Abstract
The establishment of the Yellow River wetland nature reserves improves the local soil structure and fertility through the long-term succession of microorganisms. However, little is known about which indigenous microbial resources can accelerate the process of soil improvement and ecology restoration. To fill this gap, exopolysaccharides-producing bacteria and phosphate-solubilizing bacteria were isolated from soil samples of the wetland nature reserve with higher soil organic matter, available phosphorus, and available nitrogen content. 16S rRNA nucleotide sequence homology analysis and physiological-biochemical assay showed that the strain PD12 with the highest phosphate solubilization activity and higher EPS production was identified as Klebsiella variicola, and other high yield EPS-producing strains (EPS12, EPS15, EPS18, and EPS19) were identified as Pseudomonas migulae, Pseudomonas frederiksbergensis, Aeromonas media, and Pseudomonas vancouverensis, respectively. These results provided new potential microbial resources for the research and development of biofertilizers and added new insights into accelerating the restoration of physical, chemical, and biological properties of soil in the Yellow River basin.
1. Introduction
The Yellow River is an important “ecological corridor” in China, which is deemed as the “Mother River”. With global climate change and human interference, ecological and environmental problems such as soil erosion, soil phosphorus (P) losses, and biodiversity reduction have occurred frequently [1]. To curb the trend, a series of wetland ecological restoration and conservation projects (restoration of farmland from lake and dredging operations) have been successively carried out in the Yellow River basin [2]. After years of active management and restoration, these wetland nature reserves have successfully protected the regional environment, increased vegetation coverage, resisted soil erosion in the region, improved soil structure and fertility, and brought ecological, environmental, social, and economic benefits.
Soil microorganisms are important parts of wetland ecosystems, and they play an important role in regulating carbon, nitrogen, phosphorus and sulfur cycling, (multifunctionality), shaping energy flow, and maintaining ecosystem stability [3]. Exopolysaccharides (EPSs) are high molecular weight natural polymers secreted into the extracellular environment by microorganisms such as members of Proteobacteria, Actinobacteria, and Cyanobacteria and microfungi [4]. As they are considered as the key indicator of soil health and stability, EPSs have many important ecological functions in improving soil structure and fertility, such as resisting erosion through enhancing soil and sediments cohesion, promoting the formation and stability of soil aggregates, ensuring moisture provision, improving nutrient and mineral accumulation, protecting nitrogenase against the harmful effects of oxygen, preventing external specific and non-specific threats, and facilitating plant and microorganism growth [4,5,6,7]. Thus, the screening and identification of EPS-producing bacteria are beneficial to improving soil’s physical and chemical properties through the above ecological functions.
P is the second most limiting macronutrient for plant growth in terrestrial ecosystems except nitrogen (N) [8]. Since most soil P compounds occur in insoluble forms (mineral salts or incorporated into organic compounds) that cannot be absorbed and utilized by plants [9,10], more than 15 million tons of P fertilizer are required every year in the world [11,12]. Meanwhile, most of the soluble P is rapidly transformed into insoluble forms by combining with metal ions and lost, which eventually leads to ecological problems, such as P pollution, soil erosion, and even soil dysfunction caused by the loss of soil microbial diversity [13,14,15]. Phosphate-solubilizing bacteria (PSB) can transform insoluble phosphates into soluble forms through the process of acidification, chelation, and exchange reactions, and become green biological agents to replace P fertilizer and promote plant growth [14,16]. Therefore, obtaining efficient PSB is of great significance to minimize P-fertilizer application, improve the soil available P content, promote plant growth, and reduce environmental pollution.
As mentioned above, in order to better promote the soil improvement and ecological restoration of the wetland ecosystem, EPS-producing bacteria and PSB were screened from soil samples of Yellow River wetland nature reserve in Luoyang city, and identified by 16S rRNA nucleotide sequence homology analysis and physiological-biochemical assay, aiming to provide new potential strains for the research and development of biofertilizers to promote soil restoration in the Yellow River basin.
2. Materials and Methods
2.1. Soil Sample Collection
Six sites in the Yellow River wetland nature reserve in Luoyang city were selected for this study: Riparian Zone (YR1, YR2, and YR3, 50 m away from the Yellow River, approximately 3 km interval), Jili Yellow River Wetland Park (JL), and Mengjin Yellow River Wetland Park (MJ1 and MJ2, approximately 1 km interval). The sites located in the riparian zone were at least 3 km away from Jili Yellow River Wetland Park and Mengjin Yellow River Wetland Park. The topsoil samples (0–10 cm) were collected using a sterile stainless shovel on 15 March 2022. In order to eliminate the influence of micro-topography on the selected samples, five sub-samples (separated by at least 20 m) were collected individually in each site, and then immediately mixed together to form one sample. Triplicate samples for each site were collected using the same method. All of the samples were randomly divided into duplicate aliquots (one for soil strain isolation and the other for analysis of soil’s physical and chemical properties) in the field and placed inside a sterile plastic reservoir. For soil strain isolation, each soil sample was transported to the laboratory in an ice-packed cooler and stored at 4 °C until further processing. For the soil physicochemical analysis, all soil of the samples were transported to the laboratory within 1 h, passed through a 2 mm sieve, and then air-dried for subsequent analysis.
