Abstract
Chlorine dioxide is used extensively as the bleaching agent in the clean pulp bleaching process. To further improve the efficiency of chlorine dioxide delignification, this study discusses the effect of a new system of a high proportion of chlorine dioxide on lignin removal. When the mass ratio of chlorine dioxide to lignin was 4 in this system, 50% of lignin was removed in a short time. To optimize the new bleaching system, the effects of temperature and pulp consistency on the new system were studied. The ratio of lignin removed was 50% when the reaction time was 30 min, temperature was 25 °C, and the pulp consistency was 5%. Compared with traditional chlorine dioxide bleaching, high proportion of chlorine dioxide bleaching can shorten the reaction time by 50%, reduce the reaction temperature to 25 °C, and greatly reduce energy consumption and adsorbable organic halogen (AOX) production by 45%. The new system provides a novel idea for clean bleaching of pulp.
1. Introduction
Clean pulp and papermaking are currently essential goals for the development of the paper industry. High-efficiency and clean bleaching methods have become an industry consensus, particularly while attempting to achieve a low carbon footprint for environmental protection. At present, 75% of bleached chemical pulp, globally, is produced by the elemental chlorine free (ECF) bleaching method [1,2]. In comparison to the total chlorine free (TCF) bleaching method, ECF has advantages of high brightness after bleaching, low chemical consumption, and low-cost operation. Chlorine dioxide is an efficient and environmentally friendly oxidant and disinfectant [3,4]. In the ECF bleaching process, chlorine dioxide acts as the main agent that possesses high delignification capacity, excellent delignification selectivity, less carbohydrate degradation, and good fiber strength [5]. Additionally, the formation of adsorbable organic halogen (AOX) in pulp bleaching wastewater is considerably reduced [6,7]. The bleached pulp is resistant to yellowing and easy to store. During ECF bleaching, chlorine dioxide preferentially oxidizes the phenolic structural lignin, and the lignin units will form structures such as o-quinone or p-quinone and soluble mucofuroic acid derivatives containing carboxyl groups [8,9].
However, in the actual bleaching process, the bleaching efficiency of chlorine dioxide is reduced because side reactions occur constantly. This is because a small amount of chlorine in the chlorine dioxide solution promotes the chlorination reaction, and the hypochlorite produced by the degradation of chlorine dioxide in the bleaching process easily reacts with lignin to produce AOX [10,11,12]. This pollutant is difficult to degrade if discharged into the natural environment. Moreover, it further condenses to form the primary carcinogen dioxin, risking human life and the living environment [13]. At present, enterprises use the reduction and comprehensive methods for the preparation of chlorine dioxide. Even with these methods, a slight amount of chlorine remains in the chlorine dioxide solution [14]. Therefore, research has been conducted to reduce the formation of AOX in the pulp bleaching process.
Parthasarathy et al. [15] converted chlorine dioxide into its gaseous state, and the gaseous chlorine dioxide delignification efficiency was found to be higher and faster than that of liquid chlorine dioxide. The physical properties of the bleached pulp by gaseous chlorine dioxide delignification were similar to those of the bleached pulp by liquid chlorine dioxide. The application of gaseous chlorine dioxide in the D0 section can reduce the loading of chlorine dioxide and bleaching time, thus reducing the formation of AOX. Using gaseous chlorine dioxide, the conventional bleaching effect can be achieved with reduction in the usage of chlorine dioxide by half and time to 5 min, thereby decreasing the formation of AOX by ~15%.
Nie et al. [16] used xylanase to pretreat the pulp before chlorine dioxide bleaching. Hexenuronic acid (HexA) in the pulp, the main source of AOX formation in the bleaching process [17], is significantly reduced after xylanase pretreatment, thereby reducing AOX in the wastewater by 21.4–26.6% after chlorine dioxide bleaching. Xylanase pretreatment can reduce the formation of AOX by removing HexA.
Alkali treatment of pulp is another common method of reducing the formation of AOX. NaOH has a low cost and offers many advantages in the extraction of lignin. Lignin is deposited on the surface of pulp, which easily facilitates its contact with the bleaching agent, thereby making it beneficial for the penetration of bleaching agents into the pulp. This reduced the AOX formation in bleaching wastewater [18]. Additionally, NaOH can break the hydrogen bond on the fiber cell wall, expand pores between the cells, which again makes it conducive for the penetration of the bleaching agent, thereby improving the dissolution efficiency of lignin [19,20]. The formation of AOX was related to the lignin content in the pulp during the bleaching process and the amount of bleaching agent [21,22]. Thus, NaOH has a dissolution effect on lignin, which can remove part of the lignin in the pulp, thereby reducing the formation of AOX [23,24].
