Abstract
In this review, collected information related to Moringa Oleifera seeds was evaluated, such as their properties and the main active components involved in their processes, as well as their dual efficiency as both antimicrobials and natural coagulants for treating contaminated effluents. Furthermore, discussions were completed about perspectives on progress related to this field of research to understand the bioactive properties of these seed compounds, including their antibacterial, antifungal, and antiviral activity. In addition to the coagulant properties that have been quantitatively assessed, studies have examined the underlying coagulating mechanism, and seed processing techniques. In addition, the challenges associated with the use of conventional coagulants (metals or polymers) have led to numerous research efforts towards the development of natural plant-based coagulants that are eco-friendly to treat wastewater and offer a large variety of other advantages, such as their wide availability, the reduction of by-product generation, the reduction of costs, and greater biodegradability. Based on the results of different researchers, and regarding the appraisals using Moringa Oleifera seeds for wastewater treatment processes, many studies encourage their use for those operations. Due to their extensive and potent properties as an antibacterial and a coagulant, Moringa Oleifera seeds are still used today as a promising wastewater treatment method. Finally, this paper provides suggestions and comments, as well as identifies the knowledge gaps, and makes recommendations for future research development strategies, such as studying the contents of Moringa Oleifera seeds, their interactions with colloids present in wastewater, understanding their stability and behavior, assessing the performance of seed-derived flocculants according to pH values, isolating and characterizing the active compounds to determine the toxicity and optimum dose to be used as effective antimicrobials, and removing heavy metals.
1. Introduction
We live on planet Earth, where more than 70% of its surface is covered with water, and the oceans occupy 97% of the Earth’s water (by volume). This leaves only 3% of the planet’s total freshwater. Regarding this fresh water, 65% is trapped in glaciers and in the ice caps of mountains, and 30.2% is groundwater, which is too costly to extract. As a result, only 1.3% of surface water of the volume available for human use of the total proportion of water on this planet remains [1,2]. Water is important in all domains of life (human, industrial, and agricultural), which are driven by socio-economic development and increasing population growth [3].
For this reason, there is a global concern about the possibility of obtaining clean and safe water [4], especially in arid and semi-arid developing countries where the provision of potable water is an enormous undertaking [5]. The quality of water and its treatment becomes a source of increasing concern, particularly where water quality is poor, as well as where proper treatment is lacking [6]. Furthermore, it has been estimated that the inefficiency of sanitation, polluted water, or lack of water is causing 80% of all diseases and sicknesses in the world [7]. These detrimental effects are more prominent especially in underdeveloped countries [8]. Nearly 90% of all diseases humans have are caused by microorganisms, which should motivate us to stop their activity [9]. Hence, the result of disease spread and increasing health problems of people leads to a high rate of early deaths correlated with the consumption of polluted water, which is considered an important issue worldwide [10]. In this century, achieving water recycling processes will be one of the main challenges to ensure a worldwide water supply [11]. This context allows for the development of scientific knowledge in the use of alternative coagulants. Three million people globally lack water and sanitation services, which has a negative impact on their quality of life and undermines their basic human rights. Consequently, the emergence of many diseases and the threat to health security place a heavy strain on economies [12]. Water is widely used in different sectors and especially in productive sectors such as urban supply, industry, livestock, and agricultural production, which has led to water overuse [13].
The protection of the environment from wastewater contaminants is very important [14]. Water and wastewater are contaminated with numerous colloidal solid particles that are difficult to settle, resulting in water turbidity and color change [15], which must be addressed using coagulants [16]. One of the biggest challenges in water treatment is removing hydrophobic colloids because they are typically present in high concentrations compared to other pollutants [17]. In this context, coagulation has been practiced since the earliest times in human history to reduce turbidity by removing these impurities [18] through sedimentation in both drinking water and wastewater treatment [19]. Thus lowering turbidity improves water quality [20,21]. The coagulants used in water treatment processes are classified as natural, inorganic, and synthetic organic polymers [16,22]. The use of natural coagulants has quite a long history [23]. Natural coagulants are plant-based and used to remove turbidity, and have been used by various civilizations and communities for at least 4000 years [24]. Recently, there has been increased interest in using natural coagulants [25], which are gaining interest in developing countries [26], for the treatment of water and wastewater [27]. This interest emerged due to the many problems created by using synthetic coagulants [28], such as their highcost and health-related issues [29]. Furthermore, environmental impacts are associated with inorganic/synthetic organic coagulants [30]. For example, aluminum sulfate, aluminum polychloride [31], and aluminum chloride [32] are among the most frequent inorganic coagulants used in the coagulation or flocculation processes. However, these have negative impacts on human health and the environment, as reported by Megersa et al. [21]. Moreover, it has been reported that alum produces a large volume of sludge [33]. In addition, its use reduces the pH of the treated water [34,35,36], it is not biodegradable [37], and it has a link to Alzheimer’s disease, as reported by Campbell et al. [38], Ribes et al. [39], Rondeau et al. [40], and Wang et al. [41]. Moreover, it possesses strong carcinogenic properties and is expensive [42].
The natural coagulation activity of seeds can be similar to or even better than alum [43]. Moreover, most people in rural communities tend to easily find available water sources, despite their low quality due to the high cost of treated water, and this exposes them to many waterborne diseases [29]. In the response to this problem, we have conducted this research on a natural coagulant that has the best properties, such as being low cost, effective, non-toxic, and having no significant effect on the pH of the water treated. Natural coagulants that are plant-based represent one renewable source in wastewater treatment because they have many positive properties such as being biodegradable, non-hazardous, widely available, and environmentally friendly [10].Their cost is low, a lesser quantity of sludge is produced after using them in water treatment [14], they have a wider effective dosage range in the flocculation process [10], and they are becoming increasingly more important in replacing chemical coagulants [24], as seen in most parts of India and some parts of Africa and China.They have been used in the treatment of water over the past 2000 years, and have been certified eco-friendly organic polymers of natural coagulants [23,44]. Moreover, these natural coagulants can be locally grown in developing countries because they are cost-effective [45]. Additionally, their production increases opportunities for employment by creating a new cash crop for farmers, especially in rural areas [46]. Basic coagulation mechanisms have been elucidated for Moringa Oleifera seeds by applying them in treating turbid water in 1995 [47]. Since then, there has been widespread interest in them [48], and arguably that the Moringa Oleifera tree is the most studied in the environmental scientific community as a natural coagulant [37]. Because the seeds of Moringa Oleifera contain a water-soluble cationic coagulant protein, they are used as a primary coagulant in drinking water clarification and wastewater treatment through their ability to reduce the turbidity of treated water [49]. These proteins are considered the most widely investigated plant-based coagulant [24,50,51,52], having been researched by several scholars [5].The seeds of Moringa Oleifera possess good coagulating properties comparable to commercial alum used in turbidity removal [53]. Their antimicrobial activity has also been studied in previous studies [6,53,54,55,56,57,58], as have their antifungal [59,60,61,62,63] and antiviral effects [53,54,55,56,57]. They also play a role as an antiparasitic, such as in Haemanthus contorts eggs [64,65]. To avoid defects associated with chemical and physical processes, such as high costs, the generation of toxic waste, and the use of toxic reagents in removing mineral contaminants of liquid waste, new methods have emerged [66] for removing heavy metals from wastewater [67], which has become a global concern for both the environment and human health [68]. The use of the seeds of Moringa Oleifera plays a role in the removal of pollution resulting from toxic metals (e.g., arsenic [69,70,71],copper, lead, cadmium, chromium [72], etc.). The goal of this work is to undertake an in-depth, clear, and comprehensive review of the scientific evidence of state-of-the-art uses of Moringa Oleifera seeds (powder or extract) for wastewater treatment. Thus, this review work will provide valuable information, which will be the data baseline necessary for researchers who will be focusing on the dual performance of Moringa Oleifera seeds in antimicrobial and coagulant activities while demonstrating its ability to remove heavy metals. Finally, this work intends to identify gaps in current knowledge, as well as determine future research directions.
2. Background
The Moringaceae family consists of 10, 12 [73], or 14 species [74] that belong to only one genus called Moringa. Among the Moringa species is Moringa Oleifera, which has been endowed with research and development funding by the National Research Council [5], and is the best known, most distributed or widespread, and most utilized species [75] in the production of food products, animal and aquaculture feed, medicinal products, renewable polymer products, and water purification processes [47,74]. Furthermore, most have been researched by Moulin et al. [76]. Moringa Oleifera is a versatile plant that is widely distributed in tropical and sub-tropical areas of the world [77,78,79,80]. The plant has advantages such as being able to live in poor soils and obtain up to three harvests each year [81]. It has the ability to withstand drought and grow under severe climatic conditions, and it thrives in soil with acidity ranges from 5.0 to 9.0, in addition to its ability to tolerate a different range of rainfall between 250 mm and over 3000 mm as a minimum and maximum, respectively [82]. It is a perfect example of a kind of “multipurpose tree” [29].
It has wide use in animal feed and vegetables in many countries such as Pakistan, the Philippines, India, and some African countries. Given its importance, it was ranked by the Chinese Green Food Development Center in 2012 as the “national first green food” [83]. During British rule in Sudan, it was considered an ornamental tree, which was planted in public parks, foreign gardens, and the alleys along the Nile [84]. The Moringa Oleifera has been well known for a long time for its ability to remove several contaminants from water effluents [85]. In the past century, it seems likely that Sudanese women have discovered the properties (as a clarifier tree) of Moringa Oleifera tree seeds [45]. They are utilized for water purification because they have strong coagulation properties useful in the sedimentation of suspended mud, and they can address turbidity, too [62,86]. Moreover, Moringa Oleifera is a non-toxic bio-coagulant according to earlier studies [29,37,86], and it is biodegradable [87]. Previously, Moringa Oleifera seeds have been used in the purification of water for several centuries, and have been ranked as one the best plant extracts in this purification process [10,88]. Furthermore, their seeds are considered a good source of high quality oil [89].
Research studying the toxicity of methanol extract of the seeds of Moringa Oleifera found that the relatively high median lethal dosage value was equal to (3870 mg kg−1), and this suggests that the seeds are safe to use in water purification of humans and livestock [90]. Studies have reported antimicrobial, anti-inflammatory, antioxidant, antipyretic, antibacterial, and antifungal properties of the seeds [91].
3. Properties of Moringa Oleifera Seeds
Moringa Oleifera is a kind of tree widely used in medicinal applications and diverse pharmacological activities [63]. Moringa Oleifera seeds are characterized by a high rate of potassium, reaching up to 38.29% of the mineral content of the seeds, according to results obtained by X-ray fluorescence analysis [92]. These high levels of potassium are beneficial for addressing the decreased-potassium levels in patients with COVID-19, which is caused by the SARS-CoV-2 virus [93]. Furthermore, the seeds are used as antimicrobials [29,62,94]. Additionally, one report has shown that the difference in antimicrobial properties can be attributed to several parameters such as the age of the plant used, the freshness of the plant material, physical factors (water, temperature, and light), incorrect preparation of the plant, and contamination by field microbes, etc. [95,96]. Research has also shown that Moringa Oleifera seeds act as a natural coagulant, flocculant, and absorbent for the treatment of drinking water without any toxic effect on human health [54], they serve as vegetables and functional food [13], and they are also considered an important source of health-promoting phytochemicals and micronutrients [97,98]. Tannins and saponins were detected in ethanol extracts of Moringa Oleifera seeds according to a report by Nepolean et al. [99]. Tannins and alkaloids were found to be highly present using methanol as a solvent [100]. Phytochemical screening on the methanol extract of seeds of Moringa Oleifera is shown in Table 1. Furthermore, Moringa Oleifera seed ethanol (MSE) extract has shown to contain saponins, alkaloids, and flavonoids, as reported by Bukar and Oyeyi [101]. They found that the highest amount of total flavonoid reached 273.7% in ethanol extract (80%), while phenolics reached 395.4% in acetone extract (40%), which was fermented using Aspergillus Niger for 168h with initial humidity equal to 50% and 70%, respectively [102].

Table 1.
The phytochemical screening on the methanol extract of seeds of Moringa Oleifera.
The content of oil from de-hulled seed (kernel) is approximately 42% of its total content, and this oil contains approximately 13% and 82% saturated and unsaturated fatty acids, respectively [104]. One study found that an increase in the temperature of the drying air led to greater volumetric contraction of grains of Moringa Oleifera and affected the final oil extraction yield [81]. The amount of total unsaturated fatty acids was more than 80% [73], while other studies found that it reached more than 76% (Lalas and Tsaknis) [105], 44% (Gu, Yang, and Wang) [106], and 78.69% (Delange et al.) [107]. Recent research found that the composition of fatty acids (%fatty acids) totaled 70.7%, 4.5%, and 18.0% monounsaturated fatty acids, polyunsaturated fatty acids and SFA, and saturated fatty acids, respectively [108]. The oil was found in some Egyptian reports analyzing Moringa Oleifera seeds to contain saturated acids (palmitic, stearic, and arachidic) (up to 12%),a large amount of fatty acids, and unsaturated acids, particularly an omega-9-Oleic (up to 76%) [109], while other studies found amounts totaling 71.60% [105], 74.50% [110], greater than 70% [106], and 70% [111].
Table 2 shows the typical content of oil in Moringa Oleifera seeds (fatty acid in Moringa Oleifera seeds). The oil extracted from the Moringa Oleifera seeds showed non-toxic effects [13,112], and high stability to oxidation rancidity [105]. An analysis of Moringa Oleifera seed oil of two cultivars in Argentina reported that the oil produced from both cultivars tested had practically identical fatty acid composition. Regarding monounsaturated fatty acids, omega 9 was discovered in both cultivars (18:1) (more than 70% of the total) [113]. A report showed in 2019 that the percentage of Oleic (18:1n-9) of three different sites (Ospina, La Rinco, and Carnero Beach of Santa Elena Province in Ecuador) reached 72.38, 75.46 and 75.52%, respectively [114]. This high level of Oleic acid content gives high stability to the oil of Moringa Oleifera seeds [115].
It has been suggested that regional conditions or harvesting practices of Moringa Oleifera seeds lead to differences in lipid content between 30% and 42% [116]. Further factors contributing to these differences include differences in the species, genetics of the plant, its cultivation, soil, region, state of ripening of the fruits, and the method of extraction and analysis [107].A report from 2009 showed that Moringa Oleifera seed extracts contained 39.3% and 37.6% of crude oil and crude proteins, respectively [117], while another study reported a total content level of 41% of oil extracted from Moringa Oleifera seeds [110]. A recent study found that seasonality has no significant effect on the yield of crude or refined oil [118]. The first report on the oil of Moringa Oleifera seeds showing the presence of pentadecanoic acid (C15: 0), carboxylic acid (C27: 0), and montanic acid (C28: 0) reaching 0.03% of the total content of the seed for each one of the three acids is cited here: [107]. It has been reported that Moringa Oleifera seed coagulation activity is not significantly affected by the presence of fatty acids [119]. So, there is no need to extract the oil from the seeds of Moringa Oleifera if they are used as coagulants [52,120]. Further, it was revealed through analyses of extracts of Moringa Oleifera seed oils that the retention of the oil could give added value because of the role played by the presence of some fatty acids (e.g., palmitoleic, oleic, linoleic, linolenic, cis-11-eicosenoic, and cis-11,14-eicosadienoic) at concentrations of 0.01% w/v that significantly inhibit the formation of S. aureus biofilm, as well as due to the potential benefit of unsaturated fatty acids for controlling the formation of biofilm and the virulence of S. aureus [121]. On the contrary, some studies have suggested that it is necessary to extract the oil from the seeds because the presence of oil would affect the activity of coagulation and heavy metal removal [43].

Table 2.
The percent composition of fatty acid in the oil of Moringa Oleifera seeds.
Table 2.
