Abstract
CO2 capture, use, and storage have been identified as significant strategies for reducing greenhouse gas emissions induced by the usage of fossil fuels. The current review focuses on the concepts of post-combustion capture technologies based on absorption mechanisms. Among all other developed technologies, researchers have proposed absorption as the most mature carbon capture technology for industrial-scale application. Absorption-based carbon capture can be classified into chemical and physical absorption, and researchers have developed different solvents and absorbent materials to investigate their performance in CO2 capture. This paper comprehensively reviewed these established solvents and absorbents with their performance parameters in the CO2 absorption approach. Besides the improvement in widely applied absorbents such as amine-based absorbents, recently, researchers have been working to develop some advanced nanomaterials such as nanofluids and nano-emulsions. This review focuses on the application of such absorption mechanisms that can contribute to capturing CO2 in a compact, environment-friendly, and safe way. This paper also provides future research direction for further development in absorption-based CO2 capture.
1. Introduction
Global warming caused by greenhouse gas emissions, especially carbon dioxide, is a major concern throughout the world. Attempts are being undertaken continually to prevent the extent of future environmental change caused by rising emissions of greenhouse gases. If not regulated, rising temperatures will eventually lead to rising sea levels, increasing the probability of flooding and storms. The Intergovernmental Panel on Climate Change (IPCC) estimates that by the year 2100, the CO2 content in the atmosphere shall reach 570 ppmv, the sea level will increase by 3.8 m, and the global mean temperature will rise by 2 °C with major consequences on the environment [1,2,3,4]. According to the ensemble-mean results of state-of-the-art Earth System Models (ESMs), climate warming throughout the 21st century is forecasted to be between 1.0 and 3.7 °C, depending on future greenhouse gas emissions [5,6]. The United Nations Framework Convention on Climate Change (UNFCCC) held its 21st Conference of the Parties (COP21) in Paris, France, where the primary goal of COP21 was to establish a lawful climate agreement among 195 countries of the United Nations to keep the global temperature rise since 1800 even below 2.0 °C (ideally 1.5 °C) by 2100 [7].
Absorption technologies can be integrated with both pre-combustion and post-combustion processes for carbon capture, and this process can be classified into chemical absorption and physical absorption. A basic chemical absorption system is composed of three main parts: solvent, absorber, and stripper. In the absorber, flue gases from various CO2 emitters, such as coal power plants, come into contact with the lean solution in a counter-current mechanism in the absorber. The solvents then absorb CO2, resulting in lower carbon dioxide levels in the exhaust gases. The stripper then regenerates the solvent-rich CO2. This regenerated light solution comes back to the absorber, and compressed CO2 is gathered and transferred to the stripper’s top. Chemical absorption has been the most reliable marketed method for many years; however, it has yet to be scaled up in CO2 capture in power plants. Numerous studies have been conducted and published for the creation of efficient gas–liquid contactor systems, solvent structures, and stripper configurations to maximize CC with little energy penalty [8,9]. The central concept was to optimize surface area and mass transfer for absorption and desorption procedures [10]. Packed bed (PB), bubble column, spray column, rotating packed bed (RPB), and tray tower absorber layouts were applied. Some modification methods, such as the addition of numerous columns, vapor recompression and heat integration in the stripping phase, split flows, and matrix stripping, have been observed to increase performance [11,12,13,14].
In the preceding introductory part, the urgency of carbon capture and the many technologically viable alternatives in obtaining a similar result to mitigate the serious threat of global warming have been highlighted. In the following sections, the development patterns in the key technologies of carbon capture are thoroughly explored, and future difficulties and possibilities in each of them are reviewed. In each section, a table summarizing the overall findings has been included.
Status of Global CO2 Emissions
CO2 is emitted from different sources, and the largest amount of CO2 has been emitted from power plants and energy sectors. Therefore, with increasing energy production, the amount of CO2 emission also increases. Figure 1 shows the top 10 CO2-emitting countries, and it is clear that in recent years, China has been emitting the largest amount of CO2 because it has been producing the largest amount of energy. From the year 1990 to 2005, CO2 emissions increased by nearly 4000 million metric tons, while a sudden increase was observed in 2015, when the amount of CO2 emissions passed 10,000 million metric tons (MMT). Finally, in 2021, China’s CO2 emissions reached 12,000 MMT, and China became the largest CO2 emitter in the world. According to CO2 emissions statistics, the United States has been the second-largest emitter for the last two decades (2000–2021). From Figure 1, it can be observed that from 1990 to 2021, the CO2 emissions in the United States were not drastic. The amount of CO2 emissions was always in the range of 4000 to 6000 MMT.
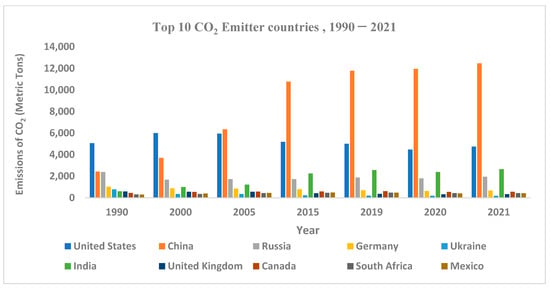
Figure 1.
Top 10 countries that emit the largest amount of CO2, 1990–2021. (Data from the EDGAR website).
Major CO2 emitters in the U.S. are listed in Figure 2, including power plants, chemical and metal processing industries, petroleum and natural gas systems, food processing, coal mining, and other different major sources. These major emitters can be promising for capturing CO2 due to the large amount of exhaust CO2 in the system.
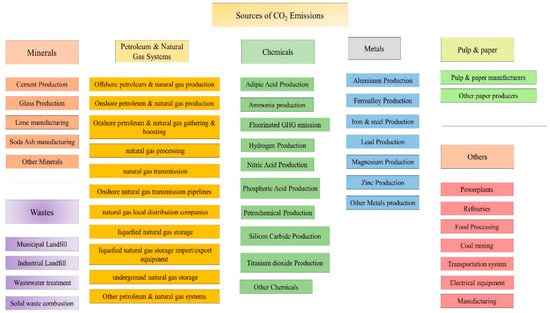
Figure 2.
Different sources of Carbon Dioxide emissions in the environment.
Power plants are the largest source of emitted CO2, which is clear from Figure 3. According to the EPA dataset, in the United States, there are 1369 facilities in total, from which 1660 MMT of CO2 was emitted in the year 2019. As populations grow, more power sectors are built, which leads to increased energy consumption. As a result, more power plants are developed, which causes a higher amount of CO2 emissions. The EPA has developed a statewide plan to control CO2 emissions from power plants, and these plans are listed in Section 111(d) of the Clean Air Act, where the EPA set different performance standards for multiple sources of pollution, which includes power plants. According to the EPA’s clean power plan, states have the responsibility to cut off 30% of CO2 emissions from power plant sectors from 2005 to 2030. To implement these acts, states have started implementing different policies to minimize power sectors’ climate impact [15]. The EPA has focused on four major opportunities to reduce CO2 emission from electricity production sectors, and these opportunities include the increased efficiency of fossil-fired power plants and fuel switching, renewable energy, increased end-use energy efficiency, and carbon capture and sequestration (CCS) [16,17].
Figure 3.
Amount of greenhouse gas emissions during the period of 1990–2020 and CO2 emissions from different sectors in the USA.
2. Absorption-Based Carbon Capture
2.1. Physical Absorption
Henry’s law is considered the base of the physical absorption process for CO2 capture. The operating condition should have high pressure and low temperature in the case of CO2 absorption, whereas it is just the opposite for CO2 desorption. Several solvents are already being used commercially to produce synthesis gas and hydrogen [18,19]. In recent years, ionic liquid (IL) is achieving much attention for physical absorption due to its uncommon characteristics such as non-toxicity, lower vapor pressure, higher thermal stability, and high polarity. Table 1 summarizes the physical absorption processes with applicable absorbent materials, advantages, and applications.

Table 1.
Several physical absorption processes with applicable absorbent materials, advantages, and applications.
2.2. Chemical Absorption
The most researched and established approach, the chemical absorption process, normally consists of two phases. In the chemical absorption process, the exhaust CO2 mixes with a chemical solvent through a chemical reaction and forms an intermediate compound whose bond is weak enough to get back the original form of CO2 and solvent by applying heat to the intermediate compound. In the first step, when CO2-containing gas is introduced from the bottom into a column of packed bed absorber, the CO2 reacts with the absorbent. In the second step, the CO2-rich absorbent enters a stripper where it is regenerated before being pumped back to the absorber for cyclic applications. The process’ compression, transportation, and storage portions receive the net CO2 emissions from the stripper. In regard to chemical absorption, CO2 capture technology, alkali absorbents, inorganic and organic solvents with amine bases, and ILs are the most employed absorbents. Amine aqueous solvents were initially applied for chemical absorption CO2 capture. After further research on chemical absorption technologies, mono-ethanolamine (MEA) appeared to be the most applicable amine solvent due to its high absorptivity of CO2.
3. Developed Chemical Absorption Processes
3.1. Amine-Based Absorption
Amines are ammonia-derived compounds where at least one hydrogen molecule is replaced by an organic compound. Different amine solvents such as MEA, DEA, and MFEA have been applied for the last few decades in natural gas industries, where they also exhibit the potential to capture CO2, making them attractive candidates in carbon capture technologies [20]. The CO2 capture process via amine-based absorption follows the reactions (Equations (1) and (2)), where R is an alkanol group. The amine reacts as a weak base to neutralize acidic CO2 and forms carbamate (R-NHCOO−) through Reaction (1) and finally forms bicarbonate in the presence of moisture that follows Reaction (2) [18].
According to this mechanism, most of the CO2 absorbed will cause the liquid amine capture system to produce bicarbonate. The binding between the absorbent and CO2 is weakened by either raising the temperature or decreasing the pressure of the solution, which removes the CO2 from the liquid amine solvent to a water stream and regenerates the solvent for further usage [21]. Higher energy requirements for solvent regeneration, slower reactivity rates, and lower absorption rates are the major barriers to conventional amine-based solvents. To withstand such limitations, Bita Karami et al. have reported that the nonporous hyper-cross-linked polymeric (HCP) networks can be employed as CO2 absorption rate promoters, and they can dramatically increase CO2 capture via absorption in N-methyldiethanolamine (MDEA) sorbents [22]. They synthesized two HCPs, namely polystyrene (HCP-S) and benzene (HCP-B), from cost-effective monomers and suspended these polymerics in MDEA solutions to form a novel slurry solvent. They discovered that by employing HCP-B and HCP-S in MDEA solution, the CO2 absorption rate was increased by 130 and 253%, respectively.
3.2. KMALC
The Kerr-McGee/AGG Lummus Crest (KMALC) is an emerging amine-based adsorption technology to capture CO2 from flue gases [23,24]. This technology utilizes 15–20 wt% MEA solution for CO2 absorption, and the low cost of MEA makes this this approach more applicable for CO2 capture. Researchers reported that a maximum of 800 tons/day of CO2 can be absorbed via KMALC processes, whereas 8000 tons/day of CO2 was emitted from the reported fossil fuel power plant [25].
3.3. Fluor EFG + Process
The Fluor Economic FG PlusSM is an experimentally proven technology that can be employed to capture CO2 from flue gases. For capturing CO2 from coal-based power plants’ flue gases, Fluor has been improved to further reduce the energy consumption, operating and capital costs, and environmental influences of CO2 capture plants in commercial applications. This technology has the license to be employed in 28 plants around the world. In EFG+ technology, a chemical solvent that absorbs CO2 via an exothermic reaction has been used. NRG Energy designed, constructed, and operated a carbon capture demonstration plant at WA Parish Electric Generating Station, and in this project, they demonstrated the ability to capture 90% inlet CO2 from a 240 MW equivalent flue gas slipstream that was exhausted from a coal-fired boiler [26]. Among recent Fluor FG+-based projects, the Electric Power Research Institute (EPRI), along with California Resources Corporation (CRC) and Fluor Corporation, has investigated Fluor’s economic FG OlusTM-based carbon capture technology on a natural gas-fired combined cycle power plant in California. This carbon capture technology was integrated with the Elk Hills Power Plant (EHPP), which is located near Kern County, California. Flue gas can be divided in two ways: in one way, 79% of flue gas moves through Fluor’s EFG+ unit, and in another way, 21% of flue gas is ventilated via the stack. The CO2 capture unit can capture 90% inlet CO2 from the flue gas with 97+% CO2 purity, and 4000 tons of CO2/day can be captured through this carbon capture technology in this plant [27].
3.4. KM-CDR Process
Mitsubishi Heavy Industries, Ltd. (MHI) and the Kansai Electric Power Co., Inc. (KEPCO, Osaka, Japan) have developed a CO2 capture technology based on the KM-CDR (Kansai Mitsubishi Carbon Dioxide Recovery) process and have integrated the carbon capture plant with the Petra Nova Project, which is considered the largest CO2 capture plant. In this project, a new solvent for CO2 capture was prepared, and this new solvent showed better performance compared to KS-1TM in terms of solvent degradation, emissions, and steam consumption rate. The new solvent possessed 50% less solvent degradation, 50% less solvent emission, and approximately 5–10% less steam requirement compared to KS-1TM [28]. MHI and the research and development activities of the Nanko Pilot Plant have started the CO2 capture operation at a 25 MW coal-based power plant at Southern Company’s Plant Barry, which is operated and developed by Alabama Power. This plant is considered the world’s first carbon capture technology integrated with a coal-based power plant. It was estimated that approximately 150,000 tons (500 tons/day) of CO2 can be captured annually with a CO2 capture rate of 90+% [29]. MHIENG’s (Mitsubishi Heavy Industries Engineering, Ltd., Singapore) latest process, the KM-CDR process with KS-1TM, investigated the near-zero emission and CO2 ratio and found a 99.5% CO2 capture ratio. With their default system, it was found that at a 99.5% CO2 capture ratio, the reboiler steam and solvent rate increased by 15% and 25%, respectively, whereas by employing 50% more absorption packing and maintaining the operating expenditure (OPEX) ($/tonne CO2) as the base case, a 50% reduction of reboiler steam and solvent rate can be achievable [30].
