MDEA | 298–373 | 0.78–140.4 | 50 | 0.01–0.49 | [102] |
| 2004 | MDEA | 298–348 | 2.7–4559.5 | 48.88, 25.73 | 0–1.3 | [103] |
| DEA | 298–348 | 4.85–357.3 | 47.78 | 0–1.09 | [103] | |
| 2006 | DEA | 323–366 | 0.4–3798 | 25 | 0.1–1.13 | [104] |
| 2010 | AMP | 313.2 | 0.89–151.9 | 28 | 0.4–0.9 | [105] |
| MDEA | 313 | 0.28–89.9 | 22.9 | 0.06–0.80 | [106] | |
| 323 | 6–434 | 50 | 0.1–0.89 | [106] | ||
| PZ | 313 | 5800–7500 | 15–60 | 0.34–0.86 | [107] | |
| TEA | 313–353 | 1.43–153.4 | 26.5 | 0.03–0.53 | [106] | |
| 2011 | PZ | 354–464.8 | 28–2583 | 29.8–40.59 | 0.23–0.45 | [108] |
| AMP | 298–328 | 0.41–1449 | 23.5–46 | 0.19–1.1 | [109] | |
| 303–328 | 0.31–1472 | 40,50 | 0.24–1.04 | [109] | ||
| 2012 | MEA | 303–323 | 0.9–335.9 | 6.7–19 | 0.35–1.16 | [110] |
| AMP | 313–393 | 6–983.5 | 30 | 0–0.97 | [111] | |
| 2013 | AEEA | 303–323 | 1.11–794.67 | 15 | 0.06–1.4077 | [112] |
| 2014 | DIPA | 313–343 | 107–4064 | 45 | 0.52–1.05 | [113] |
| 2017 | NH3 | 335–395 | 0.01–1000 | 20.4 | 1 | [114] |
| DIPA | 313–343 | 91.2–3826.6 | 30 | 0.89–1.14 | [113] | |
| DIPA + AEEA | 313.15–343.15 | 105–3819.7 | DIPA: 20.25/AEEA: 5–10 | 0.5837–1.251 | [113] | |
| MDEA + PZ | 313–375.15 | 0.033–95.78 | MDEA:22.6–47.6/PZ: 0.4–21.3 | 0.027–0.37 | [115] | |
| DIPA + AMP + PZ | 313.15–343.15 | 112.9–3709.7 | DIPA: 24–36/AMP: 7–13/PZ: 2–8 | 0.502–1.091 | [113] | |
| 2019 | MEA | 303–353 | 0–50.65 | 12–15 | 0.017–0.577 | [116] |
| AMP + PZ | 293.15–323.15 | 0.127–140.4 | AMP: 8.9–38/PZ: 0.87–8 | 0.1511–0.9405 | [117] | |
| MEA + DAP | 315.15–333.15 | 13.24–215.46 | MEA: 10–12.5/DAP: 2.5–5 | 0.22–0.711 | [118] | |
| 2021 | MEA | 307.9 | - | 24.9 | 2.5–32.5 | [119] |
| MEA + DEA | 308 | - | 24.8 | 2.5–32.5 | [119] | |
| MEA + TEA | 308 | - | 25 | 2.5–32.5 | [119] | |
| 2022 | DA2MP/AMP/PrOH | 313.15–383.15 | - | 20 | 0.91–0.95 | [120] |
4.8. Triethylenetetramine (TETA)/Ethanol Solution as Absorbent
4.9. Amino Acid Salt as Liquid-Solid Phase Changes Absorbent
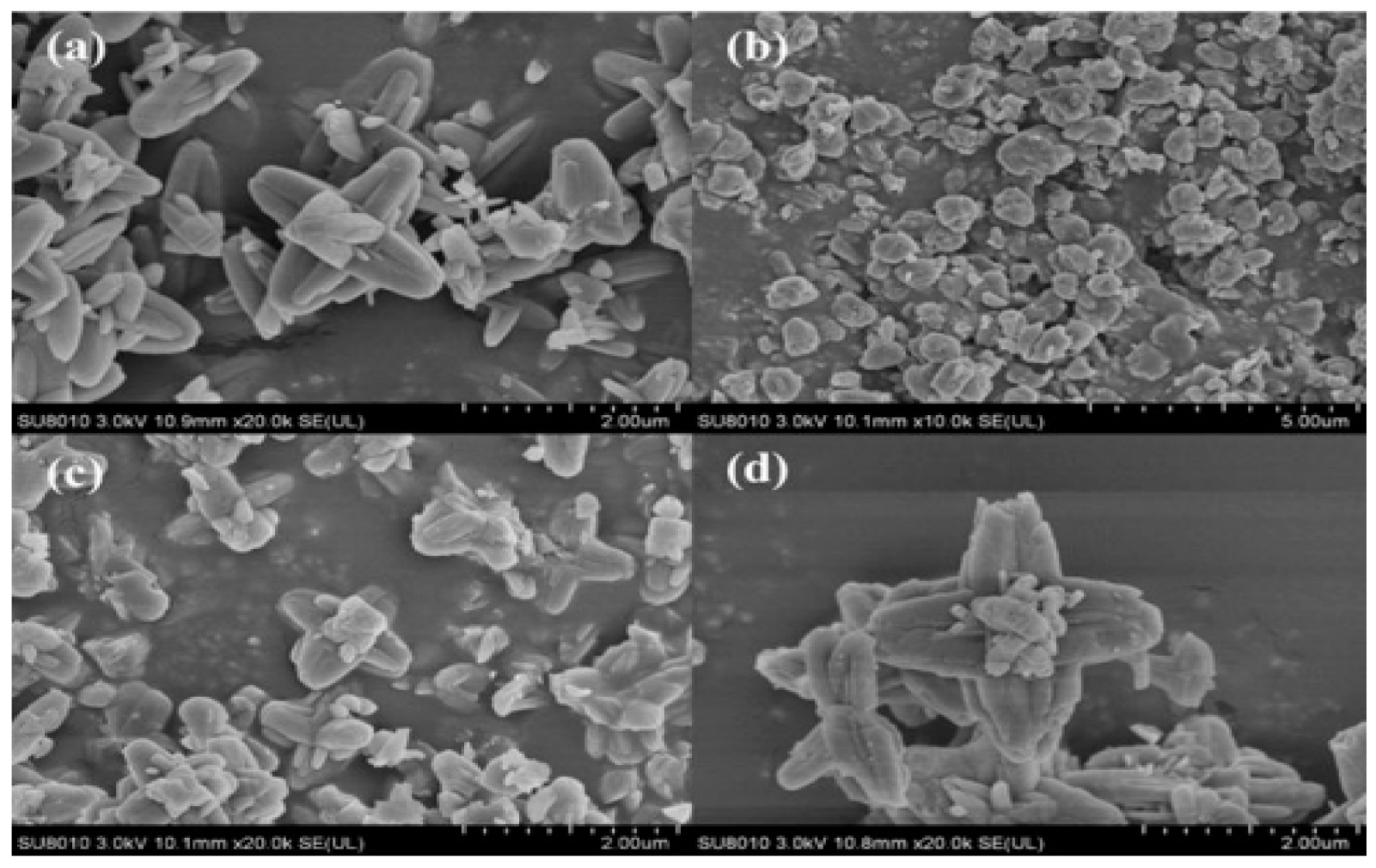
4.10. Encapsulated Absorbents
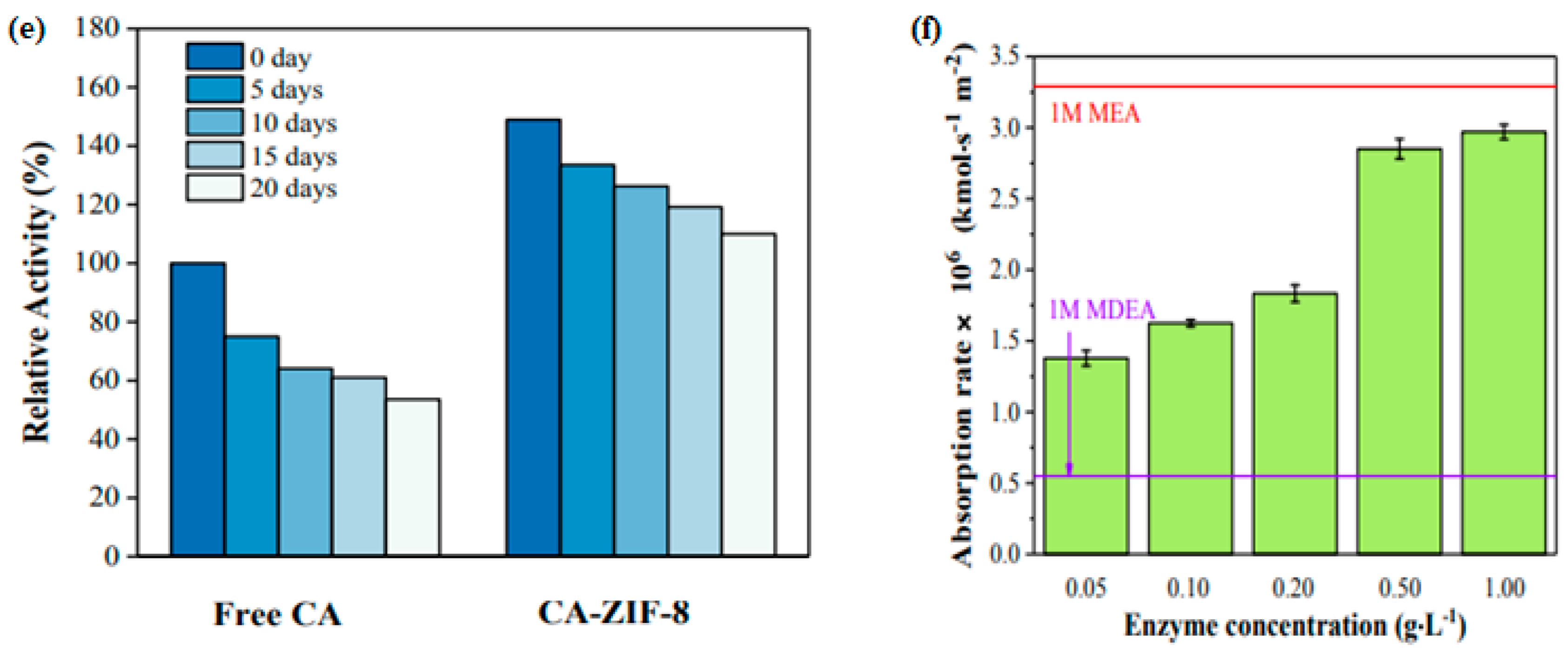
4.11. Enzymatically Catalyzed Absorbent Systems
4.12. Deep Eutectic Solvents (DESs)

5. Outlook and Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McInnes, K.; Walsh, K.; Hubbert, G.D.; Beer, T. Impact of Sea-level Rise and Storm Surges on a Coastal Community. Nat. Hazards 2003, 30, 187–207. [Google Scholar] [CrossRef]