The percent composition of fatty acid in the oil of Moringa Oleifera seeds.
| Fatty Acids | Reported Values % | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [122] | [104] * | [73] | [115] | [105] | [123] | [124] | [107] | [117] | [108] | [114] | [125] | [126] | [127] | |||
| a | b | c | ||||||||||||||
| Caprylic Acid (C8:0) | - | - | 0.03 | - | 0.03 | 0.03 | 0.02 | - | - | - | - | - | - | - | - | - |
| Lauric acid (C12:0) | 0.1 | Trace | - | - | - | - | - | 0.06 | - | 0.2 | - | - | - | - | - | - |
| Myristic acid (C14:0) | 0.1 | 0.08 | 0.11 | 0.72 | 0.13 | 0.11 | 0.1 | 0.08 | - | 0.3 | 0.42 | 0.17 | 0.15 | 0.11 | - | - |
| Pentadecanoic (C15:0) | - | Trace | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - | -- |
| Palmitic acid (C16:0) | 7.8 | Trace | 6.04 | 6.1 | 6.46 | 6.17 | 5.51 | 7.43 | 6.24 | 11.5 | 5.73 | 5.43 | 5.75 | 5.80 | 6.18 | 6.24 |
| Palmitoleic acid (C16:1) | 2.2 | 5.54 | 1.46 | 1.2 | 1.36 | 1.10 | 1.10 | 1.50 | 1.6 | 4.3 | 1.39 | 1.41 | 1.72 | 1.20 | 1.31 | 1.60 |
| Heptadecanoicacid (C17:0) | - | - | 0.09 | - | 0.08 | 0.09 | 0.04 | 0.15 | - | - | - | - | - | 0.086 | - | - |
| Stearic acid (C18:0) | 7.6 | 5.42 | 4.14 | 4.6 | 5.88 | 4.77 | 5.86 | 4.01 | 4.71 | 2.3 | 5.40 | 4.12 | 3.95 | 5.80 | 4.01 | 4.71 |
| Oleic acid (C18:1) | 67.9 | 72.9 | 73.6 | 78.7 | 71.21 | 74.4 | 67.79 | 73.10 | 74.93 | 69.7 | 72.38 | 75.46 | 75.52 | 72.30 | 74.87 | 74.93 |
| Linoleic acid (C18:2) | 1.1 | 0.76 | 0.73 | 0.65 | 1.21 | 0.71 | 0.95 | 0.72 | 2.2 | 0.69 | 0.61 | 0.69 | 0.68 | 0.68 | 0.72 | |
| Linolenic acid (C18:3) | 0.2 | 0.14 | 0.22 | 1.8 | 0.18 | 0.24 | 0.21 | 0.19 | 1 | 0.18 | 0.17 | 0.16 | 0.17 | - | - | |
| Arachidic acid (C20:0) | 4.0 | 3.39 | 2.76 | 2.3 | 3.62 | 3.51 | 3.78 | 2.48 | 3.09 | 1.2 | 3.14 | 2.74 | 2.68 | 3.70 | 2.69 | 3.09 |
| Gadoleic acid (C20:1) | 1.5 | 2.2 | 2.40 | 2.22 | 1.61 | 2.6 | 2.39 | 2.32 | 1.1 | 3.40 | 2.40 | 2.31 | 1.90 | - | 2.32 | |
| Heneicosanoicacid (C21:0) | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.067 | - | - |
| Behenic acid (C22:0) | 6.2 | 6.88 | 6.73 | 4.5 | 6.41 | 6.16 | 6.81 | 5.84 | 5.33 | 1.5 | 5.56 | 5.68 | 5.90 | 6.50 | 5.43 | 5.33 |
| Erucic acid (C22:1) | - | 0.14 | 0.14 | - | 0.12 | 0.14 | 0.11 | 0.13 | - | - | - | - | 0.73 | - | - | |
| Docosadienoicacid (C22:2) | - | - | - | - | - | - | - | - | - | - | - | 0.065 | - | - | ||
| Lignoceric acid (C24:0) | 1.3 | 0.92 | - | - | - | - | 0.87 | 1.05 | 0.5 | 1.14 | 1.06 | 0.83 | 0.92 | - | 1.05 | |
| Nurvonic (C24:1) | - | Trace | 1.08 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Cerotic (C26:0) | - | - | - | - | 1.18 | 1.08 | 0.98 | 0.06 | - | - | - | - | - | - | -- | |
(*): Analysis: Thionville Laboratories, Inc. New Orleans, LA, USA (March 1994) Values in parentheses (Becker and Siddhuraju, unpublished). (-) Non-available. (a,b,c): refers to the fatty acids of Moringa seeds oils in three different sites (Ospina, La Rinco, and Carnero Beach of Santa Elena Province in Ecuador, respectively.
4. The Activities of Moringa Oleifera Seeds
4.1. Antimicrobial Activity
4.1.1. Antibacterial Activities of Moringa Oleifera Seeds
The discovery of novel antimicrobial agents plays a very important role in the control of pathogenic microbes, particularly in the treatment of infections that are caused by resistant microbes. This antibacterial activity of Moringa Oleifera has been extensively studied and described in multiple studies with some of these presented in Table 3. For example, it has been reported that Moringa Oleifera seed flour extracts have shown antibacterial activity [128,129], though it was tested using smaller concentrations on four different bacteria (i.e., Escherichia coli (clinical isolate), E. coli (ATCC2592), Shigella dysenteriae (clinical isolate), and Salmonella typhi (clinical isolate)) [7]. It also retarded the growth of some bacteria such as Candida spp., Hortaeawerneckii, and some other food-borne microorganisms [101,130]. WEMOS plays an important role in the aggregation of coccoid bacteria and the growth of nuclei of microbials too, which are considered precursors of anaerobic granulation [131]. Furthermore, the seeds showed antimicrobial activity with a recombinant protein that has the ability to flocculate both the gram-positive and negative bacterial cells [132]. Additionally, the seeds’ aqueous extracts showed strong and admirable qualities against all bacterial strains that were tested, and especially with gram-positive bacteria (Bacillus subtilis and S. aureus) as compared to other solvents such as methanol or petroleum ether [133]. Previous studies have shown a suite of bioactive compounds in the chemical composition of Moringa Oleifera. Moreover, it has shown an effect against E. coli, S. aureus, P. aeruginosa [56].

Table 3.
Summary of the inhibitory activity of Moringa Oleifera seeds against different groups of microbials.
The antibacterial activity of steam distillate of Moringa Oleifera Lam was observed to play the main role in a considerable reduction in the growth of test bacteria. It mainly inhibited E. coli, and to a lesser degree inhibited S. aureus, K. pneumoniae, P. aeruginosa and B. subtilis [60]. Furthermore, one study proved that S. aureus, S. marcescens, and B. subtilis are sensitive to Moringa Oleifera seed extraction(MOSE) with ethanol 80% (humidity initial = 50%) fermented for 168 h [102].
One study mentioned that one of the peptides of Moringa Oleifera seeds plays a role in mediating both the sedimentation of suspended particles such as bacterial cells and antibacterial activities as well [143]. Reductions in the concentration of specific human pathogens may be attributable to the coagulative ability of the bioactive components related to a peptide contained in the seeds of Moringa Oleifera, which had potent antibacterial properties [75]. The seeds may have an effect directly upon microorganisms through antimicrobial peptides that are thought to have a role in in habiting essential enzymes or acting to disrupt the membranes of cells, thus resulting in growth inhibition [55]. Damage to bacterial cell membranes is a result of membrane fusion [129]. One previous study pointed out that in low or moderate temperatures, some protein antibacterial compounds might result in membrane permeabilization via the binding of cationic proteins to the negatively charged membrane surface followed by subsequent pore-formation [140]. Furthermore, Moringa Oleifera seed extract the replication of bacteriophages [144], and decreases the number of helminth eggs in irrigation water [65]. It has been shown in some previous studies that Moringa Oleifera extract can reduce the number of fecal coliforms by up to 99.9% [94,132,145] and the number of schistosome cercariae up to 90% [146]. The bioactive compounds that have been found in the seed extract of Moringa Oleifera have anthelmintic abilities against the eggs and infective-stage larvae of Haemanthus contortus [64]. For example, tannins detected from Moringa Oleifera seeds play an important role in inhibiting the formation of larvae and reducing the motility of infective-stage larvae (L3s) inside Haemanthus contortus eggs, which may indicate that they generate paralysis and interfere with the neuromuscular coordination of the larvae [147,148]. In one recent study, the results of phyto-chemical screening showed that its seeds contain a high percentage of phyto-molecules (in particular, terpenoids and tannins), which have antibacterial and antifungal characteristics [149]. One study suggested that the effectiveness of the seeds as antibacterial measures could be due to the presence of many bioactive chemical complexes, for example:D-allose, ethyl ester, and hexadecenoic, palmitic, and oleic acids) [150]. Given previous research, another knowledge gap that may require further studies is that concerning the role of bioactive components of Moringa Oleifera seeds in the growth inhibition of microorganisms.
4.1.2. Antifungal Activity of Moringa Oleifera Seeds
Multiple studies have described the antifungal activity of Moringa Oleifera seeds, and some of these are presented in Table 3. In this section, Mo-CBP3, a chitin-binding protein (14.3 kDa), was isolated from Moringa Oleifera seeds that showed evidence of inhibiting g ermination and mycelial growth (e.g., Fusariumsolani, F. oxysporum, Colletotrichummusae, and C. gloesporioides) [139]. Contrarily, Mo-CBP3 did not affect Pythium oligandrum [151]. Previously, it was thought that Mo-CBP3 was a genuine member of Moringa Oleifera seeds in the 2S albumin family, thanks to a comparative analysis of the deduced amino acid sequences, for this reinforces the hypothesis that these seed storage proteins are involved in plant defense [152]. Now, Mo-CBP3 is considered a new drug against fungi, which can be used in the development of transgenic crops for traits, (e.g., thermo-stability, broad antifungal spectrum, and low toxicity as well) [153]. It has been reported that water extracts of Moringa Oleifera seed could be effective in growth inhibition of A. niger, F. oxysporum, and Colletotrichum app. [154]. In Egypt, a preliminary study was carried out on the use of Moringa Oleifera extracts as natural fungicides against plant pathogens because much of the evidence confirms that extracts of Moringa Oleifera plants are cheap, naturally available, environmentally friendly, less harmful to seed viability and quality, and are a safe and alternative means for chemical control methods, which often have a harmful effect on the ecosystem and are expensive [61,154]. One study showed that the cytoplasmic membrane of fungal cells treated with a crude extract of 70% ethanol of Moringa Oleifera seeds ruptured and seriously damaged intracellular components, which caused a decrease in the percentage of water in the cell and thus produced cell swell, leading to the death of the cell [155]. Antifungal activity was observed in the steam distillate of Moringa Oleifera Lam, among fungi tested, with more inhibition observed in the case of A. niger, and less observed in A. oryzae, A. terreus, and A. nidulans [60]. In order to increase the exploitation of naturally available chemicals from seeds of Moringa Oleifera, further studies that focus on bioprospecting for fungicides are needed to isolate and characterize the antifungal agent, which makes this plant a good candidate for use as an antifungal agent.
4.1.3. Antiviral Activity of Moringa Oleifera Seeds
Human inability and deficiency clearly and unfortunately appeared in the weak scientific knowledge or technological control of the Coronavirus disease epidemic (COVID-19), despite tremendous developments in microbiology [156]. Nevertheless, great efforts have been made to develop alternative antiviral therapeutic agents in order to discover potential drugs in natural compounds of plant origin that are bioactive. One global health problem is that of viral infections, and so far, few antiviral agents are available, along with problems of emerging resistance [157]. In this domain, the Moringa Oleifera (MO) plant is one of the important medicinal plants that have proven antiviral activities against various viruses, as shown in several studies [158,159,160,161,162,163,164,165,166,167,168]. The remarkable inhibitory activity of Moringa Oleifera seeds against some virus types is shown in Table 3. Moringa Oleifera seeds possess some biochemical compounds that are considered as an indicative number according to a GC-MS analysis. These compounds include oleic acid (84%), L-(+)-ascorbic acid-2, 6-dihexadecanoate (9.80%), 9-octadecenoic acid (1.88%), methyl ester-hexadecanoic acid (1.31%), and 9-octadecenamide (0.78%). These chemical constituents that are relatively diverse may be responsible for properties of Moringa Oleifera seeds in the medical field [169]. Furthermore, the isolates tested revealed the compounds’ inhibitory effects on the induction of EBV-EA (Epstein–Barr virus EA) activation without significant cytotoxicity on Raji cells. These tested compounds include: 4-(α-L-rhamnosyloxy) benzyl isothiocyanate, Niazimicin, β-sitosterol-3-O-β-D-glucopyranoside [142], and Niaziminin [170]. In addition, Moringa Oleifera seed aqueous extract showed strong antiviral activity against Newcastle disease Virus in ovo assays [103]. In a recent study, it has been suggested to use the phytochemical extracts of Moringa Oleifera as flavonoids and anthraquinone as new effective compounds because these contain antiviral properties against COVID-19, which work to improve the immune system through the production of antibodies against SARS-CoV-2. For this reason, it is suggested that these compounds be considered and clinically tested against COVID-19 [171]. The effect of aqueous methanolic extract on herpes simplex virus type 1(HSV1) and polio virus type 1 (PV1) was observed by Ali, El Taweel, and Ali (2004) [172]. Further, the seeds were effective against several cancer lines [173]. By reviewing the various studies discussed in this part, Moringa Oleifera seeds’ high and unique immunological capabilities may leads to opening another knowledge gap in examining the antiviral efficacy against COVID-19 illness.
4.2. Moringa Oleifera Seeds’ Coagulant-Flocculant Activity
4.2.1. The Techniques for Processing Moringa Oleifera Seeds
Nowadays, three main steps are involved in the preparation of Moringa Oleifera seed coagulant. These steps are classified as follows: the first step is concentrated on flour preparation, (the second step specializes in protein extract, and the third step plays an important role in purification [30]. Therefore, the properties of the coagulant and its performance in removing pollutants from wastewater are directly correlated with these steps and their sequence (Figure 1). The primary processing stage is carried out according to a series of steps that begin by allowing the maturation of the pods of seeds on the tree before harvesting, then drying and peeling are performed manually to access the kernel. The flour of Moringa Oleifera with a specific particle size was obtained using different equipment such as a pestle, grinder, mixer, and blender. It was reported that researchers preferred using pestles and grinders because these were more effective at obtaining a small particle size of Moringa Oleifera seed flour, removing part of the lipids present in the seeds, and reducing turbidity and apparent color [174]. The particle size of seeds of Moringa Oleifera plays a main role in achieving a better coagulation process [175]. Furthermore, researchers pointed out the effects of the seed sizes and cylinder speed on the capacity of the machine used and its energy consumption (SEC) [176]. Next comes the role of a sieve with a decreased distribution of particle size according to the range most recommended in applications of water treatment, which is between 0.25 mm and 1.25 mm [13]. Figure 1 shows the sequence of preliminary process steps beginning with a harvest of mature and dry pods and finishing with sieving the powder of Moringa Oleifera seeds according to the required range. In the past 2030 years, increasing scientific interest has emerged especially in the field of extraction and purification improvements for the active component through its isolation from Moringa Oleifera seed [177]. The results reveal that the operating range of pH of natural flocculant derived from seeds of Moringa Oleifera increased from below 4.8 to below 9.8 after oil extraction with a decreased number of fatty acids and aromatic and phenolic compounds, but the content of the proteins were retained, including those with coagulant properties [178].The extract of MOS-WD contains main component of the active coagulation agent according to results from several studies [84,146,179,180,181]. Alternatively, the extract obtained by salt solutions or carried out via different organic solvents are propounded as a secondary processing stage [30,182]. It is suggested that the use of salt solutions is preferred for extraction of the active coagulation component from Moringa Oleifera seeds [116], because this medium plays a role in the potentiating of proteins that are responsible for the active coagulant of Moringa Oleifera seeds [183]. Moreover, the high value of chemical oxygen demand (COD) associated with using the extract of Moringa Oleifera seeds with distilled water discourages their use in treating drinking water [184,185]. Nonetheless, due to the fact that the prepared powder contains plant tissue and coagulating active agents, and these tissues are rich in organic constituents, they will increase the organic loads in the treated water, which may lead to a decrease in the efficiency of treatment instead of improving it [182]. Although MOC-DW, the highly effective coagulant of MOS-WD, has been proven effective, it l performs poorly in low-turbidity water [36]. In this context, one study suggested that the performance of MOC-SC-pc will be suitable for low-turbidity [186], and it has been reported that there was an increase in the content of dissolved organic carbon (DOC) in water after being treated, considering a change in source color, odor, and taste [186,187]. Moreover, it was observed that using WEMOS in filter units did not increase the efficiency of TSS removal [188]. It was suggested that one must adequately purify the Moringa Oleifera seeds from the active proteins for use as a coagulant in treating drinking water and wastewater in order to decrease organic matter that is considered a precursor to chlorination by-products during disinfection with chlorine [13], which means that organic matter resulting from MOE might be a precursor for a disinfection by-product. For this purpose, we suggest using an appropriate amount of MOE in order to reduce the amount of residual organic compound, attain higher protein content, and realize more positive charge. To achieve all this, a short extraction time for the extraction of Moringa Oleifera seeds is strongly recommended [189]. In addition, the active component of MOC-DW was different from that of MOC-SC and was not a protein such as in MOC-DW [190,191]. The coagulant properties of seeds extracted with salt solution (MOC-SC) were better than those extracted with water extraction (MOC-DW) [36], and we ascribe the efficiency of MOC-SC to an increase in ionic strength that increases active ingredient solubility [30]. Table 4 shows the characterization of the extract of Moringa Oleifera seeds with NaCl or Water.
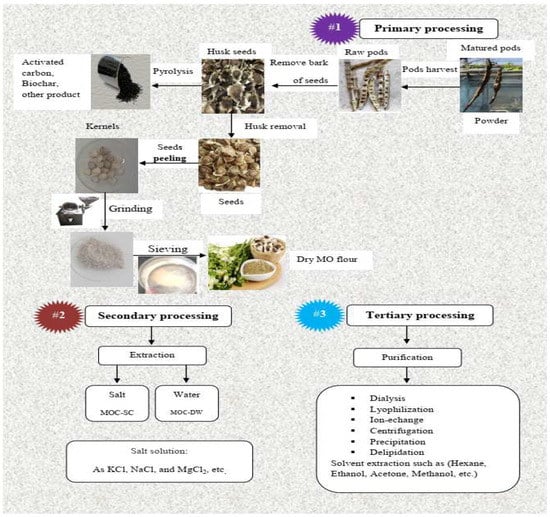
Figure 1.
Diagram of the main processing steps in the preparation of Moringa Oleifera seeds.

Table 4.
Characterization of the extract of Moringa Oleifera seeds with NaCl or water.
One study suggests that it is necessary to the extract seeds in saline solution, and seawater can be a replacement for saline solutions to attain similar effects in turbidity removal in raw water, particularly when the concentration of turbidity is greater than 150 NTU [183]. One research study showed that the efficiency of coagulation of Moringa Oleifera had no variation across different salt extracts, such as KCl, NaCl, and MgCl2, but was higher than that of the water extract [30]. However, another study by Mageshkumar and Karthikeyan (2015) found that the efficiency of removal of turbidity using MOC-SC extracts in KCl solution was lower compared to NaCl-based MOC-SC extracts [195]. On the other hand, a recent study on textile wastewater treatment found that the best results were obtained with MOSE in 1 M KCl, reaching 82.2% and 83.05% for color and COD, respectively [196]. Furthermore, coagulation with 1 M CaCl2 Moringa Oleifera seed extracts resulted in better removal of turbidity and color compared to 1 M NaCl extracts [197]. In one study, coagulant proteins of Moringa Oleifera seeds were purified of oil and then followed the salting out method at 40% (NH4)2SO4 combined with ensuing dialysis and heat treatment [50]. The purification method plays an important role in the components of the final extract of Moringa Oleifera, and therefore the use of different solvents for extraction, as well as the fragmentation of the extract, will yield different types of biologically active compounds with varying quantities from the plant material [198,199]. Another study pointed out that it is preferably to use a salt solution in the extraction of the active component of coagulation of Moringa Oleifera seeds because organic solvents such as hexane, ethanol, acetone, and methanol could not improve this extraction [146]. In view of overcoming the limitations of the crude extraction process, numerous researchers have worked on some purification methods, in addition to dilapidation. For example: dialysis, ultrafiltration, ion exchange, centrifugation, and Lyophilization have been tested, as shown in Figure 1 [47,187].