The adjustment and optimization of the reaction time, temperature, and chemicals in the bleaching process are necessary to minimize the damage to the pulp and maximize the reduction of AOX. Through a variety of pretreatment methods, the substances in the pulp that generate AOX in the bleaching process are degraded before bleaching. However, these treatment methods are relatively cumbersome and additional bleaching sections and chemicals increase the bleaching cost. Thus, the reduction of AOX and improvement of the reaction efficiency of the bleaching agents, such as chlorine dioxide, still pose major challenges in clean bleaching technology.
When our research team studied the formation law of intermediate products in the chlorine dioxide bleaching process, it was difficult to study the formation law of intermediate products due to the fast reaction rate of lignin and chlorine dioxide. The structure of phenolic and non-phenolic lignin is similar to that of chlorine dioxide. In addition, phenolic lignin has high structural activity, but the research is relatively limited. Therefore, it is of great significance to study the reaction process between phenolic lignin structure and chlorine dioxide. This was done by selecting the representative phenolic lignin model compound as the reaction substrate and analyzing the absorbance change in the reaction system of lignin model compound and chlorine dioxide by ultraviolet spectrophotometer (UV). The relationship between the consumption of chlorine dioxide in the reaction system and the concentration of added lignin model compound was studied, and the reaction mechanism of lignin and chlorine dioxide at room temperature and high concentration of chlorine dioxide was clarified. It is speculated that the lignin structure was activated by a high concentration of chlorine dioxide. In the reaction process, phenolic oxygen radicals are first generated, and then a series of oxidation reactions are carried out to generate p-benzoquinone, o-benzoquinone, or phthalic acid intermediates. It is deduced that the molar ratio of different lignin model substances to chlorine dioxide consumption is not fixed [25,26]. However, conventional chlorine dioxide bleaching considers that the molar ratio of lignin to chlorine dioxide consumption is fixed 1:2. When the concentration of chlorine dioxide in the system is high, it is conducive to the formation of p-benzoquinone, o-benzoquinone, or phthalic acid intermediates, which promote the rapid removal of lignin. On the basis of this theory, we try to use this discovery to explore new bleaching processes. We changed the amount of chlorine dioxide in the bleaching system and explored the removal effect of lignin in the new system. Then, we provide theoretical support for efficient and clean bleaching process. At the same time, we pay attention to the consumption of chlorine dioxide and have put forward corresponding solutions. The new bleaching system with low temperature and low pulp consistency can realize the rapid removal of lignin and reduce the consumption of chlorine dioxide. Additionally, the research of Yao et al. [27] showed that chlorine dioxide in the solution can be separated by air stripping and reused after reabsorption. This method enables recycling of chlorine dioxide.
This study discussed the change in lignin removal ratios in systems that contain a high proportion of chlorine dioxide. The relationship between the removed ratios of lignin and the amount of chlorine dioxide present in the system was analyzed. When the chlorine dioxide/lignin mass ratio in the system reaches a ratio, the removed ratios of lignin were significantly improved in a short time. Under the condition of low pulp consistency and at 25 °C, 50% of lignin was removed in 30 min from a system containing a high proportion of chlorine dioxide. The optimized high proportion of chlorine dioxide bleaching system can rapidly remove lignin and reduce the formation of AOX under the conditions of low temperature and low pulp consistency. The high proportion of chlorine dioxide bleaching system has the advantages of high efficiency and cleanness, which will further improve the efficiency of chlorine dioxide to remove lignin and reduce the generation of harmful pollutants. The reaction conditions of low temperature and short time make it easier to scale. This provided a new idea for the clean bleaching of pulp.
2. Materials and Methods
2.1. Experimental Materials
The eucalyptus pulp used in the experiment was provided by a paper mill in Guangxi. It was treated by oxygen delignification process. The kappa number was 9.70, brightness was 45.89% ISO, lignin content was 1.455%, and moisture content was 10.50%. The pulp was sealed after screening and air drying. Chlorine dioxide solution was provided by a paper mill in Guangxi with an initial concentration of 7.2 g/L. NaOH (analytical pure) that was purchased from Jiangtian Chemical Technology Co., Ltd. (Nantong, China).
