The Impact of Environmental Stress on the Secondary Metabolites and the Chemical Compositions of the Essential Oils from Some Medicinal Plants Used as Food Supplements
Abstract
1. Introduction
2. Materials and Methods
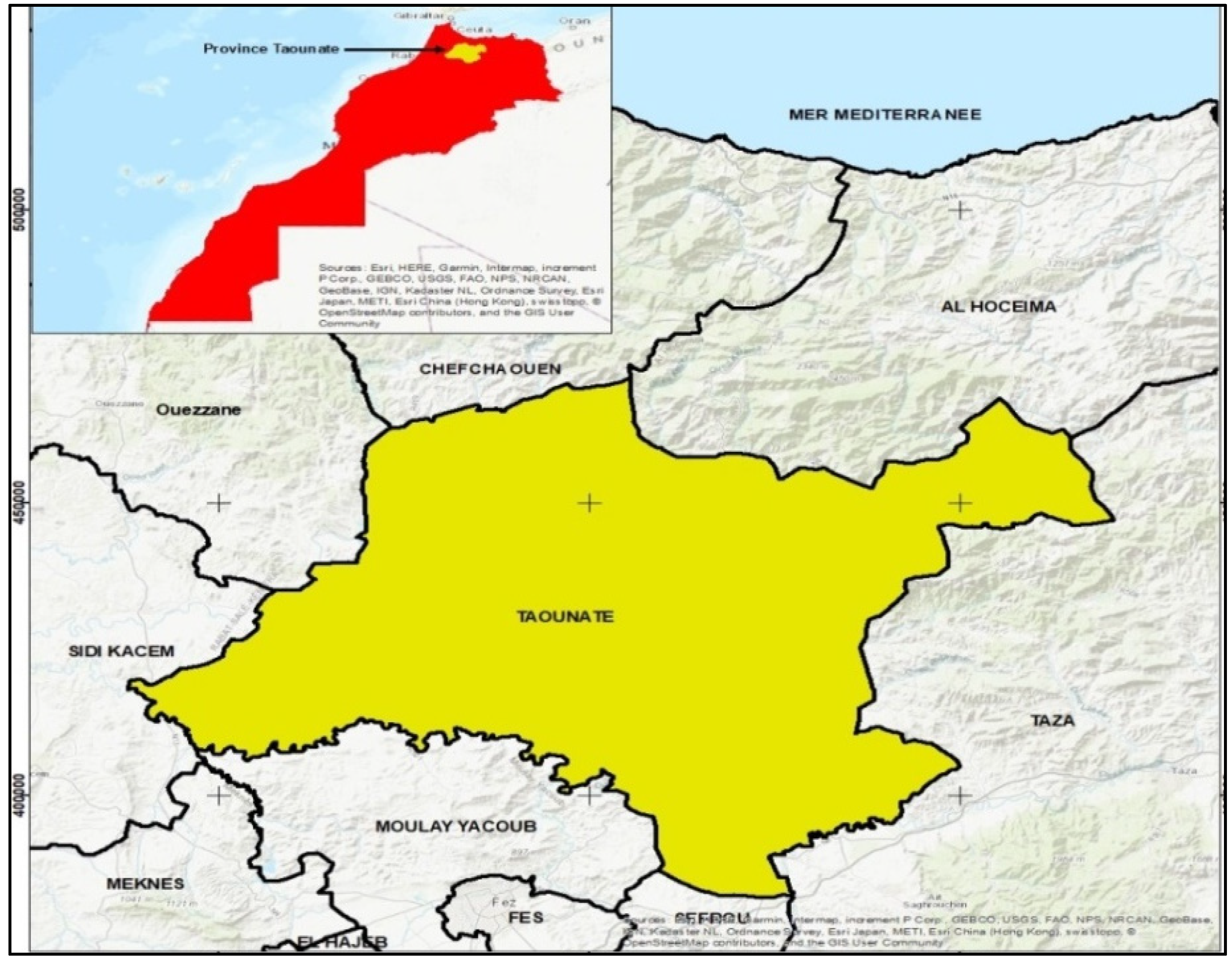
2.1. Study Area
2.2. Methodology
2.2.1. Origin of Plant Material
2.2.2. Transplantation of Sample
- Climatic conditions of transplantation
2.2.3. Quantitative Analyses
2.2.4. Essential Oil
3. Results and Discussion
3.1. Secondary Metabolites
3.2. Essential Oil
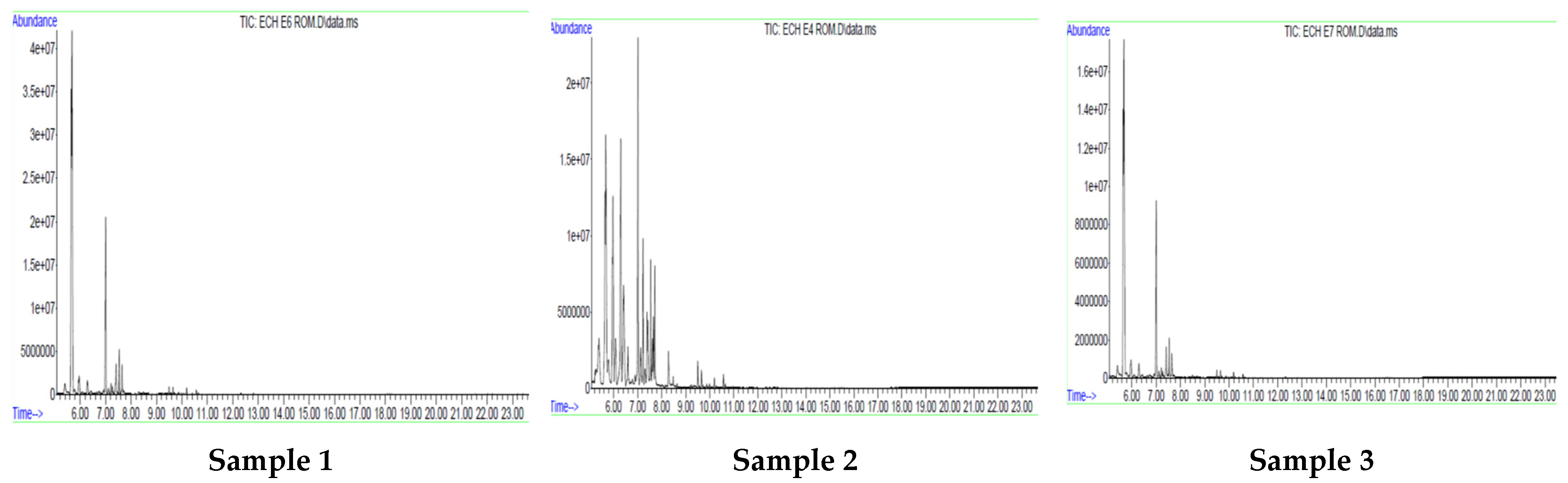
3.3. Gas Chromatography (GC)
- Mentha pulegium
- Rosmarinus officinalis:
- Thymus vulgaris:
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yasser, K.; Abdallah, M.; Abdelmadjid, B. Étude ethnobotanique de quelques plantes médicinales dans une région hyper aride du Sud-ouest Algérien «Cas du Touat dans la wilaya d’Adrar». J. Anim. Plant Sci. 2018, 36, 5844–5857. [Google Scholar]
- Rodrigues, A.M.; Jorge, T.; Osorio, S.; Pott, D.M.; Lidon, F.C.; DaMatta, F.M.; Marques, I.; Ribeiro-Barros, A.I.; Ramalho, J.C.; António, C. Primary Metabolite Profile Changes in Coffea Spp. Promoted by Single and Combined Exposure to Drought and Elevated CO2 Concentration. Metabolites 2021, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe Drought Stress Is Affecting Selected Primary Metabolites, Polyphenols, and Volatile Metabolites in Grapevine Leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Rahman, S.; Iqbal, M.; Husen, A. Medicinal Plants and Abiotic Stress: An Overview. In Medicinal Plants; Husen, A., Iqbal, M., Eds.; Springer Nature: Singapore, 2023; pp. 1–34. ISBN 978-981-19561-0-2. [Google Scholar]
- Talebi, S.M. Changes in Plant Secondary Metabolite Profiles in Response to Environmental Stresses. In Plant Stress Mitigators; Elsevier: Amsterdam, The Netherlands, 2023; pp. 325–339. ISBN 978-0-323-89871-3. [Google Scholar]
- Liu, X.; Li, Y.; Micallef, S.A. Natural Variation and Drought-Induced Differences in Metabolite Profiles of Red Oak-Leaf and Romaine Lettuce Play a Role in Modulating the Interaction with Salmonella Enterica. Int. J. Food Microbiol. 2023, 385, 109998. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant Responses to Climate Change: Metabolic Changes under Combined Abiotic Stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Mareri, L.; Parrotta, L.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef]