2.2. Soil Characteristics Analysis
Soil pH was determined in a soil–water suspension (1:5 w/v) with a pH meter (Thermo Fisher Scientific, Waltham, MA, USA), organic matter content was determined using the dichromate oxidation method [17]. The available P was extracted with 0.5 mol/L NaHCO3 in a 1:20 soil-extracting solution ratio, and determined by ammonium molybdate method [18]. The available N was determined by the alkali diffusion method [19].
2.3. Isolation and Selection of Candidate PSB
1 g of each soil sample was suspended in 9 mL of sterile saline solution (0.9% NaCl) and shaken vigorously (Stomacher 400, Seward Medical, London, UK) for 10 min. Next, ten-fold serial dilutions of 200 μL of the suspensions were spread on plates containing National Botanical Research Institute’s phosphate growth medium (NBRIP; 10 g/L glucose, 0.1 g/L (NH4)2SO4, 0.25 g/L MgSO4•7H2O, 5 g/L MgCl2•6H2O, 0.2 g/L KCl, 5 g/L Ca3(PO4)2, pH 7.0) agar [20]. The inoculated plates were incubated at 28 °C for 6 days, isolates with clear P solubilization halos were picked and purified further by streaking on Luria-Bertani (LB) agar (10 g/L tryptone, 10 g/L NaCl, 5 g/L yeast extract, pH 7.0), and the effective isolates were stored at 4 °C for future use. To further select the candidate PSB, isolates were cultured in 250 mL Erlenmeyer flask containing 30 mL of Pikovskaya (PVK; 10 g/L glucose, 0.5 g/L yeast extract, 0.5 g/L (NH4)2SO4, 0.1 g/L MgSO4•7H2O, 0.002 g/L MnSO4•4H2O, 0.002 g/L FeSO4•7H2O, 0.2 g/L KCl, 0.2 g/L NaCl, 2.5 g/L Ca3(PO4)2, pH 7.0) medium at 28 °C with shaking at 180 rpm for 6 days [20], and then their phosphate-solubilizing characteristics were determined by the molybdenum antimony resistance colorimetric method [19]. Uninoculated medium served as control.
The effects of different factors on phosphate solubilization by candidate PSB were further determined, including phosphate sources (Ca3(PO4)2, AlPO4 and Fe PO4), initial pH (5, 6, 7, and 8), carbon sources (glucose, soluble starch and cellulose) and N sources ((NH4)2SO4, NH4Cl and urea). Based on PVK medium without Ca3(PO4)2, glucose, and (NH4)2SO4, the effects of phosphate, carbon, and nitrogen sources on phosphate solubilization of candidate strain were tested respectively. The factors with the same substantial amount were added to each bottle. The candidate strain was cultured at 28 °C with shaking at 180 rpm for 6 days, and then their phosphate-solubilizing characteristics were determined by the molybdenum antimony resistance colorimetric method [19].
2.4. Isolation and Selection of Candidate EPS-Producing Strains
The EPS-producing strains were isolated and selected by the method described by Zhao et al. [5]. Briefly, 1 g of each soil sample was suspended in 9 mL of sterile saline solution (0.9% NaCl) and shaken vigorously for 10 min. Next, ten-fold serial dilutions of 200 μL of the suspensions were spread on plates containing LB agar to isolate EPS-producing strains. The inoculated plates were incubated at 28 °C for 48 h and slimy colonies were picked and purified further by streaking on LB agar. The primary screened colonies were purified three times and stored at 4 °C for future use. In order to further select the candidate EPS-producing strains, EPSs of cultured strains were extracted by the method described in the previous study [21], and the total sugar content of the precipitated EPSs was assessed by utilizing the phenol-sulfuric acid method from Dubois et al. [22].