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, V.; Carraro, C.; Massetti, E.; Tavoni, M. International energy R&D spillovers and the economics of greenhouse gas atmospheric stabilization. Energy Econ. 2008, 30, 2912–2929. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D.; Akanda, A.M.; Islam, A.R.M.T. Peer-to-Peer Energy Trading Pricing Mechanisms: Towards a Comprehensive Analysis of Energy and Network Service Pricing (NSP) Mechanisms to Get Sustainable Enviro-Economical Energy Sector. Energies 2023, 16, 2198. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.M.M.B.; Allen, S.K.; Boschung, J.; Midgley, P.M. The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Clim. Chang. 2013, 1535, 2013. [Google Scholar]
- Das, A.; Peu, S.D. A Comprehensive Review on Recent Advancements in Thermochemical Processes for Clean Hydrogen Production to Decarbonize the Energy Sector. Sustainability 2022, 14, 11206. [Google Scholar] [CrossRef]
- UNFCCC. ADOPTION OF THE PARIS AGREEMENT. Proposal by the President. 2015. Available online: https://unfccc.int/documents/9064 (accessed on 12 December 2015).
- Zhang, S.; Lu, Y.; Ye, X. Catalytic behavior of carbonic anhydrase enzyme immobilized onto nonporous silica nanoparticles for enhancing CO2 absorption into a carbonate solution. Int. J. Greenh. Gas Control 2013, 13, 17–25. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, Y.T. CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution. Energy 2013, 53, 206–211. [Google Scholar] [CrossRef]
- Wang, T.; Yu, W.; Liu, F.; Fang, M.; Farooq, M.; Luo, Z. Enhanced CO2 Absorption and Desorption by Monoethanolamine (MEA)-Based Nanoparticle Suspensions. Ind. Eng. Chem. Res. 2016, 55, 7830–7838. [Google Scholar] [CrossRef]
- Sahoo, P.C.; Kumar, M.; Singh, A.; Singh, M.P.; Puri, S.K. Biocatalyzed Accelerated Post-combustion CO2 Capture and Stripping in Monoethanolamine. Energy Fuels 2017, 31, 11007–11012. [Google Scholar] [CrossRef]
- Liang, Z.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.Z.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar] [CrossRef]
- Aboudheir, A.; Tontiwachwuthikul, P.; Idem, R. Rigorous Model for Predicting the Behavior of CO2 Absorption into AMP in Packed-Bed Absorption Columns. Ind. Eng. Chem. Res. 2005, 45, 2553–2557. [Google Scholar] [CrossRef]
- Oyenekan, B.A.; Rochelle, G.T. Energy Performance of Stripper Configurations for CO2 Capture by Aqueous Amines. Ind. Eng. Chem. Res. 2005, 45, 2457–2464. [Google Scholar] [CrossRef]
- Grant, D.; Bergstrand, K.; Running, K. Effectiveness of US state policies in reducing CO2 emissions from power plants. Nat. Clim. Chang. 2014, 4, 977–982. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Fifth Assessment Report, Working Group III, Summary for Policymakers. 2014. Available online: http://www.ipcc.ch/report/ar5/wg3/ (accessed on 27 October 2018).
- Electricity in the U.S.—U.S. Energy Information Administration (EIA) n.d. Available online: https://www.eia.gov/energyexplained/electricity/electricity-in-the-us.php (accessed on 30 October 2022).
