Abstract
Fenton technology has excellent performance in the treatment of hard-to-degrade organics but tends to cause secondary pollution to the environment. Given its excellent adsorption capacity and the availability of mature adsorption modification studies, poly(chloromethyl styrene)-based resin (PS-Cl) has received much attention for the adsorption of heavy metal ions. However, combining the mature Fenton technology with the highly popular PS-Cl through a stable bridge to exploit the advantages of catalytic degradation performance of Fenton-like technology is the main focus of our work. The PS-NH2@FeSe2 catalyst with a core–shell structure was synthesized. The catalytic degradation of tetracycline solution in the presence of PS-NH2@FeSe2 and persulfate achieved a satisfactory effect. The removal efficiency was as high as 85.94% within 2 h, and the degradation rate constant was 0.02352 min−1. The main advantages of the PS-NH2@FeSe2 catalyst were high stability and recyclability. Thus, the catalyst would not cause secondary pollution to the environment and could still achieve a degradation efficiency of nearly 70% for TC after five times of reuse. The possible catalytic degradation pathways and potential removal mechanisms were investigated through free-radical quenching experiments and HPLC-MS detection of intermediates generated through catalytic degradation. Column experiments were conducted to investigate the effect of the catalyst on the actual removal of wastewater, and a simple flow model was developed using Yellow River water to make the integration of theory and practice possible. In conclusion, the new idea constructed with FeSe2-loaded modified resin offers promising prospects in the removal of refractory organic compounds, such as tetracycline.
1. Introduction
Antibiotics play an extremely important role in the production and development of human beings [1]. The scientific and rational application of various types of antibiotics has become a top priority for human development and progress when preventing major diseases and responding to unexpected epidemic situations. However, the excessive abuse of antibiotics and the lack of environmental protection bring a heavy burden to the environment and will also bring serious threats to the survival and development of humans, animals, and plants over time [2]. Tetracycline (TC), as a member of the antibiotic family, is widely recognized for its inhibition of bacteria and viruses. Therefore, TCs are used in large quantities in the treatment of various diseases and in the development of agricultural animal husbandry [3]. Nevertheless, TC has high stability and a certain degree of toxicity. As the environment continues to accumulate human and animal excreta, some excreta are carried into other environments with surface runoff [4]. The phenomenon has led to a steady accumulation of TC levels in the environment. Considering that the conventional means of wastewater treatment can hardly meet the demand of degrading TC in the environment [5], the effective removal of TC from environmental water bodies becomes a scientific problem in the process of protecting the water environment.
In recent years, advanced oxidation technologies (AOPs) are favored by researchers because of their simplicity, effectiveness, and mature practical applications especially for the protection of the water environment and the treatment of difficult-to-degrade organic wastewater [6,7]. AOPs can be used to generate highly active oxide species in place of the complete mineralization of organic wastewater, such as TC [8]. AOPs can be classified as electrocatalytic, cavitation, photocatalytic, and Fenton reactions. These reactions can produce highly reactive oxide species such as ·OH and SO4·− [9,10]. Among AOPs, Fenton chemistry has become a frequently discussed topic of current research. Fenton reaction has become the main force in degrading TC-based organic wastewater due to its outstanding advantages, such as good safety, high efficiency, fast speed, and continuous oxidation capacity [11,12]. However, the traditional Fenton reaction also has many disadvantages, such as difficulty of recycling, poor material stability, secondary pollution, and high catalyst consumption [13,14]. Making use of the outstanding advantages of Fenton reaction to achieve efficient and environmentally friendly degradation for TC organic wastewater is the primary focus of our work.
The removal of heavy metal ions from environmental waters through adsorption is one of the common scientific approaches [15]. The adsorption method is favored by researchers because of its simplicity, safety, and independence from the toxicity of the environment to be treated, making the development of new adsorbent materials with advantages an increasingly important topic for further investigation in the field of adsorption degradation [16]. Chloromethylstyrene-based resin (PS-Cl) is a common and appropriately modified adsorbent material. The resin material is relatively low-cost and easy to obtain [17,18]. Crucially, the resin material can be chemically modified with a certain number of amino or carboxyl groups in accordance with our specific needs, resulting in a simple and easy modification process and satisfactory results [19]. This phenomenon is made possible by the excellent characteristics of PS-Cl, such as adjustable porosity and high stability [20]. Research directions and articles related to the use of this PS-Cl with excellent adsorption properties as a catalyst-loading material to catalyze the degradation of organic wastewater are few. Heterogeneous nanomaterials have been the rising stars in the field of environmental materials in the last few years [21]. With the advantages of tunable catalytic performance, excellent electron transfer capability, and high dispersion on the carrier surface, heterogeneous nanostructured materials have a high presence in various types of catalytic degradation experiments [22,23,24]. Given their unique properties and practical applications, multiphase nanostructured materials have been recognized as promising nanomaterials that have been widely developed as multifunctional materials for various applications [25].
For the past few years, the nanomaterial industry has gained momentum, and various types of nanomaterials have emerged [26,27]. Transition-metal complexes have received remarkable attention from current researchers in the removal of organic wastewater through Fenton-like chemical reactions. During the process of nonhomogeneous Fenton reactions, the stability and efficient electron migration ability of nonhomogeneous catalysts are utilized to achieve the complete removal of refractory organic compounds without bringing Fe3+ from the catalyst into the environment to cause secondary pollution [28]. Common nonhomogeneous Fenton reaction catalysts include Fe3O4, Fe2O3, FeOCl, and XFe2O4 (X: Cu, Co, Zn, or Mn) [29,30,31,32,33]. These common Fenton catalysts have been widely studied and utilized because of their low cost, good stability, simple preparation process, and easy availability of synthetic materials [34]. Nowadays, transition-metal sulfides have emerged as nonhomogeneous Fenton catalysts represented by FeS2, which have shown satisfactory degradation effects on organic wastewater, such as organic arsenic compounds, TC wastewater, and organic dye wastewater, through the activation of the PMS system [35,36,37]. Based on the structure and characteristics of metal sulfides, selenium, which is in the same main group as sulfur, has shown superior performance [38]. Transition-metal selenides, as emerging nanomaterials, have received extensive attention in the conversion and storage of energy [39,40]. Combined with the essential characteristics of Fenton reaction, FeSe2 is gradually becoming a new nanomaterial with long-term prospects by taking advantage of its excellent properties [41], such as a wide range of constituent elements, low cost, wide field of material availability, powerful redox ability, and high durability with remarkable catalytic ability [42,43]. In terms of catalytic degradation, FeSe2 can efficiently activate the PMS system to activate the persulfate by using its own Fe2+. In the process of activating persulfate, the role of Se element as an electron donor becomes increasingly evident. With the continuous regeneration of Fe2+ and conversion of electron-acquired Fe2+, the whole reaction process is rapid and efficient [39]. Interestingly, Se provides electrons to the reaction, and the formed selenium vacancies can further promote the formation and conversion of Fe2+ due to the reduction in selenium species on the catalyst surface, thus further realizing the dual pathway of promoting the recycling of Fe2+ and Fe3+ [44].
In this work, the PS-Cl resin is simply modified to have a certain amount of amino groups. On the basis of the advantage, that the amino groups can provide strong chelating sites for heavy metal ions, FeSe2 is fully loaded on ammoniated resin spheres to reach loading saturation, forming a new composite material PS-NH2@FeSe2. The composite material has excellent chemical properties and practical application value. In the process of studying the degradation of TC using this catalyst, some conditions, such as solution pH, amount of persulfate, reaction temperature, catalyst dosage, and TC concentration, are experimented upon to explore the optimal conditions and application range of PS-NH2@FeSe2 material. Interestingly, the prepared composite catalyst PS-NH2@FeSe2 can degrade most of the different types of antibiotic wastewater. The composite catalyst shows a particularly remarkable effect on TC removal, and the catalytic performance is excellent. By simply separating and recycling the reacted catalyst five times, satisfactory results can still be achieved. The catalyst is found to be effective and stable for the degradation of flowing wastewater with the aid of column experiments. Results indicate that the prepared composite catalysts are of practical application in the remediation of environmental waters and the removal of refractory organic wastewater.
2. Experiment Section
2.1. Regents and Materials
Chloromethyl styrene-based resin material (PS-Cl) was purchased from Xi’an Hanxing Resin Technology Co. Ferrous chloride (FeCl2), ferric chloride hexahydrate (FeCl3-6H2O), sodium hydroxide (NaOH) were purchased from Guangfu Technology Development Co. Ethanolamine, tetrabutylammonium bromide (TBAB) was purchased from Tianjin Damao Chemical Reagent Co. Potassium persulfate was purchased from China Pharmaceutical Group Shanghai Chemical Reagent Co. Methanol, ethanol was from Tianjin Tiantai Fine Chemical Co. Tetracycline (TC) was purchased from Adamas-Beta Reagent Co. 1,4-dioxane, 1,2-ethylenediamine was purchased from Shanghai Aladdin Chemical Co. Selenium powder was obtained from Beijing Pharmaceutical Purchasing and Supply Station.