4.2.2. Moringa Oleifera Seeds as a Coagulant-Flocculant
Recently, researchers have been focusing on alternative coagulants that are based on natural elements. Detailed previous studies have been carried out on the use of Moringa Oleifera seed extract in water treatment [84,116,180,200,201,202]. Moringa Oleifera is a natural polymer that is composed of cationic proteins [203]. Its seeds have strong natural coagulative and antimicrobial properties [62,204]. These seeds contain an active coagulating compound [6,187,205]. This compound is a water-soluble protein [47]. This protein has the ability to remove impurities that are suspended from treated water through aggregation and then precipitation, finally obtaining clear water [128].
Many previous studies suggest that these proteins are responsible for the coagulation and flocculation processes [191,206]. Further, these proteins have the ability to flocculate gram-positive and gram-negative bacterial cells [168]. Furthermore, other studies suggest that the properties of the coagulant in mature, dry Moringa Oleifera seeds emerge from the availability of cationic and water-soluble proteins, while other authors suggest that the properties of the coagulant may be improved by adding cations [47,48]. This protein is a zwitterion and a polyelectrolyte compound that contains an amine group and carboxylic acid [87]. Furthermore, it has the ability to be soluble and plays the role of natural cationic polyelectrolyte that causes coagulation in water that contains turbidity [5,88]. It has been reported that the active ingredient, which is a polyelectrolyte, was isolated in the laboratory where about one kilogram of (nearly pure) polyelectrolyte was produced from one hundred kilos of Moringa Oleifera kernels [207]. It was reported that the protein isolated from these seeds has a characteristic flocculating property [128,190], and the level of polyelectrolyte in the kernels varies according to the harvesting season. Therefore, it is preferable to harvest seeds during the dry season, because they contain a high percentage of polyelectrolyte [207]. It is worth noting that Moringa Oleifera seeds may have different constitutions according to the growing conditions of the trees in different geographical locations, which will affect the protein concentration and eventually the coagulating properties [208]. These proteins play a role in improving the quality of water by promoting the coagulation process [47,182,209]. In addition, this is considered an inexpensive method and an accessible facility for the purification process [129]. The Moringa Oleifera seeds are used in wastewater treatment as a substitute because of their effectiveness as a purifier of water that was tested as a flocculant [146]. Moreover, it has been reported that the polypeptide contained in seeds acts as cationic polymers, which cause the process of coagulation [94], and bacteria in suspension [55,210]. A recent study recommends the immediate removal of the coagulant after an appropriate treatment time in order to control the regrowth of pathogenic bacteria, especially that which is gram-negative, in treated water, such as salmonella, shigella, and Vibrios, due to the nutrients in Moringa Oleifera extract [211]. Moringa Oleifera seeds used as a natural coagulant in developing countries are recommended by Jahn (1988) [84]. Coagulant extracts from Moringa Oleifera seeds have been recommended for water treatment in African and South Asian countries [116]. Comparing coagulants resulting from the use of Moringa Oleifera seeds with other natural coagulants, it is considered one of the most efficient and environmentally friendly low-cost coagulants [212,213]. Moringa Oleifera seeds were traditionally applied as a coagulant in the treatment of drinking water [86,143] and surface waters [180]. Its crude seed extracts have been used in rural communities of African countries to purify turbid river water [48]. It has been reported that the conductivity and pH value of water is not significantly affected after treatment [214]. Furthermore, the powder of seeds was used in the system of water treatment in one village in Nicaragua by BIOMASA [207]. It was proven that the shelled seeds are more efficient in reducing turbidity from waters than unshelled ones [208].Further, the unshelled flour of seeds contains a high percentage of crude fiber (% w/w) compared to the kernel, while the kernel is richer in protein compared to the shells [108].In addition, the activity of the water extract of Moringa Oleifera seeds demonstrated that it contains lectin that has a coagulant and insecticidal properties [57,135,215,216]. A research study was carried out using lectin (cMoL), a new thermoresistant coagulant for purification extracted from Moringa Oleifera seed flour using a simple technique [209]. Recent research encourages the safe use of water-soluble Moringa Oleifera seed lectin (WSMoL) to treat water for human use [217,218].
4.2.3. Mechanisms of Coagulation and Flocculation
The zeta potential measure aims to determine the mechanism of the coagulation process, which depends on electrostatic forces between charges that are carried by colloidal particles [47]. Numerous studies showed that the dominant mechanisms of pollutant removal by Moringa Oleifera seed extract appear to consist of adsorption and charge neutralization of colloidal as well as destabilized colloids/particles [47,219], with a PI (Isoelectric point) active protein value higher than 9.6, and weight of molecule at < 6.5 kDa) [145]. Figure 2 shows the properties of cMoL coagulant through the binding of proteins positively charged with parts of the surface having negatively charged particles. Thus, the formation of floes caused by the particle collision will be led to interparticle saturation of the different sectors charged with observed coagulation [190,209,220]. The mechanism of coagulation of nonproteinic organic components proposed is that of a net-like process, similar to sweep coagulation processes [13]. It has been suggested that in the aqueous system, increasing pH values due to the availability of basic amino acids in the Moringa Oleifera seed protein play a role in accepting a proton from the water with the release of a hydroxyl group, which increases pH values [221]. The proteins found in Moringa Oleifera seeds play a main role in the coagulation process, and maintain adsorption power of the pH level ranging from 5 to 8.67. Furthermore, it was reported that seed kernels of Moringa Oleifera consist of large quantities of positive protein charges that behave like magnets and attract negatively charged particles such as silt, clay, and other harmful particles [175,221]. Some researchers have indicated that binding sites in Moringa Oleifera proteins may be linked to changes in pH due to competition between H+/H3O+ and OH− ions [13]. Further, in the case of low pH values, the competition is between H+/H3O+ and metal ions on the binding of Moringa Oleifera proteins [222]. It is reported that bivalent cations like Ca2+and Mg2+ play a role informing a structured net-like insoluble that has the ability to capture suspended particles, which leads to a highly enhanced effect of coagulate in Moringa Oleifera seed extracts [186]. Further, due to this phenomenon, the textile effluent contains a high percentage of hazardous substances, especially Azodye compounds, reaching 60–70%. Researchers have been investigating the removal of dyes via the use of biomaterials such as Moringa Oleifera seeds as an interesting natural coagulant [30]. Previous studies reported that the Moringa Oleifera seed extract has demonstrated a high ability to remove several different types of dyes by a change in pH values [85,192,223,224,225]. It was reported that a higher dye removal spent wash rate from the distillery was achieved with lower pH values in the use of Moringa Oleifera seeds extraction (MOSE) as a coagulant aid [226]. Nevertheless, in other cases, the low pH conditions do not give higher removals, as in tannery wastewater [195]. It was also mentioned that the efficiency of C-F processes is not significantly influenced by the pH values due to the complex mixture of cationic and anionic species. Additionally, the effect of wastewater can play an important role between hydrophobic colloids and Moringa Oleifera proteins [13,227]. It was reported that coagulation was the proper mechanism for removing tetracycline from polluted water resulting from its interaction with sites of lectin binding [13,228]. The Coagulation mechanism of Moringa Oleifera lectin coagulant was presented in Fig.2. However, removal mechanisms are one of the most interesting gaps that need to be filled by other related research.
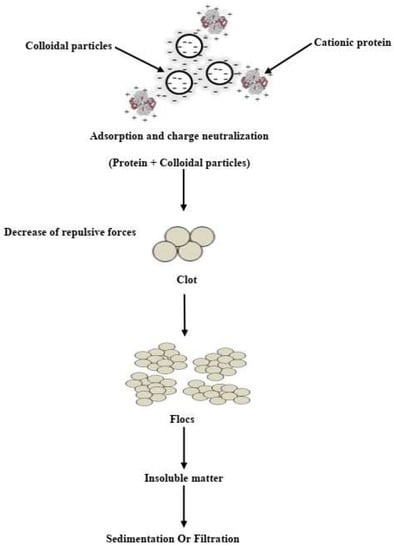
Figure 2.
Coagulation mechanism of Moringa Oleifera lectin coagulant (cMoL) [220].
4.2.4. Treatment of Wastewater Using Moringa Oleifera Seeds as a Coagulant
Municipal Wastewater Effluents
Employing Moringa Oleifera seeds (as powder or extracts) has been tested to remove pollutants from municipal wastewater effluent.
Table 5 shows detailed information on the use of Moringa Oleifera seeds and their extracts to treat municipal and industrial wastewater. It was reported that the turbidity reduced from 287 to 38.8 NTU when using Moringa Oleifera seed powder [229]. Many authors suggested that combining commercial coagulants with Moringa Oleifera seeds is a common practice in order to obtain the best results, as shown in Table 5. One of the reports stated that 64% of the COD was reduced and the amount of sludge produced after treatment of municipal effluents when coupling 100 mg/L of Moringa Oleifera with 10 mg/L (Alum) followed by a sandy filtration was lowered [17]. It was reported that a powder made from seeds of Moringa Oleifera possessed a higher efficacy reduction in the total bacteria population compared to alum and filters of sand applied in a stabilization pond [141]. Furthermore, the removal of turbidity reached its highest value (96.8%) with a combination of alum followed by WSMoL [230]. There is a substantial information gap in this area that is worth exploring, and new research directions should concentrate on real wastewater samples that contain the target pollutants.

Table 5.
Flocculating efficiencies of Moringa Oleifera seeds in treating municipal effluents.
Food Industry and Livestock Wastewater
Dairy industry wastewater treated by Moringa Oleifera seed was reported to have no better removal results. Using an increased dose of coagulant of 3 g/L (11% removal of COD, 53% removal of color, and 60%turbidity) is shown in Table 6 [234]. Reduction in the removal rate of COD can be caused by a transfer of a portion of the organic compounds from Moringa Oleifera seed to the effluent [13]. To solve this problem, some studies showed that ultrafiltration separation functioned well as a complementary treatment process. For example, one study reported an increase in COD removal from 39.4% to 98.5%, along with significant removal of both turbidity and color at 99.9% [235]. Bamboo leaves showed the best performance, among different organic filter materials (sawdust, coal, eucalyptus, gliricidia branches, and bamboo leaves), for wastewater treatment results from dairy cattle breeding [188]. Moringa Oleifera seeds Extraction (MOSE) showed great efficiency in turbidity removal of coffee cherry pulping wastewater reaching up to 93.66% and a pH of 4.27 [236].

Table 6.
Treated agro-industrial wastewater using Moringa Oleifera seeds (flour and extract).
Moreover, a better performance was obtained, as shown in Table 7, using Moringa Oleifera seeds (150 mg/L) and natural filter media in tapioca starch wastewater treatment after passing through a primary sedimentation tank followed by a two-stage clarifier (i.e., coconut fiber followed by sand) [242]. The use of MOSE as a flocculant increased the productivity of factories of cassava starch and increased efficiency by reducing sedimentation time by about 80%, thus allowing for a quick batch operation [243]. Coagulation tests with doses 0–4 g using MOSE for coffee wastewater treatment at pH range 3–7 revealed that the removal of TSS went from 8% to 54% [244]. Furthermore, the combined use of MOSE 3469 mg/L and flocculant (NALCO 7751) 6736 mg/L showed improvement in removing suspended particles, as shown in Table 7 [245]. In this case, understanding the removal of organic contaminants in wastewater treatment using Moringa Oleifera seeds is one of the most interesting gaps identified.

Table 7.
Use of seeds of Moringa Oleifera in the treatment of food industry effluent.
Treatment of Specific Wastewater
Moringa Oleifera is an important natural coagulant from environmental and economic points of view in treating refinery wastewater [247]. The removal of color and turbidity of surface water reaches above 90% along with decreased dissolved organic carbon (DOC) using Moringa Oleifera seed extract [89], as shown in Table 8. Although, protein rates in CaCl2 coagulant extract of seeds of Moringa Oleifera decreased compared to the NaCl extract. However, CaCl2 extract gives better treatment efficiency, reaching 78.9% for Microcystis Aeruginosa. This could be interpreted as being caused by calcium ions contributing to the coagulation-flocculation process that likely forms a net-like structure with coagulating compounds of Moringa Oleifera seeds [197]. The coagulation/flocculation (C/F) process using Moringa Oleifera seed powder followed by dissolved air flotation (DAF) gave the best removal value, reaching 96.5% for chlorophyll, and the sludge generated was rich in lipids and biodegradable, which means it could be a suitable alternative in biodiesel production [248]. Hospital wastewater treatment showed high efficiency performances in the removal of pollutants tested using MOP and MOP-PACl composite coagulant [138]. Given the expanding number of substances that are being considered for inclusion on the list of emerging contaminants, more studies are needed on this subject, which represents a significant knowledge gap.

Table 8.
Moringa Oleifera seeds as a coagulant for wastewater treatment.
Textile and Dye Industry Wastewater
Annual global production of commercial dyes and pigments is increasing, with Azo dyes representing the largest class of these dyes [254]. Textile effluent might damage aquatic and vegetable life in those cases when it is directly discharged into the environment [30]. In Table 9 presents the removal of turbidity and color from the textile and dye industry effluents and Table 10 shows treatment results of some textile effluents with the rate of removal efficiencies related to the difference in organic and inorganic loads for that wastewater. The Moringa Oleifera seeds were highly effective in removing several different types of dyes, such as Azo dyes, and not just anthraquinonic dyes [255]. The effectiveness of Moringa Oleifera seed in removing Rh-B dye from wastewater was reported [256]. Moreover, Moringa Oleifera demonstrated a high ability to remove anionic dyes, as shown in Table 11 [257]. Furthermore, it was suggested that pH adjustment is not needed in the treatment of reactive dyeing effluents and subsequent alkaline washing baths [223]. Many studies focus on dye removal using Moringa Oleifera seed salt extract (among them anionic dyes) because of its high ability to remove dyes in comparison with using just Moringa Oleifera seed powder [13]. It was reported that it is also used in the treatment of textile wastewater [258]. It was also reported that the combination of magnetic nanoparticles with the Moringa Oleifera seed protein as a coagulant decreases the measured values of the physico-chemical parameters and reduces settling time [259]. In addition, it was shown that applying iron oxide nanoparticles plays a role in accelerating separation, and increases the efficiency of removing four anionic synthetic dyes with a coagulation/flocculation process followed by magnetic sedimentation, as shown in Table 9 [260]. On the other hand, it was reported the ability of protein fractions derived from Moringa Oleifera seeds (albumin and globulin) as a natural coagulant functionalized with iron oxide magnetic nanoparticles and albumin (SlbFe) and was more able than globulin (GloVe) to remove RB5 [261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289]. However, the lack of information on the full potential of Moringa Oleifera seed extracts in nanoparticle synthesis is a significant knowledge gap that is worth exploring.

Table 9.
Removal of turbidity and color from the textile and dye industry effluents using Moringa Oleifera seed.

Table 10.
Treating textile industry effluents using seeds of Moringa Oleifera.

Table 11.
Efficient Removal dyes using Moringa Oleifera seed extract.
Metallic Ions Removal Using Moringa Oleifera Seeds as a Coagulant
Table 12 shows some studies that have been reported in the literature on the main metals and metalloids removed by seeds of Moringa Oleifera. The As (III) and As (V) removal processes are more sensitive to pH changes with Moringa Oleifera seed flour. The results proved that As (III) was absorbed at pH 7.0 while As (V) was weakly retained [69]. In the case of Cd, adsorption using Moringa Oleifera seed flour was a favorable effect with the decrease in particle size [270].Characterization of the Moringa Oleifera seed powder (MOSP) by Fourier-transform infrared spectrometry (FTIR) demonstrates that Cd and Cr removal with MOSP involves Cd, Cr (-amino acid) interactions that play the main role in the adsorption process of the contaminants [271,272]. Double filtration after treatment with seeds of Moringa Oleifera was suggested for the removal of heavy metals (Cu, Pb, Cd, Cr) from contaminated water, and the removal efficiencies were 95%, 93%, 76%, and 70%, respectively [72]. Significant improvement was reported in the removal rate reached for Zn at 84%, and 99% for both Mn and Ni using a bed column made of Pyrex glass that has an internal diameter of 4.5 cm and height of 55 cm, while a slight improvement in Cu removal was observed [273]. The removal of cadmium (Cd) using Moringa Oleifera seed flour has been reported to be in the range of 85.1% to 100% [72,270,274,275]. It has been reported that the range of removal of trivalent chromium (Cr (III)) was that of 70% to 99.29% using Moringa Oleifera seed flour [72,272,274,276,277,278,279,280,281,282,283,284,285,286,287,288,289], whereas removals of hexavalent chromium (Cr (VI)) were in the range of 47 to 97.5%, as reported [277,278,279]. Moringa Oleifera seed flour showed better coagulant performance in the removal of lead (Pb) with a percentage of more than 90% [72,274]. The removal of lead (Pb) increased when reducing the particle size of the powder of Moringa Oleifera seeds [271]. This could cause an accumulation of heavy metal making sludge and sediments unusable for agricultural purposes. This presents another knowledge gap that needs to increase further studies.

Table 12.
Optimal Metal Ion Reductions Using Moringa Oleifera seed flour and extract.
5. Conclusions and Recommendations
5.1. Conclusions
For decades, the search for natural-based products as an alternative to conventional synthetic coagulants has continued, and the uses of these in wastewater treatment are synonymous with plenty of challenges. Moringa Oleifera seed products are preferred if taking an environmentally friendly approach that avoids environmental contamination, because they possess more advantages over alternatives not only in terms their high efficiency in water treatment, but also for being safe, environmentally friendly, biodegradable, and low-cost. These benefits encourage their use in wastewater treatment.