2.2. Bleaching Method for the System with a High Proportion of Chlorine Dioxide
In total, 15 g of oven-dried (o.d.) pulp was weighed in a polythene sealed bag and its lignin content was calculated [28]. Chlorine dioxide was added to the pulp in the polythene sealed bag such that the chlorine dioxide/lignin mass ratio was 0.5, 1, 2, 4, 6, and 8. The pulp consistency was adjusted to 8%. The concentration of chlorine dioxide in the system was 0.47 g/L, 0.95 g/L, 1.89 g/L, 3.78 g/L, 5.67 g/L, and 7.20 g/L. The sealed bag was placed in a thermostatic water bath set at 60 °C and left to react for 3–60 min.
2.3. Conventional Chlorine Dioxide Bleaching Methods
The conventional D0 stage bleaching method [29,30] is as follows: 15 g of o.d. pulp was weighed and put into a polythene sealed bag. The amount of chlorine dioxide (ClO2) was 0.5% (on o.d. pulp weight). The pulp consistency was adjusted to 10% and the concentration of chlorine dioxide in the system was 0.40 g/L. The bag was placed in a thermostatic water bath at 70 °C for 40 min.
The conventional E0 stage bleaching method [31] is as follows: 10 g of the o.d. pulp was weighed after bleaching in the conventional D0 section and placed in the polythene sealed bag. The amount of NaOH added was 1.0% (on o.d. pulp weight). The pulp consistency was adjusted to 10%, and the sealed bag was placed in a thermostatic water bath at 75 °C for 120 min.
The conventional D1 stage bleaching method is as follows: 5 g of o.d. pulp was weighed after bleaching in the conventional E0 section and put it into a sealed polythene bag. The amount of ClO2 was 0.2% (on o.d. pulp weight) and the pulp consistency was adjusted to 10%. The concentration of chlorine dioxide in the system was 0.22 g/L, and the sealed bag was placed in a thermostatic water bath at 70 °C for 150 min.
2.4. High Proportion of Chlorine Dioxide Pre-impregnation (DPI) and Multi-Stage Bleaching Method
The DPI stage bleaching method is as follows: 15 g of o.d. pulp was weighed and placed in a sealed polythene bag. The chlorine dioxide was added into the bag containing the pulp such that the chlorine dioxide to lignin mass ratio in the system was maintained at a value of 4. The pulp consistency was adjusted to 5%. The concentration of chlorine dioxide in the system was 2.29 g/L, and the sealed bag was placed in a thermostatic water bath at 25 °C for 30 min. After bleaching, the chlorine dioxide bleach was filtered out using a Buchner funnel and a vacuum pump for recycling. This stage of bleaching is called pre-impregnation (DPI).
The D0 stage bleaching method is as follows: 15 g of the pre-impregnated o.d. pulp was weighed and 0.0%, 0.5%, or 0.75% (on o.d. pulp weight) of ClO2 was added to the pre-impregnated pulp. After the measurement of the chlorine dioxide solution, the pulp was mixed with the chlorine dioxide solution. The pulp consistency was adjusted to 10%. The concentration of chlorine dioxide in the system was 0.00 g/L, 0.56 g/L, or 0.83 g/L. Thereafter, the polythene sealed bag was placed in a thermostatic water bath set at 70 °C for 40 min.
The E0 and D1 sections were the same as the conventional bleaching methods. The specific bleaching steps are shown in Figure 1.
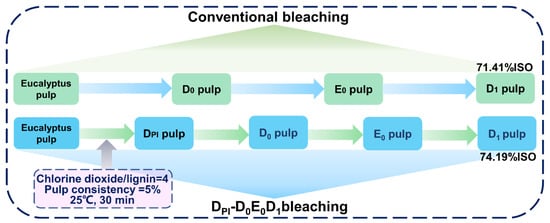
Figure 1.
Flow chart of a conventional bleaching process and high proportion of chlorine dioxide process.
2.5. Determination of Kappa Number
The kappa number of a pulp is determined using the micro kappa number method according to TAPPI UM246 [32].
2.6. Determination of Brightness
The pulp brightness is measured in units of ISO 2471 [33]. The bleached pulp is made into 200 g/m2 paper pieces with a Buchner funnel and placed in a cool and ventilated place for air drying. The brightness was measured using the Colortouch PC.
2.7. Determination of Chlorine Dioxide Reaction Efficiency
The determination of chlorine dioxide reaction efficiency was conducted as follows: the reduction of the kappa number and chlorine dioxide in the bleaching system, before and after the bleaching process, is calculated. The efficiency of the chlorine dioxide reaction is expressed as the kappa number reduced by 1 g chlorine dioxide. The chlorine dioxide reaction efficiency was calculated by Equation (1).
where η represents the kappa number reduced by 1 g chlorine dioxide, ΔΚ represents the change in kappa number before and after pulp bleaching, and ΔΜ represents the change in chlorine dioxide before and after the reaction (g).