2.5. Identification of Candidate Strains
Genetic identification of each isolate was performed by the 16S rRNA gene according to Sambrook et al. [23]. Total genomic DNA of each isolate was performed by using a bacterial DNA extraction kit (Omega Bio-Tek, Norcross, GA, USA) according to the procedure given by the manufacturer. The 16S rRNA gene was amplified by a polymerase chain reaction (PCR) using the following primers: 27 F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492 R (5′-GGTTACCTTGTTACGACTT-3′). PCR was performed using a 20 μL reaction mixture containing 5 μL of 2 × Taq PCR Master Mix (Sangon Biotech Shanghai Co., Ltd., Shanghai, China), 1 μL of each primer (10 μM), 1 μL of total DNA (10 ng), and sterilized ultrapure water. The PCR conditions were as follows: 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and a final extension at 72 °C for 10 min. The amplicons were separated by 1% agarose gel electrophoresis, purified using an SanPrep Column DNA Gel Extraction Kit (Sangon Biotech Shanghai Co. Ltd.) and quantified with QuantiFluor™-ST (Promega Corporation, Madison, WI, USA). The purified PCR product was sequenced by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). DNA sequences were submitted to the NCBI GenBank and compared with previously published gene sequences using the NCBI BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/ (accessed on 2 January 2023)). Multiple sequence alignment of DNA was performed by the Clustal W program implemented in MEGA 5.0 software [24]. The phylogenetic tree was constructed using the neighbor joining (NJ) method with 1000 bootstrap replications. In addition, physiological-biochemical characteristics (the methyl red test, Voges–Proskauer reaction, amylolysis test, gelatin liquefaction, catalase test, indole production, citrate utilization test, nitrate reduction test, acid production, and gas production) of the isolates were determined according to Bergey’s Manual of Systemic Bacteriology [25] and Laboratory manual of microbiology [26].
2.6. Statistical Analysis
One-way analysis of variance and Tukey’s honestly significant difference analysis were carried out using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) to determine the differences in soil properties of different soil samples, EPS yield, and phosphate-solubilizing characteristics of different isolates, as well as the influences of different factors on phosphate solubilization, which all meet the assumptions of normality and homoscedasticity. Figures were generated using Origin 8.0 (Origin Lab Corporation, Northampton, MA, USA).
3. Results and Discussion
3.1. Soil Characteristics
The soil pH of the wetland parks was lower than that of the riparian zone, having the highest in YR3 (8.81) and the lowest in JL (8.29) (Figure 1A). However, the contents of organic matter (Figure 1B; 5.77 g/kg in JL; 5.05 in MJ1), available P (Figure 1C; 47.73 mg/kg in JL; 45.34 mg/kg in MJ1), and available N (Figure 1D; 49.00 mg/kg in JL; 46.67 mg/kg in MJ1) in JL and MJ1 were higher than those in other soil samples, which provided guidance for the selection of soil samples for subsequent soil bacterial isolation and screening in this study. This phenomenon indicated that the active implementation and management of restoration projects such as the wetland parks in the Yellow River wetland nature reserve of Luoyang Section effectively promoted the soil improvement and ecological restoration [27].
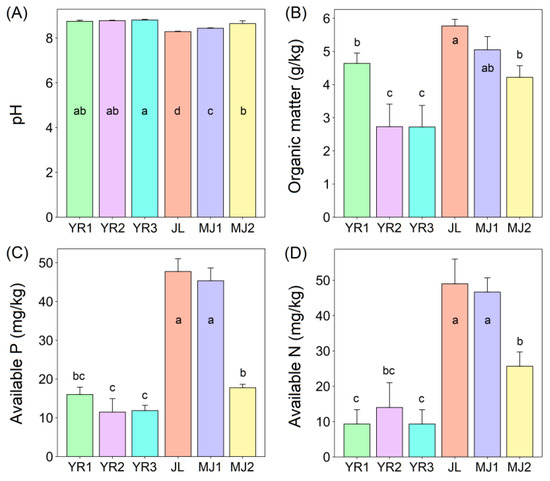
Figure 1.
Soil characteristics of six different sampling sites in the Yellow River wetland nature reserve in Luoyang city. Different lowercase letters denote significant differences between samples (p < 0.05). Error bars represent the standard deviation of the mean (n = 3). (A) soil pH, (B) soil organic matter contents, (C) soil available P contents, and (D) soil available N contents of six different sampling sites in the Yellow River wetland nature reserve in Luoyang City.
3.2. Screening and Isolation of Soil Bacteria
According to the results of soil physiochemical characteristics, JL and MJ1 were selected for the isolation of soil bacteria (Figure 1). Approximately 38 strains of soil bacteria were isolated from six soil samples (30 sub-samples) in two sampling sites, aiming to reflect different ecological protection plans and recovery times of selected wetland parks. Some strains cultured simultaneously in the same medium were ignored due to the similarity in phenotype, thus reducing the number of selected bacteria.
