Biochemical Response of Okra (Abelmoschus esculentus L.) to Selenium (Se) under Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochemical Attributes
2.2. Statistical Analysis
3. Results
3.1. Chlorophyll Content
3.2. Carotenoid Content
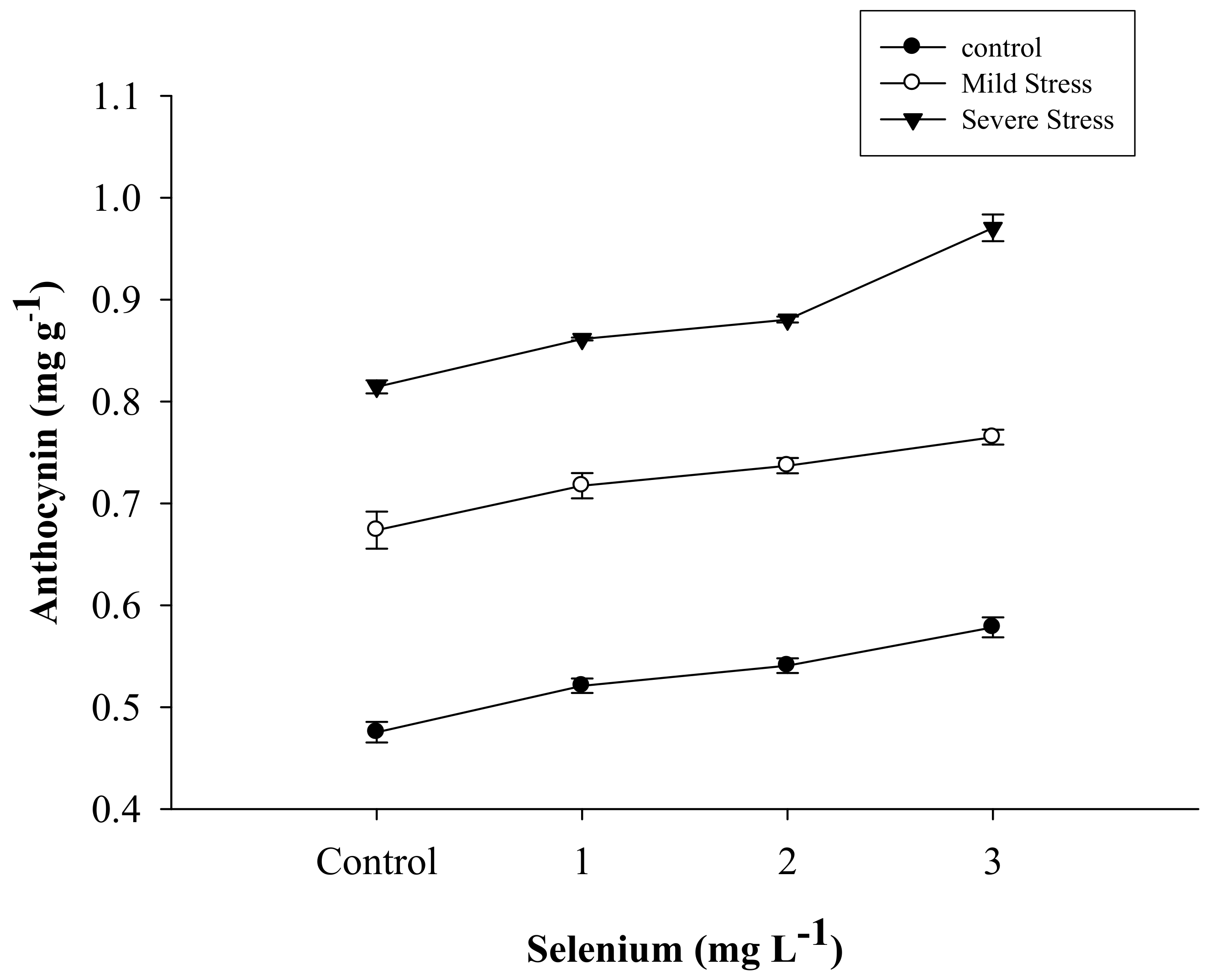
3.3. Anthocyanin Content
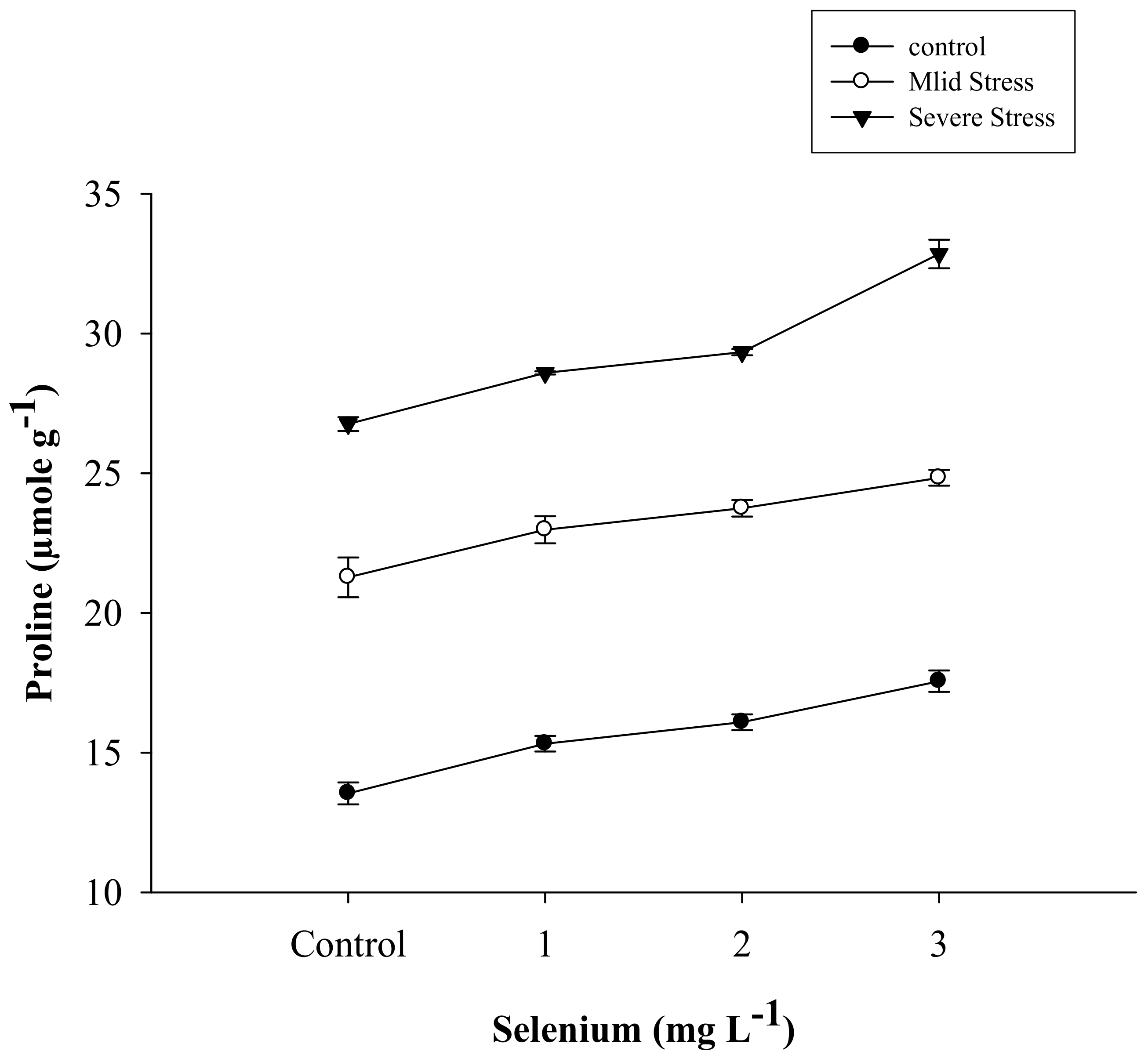
3.4. Proline Content
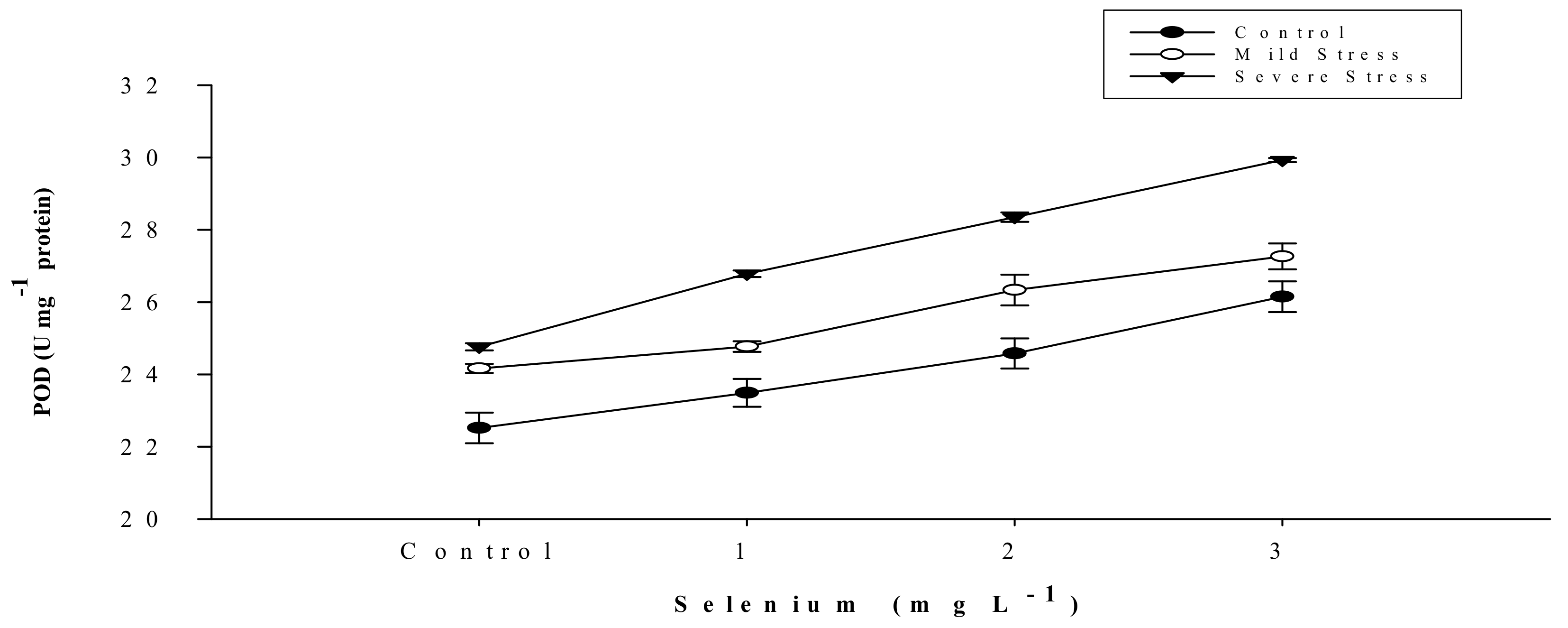
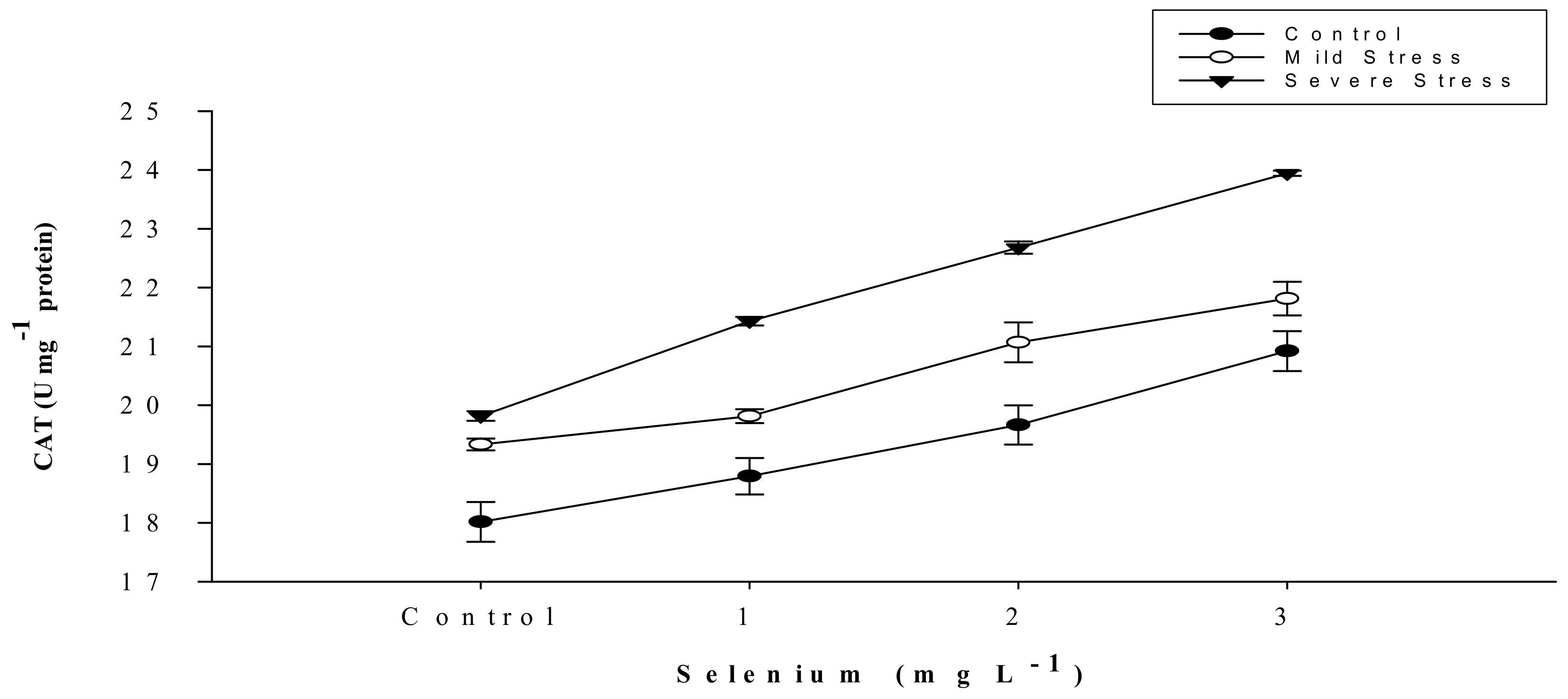
3.5. Antioxidant Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, J.; Moser, S.C. Individual understandings, perceptions, and engagement with climate change: Insights from in-depth studies across the world. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 547–569. [Google Scholar] [CrossRef]
- Amna; Ali, B.; Azeem, M.A.; Qayyum, A.; Mustafa, G.; Ahmad, M.A.; Javed, M.T.; Chaudhary, H.J. Bio-Fabricated Silver Nanoparticles: A Sustainable Approach for Augmentation of Plant Growth and Pathogen Control. In Sustainable Agriculture Reviews 53; Springer: Berlin/Heidelberg, Germany, 2021; pp. 345–371. [Google Scholar]
- Bibi, S.; Ullah, S.; Hafeez, A.; Khan, M.N.; Javed, M.A.; Ali, B.; Din, I.U.; Bangash, S.A.K.; Wahab, S.; Wahid, N. Exogenous Ca/Mg quotient reduces the inhibitory effects of PEG induced osmotic stress on Avena sativa L. Braz. J. Biol. 2022, 84, e264642. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Khan, M.S.; Hafeez, A.; Fazil, M.; Khan, M.N.; Ali, B.; Javed, M.A.; Imran, M.; Shati, A.A.; Alfaifi, M.Y. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted industrial soils. Braz. J. Biol. 2022, 84, e264473. [Google Scholar] [CrossRef]
- AL-Huqail, A.A.; Saleem, M.H.; Ali, B.; Azeem, M.; Mumtaz, S.; Yasin, G.; Marc, R.A.; Ali, S. Efficacy of priming wheat (Triticum aestivum) seeds with a benzothiazine derivative to improve drought stress tolerance. Funct. Plant Biol. 2023. [Google Scholar] [CrossRef]
- Dola, D.B.; Mannan, M.A.; Sarker, U.; Mamun, M.A.A.; Islam, T.; Ercisli, S.; Saleem, M.H.; Ali, B.; Pop, O.L.; Marc, R.A. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022, 13, 992535. [Google Scholar] [CrossRef]
- Asma; Hussain, I.; Ashraf, M.Y.; Saleem, M.H.; Ashraf, M.A.; Ali, B.; Shereen, A.; Farid, G.; Ali, M.; Shirazi, M.U.; et al. Alleviating effects of salicylic acid spray on stage-based growth and antioxidative defense system in two drought-stressed rice (Oryza sativa L.) cultivars. Turk. J. Agric. For. 2023, 47, 79–99. [Google Scholar] [CrossRef]