3.5. Chilled Ammonia Process (CAP)
Eli Gal has patented his research on the chilled ammonia process (CAP) that can absorb CO2 at much lower temperatures, and this technique also minimized the density of moisture and volatile and acidic elements existing in the gas. CAP also minimizes NH3 slip at a lower amount and controls the NH3 loss to less than 6% of the solvent compared with the conventional aqueous ammonia process [31]. Daniel Sutter et al. developed an advanced ammonia-based CO2 capture technology that controlled the solid formation chilled ammonia process (CSF-CAP). A comparison of CSF-CAP was carried out with conventional CAP (L-CAP) technology since a solid handling section, and from the scrutinized comparison it was found that in the CSF-CAP process reduction of steam, the requirement was minimized by 30% for CO2 desorption and the SPECCA (Specific Primary Energy Consumption for CO2 Avoided) by 17% [32]. There are some existing limitations of CAP technologies, including low absorption rates, the requirement of multiple absorber vessels, and the volatility of ammonia [33].
3.6. Aqueous Ammonia Scrubbing
Ammonia is considered a potential alternative to MEA, as it possesses higher availability, higher corrosion resistance, lower solvent degradation, lower expense, and higher CO2 absorption capacity compared to MEA. Aqueous ammonia can capture CO2 with higher purity, and it also captures other coexisting elements in flue gases such as SO2, NOX, HCL, HF, and different acidic gases. This process can minimize the cost of a plant by eliminating the acidic gas clean-up system, and the SOX and NOX can be converted to fertilizer that can make money and reduce the plant’s cost. The CO2 absorption by ammonia is carried out following Equations (1)–(3), where in the first step, ammonia reacts with CO2 and produces ammonium carbamate in dry conditions (Reaction (3)). After adding some moisture, ammonium carbonate is formed from ammonium carbamate (Reaction (4)), and then it is converted into ammonium bicarbonate (Reaction (5)).
Aqueous ammonia scrubbing is more advantageous than amine-based processes, as ammonia-based solvents consume a lower amount of energy for solvent regeneration and are less expensive than amine-based processes [34]. There are also some limitations of aqueous ammonia solvents compared to amine-based solvents, including the requirement for a larger absorber area, higher initial capital cost, and lower CO2 absorption capacity [35]. Moreover, a higher loss of ammonia causes higher uses of wash water, which results in a higher recovery cost of ammonia. To solve the problem of ammonia loss, Hamed Rashidi et al. developed an ammonia-glycerol hybrid solvent to capture CO2 glycerol, which is a byproduct of biodiesel has hydroxyl groups which can bind ammonia molecules and hence the vaporization of ammonia reduces. Their experimental output reveals that applying glycerol with aqueous ammonia causes an increase in the mass transfer coefficient and a reduction in the vapor pressure of ammonia that results in lower losses of ammonia in the CO2 capture system [36].
3.7. Amino Acid Absorption
The usability of amino acids in CO2 capture systems makes them a potential alternative to amine-based absorption systems. There are some crucial limitations in conventional amine-based solvents, such as the volatility of solvents [37,38], degradation of solvents [39], lower corrosive resistance [38,39], higher energy requirement for regeneration [38,40], and lower CO2 loading capacity [41]. On the other side, amino acid solvents possess some attractive features, such as a higher corrosion resistance, higher degradation resistance, lower volatility [37,38], and lower energy requirements compared to amine-based solvents. Moreover, the utilization of amino acids in CO2 is also preferable due to their natural availability and biodegradability, and so, these solvents can minimize environmental hindrances. Additionally, hydrophilic amino acids are capable of operating under a wide range of temperatures and pressure conditions required for carbon capture [42]. Cheng et al. reported an ion pair arginine-arginine carbamate derived from L-arginine (amino acid) to capture CO2 from gases, and their study expanded the understanding behavior of L-arginine and other amino acids for CO2 absorption [43]. The application of amino acid anions in CO2 absorption can enhance the CO2 uptake twofold for some amino groups, and possible reactions were reported by Stefano et al. [44]. Maria Castro analyzed the use of sodium (Na) salts of aqueous solutions of different amino acids such as glycinate and prolinate for CO2 capture via chemical absorption, and they experimented with this solution in a bubble column reactor under various testing conditions such as flow rates of flue gas and solvent concentrations [45]. Carboxylic groups have caused the major differences in reactions and absorption rates, and it has also been reported that glycinate possessed greater CO2 loading than mono-ethanolamine (MEA) due to higher destabilization of carbamate of glycinate. To minimize the energy requirement for solvent regeneration, different crystallization processes can be employed with aqueous amino acid solvents. The crystallization of bis-iminoguanidines (Glyoxal-bis-iminoguanidine or GBIG) employed with aqueous amino acids is being developed as a potential technology to reduce the energy penalty from a CO2 capture plant by minimizing the energy consumption for solvent regeneration, and the CO2 loading capacity was reported as 1.36 mol per mol of GBIG [46].
3.8. Dual Alkali Absorption (DAA)
Alkali metal carbonate solvents are a potential alternative to the conventional solvents applied in CO2 capture technologies, and these alkali-based solvents can be employed through dual alkali absorption (DAA) processes. There are some attractive advantages to applying this CO2 process, including lower degradation, lower emissions, and lower costs, whereas the limitations are a lower rate of CO2 mass transfer and a lower and slower rate of reaction. To overcome these limitations, Yang Li et al. developed a dual alkali solvent (DAS) that is different from the conventional solvents in terms of phases as DAS has two aqueous phases. In the first phase, an organic alkali 1-(2-hydroxyethyl) piperazine (HEP) was employed for CO2 absorption, while in the second phase, a mixture of K2 CO3/KHCO3 aqueous solution and KHCO3 precipitate was employed for CO2 stripping. From their experimental investigation, they revealed that by implementing DAS to capture CO2, 55.7% of energy can be saved without lowering the CO2 absorption efficiency from 90% [47]. This novel system has faster CO2 absorption kinetics and a low energy need for solvent regeneration, which are advantages of both amine and alkali metal carbonate, respectively.
3.9. Alkaline Solvent Absorption
Alkaline solvents such as sodium hydroxide or potassium hydroxide are potential solvents that are widely applied for CO2 from the air. When air meets such absorbents, then CO2 is captured via a chemical reaction following Reaction (6), where CO2 reacting with NaOH forms sodium carbonate. Normally, the regeneration of this type of solvent (NaOH) is carried out by dosing another alkaline solvent (Ca(OH)2), and calcite forms due to this Reaction (7). For further treatment, CaCO3 is heated at 700 °C to form calcium oxide (CaO) and CO2 through Reaction (8), and then, by rehydrating CaO, regenerated Ca(OH)2 can be obtained through Reaction (9) [48].
Alkali absorption is advantageous in terms of solvent availability and the cost-effectiveness of the solvent, but the treatment of CaCO3 is much more expensive, which hinders the economic feasibility of these absorbents.
4. Different Types of Absorbents
4.1. Polymeric Solvent
In recent times, polymeric solvents have attracted much attention for further research due to their applicability in CO2 capture technologies. There are some potential conveniences of these technologies, including lower viscosity, thermal stability, hydrophobicity, lower capital and operation costs, simplified installation and maintenance, and lower volatility. Among several polymeric solvents, a few that have emerged, such as polydimethylsiloxane (PDMS), polyesters, and polyethers (Pes), are remarkable. Miller et al. experimented with several solvents to compare their performances regarding the selectivity of CO2, and these solvents included polypropylene glycol dimethyl ether (PPGDME), polyethylene glycol dimethyl ether (PEGDME), poly-dimethyl siloxane (PDMS), perfluorpoly ether (PFPE), polybutylene glycol diacetate (PBGDAc), and polypropylene glycol diacetate (PPGDAc) [49]. Their experiment revealed that PDMS and PPGDME were the most promising alternative for CO2 capture from a mixed stream. Xingguang et al. prepared novel absorbent amine-infused hydrogels (AIHs) by adding hydrogels with organic amine solutions, and they found where hydrogels ensure the increased interfacial area to capture the higher amount of CO2. Their result also showed that the CO2 uptake capacity of AIHs is higher than that of aqueous amine solutions operating under the same conditions. Kim et al. prepared AIH in a disparate way than before by just adding a monoethanolamine (MEA) solution with dried hydrogel particles, and their results showed that AIHs showed higher CO2 uptake capacity and selectivity than MEA solutions [50]. Dimethyl ether of polyethylene glycol (DMEPEG) is a novel polymeric solvent that offers potential conveniences such as being suitable for both carbon and sulfur contents, less corrosion, and a lower power consumption rate. In recent times, researchers have developed several integrated systems where they prepared carbon capture technologies by adding several polymers and copolymers with membrane-based materials. Tao et al. prepared such technologies to examine the effect of several copolymers by adding them during membrane fabrication time; they applied commercially available poly(2,6-dimethyl-1,4-phenylene oxide) (PPE) and additives including polyethylene glycol (PEG) and a PEG–PDMS copolymer (commercially known as IM22). After the experiment, they compared with pure PPE membrane, and they found that CO2 permeability rose nearly 5 times after adding 50 wt% IM22 and rose 4 times after adding 40 wt% PEG. Table 2 summarizes the merits and demerits of different polymeric solvents.

Table 2.
Merits and demerits of different polymeric solvents.
4.2. Poly-Ionic Liquid
Poly-ionic liquids (PILs) are a class of ionic liquids (ILs) that are effective as CO2 absorbents. These liquids are characterized by the presence of multiple ionic groups in the molecule, which allows for a high level of CO2 binding. PILs have been studied extensively in recent years due to their potential use in carbon capture and storage (CCS) technologies. One of the main advantages of PILs as CO2 absorbents is their high CO2 binding capacity. This is due to the presence of multiple ionic groups in the molecule, which can form multiple complexation sites for CO2. Additionally, PILs are more effective at binding CO2 than traditional amine-based absorbents, as they can form stronger complexes with CO2. Another advantage of PILs is their thermal stability. Unlike traditional amine-based absorbents, which can degrade at high temperatures, PILs can maintain their CO2 binding capacity even at high temperatures. This makes them well-suited for use in CCS technologies that involve high-temperature processes, such as post-combustion capture. PILs have also been found to be highly selective for CO2, which means that they have a low affinity for other gases, such as N2 and O2. This is important in CCS applications, as it allows for a higher concentration of CO2 to be captured. Additionally, PILs are non-corrosive, which is a significant advantage over traditional amine-based absorbents that can be corrosive to certain metals. Chau et al. developed a novel cyclic -5-valve pressure swing membrane absorption where they applied ionic liquid (1-butyl-3-methyl-imidazolium dicyanamide) as an absorbent, aiming to separate CO2 from lower temperature syngas [63]. Their experimental results revealed the potentiality of their system, and 95.5% CO2 can be yielded via that mechanism. Ionic liquid membranes are potential elements for CO2 absorption, and these can be classified into supported ionic liquid membranes (SILMs) and quasi-solidified ionic liquid membranes (QSILMs) [64]. Two approaches to utilize ionic liquid as membrane material for CO2 absorption are shown in Figure 4.
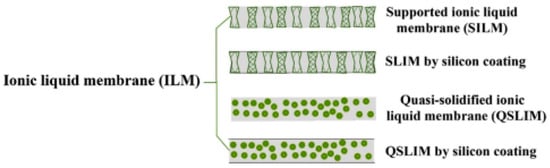
Figure 4.
Utilizing ionic liquid as membrane material [64]. © 2016, copyright permission, Elsevier.
In addition, PILs are highly reusable, which is a major advantage over traditional amine-based absorbents that must be replaced after a certain number of usages. PILs can be regenerated by heating them to release the absorbed CO2, which can then be captured and stored. This process can be repeated multiple times, which makes PILs a cost-effective option for CCS. Several different types of PILs have been studied for use as CO2 absorbents. These include quaternary ammonium-based PILs, phosphonium-based PILs, and imidazolium-based PILs. Each of these types of PILs has its unique properties, and researchers are working to optimize the CO2 binding capacity and selectivity of each type.
4.3. Nano Sorbent
4.3.1. Nanofluids for CO2 Absorption
Nanotechnology is a novel technology that is broadly utilized in numerous energy systems to produce energy in an energy-friendly, economical way. In recent years, CO2 absorption via nanofluids has attracted much attention due to this method having a higher capacity for CO2 absorption. The concept of nanofluids was first proposed by Choi, and he defined nanofluids as dispersed nano-sized materials into the soluble base material. Numerous nanomaterials, such as nanorods, droplets, nanowires, nanoparticles, and nanofibers, can be applied to prepare nanofluids, whereas water-soluble or non-water-soluble liquids (Al2O3, TiO2, SnO2) can be employed as base materials [65].
4.3.2. Nano-Emulsions for CO2 Capture
Nano-emulsions are a new class of materials that have recently been proposed as potential absorbents for carbon dioxide (CO2) capture. These materials are composed of small droplets of one liquid suspended in another liquid, and they have unique properties that make them well-suited for CO2 capture applications. One of the main advantages of nano-emulsions as CO2 absorbents is their high CO2 uptake capacity. The small droplets of the nano-emulsion have a large surface area to volume ratio, which allows them to efficiently capture CO2. Additionally, nano-emulsions can be formulated to have a high selectivity for CO2, meaning that they can effectively capture CO2 while leaving other gases such as N2, O2, and CH4 behind. Another advantage of nano-emulsions is their stability. These materials are thermodynamically stable and can remain stable over a wide range of temperatures and pressures. This makes them well-suited for use in CCS applications, which often involve capturing CO2 at high temperatures and pressures.