After incubation in NBRIP agar for 6 days, three strains (designated as PD3, PD12, and PD14) with clear P solubilization halo were isolated as the positive bacteria, and their ability to solubilize Ca3(PO4)2 was quantitatively analyzed in PVK medium. As shown in Figure 2, strain PD12 showed the most pronounced solubilization ability (39.68 mg/L; p < 0.05), followed by PD14 (11.67 mg/L) and PD3 (1.85 mg/L). Previous studies have found that there is a significant positive correlation between phosphate solubilization and PSB in soils [28]. Thus, our results suggested that PD12 had greater potential in increasing soil P availability and P concentration in plant tissues and might be a promising biological agent for improving soil fertility and promoting plant growth [29,30]. Furthermore, the growth promotion effects of PSB on plants was mainly to modify root functioning by modulation of the expression of auxin-responsive genes, thus playing a crucial role in regulation of plant hormone indole-acetic acid level with positive consequences on P acquisition and plant physiological status [31,32,33].
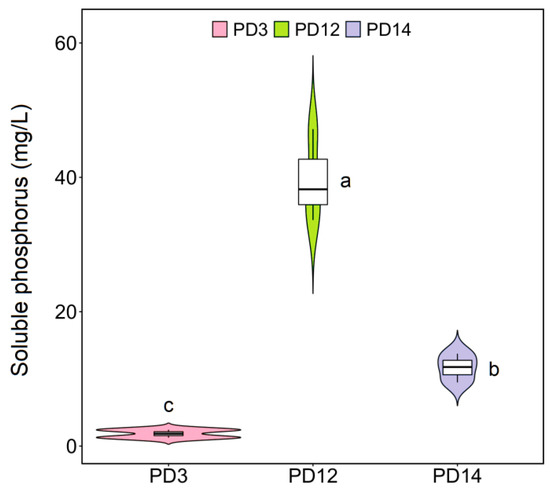
Figure 2.
Phosphate solubilizing capacity of isolated strains. Different lowercase letters denote significant differences between samples (p < 0.05). Error bars represent standard deviation of the mean (n = 3).
The effects of different factors (phosphate sources, initial pH, carbon sources, and N sources) on phosphate solubilization by strain PD12 were further determined. The solubilization of AlPO4 (51.30 mg/L) and FePO4 (52.30 mg/L) by strain PD12 was pronounced higher than Ca3(PO4)2 (Figure 3A). This finding was contrary to previous studies which suggested that the PSB solubilized Ca3(PO4)2 more than AlPO4 and FePO4 [11,34], indicating that PD12 had no specific preference for solubilizing phosphates, and that strain PD12 could not only increase the available P content in soil containing Ca3(PO4)2, but also had the potential to increase available P content in soil with high AlPO4 and FePO4 content. In addition, the maximum soluble P content was at 33.75 mg/L at pH 8.0 after 6 days (Figure 3B), and solubilization activity was detected with all selected carbon and N sources, with glucose and (NH4)2SO4 being the best carbon and N source (Figure 3C,D).
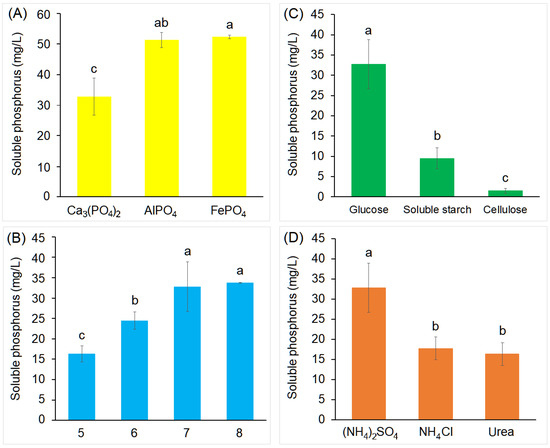
Figure 3.
Effects of different factors on phosphate solubilization by strain PD12. (A) phosphate sources, (B) initial pH, (C) carbon sources, and (D) nitrogen sources. Different lowercase letters denote significant differences between samples (p < 0.05). Error bars represent standard deviation of the mean (n = 3).
Moreover, five slimy colonies (designated as PD12, EPS12, EPS15, EPS18, and EPS19) were picked to further analyze the capacities of EPS production (Figure 4). The EPS yield of EPS18 (3.83 g/L) and PD12 (3.76 g/L) was significantly higher than that of others (p < 0.05), indicating that these strains could enhance soil cohesion, ensure moisture supply, and improve nutrients through EPS secreted and accumulated, which underlined the importance of EPS-producing bacteria for the improvement of soil quality and structure stabilization, and highlighting their potential as improvers of soil structure and fertility [4,5,6].