η = ΔΚ/ΔΜ
2.8. Determination of Chlorine Dioxide Concentration
The determination of chlorine dioxide content was conducted as follows: chlorine dioxide content was determined by the ultraviolet method as found in the literature [25]. A sodium thiosulfate titration method was used to titrate chlorine dioxide solutions of different concentrations. The absorbance at 360 nm was measured by an ultraviolet spectrophotometer. A standard curve was obtained as well, as shown in Table 1. The absorbance of the chlorine dioxide was measured before and after bleaching, and the value was substituted into the formula for calculation. The chlorine dioxide concentration was calculated using Equation (2).

Table 1.
Standard curve of chlorine dioxide concentration and absorbance.
y = 0.4411x, R² = 0.9996.
where ρ(ClO2) represents the chlorine dioxide content (g/L), k represents the standard curve coefficient of chlorine dioxide at 360 nm, and A360 represents absorbance at 360 nm measured by UV.
ρ(ClO2) = A360/k
2.9. AOX Determination of Bleaching Wastewater
AOX was detected using the microcoulometric method [34], using a Jena multi-x-2500 total organic halogen analyzer(Analytik Jena AG, Jena, Germany). The experimental steps were as follows: 1 mL of bleached waste liquid was added to 5 mL of sodium nitrate stock solution in a 100 mL volumetric flask. Then, two single activated carbon columns in series were put into a filter press and 100 mL of the configured sample dilution solution was poured. This was then filtered at a flow rate of 3 mL/min, and then taken out to be tested. 3–5 parallel tests were conducted, and data were collected. The relative error was controlled within 0.03.
2.10. Calculation of Chlorine Dioxide/Lignin Mass Ratio
The amount of chlorine dioxide used in this study was calculated according to the lignin content in the pulp participating in the reaction, and the chlorine dioxide/lignin mass ratio was calculated using Equation (3).
where 1.455% represents the lignin content in the pulp, m represents o.d. pulp quality, (g). ρ(ClO2) represents the chlorine dioxide content, (g/L), and V represents chlorine dioxide loading amount.
r = 1.455%·m/ρ(ClO2) × V
3. Results and Discussion
3.1. Effect of Chlorine Dioxide Loading on Pulp Delignification
The number of reactants and reaction time are the main factors that affect the reaction process. Lignin is degraded and dissolved by the oxidation of chlorine dioxide [24]. In this experiment, the effects of chlorine dioxide/lignin mass ratio and time on the delignification efficiency of unbleached pulp were investigated. The results are presented in Figure 2. The final data are the average of three parallel experiments.
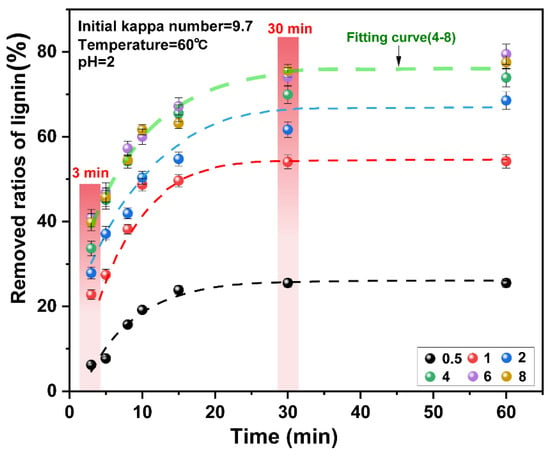
Figure 2.
Effect of chlorine dioxide/lignin mass ratio on the removed ratios of lignin from pulp under different reaction times.
The initial 0–3 min was the initial delignification stage of chlorine dioxide. When the chlorine dioxide/lignin mass ratio was increased from 0.5 to 4, the ratios of lignin removed increased from 12.97% to 33.66%. On further increase of the consumption of chlorine dioxide, the ratios of lignin removed did not change significantly. The mass ratio of chlorine dioxide/lignin in the system reached “saturation.” In the initial stages of the reaction, the ratios of lignin removed increased with the increase in the chlorine dioxide/lignin mass ratio. This indicated that the removal of lignin at the initial stage of the reaction corresponded to the residual lignin content and the amount of chlorine dioxide in the pulp. This was consistent with the previous findings on chlorine dioxide delignification [35,36]. The ratios of lignin removed was related to the amount of residual lignin in the pulp. As the reaction proceeds under the oxidation of chlorine dioxide, the amount of lignin in the pulp decreases rapidly, so the ratios of lignin removed also decreases at a rapid rate [37,38].