2.2. Synthesis of PS-NH2
According to the literature, the preparation method of amination resin is relatively mature [19]. Firstly, about 2 g of PS-Cl resin spheres was weighed into a beaker, and then 20 mL of 1,4-dioxane was precisely measured and poured into the beaker where the resin spheres were placed, so that the resin spheres were soaked for 2 h. Then, it was all poured into a 100 mL three-necked glass flask with a condensation refluxer and mechanical stirrer. After that, sodium hydroxide (2.5 g, 0.0625 mol) and phase transfer catalyst TBAB (0.1 g, 0.66 mmol) were dissolved together in 20 mL of deionized water, which was poured into the three-necked flask after being fully dissolved. Finally, 25 mL of 1,2-ethylenediamine was quickly added dropwise to the flask, followed by heating the reaction mixture to 85 °C while stirring continuously for 6 h on a magnetic stirrer. The polymer beads were filtered and rinsed repeatedly with ethanol and deionized water. After removing the residual impurities, the product was transferred to freeze-drying at −40 degrees Celsius for 24 h. The resulting resin product was named PS-NH2.
2.3. Preparation of PS-NH2@FeSe2
The resulting product PS-NH2 was placed in aqueous FeCl3 solution with a certain concentration and stirred at room temperature to allow the PS-NH2 resin spheres to fully adsorb Fe3+, thus obtaining the resin after chelation with metal ions. Subsequently, the obtained product was freeze-dried at −40 °C and transferred to a hydrothermal kettle. Then, 0.2 g of selenium powder with 30 mL of ethanolamine solvent was added to the hydrothermal kettle under 160 °C for 12 h to obtain the target product, which was named PS-NH2@FeSe2.
2.4. Catalytic Activity Evaluation
Catalytic degradation experiments were carried out by varying the parameters, such as catalyst dosage, potassium persulfate dosage, reaction temperature, substrate (TC) concentration, and solution pH. The concentration of TC at a specific time interval was detected using UV–Vis spectroscopy at the set time period. The TC removal rate of PS-NH2@FeSe2 was calculated on the basis of the difference of TC concentrations at the beginning and end of the time interval set for the catalytic reaction. In addition, PS-NH2@FeSe2 was recovered through simple filtration and washed thrice with ethanol and ultrapure water before being used in the recycling test to investigate the reusability of the PS-NH2@FeSe2 material. Methanol (MeOH) and tert-butanol (TBA) were utilized as effective scavengers of ·OH and SO4·−, respectively, which were finally used to investigate the mechanism of catalytic degradation by quenching experiments.
2.5. Characterization
The external morphology of the materials was observed using scanning electron microscopy (SEM). Analysis of the chemical components and functional groups of the products using thermogravimetric analysis (TG) and Fourier-transform infrared spectroscopy (FT-IR). X-ray diffraction (XRD) was used to examine the structural composition of the materials. The concentration of tetracycline was detected using ultraviolet–visible spectroscopy (UV–Vis). High performance liquid chromatography mass spectrometry (HPLC-MS) was used to probe the intermediate products produced during the degradation of TC. The pore size distribution and specific surface area of the synthesized materials were characterized using BET. The photoluminescence (PL) spectra of the catalysts were determined using an F97 Pro fluorescence spectrometer. Detection of free radical classes in the reaction environment was carried out using electron paramagnetic resonance (EPR) technique. The chemical information of the synthesized materials was characterized using X-ray photoelectron spectroscopy (XPS). Ultraviolet–visible diffuse reflectance spectroscopy (UV-Vis DRS) detects the reflectance of materials.
3. Result and Discussion
3.1. Preparation of PS-NH2 Resin and In Situ Growth of FeSe2
Scheme 1 demonstrates the formation of PS-NH2 resin by chemically modifying the PS-Cl resin as a substrate with an amino group. The PS-NH2 resin is placed in Fe2+ solution, and the ammoniated resin is allowed to fully adsorb Fe2+ to saturation by stirring at room temperature and chelate Fe2+ with -NH2. Finally, the target product PS-NH2@FeSe2 is synthesized using a hydrothermal method by placing the PS-NH2 resin and selenium powder in a hydrothermal kettle. Under optimized conditions, the removal of TC using the catalyst can be as high as 85.94%. Interestingly, the degradation of TC using the catalyst remains high after simple filtration for reuse. In short, the PS-NH2@FeSe2 catalyst can be obtained using a simple preparation method, confirming the reasonable presence of due elements by using various characterization means and analytical methods. In conclusion, the performance of the combined PS-NH2@FeSe2 catalysts is investigated under optimal conditions.
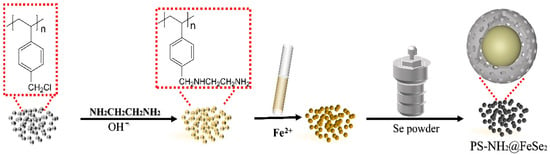
Scheme 1.
Synthesis and preparation route of PS-NH2@FeSe2 catalyst.
3.2. Physicochemical Characterization of PS-NH2@FeSe2
The functional groups of the catalysts are detected using FT-IR spectroscopy. As shown in Figure 1b, in the spectrum of PS-NH2@FeSe2 catalyst, the characteristic peak is located at 1670 cm−1, which is attributed to the stretching vibration of C=O [18,19]. In addition, the peak shows the formation of elemental Fe and Se bonds identifiable at 1060 cm−1, which indicates the successful coordination of elemental Fe and Se [45,46]. The broad characteristic peak at 3420 cm−1 is attributed to the stretching vibration of the N-H bond in imino (-NH) and amino (-NH2) groups, indicating the successful synthesis of imino and amino groups [47]. The characteristic peak located at 1620 cm−1 is attributed to the formation of -NH2 and ortho C=C bonds.
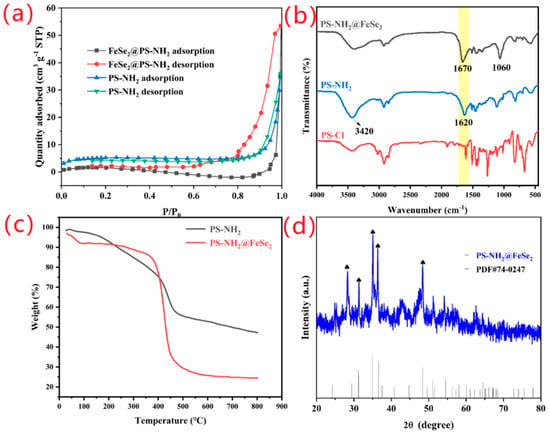
Figure 1.
(a) Nitrogen adsorption–desorption isotherms. (b) FT-IR spectra of PS-Cl, PS-NH2 and PS-NH2@FeSe2. (c) TG analysis curve patterns of PS-NH2 and PS-NH2@FeSe2. (d) XRD patterns of PS-NH2@FeSe2.
As shown in Figure 1a and Table S1, the specific surface area of the catalyst is studied using the N2 adsorption–desorption isotherm. The specific surface areas of PS-NH2 and PS-NH2@FeSe2 are 18.06 and 24.07 m2 g−1, respectively, which evidently increase significantly after loading the iron fund as selenide on the PS-NH2 resin microsphere. In addition, through the auxiliary testing of material pores, PS-NH2 resin and PS-NH2@FeSe2 materials have pore sizes of 17.8 and 16.1 nm, respectively, and pore volumes of 0.1574 and 0.1836 cm3 g−1, respectively. Although the pore size of the material is similar, the pore volume of the surface is significantly increased, indicating that the binding of selenide and ammoniated resin gives the carrier increased pore volume. By analyzing the specific surface area, pore size, and pore volume of the PS-NH2@FeSe2 catalyst, the catalyst is a mesoporous material.
As shown in Figure 1c, the PS-NH2 resin and PS-NH2@FeSe2 catalyst are characterized via thermogravimetric analysis under N2 atmosphere. Figure 1c shows that the pyrolysis of PS-NH2@FeSe2 catalytic material can be divided into three stages. The first stage is from 30 °C to 90 °C with weight loss of 5.02%, which is probably due to the evaporation of ethanol and water contained on the catalyst surface. The second stage is from 95 °C to 360 °C, with a weight loss of only 5.8%, which is probably due to the alternation of high and low and loss of deep water, solvent, and part of the organic carrier. The overall loss is lower in this stage. The third stage is from 370 °C to 800 °C with a weight loss as high as 60.84%, which may be due to the loss of almost all of the organic carrier via high-temperature pyrolysis.