This review of multiple previous studies provides valuable information related to the use of products related to Moringa Oleifera seeds in wastewater treatment which can be summarized as follows:
- Treating wastewater using Moringa Oleifera seed flour is an alternative method and highly interesting in developing countries, especially in small plants, as they have high cost-efficiency, in addition to their low toxicity;
- Moringa Oleifera seed flour shows good results in lowering turbidity and removing pollutants from municipal wastewater;
- Studies notice a significant improvement when purifying water, and in protein extraction of Moringa Oleifera seed flour when removing some contaminants, but the removal rate increases when specific pollutants are studied in samples of industrial wastewater;
- Many studies have proved that Moringa Oleifera seeds have shown antimicrobial activity against various pathogens such as bacteria, fungi, and viruses;
- Cationic proteins from seeds of Moringa Oleifera are viewed as sustainable materials possessing coagulation efficiency similar to chemical coagulants for treating a variety of wastewater samples for the removal of turbidity and toxic water ions without the development of dangerous sludge;
- Moringa Oleifera seeds improve efficiency and reduce the ultimate toxicity of treated wastewater when they are used in conjunction with chemical coagulants;
- Several studies have shown that Moringa Oleifera seed powder is promising and more effective compared to whole seeds of Moringa Oleifera;
- In order to avoid increasing the organic matter after wastewater treatment with Moringa Oleifera seed flour, it is recommended to purify the seeds before use;
- No significant effect on changes in pH was observed in the performance of Moringa Oleifera seeds when treating real wastewater. On the contrary, the pH has been found to have an effect on removing specific contaminants from synthetic wastewater;
- Economically, the increased demand for the use of Moringa Oleifera seeds in processing will help rural, poor people, and will benefit Moringa growers economically.
5.2. Recommendations
Despite the encouraging results reported for using Moringa Oleifera seeds in wastewater, there are some questions that still need to be answered that require further investigation:
- Further studies are needed to find out how the contents of seeds of Moringa Oleifera interact with colloids present in wastewater, and to understand their stability and behavior. Additionally, the study of the effect of grain size of seeds of Moringa Oleifera on the extraction of active substances need to be conducted;
- More studies should be performed to assess the performance of seed-derived flocculants according to pH values;
- Further, it is important to determine the detailed molecular mechanism of antimicrobial compounds from Moringa Oleifera seeds, and the method of attacking microbes;
- More studies should isolate and characterize the active compounds in Moringa oleifera seeds to determine the toxicity and optimum dose to be used as an effective antimicrobial;
- Although Moringa Oleifera seed flour has antimicrobial effects, these effects have not been found to significantly stop the growth of microbes for long time periods of detention. To find these, more studies are needed;
- Finally, further future work should be conducted on the use of products of Moringa Oleifera seed flour in removing heavy metals from wastewater.
Author Contributions
N.A.-J. performed conceptualization, data collection, sample analysis, and data analysis and wrote the first original draft of the manuscript. J.M. supervised the data collection, laboratory analysis, and provision of materials and reagents. M.B. contributed to project administration. D.D. contributed to the data analysis. S.E.H. and M.L. supervised and validated the research activity and review the final paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No Availability of data and materials.
Acknowledgments
This research was supported by International Institute of Water and Sanitation, National Office of Electricity and Drinking Water (IEA-ONEE).
Conflicts of Interest
The authors declare that there is not any conflict of interests regarding the publication of this manuscript.
Nomenclature
| CFS | Coagulation-Flocculation/Sedimentation |
| MO | Moringa Oleifera |
| MOS | Moringa Oleifera Seeds |
| WSP | Whole Seed Powder |
| WSMoL | The water-soluble Moringa Oleifera lectin |
| MOC-DW | Moringa Oleifera coagulant-distilled water |
| MOC-SC | Moringa Oleifera Coagulant-Sodium Chloride |
| MOC-SC-pc | Moringa Oleifera Coagulant- Sodium Chloride-purified coagulant solution |
| cMoL | coagulant Moringa Oleifera lectin |
| TDS | Total Dissolved Solids |
| WE | Water extract |
| MOSP | Moringa Oleifera seed powder |
| MOCP | Moringa Oleifera coagulant protein |
| DW | distilled water |
| MOSE | Moringa Oleifera Seeds Extraction |
| RaCE | Residue after Coagulant Extraction |
| WEMOS | Water extract of Moringa Oleifera seeds |
| MOP | Moringa Oleifera seeds protein |
| MOP-PACl | Moringa Oleifera seeds protein-polyaluminum chloride |
| FD-DW MO | Distilled water-extracted freeze-dried Moringa Oleifera |
| Mo-CBP3 | Chitin-binding protein purified from Moringa Oleifera Lam |
| CBD | 4-(β-D-glucopyranosyl-1→4-α-L-rhamnopyranosyloxy) benzylthiocarboxamide |
| CBA | 4-(α-L-rhamnosyloxy)benzyl isothiocyanate |
| CBC | Methyl N-4-(α-Lrhamnopyranosyloxy)benzyl carbamate |
| F. oxysporum | Fusariumoxysporum |
| F. solani | Fusariumsolani |
| P. multocida | Pasturellamultocida |
| C. musae | Colletotrichummusae |
| C. gloeosporioides | Colletotrichumgloeosporioides |
| A. oryzae | Aspergillus oryzae |
| A. terreus | Aspergillus terreus |
| A. nidulans | Aspergillus nidulans |
| A. niger | Aspergillus niger |
| E. coli | Escherichia coli |
| V. cholerae | Vibriocholerae |
| S. aureus | Staphylococcus aureus |
| K. pneumonia | Klebsiellapneumonia |
| P. aeruginosa | Pseudomonas aeruginosa |
| B. subtilis | Bacillus subtilis |
| B. pumillus | Bacillus pumillus |
| B. megaterium | Bacillus megaterium |
| Ps. Fluorescens | Pseudomonas Fluorescens |
| Ser. Marcescens | Serratiamarcescens |
| S. dysenteriae | Shigelladysenteriae |
| S. zeamais | Sitophiluszeamais |
| C. famata | Candida famata |
| C. guilliermondii | Candida guilliermondii |
| C. parapsilosis | Candida parapsilosis |
| C. tropicalis | Candida tropicalis |
| C. ciferrii | Candida ciferrii |
| H. werneckii | Hortaeawerneckii |
| EBV-EA | Epstein-Barr virus EA |
| NDV | Newcastle Disease Virus |
References
- Dwarapureddi, B.K.; Saritha, V. Plant based Coagulants for Point of Use Water Treatment—A Review. Curr. Environ. Eng. 2016, 3, 61–76. [Google Scholar] [CrossRef]
- Hlaing, T.T.; Htwe, K.T.T.; Htike, K.M.M. A Study of The Some Physicochemical Properties of Water Sample from Ngamoeyeik Creek near North Okkalapa Township and Treated with Moringa oleifera L. (Dant-Da-Lun) Seed. 3rd Myanmar Korea Conf. Res. J. 2020, 3, 1937–1943. [Google Scholar]
- Armeloni, J.P.N.; de Oliveira, D.S.; Donadel, C.B. Natural Agents as Auxiliaries in Water Clarification: Literature Review and Experimental Evaluation. Acta Sci. Technol. 2020, 42, e44800. [Google Scholar] [CrossRef]
- Adesina, O.A.; Abdulkareem, F.; Yusuff, A.S.; Lala, M.; Okewale, A. Response Surface Methodology Approach to Optimization of Process Parameter for Coagulation Process of Surface Water Using Moringa oleifera Seed. S. Afr. J. Chem. Eng. 2019, 28, 46–51. [Google Scholar] [CrossRef]
- Bichi, M.H. A Review of the Applications of Moringa oleifera Seeds Extract in Water Treatment. Civ. Environ. Res. 2013, 3, 10. [Google Scholar]
- Alo, M.N.; Anyim, C.; Elom, M. Coagulation and Antimicrobial Activities of Moringa oleifera Seed Storage at 3 °C Temperature in Turbid Water. Adv. Appl. Sci. Res. 2012, 3, 887–894. [Google Scholar]
- Delelegn, A.; Sahile, S.; Husen, A. Water Purification and Antibacterial Efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018, 7, 10. [Google Scholar] [CrossRef]
- Montgomery, M.A.; Elimelech, M. Water And Sanitation in Developing Countries: Including Health in the Equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef]
- Isah, Z.; Sanni, F.; Talle, M.; Joseph, M. In Vitro Antimicrobial Activity of Water Extract of Moringa oleifera Leaf Stalk on Bacteria Normally Implicated in Eye Diseases. Acad. Arena 2010, 2, 80–82. [Google Scholar]
- Pritchard, M.; Mkandawire, T.; Edmondson, A.; O’Neill, J.G.; Kululanga, G. Potential of Using Plant Extracts for Purification of Shallow Well Water in Malawi. Phys. Chem. Earth Parts ABC 2009, 34, 799–805. [Google Scholar] [CrossRef]
- Sustainable Sanitation in Cities: A Framework for Action—Resources SuSanA. Available online: https://www.susana.org/en/knowledge-hub/resources-and-publications/library/details/1019 (accessed on 16 November 2022).
- Water, Sanitation, Hygiene and Health: A Primer for Health Professionals; 2019 (WHO/CED/PHE/WSH/19.149). Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019.
- Villaseñor-Basulto, D.L.; Astudillo-Sánchez, P.D.; del Real-Olvera, J.; Bandala, E.R. Wastewater Treatment Using Moringa oleifera Lam Seeds: A Review. J. Water Process Eng. 2018, 23, 151–164. [Google Scholar] [CrossRef]
- Ravikumar, K.; Sheeja, A.K. Water Clarification Using Moringa oleifera Seed Coagulant. In Proceedings of the 2012 International Conference on Green Technologies (ICGT), Trivandrum, India, 18–20 December 2012; pp. 064–070. [Google Scholar] [CrossRef]
- Pritchard, M.; Craven, T.; Mkandawire, T.; Edmondson, A.S.; O’Neill, J.G. A Comparison between Moringa oleifera and Chemical Coagulants in the Purification of Drinking Water—An Alternative Sustainable Solution for Developing Countries. Phys. Chem. Earth Parts ABC 2010, 35, 798–805. [Google Scholar] [CrossRef]
- Vieira, A.M.S.; Vieira, M.F.; Silva, G.F.; Araújo, Á.A.; Fagundes-Klen, M.R.; Veit, M.T.; Bergamasco, R. Use of Moringa oleifera Seed as a Natural Adsorbent for Wastewater Treatment. Water. Air. Soil Pollut. 2010, 206, 273–281. [Google Scholar] [CrossRef]
- Bhuptawat, H.; Folkard, G.K.; Chaudhari, S. Innovative Physico-Chemical Treatment of Wastewater Incorporating Moringa oleifera Seed Coagulant. J. Hazard. Mater. 2007, 142, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, J.; Priyadharshini, D.; Soundammal, A.; Sudha, G.; Suriyakala, K. Wastewater Treatment Using Natural Coagulants. Int. J. Civ. Eng. 2017, 4, 40–42. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S. Use of Moringa oleifera Seeds as a Primary Coagulant in Wastewater Treatment. Environ. Technol. 1998, 19, 789–800. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Panico, A.; Pirozzi, F. Use of Acorn Leaves as a Natural Coagulant in a Drinking Water Treatment Plant. Water 2018, 11, 57. [Google Scholar] [CrossRef]
- Megersa, M.; Gach, W.; Beyene, A.; Ambelu, A.; Triest, L. Effect of Salt Solutions on Coagulation Performance of Moringa Stenopetala and Maerua Subcordata for Turbid Water Treatment. Sep. Purif. Technol. 2019, 221, 319–324. [Google Scholar] [CrossRef]
- Katayon, S.; Ng, S.C.; Johari, M.M.N.M.; Ghani, L.A.A. Preservation of Coagulation Efficiency of Moringa oleifera, a Natural Coagulant. Biotechnol. Bioprocess Eng. 2006, 11, 489–495. [Google Scholar] [CrossRef]
- Vijayaraghavan, G.; Rajasekaran, R.; Shanthakumar, S. Removal of Reactive Yellow Dye Using Natural Coagulants in Synthetic Textile Wastewater. Int. J. Chem. Sci. 2013, 11, 1824–1830. [Google Scholar]
- Saleem, M.; Bachmann, R.T. A Contemporary Review on Plant-Based Coagulants for Applications in Water Treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Jahn, S.A.A. Sudanese Native Methods for the Purification of Nile Water During the Flood Season; University of Khartoum: Khartoum, Sudan, 1976. [Google Scholar]
- Sajidu, S.M.I.; Henry, E.M.T.; Persson, I.; Masamba, W.R.L.; Kayambazinthu, D. pH dependence of sorption of Cd2+, Zn2+, Cu 2+ and Cr3+ on crude water and sodium chloride extracts of Moringa stenopetala and Moringa oleifera. Afr. J. Biotechnol. 2006, 5, 2397–2401. [Google Scholar] [CrossRef]
- Mataka, L.M.; Henry, E.M.T.; Masamba, W.R.L.; Sajidu, S.M. Lead Remediation of Contaminated Water Using Moringa Stenopetala and Moringa oleifera Seed Powder. Int. J. Environ. Sci. Technol. 2006, 3, 131–139. [Google Scholar] [CrossRef]
- Ali, E.N.; Muyibi, S.A.; Salleh, H.M.; Alam, Z.; Salleh, M.R.M. Production Technique of Natural Coagulant from Moringa Oleifra Seeds. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=2377e25b98e99943c1765e0091544078ece06bac (accessed on 11 January 2023).