Nano-emulsions can be prepared by a variety of methods, including high-pressure homogenization, ultrasonication, and micro-fluidization. The properties of the nano-emulsion can be tailored by adjusting the composition and concentration of the different components, such as the type of oil, surfactant, and co-surfactant used. One of the most common types of nano-emulsion used as CO2 absorbent is oil-in-water (O/W) nano-emulsion, which is composed of small droplets of oil suspended in water. The oil droplets can be formulated to have a high CO2 uptake capacity, and the water can act as a solvent for the CO2. Research has shown that O/W nano-emulsion can adsorb up to 40 times more CO2 than bulk oil, due to the high surface area to volume ratio of the droplets [1]. Another type of nano-emulsion that has been proposed as a potential CO2 absorbent is the water-in-oil (W/O) nano-emulsion, which is composed of small droplets of water suspended in oil. These nano-emulsions have a higher density than the O/W nano-emulsion, which makes them more suitable for use in CCS applications where the CO2 is being captured at high pressures. W/O nano-emulsions have been reported to have a high CO2 adsorption capacity and stability at high pressures and temperatures. A third type of nano-emulsion that has been studied for CO2 absorption is the multiple emulsion, which is composed of droplets of one liquid (typically water) suspended within droplets of another liquid (typically oil), which are then suspended in a third liquid (typically water). These emulsions have been found to have high CO2 adsorption capacity and stability, as well as the ability to separate CO2 from other gases, making them promising candidates for CCS applications.
Despite the promising properties of nano-emulsions for CO2 absorption, there are still some challenges that need to be overcome before they can be used in real-world applications. One of the main challenges is the cost of producing nano-emulsions on a large scale. The methods used to prepare nano-emulsions are often energy-intensive, which can make them more expensive than other CO2 absorbents. Additionally, nano-emulsions can be difficult to separate from the captured CO2, which can also add to the cost of the overall process.
4.4. Amino Acid Solution
Amino acid salt solutions are being recognized as promising absorbents to be used in CO2 capture technologies due to their several amenities, such as higher surface tension, minor absorbent loss, lower oxidative degradation, and evaporation rate while being environmental [37,38,39]. AASs are normally produced through the reaction of alkaline substances and amino acids, while there are more than 20 standard amino acids for which diverse AASs can be observed. The most common amino acids that are employed to produce AASs include arginine, glycine, glutamine, lysine, and taurine, while potassium is the most common ingredient used to produce the AAS solution. Potassium lysine was recognized as a more effective absorbent solution due to having a higher CO2 loading capacity compared to MEA and other AASs. In the case of AASs, the environmental pollution is lower because of the easy disposal of absorbed solutions due to their natural reaction, ionic nature, and extraordinary biodegradation possess. Ramazani et al. experimented with a MEA+PL solution to investigate the effect of the addition of PL and MEA on CO2 loading capacity and found that with an increasing PL/MEA ratio, the loading capacity and corrosion rate of the blend solution increased [66].
4.5. Hydroxide Absorbent
4.5.1. Potassium Hydroxide
The aqueous solutions of alkali metal hydroxides have gathered much attention from scientists and researchers for employing CO2 capture technologies due to their lower energy consumption and minimal negative influence on the environment and ecosystem. Rastegar et al. [67] experimented with aqueous KOH to investigate the effects of stirring and temperature on CO2 absorption, and their results revealed that just by increasing the stirring of the mixer from 50 to 150 rpm, the absorption rate raised by 32%, whereas when they increased the temperature from 22 °C to 65 °C, CO2 absorption dropped by 2.4%. Mourad et al. experimented with different effects on CO2 absorption, such as CO2 inlet concentration, KOH concentration, temperature, gas flow rate, pressure, and found that KOH concentration did not influence CO2 absorption [68]. However, Firman et al. found different results from their experimental investigation, and their results revealed that an increment occurred in CO2 absorption when KOH concentration was increased [69].
4.5.2. Sodium Hydroxide-Based Hybrid Absorbents
Sodium hydroxide (NaOH) has been an extensively studied topic for CO2 absorption since the 1940s; however, at that time, NaOH was not studied for CO2 capture [70,71]. Sodium hydroxide is considered an alkaline absorbent of acidic gases due to its ability to capture CO2 at ambient temperature as well as its fast kinetics, availability, and reasonable price [72]. Traditional procedures followed a water wash with caustic solutions of NaOH (5–15 wt%). Since the wasted caustic creates a disposal problem as expected, it must first be neutralized with acid before being properly disposed of following current environmental and hazardous waste requirements. Aside from that, the annual production of hundreds of millions of tons of sodium carbonate and sodium bicarbonate makes the CO2 absorption from flue gases commercially relevant. However, NaOH is generated as waste in some chemical processes, such as the manufacturing of chlorine, which lowers the price of CO2 removal from flue gases [73]. According to study findings, increasing the concentration of NaOH reduces energy loss but increases corrosion rate and solution viscosity. Alkali metal hydroxides such as sodium hydroxide are considered a well-established technology for CO2 capture; however, the major drawbacks of it are its high corrosion rate and energy loss. In this regard, glycerol can be applied as a potential element in aqueous NaOH solutions which intensifies the mass transfer performance as well as minimizes the offensive wastes and related pollutions. Sheyda et al. experimented with such NaOH-Gly solution to investigate the effect of glycerol on the CO2 capture performance of aqueous NaOH solution and found that the presence of glycerol in the solution caused CO2 absorption efficiency of more than 97%, as shown in Figure 5 [74].
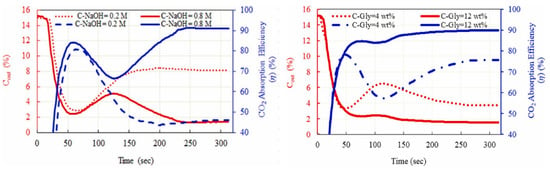
Figure 5.
CO2 outlet concentration and CO2 absorption efficiency versus time under different NaOH concentrations (Qg = 200 mL/min, C-Gly = 8 wt%, T = 25 °C) and glycerol concentration at at (Qg = 200 mL/min, C NaOH = 0.5 M and T = 25 °C) [74]. Copyright permission © 2022 Published by Elsevier Ltd.
4.6. Carbonate Absorbent
4.6.1. Potassium Carbonate
Potassium carbonate (K2CO3) is a favorable CO2 absorbent due to it having some attractive properties such as relevant cost, low degradation rate, minimal toxicity, solubility in carbonate/bicarbonate solution, and less energy consumption. Zhao et al. caused the formation of sesqui-hydrated potassium carbonate crystal (K2CO3.1.5H2O) for the lower energy requirement of K2CO3 [75]. Moreover, Thee et al. stated that 37% of the energy requirement can be minimized by utilizing potassium carbonate during regeneration processes [76]. Despite having such advantages, this absorbent also brings a limitation, which is its poor mass transfer rate. To overcome this limitation, researchers have c arried out several experiments to investigate the effect of different promoters that can be applied with K2CO3 to increase its performance.
The overall reaction of CO2 and potassium carbonate is as follows:
Bicarbonate formation:
Carbonate formation:
Hu et al. discussed detailed information on different promoters for potassium carbonate to be applied in CO2 capture technologies [77]. Zheng et al. proposed a novel [Cho] [Pro] + K2CO3 absorbent for CO2 capture and experimented with different weights of the solution to investigate CO2 absorption and desorption performances [78]. Choi et al. experimented with K2CO3-based absorbents for CO2 absorption to study the influences of promoters, evaluating the mass transfer coefficient for a specific absorbent [79]. Their experimental results revealed that the mass transfer rate was highest for K2CO3+CL-4 among other promoted K2CO3 solutions, and it was quite similar to commercial MEA solutions.
4.6.2. Sodium Carbonate
Sodium carbonate, also known as soda ash, is a chemical compound that has been used as a CO2 absorbent in various industrial processes. Sodium carbonate has been found to be an effective and low-cost option for removing CO2 from gas streams. The CO2 capture process using sodium carbonate typically involves the reaction of CO2 with sodium carbonate to form sodium bicarbonate and carbonate ions. This reaction is exothermic, which means it releases heat, and it can be represented by the following equation:
Na2CO3 is a strong base, and it can effectively capture CO2 from flue gas, biogas, and other sources. It has been found to be highly selective for CO2 over other gases, which makes it an efficient absorbent. Additionally, sodium carbonate can be easily regenerated for reuse, which makes it an attractive option for CO2 capture. Research has shown that it can capture up to 90% of CO2 from flue gas and biogas. It has also been found to be stable and durable, with a service life of up to 10 years. There are several ways to regenerate sodium carbonate after it has been used to capture CO2. One method is to heat the sodium bicarbonate, which releases CO2 and regenerates the sodium carbonate. This process is known as thermal regeneration, and it can be represented by the following equation:
Another method for regenerating sodium carbonate is to treat it with an acid. This process is known as chemical regeneration, and it can be represented by the following equation:
Both methods are effective for regenerating sodium carbonate, but thermal regeneration has been found to be more energy-efficient. Sodium carbonate has also been used in combination with other absorbents to enhance its CO2 capture capabilities. For example, research has shown that sodium carbonate can be used in combination with amines to capture CO2 from flue gas. The use of amines in combination with sodium carbonate has been found to increase the CO2 capture capacity and improve the efficiency of the process. Valluri et al. [80] carried out experiments to investigate the performance of several reagents with frother-assisted NaCO3 slurry in a pilot gas–liquid column. The added surfactant increased the surface area of NaCO3 to absorb more CO2. Hornbostel et al. experimented with CO2 absorption with Na2CO3-filled capsules and compared them with the amine solvent capsules [81]. They found that for similar dimensions, Na2CO3-filled capsules caused less energy penalties, and they also revealed that carbonate-based solvents could compete with fast-reacting amine-based solvents.
4.6.3. Calcium Carbonate
Calcium carbonate (CaCO3) is a naturally occurring compound that is commonly found in rocks such as limestone, marble, and chalk. It has recently been proposed as a potential CO2 absorbent for carbon capture and storage (CCS) technologies. The use of calcium carbonate as a CO2 absorbent is attractive due to its low cost, non-toxicity, wide availability, and environmentally friendly characteristics. Another advantage of calcium carbonate is its high CO2 uptake capacity. Calcium carbonate can effectively capture CO2 through a process known as carbonation. When CO2 comes into contact with calcium carbonate, it reacts to form calcium bicarbonate and water. This reaction is exothermic, meaning it releases heat, which can be recovered and used to generate electricity. Moreover, the reaction is reversible, which means that the captured CO2 can be released from the calcium carbonate by heating it, making it a potential option for CO2 utilization. Hong et al. [82] studies CaCO3 polymorphs to evaluate their performance as CO2 absorbents and compared this absorbent with several amine-based absorbents such as MEA, MDEA, and DEA. They found that CaCO3 had a 100% recovery rate in the MEA solution. They reported that when amine solutions came into contact with the CaCO3 surface, they restricted crystal growth. Calcium carbonate can be used in different forms as a CO2 absorbent. The most common form is precipitated calcium carbonate (PCC), which is made by reacting calcium oxide (CaO) and CO2. PCC can be used in a variety of industrial applications, including as a filler and coating material, and it can be produced in a variety of particle sizes and shapes. Additionally, there are other forms of calcium carbonate, such as ground calcium carbonate (GCC), which is made by grinding natural limestone and marble, and nano calcium carbonate (NCC) which is made by grinding PCC to a very fine powder. Each form has its unique properties and applications.
One of the main challenges in using calcium carbonate as a CO2 absorbent is its low reactivity and solubility compared to some other absorbents. This means that it requires a longer contact time and higher concentrations of CO2 to effectively capture the gas. Researchers are working to overcome this challenge by developing new methods to increase the reactivity of calcium carbonate, such as by modifying its surface or adding catalysts. However, CaCO3 takes many acids for CO2 absorption. To find out a suitable solution for this limitation Chen et al. reported adding a molten carbonate electrolyzer to capture CO2 without using other additives such as lithium salts [83]. They also stated that their study provided a guideline to utilize CaCO3 as a mediator to get pure O2 from CO2 in an enviro-economically friendly way.
4.7. Amine-Based Absorbents
4.7.1. Primary Amines
Primary amines have been studied as a potential solution for capturing carbon dioxide (CO2) from industrial flue gases. These amines are a class of organic compounds that contain a nitrogen atom with a lone pair of electrons and at least one hydrogen atom bonded to it. They are effective CO2 absorbents due to their chemical reactivity towards CO2. One of the main advantages of using primary amines for CO2 capture is their high capacity for CO2 absorption. They can effectively remove CO2 from gas streams at low concentrations, making them suitable for use in industrial settings. Additionally, primary amines are relatively low-cost and easily available, making them a cost-effective option for CO2 capture. The process of CO2 capture using primary amines typically involves the use of an amine solution, such as monoethanolamide (MEA), which is passed through the flue gas stream. As the flue gas comes into contact with the amine solution, the CO2 reacts with the amine, forming a carbamate species. This carbamate species can then be separated from the flue gas stream, allowing the CO2 to be captured. The captured CO2 can then be further processed and utilized for various industrial purposes. Akram et al. studied a 30% MEA solution and found the absorption efficiency to be 90%, which was decreased to 89.6% due to an increase in the MEA at 40 wt% [84]. They also reported that the energy requirement was increased by 12.3%, and the solvent degraded thermally due to increasing the regeneration temperature.