- Yasmeen, S.; Wahab, A.; Saleem, M.H.; Ali, B.; Qureshi, K.A.; Jaremko, M. Melatonin as a Foliar Application and Adaptation in Lentil (Lens culinaris Medik.) Crops under Drought Stress. Sustainability 2022, 14, 16345. [Google Scholar] [CrossRef]
- Farooq, T.H.; Rafy, M.; Basit, H.; Shakoor, A.; Shabbir, R.; Riaz, M.U.; Ali, B.; Kumar, U.; Qureshi, K.A.; Jaremko, M. Morpho-Physiological Growth Performance and Phytoremediation Capabilities of Selected Xerophyte Grass Species Towards Cr and Pb Stress. Front. Plant Sci. 2022, 13, 997120. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2009; pp. 153–188. [Google Scholar]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Afridi, M.S.; Dawoud, T.M.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; Alkhalifah, D.H.M. Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Javed, M.A.; Afridi, M.S.; Abbasi, H.A.; Qayyum, A.; Batool, T.; Ullah, A.; Marc, R.A.; Al Jaouni, S.K.; et al. Role of endophytic bacteria in salinity stress amelioration by physiological and molecular mechanisms of defense: A comprehensive review. S. Afr. J. Bot. 2022, 151, 33–46. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A. Bacillus mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 2022, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, X.; Saleem, M.H.; Hafeez, A.; Afridi, M.S.; Khan, S.; Ullah, I.; do Amaral Júnior, A.T.; Alatawi, A.; Ali, S.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Azeem, M.; Pirjan, K.; Qasim, M.; Mahmood, A.; Javed, T.; Muhammad, H.; Yang, S.; Dong, R.; Ali, B.; Rahimi, M. Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Sci. Rep. 2023, 13, 2895. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.S.; Ali, S.; Salam, A.; César Terra, W.; Hafeez, A.; Ali, B.; AlTami, M.S.; Ameen, F.; Ercisli, S.; Marc, R.A. Plant Microbiome Engineering: Hopes or Hypes. Biology 2022, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.S.; Javed, M.A.; Ali, S.; De Medeiros, F.H.V.; Ali, B.; Salam, A.; Sumaira; Marc, R.A.; Alkhalifah, D.H.M.; Selim, S. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 899464. [Google Scholar] [CrossRef]
- Salam, A.; Afridi, M.S.; Javed, M.A.; Saleem, A.; Hafeez, A.; Khan, A.R.; Zeeshan, M.; Ali, B.; Azhar, W.; Sumaira. Nano-Priming against Abiotic Stress: A Way Forward towards Sustainable Agriculture. Sustainability 2022, 14, 14880. [Google Scholar] [CrossRef]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-Capped Nanoapatites Amplify Osmotic Stress Tolerance in Zea mays L. by Conserving Photosynthetic Pigments, Osmolytes Biosynthesis and Antioxidant Biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, S.; Khan, M.N.; Khan, W.M.; Razak, S.A.; Wahab, S.; Hafeez, A.; Khan Bangash, S.A.; Poczai, P. The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability 2022, 14, 12924. [Google Scholar] [CrossRef]