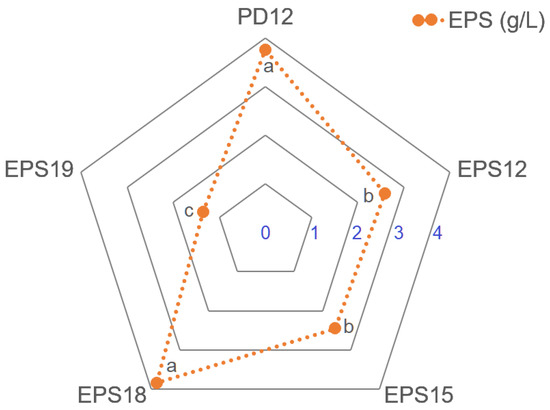
Figure 4.
EPS production of isolated strains. Different lowercase letters denote significant differences between samples (p < 0.05). Error bars represent standard deviation of the mean (n = 3).
3.3. Identification of Soil Bacteria
Phylogenic analysis was conducted with MEGA 5.0 software using the NJ method. The 16S rRNA sequences of the isolated strains were compared with available data in NCBI database. The results showed that the seven isolates of soil samples from the wetland parks in Luoyang section of the Yellow River were mainly divided into two genera, Klebsiella spp. and Pseudomonas spp., and the third most dominant genus was Aeromonas sp. (Figure 5). A variety of phosphate-solubilizing microorganisms, such as the members of Bacillus, Cedecea, Paenibacillus, Serratia, Pantoea, Aspergillus, Pseudomonas, Burkholderia, and Penicillium, have been isolated from a soil ecosystem [11,28]. In this study, all of the PSB were from the Klebsiella spp. (Figure 5), which have proven to be effective at dissolving elemental phosphate from insoluble phosphate [35]. In addition, previous studies have found that it is difficult to distinguish strains at the species level through biochemical and phenotypic tests, such as distinguishing K. pneumoniae from K. variicola and K. quasipneumoniae [36,37]. However, strains could be more reliably differentiated by genotyping and genomics methods [37,38,39]. In this study, based on the 16S rRNA nucleotide sequence homology analysis, strain PD12 was preliminary identified as K. variicola, and strains PD3 and PD14 were preliminary identified as K. quasipneumoniae (Figure 5). Moreover, strains EPS12, EPS15, EPS18, and EPS19 were preliminary identified as P. migulae, P. frederiksbergensis, A. media, and P. vancouverensis, respectively (Figure 5). Previous studies have found that inoculation of EPS-producing Pseudomonas spp. enhanced the yield of sunflower crop and the growth of wheat, which might be related to the formation of biofilm to improve nutrient utilization and water uptake of the plants [40,41,42].
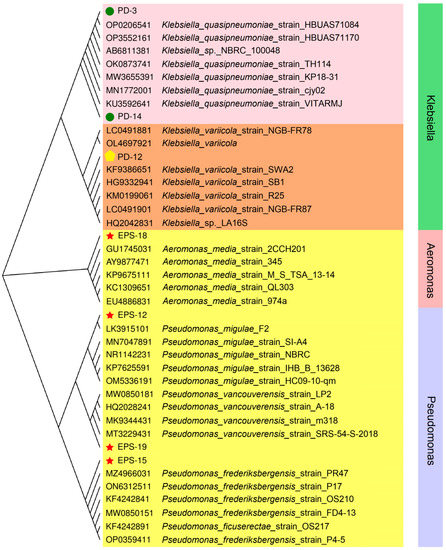
Figure 5.
Phylogenetic analysis based on 16S rRNA gene sequences available from the National Center for Biotechnology Information.