- Bencheikh, I.; Azoulay, K.; Mabrouki, J.; El Hajjaji, S.; Dahchour, A.; Moufti, A.; Dhiba, D. The adsorptive removal of MB using chemically treated artichoke leaves: Parametric, kinetic, isotherm and thermodynamic study. Sci. Afr. 2020, 9, e00509. [Google Scholar] [CrossRef]
- Kansal, S.K.; Kumari, A. Potential of M. Oleifera for the Treatment of Water and Wastewater. Chem. Rev. 2014, 114, 4993–5010. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.T.A.; Coldebella, P.F.; Cardines, P.H.F.; Gomes, R.G.; Vieira, M.F.; Bergamasco, R.; Vieira, A.M.S. Coagulation–Flocculation Process with Ultrafiltered Saline Extract of Moringa oleifera for the Treatment of Surface Water. Chem. Eng. J. 2015, 276, 166–173. [Google Scholar] [CrossRef]
- Ersoy, B.; Tosun, I.; Günay, A.; Dikmen, S. Turbidity Removal from Wastewaters of Natural Stone Processing by Coagulation/Flocculation Methods. CLEAN-Soil Air Water 2009, 37, 225–232. [Google Scholar] [CrossRef]
- Mabrouki, J.; Moufti, A.; Bencheikh, I.; Azoulay, K.; El Hamdouni, Y.; El Hajjaji, S. Optimization of the coagulant flocculation process for treatment of leachate of the controlled discharge of the city Mohammedia (Morocco). In Advanced Intelligent Systems for Sustainable Development (AI2SD’2019) Volume 7-Advanced Intelligent Systems for Sustainable Development Applied in Energy and Electrical Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 200–212. [Google Scholar]
- Mabrouki, J.; Benbouzid, M.; Dhiba, D.; El Hajjaji, S. Simulation of wastewater treatment processes with Bioreactor Membrane Reactor (MBR) treatment versus conventional the adsorbent layer-based filtration system (LAFS). Int. J. Environ. Anal. Chem. 2022, 102, 7458–7468. [Google Scholar] [CrossRef]
- de Paula, H.M.; de Oliveira Ilha, M.S.; Andrade, L.S. Concrete Plant Wastewater Treatment Process by Coagulation Combining Aluminum Sulfate and Moringa oleifera Powder. J. Clean. Prod. 2014, 76, 125–130. [Google Scholar] [CrossRef]
- Gidde, M.R.; Bhalerao, A.R. Optimisation of Physical Parameters of Coagulation-Flocculation Process in Water Treatment. J. Environ. Res. Dev. 2011, 6, 99–110. [Google Scholar]
- Mabrouki, J.; Fattah, G.; Al-Jadabi, N.; Abrouki, Y.; Dhiba, D.; Azrour, M.; Hajjaji, S.E. Study, simulation and modulation of solar thermal domestic hot water production systems. Model. Earth Syst. Environ. 2022, 8, 2853–2862. [Google Scholar] [CrossRef]
- Oladoja, N.A. Headway on Natural Polymeric Coagulants in Water and Wastewater Treatment Operations. J. Water Process Eng. 2015, 6, 174–192. [Google Scholar] [CrossRef]
- Vijayaraghavan, G.; Sivakumar, T.; Kumar, A.V. Application of Plant Based Coagulants For Waste Water Treatment. Int. J. Adv. Eng. Res. Stud. 2011, 1, 88–92. [Google Scholar]
- Campbell, A.; Becaria, A.; Lahiri, D.K.; Sharman, K.; Bondy, S.C. Chronic Exposure to Aluminum in Drinking Water Increases Inflammatory Parameters Selectively in the Brain. J. Neurosci. Res. 2004, 75, 565–572. [Google Scholar] [CrossRef]
- Ribes, D.; Colomina, M.T.; Vicens, P.; Domingo, J.L. Effects of Oral Aluminum Exposure on Behavior and Neurogenesis in a Transgenic Mouse Model of Alzheimer’s Disease. Exp. Neurol. 2008, 214, 293–300. [Google Scholar] [CrossRef]
- Rondeau, V.; Jacqmin-Gadda, H.; Commenges, D.; Helmer, C.; Dartigues, J.-F. Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings from 15-Year Follow-up of the PAQUID Cohort. Am. J. Epidemiol. 2009, 169, 489–496. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, X.; Yang, J.; Suo, J.; Chen, J.; Liu, X.; Zhao, X. Chronic Exposure to Aluminum and Risk of Alzheimer’s Disease: A Meta-Analysis. Neurosci. Lett. 2016, 610, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Adie, P.A.; Igbum, O.G.; Shenge, G.A. Comparative Study of the Disinfection Effectiveness of Processed Moringa oleifera Seed Extract and Aluminium Sulphate in the Disinfection of Raw and Waste Waters. IOSR J. Appl. Chem. IOSR-JAC 2016, 9, 4–9. [Google Scholar]
- Ali, E.N. Removal of Heavy Metals from Water and Wastewater Using Moringa oleifera. Trace Elem. Environ.-New Approaches Recent Adv. 2020, 23, 64. [Google Scholar] [CrossRef]
- Bencheikh, I.; Azoulay, K.; Mabrouki, J.; El Hajjaji, S.; Moufti, A.; Labjar, N. The use and the performance of chemically treated artichoke leaves for textile industrial effluents treatment. Chem. Data Collect. 2021, 31, 100597. [Google Scholar] [CrossRef]
- Azoulay, K.; Bencheikh, I.; Mabrouki, J.; Samghouli, N.; Moufti, A.; Dahchour, A.; El Hajjaji, S. Adsorption mechanisms of azo dyes binary mixture onto different raw palm wastes. Int. J. Environ. Anal. Chem. 2021, 1–20. [Google Scholar] [CrossRef]
- Shan, T.C.; Matar, M.A.; Makky, E.A.; Ali, E.N. The Use of Moringa oleifera Seed as a Natural Coagulant for Wastewater Treatment and Heavy Metals Removal. Appl. Water Sci. 2017, 7, 1369–1376. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S.; Talbot, B.G. Active Agents and Mechanism of Coagulation of Turbid Waters Using Moringa oleifera. Water Res. 1995, 29, 703–710. [Google Scholar] [CrossRef]
- Yin, C.-Y. Emerging Usage of Plant-Based Coagulants for Water and Wastewater Treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef]
- Gidde, M.R.; Bhalerao, A.R.; Malusare, C.N. Comparative Study of Different Forms of Moringa oleifera Extracts for Turbidity Removal. Int. J. Eng. Res. Dev. 2012, 2, 14–21. [Google Scholar]
- Dezfooli, S.M.; Uversky, V.N.; Saleem, M.; Baharudin, F.S.; Hitam, S.M.S.; Bachmann, R.T. A Simplified Method for the Purification of an Intrinsically Disordered Coagulant Protein from Defatted Moringa oleifera Seeds. Process Biochem. 2016, 51, 1085–1091. [Google Scholar] [CrossRef]
- Mabrouki, J.; Azrour, M.; Hajjaji, S.E. Use of internet of things for monitoring and evaluating water’s quality: A comparative study. Int. J. Cloud Comput. 2021, 10, 633–644. [Google Scholar] [CrossRef]
- Camacho, F.P.; Sousa, V.S.; Bergamasco, R.; Ribau Teixeira, M. The Use of Moringa oleifera as a Natural Coagulant in Surface Water Treatment. Chem. Eng. J. 2017, 313, 226–237. [Google Scholar] [CrossRef]
- Egbuikwem, P.; Sangodoyin, A. Coagulation Efficacy of Moringa oleifera Seed Extract Compared to Alum for Removal of Turbidity and E. Coli in Three Different Water Sources. Eur. Int. J. Sci. Technol. 2013, 2, 13–20. [Google Scholar]
- Omodamiro, O.D.; Nwankwo, C.I.; Ejiofor, E.U. Antimicrobial and Coagulant Property of Moringa oleifera Seed in Water Purification. Sch. J. Agric. Vet. Sci. 2014, 1, 279–287. [Google Scholar]
- Mangundayao, K.; Yasurin, P. Bioactivity of Moringa oleifera and Its Applications: A Review. J. Pure Appl. Microbiol. 2017, 11, 43–50. [Google Scholar] [CrossRef]
- Oluduro, O.A.; Aderiye, B.I.; Connolly, J.D.; Akintayo, E.T.; Famurewa, O. Characterization and Antimicrobial Activity of 4-(β-d-Glucopyranosyl-1→4-α-l-Rhamnopyranosyloxy)-Benzyl Thiocarboxamide; a Novel Bioactive Compound from Moringa oleifera Seed Extract. Folia Microbiol. 2010, 55, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.S.; Napoleão, T.H.; Santos, A.F.S.; Sá, R.A.; Carneiro-da-Cunha, M.G.; Morais, M.M.C.; Silva-Lucca, R.A.; Oliva, M.L.V.; Coelho, L.C.B.B.; Paiva, P.M.G. Coagulant and Antibacterial Activities of the Water-Soluble Seed Lectin from Moringa oleifera: Coagulant and Antibacterial M. Oleifera Lectin. Lett. Appl. Microbiol. 2011, 53, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Mabrouki, J.; El Yadini, A.; Bencheikh, I.; Azoulay, K.; Moufti, A.; El Hajjaji, S. Hydrogeological and hydrochemical study of underground waters of the tablecloth in the vicinity of the controlled city dump mohammedia. In Advanced Intelligent Systems Applied to Environment; Springer International Publishing: Cham, Switzerland, 2019; pp. 22–33. [Google Scholar]
- Chuang, P.-H.; Lee, C.-W.; Chou, J.-Y.; Murugan, M.; Shieh, B.-J.; Chen, H.-M. Anti-Fungal Activity of Crude Extracts and Essential Oil of Moringa oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef]
- Kekuda, T.R.P.; Mallikarjun, N.; Swathi, D.; Nayana, K.V.; Aiyar, M.B.; Rohini, T.R. Antibacterial and Antifungal Efficacy of Steam Distillate of Moringa oleifera Lam. J. Pharm. Sci. 2010, 2, 34–37. [Google Scholar]
- El, R.S.R.; Abdalla, A.M. Evaluation of Antifungal Activity of Moringa oleifera Extracts as Natural Fungicide against Some Plant Pathogenic Fungi In-Vitro. J. Agric. Technol. 2014, 10, 963–982. [Google Scholar]
- Eilert, U.; Wolters, B.; Nahrstedt, A. The Antibiotic Principle of Seeds of Moringa oleifera and Moringa Stenopetala. Planta Med. 1981, 42, 55–61. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Wu, A. Mini Review on Antimicrobial Activity and Bioactive Compounds of Moringa oleifera. Med. Chem. 2016, 6, 578–582. [Google Scholar] [CrossRef]
- Rahmani, M.; Mabrouki, J.; Regraguy, B.; Moufti, A.; El’Mrabet, M.; Dahchour, A.; El Hajjaji, S. Adsorption of (methylene blue) onto natural oil shale: Kinetics of adsorption, isotherm and thermodynamic studies. Int. J. Environ. Anal. Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Sengupta, M.E.; Keraita, B.; Olsen, A.; Boateng, O.K.; Thamsborg, S.M.; Pálsdóttir, G.R.; Dalsgaard, A. Use of Moringa oleifera Seed Extracts to Reduce Helminth Egg Numbers and Turbidity in Irrigation Water. Water Res. 2012, 46, 3646–3656. [Google Scholar] [CrossRef]
- Mabrouki, J.; Bencheikh, I.; Azoulay, K.; Es-Soufy, M.; El Hajjaji, S. Smart monitoring system for the long-term control of aerobic leachate treatment: Dumping case Mohammedia (Morocco). In Big Data and Networks Technologies; Springer International Publishing: Cham, Switzerland, 2020; Volume 3, pp. 220–230. [Google Scholar]
- Basra, S.; Iqbal, Z.; Ur-Rehman, H.; Ejaz, M.F. Time Course Changes in PH, Electrical Conductivity and Heavy Metals (Pb, Cr) of Wastewater Using Moringa oleifera Lam. Seed and Alum, a Comparative Evaluation. J. Appl. Res. Technol. 2014, 12, 560–567. [Google Scholar] [CrossRef]
- Gautam, S.; Saini, G. Use of Natural Coagulants for Industrial Wastewater Treatment. Glob. J. Environ. Sci. Manag. 2020, 6, 553–578. [Google Scholar] [CrossRef]
- Regraguy, B.; Rahmani, M.; Mabrouki, J.; Drhimer, F.; Ellouzi, I.; Mahmou, C.; Dahchour, A.; Mrabet, M.E.; Hajjaji, S.E. Photocatalytic degradation of methyl orange in the presence of nanoparticles NiSO4/TiO2. Nanotechnol. Environ. Eng. 2022, 7, 157–171. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, P.; Srivastava, S.; Srivastava, M.M. Arsenic Removal from the Aqueous System Using Plant Biomass: A Bioremedial Approach. J. Ind. Microbiol. Biotechnol. 2005, 32, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Sharma, P.; Srivastava, S.; Srivastava, M.M. Biosorption Studies on Shelled Moringa oleifera Lamarck Seed Powder: Removal and Recovery of Arsenic from Aqueous System. Int. J. Miner. Process. 2006, 78, 131–139. [Google Scholar] [CrossRef]
- Ravikumar, K.; Sheeja, A.K. Heavy Metal Removal from Water Using Moringa oleifera Seed Coagulant and Double Filtration. Int. J. Sci. Eng. Res. 2013, 4, 10–13. [Google Scholar]
- Tsaknis, J.; Lalas, S.; Gergis, V.; Dourtoglou, V.; Spiliotis, V. Characterization of Moringa oleifera Variety Mbololo Seed Oil of Kenya. J. Agric. Food Chem. 1999, 47, 4495–4499. [Google Scholar] [CrossRef]
- Abrouki, Y.; Mabrouki, J.; Anouzla, A.; Rifi, S.K.; Zahiri, Y.; Nehhal, S.; El Yadini, A.; Slimani, R.; El Hajjaji, S.; Loukili, H.; et al. Optimization and modeling of a fixed-bed biosorption of textile dye using agricultural biomass from the Moroccan Sahara. Desalin Water Treat. 2021, 240, 144–151. [Google Scholar] [CrossRef]
- Oluduro, A.O.; Aderiye, J. Impact of Moringa Seed Extract on the Physicochemical Properties of Surface and Underground Water. Int. J. Biol. Chem. 2007, 1, 244–249. [Google Scholar] [CrossRef]
- Moulin, M.; Mossou, E.; Signor, L.; Kieffer-Jaquinod, S.; Kwaambwa, H.; Nermark, F.; Gutfreund, P.; Mitchell, E.; Haertlein, M.; Forsyth, V.; et al. Towards a Molecular Understanding of the Water Purification Properties of Moringa Seed Proteins. J. Colloid Interface Sci. 2019, 554, 296–304. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Ahmed, F.A.; Kurimoto, S.; Kim, S.-Y.; Shibata, H.; Fujioka, T.; Takaishi, Y. New α-Glucosides of Caffeoyl Quinic Acid from the Leaves of Moringa oleifera Lam. J. Nat. Med. 2012, 66, 217–221. [Google Scholar] [CrossRef]
- Maldini, M.; Maksoud, S.A.; Natella, F.; Montoro, P.; Petretto, G.L.; Foddai, M.; De Nicola, G.R.; Chessa, M.; Pintore, G. Moringa oleifera: Study of Phenolics and Glucosinolates by Mass Spectrometry’: Moringa oleifera, Glucosinolates, PCA, LC-MS. J. Mass Spectrom. 2014, 49, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Rashid, U. Physico-Chemical Characteristics of Moringa oleifera Seeds And Seed Oil From A Wild Provenance of Pakistan. Pak. J. Bot. 2007, 39, 1443–1453. [Google Scholar]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; Wadaan, M. In Vitro Evaluation of Cytotoxic Activities of Essential Oil from Moringa oleifera Seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 Cell Lines. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 4671–4675. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.L.O.; de Andrade, E.T.; Nolasco, K.C.V.; Castro, R.P.; Neto, P.C. Efficiency of mechanical extraction of Moringa oleifera according to different grain drying conditions. Res. Soc. Dev. 2020, 9, e937975133. [Google Scholar] [CrossRef]
- Khan, R.U.; Khan, A.; Naz, S.; Ullah, Q.; Laudadio, V.; Tufarelli, V.; Ragni, M. Potential Applications of Moringa oleifera in Poultry Health and Production as Alternative to Antibiotics: A Review. Antibiotics 2021, 10, 1540. [Google Scholar] [CrossRef]
- Coppin, J.P.; Juliani, H.R.; Wu, Q.; Simon, J.E. Variations in Polyphenols and Lipid Soluble Vitamins in Moringa oleifera. In Processing and Impact on Active Components in Food; Elsevier: Amsterdam, The Netherlands, 2015; pp. 655–663. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Muñoz-Serrano, A.; Peres, J.A. Towards Overcoming TOC Increase in Wastewater Treated with Moringa oleifera Seed Extract. Chem. Eng. J. 2012, 188, 40–46. [Google Scholar] [CrossRef]
- Madsen, M.; Schlundt, J.; Omer, E.F.E. Effect of Water Coagulation by Seeds of Moringa oleifera on Bacterial Concentrations. J. Ethnopharmacol. 1988, 22, 101–109. [Google Scholar] [CrossRef]
- Grabow, O.; Slabbert, J.; Morgan, W.; Jahn, A. Toxicity and Mutagenicity Evaluation of Water Coagulated with Moringa oleifera Seed Preparations Using Fish, Protozoan, Bacterial, Coliphage, Enzyme and Ames Salmonella Assays. Water SA 1985, 11, 9–14. [Google Scholar]
- Putra, R.S.; Amri, R.Y.; Ayu, M. Turbidity Removal of Synthetic Wastewater Using Biocoagulants Based on Protein and Tannin. AIP Conf. Proc. 2020, 2242, 040028. [Google Scholar] [CrossRef]
- Mabrouki, J.; Azoulay, K.; Elfanssi, S.; Bouhachlaf, L.; Mousli, F.; Azrour, M.; El Hajjaji, S. Smart system for monitoring and controlling of agricultural production by the IoT. In IoT and Smart Devices for Sustainable Environment; Springer International Publishing: Cham, Switzerland, 2022; pp. 103–115. [Google Scholar]
- Feihrmann, A.C.; Baptista, A.T.; Lazari, J.P.; Silva, M.O.; Vieira, M.F.; Vieira, A.M. Evaluation of Coagulation/Floculation Process for Water Treatment Using Defatted Cake from Moringa oleifera. Chem. Eng. Trans. 2017, 57, 1543–1548. [Google Scholar] [CrossRef]
- Ajibade, T.O.; Arowolo, R.; Olayemi, F.O. Phytochemical screening and toxicity studies on the methanol extract of the seeds of Moringa oleifera. J. Complement. Integr. Med. 2013, 10, 1–6. [Google Scholar] [CrossRef]
- Kaur, P.; Pandey, N. Pharmaceutical Properties of Moringa oleifera: A Review. J. Biol. Nat. 2020, 10, 18–22. [Google Scholar]
- Jahn, S.A.A. Using Moringa Seeds as Coagulants in Developing Countries. J.-Am. Water Works Assoc. 1988, 80, 43–50. [Google Scholar] [CrossRef]
- Zaid, A.Q.; Ghazali, S.B. Dataset on physicochemical properties of particle-sized Moringa oleifera seed cake and its application as bio-coagulants in water treatment application. Chem. Data Collect. 2019, 24, 100284. [Google Scholar] [CrossRef]
- Ignatov, I. Anti Inflammatory and Anti Viral Effects of Potassium (K) and Chemical Composition of Moringa. Asian J. Biol. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Okigbo, R.; Mmeka, E. Antimicrobial Effects of Three Tropical Plant Extracts On Staphylococcus aureus, Escherichia coli and Candida albicans. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 226–229. [Google Scholar] [CrossRef]
- Stafford, G.I.; Jäger, A.K.; van Staden, J. Effect of storage on the chemical composition and biological activity of several popular South African medicinal plants. J. Ethnopharmacol. 2005, 97, 107–115. [Google Scholar] [CrossRef]
- Zeng, K.; Li, Y.; Yang, W.; Ge, Y.; Xu, L.; Ren, T.; Zhang, H.; Zhuo, R.; Peng, L.; Chen, C.; et al. Moringa oleifera seed extract protects against brain damage in both the acute and delayed stages of ischemic stroke. Exp. Gerontol. 2019, 122, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Nepolean, P.; Anitha, J.; Renitta, R.E. Isolation, analysis and identification of phytochemicals of antimicrobial activity of Moringa oleifera Lam. Curr. Biot. 2009, 3, 33–39. [Google Scholar]
- Onuoha, V.C.; Enweani, I.B.; Okereke, O.E. Susceptibility Pattern of Different Parts of Moringa oleifera against Some Pathogenic Fungi, Isolated from Sputum Samples of HIV Positive Individuals Co-Infected with Pulmonary Tuberculosis. J. Adv. Microbiol. 2020, 20, 56–82. [Google Scholar] [CrossRef]
- Bukar, A.; Oyeyi, T. Antimicrobial profile of Moringa oleifera Lam. extracts against some food-borne microorganisms. Bayero J. Pure Appl. Sci. 2010, 3, 43–48. [Google Scholar] [CrossRef]
- Feitosa, P.R.B.; Santos, T.R.J.; Gualberto, N.C.; Narain, N.; de Aquino Santana, L.C.L. Bioactive potential of Moringa seeds (Moringa oleifera Lamarck) after solid-state fermentation process. Res. Soc. Dev. 2020, 9, 16. [Google Scholar] [CrossRef]
- Chollom, S.C.; Agada, G.O.A.; Gotep, J.G.; Mwankon, S.E.; Dus, P.C.; Bot, Y.S.; Nyango, D.Y.; Singnap, C.L.; Fyaktu, E.J.; Okwori, A.E.J. Investigation of aqueous extract of Moringa oleifera lam seed for antiviral activity against newcastle disease virus in ovo. J. Med. Plant Res. 2010, 6, 3870–3875. [Google Scholar] [CrossRef]
- Foild, N.; Makkar, H.P.S.; Becker, K. The Potential of Moringa oleifera For Agricultural and Industrial Uses. Available online: https://moringatrees.org/moringa-doc/the_potential_of_moringa_oleifera_for_agricultural_and_industrial_uses.pdf (accessed on 12 January 2023).