- Balfagón, D.; Rambla, J.L.; Granell, A.; Arbona, V.; Gómez-Cadenas, A. Grafting Improves Tolerance to Combined Drought and Heat Stresses by Modifying Metabolism in Citrus Scion. Environ. Exp. Bot. 2022, 195, 104793. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A. Drought-Stress Induced Physiological and Molecular Changes in Plants. Int. J. Mol. Sci. 2022, 23, 4698. [Google Scholar] [CrossRef]
- Amine, D.; Lamiae, B.; Mohamed, B.; Jamal, P.I.; Laila, P.N. Etude Ethnobotanique au Moyen Atlas Central. Eur. Sci. J. 2015, 11, 226–242. [Google Scholar]
- Bouterfas, K.; Mehdadi, Z.; Latreche, A.; Hazem, Z. Quantification de quelques polyphénols de Marrubium vulgare L. du mont de Tessala (Algérie occidentale) pendant les deux périodes de végétation et de floraison. Les Technol. Lab. 2013, 8, 34–41. [Google Scholar]
- Togola, I.; Konaré, M.; Diakité, M.; Diarra, N.; Tounkara, F.; Sanogo, R.; Dembélé, D. Evaluation of total alkaloid content at different developmental stages of Datura innoxia Mill., a plant used in traditional medicine in Mali. Am. J. Innov. Res. Appl. SCiences. French. 2019, 9, 200–207. [Google Scholar]
- Soulama, S.; Nacoulma, O.; Nagtiero Meda, R.; Boussim, J.; Millogo-Rasolodimby, J. Teneurs En Coumarines de 15 Ligneux Fourragers Du Burkina Faso. Int. J. Biol. Chem. Sci. 2014, 7, 2283. [Google Scholar] [CrossRef]
- Baguia-Broune, F.D.M.; N’Gaman, K.C.C.; Mamyrbekova-Békro, J.A.; Virieux, D. Saponines des racines de Securidaca longipedunculata (Polygalaceae): Quantification et évaluation anti-oxydante. Rev. Nat. Technol. 2018, 10, 25–30. [Google Scholar]
- Gorgini Shabankareh, H.; Khorasaninejad, S.; Soltanloo, H.; Shariati, V. Physiological Response and Secondary Metabolites of Three Lavender Genotypes under Water Deficit. Sci. Rep. 2021, 11, 19164. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Sanz, M.Á.; Peguero-Pina, J.J.; Niinemets, Ü.; Gil-Pelegrín, E. Changes of Secondary Metabolites in Pinus sylvestris L. Needles under Increasing Soil Water Deficit. Ann. For. Sci. 2017, 74, 24. [Google Scholar] [CrossRef]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized Metabolites: Physiological and Biochemical Role in Stress Resistance, Strategies to Improve Their Accumulation, and New Applications in Crop Breeding and Management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Mumivand, H.; Ebrahimi, A.; Morshedloo, M.R.; Shayganfar, A. Water Deficit Stress Changes in Drug Yield, Antioxidant Enzymes Activity and Essential Oil Quality and Quantity of Tarragon (Artemisia dracunculus L.). Ind. Crops Prod. 2021, 164, 113381. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of Water Stress on Lavender and Sage Biomass Production, Essential Oil Composition and Biocidal Properties against Tetranychus Urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Mohammadi, H.; Akhondzadeh, M.; Ghorbanpour, M.; Aghaee, A. Physiological Responses and Secondary Metabolite Ingredients in Sage Plants Induced by 24-Epibrassinolide Foliar Application under Different Water Deficit Regimes. Sci. Hortic. 2020, 263, 109139. [Google Scholar] [CrossRef]