- Saleem, A.; Zulfiqar, A.; Ali, B.; Naseeb, M.A.; Almasaudi, A.S.; Harakeh, S. Iron Sulfate (FeSO4) Improved Physiological Attributes and Antioxidant Capacity by Reducing Oxidative Stress of Oryza sativa L. Cultivars in Alkaline Soil. Sustainability 2022, 14, 16845. [Google Scholar] [CrossRef]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-Asbestos-Induced Damage in Panicum virgatum and Phleum pretense Species and Its Alleviation by Organic-Soil Amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Ma, J.; Ali, S.; Saleem, M.H.; Mumtaz, S.; Yasin, G.; Ali, B.; Al-Ghamdi, A.A.; Elshikh, M.S.; Vodnar, D.C.; Marc, R.A. Short-term responses of Spinach (Spinacia oleracea L.) to the individual and combinatorial effects of Nitrogen, Phosphorus and Potassium and silicon in the soil contaminated by boron. Front. Plant Sci. 2022, 13, 983156. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Saleem, M.H.; Yasin, G.; Mumtaz, S.; Qureshi, F.F.; Ali, B.; Ercisli, S.; Alhag, S.K.; Ahmed, A.E.; Vodnar, D.C. Individual and combinatorial effects of SNP and NaHS on morpho-physio-biochemical attributes and phytoextraction of chromium through Cr-stressed spinach (Spinacia oleracea L.). Front. Plant Sci. 2022, 13, 973740. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Ali, B.; Rasheed, R.; Ashraf, M.A.; Aziz, H.; Ercisli, S.; Riaz, S.; Elsharkawy, M.M.; Hussain, I. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 2022, 13, 950120. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Elkhlifi, Z.; Iftikhar, J.; Sarraf, M.; Ali, B.; Saleem, M.H.; Ibranshahib, I.; Bispo, M.D.; Meili, L.; Ercisli, S.; Torun Kayabasi, E.; et al. Potential Role of Biochar on Capturing Soil Nutrients, Carbon Sequestration and Managing Environmental Challenges: A Review. Sustainability 2023, 15, 2527. [Google Scholar] [CrossRef]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Zafar, M.; Ahmed, S.; Munir, M.K.; Zafar, N.; Saqib, M.; Sarwar, M.A.; Iqbal, S.; Ali, B.; Akhtar, N.; Ali, B.; et al. Application of Zinc, Iron and Boron enhances productivity and grain biofortification of Mungbean. Phyton-Int. J. Exp. Bot. 2023, 92, 983–999. [Google Scholar] [CrossRef]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Nawaz, H.; Ali, A.; Saleem, M.H.; Ameer, A.; Hafeez, A.; Alharbi, K.; Ezzat, A.; Khan, A.; Jamil, M.; Farid, G. Comparative effectiveness of EDTA and citric acid assisted phytoremediation of Ni contaminated soil by using canola (Brassica napus). Braz. J. Biol. 2022, 82, e261785. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.-L.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated deficit irrigation for crop production under drought stress. A review. Agron. Sustain. Dev. 2016, 36, 3. [Google Scholar] [CrossRef]
- Mehmood, S.; Khatoon, Z.; Amna; Ahmad, I.; Muneer, M.A.; Kamran, M.A.; Ali, J.; Ali, B.; Chaudhary, H.J.; Munis, M.F.H. Bacillus sp. PM31 harboring various plant growth-promoting activities regulates Fusarium dry rot and wilt tolerance in potato. Arch. Agron. Soil Sci. 2021, 69, 197–211. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Munis, M.F.H.; Hashem, M. Pgpr-mediated plant growth attributes and metal extraction ability of sesbania sesban L. In industrially contaminated soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Hajiboland, R. Effect of Micronutrient Deficiencies on Plants Stress Responses. In Abiotic Stress Responses in Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 283–329. [Google Scholar]
- Yasin, M.; Anwar, F.; Noorani, H.; Muhammad, S.; Mahmood, A.; Javed, T.; Ali, B.; Alharbi, K.; Saleh, I.A.; Abu-Harirah, H.A. Evaluating Non-Composted Red Cotton Tree (Bombax ceiba) Sawdust Mixtures for Raising Okra (Abelmoschus esculentus (L.) Moench) in Pots. Agronomy 2022, 13, 97. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; Wang, G. Effects of selenium on wheat seedlings under drought stress. Biol. Trace Elem. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef]
- Yang, C.-M.; Chang, K.-W.; Yin, M.-H.; Huang, H.-M. Methods for the determination of the chlorophylls and their derivatives. Taiwania 1998, 43, 116–122. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Asada, K.; Takahashi, M. Production and Scavenging of Active Oxygen in Photosynthesis. In Photoinhibition; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–287. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Biochem. Anal 1955, 1, 357–424. [Google Scholar]
- Zhang, D.; Quantick, P.C.; Grigor, J.M. Changes in phenolic compounds in Litchi (Litchi chinensis Sonn.) fruit during postharvest storage. Postharvest Biol. Technol. 2000, 19, 165–172. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, A Biometrical Approach; McGraw-Hill Kogakusha, Ltd.: Tokyo, Japan, 1980; ISBN 0070609268. [Google Scholar]