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Gielen, D. CO2 removal in the iron and steel industry. Energy Convers. Manag. 2003, 44, 1027–1037. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, B.; Li, L. Advance in Post-Combustion CO2 Capture with Alkaline Solution: A Brief Review. Energy Procedia 2012, 14, 1515–1522. [Google Scholar] [CrossRef]
- Paul, H.M.; Cousins, F.A.; Jiang, K.; Zhai, R.; Garcia, M. An update of the benchmark post-combustion CO2-capture technology. Fuel 2020, 273, 117776. [Google Scholar] [CrossRef]
- Karami, B.; Ghaemi, A. Cost-effective nanoporous hypercross-linked polymers could drastically promote the CO2absorption rate in amine-based solvents, improving energy-efficient CO2capture. Ind. Eng. Chem. Res. 2021, 60, 3105–3114. [Google Scholar] [CrossRef]
- Arunachalam, R.; Chinnaraja, E.; Subramanian, P.S. Efficient Homogeneous Catalysts for Conversion of CO2 to Fine Chemicals. In Catalysis for Clean Energy and Environmental Sustainability; Pant, K.K., Gupta, S.K., Ahmad, E., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Yuan, Z.; Eden, M.R.; Gani, R. Toward the Development and Deployment of Large-Scale Carbon Dioxide Capture and Conversion Processes. Ind. Eng. Chem. Res. 2016, 55, 3383–3419. [Google Scholar] [CrossRef]
- Barchas, R.; Davis, R. The Kerr-McGee/ABB Lummus Crest technology for the recovery of CO2 from stack gases. Energy Convers. Manag. 1992, 33, 333–340. [Google Scholar] [CrossRef]
- Scherffius, J.R.; Reddy, S.; Klumpyan, J.P.; Armpriester, A. Large-Scale CO2 Capture Demonstration Plant Using Fluor’s Econamine FG PlusSM Technology at NRG’s WA Parish Electric Generating Station. Energy Procedia 2013, 37, 6553–6561. [Google Scholar] [CrossRef]
- Bhown, A.; Dillon, D.; Berger, A.H.; Du, Y.; Haney, K.; Carroll, B.; Gilmartin, J.; Simonson, T.; Reddy, S. Front End Engineering Design Study for Carbon Capture at a Natural Gas Combined Cycle Power Plant in California. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Miyamoto, O.; Maas, C.; Tsujiuchi, T.; Inui, M.; Hirata, T.; Tanaka, H.; Yonekawa, T.; Kamijo, T. KM CDR ProcessTM Project Update and the New Novel Solvent Development. Energy Procedia 2017, 114, 5616–5623. [Google Scholar] [CrossRef]
- Kadono, K.; Suzuki, A.; Iijima, M.; Ohishi, T.; Tanaka, H.; Hirata, T.; Kondo, M. New Energy Efficient Processes and Newly Developed Absorbents for Flue Gas CO2 Capture. Energy Procedia 2013, 37, 1785–1792. [Google Scholar] [CrossRef]
- Hirata, T.; Tsujiuchi, T.; Kamijo, T.; Kishimoto, S.; Inui, M.; Kawasaki, S.; Lin, Y.-J.; Nakagami, Y.; Nojo, T. Near-zero emission coal-fired power plant using advanced KM CDR process™. Int. J. Greenh. Gas Control 2019, 92, 102847. [Google Scholar] [CrossRef]
- Molina, C.T.; Bouallou, C. Assessment of different methods of CO 2 capture in post-combustion using ammonia as solvent. J. Clean. Prod. 2015, 103, 463–468. [Google Scholar] [CrossRef]
- Hughes, R.; Kotamreddy, G.; Bhattacharyya, D.; Omell, B.; Matuszewski, M. Modeling and Bayesian Uncertainty Quantifica-tion of a Membrane-Assisted Chilled Ammonia Process for CO2Capture. Ind. Eng. Chem. Res. 2022, 61, 4001–4016. [Google Scholar] [CrossRef]
- Darde, V.; Thomsen, K.; Van Well, W.J.M.; Stenby, E.H. Chilled ammonia process for CO2 capture. Energy Procedia 2009, 1, 1035–1042. [Google Scholar] [CrossRef]
- Bonalumi, D.; Lillia, S.; Valenti, G. Rate-based simulation and techno-economic analysis of coal-fired power plants with aqueous ammonia carbon capture. Energy Convers. Manag. 2019, 199, 111966. [Google Scholar] [CrossRef]
- Yu, H.; Qi, G.; Xiang, Q.; Wang, S.; Fang, M.; Yang, Q.; Wardhaugh, L.; Feron, P. Aqueous Ammonia Based Post Combustion Capture: Results from Pilot Plant Operation, Challenges and Further Opportunities. Energy Procedia 2013, 37, 6256–6264. [Google Scholar] [CrossRef]
- Rashidi, H.; Rasouli, P.; Azimi, H. A green vapor suppressing agent for aqueous ammonia carbon dioxide capture solvent: Microcontactor mass transfer study. Energy 2021, 244, 122711. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, W.; Wang, J.; Soltanian, M.R.; Olabi, A.G. Effectiveness of amino acid salt solutions in capturing CO2: A review. Renew. Sustain. Energy Rev. 2018, 98, 179–188. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Gray, M.L.; Duan, Y.; Luebke, D.; Li, B. Development of amino acid and amino acid-complex based solid sorbents for CO2 capture. Appl. Energy 2013, 109, 112–118. [Google Scholar] [CrossRef]
- Majchrowicz, M.E.; Brilman, D.; Groeneveld, M.J. Precipitation regime for selected amino acid salts for CO2 capture from flue gases. Energy Procedia 2009, 1, 979–984. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A.; Ho, M.T.; Wiley, D.E. A comparison between amino acid based solvent and traditional amine solvent processes for CO2 removal. Chem. Eng. Res. Des. 2019, 146, 509–517. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, E.; Heffernan, K.; van der Ham, L.; Linders, M.J.; Goetheer, E.L.; Vlugt, T.J. Precipitating Amino Acid Solvents for CO2 Capture. Opportunities to Reduce Costs in Post Combustion Capture. Energy Procedia 2014, 63, 727–738. [Google Scholar] [CrossRef]
- Kim, K.; Cho, S.-G.; Sa, J.-H. Natural Hydrophilic Amino Acids as Environment-Friendly Gas Hydrate Inhibitors for Carbon Capture and Sequestration. ACS Sustain. Chem. Eng. 2021, 9, 17413–17419. [Google Scholar] [CrossRef]
- Lyu, H.; Chen, O.I.-F.; Hanikel, N.; Hossain, M.I.; Flaig, R.W.; Pei, X.; Amin, A.; Doherty, M.D.; Impastato, R.K.; Glover, T.G.; et al. Carbon Dioxide Capture Chemistry of Amino Acid Functionalized Metal–Organic Frameworks in Humid Flue Gas. J. Am. Chem. Soc. 2022, 144, 2387–2396. [Google Scholar] [CrossRef]
- Onofri, S.; Bodo, E. CO2 Capture in Biocompatible Amino Acid Ionic Liquids: Exploring the Reaction Mechanisms for Bimolecular Absorption Processes. J. Phys. Chem. B 2021, 125, 5611–5619. [Google Scholar] [CrossRef]
- Castro, M.; Gómez-Díaz, D.; Navaza, J.M.; Rumbo, A. Carbon Dioxide Capture by Chemical Solvents Based on Amino Acids: Absorption and Regeneration. Chem. Eng. Technol. 2020, 44, 248–257. [Google Scholar] [CrossRef]