Moreover, physiological–biochemical characteristics (the methyl red test, Voges–Proskauer reaction, amylolysis test, gelatin liquefaction, catalase test, indole production, citrate utilization test, nitrate reduction test, acid production, and gas production) of PD3, PD12, PD14, EPS12, EPS15, EPS19, and EPS18 were identified (Table 1) and further compared with Bergey’s Manual of Systemic Bacteriology. As shown in Table 1, the results of the Voges–Proskauer reaction, amylolysis test, catalase test, citrate utilization test, nitrate reduction test, and gas production from glucose, as well as acid production from glucose, galactose, D-mannose, maltose, sucrose, and fructose of strains PD3, PD12, and PD14, were all positive. In contrast, the results of the methyl red test, gelatin liquefaction, and indole production of strains PD3, PD12, and PD14 were negative (Table 1). The results of the catalase test, nitrate reduction test, and acid production from glucose of strains EPS12, EPS15, and EPS19 were positive (Table 1). However, the results of methyl red test, Voges–Proskauer reaction, amylolysis test, gelatin liquefaction, indole production, citrate utilization test, and gas production from glucose, as well as acid production from galactose, D-mannose, maltose, sucrose, and fructose of strains EPS12, EPS15, and EPS19, were all negative (Table 1). In addition, various biochemical tests of strain EPS18 were performed and recorded as methyl red test—positive, Voges–Proskauer reaction—negative, amylolysis test—positive, gelatin liquefaction—positive, catalase test—positive, indole production—negative, citrate utilization test—negative, nitrate reduction test—positive, and acid production from glucose, galactose, D-mannose, maltose, sucrose, and fructose—positive, as well as gas production from glucose—negative (Table 1). Based on these biochemical characteristics, the strains PD3, PD12, and PD14 were homologous with Klebsiella spp., the strains EPS12, EPS15, and EPS19 were homologous with Pseudomonas spp., and strain EPS18 was homologous with Aeromonas sp.

Table 1.
Physiological and biochemical characteristics of the isolated strains.
Based on the results of 16S rRNA gene sequence analysis and physiological-biochemical identification, strains PD12, EPS12, EPS15, EPS18, and EPS19 were identified as K. variicola, P. migulae, P. frederiksbergensis, A. media, and P. vancouverensis, respectively, and strains PD3 and PD14 were identified as K. quasipneumoniae. Consistent with previous studies [23,43], our research identified the function of Klebsiella spp. in enhancing soil available P and revealed high solubilization activity of K. variicola for Ca3(PO4)2 (Figure 2). In addition, K. variicola and K. quasipneumoniae are well-known nitrogen-fixing species, especially as all of the members of K. variicola are able to fix N2 [44,45]. Therefore, K. variicola PD12 not only has the potential to regulate the P cycle in the soil environment but also can promote the soil N cycle through its nitrogen-fixation ability. This regulation effect on the key process of the biogeochemical cycle will directly affect soil fertility and indirectly promote the structure and function succession of soil microorganisms. At present, Pivot Bio has created the first corn biofertilizer PROVEN based on K. variicola KV137 that reduces fertilizer requirements by 25 lbs./acre while increasing yields by 5.8 bushels [46].
Moreover, these EPS-producing strains have proven to play important roles in preventing soil erosion and improving soil structure by cell adhesion/cohesion and having an influence on the physicochemical properties of cell aggregates (charge, viscosity, and flocculation), accumulating minerals and nutrients by ionic interactions, and thereby accelerating soil ecological restoration and plant growth by affecting soil’s physical, chemical, and biological properties [4,5,6,7]. For example, our previous work on Bacillus tequilensis CGMCC 17603 isolated from the biological soil crust (BSCs) of a desert ecosystem found that EPSs secreted by B. tequilensis CGMCC 17603 effectively promoted the formation of soil aggregates and BSCs and improved the stability of the sand surface, which would further contribute to the resistance to wind erosion and the restoration of the desert ecosystems [5]. In addition, there was another interesting phenomenon observed in our study. K. variicola PD12 not only had greater phosphate (Ca3(PO4)2, AlPO4 and FePO4; Figure 2 and Figure 3A) solubilization activity but it also had a superior EPS yield, this it is a promising bacterial species for regulating biogeochemical cycles (P cycle and N cycle), enhancing the capability of cells to bind to solid substrates, and thus improving soil structure and fertility.
4. Conclusions
In summary, two phosphate-solubilizing species (K. variicola and K. quasipneumoniae) and five EPS-producing species (K. variicola, P. migulae, P. frederiksbergensis, A. media, and P. vancouverensis) were isolated from soil samples of the Yellow River wetland nature reserve in Luoyang city. Intriguingly, the new strain of K. variicola PD12 had the highest phosphate solubilization activity, higher EPS yield, and N2-fixing capacity, which laid the foundation for it to be a candidate soil probiotic. Our work provides potential strains for the development of simple, low-cost and environment-friendly biofertilizers to promote soil structure and fertility restoration. However, more in-depth studies are needed to determine the safety and effectiveness of these strains for the improvement of the soil’s physical, chemical, and biological characteristics. For example, greenhouse experiments, pot experiments, and even field experiments targeting the inoculation of K. variicola PD12 and other strains (EPS18, EPS12, EPS15, EPS19, PD3, and/or PD14) on different crops (wheat and corn are common crops in the study area) and soil types in the Yellow River basin will be of verifiable significance for determining the positive effects of these strain on the physical, biological, and chemical properties of soil, such as promoting the formation of soil aggregates to prevent soil erosion, regulating the biological cycle process to improve soil fertility, and enhancing microbial interaction to stabilize microbial structure [5,40]. In addition, in order to realize the efficient application of the strains (mainly K. variicola PD12), the high-density culture scheme and preparation process of the strain need to be further studied.