- Tahir, O.; Bangash, S.A.K.; Ibrahim, M.; Shahab, S.; Khattak, S.H.; Ud Din, I.; Khan, M.N.; Hafeez, A.; Wahab, S.; Ali, B.; et al. Evaluation of Agronomic Performance and Genetic Diversity Analysis Using Simple Sequence Repeats Markers in Selected Wheat Lines. Sustainability 2023, 15, 293. [Google Scholar] [CrossRef]
- Saini, A.; Manuja, S.; Kumar, S.; Hafeez, A.; Ali, B.; Poczai, P. Impact of Cultivation Practices and Varieties on Productivity, Profitability, and Nutrient Uptake of Rice (Oryza sativa L.) and Wheat (Triticum aestivum L.) Cropping System in India. Agriculture 2022, 12, 1678. [Google Scholar] [CrossRef]
- Antwi-Agyei, P.; Fraser, E.D.G.; Dougill, A.J.; Stringer, L.C.; Simelton, E. Mapping the vulnerability of crop production to drought in Ghana using rainfall, yield and socioeconomic data. Appl. Geogr. 2012, 32, 324–334. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Akram, N.A.; Saleem, M.H.; Shafiq, S.; Naz, H.; Farid-ul-Haq, M.; Ali, B.; Shafiq, F.; Iqbal, M.; Jaremko, M.; Qureshi, K.A. Phytoextracts as Crop Biostimulants and Natural Protective Agents—A Critical Review. Sustainability 2022, 14, 14498. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef]
- Jagtap, V.; Bhargava, S.; Streb, P.; Feierabend, J. Comparative effect of water, heat and light stresses on photosynthetic reactions in Sorghum bicolor (L.) Moench. J. Exp. Bot. 1998, 49, 1715–1721. [Google Scholar]
- Jan, M.; Haq, M.A.; Javed, T.; Hussain, S.; Ahmad, I.; Sumrah, M.A.; Iqbal, J.; Babar, B.H.; Hafeez, A.; Aslam, M.; et al. Response of contrasting rice genotypes to zinc sources under saline conditions. Phyton-Int. J. Exp. Bot. 2023, 92, 1361–1375. [Google Scholar] [CrossRef]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, A.; Ullah, S.; Noor, J.; Ali, B.; Khan, M.N.; Hashem, M.; Mostafa, Y.S.; Alamri, S. Validating the Impact of Water Potential and Temperature on Seed Germination of Wheat (Triticum aestivum L.) via Hydrothermal Time Model. Life 2022, 12, 983. [Google Scholar] [CrossRef]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Gong, X.; Zeng, G.; Zheng, B.; Wang, D.; Sun, Z.; Zhou, L.; Zeng, X. Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 9999–10008. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Qadir, G.; Ansar, M.; Wattoo, F.M.; Javed, T.; Ali, B.; Marc, R.A.; Rahimi, M. Shattering and yield expression of sesame (Sesamum indicum L) genotypes influenced by paclobutrazol concentration under rainfed conditions of Pothwar. BMC Plant Biol. 2023, 23, 137. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, Y.; Wang, S.H.; Yin, L.P.; Xu, G.J.; Zheng, C.; Lei, C.; Zhang, M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lycium chinense leaves. J. Sci. Food Agric. 2013, 93, 310–315. [Google Scholar] [CrossRef]