- Kasturi, A.; Gabitto, J.; Tsouris, C.; Custelcean, R. Carbon dioxide capture with aqueous amino acids: Mechanistic study of amino acid regeneration by guanidine crystallization and process intensification. Sep. Purif. Technol. 2021, 271, 118839. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.P.; Liao, C.-Y.; Zhao, X.; Hsiung, T.-L.; Liu, S.-H.; Chang, S.-G. Dual Alkali Solvent System for CO2Capture from Flue Gas. Environ. Sci. Technol. 2017, 51, 8824–8831. [Google Scholar] [CrossRef]
- Shu, Q.; Legrand, L.; Kuntke, P.; Tedesco, M.; Hamelers, H.V.M. Electrochemical Regeneration of Spent Alkaline Absorbent from Direct Air Capture. Environ. Sci. Technol. 2020, 54, 8990–8998. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Luebke, D.R.; Enick, R.M. CO2-philic Oligomers as Novel Solvents for CO2Absorption. Energy Fuels 2010, 24, 6214–6219. [Google Scholar] [CrossRef]
- Kim, H.; Jung, J.Y.; Park, K.H.; Linga, P.; Seo, Y.; Wood, C.D. Enhanced Kinetic Performance of Amine-Infused Hydrogels for Separating CO2from CH4/CO2Gas Mixture. Energy Fuels 2021, 35, 13889–13899. [Google Scholar] [CrossRef]
- Enick, R.M.; Koronaios, P.; Stevenson, C.; Warman, S.; Morsi, B.; Nulwala, H.; Luebke, D. Hydrophobic Polymeric Solvents for the Selective Absorption of CO2 from Warm Gas Streams that also Contain H2 and H2O. Energy Fuels 2013, 27, 6913–6920. [Google Scholar] [CrossRef]
- Xu, X.; Heath, C.; Pejcic, B.; Wood, C.D. CO2 capture by amine infused hydrogels (AIHs). J. Mater. Chem. A 2018, 6, 4829–4838. [Google Scholar] [CrossRef]
- White, C.; Adam, E.; Sabri, Y.; Myers, M.B.; Pejcic, B.; Wood, C.D. Amine-Infused Hydrogels with Nonaqueous Solvents: Facile Platforms to Control CO2 Capture Performance. Ind. Eng. Chem. Res. 2021, 60, 14758–14767. [Google Scholar] [CrossRef]
- Dave, A.; Pathak, B.; Dave, M.; Rezvani, S.; Huang, Y.; Hewitt, N. Process design of CO2 desorption from physical solvent di-methyl-ether of poly-ethylene-glycol. Mater. Sci. Energy Technol. 2019, 3, 209–217. [Google Scholar] [CrossRef]
- Dave, A.; Dave, M.; Huang, Y.; Rezvani, S.; Hewitt, N. Process design for CO 2 absorption from syngas using physical solvent DMEPEG. Int. J. Greenh. Gas Control 2016, 49, 436–448. [Google Scholar] [CrossRef]
- Sattari, A.; Ramazani, A.; Aghahosseini, H.; Aroua, M.K. The application of polymer containing materials in CO2 capturing via absorption and adsorption methods. J. CO2 Util. 2021, 48, 101526. [Google Scholar] [CrossRef]
- Zheng, W.-T.; Zhang, J.-B.; Liu, Y.; Huang, K. Reversible Chemical Absorption of CO2 in Polyethylenimine Supported by Low-Viscous Tetrabutylphosphonium 2-Fluorophenolate. Energy Fuels 2020, 34, 3493–3500. [Google Scholar] [CrossRef]
- Rolker, J.; Seiler, M.; Mokrushina, L.; Arlt, W. Potential of Branched Polymers in the Field of Gas Absorption: Experimental Gas Solubilities and Modeling. Ind. Eng. Chem. Res. 2007, 46, 6572–6583. [Google Scholar] [CrossRef]
- Hossain, I.; Kim, D.; Al Munsur, A.Z.; Roh, J.M.; Park, H.B.; Kim, T.-H. PEG/PPG–PDMS-Based Cross-Linked Copolymer Membranes Prepared by ROMP and In Situ Membrane Casting for CO2 Separation: An Approach to Endow Rubbery Materials with Properties of Rigid Polymers. ACS Appl. Mater. Interfaces 2020, 12, 27286–27299. [Google Scholar] [CrossRef] [PubMed]
- Shamshiri, M.; Jafari, R.; Momen, G. Icephobic properties of aqueous self-lubricating coatings containing PEG-PDMS copolymers. Prog. Org. Coat. 2021, 161, 106466. [Google Scholar] [CrossRef]
- Ramalingame, R.; Chandraker, P.; Kanoun, O. Investigation on the Influence of Solvents on MWCNT-PDMS Nanocomposite Pressure Sensitive Films. Proceedings 2017, 1, 384. [Google Scholar] [CrossRef]
- Rumens, C.V.; Ziai, M.A.; Belsey, K.E.; Batchelor, J.C.; Holder, S.J. Swelling of PDMS networks in solvent vapours; applications for passive RFID wireless sensors. J. Mater. Chem. C 2015, 3, 10091–10098. [Google Scholar] [CrossRef]
- Chau, J.; Jie, X.; Sirkar, K.K. Polyamidoamine-facilitated poly(ethylene glycol)/ionic liquid based pressure swing membrane absorption process for CO2 removal from shifted syngas. Chem. Eng. J. 2016, 305, 212–220. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef]
- McGrail, B.; Thallapally, P.; Blanchard, J.; Nune, S.; Jenks, J.; Dang, L. Metal-organic heat carrier nanofluids. Nano Energy 2013, 2, 845–855. [Google Scholar] [CrossRef]
- Ramazani, R.; Samsami, A.; Jahanmiri, A.; Van der Bruggen, B.; Mazinani, S. Characterization of monoethanolamine + potassium lysinate blend solution as a new chemical absorbent for CO2 capture. Int. J. Greenh. Gas Control 2016, 51, 29–35. [Google Scholar] [CrossRef]
- Rastegar, Z.; Ghaemi, A.; Shirvani, M. Experimental Study of Carbon Dioxide Absorption Using Aqueous Potassium Hydroxide Solutions. Nashrieh Shimi Mohandesi Shimi Iran 2021, 40, 115–126. [Google Scholar]
- Mourad, A.A.-H.; Mohammad, A.F.; Al-Marzouqi, A.H.; Altarawneh, M.; Al-Marzouqi, M.H.; El-Naas, M.H. Carbon dioxide capture through reaction with potassium hydroxide and reject brine: A kinetics study. Int. J. Greenh. Gas Control 2022, 120, 103768. [Google Scholar] [CrossRef]
- Firman, N.; Noor, A.; Zakir, M.; Maming, M.; Fathurrahman, A.F. Absorption of Carbon Dioxide into Potassium Hydroxide: Preliminary Study for its Application into Liquid Scintillation Counting Procedure. Egypt. J. Chem. 2021, 64, 4907–4912. [Google Scholar] [CrossRef]
- Spector: Removal of Carbon Dioxide from Atmospheric Air-Google Scholar n.d. Available online: https://scholar.google.com/scholar_lookup?title=Removal%20of%20carbon%20dioxide%20from%20atmospheric%20air&publication_year=1946&author=N.A.%20Spector&author=B.F.%20Dodge (accessed on 26 January 2023).
- Tepe: Absorption of Carbon Dioxide by Sodium Hydroxide...-Google Scholar n.d. Available online: https://scholar.google.com/scholar_lookup?title=Absorption%20of%20carbon%20dioxide%20by%20sodium%20hydroxide%20solutions%20in%20a%20packed%20column&publication_year=1943&author=J.B.%20Tepe&author=B.F.%20Dodge (accessed on 26 January 2023).