As shown in Figure 1d, the phase composition and crystal structure of the catalytic material are analyzed using XRD. Comparison with the XRD pattern of FeSe2 standard card shows that the position of the diffraction peak of PS-NH2@FeSe2 catalyst is the same as the position of FeSe2 standard pattern. In addition, the literature showed that the structure of PS-NH2 resin is amorphous. In summary, we can conclude that the FeSe2 material has a highly crystalline structure by comparing the number and intensity of the diffraction peaks of the PS-NH2@FeSe2 catalyst and the FeSe2 standard pattern in Figure 1d [48,49]. FeSe2 is successfully loaded on the PS-NH2 resin.
The content and composition of the PS-NH2@FeSe2 catalyst are analyzed by XPS. As shown in Figure 2a, the peaks at 55.3, 284.3, 399.3, 531.3, and 724.3 eV are attributed to Se 3d, C 1s, N 1s, O 1s, and Fe 2d, respectively. Therefore, XPS spectra of the PS-NH2@FeSe2 catalyst confirms the presence of Se 3d, C 1s, N 1s, O 1s, and Fe 2d elements. As shown in Figure 2b, the high-resolution XPS spectra of the C 1s elements contained in the PS-NH2@FeSe2 catalyst show two peaks at binding energies of 284.6 and 286.1 eV position points, which correspond to C = C and C = O, respectively. The analysis of elemental species allows the determination of the successful introduction of the -NH2 group onto the PS-Cl resin [19]. As shown in Figure 2c, the high-resolution XPS spectra of the Fe 2d element contained in the PS-NH2@FeSe2 catalyst reveal two peaks at binding energies of 711.9 and 725.3 eV, which can be attributed to the spin–orbit pairs of Fe 2p3/2 and Fe 2p1/2, respectively [50]. In addition, the peak at 718.8 eV belongs to the satellite peak and is labeled as Sat. As shown in Figure 3d, the high-resolution XPS spectra of the Se 3d elements contained in the PS-NH2@FeSe2 catalyst show peaks at binding energies of 53.7, 55.7, and 58.7 eV, which can be attributed to the Se 3d5/2, Se 3d3/2, and Se 3d spin–orbit pairs [51,52].
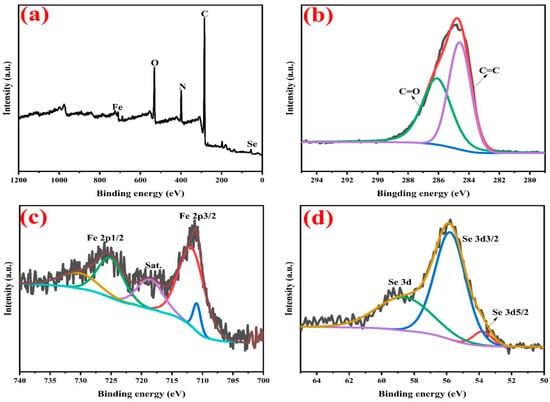
Figure 2.
XPS survey spectra of PS-NH2@FeSe2 (a), high resolution of C 1s (b), Fe 2p (c) and Se 3d (d) spectra of as-prepared PS-NH2@FeSe2.
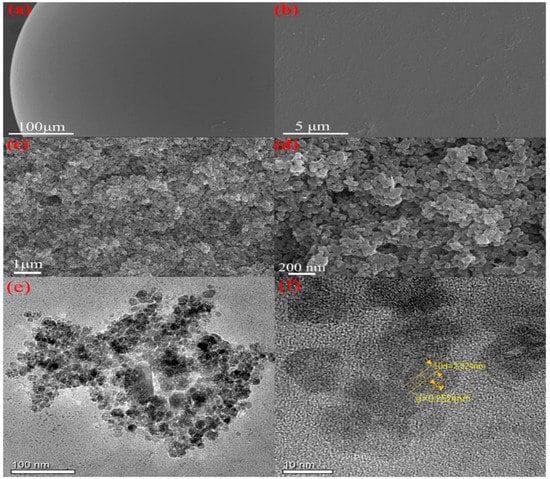
Figure 3.
SEM images of PS-NH2 resin (a,b) and PS-NH2@FeSe2 catalyst (c,d). TEM images of PS-NH2@FeSe2 catalyst (e,f).
Scanning electron microscopy (SEM) is used to study the morphological characteristics and surface structure of the material required for the preparation of the catalyst. Figure 3a,b shows the micrographs of the catalyst carrier PS-NH2. Externally, PS-NH2 has a smooth surface, uniform texture, and no defect. Such a structure ensures uniform and continuous pore size distribution. The introduced -NH2 is uniformly distributed on the surface of resin spheres without agglomeration, ensuring the uniform adsorption of metal ions on the outer surface of the resin. Figure 3c,d shows the micrographs of the in situ growth of FeSe2 on PS-NH2 resin spheres. The comparison of the photos of unloaded PS-NH2 reveals that the introduction of FeSe2 significantly changes the surface morphology of PS-NH2. SEM images show that FeSe2 is stacked on the surface of PS-NH2, making the accumulated sites exhibit a multidimensional three-dimensional spatial structure generated by the stacked aggregation of FeSe2. This multilayer mesh structure is beneficial to increase the reaction area of the catalyst and the electron transfer efficiency during the reaction. Furthermore, the FeSe2 loading is uniform and almost loaded on the complete resin spherical surface. The specific surface area and volume of the PS-NH2 resin spheres are fully expanded. Transmission electron microscopy (TEM) shows that FeSe2 particles are relatively uniformly distributed (Figure 3e,f). The amount of stacking formed by FeSe2 loaded at each position is different, resulting in a multiangle three-dimensional mesh-like irregular configuration. Forming increased defects and edge structures and accelerating the transfer between electrons and holes are favorable. As shown in Figure 3e,f, the particle size of FeSe2 particles is about 5–10 nm with evident lattice stripes [43,53]. As shown in Figure S1, the elemental map corresponding to the PS-NH2@FeSe2 catalyst is analyzed and reveals that C, N, Cl, Fe, and Se elements appear simultaneously with dominant C and N. Overall, FeSe2 is successfully loaded onto the surface of PS-NH2 resin spheres, and the synthesized products can achieve the expected results.
3.3. Catalytic Activity between the Different Factors
3.3.1. Effect of Catalytic System
The different effects of catalyst dosage, substrate (TC) concentration, reaction temperature, initial solution pH, K2S2O8 concentration, and adsorption degradation are investigated to determine the most suitable catalytic reaction conditions. According to the analysis of the control test, as shown in Table S2, the removal of TC using potassium persulfate is only 13.27% under the same conditions, showing the importance of catalyst addition into the reaction system. The control test shows that PS-NH2 in combination with potassium persulfate can remove up to 37.96% of TC. Interestingly, the loading effect of PS-Cl resin is much less powerful than that of PS-NH2. However, when FeSe2 is loaded directly onto the unmodified PS-Cl resin and then degrades TC through a combination with the potassium persulfate system, the removal rate is surprisingly high at 61.44%. In contrast, the PS-NH2@FeSe2 catalyst is formed by modifying the PS-Cl resin with the modified -NH2 with strong chelating and coordination abilities and then loading FeSe2 on the spherical surface of PS-NH2 resin spheres. Interestingly, the combination of PS-NH2@FeSe2 and potassium persulfate for TC degradation can remove TC up to 85.94% within 120 min. Moreover, compared with FeSe2 material alone, the removal rate of TC in the potassium persulfate system can reach 60.37%, indicating that the combination of PS-NH2 resin and FeSe2 can maximize the removal rate of TC.