One of the main challenges in using primary amines for CO2 capture is their tendency to degrade over time. This can lead to a decrease in their effectiveness as CO2 absorbents, as well as the formation of byproducts that can be harmful to the environment. To mitigate this, research is ongoing to develop more stable amine solutions and to improve the overall process of CO2 capture using primary amines. Another challenge is the energy consumption associated with the regeneration of the amine solution. Amine solutions used in CO2 capture are typically heated to high temperatures to release the CO2, which can be energy-intensive. However, researchers are exploring various methods to reduce energy consumption, such as the use of membrane separation or pressure swing adsorption.
4.7.2. Secondary Amines
Secondary amines, like primary amines, have been studied as a potential solution for capturing carbon dioxide (CO2) from industrial flue gases. These amines are a class of organic compounds that contain a nitrogen atom with two hydrogen atoms or alkyl groups bonded to it and they are effective CO2 absorbents due to their chemical reactivity towards CO2 [85]. One of the main advantages of using secondary amines for CO2 capture is their high selectivity towards CO2. They can selectively remove CO2 from a gas stream containing other gases such as nitrogen, oxygen, and water vapor. Additionally, secondary amines have higher thermal stability compared to primary amines, which means they are less prone to degradation and can have longer lifetimes. The process of CO2 capture using secondary amines typically involves the use of an amine solution, such as diisopropanolamine (DIPA) or methyl diethanolamine (MDEA), which is passed through the flue gas stream. As the flue gas meets the amine solution, the CO2 reacts with the amine, forming a carbamate species. This carbamate species can then be separated from the flue gas stream, allowing the CO2 to be captured. The captured CO2 can then be further processed and utilized for various industrial purposes. For example, it can be used as a feedstock for producing chemicals and fuels, or for enhanced oil recovery. Wang et al. carried out experimental investigations to compare the performance of n-methyl-2-hydroxy ethylamine (MAE)+H2O with several water-soluble alcohols [86]. They reported that the MAE/n-butanol/H2O system with a ratio of 3:4:3 possessed great potential to be an attractive phase change absorbent for CO2 capture, and they found excellent absorbent stability during CO2 absorption.
One of the main challenges in using secondary amines for CO2 capture is their relatively high cost compared to primary amines. However, secondary amines are more selective and have better thermal stability, which can lead to reduced costs associated with amine degradation and fewer emissions of byproducts. Another challenge is the energy consumption associated with the regeneration of the amine solution. Amine solutions used in CO2 capture are typically heated to high temperatures to release the CO2, which can be energy-intensive. However, researchers are exploring various methods to reduce energy consumption, such as the use of membrane separation or pressure swing adsorption. A comprehensive list depicting the advancements of alkanol amines during the period from 1970 to 2022 can be observed from Table 3.
4.7.3. Tertiary Amines
Tertiary amines have been studied as a potential solution for capturing carbon dioxide (CO2) from industrial flue gases. They are a class of amines that contain a nitrogen atom with three alkyl or aryl groups bonded to it and these amines are effective CO2 absorbents due to their chemical reactivity towards CO2 [87]. One of the main advantages of using tertiary amines for CO2 capture is their high selectivity towards CO2. They can selectively remove CO2 from a gas stream containing other gases such as nitrogen, oxygen, and water vapor. Additionally, tertiary amines have higher thermal stability compared to primary and secondary amines, which means they are less prone to degradation and can have longer lifetimes.
The process of CO2 capture using tertiary amines typically involves the use of an amine solution, such as trimethylamine (TMA), which is passed through the flue gas stream. As the flue gas comes into contact with the amine solution, the CO2 reacts with the amine, forming a carbamate species. This carbamate species can then be separated from the flue gas stream, allowing the CO2 to be captured. The captured CO2 can then be further processed and utilized for various industrial purposes. For example, it can be used as a feedstock for producing chemicals and fuels or for enhanced oil recovery.
Sharif et al. [88] conducted a study to compare the intermolecular reaction of single DMAE, 2EAE, and blended solvent (2DMAE/PZ, 2EAE/PZ) with CO2. They used a material studio application to carry out molecular dynamic simulations, and their results revealed that the mixture of secondary and tertiary amines showed better intermolecular reaction with CO2 compared to single amines where PZ acted as a promoter on 2DMAE and 2EAE with carbon dioxide.
One of the main challenges in using tertiary amines for CO2 capture is their relatively high cost compared to primary and secondary amines. However, the higher selectivity and thermal stability of tertiary amines can lead to reduced costs associated with amine degradation and fewer missions of byproducts. Another challenge is the energy consumption associated with the regeneration of the amine solution. Amine solutions used in CO2 capture are typically heated to high temperatures to release the CO2, which can be energy-intensive. However, researchers are exploring various methods to reduce energy consumption, such as the use of membrane separation or pressure swing adsorption.


Table 3.
Development of several alkanol amines as CO2 absorbents (from 1970–2022).
Table 3.
Development of several alkanol amines as CO2 absorbents (from 1970–2022).
| Year | Alkanolamine | Temperature, K | The Partial Pressure of CO2, kPa | Amine Concentration, % | CO2 Loading, α | Ref. |
|---|---|---|---|---|---|---|
| 1972 | DEA | 323 | 7–3370 | 19.2 | 0.45–1.13 | [89] |
| 1976 | DEA | 338.5–366.9 | 32–767 | 25 | 0.4–0.79 | [90] |
| 1977 | DIPA | 313–373 | 2.7–5888 | 33.63 | 0.07–1.11 | [91] |
| 1978 | DGA | 323–373 | 1.58–4720 | 60 | 0.13–0.62 | [92] |
| 1988 | AMP | 313.2 | 1.25–144 | 28 | 0.4–0.9 | [93] |
| 1990 | AMP | 313, 343 | 0.16–5279 | 18.8 | 0.03–1.65 | [94] |
| 1991 | AMP | 293–353 | 1.59–94 | 18.76, 28.14 | 0.13–0.94 | [95] |
| MDEA | 313 | 0.18–92.8 | 22.9 | 0.04–0.84 | [96] | |
| 1992 | MDEA+MEA | 313.15–373.15 | 1.12–2080 | MDEA: 12–24/MEA: 6–18 | 0.188–1.015 | [97] |
| 1996 | AMP | 313–353 | 3.94–336.6 | 30 | 0.28–0.9 | [98] |
| DEA | 313–353 | 4.85–357.3 | 30 | 0.4–0.73 | [98] | |
| 1998 | DEA+AMP | 313.15–373.15 | 22–2838 | DEA: 20–25/AMP: 5–10 | 0.337–1.2 | [99] |
| DEA+MDEA | 313.15–393.15 | 0.4–2833.6 | DEA: 10–32.5/MDEA: 10–35 | 0.038–1.119 | [99] | |
| 2000 | MDEA | 297.7 | 0.02–1.64 | 23.63 | 0.02–0.26 | [100] |
| PZ | 313–343 | 0.03–40 | 4.7 | 0.16–0.96 | [101] | |
| 313–343 | 29–40,200 | 4.7 | 0.6–0.96 | [101] | ||
| 2001 | MDEA | 298–373 | 0.78–140.4 | 50 | 0.01–0.49 | [102] |
| 2004 | MDEA | 298–348 | 2.7–4559.5 | 48.88, 25.73 | 0–1.3 | [103] |
| DEA | 298–348 | 4.85–357.3 | 47.78 | 0–1.09 | [103] | |
| 2006 | DEA | 323–366 | 0.4–3798 | 25 | 0.1–1.13 | [104] |
| 2010 | AMP | 313.2 | 0.89–151.9 | 28 | 0.4–0.9 | [105] |
| MDEA | 313 | 0.28–89.9 | 22.9 | 0.06–0.80 | [106] | |
| 323 | 6–434 | 50 | 0.1–0.89 | [106] | ||
| PZ | 313 | 5800–7500 | 15–60 | 0.34–0.86 | [107] | |
| TEA | 313–353 | 1.43–153.4 | 26.5 | 0.03–0.53 | [106] | |
| 2011 | PZ | 354–464.8 | 28–2583 | 29.8–40.59 | 0.23–0.45 | [108] |
| AMP | 298–328 | 0.41–1449 | 23.5–46 | 0.19–1.1 | [109] | |
| 303–328 | 0.31–1472 | 40,50 | 0.24–1.04 | [109] | ||
| 2012 | MEA | 303–323 | 0.9–335.9 | 6.7–19 | 0.35–1.16 | [110] |
| AMP | 313–393 | 6–983.5 | 30 | 0–0.97 | [111] | |
| 2013 | AEEA | 303–323 | 1.11–794.67 | 15 | 0.06–1.4077 | [112] |
| 2014 | DIPA | 313–343 | 107–4064 | 45 | 0.52–1.05 | [113] |
| 2017 | NH3 | 335–395 | 0.01–1000 | 20.4 | 1 | [114] |
| DIPA | 313–343 | 91.2–3826.6 | 30 | 0.89–1.14 | [113] | |
| DIPA + AEEA | 313.15–343.15 | 105–3819.7 | DIPA: 20.25/AEEA: 5–10 | 0.5837–1.251 | [113] | |
| MDEA + PZ | 313–375.15 | 0.033–95.78 | MDEA:22.6–47.6/PZ: 0.4–21.3 | 0.027–0.37 | [115] | |
| DIPA + AMP + PZ | 313.15–343.15 | 112.9–3709.7 | DIPA: 24–36/AMP: 7–13/PZ: 2–8 | 0.502–1.091 | [113] | |
| 2019 | MEA | 303–353 | 0–50.65 | 12–15 | 0.017–0.577 | [116] |
| AMP + PZ | 293.15–323.15 | 0.127–140.4 | AMP: 8.9–38/PZ: 0.87–8 | 0.1511–0.9405 | [117] | |
| MEA + DAP | 315.15–333.15 | 13.24–215.46 | MEA: 10–12.5/DAP: 2.5–5 | 0.22–0.711 | [118] | |
| 2021 | MEA | 307.9 | - | 24.9 | 2.5–32.5 | [119] |
| MEA + DEA | 308 | - | 24.8 | 2.5–32.5 | [119] | |
| MEA + TEA | 308 | - | 25 | 2.5–32.5 | [119] | |
| 2022 | DA2MP/AMP/PrOH | 313.15–383.15 | - | 20 | 0.91–0.95 | [120] |
4.8. Triethylenetetramine (TETA)/Ethanol Solution as Absorbent
Triethylenetetramine (TETA) is a type of tertiary amine that has been studied as a potential CO2 absorbent as when dissolved in ethanol, it forms a solution that has been found to be effective at capturing CO2 from industrial flue gases. One of the main advantages of using TETA/ethanol solution for CO2 capture is its high selectivity towards CO2 [121]. The TETA/ethanol solution can selectively remove CO2 from a gas stream containing other gases, such as nitrogen and oxygen [122]. Additionally, the TETA/ethanol solution has a high absorption capacity for CO2 and can be regenerated easily by heating, which allows for the captured CO2 to be released and reused. The process of CO2 capture using TETA/ethanol solution typically involves passing the flue gas through a TETA/ethanol solution. As the flue gas encounters the TETA/ethanol solution, the CO2 reacts with the TETA, forming a carbamate species. This carbamate species can then be separated from the flue gas stream, allowing the CO2 to be captured. The captured CO2 can then be further processed and utilized for various industrial purposes, such as being used as a feedstock for producing chemicals and fuels. Additionally, TETA/ethanol solution has a high potential for enhanced oil recovery. From several experiments, it was found that TETA/ethanol solution having lower concentration polyamine showed better CO2 desorption and cycle loading compared to higher concentration polyamines [123].
One of the main challenges in using TETA/ethanol solution for CO2 capture is its relatively high cost. However, the high selectivity and absorption capacity of TETA/ethanol solution can lead to reduced costs associated with amine degradation and fewer emissions of byproducts. Another challenge is the energy consumption associated with the regeneration of the TETA/ethanol solution. TETA/ethanol solutions used in CO2 capture are typically heated to high temperatures to release the CO2, which can be energy-intensive. However, researchers are exploring various methods to reduce energy consumption, such as the use of membrane separation or pressure swing adsorption. One typical challenge for applying this technology is the generation of gelatinous products during absorption. To provide a potential solution to this problem, Zhifang et al. introduced an amine AMP into the TETA-based SLPCAs which successfully ignored such solid byproducts via yielding crystalline powders that could be easily separated [124].
4.9. Amino Acid Salt as Liquid-Solid Phase Changes Absorbent
Amino acid salt as a liquid-solid phase change absorbent of CO2 is a novel technology that utilizes amino acid salts as a material for CO2 capture [125]. These absorbents can capture CO2 through a liquid-solid phase change process. When in contact with CO2, the absorbent changes from a solid state to a liquid state, allowing for the absorption of CO2. Amino acid salts have been found to have high selectivity and capacity for CO2 capture. They can also be regenerated and reused, making them a sustainable option for CO2 capture. The absorbents can capture CO2 at a lower energy cost compared to traditional absorbents, which can lead to a more cost-effective CO2 capture process. One of the advantages of using amino acid salts as liquid-solid phase change absorbents is that they can be used in a wide range of applications, such as power plants, industrial facilities, and transportation. Additionally, they can be used in post-combustion CO2 capture processes, which is a common method for capturing CO2 from power plants and other large emitters [126].
Li et al. [127] developed novel biphasic absorbent based on water-lean amino acid salt to enhance CO2 capture performance and energy efficiency. They employed potassium prolinate and potassium sarcosinate with a secondary amino group as active elements, while 2-alkoxy ethanol possessing low specific heat, volatility, and viscosity were employed as physical antisolvents and accelerated the generation of the solid phase during CO2 absorption. They used 13C NMR and XRD to characterize the phase change behavior and separation of CO2 in the solid and liquid phases and to identify the product species in CO2-rich solid phase. Their experiment revealed that 50–80% CO2 could be captured via the solid slurry with 2.5 to 3.5 mol kg−1 CO2 loading.