- Naeem, S.; Shahhosseini, S.; Ghaemi, A. Simulation of CO 2 capture using sodium hydroxide solid sorbent in a fluidized bed reactor by a multi-layer perceptron neural network. J. Nat. Gas Sci. Eng. 2016, 31, 305–312. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Valeh-E-Sheyda, P.; Nafchi, N.F. Carbon dioxide capture by the green aqueous sodium hydroxide-glycerol solution in a gas-liquid microchannel contactor. J. Environ. Chem. Eng. 2022, 10, 108666. [Google Scholar] [CrossRef]
- Zhao, W.; Sprachmann, G.; Li, Z.; Cai, N.; Zhang, X. Effect of K2CO3·1.5H2O on the regeneration energy consumption of potassium-based sorbents for CO2 capture. Appl. Energy 2013, 112, 381–387. [Google Scholar] [CrossRef]
- Thee, H.; Smith, K.H.; da Silva, G.; Kentish, S.E.; Stevens, G.W. Carbon dioxide absorption into unpromoted and borate-catalyzed potassium carbonate solutions. Chem. Eng. J. 2011, 181–182, 694–701. [Google Scholar] [CrossRef]
- Hu, G.; Smith, K.H.; Wu, Y.; Kentish, S.E.; Stevens, G.W. Screening Amino Acid Salts as Rate Promoters in Potassium Carbonate Solvent for Carbon Dioxide Absorption. Energy Fuels 2017, 31, 4280–4286. [Google Scholar] [CrossRef]
- Li, Z.; Ji, X.; Yang, Z.; Lu, X. Study of CO2 absorption/desorption behaviors in aqueous (2-hydroxyethyl)-trimethyl-ammonium (S)-2-pyrrolidine-carboxylic acid salt ([Cho][Pro]) + K2CO3 solutions. Int. J. Greenh. Gas Control 2019, 83, 51–60. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.E.; Nam, S.C.; Park, S.Y.; Chun, I.S.; Yoon, Y.I.; Lee, J.-H. Promoter Characteristic Study on the K2CO3 Absorbents for CO2 Capture: Mass Transfer According to Functional Group and Chain Length of Promoter. Energy Procedia 2017, 114, 898–905. [Google Scholar] [CrossRef]
- Valluri, S.; Kawatra, S. Use of frothers to improve the absorption efficiency of dilute sodium carbonate slurry for post combustion CO2 capture. Fuel Process. Technol. 2020, 212, 106620. [Google Scholar] [CrossRef]
- Hornbostel, K.; Nguyen, D.; Bourcier, W.; Knipe, J.; Worthington, M.; McCoy, S.; Stolaroff, J. Packed and fluidized bed absorber modeling for carbon capture with micro-encapsulated sodium carbonate solution. Appl. Energy 2019, 235, 1192–1204. [Google Scholar] [CrossRef]
- Hong, S.; Sim, G.; Moon, S.; Park, Y. Low-Temperature Regeneration of Amines Integrated with Production of Structure-Controlled Calcium Carbonates for Combined CO2 Capture and Utilization. Energy Fuels 2020, 34, 3532–3539. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, H.; Qu, J.; Tang, D.; Zhao, Z.; Xie, H.; Wang, D.; Yin, H. A molten calcium carbonate mediator for the electrochemical conversion and absorption of carbon dioxide. Green Chem. 2020, 22, 7946–7954. [Google Scholar] [CrossRef]
- Aliyu, A.A.; Akram, M.; Hughes, K.J.; Ma, L.; Ingham, D.B.; Pourkashanian, M. Investigation into simulating Selective Exhaust Gas Recirculation and varying Pressurized Hot Water temperature on the performance of the Pilot-scale Advanced CO2 Capture Plant with 40 wt(%) MEA. Int. J. Greenh. Gas Control 2021, 107, 103287. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Liu, H.; Li, W.; Xiao, M.; Gao, H.; Liang, Z. Reduction of energy requirement of CO2 desorption from a rich CO2-loaded MEA solution by using solid acid catalysts. Appl. Energy 2017, 202, 673–684. [Google Scholar] [CrossRef]
- Wang, N.; Peng, Z.; Gao, H.; Sema, T.; Shi, J.; Liang, Z. New insight and evaluation of secondary Amine/N-butanol biphasic solutions for CO2 Capture: Equilibrium Solubility, phase separation Behavior, absorption Rate, desorption Rate, energy consumption and ion species. Chem. Eng. J. 2021, 431, 133912. [Google Scholar] [CrossRef]
- Rozanska, X.; Wimmer, E.; de Meyer, F. Quantitative Kinetic Model of CO2 Absorption in Aqueous Tertiary Amine Solvents. J. Chem. Inf. Model. 2021, 61, 1814–1824. [Google Scholar] [CrossRef]
- Sharif, M.; Zhang, T.; Wu, X.; Yu, Y.; Zhang, Z. Evaluation of CO2 absorption performance by molecular dynamic simulation for mixed secondary and tertiary amines. Int. J. Greenh. Gas Control 2020, 97, 103059. [Google Scholar] [CrossRef]
- Lee, J.I.; Otto, F.D.; Mather, A.E. Solubility of carbon dioxide in aqueous diethanolamine solutions at high pressures. J. Chem. Eng. Data 1972, 17, 465–468. [Google Scholar] [CrossRef]
- Lawson, J.D.; Garst, A.W. Gas sweetening data: Equilibrium solubility of hydrogen sulfide and carbon dioxide in aqueous monoethanolamine and aqueous diethanolamine solutions. J. Chem. Eng. Data 1976, 21, 20–30. [Google Scholar] [CrossRef]
- Isaacs, E.E.; Otto, F.D.; Mather, A.E. Solubility of hydrogen sulfide and carbon dioxide in an aqueous diisopropanolamine solution. J. Chem. Eng. Data 1977, 22, 71–73. [Google Scholar] [CrossRef]
- Martin, J.L.; Otto, F.D.; Mather, A.E. Solubility of hydrogen sulfide and carbon dioxide in a diglycolamine solution. J. Chem. Eng. Data 1978, 23, 163–164. [Google Scholar] [CrossRef]
- Roberts, B.E.; Mather, A.E. Solubility of CO2 and H2S in a hindered amine solution. Chem. Eng. Commun. 1988, 64, 105–111. [Google Scholar] [CrossRef]
- Teng, T.T.; Mather, A.E. Solubility of CO2in an AMP Solution. J. Chem. Eng. Data 1990, 35, 410–411. [Google Scholar] [CrossRef]
- Tontlwachwuthikul, P.; Meisen, A.; Llm, C.J. Solubility of CO2 in 2-Amino-2-methyl-1-propanol Solutions. J. Chem. Eng. Data 1991, 36, 130–133. [Google Scholar] [CrossRef]
- Austgen, D.M.; Rochelle, G.T.; Chen, C.C. Model of Vapor-Liquid Equilibria for Aqueous Acid Gas-Alkanolamine Systems. 2. Representation of H2S and CO2 Solubility in Aqueous MDEA and CO2 Solubility in Aqueous Mixtures of MDEA with MEA or DEA. Ind. Eng. Chem. Res. 1991, 30, 543–555. [Google Scholar] [CrossRef]
- Li, M.H.; Shan, K.P. Densities and Solubilities of Solutions of Carbon Dioxide in Water + Monoethanolamine + N-Methyldiethanolamine. J. Chem. Eng. Data 1992, 37, 288–290. [Google Scholar] [CrossRef]
- Seo, D.-J.; Hong, W.-H. Solubilities of Carbon Dioxide in Aqueous Mixtures of Diethanolamine and 2-Amino-2-methyl-1-Propanol. J. Chem. Eng. Data 1996, 41, 258–260. [Google Scholar] [CrossRef]
- Murrieta-Guevara, F.; Rebolledo-Libreros, M.; Romero-Martínez, A.; Trejo, A. Solubility of CO2 in aqueous mixtures of diethanolamine with methyldiethanolamine and 2-amino-2-methyl-1-propanol. Fluid Phase Equilibria 1998, 150–151, 721–729. [Google Scholar] [CrossRef]
- Lemoine, B.; Li, Y.-G.; Cadours, R.; Bouallou, C.; Richon, D. Partial vapor pressure of CO2 and H2S over aqueous methyldiethanolamine solutions. Fluid Phase Equilibria 2000, 172, 261–277. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of carbon dioxide into aqueous piperazine: Reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 2000, 55, 5531–5543. [Google Scholar] [CrossRef]