3.3.2. Effect of Adsorption and Degradation
To investigate the difference between adsorption and catalytic degradation, we further investigate the practical feasibility of TC removal using adsorption. As shown in Table S2, the PS-Cl resin has almost no adsorption effect on TC. Nevertheless, the adsorption performance of the PS-NH2 resin obtained after modification is improved, which can reach 16.77% adsorption rate. The main reason is because -NH2 has excellent chelating and coordination abilities compared to -Cl [19]. The addition of FeSe2 with the same content as PS-NH2@FeSe2 alone to TC as a comparison shows that the adsorption capacity of FeSe2 on TC is only 4.36%. This result indicates that the possibility of removing TC through adsorption is extremely weak. In contrast, the addition of a certain amount of potassium persulfate into PS-Cl and modified PS-NH2 results in an exponential increase in TC removal, thus reaching 17.81% and 37.96%, respectively. Considering the strong oxidizing property of potassium persulfate, we conduct a control test with potassium persulfate alone. At room temperature (298 K), potassium persulfate has a small degradation effect on TC, which can reach about 13.27%. This considerable catalytic degradation effect remarkably stimulates our research interest. Compared with the adsorption rate of TC through PS-NH2, PS-NH2 resin with potassium persulfate alone can increase the TC removal rate to 37.96%, which is higher than the sum of the single removal rate with PS-NH2 adsorption and potassium persulfate. The mesoporous structure of PS-NH2 and excellent adsorption properties for TC molecules in the water column accelerate the electron transfer of free radicals and remarkably reduce the removal path of TC via potassium persulfate [16,54]. Therefore, the removal efficiency increases significantly. After analyzing the TC degradation rate of PS-NH2@FeSe2 with potassium persulfate and comparing the TC adsorption rate of PS-NH2@FeSe2 alone, the actual effect of catalytic degradation with the persulfate system is outstanding and far superior to that of adsorption.
3.3.3. Effect of TC Concentration
The catalytic degradation efficiency of catalysts is investigated by analyzing TC solutions with different initial concentrations. As shown in Figure 4a, the corresponding degradation rate (K) and catalytic degradation efficiency decrease as the initial concentration of TC solution increases. At TC concentration of 10–30 mg L−1, the degradation rates of 10 and 20 mg L−1, i.e., 0.02089 and 0.02103 min−1, respectively, are relatively close to each other. At a concentration of 30 mg L−1, the degradation rate decreases significantly to 0.01316 min−1. The main reason is that at low initial concentration, the production rate of reactive radicals is superior because the production rate of reactive radicals is higher than the consumption rate at low initial concentrations. The degradation effect is similar, and the degradation rate is high at TC concentrations of 10 and 20 mg L−1 because the quantitative PS-NH2@FeSe2 catalyst has a limited number of active catalytic sites, leading to a low degradation rate for a high concentration of TC molecules. This phenomenon is because active sites are not utilized in time [32,39]. Therefore, the catalytic performance of the catalyst can be optimized at the appropriate TC concentration range.
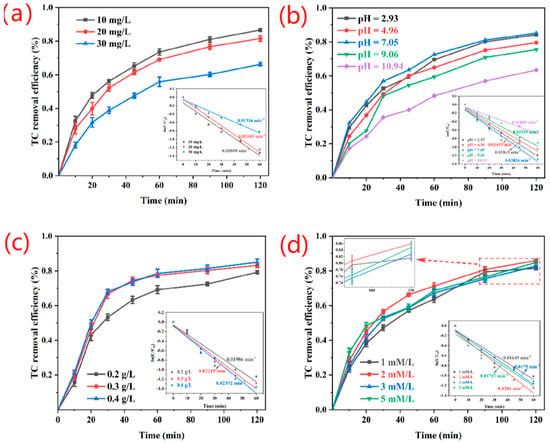
Figure 4.
Effect of initial TC concentration (a), pH (b), catalyst dosage (c) and K2S2O8 amount (d) on the catalytic degradation of TC over PS-NH2@FeSe2. The inserts are catalytic degradation rates (k) under different conditions: (a) [K2S2O8] = 2 mM, [catalyst] = 0.2 g/L, pH = 7.05, T = 298 K. (b) [TC]0 = 20 mg/L, [K2S2O8] = 2 mM, [catalyst] = 0.2 g/L, T = 298 K. (c) [TC]0 = 20 mg/L, [K2S2O8] = 2 mM, pH = 7.05, T = 298 K. (d) [TC]0 = 20 mg/L, [catalyst] = 0.2g/L, pH = 7.05, T = 298 K.
3.3.4. Effect of pH
The initial pH of the TC solution influences the stability of the oxidant and the catalytic degradation properties of the catalyst. In accordance with the different initial pH values of the TC solution, the most suitable pH condition for TC degradation through the PS-NH2@FeSe2 catalyst is explored. As shown in Figure 4b, the PS-NH2@FeSe2 catalyst shows excellent catalytic degradation in a wide pH range of 2.93–9.06. However, when the initial pH of the TC solution reaches 10.94, the effect of the catalytic degradation decreases to 63.41%. A small number of Fe3+ forms precipitates under alkaline conditions, resulting in Fe3+ deficiency in the reaction system. Thus, the Fe3+/Fe2+ cycle gradually weakens, resulting in a decreased TC removal rate. However, at pH 7.05, the PS-NH2@FeSe2 catalyst shows strong catalytic properties. The performance transition is due to the role of -NH2 in PS-NH2. In the synthesis of PS-NH2@FeSe2 catalyst, Fe3+ and PS-NH2 should be mutually coordinated so that Fe3+ can be trapped by -NH2, and Fe3+ should be fixed to the surface of PS-NH2 through the coordination bond formed between Fe3+ and -NH2. Afterward, Fe3+ is coordinated with the Se element on the surface of PS-NH2 to form FeSe2. In this way, the stability of Fe3+ on the catalyst surface is strengthened. Fe3+ is subject to binding by -NH2 and bound by the bonding position of Se elements. Thus, when the ionization influence of Fe3+ is reduced, the amount of precipitation in an inappropriate environment is also reduced. At the same time, the optimal pH conditions of the catalyst are subject to migration, forming a wide pH application range [55,56]. Moreover, the nearly neutral reaction conditions are friendly to the environment and do not easily cause secondary pollution and harm to the surrounding environment.
3.3.5. Effect of Catalyst Dosage
The effects of different doses of PS-NH2@FeSe2 catalyst on the degradation effect and rate (K) of TC are investigated under the conditions of constant influencing factors, such as potassium persulfate, TC concentration, and temperature. As shown in Figure 4c, the degradation effect and rate K on TC increase with increasing catalyst amount. When the dosage of PS-NH2@FeSe2 catalyst added increases from 20 mg to 30 mg, the removal rate increases from approximately 80% to 83%, and the rate constant K also increases from 0.01986 min−1 to 0.02215 min−1. When 40 mg catalyst is added, the removal rate increases to 85%, and the rate constant K changes to 0.02352 min−1. This result indicates that a high amount of catalyst provides increased active catalytic sites. The rate of degradation of TC molecules is accelerated by generating more radicals [57]. By analyzing the effect of the dose difference of the added catalyst on the removal rate and the floating change of the degradation rate (K) and considering the cost-effectiveness of its amount of potassium persulfate, the 20 mg catalyst is chosen as the most reasonable experimental parameter.
3.3.6. Effect of K2S2O8 Concentration
As shown in Figure 4d, the effect of the amount of potassium persulfate oxidant on the TC removal rate is investigated under the same reaction conditions. When the K2S2O8 concentration increases from 1 mM L−1 to 2 mM L−1, the catalytic degradation efficiency slightly increases from 81.55% to 85.94%, and the catalytic rate K increases from 0.01649 min−1 to 0.0206 min−1. This result indicates that a high K2S2O8 concentration improves the degradation effect and rate within a certain concentration range. However, by increasing K2S2O8 concentration to 3 and 5 mM L−1, the catalytic removal rate decreases slightly to 82.79% and 84.82%, respectively, and the catalytic rate K decreases to 0.0179 and 0.01737 min−1, respectively. This result is because increasing the K2S2O8 concentration can increase the generation of free radicals. However, the fixed PS-NH2@FeSe2 catalyst content is the rate-limiting factor controlling the generation of active radicals. Interestingly, a difference in the efficiency of radical utilization at different reaction stages is observed. Therefore, after the initial K2S2O8 concentration exceeds a certain range, the excess K2S2O8 decomposes rapidly to produce a large number of free radicals. Considering that the large number of free radicals generated in the initial stage increases the quenching reaction with K2S2O8, FeSe2, and other free radicals, the concentration of excessive K2S2O8 has a low utilization of free radicals in the initial stage of catalysis, and the excess K2S2O8 consumes the generated free radicals and slightly inhibits the degradation of TC [13,39]. Finally, considering the degradation rate and degradation rate constant (K) of the catalyst and the scientific nature of K2S2O8 dosage, 2 mM L−1 potassium persulfate oxidant is chosen as an experimental parameter for TC degradation.