- Rahimi, A.; Mohammadi, M.M.; Siavash Moghaddam, S.; Heydarzadeh, S.; Gitari, H. Effects of Stress Modifier Biostimulants on Vegetative Growth, Nutrients, and Antioxidants Contents of Garden Thyme (Thymus vulgaris L.) Under Water Deficit Conditions. J. Plant Growth Regul. 2022, 41, 2059–2072. [Google Scholar] [CrossRef]
- Saber, M.; Harhar, H.; El Hattabi, L.; Zengin, G.; Bouyahya, A.; Tabyaoui, M. Chemical Composition and Antioxidant Activities of Essential Oils and Extracts from Cones of Tetraclinis articulata (Vahl) Masters. Int. J. Second. Metab. 2021, 8, 352–363. [Google Scholar] [CrossRef]
- Farhoudi, R. Effect of Drought Stress on Chemical Constituents, Photosynthesis and Antioxidant Properties of Rosmarinus officinalis Essential Oil. J. Med. Plants By-Prod. 2013, 2, 17–22. [Google Scholar]
- Department of Watershed and Rangeland Management, University of Kashan, Iran; Bidgoli, R.D. Effect of Drought Stress on Some Morphological Characteristics, Quantity and Quality of Essential Oil in Rosemary (Rosmarinus officinalis L.). Adv. Med. Plant Res. 2018, 6, 40–45. [Google Scholar] [CrossRef]
- Khalil, N.; Fekry, M.; Bishr, M.; El-Zalabani, S.; Salama, O. Foliar Spraying of Salicylic Acid Induced Accumulation of Phenolics, Increased Radical Scavenging Activity and Modified the Composition of the Essential Oil of Water Stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef]
- Zekri, N.; Amalich, S.; Boughdad, A.; Alaoui El Belghiti, M.; Zair, T. Phytochemical Study and Insecticidal Activity of Mentha pulegium L. Oils from Morocco against Sitophilus oryzae. Mediterr. J. Chem. 2013, 2, 607–619. [Google Scholar] [CrossRef]
- Orch, H.; Douira, A.; Zidane, L. Étude ethnobotanique des plantes médicinales utilisées dans le traitement du diabète, et des maladies cardiaques dans la région d’Izarène (Nord du Maroc). J. Appl. Biosci. 2015, 86, 7940. [Google Scholar] [CrossRef]
- Zeggwagh, A.A.; Lahlou, Y.; Bousliman, Y. Enquete sur les aspects toxicologiques de la phytotherapie utilisee par un herboriste à Fes, Maroc. Pan Afr. Med. J. 2013, 14, 125. [Google Scholar] [CrossRef]