- Park, M.K.; Sandall, O.C. Solubility of Carbon Dioxide and Nitrous Oxide in 50 mass Methyldiethanolamine. J. Chem. Eng. Data 2000, 46, 166–168. [Google Scholar] [CrossRef]
- Sidi-Boumedine, R.; Horstmann, S.; Fischer, K.; Provost, E.; Fürst, W.; Gmehling, J. Experimental determination of carbon dioxide solubility data in aqueous alkanolamine solutions. Fluid Phase Equilibria 2004, 218, 85–94. [Google Scholar] [CrossRef]
- Barreau, A.; le Bouhelec, E.B.; Tounsi, K.H.; Mougin, P.; Lecomte, F. Absorption of H2S and CO2in Alkanolamine Aqueous Solution: Experimental Data and Modelling with the Electrolyte-NRTL Model. Oil Gas Sci. Technol. Rev. d’IFP Energies Nouv. 2006, 61, 345–361. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Soriano, A.N.; Caparanga, A.; Li, M.-H. Equilibrium solubility of carbon dioxide in (2-amino-2-methyl-1-propanol+piperazine+water). J. Chem. Thermodyn. 2010, 42, 659–665. [Google Scholar] [CrossRef]
- Chung, P.-Y.; Soriano, A.N.; Leron, R.B.; Li, M.-H. Equilibrium solubility of carbon dioxide in the amine solvent system of (triethanolamine+piperazine+water). J. Chem. Thermodyn. 2010, 42, 802–807. [Google Scholar] [CrossRef]
- Nguyen, T.; Hilliard, M.; Rochelle, G.T. Amine volatility in CO2 capture. Int. J. Greenh. Gas Control 2010, 4, 707–715. [Google Scholar] [CrossRef]
- Xu, Q.; Rochelle, G. Total pressure and CO2 solubility at high temperature in aqueous amines. Energy Procedia 2011, 4, 117–124. [Google Scholar] [CrossRef]
- Dash, S.K.; Samanta, A.N.; Bandyopadhyay, S.S. (Vapour+liquid) equilibria (VLE) of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol: New data and modelling using eNRTL-equation. J. Chem. Thermodyn. 2011, 43, 1278–1285. [Google Scholar] [CrossRef]
- Kumar, G.; Kundu, M. Vapour-liquid equilibrium of CO2 in aqueous solutions of N-methyl-2-ethanolamine. Can. J. Chem. Eng. 2011, 90, 627–630. [Google Scholar] [CrossRef]
- Tong, D.; Trusler, J.M.; Maitland, G.C.; Gibbins, J.; Fennell, P.S. Solubility of carbon dioxide in aqueous solution of monoethanolamine or 2-amino-2-methyl-1-propanol: Experimental measurements and modelling. Int. J. Greenh. Gas Control 2012, 6, 37–47. [Google Scholar] [CrossRef]
- Guo, C.; Chen, S.; Zhang, Y. Solubility of Carbon Dioxide in Aqueous 2-(2-Aminoethylamine)ethanol (AEEA) Solution and Its Mixtures with N-Methyldiethanolamine/2-Amino-2-methyl-1-propanol. J. Chem. Eng. Data 2013, 58, 460–466. [Google Scholar] [CrossRef]
- Haghtalab, A.; Eghbali, H.; Shojaeian, A. Experiment and modeling solubility of CO2 in aqueous solutions of Diisopropanolamine+2-amino-2-methyl-1-propanol+Piperazine at high pressures. J. Chem. Thermodyn. 2014, 71, 71–83. [Google Scholar] [CrossRef]
- Lu, R.; Li, K.; Chen, J.; Yu, H.; Tade, M. CO 2 capture using piperazine-promoted, aqueous ammonia solution: Rate-based modelling and process simulation. Int. J. Greenh. Gas Control 2017, 65, 65–75. [Google Scholar] [CrossRef]
- Ghalib, L.; Ali, B.S.; Ashri, W.M.; Mazari, S.; Saeed, I.M. Modeling the effect of piperazine on CO2 loading in MDEA/PZ mixture. Fluid Phase Equilibria 2017, 434, 233–243. [Google Scholar] [CrossRef]
- Tzirakis, F.; Tsivintzelis, I.; Papadopoulos, A.I.; Seferlis, P. Experimental measurement and assessment of equilibrium behaviour for phase change solvents used in CO2 capture. Chem. Eng. Sci. 2019, 199, 20–27. [Google Scholar] [CrossRef]
- Jahangiri, A.; Hassankiadeh, M.N. Effects of piperazine concentration and operating conditions on the solubility of CO2 in AMP solution at low CO2 partial pressure. Sep. Sci. Technol. 2018, 54, 1067–1078. [Google Scholar] [CrossRef]
- Khodadadi, M.J.; Abbasi, M.; Riahi, S.; Shokrollahzadeh, H. Investigation on kinetics of carbon dioxide absorption in aqueous solutions of monoethanolamine + 1, 3-diaminopropane. Sep. Sci. Technol. 2019, 54, 2800–2808. [Google Scholar] [CrossRef]
- Janati, S.; Aghel, B.; Shadloo, M.S. The effect of alkanolamine mixtures on CO2 absorption efficiency in T-Shaped microchannel. Environ. Technol. Innov. 2021, 24, 102006. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Chen, Y.; Jing, G.; Lv, B.; Zhou, Z.; Zhang, S. Regulatory mechanism of a novel non-aqueous absorbent for CO2 capture using 2-amino-2-methyl-1-propanol: Low viscosity and energy efficient. J. CO2 Util. 2023, 67. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, C.; Tu, R.; Xie, P.; He, Y.; Shi, Y. CO 2 absorption and microwave regeneration with high-concentration TETA nonaqueous absorbents. Greenh. Gases Sci. Technol. 2022, 12, 362–375. [Google Scholar] [CrossRef]
- Tao, M.; Gao, J.; Zhang, W.; Li, Y.; He, Y.; Shi, Y. A Novel Phase-Changing Nonaqueous Solution for CO2 Capture with High Capacity, Thermostability, and Regeneration Efficiency. Ind. Eng. Chem. Res. 2018, 57, 9305–9312. [Google Scholar] [CrossRef]
- Liu, J.; Qian, J.; He, Y. Water-lean triethylenetetramine/N,N-diethylethanolamine/n-propanol biphasic solvents: Phase-separation performance and mechanism for CO2 capture. Sep. Purif. Technol. 2022, 289, 120740. [Google Scholar] [CrossRef]
- Tu, Z.; Han, F.; Liu, C.; Wang, Y.; Wei, J.; Zhou, X. 2-Amino-2-methyl-1-propanol regulated triethylenetetramine-based nonaqueous absorbents for solid-liquid phase-change CO2 capture: Formation of crystalline powder products and mechanism analysis. Sep. Purif. Technol. 2023, 307, 122722. [Google Scholar] [CrossRef]
- Machida, H.; Oba, K.; Tomikawa, T.; Esaki, T.; Yamaguchi, T.; Horizoe, H. Development of phase separation solvent for CO2 capture by aqueous (amine + ether) solution. J. Chem. Thermodyn. 2017, 113, 64–70. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Xu, L. Screening of physical-chemical biphasic solvents for CO2 absorption. Int. J. Greenh. Gas Control 2019, 85, 199–205. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Shen, S. Low-Energy-Consumption CO2 Capture by Liquid–Solid Phase Change Absorption Using Water-Lean Blends of Amino Acid Salts and 2-Alkoxyethanols. ACS Sustain. Chem. Eng. 2020, 8, 12956–12967. [Google Scholar] [CrossRef]
- Lail, M.; Tanthana, J.; Coleman, L. Non-Aqueous Solvent (NAS) CO2 Capture Process. Energy Procedia 2014, 63, 580–594. [Google Scholar] [CrossRef]
- Perry, R.J.; Wood, B.R.; Genovese, S.; O’Brien, M.J.; Westendorf, T.; Meketa, M.L.; Farnum, R.; McDermott, J.; Sultanova, I.; Perry, T.M.; et al. CO2 Capture Using Phase-Changing Sorbents. Energy Fuels 2012, 26, 2528–2538. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Wang, L.; Chen, J.; Lu, Y. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges. Appl. Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Novel water-free biphasic absorbents for efficient CO 2 capture. Int. J. Greenh. Gas Control 2017, 60, 100–109. [Google Scholar] [CrossRef]