3.3.7. Effect of Reaction Temperature
The effect on the TC degradation effect is investigated by setting different catalytic temperatures. As shown in Figure S3a,b, when the reaction temperature is increased from 288 K to 308 K, the corresponding TC removal rate and degradation rate K also increase. When the reaction temperature is increased from 288 K to 298 K, the TC removal rate increases from 67.30% to 84.96%, and the degradation rate increases from 0.01146 min−1 to 0.018926 min−1. This finding is attributed to the ability of potassium persulfate to produce reactive radicals through autolytic decomposition under heat. Therefore, a large number of reactive radicals available in the liquid environment effectively improve the TC removal rate. The degradation rate of TC is accelerated in a limited time. Additionally, with increasing temperature, the collision frequency between catalyst and active radicals is gradually accelerated, effectively improving the TC removal rate and degradation rate (K). When the reaction temperature is increased from 298 K to 308 K, the removal rate of TC increases from 84.96% to 87.26%, and K increases from 0.0189266 min−1 to 0.019126 min−1. The main reason for the gentle increases in removal and degradation rates is that a high temperature causes autolytic decomposition of potassium persulfate, generating a large amount of reactive radicals [58]. However, the catalyst surface is saturated with active sites capable of generating active radicals. Excess radicals degrade TC while being attacked by the undecomposed K2S2O8, FeSe2, and other reactive groups and quenched. This phenomenon directly leads to the low utilization of free radicals at this stage and causes no increase in the degradation (K) and removal rates of TC. In addition, the Arrhenius equation enables the calculation of the activation energy (Ea) required for the reaction based on the catalytic reaction at different temperatures [39]. As shown in Figure S2, the calculated Ea of the reaction for the PS-NH2@FeSe2 catalyst is about 13.63 KJ mol−1. The Ea of the PS-NH2@FeSe2 catalyst is lower than those reported in other literature. This result indicates that the PS-NH2@FeSe2 catalyst has excellent catalytic ability and that the catalytic degradation rate can be easily increased.
As shown in Table S3, the excellent catalytic performance of PS-NH2@FeSe2 catalysts is highlighted by compiling the degradation effects of various Fenton-like catalysts on TC. Compared with currently reported relevant catalytic materials, PS-NH2@FeSe2 catalysts show excellent removal and degradation rates in the degradation of TC. The main reasons are the mesoporous structure of the PS-NH2@FeSe2 catalyst, high comparative area, loading and high stability of FeSe2 on top of PS-NH2, and the excellent electron transfer efficiency of the catalytic material. Furthermore, due to the robustness of the resin material, the PS-NH2@FeSe2 catalyst with PS-NH2 resin as carrier can be stored for a long time and easily recycled [19].
3.4. Catalytic Mechanism
The type of free radicals by EPR should be first determined to investigate the catalytic mechanism of TC degradation by PS-NH2@FeSe2 catalyst and elucidate the mechanism of K2S2O8 activation by PS-NH2@FeSe2. DMPO is often used in free-radical scavengers to stabilize the system’s free radicals [19]. As shown in Figure S3c, a large amount of DMPOX generated through DMPO is present in the reaction system, thus agreeing with the reports about DMPO as a trapping agent. Interestingly, relevant literature revealed that hydroxyl radicals are the main cause of induced DMPOX production. The standard peak intensity ratio shown in Figure S3c is 1:2:2:1, which is the typical signal of DMPO-OH. Comparing the peak signal intensities generated shows that at the beginning, the sulfate radical (SO4·−) generated by the decomposition of potassium persulfate is continuously converted into hydroxyl radical (·OH). The peak intensity of DMPO-OH increases significantly with time and reaches a stable value in a short period [13]. This finding fully demonstrates the good stability of PS-NH2@FeSe2 catalyst in activating K2S2O8 to generate free radicals. Results indicate that the PS-NH2@FeSe2 catalyst is an excellent activator capable of activating K2S2O8 to generate free radicals. As shown in Scheme S1, the degradation mechanism of PS-NH2@FeSe2 catalyst by activating K2S2O8 to remove TC is demonstrated. On the basis of the relevant literature and EPR assay results, the possible mechanism of the reaction is inferred as follows.
Fe(II) + S2O82− → Fe(III) + SO4·− + SO42−
SO4·− + H2O → SO42− + ·OH + H+
SO4·−/·OH + TC → Intermediates + CO2 + H2O
According to relevant studies, TBA and MeOH are used to quench sulfate and hydroxyl radicals, respectively, which are effective in quenching radicals [39]. As shown in Figure S3d, the TC removal rate using the PS-NH2@FeSe2/K2S2O8 system reaches 85.94% when no quencher is added to the reaction environment. This result shows that the active free radicals during the reaction are in a coexisting state and play a role together. After the addition of TBA to the reaction environment, the degradation rate of TC using the PS-NH2@FeSe2/K2S2O8 system is decreased to 54.59%. This result indicates that SO4·− exists in the reaction process. The addition of MeOH to the reaction environment reduces the removal of TC to 64.25%. The existence of ·OH is demonstrated during the reaction.
3.5. Possible Catalytic Pathways for TC Degradation
A large number of intermediates produced during the degradation of TC are detected using HPLC-MS. As shown in Figure S4, the major intermediates with m/z values are 445, 431, 427, 359, 401, 369, 316, 321, 304, 302, 281, 272, and 242. The main reactive group that produced these intermediates by attacking the TC molecule is ·OH. After analyzing the structure of TC and the produced intermediates, several possible degradation pathways are postulated [59,60]. Initially, the TC molecule is demethylated due to the attack of ·OH on the C4 tertiary amine of the TC molecule, and the process is achieved through two pathways, i.e., TC-M1-M2 and TC-M9. With the breakage of the hydroxyl group during the reaction, the formation of C=C starts at the C6 position, and the process is finished through two pathways, i.e., TC-M5 and TC-M9. Then, the generated intermediates further form intermediates, labeled M3 and M10, at C2 and C3 positions. Considering that the C=C between C11a and C12 is easily oxidized by hydroxyl radicals, the intermediate structure labeled M4 is generated [19]. Another degradation pathway is observed for M2. M2 is further degraded into M7 by bond breaking due to hydroxyl radicals attacking the C6 position of M7, causing M7 to shed its hydroxyl and methyl groups at the C6 position and forming M8 possessing C=C [61,62]. Afterwards, M8 is further decomposed to the M12 intermediate with a small mass fraction by breaking the C1 bond position under the attack of reactive radicals. In another degradation pathway, the C4 position of M10 continues to be attacked by oxidation of ·OH to become M11. M11 becomes further decomposed into the low-mass fraction M12 intermediate. As the reaction continues, macromolecules are decomposed layer by layer into intermediates with small mass fractions due to the strong oxidative properties of ·OH [63,64]. Eventually, TC molecules are mineralized into nonhazardous H2O, CO2, and NH4+.
3.6. Reusability and Stability
The reusability of PS-NH2@FeSe2 catalyst is investigated. The changes in the content of the catalyst elements and the possible causative factors when the catalyst is used for the first time and reused again are analyzed using XPS. As shown in Figure S5a, the peak changes of Fe elements contained in the PS-NH2@FeSe2 catalyst during the first use and the second reuse are illustrated. Combined with the peak areas of Fe elements contained at different peak positions listed in Table S4, results demonstrate that the electron migration due to the interconversion of Fe2+ and Fe3+ can contribute to the effective decomposition of K2S2O8 to produce reactive radicals. In addition, the changes in XPS and peak areas before and after the catalyst reaction can prove that the electron migration caused by the conversion of Fe2+ and Fe3+ is the most important factor that promotes the efficient decomposition of K2S2O8 to produce ·OH. As shown in Figure S5b, the peak changes in the contained Se elements are responded during the first use and the second reuse of the catalyst. Interestingly, Se may be involved in the reaction system through another pathway, as documented in the literature and in combination with the change in the peak profile of Se elements. First, XPS shows that the proportion of Se2− decreases gradually during the activation of the K2S2O8. The Se element is indicated to be an important electron donor for Fe2+ regeneration. Subsequently, the Se atoms on the outer surface of the PS-NH2@FeSe2 catalyst readily ligate with metal atoms to capture protons during the activation of K2S2O8. The formation of Se vacancies directly exposes the active sites of the metal and accelerates the electron migration, effectively promoting the catalytic reaction activity. The formation of Se vacancies accelerates the generation of Fe2+, which promotes the regeneration of Se vacancies [39]. The process fully illustrates the excellent synergistic effect between Se and Fe elements and effectively enhances the activation of K2S2O8.