Amino acid salts have also been found to have high thermal stability, which allows them to be used in high-temperature CO2 capture processes [128,129]. They are also non-toxic and biocompatible, making them a safe and environmentally friendly option for CO2 capture. In addition, amino acid salts can be produced from renewable sources such as waste or byproducts of agriculture, food, and the chemical industry. This makes them a sustainable alternative to traditional absorbents, which are usually produced from fossil fuels. Research on amino acid salt as liquid-solid phase change absorbents is ongoing, and further advancements are expected in the future. For example, researchers are working on developing new amino acid salts with improved properties and finding new ways to integrate them into CO2 capture processes [130,131].
4.10. Encapsulated Absorbents
Encapsulated absorbents for CO2 capture are technologies that involve enclosing an absorbent material within a protective shell or capsule. The main benefit of this technology is that it improves the stability, selectivity, and overall performance of the absorbent material by protecting it from degradation and chemical reactions. This can lead to a more efficient and cost-effective CO2 capture process. Encapsulation can be achieved through a variety of methods, such as coating, impregnation, and entrapment. Coating involves applying a thin layer of protective material on the surface of the absorbent, impregnation involves filling the pores of the absorbent with a protective material, and entrapment involves enclosing the absorbent within a protective shell or capsule. The selection of any approach from these usually depends on the application and the properties of the absorbent. Polesso et al. experimented with a novel CO2 absorption system where they investigated the performance of encapsulated poly-ionic liquids (PIL) as green solvents [132]. From this experiment, by employing the nano spray dryer B-90, encapsulated ionic liquids Emim [X], and capsules of water-based PIL P[DADMA][BF4] were achieved, and the stability of this system and high CO2 selectivity emphasized its potentiality for CO2 absorption. Other encapsulated PILs were also compared, and P[DADAM]/BF4 showed the highest performance based on CO2 absorption rate and CO2/N2 selectivity, which is clear from Figure 6.
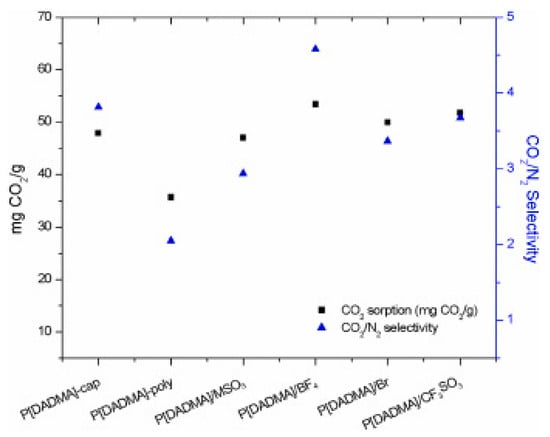
Figure 6.
CO2 sorption (mg CO2/g) and CO2/N2 selectivity [132].
One of the significant advantages of encapsulated absorbents is that they can improve the stability of the absorbent. The protective shell or capsule can shield the absorbent from environmental factors, such as heat and light, which can cause degradation. This can increase the service life of the absorbent and reduce the need for frequent replacement. Additionally, encapsulation can also protect the absorbent from chemical reactions that may occur during the absorption process, which can improve its performance. Encapsulation can also improve the selectivity of the absorbent material for CO2. For example, encapsulating amines within a porous material can increase their selectivity for CO2 over other gases. This leads to a more efficient and specific CO2 capture process. Some encapsulated absorbents are also designed to be regenerated and reused after the CO2 is captured. This can reduce the cost of the CO2 capture process and make it more sustainable.
4.11. Enzymatically Catalyzed Absorbent Systems
Enzymatically catalyzed absorbent systems for CO2 capture are novel technologies that utilize enzymes to catalyze the capture and conversion of CO2. Enzymes are biomolecules that act as catalysts, increasing the rate of a chemical reaction without being consumed in the process. This technology has been studied as a way to improve the efficiency and sustainability of CO2 capture processes.
Enzymes that have been studied for CO2 capture include carbonic anhydrase, which catalyzes the conversion of CO2 and water to bicarbonate and protons, and formate dehydrogenase, which catalyzes the conversion of CO2 and formate to carbon monoxide and water. These enzymes are typically immobilized on a support material, such as a polymer or a nanoparticle, to increase their stability and reuse. Hannaneh et al. proposed a hybrid enzymatic CO2 absorption process integrated with carbonic anhydrase II enzyme in a membrane, and they found from their experiment that such enzymatic approaches improve CO2 absorption [133]. In another article, Hannaneh reported a promising enzymatic CO2 absorption approach in a packed bed reactor, and based on their experimental and theoretical approaches, they reported a packed bed bioreactor with immobilized carbonic anhydrase enzyme on magnetic nanoparticles and packing surface as an attractive process for green CO2 capture process [134]. Several experiments have revealed that applying CA with absorbents (such as MDEA) can enhance absorption performance significantly [135]. Mthias et al. experimented with an enzyme-enhanced CO2 absorption mechanism in which they incorporated CA in the biocatalyst delivery system to investigate with an aqueous MDEA solvent. Their result showed a sixfold improvement in the total absorbed CO2 moles [136].
One of the advantages of enzymatically catalyzed absorbent systems is that they can improve the efficiency of CO2 capture. Enzymes can catalyze the conversion of CO2 to a more easily captured and stored form, such as bicarbonate or formate. This can lead to a more efficient and cost-effective CO2 capture process. Enzymatically catalyzed absorbent systems can also improve the sustainability of CO2 capture processes. Enzymes are biocompatible and can be produced from renewable sources, such as bacteria or plants. Additionally, enzymes can be recycled and reused after the CO2 is captured, reducing the need for frequent replacement. Another advantage of enzymatically catalyzed absorbent systems is that they can be used in a wide range of applications, such as power plants and industrial facilities, and can be integrated into existing CO2 capture processes. This technology is an active area of research, and further advancements are expected in the future.
The major barrier of this novel technology is the stability and activity of carbonic anhydrase enzyme under typical flue gas operating conditions. To propose a solution to this issue, Zhang et al. [137] proposed a novel CA/ZIF-L-1 composite by embedding CA into ZIF-L (zeolitic imidazolate framework). They found that the novel composite caused high enzyme activity retention and exhibited high thermal stability, which was improved by 100% at 40 °C, which can be observed from Figure 7.
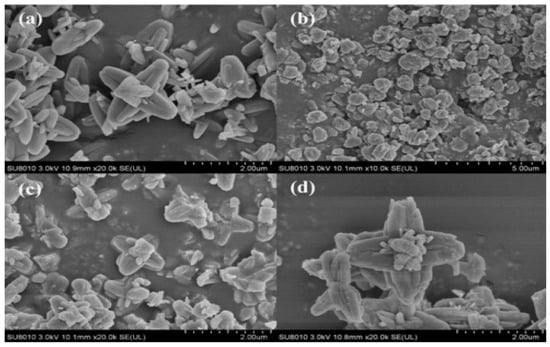
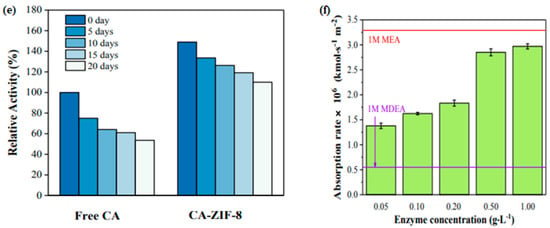
Figure 7.
SEM images of (a) ZIF-L, (b) ZIF-L (5 min), (c) CA/ZIF-L-1, and (d) CA/ZIF-L-2. (e) The stability of the free enzyme and CA/ZIF-L-1 and reusability of CA/ZIF-L-1; (f) Performance of CO2 absorption into 1M MDEA solution with different 604 concentrations of CA/ZIF-L-1 at 40 °C and a CO2 partial pressure of 15 kPa [137]. Copyright © 2018, American Chemical Society.
4.12. Deep Eutectic Solvents (DESs)
Deep eutectic solvents (DESs) are mixtures of two or more components that exhibit lower melting points and higher solubility compared to individual components. In recent years, they have gained attention as a potential solution for CO2 capture and utilization due to their unique properties, such as low toxicity and high CO2 solubility. Research advancements in the field of DESs for CO2 absorption have focused on optimizing the composition and properties of DESs to increase their efficiency and cost-effectiveness. For example, studies have explored the use of different hydrogen bond donors and acceptors to improve CO2 solubility and selectivity. The Lewis or Bronsted acids and bases are used to create the DESs as novel ionic solvents that can contain a wide range of anionic and cationic species [138]. In more detail, DESs are created by combining a hydrogen-bond donor (HBD) and a hydrogen-bond acceptor (HBA) at the proper molar ratio. These materials are inexpensive, reliable, and simple to make from a variety of readily accessible beginning ingredients. The most typical and extensively utilized DESs are those based on cholinium chloride (ChCl). ChCl-based DESs are well known for sharing characteristics and behavior with traditional ILs, as well as having the same CO2 order. capability for absorption [139,140,141]. Ruan et al. experimented with CO2 absorption using DES, which was formed by superbase 1,5-diazabicyclo [4.3.0] non-5-ene (DBN) and 1,2,4-triazole (Tz), and they investigated the impacts of molar ratios of DBN and TZ for CO2 absorption. Their experimental results revealed that DES [2DBN:Tz] showed the highest performance [142], which is clear in Figure 8.
DESs have potential applications in various industries, including the power generation and chemical industries, where they can be used to capture and utilize CO2 emissions [138]. Additionally, they have been proposed to enhance oil recovery and as a solvent for chemical reactions. DESs work by dissolving CO2 in the solvent mixture, forming a stable complex. This allows for the efficient separation of CO2 from other gases, such as nitrogen and oxygen, in flue gas streams. The captured CO2 can then be utilized or stored for later use. The advantages of using DESs for CO2 capture include their low cost, low toxicity, and high CO2 solubility. Additionally, they can be regenerated and reused multiple times, making them a sustainable solution for CO2 capture. However, there are also limitations to the use of DESs for CO2 capture. For example, the efficiency of CO2 capture can be affected by temperature and pressure changes, and the stability of DESs can be impacted by impurities in the gas stream. Additionally, the production and use of DESs can also have an impact on the environment. Table 4 summarizes different absorbent materials with their advantages and disadvantages.
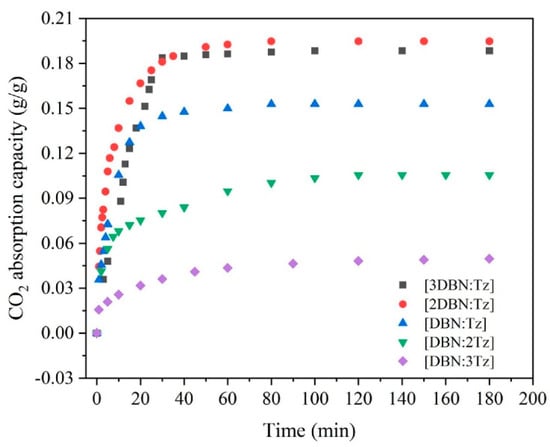

Figure 8.
CO2 gravimetric absorption capacity by DBN-Tz DESs under different molar ratios at 25 °C and 100 kPa [142]. Copyright permission © 2022 Elsevier.

Table 4.
Different absorbent materials with their advantages and disadvantages.
5. Outlook and Prospects
Carbon capture and storage (CCS) is a technology that aims to capture carbon dioxide (CO2) emissions from power plants and other industrial sources and store them underground. The use of CO2 absorbents is a crucial component of CCS, as they are responsible for capturing the CO2 from the emissions. The future of CCS and CO2 absorbents is of great interest, as it is expected to play a significant role in reducing greenhouse gas emissions and slowing down global warming. One of the main prospects of CO2 absorption and absorbents is the increased use of CCS in power generation. As countries around the world work to reduce their greenhouse gas emissions, the use of CCS in power generation is expected to grow. This will lead to an increased demand for CO2 absorbents, and researchers are working to develop new and improved absorbents that are more effective and efficient at capturing CO2.
Another prospect is the use of CCS in industrial processes. Currently, many industrial processes, such as cement and steel production, release large amounts of CO2 into the atmosphere. CCS technologies, including CO2 absorbents, can be used to capture and store these emissions, reducing their impact on the environment. As the demand for these technologies grows, researchers are working to develop new absorbents that are specifically designed for industrial applications. In addition, the use of CO2 absorbents in direct air capture (DAC) is an emerging field. DAC technology captures CO2 directly from the atmosphere rather than from industrial emissions [143]. This technology has the potential to significantly reduce the amount of CO2 in the atmosphere and is seen as a promising solution for addressing climate change. The use of CO2 absorbents in DAC is still in the early stages of development, but it is expected to grow in the future as researchers work to improve the efficiency and effectiveness of the technology [144].
Another prospect is the integration of CO2 absorption and utilization. While traditional CCS technologies focus on capturing and storing CO2, there is also a growing interest in utilizing the captured CO2 for various applications such as producing chemicals, fuels, and even food. Researchers are working to develop new absorbents that can capture CO2 for utilization, which would increase the value of CCS and make it more economically viable. Additionally, the development of sustainable and biobased absorbents is also an area of research. Traditional CO2 absorbents are mostly based on fossil fuels, which are non-renewable and environmentally damaging. There is a growing interest in the development of absorbents that are based on renewable and sustainable resources such as biomass, which can reduce the environmental impact of CCS.
Another prospect for CO2 absorption and absorbents is the development of hybrid absorbents. These absorbents combine two or more different types of absorbents to improve the overall efficiency and effectiveness of the CO2 capture process. For example, a hybrid absorbent could consist of a traditional amine-based absorbent combined with a PIL (poly-ionic liquid) absorbent. The amine-based absorbent would provide a high CO2 binding capacity, while the PIL absorbent would provide improved thermal stability and selectivity for CO2. Researchers are also working on developing hybrid absorbents that integrate solid and liquid absorbents to achieve better performance.