- Polesso, B.B.; Duczinski, R.; Bernard, F.L.; Faria, D.J.; dos Santos, L.M.; Einloft, S. New water-based nanocapsules of poly(diallyldimethylammonium tetrafluoroborate)/ionic liquid for CO2 capture. Heliyon 2023, 9, e13298. [Google Scholar] [CrossRef]
- Rasouli, H.; Iliuta, I.; Bougie, F.; Garnier, A.; Iliuta, M.C. Hybrid enzymatic CO2 capture process in intensified flat sheet membrane contactors with immobilized carbonic anhydrase. Sep. Purif. Technol. 2022, 287, 120505. [Google Scholar] [CrossRef]
- Rasouli, H.; Iliuta, I.; Bougie, F.; Garnier, A.; Iliuta, M.C. Enhanced CO2 capture in packed-bed column bioreactors with immobilized carbonic anhydrase. Chem. Eng. J. 2022, 432, 134029. [Google Scholar] [CrossRef]
- Wojtasik-Malinowska, J.; Piątkowski, M.; Blatkiewicz, M.; Jaskulski, M.; Wawrzyniak, P.; Górak, A. Reactive absorption of carbon dioxide in aqueous n-methyldiethanoloamine solutions catalysed with carbonic anhydrase in a rotating packed bed (RPB). Chem. Eng. Process. Process. Intensif. 2023, 184, 109266. [Google Scholar] [CrossRef]
- Leimbrink, M.; Nikoleit, K.G.; Spitzer, R.; Salmon, S.; Bucholz, T.; Górak, A.; Skiborowski, M. Enzymatic reactive absorption of CO2 in MDEA by means of an innovative biocatalyst delivery system. Chem. Eng. J. 2018, 334, 1195–1205. [Google Scholar] [CrossRef]
- Zhang, S.; Du, M.; Shao, P.; Wang, L.; Ye, J.; Chen, J.; Chen, J. Carbonic Anhydrase Enzyme-MOFs Composite with a Superior Catalytic Performance to Promote CO2 Absorption into Tertiary Amine Solution. Environ. Sci. Technol. 2018, 52, 12708–12716. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, C.; Fu, T.; Gao, X.; Ma, Y. CO2 absorption performance of ChCl-MEA deep eutectic solvent in microchannel. J. Environ. Chem. Eng. 2022, 10, 108792. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Leron, R.B.; Li, M.-H. Solubility of carbon dioxide in aqueous mixtures of (reline+monoethanolamine) at T=(313.2 to 353.2)K. J. Chem. Thermodyn. 2014, 72, 94–99. [Google Scholar] [CrossRef]
- Ullah, R.; Atilhan, M.; Anaya, B.; Khraisheh, M.; García, G.; ElKhattat, A.; Tariq, M.; Aparicio, S. A detailed study of cholinium chloride and levulinic acid deep eutectic solvent system for CO2 capture via experimental and molecular simulation approaches. Phys. Chem. Chem. Phys. 2015, 17, 20941–20960. [Google Scholar] [CrossRef]
- Ruan, J.; Ye, X.; Wang, R.; Chen, L.; Deng, L.; Qi, Z. Experimental and theoretical study on efficient CO2 absorption coordinated by molecules and ions of DBN and 1,2,4-triazole formed deep eutectic solvents. Fuel 2023, 334, 126709. [Google Scholar] [CrossRef]
- Dods, M.N.; Weston, S.C.; Long, J.R. Prospects for Simultaneously Capturing Carbon Dioxide and Harvesting Water from Air. Adv. Mater. 2022, 34, 2204277. [Google Scholar] [CrossRef]
- Stuckenberg, D.J.; Kode, V.R.; Went, E.K. ATMOSPHERIC WATER GENERATION SYSTEMS AND METHODS UTILIZING MEMBRANE-BASED WATER EXTRACTION. 2023. Available online: https://www.freepatentsonline.com/y2023/0010090.html (accessed on 1 March 2023).

| Absorption Process | Used Absorbent | Advantage | Application |
|---|---|---|---|
| Selexol | Dimethyl ether/propylene glycol | Low vapor pressure, low toxicity, and less corrosive solvent | Remove both CO2 and H2S at low temperature |
| Rectisol | Methanol | Less corrosive and more stable absorbent | CO2 removal from sulfur-containing gas |
| Purisol | N-methyl pyrrolidone | Low energy consumption | CO2 removal from sulfur-containing gas |
| Morphysorb | Morpholine | Operation cost is 30% to 40% lower than that for Selexol | Selective removal of H2S from CO2 |
| Fluor | Propylene carbonate | Solubility of CO2 in the solvent is high | Suitable for gases containing CO2 partial pressure higher than 60 psig |
| Polymeric Solvent | Merits | Demerits | Ref. |
|---|---|---|---|
| Hydrophobic polymeric solvents (PPGDME & PDMS) | Selective CO2 absorption. | Requires higher temperature. | [49,51] |
| Amine-infused hydrogels (AIHs) | Solid sorbents, can be easily manufactured at a large scale, fast kinetics, minimal performance degradation after recycling. | Tendency to agglomerate with each other without mixing, which resulted in decreased CO2 absorption capacity. | [50,52,53] |
| Di-Methyl-Ether of poly-Ethylene-Glycol (DMEPEG) | Suitable for both CO2 and H2S. Less corrosive than chemical solvents. Consume a lower amount of power. Lower heat duty for gas desorption. | Varieties in solvent processing ability within a specific tower. | [54,55] |
| Low-viscous branched polymers | High capacity for CO2. Large selectiveness. | For post-combustion CO2 capture, poly ethers are not suitable. | [56,57,58] |
| PEG-PDMS copolymer | Well-suited for precombustion carbon capture. Selective CO2 capture. | The existence of flowing gas may cause severe foaming, | [59,60] |
| PDMS solvents | Selective uptake of CO2 from different gases (H2S, H2O). Higher solubility and thermal stability. | A fall in CO2 solubility may be caused due to increased temperature. High cost for operational processes. | [61,62] |
| Solvent/Absorbent | Advantages | Limitations/Disadvantages | Remarks |
|---|---|---|---|
| DEA | The absorption rate is high. Less expensive. | Lower capacity. Risk of corrosion due to the presence of atmospheric oxygen. | DEA is a liquid at room temperature, making it easy to handle and transport. It also has a high CO2 solubility, making it an attractive option for use in post-combustion CO2 capture and air purification applications. |
| K2CO3 | Degradation resistance is high, less expensive, and less enthalpy is needed for this solvent. | The mass transfer rate is low. | Organic/inorganic salts are mixed to enhance the mass transfer rate. Reactivity drops down at 40–200 °C. |
| AMP | Selectivity rate is high, simple regeneration, good absorption capacity, and degradation resistance. | The absorption rate is low. | Temperature decreases linearly with column height. |
| Di-isopropylamine (DIPA) | Less corrosion, cost-effective regeneration. | The absorption rate is low. | Dipa is a tertiary amine that can react with CO2 to form a stable carbamate, making it a viable option for removing CO2 from gas streams. |
| MEA | The cost-effective, high reaction rate | Lower capacity. Risk of corrosion due to the presence of atmospheric oxygen. | Among pilot, spray, and packed columns, pilot and spray columns show superior performance. |
| 2PE | Rigid thermal and chemical behavior. Non-toxic and environmentally friendly. | Its high volatility can result in significant losses of 2PE during the CO2 capture process. | 2PE has a high CO2 solubility and is a liquid at room temperature, making it easy to handle and transport. |