As shown in Figure S6a, the PS-NH2@FeSe2 catalyst is cycled five times, and the removal rate of TC still reaches 66%. The removal rate of TC decreases gently with each cycle without large fluctuations. Results fully demonstrate the good stability and reusability of the catalyst. As shown in Figure S6b, the removal effect of PS-NH2@FeSe2 catalyst on total organic carbon (TOC) in TC solution is visually shown. The amount of Fe ions leached from the catalyst surface is also demonstrated. The removal of TOC also shows a gentle decrease during five cycles of use. After five consecutive uses, the removal rate of TOC can still reach more than half, indicating that the catalyst is effective in the mineralization of organic matter. Moreover, the stability and reusability of the PS-NH2@FeSe2 catalyst are verified by the degradation experiments on TOC. To further investigate the stability of the catalyst, we examine the leaching of Fe ions after each cycle. Notably, the leaching of Fe ions decreases significantly during each cycle. In the fifth cycle, the Fe ion leaching decreases to 0.017 mg L−1. In summary, the FeSe2 loaded on PS-NH2 is the main factor affecting the TC removal rate. In addition, the PS-NH2@FeSe2 catalyst can be recovered through simple filtration and washing and can be easily reused.
3.7. Comparison of Similar Materials
Metal sulfides also have an advantage in degrading organic pollutants. Elemental sulfur and elemental selenium belong to the same main group of elements and have similar chemical properties. Thus, we load FeS2 and FeSe2 onto PS-NH2 to determine the difference between the two catalysts in degrading the TC solution [35]. As shown in Figure S7a, PS-NH2@FeS2 paired with K2S2O8 achieves 65% removal of TC under the same reaction conditions. However, PS-NH2@FeSe2 paired with K2S2O8 achieves 85% removal of TC. Hence, the PS-NH2@FeSe2 catalyst shows a powerful reaction power in activating persulfate for TC degradation. As shown in Figure S7b, in addition to catalytic persulfate degradation of TC, the removal of organic matter by adsorption is a means. The effect of TC adsorption of PS-NH2@FeSe2 can reach 35.23% under the same conditions. The adsorption rate of TC by the PS-NH2@FeS2 catalyst is 28.05%, which is slightly lower than that of PS-NH2@FeSe2. In summary, the PS-NH2@FeSe2 catalyst has excellent performance in degrading TC solutions. This result may be attributed to the fact that S and Se are both oxygen group elements, but Se is more metallic than S. Therefore, the Se element has a strong ability to transfer electrons, which is more favorable for the valence transformation of Fe atoms.
3.8. Column Experiment
The PS-NH2@FeSe2 catalyst is subjected to column experiments to dynamically simulate the actual performance of the catalyst in practice. The use of loaded column experiments is a practical method in the actual treatment of wastewater. As shown in Figure S8a, 250 mg TC is first dissolved in 2500 mL Yellow River water. The Yellow River water with a packed column containing 5 g PS-NH2@FeSe2 catalyst is purified. Afterwards, the Yellow River water containing the TC solution is flowed through a peristaltic pump (pump rate of 11.127 mL min−1). According to the UV–vis spectra, the concentration of TC decreases from 100 mg L−1 to 18.6 mg L−1 when passing through the reaction column containing PS-NH2@FeSe2 catalyst. As shown in Figure S8b, the concentration of TC decreases significantly when TC flows through the reaction column within 10 min. In addition, the PS-NH2@FeSe2 catalyst can maintain a good degradation effect on the first day. The absorption peak at 275 nm is weakened, which is attributed to the amide and hydroxyl groups attached to the aromatic ring [65]. The cleavage of the phenolic group on the aromatic ring of the TC molecule due to the attack of ·OH causes the destruction of the TC molecular structure, resulting in decreased absorption peak at 358 nm [66]. The sufficient degradation of TC in the water sample may be the reason for the disappearance of the two main absorption peaks. In conclusion, the PS-NH2@FeSe2 catalyst with column experimental setup is promising in degrading antibiotic wastewater.
4. Conclusions
In this work, we have assembled FeSe2 with PS-NH2 resin into a PS-NH2@FeSe2 catalyst for Fenton-like processes to degrade TC. Through the above characterization results and the catalytic degradation effect, we prove that the PS-NH2@FeSe2 catalyst can be successfully prepared. Furthermore, the PS-NH2@FeSe2 catalyst shows excellent catalytic activity within 120 min, removing the TC by up to 85.94%. At the same time, the maximum degradation rate constant of the TC can reach 0.02352 min−1 at around 30 min. In brief, we simply modify the PS-Cl resin with -NH2 to change the PS-Cl resin into an improved PS-NH2 resin. Later, FeSe2 is grown in situ on PS-NH2 resin through water–thermal synthesis, and the excellent PS-NH2@FeSe2 catalyst is successfully synthesized through simple washing and drying. The best catalytic performance of the catalyst is determined by examining the required catalytic degradation conditions, such as pH, catalyst dosage, reaction temperature, K2S2O8 dosage, and TC concentration. Using the explored catalyst performance parameters and similar catalytic materials, the PS-NH2@FeSe2 catalyst shows a relatively excellent catalytic degradation performance for TC. EPR techniques and XPS prove that the active species ·OH plays a dominant role throughout the catalytic degradation process, which is because ·OH has a strong oxidation capacity. Overall, the study has successfully prepared PS-NH2@FeSe2 catalysts with excellent performance through a simple chemical modification and a one-step hydrothermal method with potential applications in the field of environmental remediation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15043657/s1, References [67,68] are cited in the supplementary materials.
Author Contributions
Conceptualization, J.M. and X.W.; methodology, J.M.; software, J.M.; validation, J.M., X.W. and X.Z.; formal analysis, S.S.; investigation, L.Z.; resources, Z.Y.; data curation, X.W.; writing—original draft preparation, J.M.; writing—review and editing, J.M.; visualization, Z.Y.; supervision, L.Z.; project administration, Z.Y.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, H.; Zeng, S.; Dong, X.; Li, D.; Zhang, Y.; He, M.; Du, P. Diverse and abundant antibiotics and antibiotic resistance genes in an urban water system. J. Environ. Manag. 2019, 231, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Long, C.; Qin, L.; Jiang, Z.; Qing, T.; Zhang, P.; Feng, B. Fluorescent and colorimetric dual-mode detection of tetracycline in wastewater based on heteroatoms-doped reduced state carbon dots. Environ. Pollut. 2021, 283, 117109. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, J.A.I.; Dong, C.-D.; Garcia-Segura, S.; Abarca, R.R.M.; Chen, C.-W.; de Luna, M.D.G. Degradation of tetracycline antibiotics by Fe2+-catalyzed percarbonate oxidation. Sci. Total Environ. 2021, 781, 146411. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sheng, G.D.; O’Connor, P. Microplastics combined with tetracycline in soils facilitate the formation of antibiotic resistance in the Enchytraeus crypticus microbiome. Environ. Pollut. 2020, 264, 114689. [Google Scholar] [CrossRef]
- Li, K.; Li, J.J.; Zhao, N.; Ma, Y.; Di, B. Removal of Tetracycline in Sewage and Dairy Products with High-Stable MOF. Molecules 2020, 25, 1312. [Google Scholar] [CrossRef]
- Nazari, P.; Tootoonchian, P.; Setayesh, S.R. Efficient degradation of AO7 by ceria-delafossite nanocomposite with non-inert support as a synergistic catalyst in electro-fenton process. Environ. Pollut. 2019, 252, 749–757. [Google Scholar] [CrossRef]