- Lalas, S.; Tsaknis, J. Characterization of Moringa oleifera Seed Oil Variety “Periyakulam 1”. J. Food Compos. Anal. 2002, 15, 65–77. [Google Scholar] [CrossRef]
- Gu, X.; Yang, Y.; Wang, Z. Nutritional, phytochemical, antioxidant, α-glucosidase and α-amylase inhibitory properties of Moringa oleifera seeds. S. Afr. J. Bot. 2020, 133, 151–160. [Google Scholar] [CrossRef]
- Delange, D.M.; Murillo, R.V.; Canavaciolo, V.L.G.; Amaro, J.G. Fatty acid composition of seed oil from Moringa oleifera grown in Havana, Cuba. Rev. Cuba. Plantas Med. 2014, 19, 197–204. [Google Scholar]
- Boukandoul, S.; Casal, S.; Mendes, E.; Zaidi, F. Moringa oleifera defatted flour: Nutritive and bioactive impact of shells. J. Food Process. Preserv. 2020, 44, e14693. [Google Scholar] [CrossRef]
- Aly, A.A.; Maraei, R.W.; Ali, H.G.M. Fatty Acids Profile and Chemical Composition of Egyptian Moringa oleifera Seed Oils. J. Am. Oil Chem. Soc. 2016, 93, 397–404. [Google Scholar] [CrossRef]
- Bencheikh, I.; Mabrouki, J.; Azoulay, K.; Moufti, A.; El Hajjaji, S. Predictive analytics and optimization of wastewater treatment efficiency using statistic approach. In Big Data and Networks Technologies; Springer International Publishing: Cham, Switzerland, 2020; Volume 3, pp. 310–319. [Google Scholar]
- dos Santos Barbosa, M.; de Carvalho Peres, A.A.; Silva Lima, Á.; Faria Soares, C.M. Contribuição Dos Insumos No Custo Total Do Bioprocesso Para produção De Biolubrificante Em Escala De laboratório. Sustentabilidade 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Faisal, M.I.; Iqbal, S.; Basra, S.M.A.; Afzal, I.; Saddiq, M.S.; Bakhtavar, M.A.; Hafeez, M.B.; Rehman, H.; Basit, A.; Habib-ur-Rahman, M. Moringa landraces of Pakistan are potential source of premium quality oil. S. Afr. J. Bot. 2020, 129, 397–403. [Google Scholar] [CrossRef]
- Kayode, R.M.O.; Afolayan, A.J. Cytotoxicity and effect of extraction methods on the chemical composition of essential oils of Moringa oleifera seeds. J. Zhejiang Univ.-Sci. B 2015, 16, 680–689. [Google Scholar] [CrossRef]
- Ayerza, R. Seed yield components, oil content, and fatty acid composition of two cultivars of moringa (Moringa oleifera Lam.) growing in the Arid Chaco of Argentina. Ind. Crops Prod. 2011, 33, 389–394. [Google Scholar] [CrossRef]
- Ayerza, R. Seed characteristics, oil content and fatty acid composition of Moringa (Moringa oleifera Lam.) seeds from three arid land locations in Ecuador. Ind. Crops Prod. 2019, 140, 111757. [Google Scholar] [CrossRef]
- Loukili, H.; Anouzla, A.; Jioui, I.; Achiou, B.; Alami Younssi, S.; Azoulay, K.; Bencheikh, I.; Mabrouki, J.; Abrouki, Y.; Sebbahi, S.; et al. Combining multiple regression and principal component analysis to evaluate the effects of ambient air pollution on children’s respiratory diseases. Int. J. Inf. Tecnol. 2022, 14, 1305–1310. [Google Scholar] [CrossRef]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Res. 1999, 33, 3373–3378. [Google Scholar] [CrossRef]
- Nzikou, J.M.; Matos, L.; Moussounga, J.E.; Ndangui, C.B.; Kimboguila, A.; Silou, T.; Linder, M.; Desobry, S. Characterization of Moringa oleifera seed oil variety Congo-Brazaville. J. Food Technol. 2009, 7, 59–65. [Google Scholar]
- Mabrouki, J.; Benbouzid, M.; Dhiba, D.; El Hajjaji, S. Internet of Things for Monitoring and Detection of Agricultural Production. In Intelligent Systems in Big Data, Semantic Web and Machine Learning; Gherabi, N., Kacprzyk, J., Eds.; Springer: Cham, Switzerland, 2021; pp. 271–282. [Google Scholar] [CrossRef]
- Nordmark, B.A.; Przybycien, T.M.; Tilton, R.D. Comparative coagulation performance study of Moringa oleifera cationic protein fractions with varying water hardness. J. Environ. Chem. Eng. 2016, 4, 4690–4698. [Google Scholar] [CrossRef]
- Mgalaa, S.; Mabrouki, J.; Elouardi, M.; El Azzouzi, L.; Moufti, A.; El Hajjaji, S.; EL Azzouzi, M.; El Belghiti, M.A. Study and evaluation of the degradation of procion blue dye by the ozonation method: Parametric and isothermal study. Nanotechnol. Environ. Eng. 2022, 7, 691–697. [Google Scholar] [CrossRef]
- Gámez, L.; Luna-delRisco, M.; Cano, R. Effect of storage and preparation methods of Moringa oleifera seeds during the coagulation process. Desalination Water Treat. 2015, 57, 16376–16383. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Abdulkarim, S.M.; Long, K.; Lai, O.M.; Muhammad, S.K.S.; Ghazali, H.M. Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem. 2005, 93, 253–263. [Google Scholar] [CrossRef]
- Rahman, I.M.M.; Barua, S.; Nazimuddin, M.; Begum, Z.A.; Rahman, M.A.; Hasegawa, H. Physicochemical Properties of Moringa oleifera Lam. Seed Oil of the Indigenous-Cultivar of Bangladesh. J. Food Lipids. 2009, 16, 540–553. [Google Scholar] [CrossRef]
- Lalas, S.; Tsaknis, J.; Gergis, V.; Spiliotis, V. A total characterisation of Moringa oleifera Malawi seed oil, Riv. Ital. Delle Sostanze Grasse. 1998, 75, 21–27. [Google Scholar]
- Chen, R.; Wang, X.-J.; Zhang, Y.-Y.; Xing, Y.; Yang, L.; Ni, H.; Li, H.-H. Simultaneous extraction and separation of oil, proteins, and glucosinolates from Moringa oleifera seeds. Food Chem. 2019, 300, 125162. [Google Scholar] [CrossRef]
- Al-Ghamdi, F.A. Fatty acids and Macroelements of Moringa (M. peregrina and M. Oleifera) Seed Oils. Pak. J. Nutr. 2018, 17, 609–614. [Google Scholar] [CrossRef]
- Bouanga-Kalou, G.; Dhellot, J.R.; Matos, L.; Kimbonguila, A.; Mountou-Tchitoula, D.; Malela, K.E.; Hounounou, C.H.; Nzikou, J.M.; Silou, T.; Desobry, S. Characteristic Physicochemical of Oil Extract from Moringa oleifera and the Kinetics of Degradation of the Oil during Heating. Res.J. Appl. Sci. Eng. Technol. 2014, 7, 3649–3655. [Google Scholar] [CrossRef]
- Santos, A.F.S.; Argolo, A.C.C.; Coelho, L.C.B.B.; Paiva, P.M.G. Detection of water soluble lectin and antioxidant component from Moringa oleifera seeds. Water Res. 2005, 39, 975–980. [Google Scholar] [CrossRef]
- Shebek, K.; Schantz, A.B.; Sines, I.; Lauser, K.; Velegol, S.; Kumar, M. The Flocculating Cationic Polypetide from Moringa oleifera Seeds Damages Bacterial Cell Membranes by Causing Membrane Fusion. Langmuir ACS J. Surf. Colloids. 2015, 31, 4496–4502. [Google Scholar] [CrossRef]
- Guevara, A.P.; Vargas, C.; Sakurai, H.; Fujiwara, Y.; Hashimoto, K.; Maoka, T.; Kozuka, M.; Ito, Y.; Tokuda, H.; Nishino, H. An antitumor promoter from Moringa oleifera Lam. Mutat. Res. 1999, 440, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Kitazono, Y.; Jiwajinda, S.; Koshimizu, K.; Ohigashi, H. Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor-promoter-induced Epstein-Barr virus activation. Planta Med. 1998, 64, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Ali, A.; Khan, S.; Ahmed, S.; Attique, Z.; Rehman, S.U.; Khan, A.; Ali, H.; Rizwan, M.; Munir, A.; et al. nCOV-19 peptides mass fingerprinting identification, binding, and blocking of inhibitors flavonoids and anthraquinone of Moringa oleifera and hydroxychloroquine. J. Biomol. Struct. Dyn. 2020, 39, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.H.; El-Taweel, G.E.; Ali, M.A. The cytotoxicity and antimicrobial efficiency of Moringa oleifera seeds extracts. Int. J. Environ. Stud. 2004, 61, 699–708. [Google Scholar] [CrossRef]
- Gupta, D.; Soin, D. Recent Advances in Health Benefits of Moringa oleifera. Int. J. Pharm. Sci. Nanotechnol. 2020, 13, 4870–4875. [Google Scholar] [CrossRef]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef]
- Kalogo, Y.; M’Bassiguié Séka, A.; Verstraete, W. Enhancing the start-up of a UASB reactor treating domestic wastewater by adding a water extract of Moringa oleifera seeds. Appl. Microbiol. Biotechnol. 2001, 55, 644–651. [Google Scholar] [CrossRef]
- Kwabena Ntibrey, R.A.; Kuranchie, F.A.; Gyasi, S.F. Antimicrobial and coagulation potential of Moringa oleifera seed powder coupled with sand filtration for treatment of bath wastewater from public senior high schools in Ghana. Heliyon 2020, 6, e04627. [Google Scholar] [CrossRef]
- Noumi, E.; Manga, P.N. Traditional Medicines for HIV/AIDS and Opportunistic Infections in North-West Cameroon: Case of Skin Infections. Int. J. Trop. Dis. Health 2011, 1, 44–64. [Google Scholar]
- Rocha, M.; Alencar, L.; Brilhante, R.; Sales, J.; Ponte, Y.; Rodrigues, P.; Sampaio, C.; Castelo-Branco, D.; Oliveira, F.; Barbosa, F.; et al. Moringa oleifera inhibits growth of Candida spp. and Hortaea werneckii isolated from Macrobrachium amazonicum prawn farming with a wide margin of safety. Cienc. Rural 2014, 44, 2197–2203. [Google Scholar] [CrossRef]
- Broin, M.; Santaella, C.; Cuine, S.; Kokou, K.; Peltier, G.; Joët, T. Flocculent activity of a recombinant protein from Moringa oleifera Lam. Seeds. Appl. Microbiol. Biotechnol. 2002, 60, 114–119. [Google Scholar] [CrossRef]
- Saadabi, A.M.; Zaid, I.E.A. An In vitro Antimicrobial Activity of Moringa oleifera L. Seed Extracts Against Different Groups of Microorganisms. Aust. J. Basic Appl. Sci. 2011, 5, 129–134. [Google Scholar]
- Suarez, M.; Haenni, M.; Canarelli, S.; Fisch, F.; Chodanowski, P.; Servis, C.; Michielin, O.; Freitag, R.; Moreillon, P.; Mermod, N. Structure-Function Characterization and Optimization of a Plant-Derived Antibacterial Peptide, Antimicrob. Agents Chemother. 2005, 49, 3847–3857. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, R.; Shahid, M.; Jamil, A.; Ashraf, M. Microscopic Evaluation of The Antimicrobial Activity of Seed Extracts of Moringa oleifera. Pak. J. Bot. 2008, 40, 1349–1358. [Google Scholar]
- Sutherland, J.P.; Folkard, G.K.; Grant, W.D. Natural coagulants for appropriate water treatment: A novel approach. Waterlines 1990, 8, 30–32. [Google Scholar] [CrossRef]
- Ghebremichael, K.A. Moringa Seed and Pumice as Alternative Natural Materials for Drinking Water Treatment. Ph.D. Thesis, Royal Institute of Technology, Division of Land and Water Resources, Stockholm, Sweden, 2004. [Google Scholar]
- Olsen, A. Low technology water purification by bentonite clay and Moringa oleifera seed flocculation as performed in Sudanese villages: Effects on Schistosoma mansoni cercariae. Water Res. 1987, 21, 517–522. [Google Scholar] [CrossRef]
- Molan, A.L.; Alexander, R.A.; Brookes, I.M.; McNabb, W.C. Effects of an extract from sulla (Hedysarum coronarium) containing condensed tannins on the migration of three sheep gastrointestinal nematodes in vitro. Proc. N. Z. Soc. Anim. Prod. 2000, 60, 21–25. [Google Scholar]
- Khayra, S.; Ahmed, M.; Nawal, O.; Ahlem, Y.; Linda, D.R.; Noura, S.; Yahia, H. Antibacterial Activity of Moringa oleifera Seeds Extract on Pathogenic Bacteria Isolated from Wastewater of Oued Bechar, Bechar Province, Southwest Algeria. S. Asian J. Exp. Biol. 2020, 10, 121–129. [Google Scholar] [CrossRef]
- Aldakheel, R.K.; Rehman, S.; Almessiere, M.A.; Khan, F.A.; Gondal, M.A.; Mostafa, A.; Baykal, A. Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy. Pharmaceuticals 2020, 13, 193. [Google Scholar] [CrossRef]
- Batista, A.B.; Oliveira, J.T.A.; Gifoni, J.M.; Pereira, M.L.; Almeida, M.G.G.; Gomes, V.; Da Cunha, M.; Ribeiro, S.F.F.; Dias, G.B.; Beltramini, L.; et al. New Insights into the Structure and Mode of Action of Mo-CBP3, an Antifungal Chitin-Binding Protein of Moringa oleifera Seeds. PLoS ONE 2014, 9, e111427. [Google Scholar] [CrossRef]
- Gifoni, J.M.; Oliveira, J.T.A.; Oliveira, H.D.; Batista, A.B.; Pereira, M.L.; Gomes, A.S.; Oliveira, H.P.; Grangeiro, T.B.; Vasconcelos, I.M. A novel chitin-binding protein from Moringa oleifera seed with potential for plant disease control. Biopolymers 2012, 98, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Freire, J.E.C.; Vasconcelos, I.M.; Moreno, F.B.M.B.; Batista, A.B.; Lobo, M.D.P.; Pereira, M.L.; Lima, J.P.M.S.; Almeida, R.V.M.; Sousa, A.J.S.; Monteiro-Moreira, A.C.O.; et al. Mo-CBP3, an Antifungal Chitin-Binding Protein from Moringa oleifera Seeds, Is a Member of the 2S Albumin Family. PLoS ONE 2015, 10, e0119871. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.E.M.; Farias, D.F.; Carvalho, A.F.U.; Oliveira, J.T.A.; Pereira, M.L.; Grangeiro, T.B.; Freire, J.E.C.; Viana, D.A.; Vasconcelos, I.M. Food safety assessment of an antifungal protein from Moringa oleifera seeds in an agricultural biotechnology perspective. Food Chem. Toxicol. 2015, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zubairu, T.; Gwa, V.I. Antifungal Activity of Azadirachta Indica A. Juss And Moringa oleifera L. Seed Extracts Against Rot Fungi of Hot Pepper (Capsicum annuum L.) Fruits In Dutsin-Ma, Katsina State, Nigeria. FUDMA JAAT 2020, 5, 254–265. [Google Scholar]
- Al-Jadabi, N.; Laaouan, M.; Mabrouki, J.; Fattah, G.; El Hajjaji, S. Comparative study of the coagulation efficacy of Moringa oleifera seeds extracts to alum for domestic wastewater treatment of Ain Aouda City, Morocco. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 314, p. 08003. [Google Scholar]
- Ghernaout, D. Water Treatment Challenges towards Viruses Removal. Open Access Libr. J. 2020, 7, 22. [Google Scholar] [CrossRef]
- Esimone, C.O.; Ofokansi, K.C.; Adikwu, M.U.; Ibezim, E.C.; Abonyi, D.O.; Odaibo, G.N.; Olaleye, D.O. In vitro evaluation of the antiviral activity of extracts from the lichen Parmelia perlata (L.). against three RNA viruses. J. Infect. Dev. Ctries 2007, 1, 315–320. [Google Scholar] [CrossRef]
- Popoola, J.O.; Obembe, O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Chadrakant Sangar, V. In Silico Approach to Combat HIV Using Phytoconstituents of Moringa oleifera Lam. Juniper Online J. ImmunoVirol. 2015, 1, 555558. [Google Scholar] [CrossRef]
- Jadabi, N.A.; Laaouan, M.; Mabrouki, J.; Hajjaji, S.E. Study of the efficacy of coagulation-flocculation process in domestic wastewater treatment plant (WWTP) from the city of Hattane (MOROCCO). J. Adv. Res. Dyn. Control. Syst. 2020, 12, 147–157. [Google Scholar] [CrossRef]
- Agoyi, E.E.; Assogbadjo, A.E.; Gouwakinnou, G.; Okou, F.A.Y.; Sinsin, B. Ethnobotanical Assessment of Moringa oleifera Lam. in Southern Benin (West Africa). Ethnobot. Res. Appl. 2014, 12, 551–560. [Google Scholar]
- Chinsembu, K.C.; Hedimbi, M. An ethnobotanical survey of plants used to manage HIV/AIDS opportunistic infections in Katima Mulilo, Caprivi region, Namibia. J. Ethnobiol. Ethnomed. 2010, 6, 25. [Google Scholar] [CrossRef]
- Lamorde, M.; Tabuti, J.R.S.; Obua, C.; Kukunda-Byobona, C.; Lanyero, H.; Byakika-Kibwika, P.; Bbosa, G.S.; Lubega, A.; Ogwal-Okeng, J.; Ryan, M.; et al. Medicinal plants used by traditional medicine practitioners for the treatment of HIV/AIDS and related conditions in Uganda. J. Ethnopharmacol. 2010, 130, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.A. Ethnobotanical field survey of medicinal plants used by traditional medicine practitioners to manage HIV/AIDS opportunistic infections and their prophylaxis in Keffi Metropolis. Asian J. Plant Sci. Res. 2014, 4, 7–14. [Google Scholar]