Another important area of research is the development of more cost-effective CO2 capture technologies. While CCS and CO2 absorbents have the potential to significantly reduce greenhouse gas emissions, the cost of these technologies remains a major barrier to their widespread adoption. Researchers are working to develop new absorbents that are more cost-effective, such as those based on sustainable and biobased resources, to make CCS more economically viable. There is also growing interest in the use of CO2 absorbents in carbon mineralization, which is a process that converts CO2 into stable carbonates, such as limestone. Carbon mineralization has the potential to permanently remove CO2 from the atmosphere and researchers are working to develop new absorbents that can capture CO2 for mineralization. Lastly, the development of advanced monitoring and control systems for CCS facilities is also an important area of research. These systems would allow the real-time monitoring of the CO2 capture process and would enable facilities to optimize the performance of their absorbents and improve the efficiency of their CCS systems.
6. Conclusions
In this paper, several absorption-based CO2 capture approaches have been studied comprehensively, considering material and mechanism improvement as well as engineering aspects. The increased amount of CO2 has been considered a curse in recent years, and researchers are working hard to develop materials for capturing CO2 to protect the environment from its negative influences. Several catalysts can be utilized to ameliorate the performance of different absorbent materials. Researchers are working to develop different types of absorbents and technologies to synthesize absorbents while considering cost, safety, absorption rate, stability, and durability. Different novel technologies such as enzyme-based absorption and nanocomposite-assisted absorption, which improve the potentiality of carbon capture, have been developed. Power plants and other GHG emitters emit a massive amount of CO2 regularly, which is harming the environment. However, these massive amounts of CO2 can be utilized for energy production by applying CO2 as feedstock material as well as CO2 as a raw material to produce the alternative fuel methanol. To properly utilize and store of CO2, it is necessary to have comprehensive knowledge of advanced CO2 capture technologies, and as absorption is the most widely applied CO2 capture technology, we reviewed and discussed previous research to assist researchers in further research.
Author Contributions
All authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McInnes, K.; Walsh, K.; Hubbert, G.D.; Beer, T. Impact of Sea-level Rise and Storm Surges on a Coastal Community. Nat. Hazards 2003, 30, 187–207. [Google Scholar] [CrossRef]
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, V.; Carraro, C.; Massetti, E.; Tavoni, M. International energy R&D spillovers and the economics of greenhouse gas atmospheric stabilization. Energy Econ. 2008, 30, 2912–2929. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D.; Akanda, A.M.; Islam, A.R.M.T. Peer-to-Peer Energy Trading Pricing Mechanisms: Towards a Comprehensive Analysis of Energy and Network Service Pricing (NSP) Mechanisms to Get Sustainable Enviro-Economical Energy Sector. Energies 2023, 16, 2198. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.M.M.B.; Allen, S.K.; Boschung, J.; Midgley, P.M. The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Clim. Chang. 2013, 1535, 2013. [Google Scholar]
- Das, A.; Peu, S.D. A Comprehensive Review on Recent Advancements in Thermochemical Processes for Clean Hydrogen Production to Decarbonize the Energy Sector. Sustainability 2022, 14, 11206. [Google Scholar] [CrossRef]
- UNFCCC. ADOPTION OF THE PARIS AGREEMENT. Proposal by the President. 2015. Available online: https://unfccc.int/documents/9064 (accessed on 12 December 2015).
- Zhang, S.; Lu, Y.; Ye, X. Catalytic behavior of carbonic anhydrase enzyme immobilized onto nonporous silica nanoparticles for enhancing CO2 absorption into a carbonate solution. Int. J. Greenh. Gas Control 2013, 13, 17–25. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, Y.T. CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution. Energy 2013, 53, 206–211. [Google Scholar] [CrossRef]
- Wang, T.; Yu, W.; Liu, F.; Fang, M.; Farooq, M.; Luo, Z. Enhanced CO2 Absorption and Desorption by Monoethanolamine (MEA)-Based Nanoparticle Suspensions. Ind. Eng. Chem. Res. 2016, 55, 7830–7838. [Google Scholar] [CrossRef]
- Sahoo, P.C.; Kumar, M.; Singh, A.; Singh, M.P.; Puri, S.K. Biocatalyzed Accelerated Post-combustion CO2 Capture and Stripping in Monoethanolamine. Energy Fuels 2017, 31, 11007–11012. [Google Scholar] [CrossRef]
- Liang, Z.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.Z.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar] [CrossRef]
- Aboudheir, A.; Tontiwachwuthikul, P.; Idem, R. Rigorous Model for Predicting the Behavior of CO2 Absorption into AMP in Packed-Bed Absorption Columns. Ind. Eng. Chem. Res. 2005, 45, 2553–2557. [Google Scholar] [CrossRef]
- Oyenekan, B.A.; Rochelle, G.T. Energy Performance of Stripper Configurations for CO2 Capture by Aqueous Amines. Ind. Eng. Chem. Res. 2005, 45, 2457–2464. [Google Scholar] [CrossRef]
- Grant, D.; Bergstrand, K.; Running, K. Effectiveness of US state policies in reducing CO2 emissions from power plants. Nat. Clim. Chang. 2014, 4, 977–982. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Fifth Assessment Report, Working Group III, Summary for Policymakers. 2014. Available online: http://www.ipcc.ch/report/ar5/wg3/ (accessed on 27 October 2018).
- Electricity in the U.S.—U.S. Energy Information Administration (EIA) n.d. Available online: https://www.eia.gov/energyexplained/electricity/electricity-in-the-us.php (accessed on 30 October 2022).
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Gielen, D. CO2 removal in the iron and steel industry. Energy Convers. Manag. 2003, 44, 1027–1037. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, B.; Li, L. Advance in Post-Combustion CO2 Capture with Alkaline Solution: A Brief Review. Energy Procedia 2012, 14, 1515–1522. [Google Scholar] [CrossRef]
- Paul, H.M.; Cousins, F.A.; Jiang, K.; Zhai, R.; Garcia, M. An update of the benchmark post-combustion CO2-capture technology. Fuel 2020, 273, 117776. [Google Scholar] [CrossRef]
- Karami, B.; Ghaemi, A. Cost-effective nanoporous hypercross-linked polymers could drastically promote the CO2absorption rate in amine-based solvents, improving energy-efficient CO2capture. Ind. Eng. Chem. Res. 2021, 60, 3105–3114. [Google Scholar] [CrossRef]
- Arunachalam, R.; Chinnaraja, E.; Subramanian, P.S. Efficient Homogeneous Catalysts for Conversion of CO2 to Fine Chemicals. In Catalysis for Clean Energy and Environmental Sustainability; Pant, K.K., Gupta, S.K., Ahmad, E., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Yuan, Z.; Eden, M.R.; Gani, R. Toward the Development and Deployment of Large-Scale Carbon Dioxide Capture and Conversion Processes. Ind. Eng. Chem. Res. 2016, 55, 3383–3419. [Google Scholar] [CrossRef]
- Barchas, R.; Davis, R. The Kerr-McGee/ABB Lummus Crest technology for the recovery of CO2 from stack gases. Energy Convers. Manag. 1992, 33, 333–340. [Google Scholar] [CrossRef]
- Scherffius, J.R.; Reddy, S.; Klumpyan, J.P.; Armpriester, A. Large-Scale CO2 Capture Demonstration Plant Using Fluor’s Econamine FG PlusSM Technology at NRG’s WA Parish Electric Generating Station. Energy Procedia 2013, 37, 6553–6561. [Google Scholar] [CrossRef]
- Bhown, A.; Dillon, D.; Berger, A.H.; Du, Y.; Haney, K.; Carroll, B.; Gilmartin, J.; Simonson, T.; Reddy, S. Front End Engineering Design Study for Carbon Capture at a Natural Gas Combined Cycle Power Plant in California. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Miyamoto, O.; Maas, C.; Tsujiuchi, T.; Inui, M.; Hirata, T.; Tanaka, H.; Yonekawa, T.; Kamijo, T. KM CDR ProcessTM Project Update and the New Novel Solvent Development. Energy Procedia 2017, 114, 5616–5623. [Google Scholar] [CrossRef]
- Kadono, K.; Suzuki, A.; Iijima, M.; Ohishi, T.; Tanaka, H.; Hirata, T.; Kondo, M. New Energy Efficient Processes and Newly Developed Absorbents for Flue Gas CO2 Capture. Energy Procedia 2013, 37, 1785–1792. [Google Scholar] [CrossRef]
- Hirata, T.; Tsujiuchi, T.; Kamijo, T.; Kishimoto, S.; Inui, M.; Kawasaki, S.; Lin, Y.-J.; Nakagami, Y.; Nojo, T. Near-zero emission coal-fired power plant using advanced KM CDR process™. Int. J. Greenh. Gas Control 2019, 92, 102847. [Google Scholar] [CrossRef]
- Molina, C.T.; Bouallou, C. Assessment of different methods of CO 2 capture in post-combustion using ammonia as solvent. J. Clean. Prod. 2015, 103, 463–468. [Google Scholar] [CrossRef]
- Hughes, R.; Kotamreddy, G.; Bhattacharyya, D.; Omell, B.; Matuszewski, M. Modeling and Bayesian Uncertainty Quantifica-tion of a Membrane-Assisted Chilled Ammonia Process for CO2Capture. Ind. Eng. Chem. Res. 2022, 61, 4001–4016. [Google Scholar] [CrossRef]
- Darde, V.; Thomsen, K.; Van Well, W.J.M.; Stenby, E.H. Chilled ammonia process for CO2 capture. Energy Procedia 2009, 1, 1035–1042. [Google Scholar] [CrossRef]
- Bonalumi, D.; Lillia, S.; Valenti, G. Rate-based simulation and techno-economic analysis of coal-fired power plants with aqueous ammonia carbon capture. Energy Convers. Manag. 2019, 199, 111966. [Google Scholar] [CrossRef]
- Yu, H.; Qi, G.; Xiang, Q.; Wang, S.; Fang, M.; Yang, Q.; Wardhaugh, L.; Feron, P. Aqueous Ammonia Based Post Combustion Capture: Results from Pilot Plant Operation, Challenges and Further Opportunities. Energy Procedia 2013, 37, 6256–6264. [Google Scholar] [CrossRef]
- Rashidi, H.; Rasouli, P.; Azimi, H. A green vapor suppressing agent for aqueous ammonia carbon dioxide capture solvent: Microcontactor mass transfer study. Energy 2021, 244, 122711. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, W.; Wang, J.; Soltanian, M.R.; Olabi, A.G. Effectiveness of amino acid salt solutions in capturing CO2: A review. Renew. Sustain. Energy Rev. 2018, 98, 179–188. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Gray, M.L.; Duan, Y.; Luebke, D.; Li, B. Development of amino acid and amino acid-complex based solid sorbents for CO2 capture. Appl. Energy 2013, 109, 112–118. [Google Scholar] [CrossRef]
- Majchrowicz, M.E.; Brilman, D.; Groeneveld, M.J. Precipitation regime for selected amino acid salts for CO2 capture from flue gases. Energy Procedia 2009, 1, 979–984. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A.; Ho, M.T.; Wiley, D.E. A comparison between amino acid based solvent and traditional amine solvent processes for CO2 removal. Chem. Eng. Res. Des. 2019, 146, 509–517. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, E.; Heffernan, K.; van der Ham, L.; Linders, M.J.; Goetheer, E.L.; Vlugt, T.J. Precipitating Amino Acid Solvents for CO2 Capture. Opportunities to Reduce Costs in Post Combustion Capture. Energy Procedia 2014, 63, 727–738. [Google Scholar] [CrossRef]
- Kim, K.; Cho, S.-G.; Sa, J.-H. Natural Hydrophilic Amino Acids as Environment-Friendly Gas Hydrate Inhibitors for Carbon Capture and Sequestration. ACS Sustain. Chem. Eng. 2021, 9, 17413–17419. [Google Scholar] [CrossRef]
- Lyu, H.; Chen, O.I.-F.; Hanikel, N.; Hossain, M.I.; Flaig, R.W.; Pei, X.; Amin, A.; Doherty, M.D.; Impastato, R.K.; Glover, T.G.; et al. Carbon Dioxide Capture Chemistry of Amino Acid Functionalized Metal–Organic Frameworks in Humid Flue Gas. J. Am. Chem. Soc. 2022, 144, 2387–2396. [Google Scholar] [CrossRef]