| PZ | Good corrosion resistance, less thermal degradation, cost-effective regeneration. | - | |
| AEP | The absorption capacity is high. | - | Include primary, secondary, and tertiary amines without formatting any carbamate. |
| Ionic liquids | Thermally and chemically stable. Cost-effective and high carbon capture selectivity. | - | By decreasing temperature and increasing concentration, an increase in density and viscosity is possible. |
| Ammonia | Low cost, high selectivity. Absorption capacity is high and available. | Slip | Pilot scale project. |
| MDEA | Good corrosion and degradation resistance. Low regeneration cost. | The absorption rate is low | Unlike primary and secondary amines, it does not bind. |
| AMP + PZ | - | A pilot plant, packed bed. | |
| MDEA + PZ | Available, less corrosion, low cost. | High cost. High corrosivity. | MDEA is a tertiary amine that reacts with CO2 to form a stable carbamate, while PZ is an acidic compound that can enhance the reaction kinetics of the MDEA-CO2 reaction |
| MDEA + glycerol | Concentration and pressure are low. Absorption capacity is high. | The solubility of carbon dioxide can be decreased due to high pressure and concentration of glycerol. | MDEA is a tertiary amine that reacts with CO2 to form a stable carbamate, while glycerol is a hydrophilic compound that can act as a solvent for the absorbent. |
| DETA | Huge loading of CO2. Promote the absorption process. | - | |
| DETA/Sulfolane | A low volume required for regeneration. Low total heat duty, improved viscosity. | - | Novel absorbent to capture CO2. |
| PEG-dicholine chlorides | CO2 intake increased with increasing pressure and temperature. Non-toxic. Fast kinetics and high absorption capacity. | Long-chain polymers have lesser mobility and CO2 loading. High cost. Form solid residue after absorption. | PEG (polyethylene glycol) is a hydrophilic polymer that is combined with a dicholine chloride molecule to form the PEG-dicholine chloride. |
| PEG-PDMS copolymer | Selective CO2 absorption: a copolymer with an imidazolium chromophore was shown to be an effective solvent for precombustion CO2 capture. | CO2 selectivity is moderate; severe foaming in the presence of flowing gas | The PEG component provides hydrophilic properties, while the PDMS component provides hydrophobic properties, making the copolymer an attractive option for CO2 removal from gas streams |
| AAM-co-AAC porous hydrogel copolymers | Excessive water content increased CO2 uptake capacity. | Water has a low CO2 solubility along polymer scaffolds. | The high swelling capacity of the copolymers allows for efficient CO2 absorption, and the porous structure increases the available surface area for CO2 absorption. |
| DMEPEG | Absorption of CO2 and H2S from syngas; recovery of co-absorbed H2; and reduction of equipment size through solvent saturation. | Process performance variation; fluctuation in gas/solvent processing capability within a packed column. | DME-PEG copolymers are composed of a combination of dimethanolamine (DME), a tertiary amine that can react with CO2 to form a stable carbamate, and polyethylene glycol (PEG), a hydrophilic polymer that increases the solubility of DME in water. |
| Amino acid poly-ionic liquids (AAPILs) | Poly-ionic liquids based on [Arg] exhibited the maximum CO2 absorption and sorption capability. | Procedures execution on an industrial scale or for commercialization. | |
| Amine-infused microgels (AIMGs) | Increased CO2 intake and absorbing kinetics. | Long-term robustness, durability, and CO2 capturing capacity at high pressure and temperature are required for large-scale applications. | AIMGs are soft, hydrogel-like particles that are infused with an amine-containing chemical, such as a tertiary amine. The amine reacts with CO2 to form a stable carbamate, which is trapped within the gel structure of the AIMG. |
| Novel Multiphase Systems of NOHMs | Because of the positive entropic impact, CO2 is readily available. At high pressure, and high CO2 capture; CO2 has a high selectivity over N2O, O2, and N2. | The high temperature has a negative impact on CO2 absorption. | NOHMs are highly branched, organic molecules that contain nitroxide radicals, which can react with CO2 to form a stable carbamate. |
| POSS containing NOHMs | High thermal stability, enhanced CO2 molecule assimilation due to high canopy/core size ratio. | More physical research and polymer chain couplings are required to assess CO2 capture efficacy. | By incorporating POSS into NOHMs, the resulting materials can exhibit unique properties, such as high stability, low toxicity, and improved CO2 uptake. |
| Polydimethylsiloxane (PDMS) | Highly soluble and thermally stable; efficient CO2 extraction from H2, H2O, and H2S. | Decreasing CO2 solubility with rising temperature causes high-cost process. | PDMS is often synthesized as a gel, which can be cut into small pieces to increase the surface area and therefore the CO2 absorption capacity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peu, S.D.; Das, A.; Hossain, M.S.; Akanda, M.A.M.; Akanda, M.M.H.; Rahman, M.; Miah, M.N.; Das, B.K.; Islam, A.R.M.T.; Salah, M.M. A Comprehensive Review on Recent Advancements in Absorption-Based Post Combustion Carbon Capture Technologies to Obtain a Sustainable Energy Sector with Clean Environment. Sustainability 2023, 15, 5827. https://doi.org/10.3390/su15075827
Peu SD, Das A, Hossain MS, Akanda MAM, Akanda MMH, Rahman M, Miah MN, Das BK, Islam ARMT, Salah MM. A Comprehensive Review on Recent Advancements in Absorption-Based Post Combustion Carbon Capture Technologies to Obtain a Sustainable Energy Sector with Clean Environment. Sustainability. 2023; 15(7):5827. https://doi.org/10.3390/su15075827
Chicago/Turabian StylePeu, Susmita Datta, Arnob Das, Md. Sanowar Hossain, Md. Abdul Mannan Akanda, Md. Muzaffer Hosen Akanda, Mahbubur Rahman, Md. Naim Miah, Barun K. Das, Abu Reza Md. Towfiqul Islam, and Mostafa M. Salah. 2023. "A Comprehensive Review on Recent Advancements in Absorption-Based Post Combustion Carbon Capture Technologies to Obtain a Sustainable Energy Sector with Clean Environment" Sustainability 15, no. 7: 5827. https://doi.org/10.3390/su15075827
APA StylePeu, S. D., Das, A., Hossain, M. S., Akanda, M. A. M., Akanda, M. M. H., Rahman, M., Miah, M. N., Das, B. K., Islam, A. R. M. T., & Salah, M. M. (2023). A Comprehensive Review on Recent Advancements in Absorption-Based Post Combustion Carbon Capture Technologies to Obtain a Sustainable Energy Sector with Clean Environment. Sustainability, 15(7), 5827. https://doi.org/10.3390/su15075827