- Hajiboland, R.; Sadeghzadeh, N.; Ebrahimi, N.; Sadeghzadeh, B.; Mohammadi, S.A. Influence of selenium in drought-stressed wheat plants under greenhouse and field conditions. Acta Agric. Slov. 2015, 105, 175–191. [Google Scholar] [CrossRef]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of selenium biofortification and beneficial microorganism inoculation on yield, quality and antioxidant properties of shallot bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.R.; da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; de Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; Dos Reis, A.R. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Iqbal, Z.; Anjum, M.A.; Garcia-Sanchez, F.; Mattson, N.S. The role of selenium in amelioration of heat-induced oxidative damage in cucumber under high temperature stress. Acta Physiol. Plant. 2016, 38, 158. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R.; Jamaati-e-Somarin, S.; Zabihi-e-Mahmoodabad, R. The leaf chlorophyll content and stress resistance relationship considering in corn cultivars (Zea mays). Adv. Environ. Biol 2011, 5, 118–122. [Google Scholar]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Selenium—An antioxidative protectant in soybean during senescence. Plant Soil 2005, 272, 77–86. [Google Scholar] [CrossRef]
- Chu, J.; Yao, X.; Zhang, Z. Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol. Trace Elem. Res. 2010, 136, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.M. Effects of low temperature and selenium application on growth and the physiological changes in sorghum seedlings. J. Stress Physiol. Biochem. 2012, 8, 268–286. [Google Scholar]
- Padmaja, K.; Somasekharaiah, B.V.; Prasad, A.R.K. Inhibition of chlorophyll synthesis by selenium: Involvement of lipoxygenase mediated lipid peroxidation and antioxidant enzymes. Photosynthetica 1995, 31, 1–7. [Google Scholar]
- Terry, N.; Zayed, A.M.; de Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol. Trace Elem. Res. 2009, 132, 259–269. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, Q. Antioxidant capacity of flavonoid in soybean seedlings under the joint actions of rare earth element La (III) and ultraviolet-B stress. Biol. Trace Elem. Res. 2009, 127, 69–80. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. The effects of selenium on some physiological traits and K, Na concentration of garlic (Allium sativum L.) under NaCl stress. Inf. Process. Agric. 2018, 5, 156–161. [Google Scholar] [CrossRef]