- Onofri, S.; Bodo, E. CO2 Capture in Biocompatible Amino Acid Ionic Liquids: Exploring the Reaction Mechanisms for Bimolecular Absorption Processes. J. Phys. Chem. B 2021, 125, 5611–5619. [Google Scholar] [CrossRef]
- Castro, M.; Gómez-Díaz, D.; Navaza, J.M.; Rumbo, A. Carbon Dioxide Capture by Chemical Solvents Based on Amino Acids: Absorption and Regeneration. Chem. Eng. Technol. 2020, 44, 248–257. [Google Scholar] [CrossRef]
- Kasturi, A.; Gabitto, J.; Tsouris, C.; Custelcean, R. Carbon dioxide capture with aqueous amino acids: Mechanistic study of amino acid regeneration by guanidine crystallization and process intensification. Sep. Purif. Technol. 2021, 271, 118839. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.P.; Liao, C.-Y.; Zhao, X.; Hsiung, T.-L.; Liu, S.-H.; Chang, S.-G. Dual Alkali Solvent System for CO2Capture from Flue Gas. Environ. Sci. Technol. 2017, 51, 8824–8831. [Google Scholar] [CrossRef]
- Shu, Q.; Legrand, L.; Kuntke, P.; Tedesco, M.; Hamelers, H.V.M. Electrochemical Regeneration of Spent Alkaline Absorbent from Direct Air Capture. Environ. Sci. Technol. 2020, 54, 8990–8998. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Luebke, D.R.; Enick, R.M. CO2-philic Oligomers as Novel Solvents for CO2Absorption. Energy Fuels 2010, 24, 6214–6219. [Google Scholar] [CrossRef]
- Kim, H.; Jung, J.Y.; Park, K.H.; Linga, P.; Seo, Y.; Wood, C.D. Enhanced Kinetic Performance of Amine-Infused Hydrogels for Separating CO2from CH4/CO2Gas Mixture. Energy Fuels 2021, 35, 13889–13899. [Google Scholar] [CrossRef]
- Enick, R.M.; Koronaios, P.; Stevenson, C.; Warman, S.; Morsi, B.; Nulwala, H.; Luebke, D. Hydrophobic Polymeric Solvents for the Selective Absorption of CO2 from Warm Gas Streams that also Contain H2 and H2O. Energy Fuels 2013, 27, 6913–6920. [Google Scholar] [CrossRef]
- Xu, X.; Heath, C.; Pejcic, B.; Wood, C.D. CO2 capture by amine infused hydrogels (AIHs). J. Mater. Chem. A 2018, 6, 4829–4838. [Google Scholar] [CrossRef]
- White, C.; Adam, E.; Sabri, Y.; Myers, M.B.; Pejcic, B.; Wood, C.D. Amine-Infused Hydrogels with Nonaqueous Solvents: Facile Platforms to Control CO2 Capture Performance. Ind. Eng. Chem. Res. 2021, 60, 14758–14767. [Google Scholar] [CrossRef]
- Dave, A.; Pathak, B.; Dave, M.; Rezvani, S.; Huang, Y.; Hewitt, N. Process design of CO2 desorption from physical solvent di-methyl-ether of poly-ethylene-glycol. Mater. Sci. Energy Technol. 2019, 3, 209–217. [Google Scholar] [CrossRef]
- Dave, A.; Dave, M.; Huang, Y.; Rezvani, S.; Hewitt, N. Process design for CO 2 absorption from syngas using physical solvent DMEPEG. Int. J. Greenh. Gas Control 2016, 49, 436–448. [Google Scholar] [CrossRef]
- Sattari, A.; Ramazani, A.; Aghahosseini, H.; Aroua, M.K. The application of polymer containing materials in CO2 capturing via absorption and adsorption methods. J. CO2 Util. 2021, 48, 101526. [Google Scholar] [CrossRef]
- Zheng, W.-T.; Zhang, J.-B.; Liu, Y.; Huang, K. Reversible Chemical Absorption of CO2 in Polyethylenimine Supported by Low-Viscous Tetrabutylphosphonium 2-Fluorophenolate. Energy Fuels 2020, 34, 3493–3500. [Google Scholar] [CrossRef]
- Rolker, J.; Seiler, M.; Mokrushina, L.; Arlt, W. Potential of Branched Polymers in the Field of Gas Absorption: Experimental Gas Solubilities and Modeling. Ind. Eng. Chem. Res. 2007, 46, 6572–6583. [Google Scholar] [CrossRef]
- Hossain, I.; Kim, D.; Al Munsur, A.Z.; Roh, J.M.; Park, H.B.; Kim, T.-H. PEG/PPG–PDMS-Based Cross-Linked Copolymer Membranes Prepared by ROMP and In Situ Membrane Casting for CO2 Separation: An Approach to Endow Rubbery Materials with Properties of Rigid Polymers. ACS Appl. Mater. Interfaces 2020, 12, 27286–27299. [Google Scholar] [CrossRef] [PubMed]
- Shamshiri, M.; Jafari, R.; Momen, G. Icephobic properties of aqueous self-lubricating coatings containing PEG-PDMS copolymers. Prog. Org. Coat. 2021, 161, 106466. [Google Scholar] [CrossRef]
- Ramalingame, R.; Chandraker, P.; Kanoun, O. Investigation on the Influence of Solvents on MWCNT-PDMS Nanocomposite Pressure Sensitive Films. Proceedings 2017, 1, 384. [Google Scholar] [CrossRef]
- Rumens, C.V.; Ziai, M.A.; Belsey, K.E.; Batchelor, J.C.; Holder, S.J. Swelling of PDMS networks in solvent vapours; applications for passive RFID wireless sensors. J. Mater. Chem. C 2015, 3, 10091–10098. [Google Scholar] [CrossRef]
- Chau, J.; Jie, X.; Sirkar, K.K. Polyamidoamine-facilitated poly(ethylene glycol)/ionic liquid based pressure swing membrane absorption process for CO2 removal from shifted syngas. Chem. Eng. J. 2016, 305, 212–220. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef]
- McGrail, B.; Thallapally, P.; Blanchard, J.; Nune, S.; Jenks, J.; Dang, L. Metal-organic heat carrier nanofluids. Nano Energy 2013, 2, 845–855. [Google Scholar] [CrossRef]
- Ramazani, R.; Samsami, A.; Jahanmiri, A.; Van der Bruggen, B.; Mazinani, S. Characterization of monoethanolamine + potassium lysinate blend solution as a new chemical absorbent for CO2 capture. Int. J. Greenh. Gas Control 2016, 51, 29–35. [Google Scholar] [CrossRef]
- Rastegar, Z.; Ghaemi, A.; Shirvani, M. Experimental Study of Carbon Dioxide Absorption Using Aqueous Potassium Hydroxide Solutions. Nashrieh Shimi Mohandesi Shimi Iran 2021, 40, 115–126. [Google Scholar]
- Mourad, A.A.-H.; Mohammad, A.F.; Al-Marzouqi, A.H.; Altarawneh, M.; Al-Marzouqi, M.H.; El-Naas, M.H. Carbon dioxide capture through reaction with potassium hydroxide and reject brine: A kinetics study. Int. J. Greenh. Gas Control 2022, 120, 103768. [Google Scholar] [CrossRef]
- Firman, N.; Noor, A.; Zakir, M.; Maming, M.; Fathurrahman, A.F. Absorption of Carbon Dioxide into Potassium Hydroxide: Preliminary Study for its Application into Liquid Scintillation Counting Procedure. Egypt. J. Chem. 2021, 64, 4907–4912. [Google Scholar] [CrossRef]
- Spector: Removal of Carbon Dioxide from Atmospheric Air-Google Scholar n.d. Available online: https://scholar.google.com/scholar_lookup?title=Removal%20of%20carbon%20dioxide%20from%20atmospheric%20air&publication_year=1946&author=N.A.%20Spector&author=B.F.%20Dodge (accessed on 26 January 2023).
- Tepe: Absorption of Carbon Dioxide by Sodium Hydroxide...-Google Scholar n.d. Available online: https://scholar.google.com/scholar_lookup?title=Absorption%20of%20carbon%20dioxide%20by%20sodium%20hydroxide%20solutions%20in%20a%20packed%20column&publication_year=1943&author=J.B.%20Tepe&author=B.F.%20Dodge (accessed on 26 January 2023).
- Naeem, S.; Shahhosseini, S.; Ghaemi, A. Simulation of CO 2 capture using sodium hydroxide solid sorbent in a fluidized bed reactor by a multi-layer perceptron neural network. J. Nat. Gas Sci. Eng. 2016, 31, 305–312. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Valeh-E-Sheyda, P.; Nafchi, N.F. Carbon dioxide capture by the green aqueous sodium hydroxide-glycerol solution in a gas-liquid microchannel contactor. J. Environ. Chem. Eng. 2022, 10, 108666. [Google Scholar] [CrossRef]
- Zhao, W.; Sprachmann, G.; Li, Z.; Cai, N.; Zhang, X. Effect of K2CO3·1.5H2O on the regeneration energy consumption of potassium-based sorbents for CO2 capture. Appl. Energy 2013, 112, 381–387. [Google Scholar] [CrossRef]
- Thee, H.; Smith, K.H.; da Silva, G.; Kentish, S.E.; Stevens, G.W. Carbon dioxide absorption into unpromoted and borate-catalyzed potassium carbonate solutions. Chem. Eng. J. 2011, 181–182, 694–701. [Google Scholar] [CrossRef]
- Hu, G.; Smith, K.H.; Wu, Y.; Kentish, S.E.; Stevens, G.W. Screening Amino Acid Salts as Rate Promoters in Potassium Carbonate Solvent for Carbon Dioxide Absorption. Energy Fuels 2017, 31, 4280–4286. [Google Scholar] [CrossRef]
- Li, Z.; Ji, X.; Yang, Z.; Lu, X. Study of CO2 absorption/desorption behaviors in aqueous (2-hydroxyethyl)-trimethyl-ammonium (S)-2-pyrrolidine-carboxylic acid salt ([Cho][Pro]) + K2CO3 solutions. Int. J. Greenh. Gas Control 2019, 83, 51–60. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.E.; Nam, S.C.; Park, S.Y.; Chun, I.S.; Yoon, Y.I.; Lee, J.-H. Promoter Characteristic Study on the K2CO3 Absorbents for CO2 Capture: Mass Transfer According to Functional Group and Chain Length of Promoter. Energy Procedia 2017, 114, 898–905. [Google Scholar] [CrossRef]
- Valluri, S.; Kawatra, S. Use of frothers to improve the absorption efficiency of dilute sodium carbonate slurry for post combustion CO2 capture. Fuel Process. Technol. 2020, 212, 106620. [Google Scholar] [CrossRef]
- Hornbostel, K.; Nguyen, D.; Bourcier, W.; Knipe, J.; Worthington, M.; McCoy, S.; Stolaroff, J. Packed and fluidized bed absorber modeling for carbon capture with micro-encapsulated sodium carbonate solution. Appl. Energy 2019, 235, 1192–1204. [Google Scholar] [CrossRef]
- Hong, S.; Sim, G.; Moon, S.; Park, Y. Low-Temperature Regeneration of Amines Integrated with Production of Structure-Controlled Calcium Carbonates for Combined CO2 Capture and Utilization. Energy Fuels 2020, 34, 3532–3539. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, H.; Qu, J.; Tang, D.; Zhao, Z.; Xie, H.; Wang, D.; Yin, H. A molten calcium carbonate mediator for the electrochemical conversion and absorption of carbon dioxide. Green Chem. 2020, 22, 7946–7954. [Google Scholar] [CrossRef]
- Aliyu, A.A.; Akram, M.; Hughes, K.J.; Ma, L.; Ingham, D.B.; Pourkashanian, M. Investigation into simulating Selective Exhaust Gas Recirculation and varying Pressurized Hot Water temperature on the performance of the Pilot-scale Advanced CO2 Capture Plant with 40 wt(%) MEA. Int. J. Greenh. Gas Control 2021, 107, 103287. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Liu, H.; Li, W.; Xiao, M.; Gao, H.; Liang, Z. Reduction of energy requirement of CO2 desorption from a rich CO2-loaded MEA solution by using solid acid catalysts. Appl. Energy 2017, 202, 673–684. [Google Scholar] [CrossRef]
- Wang, N.; Peng, Z.; Gao, H.; Sema, T.; Shi, J.; Liang, Z. New insight and evaluation of secondary Amine/N-butanol biphasic solutions for CO2 Capture: Equilibrium Solubility, phase separation Behavior, absorption Rate, desorption Rate, energy consumption and ion species. Chem. Eng. J. 2021, 431, 133912. [Google Scholar] [CrossRef]
- Rozanska, X.; Wimmer, E.; de Meyer, F. Quantitative Kinetic Model of CO2 Absorption in Aqueous Tertiary Amine Solvents. J. Chem. Inf. Model. 2021, 61, 1814–1824. [Google Scholar] [CrossRef]
- Sharif, M.; Zhang, T.; Wu, X.; Yu, Y.; Zhang, Z. Evaluation of CO2 absorption performance by molecular dynamic simulation for mixed secondary and tertiary amines. Int. J. Greenh. Gas Control 2020, 97, 103059. [Google Scholar] [CrossRef]
- Lee, J.I.; Otto, F.D.; Mather, A.E. Solubility of carbon dioxide in aqueous diethanolamine solutions at high pressures. J. Chem. Eng. Data 1972, 17, 465–468. [Google Scholar] [CrossRef]
- Lawson, J.D.; Garst, A.W. Gas sweetening data: Equilibrium solubility of hydrogen sulfide and carbon dioxide in aqueous monoethanolamine and aqueous diethanolamine solutions. J. Chem. Eng. Data 1976, 21, 20–30. [Google Scholar] [CrossRef]
- Isaacs, E.E.; Otto, F.D.; Mather, A.E. Solubility of hydrogen sulfide and carbon dioxide in an aqueous diisopropanolamine solution. J. Chem. Eng. Data 1977, 22, 71–73. [Google Scholar] [CrossRef]
- Martin, J.L.; Otto, F.D.; Mather, A.E. Solubility of hydrogen sulfide and carbon dioxide in a diglycolamine solution. J. Chem. Eng. Data 1978, 23, 163–164. [Google Scholar] [CrossRef]
- Roberts, B.E.; Mather, A.E. Solubility of CO2 and H2S in a hindered amine solution. Chem. Eng. Commun. 1988, 64, 105–111. [Google Scholar] [CrossRef]