- Lazli, A.; Beldi, M.; Ghouri, L.; Nouri, N.E.H. Étude ethnobotanique et inventaire des plantes médicinales dans la région de Bougous: (Parc National d’El Kala,-Nord-est algérien). Bull. Société R. Sci. Liège 2019, 88, 22–43. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Fadli, M.; El Ibaoui, H.; Zidane, L. Ethnobotanical and Ethnopharmacological Studies of Medicinal and Aromatic Plants Used in the Treatment of Metabolic Diseases in the Moroccan Rif. Heliyon 2019, 5, e02191. [Google Scholar] [CrossRef]
- de Macedo, L.M.; dos Santos, É.M.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., Syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- Dheyab, A.S.; Kanaan, M.Q.; Hussein, N.A.; AlOmar, M.K.; Sabran, S.F.; Abu Bakar, M.F. Antimycobacterial Activity of Rosmarinus officinalis (Rosemary) Extracted by Deep Eutectic Solvents. Separations 2022, 9, 271. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Ez Zoubi, Y.; Lairini, S.; El Amrani, S.; El-Akhal, F.; Farah, A.; Bouslamti, R.; El Ouali Lalami, A. Ethnobotanical Survey of Herbs Used in the Preservation of Food Products in Fez, Morocco. J. Ethn. Food 2022, 9, 29. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef]
- Mikou, K.; Rachiq, S.; Jarrar Oulidi, A. Étude ethnobotanique des plantes médicinales et aromatiques utilisées dans la ville de Fès au Maroc. Phytothérapie 2016, 14, 35–43. [Google Scholar] [CrossRef]
- Slimani, I.; Najem, M.; Belaidi, R.; Bachiri, L.; Bouiamrine, E.H.; Nassiri, L. Étude ethnobotanique des plantes médicinales utilisées dans la région de Zerhoun-Maroc-[Ethnobotanical Survey of medicinal plants used in Zerhoun region-Morocco-]. Int. J. Innov. Appl. Stud. 2016, 15, 846. [Google Scholar]
- Boudjabi, S.; Kribaa, M.; Chenchouni, H. Sewage Sludge Fertilization Alleviates Drought Stress and Improves Physiological Adaptation and Yield Performances in Durum Wheat (Triticum Durum): A Double-Edged Sword. J. King Saud Univ.-Sci. 2019, 31, 336–344. [Google Scholar] [CrossRef]
- Degla, L.H.; Olounlade, P.A.; Lagnika, L.; Amoussa, A.M.O.; Dansou, C.C.; Konmy, S.B.; Azando, E.V.B.; Hounzangbe, S.M. Ethnobotanical Survey on Medicinal Plants Traditionally Used for Treatment of Intestinal Parasitosis of Animals and Humans in Northern Benin. J. Med. Plants Res. 2021, 5, 466–478. [Google Scholar]
- Tamert, A.; Latreche, A.; Aouad, L. Criblage phytochimique et activité antimicrobienne des extraits de Thymus serpyllum et de Thymus vulgaris du mont de Tessala (Algérie occidentale). Phytothérapie 2017, 15, 384–394. [Google Scholar] [CrossRef]
- Cheurfa, M.; Allem, R. Screening Phytochimique et Détermination du Pouvoir Antiinflammatoire in Vivo des Extraits de Thymus vulgaris L., d’ Olea europaea L. et de Pistacia lentiscus L. Phytothérapie 2022, 20, 315–319. [Google Scholar] [CrossRef]