- Asiimwe, S.; Kamatenesi-Mugisha, M.; Namutebi, A.; Borg-Karlsson, A.-K.; Musiimenta, P. Ethnobotanical study of nutri-medicinal plants used for the management of HIV/AIDS opportunistic ailments among the local communities of western Uganda. J. Ethnopharmacol. 2013, 150, 639–648. [Google Scholar] [CrossRef]
- Ohemu, T.L.; Agunu, A.; Olotu, P.N.; Ajima, U.; Dafam, D.G.; Azila, J.J. Ethnobotanical Survey of Medical Plants Used in the Traditional Treatment of Viral Infections in Jos, Plateau State, Nigeria. Int. J. Med. Arom. Plants 2014, 4, 74–81. [Google Scholar]
- Monera, T.G.; Maponga, C.C. Prevalence and patterns of Moringa oleifera use among HIV positive patients in Zimbabwe: A cross-sectional survey. J. Public Health Afr. 2012, 3, 22–24. [Google Scholar] [CrossRef]
- Sonika, S.D.; Singh, T.G.; Arora, G.; Arora, S. Moringa Gum: A Comprehensive Review On Its Physicochemical And Functional Properties. Plant Arch. 2020, 20, 3794–3805. [Google Scholar]
- Aja, P.M.; Nwachukwu, N.; Ibiam, U.A.; Igwenyi, I.O.; Offor, C.E.; Orji, U.O. Chemical Constituents of Moringa oleifera Leaves and Seeds from Abakaliki, Nigeria. Am. J. Phytomed. Clin. Ther. 2014, 2, 310–321. [Google Scholar]
- Moura, M.; Napoleão, T.; Coriolano, M.; Paiva, P.; Figueiredo, R.; Coelho, L. Water-soluble Moringa oleifera lectin interferes with growth, survival and cell permeability of corrosive and pathogenic bacteri. J. Appl. Microbiol. 2015, 119, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Ruttarattanamongkol, K.; Petrasch, A. Antimicrobial activities of Moringa oleifera seed and seed oil residue and oxidative stability of its cold pressed oil compared with extra virgin olive oil. Songklanakarin J. Sci. Technol. 2015, 37, 587–594. [Google Scholar]
- Monaco, P.A.V.L.; Matos, A.; Ribeiro, I.; Nascimento, F.D.S.; Sarmento, A.P. Use of extract of Moringa seeds as coagulant agent in treatment of water supply and wastewater. Ambient. Agua-Interdiscip. J. Appl. Sci. 2010, 5, 222–231. [Google Scholar] [CrossRef]
- Nonfodji, O.M.; Fatombi, J.K.; Ahoyo, T.A.; Osseni, S.A.; Aminou, T. Performance of Moringa oleifera seeds protein and Moringa oleifera seeds protein-polyaluminum chloride composite coagulant in removing organic matter and antibiotic resistant bacteria from hospital wastewater. J. Water Process Eng. 2020, 33, 101103. [Google Scholar] [CrossRef]
- Regraguy, B.; Ellouzi, I.; Mabrouki, J.; Rahmani, M.; Drhimer, F.; Mahmou, C.; Dahchour, A.; El Mrabet, M.; El Hajjaji, S. Zinc doping of different nanoparticles of TiO2 Sachtopore for improved elimination of the methyl orange by photocatalysis. Emergent Mater. 2022, 5, 1945–1958. [Google Scholar] [CrossRef]
- Arantes, C.C.; Ribeiro, T.A.P.; Paterniani, J.E.S. Processing of Moringa oleifera seeds using different equipments to obtain coagulant solution. Rev. Bras. Eng. Agríc. Ambient. 2012, 16, 661–666. [Google Scholar] [CrossRef]
- Abdulsalam, S.; Gital, A.; Misau, I.; Suleiman, M.; Rastilantie, M. Water clarification using Moringa oleifera seed coagulant: Maiduguri raw water as a case study WFL Publisher Science and Technology. J. Food Agric. Environ. 2007, 5, 302–306. [Google Scholar]
- Fadele, O.; Afolabi, A.; Oloyede, D.; Adedire, O.; Bankole, H.; Adetunji, A. Specific energy consumption of a Moringa oleifera seed shelling machine. Res. Agric. Eng. 2020, 66, 104–111. [Google Scholar] [CrossRef]
- Okuda, T.; Ali, E.N. Application of Moringa oleifera Plant in Water Treatment. In Water and Wastewater Treatment Technologies; Bui, X.-T., Chiemchaisri, C., Fujioka, T., Varjani, S., Eds.; Springer: Singapore, 2019; pp. 63–79. [Google Scholar] [CrossRef]
- Magalhães, E.R.B.; de Menezes, N.N.F.; Silva, F.L.; Garrido, J.W.A.; Sousa, M.A.D.S.B.; dos Santos, E.S. Effect of oil extraction on the composition, structure, and coagulant effect of Moringa oleifera seeds. J. Clean. Prod. 2020, 279, 123902. [Google Scholar] [CrossRef]
- Muyibi, S.A.; Evison, L.M. Coagulation of turbid water and softening of hardwater with Moringa oleifera seeds. Int. J. Environ. Stud. 1996, 3, 247–259. [Google Scholar] [CrossRef]
- Muyibi, S.A.; Okuofu, C.A. Coagulation of low turbidity surface waters with Moringa oleifera seeds. Int. J. Environ. Stud. 1995, 48, 263–273. [Google Scholar] [CrossRef]
- Schulz, C.R.; Okun, D.A. Treating surface waters for communities in developing countries. J. AWWA 1983, 75, 212–219. [Google Scholar] [CrossRef]
- Ghebremichael, K.A.; Gunaratna, K.R.; Henriksson, H.; Brumer, H.; Dalhammar, G. A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res. 2005, 39, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.J.F.; Wilches, F.J.; Fernandez, T.M. Use of Seawater and Moringa oleifera Seeds for Turbidity Removal in Water Treatment. Int. J. Eng. Res. Technol. 2020, 13, 66–72. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Subba Narasiah, K. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998, 32, 781–791. [Google Scholar] [CrossRef]
- Samia, A.A.J. Proper Use of African Natural Coagulants for Rural Water Supplies: Research in the Sudan and a Guide for New Projects; Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ) GmbH: Eschborn, Germany, 1986. [Google Scholar]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Coagulation Mechanism of Salt Solution-Extracted Active Component in Moringa oleifera Seeds. Water Res. 2001, 35, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 2001, 35, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.P.; Silva, J.B.G.; Roque, O.C.C.; Nascentes, A.L.; Silva, L.D.B. Evaluation of the effect of the seed extract of Moringa oleifera Lam over the efficiency of organic filters in wastewater treatment of dairy cattle breeding. Eng. Agrícola. 2014, 34, 143–152. [Google Scholar] [CrossRef]
- Jung, Y.; Jung, Y.; Kwon, M.; Kye, H.; Abrha, Y.W.; Kang, J.-W. Evaluation of Moringa oleifera seed extract by extraction time: Effect on coagulation efficiency and extract characteristic. J. Water Health 2018, 16, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Gassenschmidt, U.; Jany, K.D.; Bernhard, T.; Niebergall, H. Isolation and characterization of a flocculating protein from Moringa oleifera Lam. Biochim. Biophys. Acta BBA-Gen. Subj. 1995, 1243, 477–481. [Google Scholar] [CrossRef]
- Gassenschmidt, U.; Jany, K.-D.; Tauscher, B. Chemical properties of flocculant-active proteins from Moringa oleifera lam. Biol. Chem. Hoppe-Seyler. 1991, 372, 659. [Google Scholar]
- Samghouli, N.; Bencheikh, I.; Azoulay, K.; Abahdou, F.Z.; Mabrouki, J.; El Hajjaji, S. Study of Piroxicam Removal from Wastewater by Artichoke Waste Using NemrodW® Software: Statistical Analysis. In IoT and Smart Devices for Sustainable Environment; Springer International Publishing: Cham, Switzerland, 2022; pp. 29–42. [Google Scholar]
- Beltrán-Heredia, J.; Martín, J.S. Azo dye removal by Moringa oleifera seed extract coagulation. Color. Technol. 2008, 124, 310–317. [Google Scholar] [CrossRef]
- Agrawal, H.; Shee, C.; Sharma, A.K. Isolation of a 66 KDa protein with coagulation activity from seeds of Moringa oleifera. Res. J. Agric. Biol. Sci. 2007, 3, 418–421. [Google Scholar]
- Madrona, G.S.; Branco, I.G.; Seolin, V.J.; Alves Filho, B.D.A.; Fagundes-Klen, M.R.; Bergamasco, R. Evaluation of extracts of Moringa oleifera Lam seeds obtained with NaCl and their effects on water treatment. Acta Sci. Technol. 2012, 34, 289–293. [Google Scholar] [CrossRef]
- Mageshkumar, M.; Karthikeyan, R. Modelling the kinetics of coagulation process for tannery industry effluent treatment using Moringa oleifera seeds protein. Desalination Water Treat. 2015, 57, 14954. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Alves, B.R.R.; Silva, M.F.; Bergamasco, R.; Coral, L.A.; Bassetti, F.J. CaCl2 applied to the extraction of Moringa oleifera seeds and the use for Microcystis aeruginosa removal. Chem. Eng. J. 2016, 304, 469–475. [Google Scholar] [CrossRef]
- Hernández-Villegas, M.M.; Borges-Argáez, R.; Rodriguez-Vivas, R.I.; Torres-Acosta, J.F.J.; Méndez-Gonzalez, M.; Cáceres-Farfan, M. Ovicidal and larvicidal activity of the crude extracts from Phytolacca icosandra against Haemonchus contortus. Vet. Parasitol. 2011, 179, 100–106. [Google Scholar] [CrossRef]
- Bhutada, P.R.; Jadhav, A.J.; Pinjari, D.V.; Nemade, P.R.; Jain, R.D. Solvent assisted extraction of oil from Moringa oleifera Lam. seeds. Ind. Crops Prod. 2016, 82, 74–80. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a] pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Muyibi, S.A.; Ahmadun, S.A.; Noor, M.J.M.M.; Ahmadun, F.R. Enhanced Coagulation Efficiency of Moringa oleifera Seeds Through Selective Oil Extraction. IIUM Eng. J. 2003, 4, 11. [Google Scholar] [CrossRef]
- Nascimento, A.C.S.; Oliveira, A.F.G.D.; Lavôr, M.B.D.; Pereira, E.C.; Amorim, M.C.C.D. Low turbidity water treated with seeds of Moringa oleífera Lam. Rev. Eletrônica Gest. Educ. E Tecnol. Ambient. 2020, 24, 15. [Google Scholar] [CrossRef]
- Rahmani, M.; Regraguy, B.; Mabrouki, J.; Moufti, A.; EL’Mrabet, M.; Dahchour, A.; Hajjaji, S.E.L. Response surface modeling of methylene blue dye removal from wastewater on natural oil shale. Desalination Water Treat. 2021, 244, 253–262. [Google Scholar] [CrossRef]
- Abdul Hamid, S.H.; Lananan, F.; Din, W.N.S.; Lam, S.S.; Khatoon, H.; Endut, A.; Jusoh, A. Harvesting microalgae, Chlorella sp. by bio-flocculation of Moringa oleifera seed derivatives from aquaculture wastewater phytoremediation. Int. Biodeterior. Biodegrad. 2014, 95, 270–275. [Google Scholar] [CrossRef]
- Modern methods in protein and nucleic acid analysis. 86th Conference of the Society for Biological Chemistry. Konstanz, September 18th, 1990. Abstracts. Biol. Chem. Hoppe Seyler. 1990, 371, 757–769.
- Fuglie, L. New Uses of Moringa Studied in Nicaragua. ECHO Development Notes no. 68. 2000. Available online: https://www.echocommunity.org/resources/15db05d7-4693-425b-9d86-468143bd0ec9 (accessed on 12 January 2023).
- Narasiah, K.S.; Vogel, A.; Kramadhati, N.N. Coagulation of turbid waters using Moringa oleifera seeds from two distinct sources. Water Supply 2002, 2, 83–88. [Google Scholar] [CrossRef]
- Santos, A.F.S.; Luz, L.A.; Argolo, A.C.C.; Teixeira, J.A.; Paiva, P.M.G.; Coelho, L.C.B.B. Isolation of a seed coagulant Moringa oleifera lectin. Process Biochem. 2009, 44, 504–508. [Google Scholar] [CrossRef]
- Suarez, M.; Entenza, J.M.; Doerries, C.; Meyer, E.; Bourquin, L.; Sutherland, J.; Marison, I.; Moreillon, P.; Mermod, N. Expression of a plant-derived peptide harboring water-cleaning and antimicrobial activities. Biotechnol. Bioeng. 2003, 81, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.M.; Shafik, H.M.; Abdelrafee, S. Selective Coagulation Mechanism of Moringa oleifera Seeds on Gram Positive and Gram Negative Bacteria. Egypt. J. Microbiol. 2020, 55, 27–35. [Google Scholar] [CrossRef]
- De Paula, H.M.; de Oliveira Ilha, M.S.; Sarmento, A.P.; Andrade, L.S. Dosage optimization of Moringa oleifera seed and traditional chemical coagulants solutions for concrete plant wastewater treatment. J. Clean. Prod. 2018, 174, 123–132. [Google Scholar] [CrossRef]
- Miranda, J.P.R.; Díaz, J.J.F.; BallutDajud, G. Influence of Storage Time of Moringa oleifera seed on the coagulant activity efficiency for raw water treatment. Indian J. Sci. Technol. 2018, 11, 4. [Google Scholar] [CrossRef]
- Jami, M.S.; Zakaria, N.S.; Ahmed, M.; Ghani, N.R.N.A.; Ngabura, M.; Ahmad, M.M. Parameter Effects of pH, Dosage And Contact Time On Boron Removal From Synthetic Sea Water Using Moringa oleifera Seeds. IIUM Eng. J. 2020, 21, 12–24. [Google Scholar] [CrossRef]
- Coelho, J.S.; Santos, N.D.L.; Napoleão, T.H.; Gomes, F.S.; Ferreira, R.S.; Zingali, R.B.; Coelho, L.C.B.B.; Leite, S.P.; Navarro, D.M.A.F.; Paiva, P.M.G. Effect of Moringa oleifera lectin on development and mortality of Aedes aegypti larvae. Chemosphere 2009, 77, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Loukili, H.; Mabrouki, J.; Anouzla, A.; Kouzi, Y.; Younssi, S.A.; Diguab, K.; Abrouki, Y. Pre-treated Moroccan natural clays: Application to the wastewater treatment of textile industry. Desalination Water Treat. 2021, 240, 124–136. [Google Scholar] [CrossRef]
- Rodríguez de Yurre, A.; Dayvid Ferreira da Silva, J.; Kalinne da Silva Torres, M.; Lopes Martins, E.; Peroba Ramos, I.; Sandoval Filho Lima da Silva, W.; da Silva Sarpa, J.; César da Silva Guedes, C.; Henrique Napoleão, T.; Cassandra Breitenbach Barroso Coelho, L.; et al. Evaluation of the Cardiac Effects of a Water-Soluble Lectin (Wsmol) from Moringa oleifera Seeds. Arq. Bras. Cardiol. 2020, 114, 1029–1037. [Google Scholar] [CrossRef]
- Koike, M.K.; Kochi, A.K.; Pinto, D.Y.G. Use of Moringa oleifera Seeds in Water Treatment. Arq. Bras. Cardiol. 2020, 114, 1038–1039. [Google Scholar] [CrossRef]
- Yarahmadi, M.; Hossieni, M.; Bina, B.; Mahmoudian, M.H.; Naimabadie, A.; Shahsavani, A. Application of Moringa Oleifer Seed Extract and Polyaluminum Chloride in Water Treatment. World Appl. Sci. J. 2009, 7, 962–967. [Google Scholar]
- Santos, A.F.; Luz, L.; Napoleão, T.H.; Paiva, P.M.; Coelho, L.C.B.B. Coagulation, Flocculation, Agglutination and Hemagglutination: Similar Properties? Adv. Chem. Res. 2014, 20, 51–70. [Google Scholar]
- Amagloh, F.K.; Benang, A. Effectiveness of Moringa oleifera seed as coagulant for water purification. Afr.J. Agric. Res. 2009, 4, 119–123. [Google Scholar]
- Kituyi, J.L.; Foulkes, M.; Worsfold, P.; Ongulu, R.A.; Kiplagat, A.; Gachanja, A. Efficiency of pre-treated Moringa oleifera for the removal of Cd2+ and Zn2+ ions from wastewaters. Ecohydrol. Hydrobiol. 2013, 13, 267–271. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants. Appl. Sci. 2018, 8, 1116. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Delgado-Regalado, A. Removal of Carmine Indigo Dye with Moringa oleifera Seed Extract. Ind. Eng. Chem. Res. 2009, 48, 6512–6520. [Google Scholar] [CrossRef]
- López-Grimau, V.; Vilaseca, M.; Gutiérrez-Bouzán, C. Comparison of different wastewater treatments for colour removal of reactive dye baths. Desalination Water Treat. 2016, 57, 2685–2692. [Google Scholar] [CrossRef]