- Teng, T.T.; Mather, A.E. Solubility of CO2in an AMP Solution. J. Chem. Eng. Data 1990, 35, 410–411. [Google Scholar] [CrossRef]
- Tontlwachwuthikul, P.; Meisen, A.; Llm, C.J. Solubility of CO2 in 2-Amino-2-methyl-1-propanol Solutions. J. Chem. Eng. Data 1991, 36, 130–133. [Google Scholar] [CrossRef]
- Austgen, D.M.; Rochelle, G.T.; Chen, C.C. Model of Vapor-Liquid Equilibria for Aqueous Acid Gas-Alkanolamine Systems. 2. Representation of H2S and CO2 Solubility in Aqueous MDEA and CO2 Solubility in Aqueous Mixtures of MDEA with MEA or DEA. Ind. Eng. Chem. Res. 1991, 30, 543–555. [Google Scholar] [CrossRef]
- Li, M.H.; Shan, K.P. Densities and Solubilities of Solutions of Carbon Dioxide in Water + Monoethanolamine + N-Methyldiethanolamine. J. Chem. Eng. Data 1992, 37, 288–290. [Google Scholar] [CrossRef]
- Seo, D.-J.; Hong, W.-H. Solubilities of Carbon Dioxide in Aqueous Mixtures of Diethanolamine and 2-Amino-2-methyl-1-Propanol. J. Chem. Eng. Data 1996, 41, 258–260. [Google Scholar] [CrossRef]
- Murrieta-Guevara, F.; Rebolledo-Libreros, M.; Romero-Martínez, A.; Trejo, A. Solubility of CO2 in aqueous mixtures of diethanolamine with methyldiethanolamine and 2-amino-2-methyl-1-propanol. Fluid Phase Equilibria 1998, 150–151, 721–729. [Google Scholar] [CrossRef]
- Lemoine, B.; Li, Y.-G.; Cadours, R.; Bouallou, C.; Richon, D. Partial vapor pressure of CO2 and H2S over aqueous methyldiethanolamine solutions. Fluid Phase Equilibria 2000, 172, 261–277. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of carbon dioxide into aqueous piperazine: Reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 2000, 55, 5531–5543. [Google Scholar] [CrossRef]
- Park, M.K.; Sandall, O.C. Solubility of Carbon Dioxide and Nitrous Oxide in 50 mass Methyldiethanolamine. J. Chem. Eng. Data 2000, 46, 166–168. [Google Scholar] [CrossRef]
- Sidi-Boumedine, R.; Horstmann, S.; Fischer, K.; Provost, E.; Fürst, W.; Gmehling, J. Experimental determination of carbon dioxide solubility data in aqueous alkanolamine solutions. Fluid Phase Equilibria 2004, 218, 85–94. [Google Scholar] [CrossRef]
- Barreau, A.; le Bouhelec, E.B.; Tounsi, K.H.; Mougin, P.; Lecomte, F. Absorption of H2S and CO2in Alkanolamine Aqueous Solution: Experimental Data and Modelling with the Electrolyte-NRTL Model. Oil Gas Sci. Technol. Rev. d’IFP Energies Nouv. 2006, 61, 345–361. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Soriano, A.N.; Caparanga, A.; Li, M.-H. Equilibrium solubility of carbon dioxide in (2-amino-2-methyl-1-propanol+piperazine+water). J. Chem. Thermodyn. 2010, 42, 659–665. [Google Scholar] [CrossRef]
- Chung, P.-Y.; Soriano, A.N.; Leron, R.B.; Li, M.-H. Equilibrium solubility of carbon dioxide in the amine solvent system of (triethanolamine+piperazine+water). J. Chem. Thermodyn. 2010, 42, 802–807. [Google Scholar] [CrossRef]
- Nguyen, T.; Hilliard, M.; Rochelle, G.T. Amine volatility in CO2 capture. Int. J. Greenh. Gas Control 2010, 4, 707–715. [Google Scholar] [CrossRef]
- Xu, Q.; Rochelle, G. Total pressure and CO2 solubility at high temperature in aqueous amines. Energy Procedia 2011, 4, 117–124. [Google Scholar] [CrossRef]
- Dash, S.K.; Samanta, A.N.; Bandyopadhyay, S.S. (Vapour+liquid) equilibria (VLE) of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol: New data and modelling using eNRTL-equation. J. Chem. Thermodyn. 2011, 43, 1278–1285. [Google Scholar] [CrossRef]
- Kumar, G.; Kundu, M. Vapour-liquid equilibrium of CO2 in aqueous solutions of N-methyl-2-ethanolamine. Can. J. Chem. Eng. 2011, 90, 627–630. [Google Scholar] [CrossRef]
- Tong, D.; Trusler, J.M.; Maitland, G.C.; Gibbins, J.; Fennell, P.S. Solubility of carbon dioxide in aqueous solution of monoethanolamine or 2-amino-2-methyl-1-propanol: Experimental measurements and modelling. Int. J. Greenh. Gas Control 2012, 6, 37–47. [Google Scholar] [CrossRef]
- Guo, C.; Chen, S.; Zhang, Y. Solubility of Carbon Dioxide in Aqueous 2-(2-Aminoethylamine)ethanol (AEEA) Solution and Its Mixtures with N-Methyldiethanolamine/2-Amino-2-methyl-1-propanol. J. Chem. Eng. Data 2013, 58, 460–466. [Google Scholar] [CrossRef]
- Haghtalab, A.; Eghbali, H.; Shojaeian, A. Experiment and modeling solubility of CO2 in aqueous solutions of Diisopropanolamine+2-amino-2-methyl-1-propanol+Piperazine at high pressures. J. Chem. Thermodyn. 2014, 71, 71–83. [Google Scholar] [CrossRef]
- Lu, R.; Li, K.; Chen, J.; Yu, H.; Tade, M. CO 2 capture using piperazine-promoted, aqueous ammonia solution: Rate-based modelling and process simulation. Int. J. Greenh. Gas Control 2017, 65, 65–75. [Google Scholar] [CrossRef]
- Ghalib, L.; Ali, B.S.; Ashri, W.M.; Mazari, S.; Saeed, I.M. Modeling the effect of piperazine on CO2 loading in MDEA/PZ mixture. Fluid Phase Equilibria 2017, 434, 233–243. [Google Scholar] [CrossRef]
- Tzirakis, F.; Tsivintzelis, I.; Papadopoulos, A.I.; Seferlis, P. Experimental measurement and assessment of equilibrium behaviour for phase change solvents used in CO2 capture. Chem. Eng. Sci. 2019, 199, 20–27. [Google Scholar] [CrossRef]
- Jahangiri, A.; Hassankiadeh, M.N. Effects of piperazine concentration and operating conditions on the solubility of CO2 in AMP solution at low CO2 partial pressure. Sep. Sci. Technol. 2018, 54, 1067–1078. [Google Scholar] [CrossRef]
- Khodadadi, M.J.; Abbasi, M.; Riahi, S.; Shokrollahzadeh, H. Investigation on kinetics of carbon dioxide absorption in aqueous solutions of monoethanolamine + 1, 3-diaminopropane. Sep. Sci. Technol. 2019, 54, 2800–2808. [Google Scholar] [CrossRef]
- Janati, S.; Aghel, B.; Shadloo, M.S. The effect of alkanolamine mixtures on CO2 absorption efficiency in T-Shaped microchannel. Environ. Technol. Innov. 2021, 24, 102006. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Chen, Y.; Jing, G.; Lv, B.; Zhou, Z.; Zhang, S. Regulatory mechanism of a novel non-aqueous absorbent for CO2 capture using 2-amino-2-methyl-1-propanol: Low viscosity and energy efficient. J. CO2 Util. 2023, 67. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, C.; Tu, R.; Xie, P.; He, Y.; Shi, Y. CO 2 absorption and microwave regeneration with high-concentration TETA nonaqueous absorbents. Greenh. Gases Sci. Technol. 2022, 12, 362–375. [Google Scholar] [CrossRef]
- Tao, M.; Gao, J.; Zhang, W.; Li, Y.; He, Y.; Shi, Y. A Novel Phase-Changing Nonaqueous Solution for CO2 Capture with High Capacity, Thermostability, and Regeneration Efficiency. Ind. Eng. Chem. Res. 2018, 57, 9305–9312. [Google Scholar] [CrossRef]
- Liu, J.; Qian, J.; He, Y. Water-lean triethylenetetramine/N,N-diethylethanolamine/n-propanol biphasic solvents: Phase-separation performance and mechanism for CO2 capture. Sep. Purif. Technol. 2022, 289, 120740. [Google Scholar] [CrossRef]
- Tu, Z.; Han, F.; Liu, C.; Wang, Y.; Wei, J.; Zhou, X. 2-Amino-2-methyl-1-propanol regulated triethylenetetramine-based nonaqueous absorbents for solid-liquid phase-change CO2 capture: Formation of crystalline powder products and mechanism analysis. Sep. Purif. Technol. 2023, 307, 122722. [Google Scholar] [CrossRef]
- Machida, H.; Oba, K.; Tomikawa, T.; Esaki, T.; Yamaguchi, T.; Horizoe, H. Development of phase separation solvent for CO2 capture by aqueous (amine + ether) solution. J. Chem. Thermodyn. 2017, 113, 64–70. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Xu, L. Screening of physical-chemical biphasic solvents for CO2 absorption. Int. J. Greenh. Gas Control 2019, 85, 199–205. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Shen, S. Low-Energy-Consumption CO2 Capture by Liquid–Solid Phase Change Absorption Using Water-Lean Blends of Amino Acid Salts and 2-Alkoxyethanols. ACS Sustain. Chem. Eng. 2020, 8, 12956–12967. [Google Scholar] [CrossRef]
- Lail, M.; Tanthana, J.; Coleman, L. Non-Aqueous Solvent (NAS) CO2 Capture Process. Energy Procedia 2014, 63, 580–594. [Google Scholar] [CrossRef]
- Perry, R.J.; Wood, B.R.; Genovese, S.; O’Brien, M.J.; Westendorf, T.; Meketa, M.L.; Farnum, R.; McDermott, J.; Sultanova, I.; Perry, T.M.; et al. CO2 Capture Using Phase-Changing Sorbents. Energy Fuels 2012, 26, 2528–2538. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Wang, L.; Chen, J.; Lu, Y. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges. Appl. Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Novel water-free biphasic absorbents for efficient CO 2 capture. Int. J. Greenh. Gas Control 2017, 60, 100–109. [Google Scholar] [CrossRef]
- Polesso, B.B.; Duczinski, R.; Bernard, F.L.; Faria, D.J.; dos Santos, L.M.; Einloft, S. New water-based nanocapsules of poly(diallyldimethylammonium tetrafluoroborate)/ionic liquid for CO2 capture. Heliyon 2023, 9, e13298. [Google Scholar] [CrossRef]
- Rasouli, H.; Iliuta, I.; Bougie, F.; Garnier, A.; Iliuta, M.C. Hybrid enzymatic CO2 capture process in intensified flat sheet membrane contactors with immobilized carbonic anhydrase. Sep. Purif. Technol. 2022, 287, 120505. [Google Scholar] [CrossRef]
- Rasouli, H.; Iliuta, I.; Bougie, F.; Garnier, A.; Iliuta, M.C. Enhanced CO2 capture in packed-bed column bioreactors with immobilized carbonic anhydrase. Chem. Eng. J. 2022, 432, 134029. [Google Scholar] [CrossRef]
- Wojtasik-Malinowska, J.; Piątkowski, M.; Blatkiewicz, M.; Jaskulski, M.; Wawrzyniak, P.; Górak, A. Reactive absorption of carbon dioxide in aqueous n-methyldiethanoloamine solutions catalysed with carbonic anhydrase in a rotating packed bed (RPB). Chem. Eng. Process. Process. Intensif. 2023, 184, 109266. [Google Scholar] [CrossRef]
- Leimbrink, M.; Nikoleit, K.G.; Spitzer, R.; Salmon, S.; Bucholz, T.; Górak, A.; Skiborowski, M. Enzymatic reactive absorption of CO2 in MDEA by means of an innovative biocatalyst delivery system. Chem. Eng. J. 2018, 334, 1195–1205. [Google Scholar] [CrossRef]
- Zhang, S.; Du, M.; Shao, P.; Wang, L.; Ye, J.; Chen, J.; Chen, J. Carbonic Anhydrase Enzyme-MOFs Composite with a Superior Catalytic Performance to Promote CO2 Absorption into Tertiary Amine Solution. Environ. Sci. Technol. 2018, 52, 12708–12716. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, C.; Fu, T.; Gao, X.; Ma, Y. CO2 absorption performance of ChCl-MEA deep eutectic solvent in microchannel. J. Environ. Chem. Eng. 2022, 10, 108792. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Leron, R.B.; Li, M.-H. Solubility of carbon dioxide in aqueous mixtures of (reline+monoethanolamine) at T=(313.2 to 353.2)K. J. Chem. Thermodyn. 2014, 72, 94–99. [Google Scholar] [CrossRef]
- Ullah, R.; Atilhan, M.; Anaya, B.; Khraisheh, M.; García, G.; ElKhattat, A.; Tariq, M.; Aparicio, S. A detailed study of cholinium chloride and levulinic acid deep eutectic solvent system for CO2 capture via experimental and molecular simulation approaches. Phys. Chem. Chem. Phys. 2015, 17, 20941–20960. [Google Scholar] [CrossRef]
- Ruan, J.; Ye, X.; Wang, R.; Chen, L.; Deng, L.; Qi, Z. Experimental and theoretical study on efficient CO2 absorption coordinated by molecules and ions of DBN and 1,2,4-triazole formed deep eutectic solvents. Fuel 2023, 334, 126709. [Google Scholar] [CrossRef]
- Dods, M.N.; Weston, S.C.; Long, J.R. Prospects for Simultaneously Capturing Carbon Dioxide and Harvesting Water from Air. Adv. Mater. 2022, 34, 2204277. [Google Scholar] [CrossRef]
- Stuckenberg, D.J.; Kode, V.R.; Went, E.K. ATMOSPHERIC WATER GENERATION SYSTEMS AND METHODS UTILIZING MEMBRANE-BASED WATER EXTRACTION. 2023. Available online: https://www.freepatentsonline.com/y2023/0010090.html (accessed on 1 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).