- Hamdani, F.Z.; Ziri, S.; Benallou, A.; Djani, H.; Belkacemi, A. Fort Potentiel Antifongique des Huiles Essentielles de Thymus vulgaris et Tetraclinis articulata. Phytothérapie 2021, 19, 190–194. [Google Scholar] [CrossRef]
- Klupsaite, D.; Zavistanaviciute, P.; Sakiene, V.; Lele, V.; Mozuriene, E.; Klementaviciute, J.; Sidlauskiene, S.; Buckiuniene, V.; Tolpeznikaite, E.; Ruibys, R.; et al. Evaluation of the Use of Lactic Acid Bacteria and Thymus vulgaris Essential Oil on Suffolk and Ile de France Lamb Breed (MuscuIus gluteus) Quality Parameters. Int. J. Food Sci. Technol. 2020, 55, 3463–3474. [Google Scholar] [CrossRef]
- Chraibi, M.; Fikri-Benbrahim, K.; Amrani, M.; Farah, A.; Bari, A.; Ouaritini, Z.B. Etude Ethnobotanique Sur L’utilisation de Mentha pulegium, Mentha piperita et Pelargonium graveolens au Nord du Maroc (Taounate) Et Évaluation de Leur Pouvoir Antimicrobien. Eur. Sci. J. 2018, 14, 113. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Bakri, Y.; Dakka, N. Screening phytochimique et évaluation de l’activité antioxydante et antibactérienne des extraits d’Origanum compactum. Phytothérapie 2017, 15, 379–383. [Google Scholar] [CrossRef]
- Lahsissene, H.; Kahouadji, A. Usages thérapeutiques traditionnels des plantes médicinales dans le Maroc occidental: Cas de la région de Zaër. Phytothérapie 2010, 8, 210–217. [Google Scholar] [CrossRef]
- Fadil, M.; Fikri-Benbrahim, K.; Rachiq, S.; Ihssane, B.; Lebrazi, S.; Chraibi, M.; Haloui, T.; Farah, A. Combined Treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. Essential Oils against Salmonella Typhimurium: Optimization of Antibacterial Activity by Mixture Design Methodology. Eur. J. Pharm. Biopharm. 2018, 126, 211–220. [Google Scholar] [CrossRef]
- Ainane, A.; Khammour, F.; El Kouali, M.; Talbi, M.; Oussaid, A.; Lemhidi, A.; Oussaid, A.; Ainane, T. Evaluation of the Toxicity of the Essential Oils of Certain Mints Grown in the Region of Settat (Morocco): Mentha piperita, Mentha pulegium and Mentha spicata against, Sitophilus granarius, Sitophilus oryzae and Sitophilus zeamais. J. Anal. Sci. Appl. Biotechnol. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Feriotto, G.; Marchetti, N.; Costa, V.; Beninati, S.; Tagliati, F.; Mischiati, C. Chemical Composition of Essential Oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and Their Effects on the HIV-1 Tat Protein Function. Chem. Biodivers. 2018, 15, e1700436. [Google Scholar] [CrossRef]