- Spyropoulos, C.G.; Mavrommatis, M. Effect of water stress on pigment formation in Quercus species. J. Exp. Bot. 1978, 29, 473–477. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Giannakoula, A.; Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P.; Moustakas, M. Anthocyanin accumulation in poinsettia leaves and its functional role in photo-oxidative stress. Environ. Exp. Bot. 2020, 175, 104065. [Google Scholar] [CrossRef]
- Hughes, N.M.; Smith, W.K. Attenuation of incident light in Galax urceolata (Diapensiaceae): Concerted influence of adaxial and abaxial anthocyanic layers on photoprotection. Am. J. Bot. 2007, 94, 784–790. [Google Scholar] [CrossRef]
- Wahid, A.; Ghazanfar, A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 2006, 163, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Pukacka, S.; Ratajczak, E.; Kalemba, E. The protective role of selenium in recalcitrant Acer saccharium L. seeds subjected to desiccation. J. Plant Physiol. 2011, 168, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Daiponmak, W.; Theerakulpisut, P.; Thanonkao, P.; Vanavichit, A.; Prathepha, P. Changes of anthocyanin cyanidin-3-glucoside content and antioxidant activity in Thai rice varieties under salinity stress. Sci. Asia 2010, 36, 286–291. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A.; Mora, M.L. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 156, 297–307. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Q. Effect of foliar application of selenium on the antioxidant activity of aqueous and ethanolic extracts of selenium-enriched rice. J. Agric. Food Chem. 2004, 52, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; McGrath, S.P.; Meharg, A.A.; Zhao, F.J. Growing rice aerobically markedly decreases arsenic accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Bijo, A.J.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Selenium and spermine alleviate cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidants and DNA methylation. Plant Physiol. Biochem. 2012, 51, 129–138. [Google Scholar] [CrossRef] [PubMed]
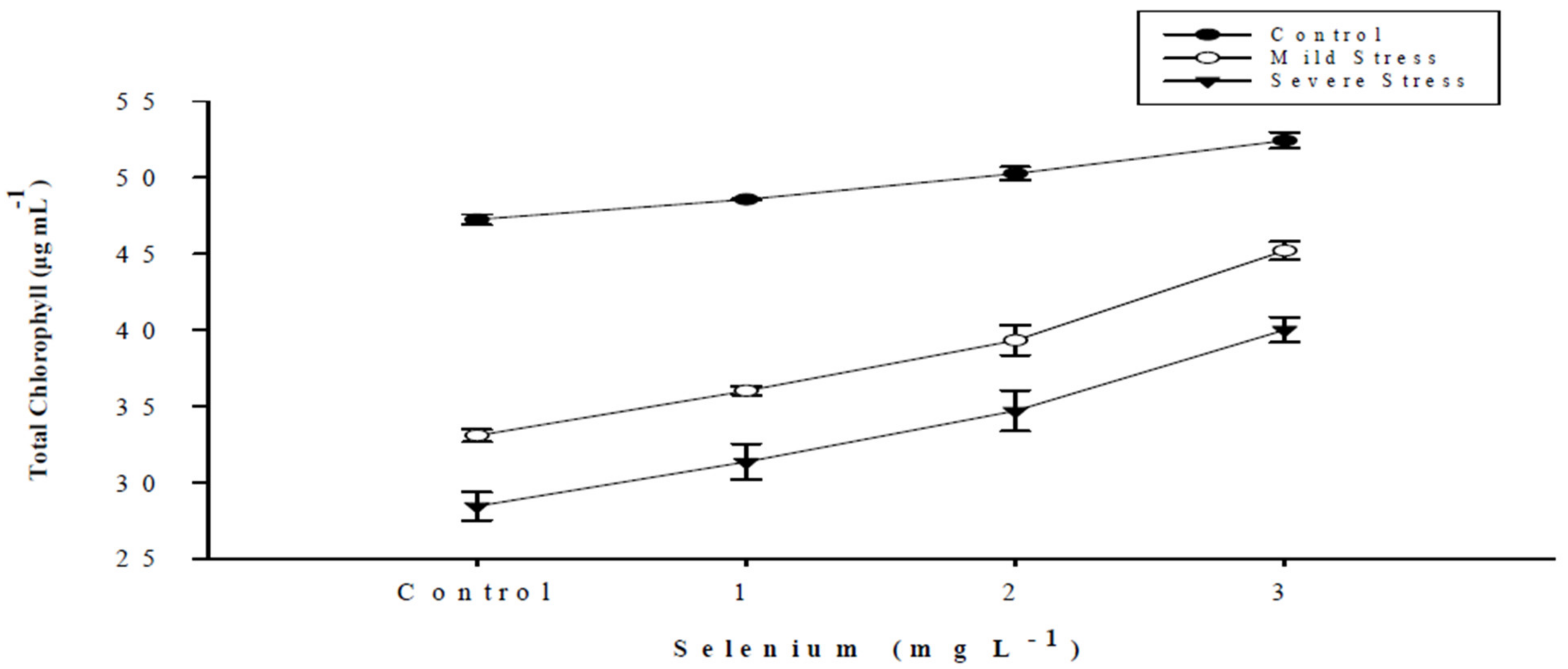
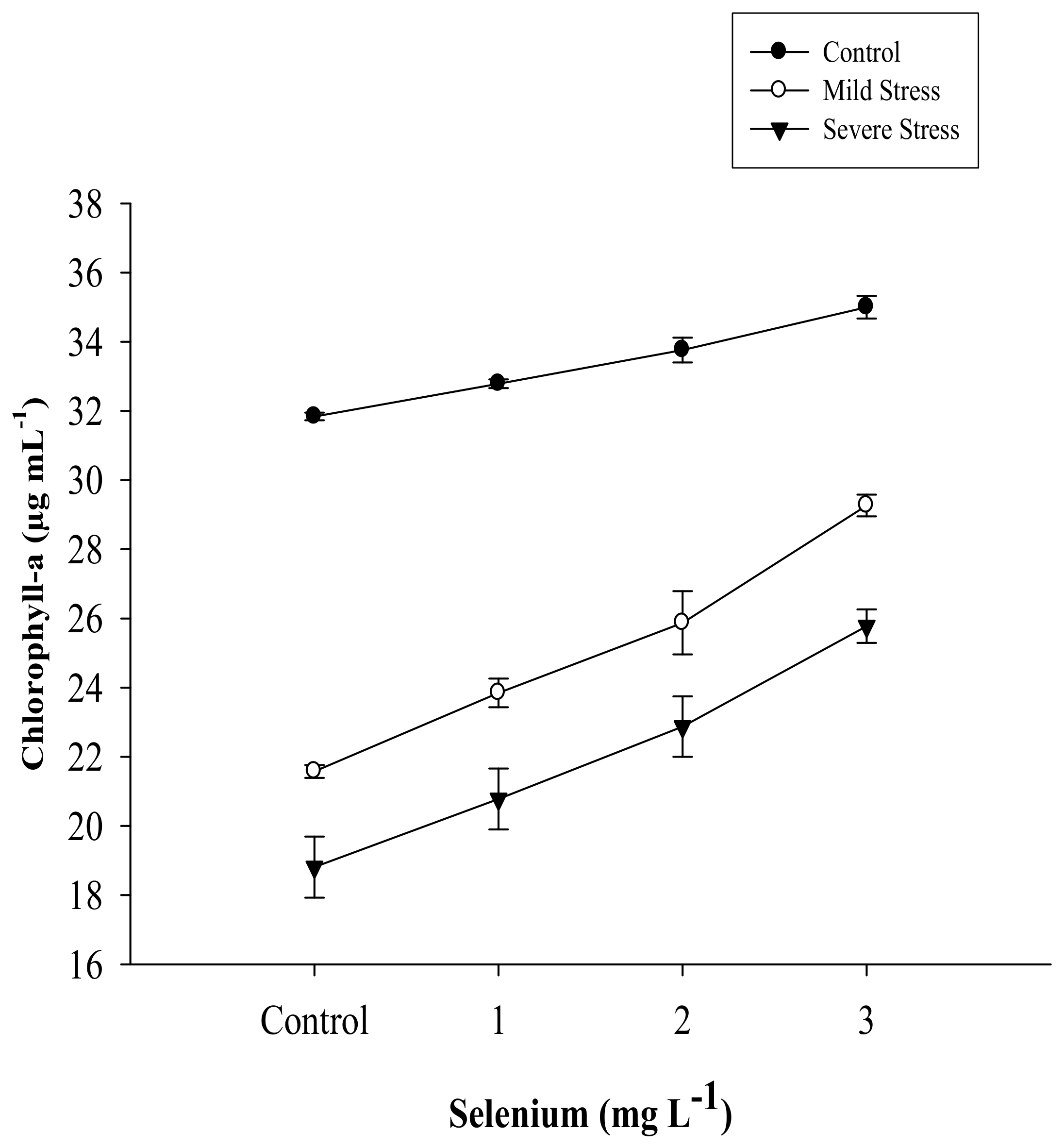
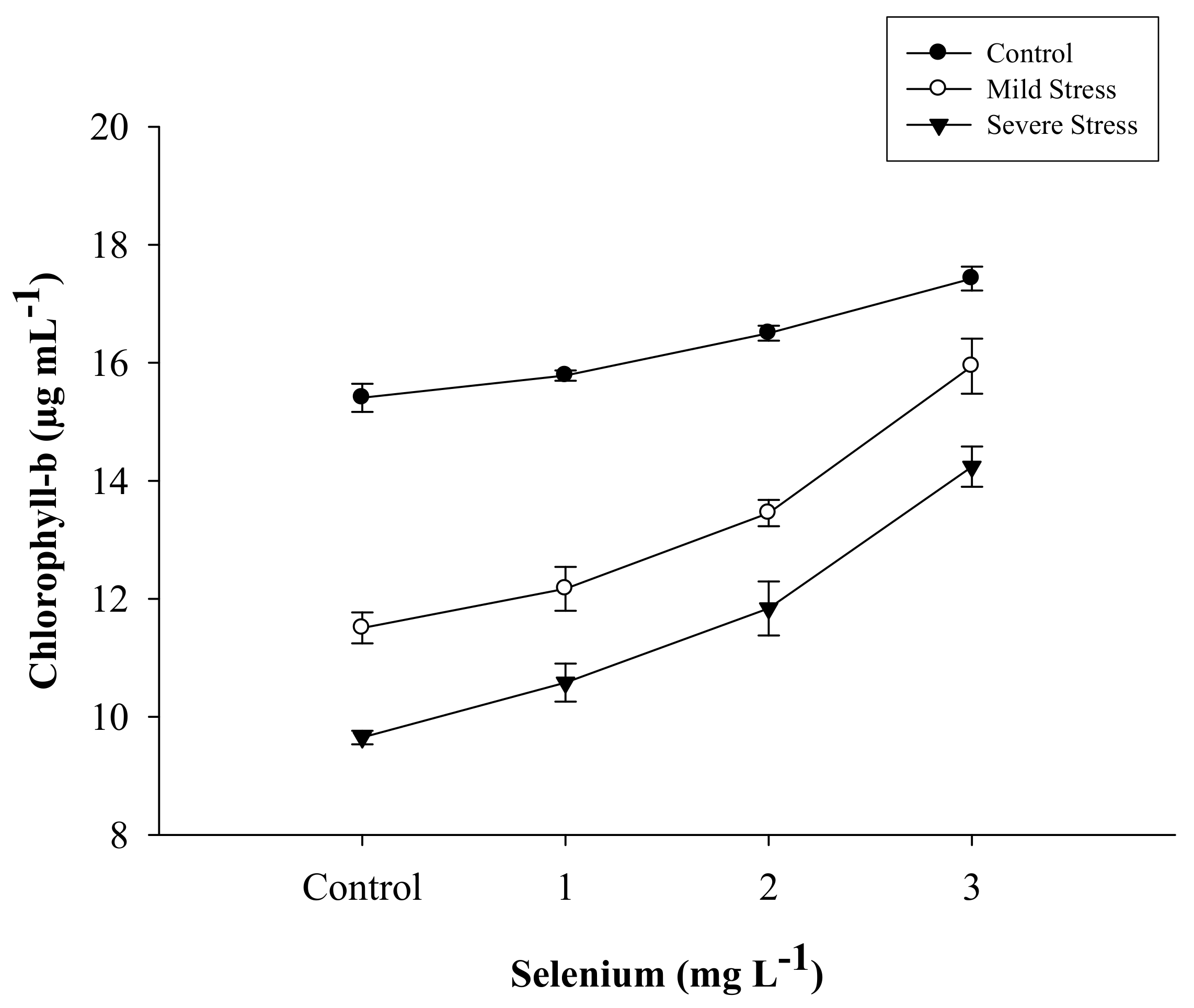
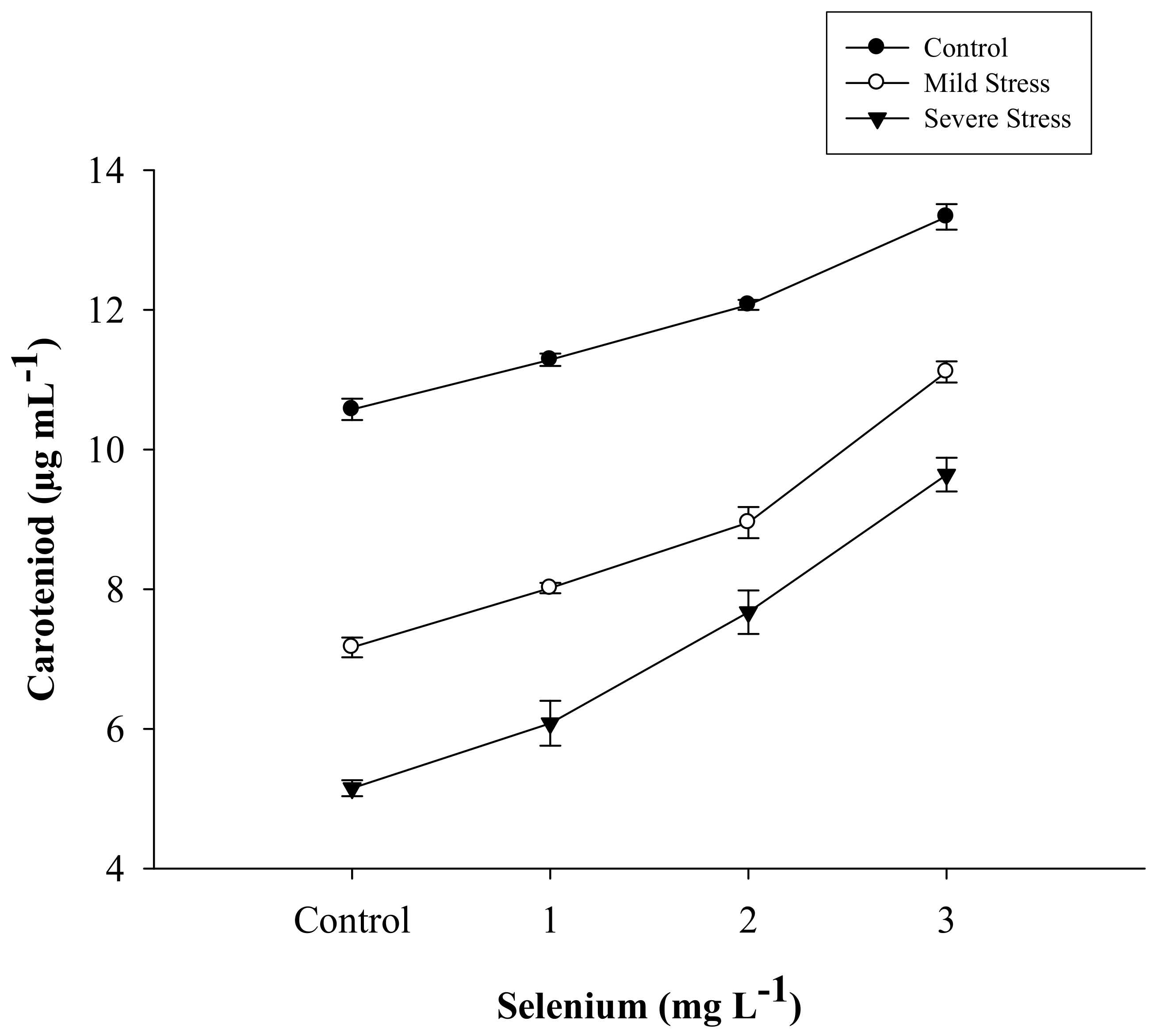

| Selenium (Se) (mg L−1) | Total Chlorophyll (µg mL−1) | Chlorophyll a (µg mL−1) | Chlorophyll b (µg mL−1) | Carotenoid (µg mL−1) |
|---|---|---|---|---|
| Control | 36.263 d | 24.076 d | 12.188 d | 7.630 d |
| 1 | 38.648 c | 25.803 c | 12.844 c | 8.460 c |