Author Contributions
X.S. and L.Z. conceived and designed the experiments. X.S., C.G. (Chenxue Geng), C.G. (Chunli Guo), Y.N. and Y.D. performed the experiments. X.S., Y.N. and Y.D. analyzed the data and prepared figures. X.S. and L.Z. wrote and participated in the revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by the Scientific and Technological Research Project of Henan Province (222102320212).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully acknowledge the editor and four anonymous reviewers for their constructive comments in improving the manuscript. We thank the lab members (Zhihao Cao, Mengyao Hou, and Minghao Jin) for their help and support with respect to many aspects of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, Y.; Hu, C.; Zhang, X.; Lv, X.; Wang, Z. Response of sediment discharge to soil erosion control in the middle reaches of the yellow river. Catena 2021, 203, 105330. [Google Scholar] [CrossRef]
- Bu, X.; Cui, D.; Dong, S.; Mi, W.; Li, Y.; Li, Z.; Feng, Y. Effects of wetland restoration and conservation projects on soil carbon sequestration in the Ningxia basin of the Yellow River in China from 2000 to 2015. Sustainability 2020, 12, 10284. [Google Scholar] [CrossRef]
- Yan, Y.W.; Jiang, Q.Y.; Wang, J.G.; Zhu, T.; Quan, Z.X. Microbial communities and diversities in mudflat sediments analyzed using a modified metatranscriptomic method. Front. Microbiol. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Mugnai, G.; Philippis, R.D. Complex role of the polymeric matrix in biological soil crusts. Plant Soil 2018, 429, 19–34. [Google Scholar] [CrossRef]
- Zhao, L.N.; Li, X.R.; Wang, Z.R.; Qi, J.H.; Zhang, W.L.; Wang, Y.S.; Liu, Y.B. A new strain of Bacillus tequilensis CGMCC 17603 isolated from biological soil crusts: A promising sand-fixation agent for desertification control. Sustainability 2019, 11, 6501. [Google Scholar] [CrossRef]
- Lehman, A.P.; Long, S.R. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J. Bacteriol. 2013, 195, 5362–5369. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Bagalkar, N.W. Isolation and Characterization of Phosphate Solubilizing Bacteria from Rhizospheric Soil of the Soybean Plants. Online Int. Interdiscip. Res. J. 2013, 3, 251–258. [Google Scholar]
- Miller, S.H.; Browne, P.; Prigent-Combaret, C.; Combes-Meynet, E.; Morrissey, J.P.; O’Gara, F. Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Environ. Microbiol. Rep. 2009, 2, 403–411. [Google Scholar] [CrossRef]
- Illmer, P.; Schinner, F. Solubilization of inorganic calcium phosphates-solubilization mechanisms. Soil Biol. Biochem. 1995, 27, 257–263. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, P.S.; Zhang, B.X.; Wang, Y.P.; Meng, J.; Gao, Y.F.; He, X.M.; Hu, X.M. Identification of phosphate-solubilizing microorganisms and determination of their phosphate-solubilizing activity and growth-promoting capability. BioResources 2020, 15, 2560–2578. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Sati, S.C.; Pant, P. Evaluation of phosphate Solubilization by root endophytic aquatic Hyphomycete Tetracladium setigerum. Symbiosis 2019, 77, 141–145. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, X.; Wen, X. Identification and characterization of the rhizosphere phosphate-solubilizing bacterium Pseudomonas frederiksbergensis JW-SD2, and its plant growth-promoting effects on poplar seedlings. Ann. Microbiol. 2016, 66, 1343–1354. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture-a review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.Q.; Ren, J.H.; Ye, J.R. Isolation and identification rhizosphere from different of phosphobacteria in Poplar regions of China. Pedosphere 2011, 21, 90–97. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc., Soil Science Society of America, Inc.: Madison, WI, USA, 1982; Volume 9, pp. 539–577. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular; US Department of Agriculture: Washington, DC, USA, 1954; Volume 939.