- Puvača, N.; Tufarelli, V.; Giannenas, I. Essential Oils in Broiler Chicken Production, Immunity and Meat Quality: Review of Thymus vulgaris, Origanum Vulgare, and Rosmarinus officinalis. Agriculture 2022, 12, 874. [Google Scholar] [CrossRef]



| Seasonal Average Temperature in °C | Seasonal Average Precipitation in mm | |||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | |
| Sample 1 | 16.25 | 34 | 21.25 | 6.75 | 42 | 21.75 | 70.25 | 55.25 |
| Sample 2 | 21.25 | 39 | 26.25 | 11.75 | 21 | 10.87 | 35.125 | 27.625 |
| Sample 3 | 26.25 | 44 | 31.25 | 16.75 | 14 | 7.25 | 23.41 | 18.41 |
| Thymus vulgaris | Mentha pulegium | Rosmarinus officinalis | |
|---|---|---|---|
| Sample 1 | 3.40% | 1.90% | 2.08% |
| Sample 2 | 3.90% | 1.98% | 2.15% |
| Sample 3 | 1.15% | 0.50% | 1.18% |
| Components | RI | Content of % | ||
|---|---|---|---|---|
| S1 | S2 | S3 | ||
| a-Pinene | 937 | 1.7 | 0.2 | 1.2 |
| Cyclohexanone-3-methyl | 952 | 0.4 | 0.1 | Tr |
| b-Pinene | 974 | 0.5 | 0.1 | 0.7 |
| Myrcene | 992 | 0.1 | 0.3 | 0.1 |
| Octanol-3 | 995 | 2.3 | 0.1 | 0.5 |
| d-2-Carene | 1003 | Tr | 0.1 | 0.9 |
| Limonene | 1030 | 1.4 | 0.5 | 0.5 |
| p-Mentha-3,8-diene | 1071 | 2.0 | 0.1 | 0.7 |
| Menthone | 1150 | 0.1 | 0.2 | 0.8 |
| Pinocarvone | 1166 | 1.8 | 0.1 | 1.9 |
| Isomenthol | 1182 | 0.3 | - | 0.1 |
| Menthol | 1171 | 2.6 | 0.1 | 3.4 |
| Dihydrocarvone | 1193 | 5.6 | - | 8.1 |
| R(+)-pulegone | 1236 | 71.1 | 73.3 | 61.8 |
| Carvone | 1240 | 5.9 | - | 10.3 |
| a-Peperitone | 1251 | 0.4 | - | 0.1 |
| Piperitenone | 1349 | 2.1 | 24.1 | 7.9 |
| Caryophyllene | 1418 | 0.2 | 0.1 | 0.3 |
| Germacrene D | 1475 | 0.1 | 0.1 | 0.2 |
| g-Eudesmol | 1630 | 0.4 | 0.1 | 0.1 |
| a-Eudesmol | 1649 | 0.6 | 0.1 | 0.1 |
| Total | 99.3% | 99.7% | 99.7% | |
| Components | RI | Content of % | ||
|---|---|---|---|---|
| S1 | S2 | S3 | ||
| Alpha-pinene | 939 | 9.30 | 8.34 | 9.17 |
| Camphene | 954 | 4.66 | 4.14 | 4.56 |
| Beta-pinene | 979 | 3.85 | 0.23 | 9.04 |
| a-Terpinene | 1017 | 0.22 | 0.04 | 0.08 |
| p-Cymene | 1025 | 2.55 | 0.71 | 2.07 |
| Limonene | 1028 | 0.09 | Tr | Tr |
| Cineole | 1030 | 45.89 | 55.36 | 43.08 |
| Beta-myrcene | 1048 | 3.99 | 1.93 | 1.99 |
| Linalool | 1097 | 0.35 | 0.12 | 0.41 |
| Camphre | 1146 | 17.44 | 21.44 | 21.56 |
| Bornéole | 1169 | 1.09 | 2.44 | 0.85 |
| a-Terpineole | 1199 | 3.92 | 4.03 | 1.66 |
| Verbenone | 1205 | 0.54 | 0.12 | 0.38 |
| Acetate de Bornyle | 1289 | 5.99 | 1.05 | 5.42 |
| B-Caryophyllene | 1419 | 0.03 | 0.03 | 0.09 |
| a-Caryophyllene | 1423 | 0.07 | Tr | 0.08 |
| Total | 99.98% | 99.98% | 99.98% | |
| Compounds | RI | Content of % | ||
|---|---|---|---|---|
| S1 | S2 | S3 | ||
| a-Pinene | 937 | 3.4 | 1.9 | 1.2 |
| Sabinene | 965 | 0.7 | 0.7 | 0.5 |