| 2 | 41.432 b | 27.502 b | 13.930 b | 9.564 b |
| 3 | 45.883 a | 30.013 a | 15.870 a | 11.360 a |
| LSD | 1.2702 * | 0.9678 * | 0.4950 * | 0.3224 * |
| Drought Stress (DS) | ||||
| Control | 49.624 a | 33.345 a | 16.279 a | 11.814 a |
| Mild stress | 38.409 b | 25.141 b | 13.268 b | 8.812 b |
| Severe stress | 33.637 c | 22.060 c | 11.577 c | 7.135 c |
| LSD | 1.1000 * | 8.8381 * | 0.4290 * | 0.2798 * |
| SE × DS | Figure 1 | Figure 2 | Figure 3 | Figure 4 |
| Selenium (Se) (mg L−1) | Anthocyanin (mg g–1) | Proline (µmole g−1) | APX | POD | CAT |
|---|---|---|---|---|---|
| µg per mg of Protein | |||||
| Control | 0.6544 d | 20.523 d | 15.879 d | 22.586 d | 15.755 d |
| 1 | 0.6999 c | 22.296 c | 16.677 c | 23.785 c | 16.711 c |
| 2 | 0.7193 b | 23.054 b | 17.614 b | 25.192 b | 17.837 b |
| 3 | 0.7712 a | 25.078 a | 18.521 a | 26.552 a | 18.925 a |
| LSD | 0.0163 * | 0.6361 * | 0.3342 * | 0.5023 * | 0.4018 * |
| Drought Stress (DS) | |||||
| Control | 0.5288 c | 15.624 c | 16.123 c | 22.955 c | 16.048 c |
| Mild stress | 0.7193 b | 23.207 b | 17.089 b | 24.404 b | 17.206 b |
| Severe stress | 0.7712 a | 29.382 a | 18.306 a | 26.227 a | 18.667 a |
| LSD | 0.0141 * | 0.5500 * | 0.2894 * | 0.4350 * | 0.3480 * |
| SE × DS | Figure 5 | Figure 6 | Figure 7 | Figure 8 | Figure 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, J.; Jan, I.; Ullah, H.; Fahad, S.; Saud, S.; Adnan, M.; Ali, B.; Liu, K.; Harrison, M.T.; Hassan, S.; et al. Biochemical Response of Okra (Abelmoschus esculentus L.) to Selenium (Se) under Drought Stress. Sustainability 2023, 15, 5694. https://doi.org/10.3390/su15075694
Ali J, Jan I, Ullah H, Fahad S, Saud S, Adnan M, Ali B, Liu K, Harrison MT, Hassan S, et al. Biochemical Response of Okra (Abelmoschus esculentus L.) to Selenium (Se) under Drought Stress. Sustainability. 2023; 15(7):5694. https://doi.org/10.3390/su15075694
Chicago/Turabian StyleAli, Jawad, Ibadullah Jan, Hidayat Ullah, Shah Fahad, Shah Saud, Muhammad Adnan, Baber Ali, Ke Liu, Matthew Tom Harrison, Shah Hassan, and et al. 2023. "Biochemical Response of Okra (Abelmoschus esculentus L.) to Selenium (Se) under Drought Stress" Sustainability 15, no. 7: 5694. https://doi.org/10.3390/su15075694
APA StyleAli, J., Jan, I., Ullah, H., Fahad, S., Saud, S., Adnan, M., Ali, B., Liu, K., Harrison, M. T., Hassan, S., Kumar, S., Khan, M. A., Kamran, M., Alwahibi, M. S., & S. Elshikh, M. (2023). Biochemical Response of Okra (Abelmoschus esculentus L.) to Selenium (Se) under Drought Stress. Sustainability, 15(7), 5694. https://doi.org/10.3390/su15075694