- Qin, H.; Yang, Y.; Shi, W.; She, Y.; Wu, S. Heterogeneous Fenton degradation of azithromycin antibiotic in water catalyzed by amino/thiol-functionalized MnFe2O4 magnetic nanocatalysts. J. Environ. Chem. Eng. 2021, 9, 106184. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, J.; Zhang, H.; Shao, Y.; Li, Y. Fabrication of magnetic carbon composites from peanut shells and its application as a heterogeneous Fenton catalyst in removal of methylene blue. Appl. Surf. Sci. 2015, 324, 490–498. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Qi, J.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Enhanced heterogeneous Fenton-like systems based on highly dispersed Fe(0)-Fe2O3 nanoparticles embedded ordered mesoporous carbon composite catalyst. Environ. Pollut. 2018, 243, 1068–1077. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, R.; Song, L.; Shi, X. Cobalt-based metal-organic frameworks for the photocatalytic reduction of carbon dioxide. Nanoscale 2021, 13, 9075–9090. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Lian, C.; Huang, K.; Liang, L.; Yu, H.; Yin, P.; Zhang, J.; Xing, M. Constructing an Acidic Microenvironment by MoS2 in Heterogeneous Fenton Reaction for Pollutant Control. Angew. Chem. Int. Ed. Engl. 2021, 60, 17155–17163. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, M.; Li, X.; Ren, F.; Sun, P.; Zhou, L. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation. Chem. Eng. J. 2020, 387, 124008. [Google Scholar] [CrossRef]
- Wang, F.; Yu, X.; Ge, M.; Wu, S.; Guan, J.; Tang, J.; Wu, X.; Ritchie, R.O. Facile self-assembly synthesis of gamma-Fe2O3/graphene oxide for enhanced photo-Fenton reaction. Environ. Pollut. 2019, 248, 229–237. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, W.; Bi, F.; Yan, S.; Miao, X.; Zhang, B.; Wang, Y.; Ge, C.; Zhang, Y. Two-step ball milling-assisted synthesis of N-doped biochar loaded with ferrous sulfide for enhanced adsorptive removal of Cr() and tetracycline from water. Environ. Pollut. 2022, 306, 119398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, F.; Yang, W.; Yang, Z.; Li, A. A comparative study on the adsorption of 8-amino-1-naphthol-3,6-disulfonic acid by a macroporous amination resin. Chem. Eng. J. 2016, 283, 1522–1533. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, P.; Liu, D.; Zhao, Q.; Zou, B.; Zhou, L.; Ye, Z. Method to fabricate porous multifunction β-cyclodextrin modified resin for ultrafast and efficient removal of Cu(II) and bisphenol A. J. Taiwan Inst. Chem. Eng. 2021, 119, 286–297. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Y.; Yang, L.; Li, X.; Zhou, L.; Li, Y. Novel adsorbent of polymeric complex derived from chaleting resin with Cu(II) and its removal properties for cyanide in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 136–146. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Zhang, X.; Ran, L.; Zhao, Q.; Zou, B.; Zhou, L.; Ye, Z. A novel design of self-assembled metal-organic frameworks MIL-53(Fe) modified resin as a catalyst for catalytic degradation of tetracycline. J. Clean. Prod. 2022, 348, 131385. [Google Scholar] [CrossRef]
- Zou, B.; Zhang, S.; Sun, P.; Ye, Z.; Zhao, Q.; Zhang, W.; Zhou, L. Preparation of a novel Poly-chloromethyl styrene chelating resin containing heterofluorenone pendant groups for the removal of Cu (II), Pb (II), and Ni (II) from wastewaters. Colloid Interface Sci. Commun. 2021, 40, 100349. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Dragoi, E.N.; Almomani, F.; Le, V.T. A comprehensive review on MXenes as new nanomaterials for degradation of hazardous pollutants: Deployment as heterogeneous sonocatalysis. Chemosphere 2022, 287, 132387. [Google Scholar] [CrossRef]
- Konopatsky, A.S.; Firestein, K.L.; Evdokimenko, N.D.; Kustov, A.L.; Baidyshev, V.S.; Chepkasov, I.y.V.; Popov, Z.I.; Matveev, A.T.; Shetinin, I.V.; Leybo, D.V.; et al. Microstructure and catalytic properties of Fe3O4/BN, Fe3O4(Pt)/BN, and FePt/BN heterogeneous nanomaterials in CO2 hydrogenation reaction: Experimental and theoretical insights. J. Catal. 2021, 402, 130–142. [Google Scholar] [CrossRef]
- Qu, J.; Ye, F.; Chen, D.; Feng, Y.; Yao, Q.; Liu, H.; Xie, J.; Yang, J. Platinum-based heterogeneous nanomaterials via wet-chemistry approaches toward electrocatalytic applications. Adv. Colloid Interface Sci. 2016, 230, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, M.; Nirmala, C.; Jawahar, N.; Arthi, G.; Vallinayagam, S.; Sharma, V.K. Role of nanomaterial’s as adsorbent for heterogeneous reaction in waste water treatment. J. Mol. Struct. 2021, 1241, 130596. [Google Scholar] [CrossRef]
- Tao, Y.; De Luca, O.; Singh, B.; Kamphuis, A.J.; Chen, J.; Rudolf, P.; Pescarmona, P.P. WO3–SiO2 nanomaterials synthesized using a novel template-free method in supercritical CO2 as heterogeneous catalysts for epoxidation with H2O2. Mater. Today Chem. 2020, 18, 100373. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Betiha, M.A.; Mohamed, S.K.; El-Sharkawy, E.A.; Ahmed, E.A. Stable and recyclable MIL-101(Cr)–Ionic liquid based hybrid nanomaterials as heterogeneous catalyst. J. Mol. Liq. 2017, 236, 385–394. [Google Scholar] [CrossRef]
- Srinivasan, S.; Vivek, C.; Sakthivel, P.; Chamundeeswari, G.; Prasanna Bharathi, S.; Amuthameena, S.; Balraj, B. Synthesis of Ag incorporated ZrO2 nanomaterials for enhanced electrochemical energy storage applications. Inorg. Chem. Commun. 2022, 138, 109262. [Google Scholar] [CrossRef]
- Skoda, D.; Hanulikova, B.; Styskalik, A.; Vykoukal, V.; Machac, P.; Urbanek, P.; Domincova Bergerova, E.; Simonikova, L.; Kuritka, I. Non-aqueous synthesis of homogeneous molybdenum silicate microspheres and their application as heterogeneous catalysts in olefin epoxidation and selective aniline oxidation. J. Ind. Eng. Chem. 2022, 107, 320–332. [Google Scholar] [CrossRef]
- Jiang, S.; Zheng, H.; Sun, X.; Zhu, M.; Zhou, Y.; Wang, D.; Zhang, D.; Zhang, L. New and highly efficient Ultra-thin g-C3N4/FeOCl nanocomposites as photo-Fenton catalysts for pollutants degradation and antibacterial effect under visible light. Chemosphere 2022, 290, 133324. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Lu, S.; Ma, Y.; Zhao, L. Synergistic photocatalysis-fenton reaction of flower-shaped CeO2/Fe3O4 magnetic catalyst for decolorization of high concentration congo red dye. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129021. [Google Scholar] [CrossRef]
- Vinosha, P.A.; Manikandan, A.; Ragu, R.; Dinesh, A.; Paulraj, P.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Madhavan, J.; Xavier, B.; et al. Exploring the influence of varying pH on structural, electro-optical, magnetic and photo-Fenton properties of mesoporous ZnFe2O4 nanocrystals. Environ. Pollut. 2021, 272, 115983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Zhou, L.; Wu, P.; Zhao, Y.; Lai, Y.; Wang, F. Heterogeneous Fenton degradation of bisphenol A using Fe3O4@beta-CD/rGO composite: Synergistic effect, principle and way of degradation. Environ. Pollut. 2019, 244, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, Z.; Wang, Y.; Xia, Q.; Chu, H.; Jiang, Z. Preparation of immobilized coating Fenton-like catalyst for high efficient degradation of phenol. Environ. Pollut. 2017, 224, 552–558. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, K.; Zhao, W.; Jiang, H.; Liu, L.; Hu, D.; Cui, B.; Li, Y.; Sun, Y. Novel nanoparticle-assembled tetrakaidekahedron Bi25FeO40 as efficient photo-Fenton catalysts for Rhodamine B degradation. Adv. Powder Technol. 2022, 33, 103579. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Lu, J.; Zhou, Y.; Zhou, Y. In-situ production and activation of H2O2 for enhanced degradation of roxarsone by FeS2 decorated resorcinol-formaldehyde resins. J. Hazard. Mater. 2022, 424, 127650. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cheng, X.; Li, H. Microwave Synthesis of High Activity FeSe2/C Catalyst toward Oxygen Reduction Reaction. Catalysts 2015, 5, 1079–1091. [Google Scholar] [CrossRef]
- Liu, T.; Hou, S.; Guo, Q.; Liang, Z.; Xiong, Z.; Zhao, L. Achieving superior sodium storage of FeSe2@NC composite via optimizing architecture. Appl. Surf. Sci. 2022, 579, 152227. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Santhoshkumar, P.; Hussain, T.; Vikraman, D.; Yim, C.J.; Hussain, S.; Shanmugam, P.; Alfantazi, A.; Manickam, S.; Kim, H.S. Influence of selenium precursors on the formation of iron selenide nanostructures (FeSe2): Efficient Electro-Fenton catalysts for detoxification of harmful organic dyestuffs. Chemosphere 2021, 272, 129639. [Google Scholar] [CrossRef]