| b-Pinene | 975 | 2.1 | 0.1 | 0.4 |
| Myrcene | 984 | 0.5 | 0.2 | 0.9 |
| α-Terpinene | 1009 | 1.2 | 0.3 | 2.1 |
| p-Cymene | 1013 | 8.1 | 0.4 | 3.4 |
| 1,8-Cineole | 1025 | 0.8 | 0.1 | 0.5 |
| Limonene | 1032 | 0.9 | 1.9 | 1.3 |
| Ƴ-Terpinene | 1050 | 0.4 | 0.7 | 22.8 |
| linalol | 1086 | 0.7 | 0.3 | 0.4 |
| Camphre | 1127 | 0.9 | 2.8 | 2.3 |
| transPinocarveol | 1127 | 0.8 | 0.5 | 0.1 |
| Borneol | 1153 | 0.2 | 0.3 | 0.2 |
| Terpinen-4-ol | 1165 | 0.7 | 0.6 | 1.7 |
| Carvacrylmethylether | 1231 | 0.1 | 0.1 | 0.1 |
| Thymol | 1290 | 6.4 | 4.6 | 34.8 |
| Carvacrol | 1298 | 69.9 | 83.3 | 26.6 |
| (E)-Caryophyllene | 1420 | 0.1 | 0.3 | 0.1 |
| Aromadendrene | 1438 | 0.1 | 0.1 | - |
| Alloaromadendrene | 1458 | 0.1 | 0.1 | - |
| Ledene | 1493 | - | 0.1 | 0.1 |
| Spathulenol | 1564 | - | 0.1 | - |
| Caryophyllene oxyde | 1571 | 0.1 | 0.1 | 0.1 |
| Total | 98.2% | 99.6% | 99.6% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laftouhi, A.; Eloutassi, N.; Ech-Chihbi, E.; Rais, Z.; Abdellaoui, A.; Taleb, A.; Beniken, M.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M.; et al. The Impact of Environmental Stress on the Secondary Metabolites and the Chemical Compositions of the Essential Oils from Some Medicinal Plants Used as Food Supplements. Sustainability 2023, 15, 7842. https://doi.org/10.3390/su15107842
Laftouhi A, Eloutassi N, Ech-Chihbi E, Rais Z, Abdellaoui A, Taleb A, Beniken M, Nafidi H-A, Salamatullah AM, Bourhia M, et al. The Impact of Environmental Stress on the Secondary Metabolites and the Chemical Compositions of the Essential Oils from Some Medicinal Plants Used as Food Supplements. Sustainability. 2023; 15(10):7842. https://doi.org/10.3390/su15107842
Chicago/Turabian StyleLaftouhi, Abdelouahid, Noureddine Eloutassi, Elhachmia Ech-Chihbi, Zakia Rais, Abdelfattah Abdellaoui, Abdslam Taleb, Mustapha Beniken, Hiba-Allah Nafidi, Ahmad Mohammad Salamatullah, Mohammed Bourhia, and et al. 2023. "The Impact of Environmental Stress on the Secondary Metabolites and the Chemical Compositions of the Essential Oils from Some Medicinal Plants Used as Food Supplements" Sustainability 15, no. 10: 7842. https://doi.org/10.3390/su15107842
APA StyleLaftouhi, A., Eloutassi, N., Ech-Chihbi, E., Rais, Z., Abdellaoui, A., Taleb, A., Beniken, M., Nafidi, H.-A., Salamatullah, A. M., Bourhia, M., & Taleb, M. (2023). The Impact of Environmental Stress on the Secondary Metabolites and the Chemical Compositions of the Essential Oils from Some Medicinal Plants Used as Food Supplements. Sustainability, 15(10), 7842. https://doi.org/10.3390/su15107842