- David, C.; Narlawar, R.; Arivazhagan, M. Performance Evaluation of Moringa oleifera Seed Extract (MOSE) in Conjunction with Chemical Coagulants for Treating Distillery Spent Wash. Indian Chem. Eng. 2016, 58, 189–200. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, J. Heterogeneous surfaces to repel proteins. Adv. Colloid Interface Sci. 2016, 228, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.S.; Matos, M.; Sousa, Â.; Costa, C.; Nogueira, R.; Teixeira, J.A.; Paiva, P.M.G.; Parpot, P.; Coelho, L.C.B.B.; Brito, A.G. Removal of tetracycline from contaminated water by Moringa oleifera seed preparations. Environ. Technol. 2016, 37, 744–751. [Google Scholar] [CrossRef]
- Vunain, E.; Masoamphambe, E.F.; Mpeketula, P.M.G.; Monjerezi, M.; Etale, A. Evaluation of coagulating efficiency and water borne pathogens reduction capacity of Moringa oleifera seed powder for treatment of domestic wastewater from Zomba, Malawi. J. Environ. Chem. Eng. 2019, 7, 103118. [Google Scholar] [CrossRef]
- Freitas, J.H.E.S.; de Santana, K.V.; do Nascimento, A.C.C.; de Paiva, S.C.; de Moura, M.C.; Coelho, L.C.B.B.; de Oliveira, M.B.M.; Paiva, P.M.G.; do Nascimento, A.E.; Napoleão, T.H. Evaluation of using aluminum sulfate and water-soluble Moringa oleifera seed lectin to reduce turbidity and toxicity of polluted stream water. Chemosphere 2016, 163, 133–141. [Google Scholar] [CrossRef]
- Formentini-Schmitt, D.M.; Alves, Á.C.D.; Veit, M.T.; Bergamasco, R.; Vieira, A.M.S.; Fagundes-Klen, M.R. Ultrafiltration Combined with Coagulation/Flocculation/Sedimentation Using Moringa oleifera as Coagulant to Treat Dairy Industry Wastewater. Water Air Soil Pollut. 2013, 224, 1682. [Google Scholar] [CrossRef]
- Matos, A.T.; Cabanellas, C.F.G.; Cecon, P.R.; Brasil, M.S.; Mudado, C.S. Efeito da concentração de coagulantes e do pH da solução na turbidez da água, em recirculação, utilizada no processamento dos frutos do cafeeiro. Eng. Agríc. 2007, 27, 544–551. [Google Scholar] [CrossRef]
- Sri, S.; Nur, H.; Esti, R. Influence of Powdered Moringa oleifera Seeds And Natural Filter Media On The Characteristics of Tapioca Starch Wastewater. Int. J. Recycl. Org. Waste Agric. 2013, 2, 91–101. [Google Scholar]
- e Souza, A.S.; dos Santos, K.G. Settling process of cassava starch using natural coagulant from Moringa oleifera Lam seed extract. Res. Soc. Dev. 2020, 9, e28953169. [Google Scholar] [CrossRef]
- Garde, W.K.; Buchberger, S.G.; Wendell, D.; Kupferle, M.J. Application of Moringa oleifera seed extract to treat coffee fermentation wastewater. J. Hazard. Mater. 2017, 329, 102–109. [Google Scholar] [CrossRef]
- Adelodun, B.; Ogunshina, M.S.; Ajibade, F.O.; Abdulkadir, T.S.; Bakare, H.O.; Choi, K.S. Kinetic and Prediction Modeling Studies of Organic Pollutants Removal from Municipal Wastewater using Moringa oleifera Biomass as a Coagulant. Water 2020, 12, 2052. [Google Scholar] [CrossRef]
- El Bakraoui, H.; Slaoui, M.; Mabrouki, J.; Hmouni, D.; Laroche, C. Recent Trends on Domestic, Agricultural and Industrial Wastewaters Treatment Using Microalgae Biorefinery System. Appl. Sci. 2022, 13, 68. [Google Scholar] [CrossRef]
- Lo Monaco, P.A.; Matos, A.T.; Pereira, M.D.S.; Eustáquio Júnior, V.; Batista, A.P.D.S.; Baker, S.A. Effect of addition of different chemical substances in Moringa seed extract used as coagulant in sewage treatment. Eng. Agríc. 2013, 33, 1038–1048. [Google Scholar] [CrossRef]
- Mateus, G.A.P.; Formentini-Schmitt, D.M.; Nishi, L.; Fagundes-Klen, M.R.; Gomes, R.G.; Bergamasco, R. Coagulation/Flocculation with Moringa oleifera and Membrane Filtration for Dairy Wastewater Treatment. Water Air Soil Pollut. 2017, 228, 342. [Google Scholar] [CrossRef]
- Bhatia, S.; Othman, Z.; Ahmad, A.L. Coagulation–flocculation process for POME treatment using Moringa oleifera seeds extract: Optimization studies. Chem. Eng. J. 2007, 133, 205–212. [Google Scholar] [CrossRef]
- Mateus, G.A.P.; Pinto, L.D.M.; Baptista, A.T.A.; Nishi, L.; Klen, M.R.F.; Gomes, R.G.; Araújo, A.A.; Bergamasco, R. Evaluation of natural coagulant Moringa oleifera Lam. in the treatment of dairy wastewater at different pH. Acta Hortic. 2017, 1158, 357–364. [Google Scholar] [CrossRef]
- Nagaraju, P.; Mahesh, S. Feasibility Study of Moringa oleifera as a Natural Coagulant for the Treatment of Dairy Wastewater. Int. J. Eng. Res. 2013, 2, 200–202. [Google Scholar]
- Mabrouki, J.; Fattah, G.; Kherraf, S.; Abrouki, Y.; Azrour, M.; El Hajjaji, S. Artificial intelligence system for intelligent monitoring and management of water treatment plants. In Emerging Real-World Applications of Internet of Things; CRC Press: Boca Raton, FL, USA, 2022; pp. 69–87. [Google Scholar]
- Kokila, P.; Yogesh, D.; Rinku, P.; Sarju, P. Effectiveness of Moringa oleifera as natural coagulant aid for waste water treatment of dairy industry. Asian J. Environ. Sci. 2012, 7, 167–171. [Google Scholar]
- del Real-Olvera, J.; Rustrian-Portilla, E.; Houbron, E.; Landa-Huerta, F.J. Adsorption of organic pollutants from slaughterhouse wastewater using powder of Moringa oleifera Seeds as a natural coagulant. Desalination Water Treat. 2016, 57, 9971–9981. [Google Scholar] [CrossRef]
- Bhatia, S.; Othman, Z.; Ahmad, A.L. Pretreatment of palm oil mill effluent (POME) using Moringa oleifera seeds as natural coagulant. J. Hazard. Mater. 2007, 145, 120–126. [Google Scholar] [CrossRef]
- Dehghani, M.; Alizadeh, M. The effects of the natural coagulant Moringa oleifera and alum in wastewater treatment at the Bandar Abbas Oil Refinery. Environ. Health Eng. Manag. 2016, 3, 225–230. [Google Scholar] [CrossRef]
- Moreti, L.; Coldebella, P.; Camacho, F.; Bongiovani, M.; Souza, A.; Gohara, A.; Matsushita, M.; Silva, M.; Nishi, L.; Bergamasco, R. Removal of Anabaena flos-aquae in water treatment process using Moringa oleifera and assessment of fatty acid profile of generated sludge. Environ. Technol. 2015, 37, 1408–1417. [Google Scholar] [CrossRef]
- Uniyal, H.; Vaishnav, S.R.; Sindhi, A.M. To study the purification of water by the use of Moringa oleifera (drumstick). J. Emerg. Technol. Innov. Res. JETIR 2019, 6, 555–559. [Google Scholar]
- Asrafuzzaman, M.; Fakhruddin, A.N.M.; Hossain, M. Reduction of Turbidity of Water Using Locally Available Natural Coagulants. ISRN Microbiol. 2011, 2011, 632189. [Google Scholar] [CrossRef]
- Noor, M.J.M.M.; Mohamed, E.H.; Mohammad, T.A.; Ghazali, A.H. Effectiveness of salt-extracted freeze-dried Moringa oleifera as a coagulant. Desalination Water Treat. 2015, 55, 3621–3627. [Google Scholar] [CrossRef]
- Araújo, G.S.; Santos, Y.P.; de Oliveira, A.G. Avaliação do uso da Moringa Oleífera no tratamento de efluente proveniente de usina de concreto / Evaluation of the use of Moringa oleifera in the treatment of wastewater from a concrete plant. Braz. J. Dev. 2020, 6, 32822–32835. [Google Scholar] [CrossRef]
- Boulaadjoul, S.; Zemmouri, H.; Bendjama, Z.; Drouiche, N. A novel use of Moringa oleifera seed powder in enhancing the primary treatment of paper mill effluent. Chemosphere 2018, 206, 142–149. [Google Scholar] [CrossRef]
- Tie, J.; Jiang, M.; Li, H.; Zhang, S.; Zhang, X. A comparison between Moringa oleifera seed presscake extract and polyaluminum chloride in the removal of direct black 19 from synthetic wastewater. Ind. Crops Prod. 2015, 74, 530–534. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Delgado-Regalado, A.; Jurado-Bustos, C. Removal of Alizarin Violet 3R (anthraquinonic dye) from aqueous solutions by natural coagulants. J. Hazard. Mater. 2009, 170, 43–50. [Google Scholar] [CrossRef] [PubMed]
- El Alouani, M.; Aouan, B.; Rachdi, Y.; Alehyen, S.; El Herradi, E.H.; Saufi, H.; Mabrouki, J.; Barka, N. Porous geopolymers as innovative adsorbents for the removal of organic and inorganic hazardous substances: A mini-review. Int. J. Environ. Anal. Chem. 2022, 13. [Google Scholar] [CrossRef]
- Bello, O.S.; Lasisi, B.M.; Adigun, O.J.; Ephraim, V. Scavenging Rhodamine B dye using Moringa oleifera seed pod. Chem. Speciat. Bioavailab. 2017, 29, 120–134. [Google Scholar] [CrossRef]
- Ali, N.F.; EL-Mohamedy, R.S.R. Evaluation of Moringa oleifera seed extract coagulation in removal of some dyes in textile wastewater. Int. J. ChemTech Res. 2016, 9, 538–545. [Google Scholar]
- Methneni, N.; Anthonissen, R.; Van de Maele, J.; Trifa, F.; Verschaeve, L.; Mansour, H.B.; Mertens, B. Assessment of natural coagulants to remediate Tunisian textile wastewater by combining physicochemical, analytical, and toxicological data. Environ. Sci. Pollut. Res. 2020, 27, 40088–40100. [Google Scholar] [CrossRef]
- Santos, T.R.T.; Silva, M.F.; Nishi, L.; Vieira, A.M.S.; Klein, M.R.F.; Andrade, M.B.; Vieira, M.F.; Bergamasco, R. Development of a magnetic coagulant based on Moringa oleifera seed extract for water treatment. Environ. Sci. Pollut. Res. 2016, 23, 7692–7700. [Google Scholar] [CrossRef]
- Reck, I.M.; Baptista, A.T.A.; Paixão, R.M.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Application of magnetic coagulant based on fractionated protein of Moringa oleifera Lam. seeds for aqueous solutions treatment containing synthetic dyes. Environ. Sci. Pollut. Res. 2020, 27, 12192–12201. [Google Scholar] [CrossRef] [PubMed]
- Reck, I.M.; Baptista, A.T.A.; Paixão, R.M.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Protein fractionation of Moringa oleifera Lam. seeds and functionalization with magnetic particles for the treatment of reactive black 5 solution. Can. J. Chem. Eng. 2019, 97, 2309–2317. [Google Scholar] [CrossRef]
- dos Santos, T.R.T.; Mateus, G.A.P.; Silva, M.F.; Miyashiro, C.S.; Nishi, L.; de Andrade, M.B.; Fagundes-Klen, M.R.; Gomes, R.G.; Bergamasco, R. Evaluation of Magnetic Coagulant (α-Fe2O3-MO) and its Reuse in Textile Wastewater Treatment. Water Air Soil Pollut. 2018, 229, 92. [Google Scholar] [CrossRef]
- Silva, M.F.; de Oliveira, L.A.S.; Ciciliati, M.A.; Silva, L.T.; Pereira, B.S.; Hechenleitner, A.A.W.; Oliveira, D.M.F.; Pirota, K.R.; Ivashita, F.F.; Paesano, A.; et al. Nanometric particle size and phase controlled synthesis and characterization of γ-Fe or (α + γ)-Fe by a modified sol-gel method. J. Appl. Phys. 2013, 114, 104311. [Google Scholar] [CrossRef]
- Azoulay, K.; Bencheikh, I.; Samghouli, N.; Mabrouki, J.; Moufti, A.; El Hajjaji, S. Modeling and Design of Water Treatment Processes by Biosorption Method Using JMP® 11 Software. In IoT and Smart Devices for Sustainable Environment; Springer International Publishing: Cham, Switzerland, 2022; pp. 53–69. [Google Scholar]
- Bedekar, P.A.; Bhalkar, B.N.; Patil, S.M.; Govindwar, S.P. Moringa oleifera-mediated coagulation of textile wastewater and its biodegradation using novel consortium-BBA grown on agricultural waste substratum. Environ. Sci. Pollut. Res. 2016, 23, 20963–20976. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. Comparison of naturally prepared coagulants for removal of COD and color from textile wastewater. Glob. NEST J. 2013, 15, 522–528. [Google Scholar] [CrossRef]
- Ramesh, S.; Mekala, L. Treatment of Textile Waste Water Using Moringa oleifera And Tamarindus Indica. Int. Res.J. Eng. Technol. 2018, 5, 3891–3895. [Google Scholar]
- Padhiyar, H.; Thanki, A.; Kumar Singh, N.; Pandey, S.; Yadav, M.; Chand Yadav, T. Parametric and kinetic investigations on segregated and mixed textile effluent streams using Moringa oleifera seed powders of different sizes. J. Water Process Eng. 2020, 34, 101159. [Google Scholar] [CrossRef]
- Al-Gheethi, A.; Mohamed, R.; Wurochekke, A.; Nurulainee, N.; Mas Rahayu, J.; Amir Hashim, M. Efficiency of Moringa oleifera Seeds for Treatment of Laundry Wastewater. MATEC Web Conf. 2017, 103, 06001. [Google Scholar] [CrossRef]
- Effendi, H.; Sari, R.; Hasibuan, S. Moringa oleifera as coagulant for batik effluent treatment. In Proceedings of the 35th Annual Conference of the International Association for Impact Assessment, Florence, Italy, 20–23 April 2015. [Google Scholar]
- Sharma, P.; Kumari, P.; Srivastava, M.M.; Srivastava, S. Removal of cadmium from aqueous system by shelled Moringa oleifera Lam. seed powder. Bioresour. Technol. 2006, 97, 299–305. [Google Scholar] [CrossRef]
- Obuseng, V.; Nareetsile, F.; Kwaambwa, H.M. A study of the removal of heavy metals from aqueous solutions by Moringa oleifera seeds and amine-based ligand 1,4-bis[N,N-bis(2-picoyl)amino]butane. Anal. Chim. Acta 2012, 730, 87–92. [Google Scholar] [CrossRef]
- Mabrouki, J.; Azroure, M.; Boubekraoui, A.; El Hajjaji, S. Simulation and Optimization of Solar Domestic Hot Water Systems. Int. J. Soc. Ecol. Sustain. Dev. (IJSESD) 2022, 13, 11. [Google Scholar] [CrossRef]
- Sharma, P.; Goyal, P.; Srivastava, S. Biosorption of trivalent and hexavalent chromium from aqueous systems using shelled Moringa oleifera seeds. Chem. Speciat.Bioavailab. 2007, 19, 175–182. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sethuraman, L.; Panjanathan, R.; Natarajan, A.; Solaiappan, V.; Thilagaraj, W.R. Biosorption of heavy metals from actual electroplating wastewater using encapsulated Moringa oleifera beads in fixed bed column. Desalination Water Treat. 2016, 57, 3572–3587. [Google Scholar] [CrossRef]
- Rachiqa, T.; Abroukia, Y.; Mabroukia, J.; Samghoulia, N.; Fersib, C.; Rahalc, S.; El Hajjajia, S. Evaluation of the efficiency of different materials to remove specific pollutants from landfill leachate. Desalination Water Treat. 2021, 238, 240–250. [Google Scholar] [CrossRef]
- Hong, A.H.; Burmamu, B.R.; Umaru, A.B.; Sadiq, U.M. Removal of Heavy Metals from Contaminated Water Using Moringa olefeira Seed Coagulant in Yola and Its Environs. Int. J. Eng. Invent. 2017, 6, 40–45. [Google Scholar]
- Sharma, P.; Kumari, P.; Srivastava, M.; Srivastava, S. Ternary biosorption studies of Cd(II), Cr(III) and Ni(II) on shelled Moringa oleifera seeds. Bioresour. Technol. 2006, 98, 474–477. [Google Scholar] [CrossRef]
- Ghebremichael, K.; Gebremedhin, N.; Amy, G. Performance of Moringa oleifera as a biosorbent for chromium removal. Water Sci. Technol. 2010, 62, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Al-Jadabi, N.; Laaouan, M.; Benbouzid, M.; Mabrouki, J.; El Hajjaji, S. Coagulation-flocculation technique for domestic wastewater treatment in the city of Ain Aouda, Rabat, Morocco. E3S Web Conf. 2022, 337, 02003. [Google Scholar] [CrossRef]
- Ahmed, S.; Aktar, S.; Zaman, S.; Jahan, R.A.; Bari, M.L. Use of natural bio-sorbent in removing dye, heavy metal and antibiotic-resistant bacteria from industrial wastewater. Appl. Water Sci. 2020, 10, 107. [Google Scholar] [CrossRef]
- Sajidu, S.M.; Henry, E.M.T.; Kwamdera, G.; Mataka, L. Removal of lead, iron and cadmium ions by means of polyelectrolytes of Moringa oleifera whole seed kernel. WIT Trans Ecol Env. 2005, 80, 251–258. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).