- Fang, G.; Zhang, T.; Cui, H.; Dionysiou, D.D.; Liu, C.; Gao, J.; Wang, Y.; Zhou, D. Synergy between Iron and Selenide on FeSe2(111) Surface Driving Peroxymonosulfate Activation for Efficient Degradation of Pollutants. Environ. Sci. Technol. 2020, 54, 15489–15498. [Google Scholar] [CrossRef]
- Fan, H.; Yu, H.; Zhang, Y.; Guo, J.; Wang, Z.; Wang, H.; Zhao, N.; Zheng, Y.; Du, C.; Dai, Z.; et al. 1D to 3D hierarchical iron selenide hollow nanocubes assembled from FeSe2@C core-shell nanorods for advanced sodium ion batteries. Energy Storage Mater. 2018, 10, 48–55. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; Che, G.; Fan, W.; Liu, C. Controlled hydrothermal synthesis and magnetic properties of three-dimensional FeSe2 rod clusters and microspheres. Chem. Eng. J. 2013, 215–216, 508–516. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, Q.; Li, H.; Xu, J.; Li, X.; Zhao, H.; Tang, Y.; Zhao, G.; Li, H.; et al. Constructing Three-Dimensional Porous Carbon Framework Embedded with FeSe2 Nanoparticles as an Anode Material for Rechargeable Batteries. ACS Appl. Mater. Interfaces 2018, 10, 38862–38871. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, Q.; Chen, W.; Zai, J.; Qiao, Q.; Qian, X. 3D hierarchical FeSe2 microspheres: Controlled synthesis and applications in dye-sensitized solar cells. Nano Energy 2015, 15, 205–215. [Google Scholar] [CrossRef]
- Zhong, Q.; Xu, C.; Liu, Y.; Ji, Q.; Xu, Z.; Sun, D.; Zhou, S.; Yang, B.; Dai, Y.; Qi, C.; et al. Defect-engineered FeSe2−x@C with porous architecture for enhanced peroxymonosulfate-based advanced oxidation processes. Appl. Catal. B Environ. 2022, 309, 121259. [Google Scholar] [CrossRef]
- Dong, S.; Su, Q.; Jiao, W.; Ding, S.; Zhang, M.; Du, G.; Xu, B. FeSe2 microspheres coated with carbon layers as anode materials for sodium-ion batteries. J. Alloys Compd. 2020, 842, 155888. [Google Scholar] [CrossRef]
- Wang, T.; Guo, W.; Wang, G.; Wang, H.; Bai, J.; Wang, B. Highly dispersed FeSe2 nanoparticles in porous carbon nanofibers as advanced anodes for sodium and potassium ion batteries. J. Alloys Compd. 2020, 834, 155265. [Google Scholar] [CrossRef]
- Xiao, G.; Wen, R.; Liu, A.; He, G.; Wu, D. Adsorption performance of salicylic acid on a novel resin with distinctive double pore structure. J. Hazard. Mater. 2017, 329, 77–83. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Ran, L.; Wang, X.; Zou, B.; Zhou, L. Mechanism and performance evaluation of FeSe2 and MoS2-loaded carbon fiber sheet as a novel heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2022, 598, 153860. [Google Scholar] [CrossRef]
- Cheng, X.-L.; Xia, X.; Xu, Q.-Q.; Wang, J.; Sun, J.-C.; Zhang, Y.; Li, S.-S. Superior conductivity FeSe2 for highly sensitive electrochemical detection of p-nitrophenol and o-nitrophenol based on synergistic effect of adsorption and catalysis. Sens. Actuators B Chem. 2021, 348, 130692. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, L.; Ding, Y.; Shi, X.; Ding, Y.L.; Mujtaba, J.; Li, Z.; Fang, Z. Rational nanostructured FeSe2 wrapped in nitrogen-doped carbon shell for high-rate capability and long cycling sodium-ion storage. J. Colloid Interface Sci. 2022, 622, 840–848. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Zeng, B.; Zhou, D.; Tai, L.; Liu, X.-D.; Lau, W.-M. Rich-oxygen-doped FeSe2 nanosheets with high pseudocapacitance capacity as a highly stable anode for sodium ion battery. Chem. Eng. J. 2022, 428, 132637. [Google Scholar] [CrossRef]
- Liang, T.; Wang, H.; Wang, R.; He, B.; Gong, Y.; Yan, C. Nitrogen-doped carbon nanotube-buffered FeSe2 anodes for fast-charging and high-capacity lithium storage. Electrochim. Acta 2021, 389, 138686. [Google Scholar] [CrossRef]
- Min, H.; Li, M.; Shu, H.; Zhang, X.; Hu, T.; Wang, W.; Zhou, Y.; Jian, J.; Wang, X. FeSe2 nanoparticle embedded in 3D honeycomb-like N-doped carbon architectures coupled with electrolytes engineering boost superior potassium ion storage. Electrochim. Acta 2021, 366, 137381. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Z.; Zhang, M.; Wang, S.; Qu, C. Synthesis of a novel magnetic multi-amine decorated resin for the adsorption of tetracycline and copper. J. Taiwan Inst. Chem. Eng. 2020, 106, 130–137. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, L.; Lei, J.; Liu, Y.; Zhang, J. Photo-Fenton degradation of phenol by CdS/rGO/Fe2+ at natural pH with in situ-generated H2O2. Appl. Catal. B Environ. 2019, 241, 367–374. [Google Scholar] [CrossRef]
- Xiang, W.; Chen, H.; Zhong, Z.; Zhang, C.; Lu, X.; Huang, M.; Zhou, T.; Yu, P.; Zhang, B. Efficient degradation of carbamazepine in a neutral sonochemical FeS/persulfate system based on the enhanced heterogeneous-homogeneous sulfur-iron cycle. Sep. Purif. Technol. 2022, 282, 120041. [Google Scholar] [CrossRef]
- Ren, H.; He, F.; Liu, S.; Li, T.; Zhou, R. Enhancing Fenton-like process at neutral pH by Fe(III)-GLDA complexation for the oxidation removal of organic pollutants. J. Hazard. Mater. 2021, 416, 126077. [Google Scholar] [CrossRef]
- Zhao, S.; Long, Y.; Su, Y.; Wang, S.; Zhang, Z.; Zhang, X. Cobalt-Enhanced Mass Transfer and Catalytic Production of Sulfate Radicals in MOF-Derived CeO2 * Co3O4 Nanoflowers for Efficient Degradation of Antibiotics. Small 2021, 17, 2101393. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Ma, J.; Li, M.; Ren, F.; Zhou, L. Metal-organic framework modified pine needle-derived N, O-doped magnetic porous carbon embedded with Au nanoparticles for adsorption and catalytic degradation of tetracycline. J. Clean. Prod. 2021, 278, 123575. [Google Scholar] [CrossRef]
- Peng, X.; Yang, Z.; Hu, F.; Tan, C.; Pan, Q.; Dai, H. Mechanistic investigation of rapid catalytic degradation of tetracycline using CoFe2O4@MoS2 by activation of peroxymonosulfate. Sep. Purif. Technol. 2022, 287, 120525. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Zhang, G.; Meng, Y.; Yin, B.; Zhao, Y.; Shi, W. Efficient and stable Nb2O5 modified g-C3N4 photocatalyst for removal of antibiotic pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, Q.; Yang, B.; Petropoulos, E.; Xue, L.; Feng, Y.; He, S.; Yang, L. Efficient photocatalysis of tetracycline hydrochloride (TC-HCl) from pharmaceutical wastewater using AgCl/ZnO/g-C3N4 composite under visible light: Process and mechanisms. J. Environ. Sci. 2023, 126, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, M.; Wang, R.; Wu, S.; Tan, X. Efficient removal TC by Zn@SnO2/PI via the synergy of adsorption and photocatalysis under visible light. Chem. Eng. J. 2022, 444, 136567. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, J.; Wang, J.; Zhang, Z.; Zhang, J.; Chang, J. Fe/Co redox and surficial hydroxyl potentiation in the FeCo2O4 enhanced Co3O4/persulfate process for TC degradation. J. Environ. Manag. 2022, 313, 114855. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Xiao, P. Catalytic degradation of tetracycline using peroxymonosulfate activated by cobalt and iron co-loaded pomelo peel biochar nanocomposite: Characterization, performance and reaction mechanism. Sep. Purif. Technol. 2022, 287, 120533. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Liu, Z.; Wang, S.; Zhang, X.; Xu, B.; Yu, P.; Xu, Y.; Sun, Y. Effective removal of tetracycline from water by catalytic peroxymonosulfate oxidation over Co@MoS2: Catalytic performance and degradation mechanism. Sep. Purif. Technol. 2022, 294, 121139. [Google Scholar] [CrossRef]
- Luo, B.; Xu, D.; Li, D.; Wu, G.; Wu, M.; Shi, W.; Chen, M. Fabrication of a Ag/Bi3TaO7 Plasmonic Photocatalyst with Enhanced Photocatalytic Activity for Degradation of Tetracycline. ACS Appl. Mater. Interfaces 2015, 7, 17061–17069. [Google Scholar] [CrossRef]
- Zhang, W.; Xing, P.; Zhang, C.; Zhang, J.; Hu, X.; Zhao, L.; He, Y. Facile synthesis of strontium molybdate coupled g-C3N4 composite for effective tetracycline and dyes degradation under visible light. Adv. Powder Technol. 2022, 33, 103573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).