- Nanjing Institute of Soil Research. Analysis of Soil Physicochemical Features; Shanghai Science and Technology Press: Shanghai, China, 1980; 360p. (In Chinese) [Google Scholar]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Abdhul, K.; Ganesh, M.; Shanmughapriya, S.; Kanagavel, M.; Anbarasu, K.; Natarajaseenivasan, K. Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. Int. J. Biol. Macromol. 2014, 70, 450–454. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T.; Garrity, G.M.; Boone, D.R.; Vos, P.; Goodfellow, M.; Rainey, F.A.; Schleifer, K.H. Bergey’s Manual of Systematic Bacteriology; The Proteobacteria; Springer: Berlin, Germany, 2006; Volume 2. [Google Scholar]
- Qin, C.L.; Li, S.B. Laboratory Manual of Microbiology; Weapon Industry Press: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Suh, J.S.; Kwon, J.S. Characterization of phosphate-solubilizing microorganisms in upland and plastic film house soils. Korean J. Soil Sci. Fert. 2008, 41, 348–353. [Google Scholar]
- Bakhshandeh, E.; Pirdashti, H.; Lendeh, K.S. Phosphate and potassium-solubilizing bacteria effect on the growth of rice. Ecol. Eng. 2017, 103, 164–169. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Meena, R.S. Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016. [Google Scholar]
- Liu, X.; Jiang, X.; He, X.; Zhao, W.; Cao, Y.; Guo, T.; Li, T.; Ni, H.; Tang, X. Phosphate-solubilizing pseudomonas sp. strain p34-l promotes wheat growth by colonizing the wheat rhizosphere and improving the wheat root system and soil phosphorus nutritional status. J. Plant Growth Regul. 2019, 38, 1314–1324. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, J.; Wu, Y.; Luo, S.; Chen, B.; Ma, L.; Pan, F.; Feng, Y.; Yang, X. Promotion of the root development and Zn uptake of Sedum alfredii was achieved by an endophytic bacterium Sasm05. Ecotoxicol. Environ. Saf. 2019, 172, 97–104. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate solubilizing rhizobacteria could have a stronger influence on wheat root traits and aboveground physiology than rhizosphere P solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef]
- Chung, H.; Park, M.; Madhaiyan, M.; Seshadri, S.; Song, J.; Cho, H.; Sa, T. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol. Biochem. 2005, 37, 1970–1974. [Google Scholar] [CrossRef]
- Widawati, S. Augmentation of potential phosphate solubilizing bacteria (PSB) stimulate growth of green mustard (Brasica caventis Oed.) in marginal soil. Biodiversitas 2006, 7, 10–14. [Google Scholar] [CrossRef]
- Brisse, S.; van Himbergen, T.; Kusters, K.; Verhoef, J. Development of a rapid identification method for Klebsiella pneumoniae phylogenetic groups and analysis of 420 clinical isolates. Clin. Microbiol. Infect. 2004, 10, 942–945. [Google Scholar] [CrossRef]
- Alves, M.S.; Dias, R.C.; de Castro, A.C.; Riley, L.W.; Moreira, B.M. Identification of clinical isolates of indole-positive and indole-negative Klebsiella spp. J. Clin. Microbiol. 2006, 44, 3640–3646. [Google Scholar] [CrossRef]
- Garza-Ramos, U.; Silva-Sánchez, J.; Martínez-Romero, E.; Tinoco, P.; Pina-Gonzales, M.; Barrios, H.; Martínez-Barnetche, J.; Gómez-Barreto, R.E.; Tellez-Sosa, J. Development of a multiplex-PCR probe system for the proper identification of Klebsiella variicola. BMC Microbiol. 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Arora, N.K. Role of salicylic acid from Pseudomonas aeruginosa PF23 EPS+ in growth promotion of sunflower in saline soils infested with phytopathogen Macrophomina phaseolina. Environ. Sustain. 2018, 1, 49–59. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 876. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Greiner, R.; Haller, E.; Konietzny, U.; Jany, K.D. Purification and characterization of a phytase from Klebsella terrigena. Arch. Biochem. Biophys. 1997, 341, 201–206. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, Y.Y.; Li, S.Y.; Tang, L.; Zheng, J.W.; An, Q.L. Genomic identification of nitrogen-fixing Klebsiella variicola, K. pneumoniae and K. quasipneumoniae. J. Basic Microbiol. 2016, 56, 78–84. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Martínez, L.; Silva, J.; Martínez-Romero, E. Klebsiella variicola, a novel species with clinical and plant-associated isolates. Syst. Appl. Microbiol. 2004, 27, 27–35. [Google Scholar] [CrossRef]
- Temme, K.; Tamsir, A.; Bloch, S.; Clark, R.; Tung, E. Methods and Compositions for Improving Plant Traits; USPTO, Pivot Bio, Inc.: Berkeley, CA, USA, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).