Lignin was rapidly degraded in the 3–30 min period. At this stage, the ratios of lignin removed were considerably improved. When the mass ratio of chlorine dioxide/lignin was increased from 0.5 to 4, the ratios of lignin removed increased from 19.31% to 36.28% respectively. Upon continually increasing the chlorine dioxide/lignin mass ratio, the ratios of lignin removed did not change significantly. The ratios of lignin removed were plotted for different chlorine dioxide/lignin mass ratios. The result is presented in Figure 3. When the chlorine dioxide/lignin mass ratio increases from 0.5 to 4, the ratios of lignin removed increases. The overall removal of lignin increased. The ratios increased from 25.52% to 73.00% at the end of bleaching. Upon continual increase of the chlorine dioxide/lignin mass ratio, the ratios of lignin removed began to slowly increase. This showed that lignin can be rapidly degraded when a high proportion of chlorine dioxide was used for bleaching. The optimal amount of chlorine dioxide was found at a chlorine dioxide/lignin mass ratio of 4.
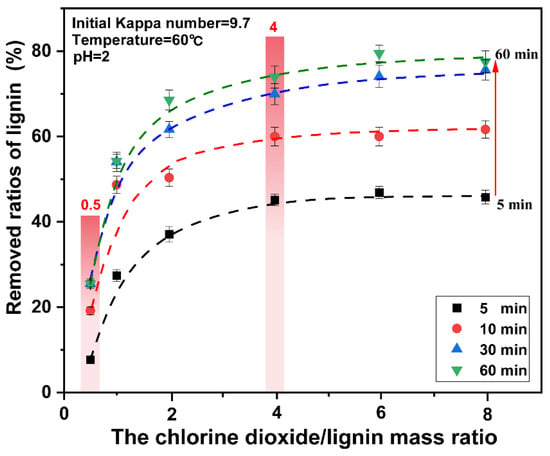
Figure 3.
Effect of reaction time on the removed ratios of lignin from pulp under different chlorine dioxide/lignin mass ratio.
The relationship between the ratios of lignin removed and the different chlorine dioxide/lignin mass ratio at 5 min, 10 min, 30 min, and 60 min is shown in Figure 3. The final data is the average of three parallel experiments. It was observed that under the same chlorine dioxide/lignin mass ratio, the bleaching time had a significant impact on the ratios of lignin removed. However, unlike the conventional chlorine dioxide bleaching process, when the time increases from 30 min to 60 min, the ratios of lignin removed did not change significantly. Additionally, the degradation of cellulose was aggravated with time, and a highly-efficient removal of lignin was obtained for a high proportion of chlorine dioxide in a short time. Therefore, the optimal treatment time for lignin removal is 30 min. In the bleaching process, when the mass ratio of chlorine dioxide/lignin was less than 4, the ratios of lignin removed increased rapidly with the increase of the chlorine dioxide/lignin mass ratio. When the mass ratio of chlorine dioxide/lignin was greater than 4, the ratios of lignin removed only changed with time, regardless of the amount of chlorine dioxide.
From the aforementioned analysis, a high proportion of chlorine dioxide bleaching system can significantly improve the ratios of lignin removed in a short time, which was consistent with the research results of the lignin model. The further increase in the amount of chlorine dioxide did not have a significant effect on the removal of lignin, when the mass ratio of chlorine dioxide/lignin in the system reaches a certain level. A high proportion of chlorine dioxide bleaching system results in rapid removal of lignin.
3.2. Effect of Pulp Consistency on a High Proportion of Chlorine Dioxide Bleaching System
In the previous studies, we demonstrated that efficient removal of lignin occurs with a high proportion of chlorine dioxide. However, when the chlorine dioxide/lignin mass ratio is relatively large, chlorine dioxide will decompose and volatilize. In particular, it is the concentration that has considerable influence on the decomposition rates of chlorine dioxide [39]. To explore the effect of pulp consistency on a high proportion chlorine dioxide bleaching system, the concentration of chlorine dioxide in the bleaching system was reduced by reducing the pulp consistency. Chlorine dioxide was added to ensure that the chlorine dioxide/lignin mass ratio in the system was 4. The pulp consistency was 2%, 3%, 4%, 5%, 6%, and 8%. The concentration of chlorine dioxide in the system was 0.89 g/L, 1.35 g/L, 1.81 g/L, 2.29 g/L, 2.79 g/L, and 3.78 g/L. The polythene sealed bag containing the paper pulp was placed in a thermostatic water bath at 60 °C for 30 min. The final data are the average of three parallel experiments.
The results showed that at low pulp consistency, the ratios of lignin removed were not affected. The decomposition of chlorine dioxide was reduced, thus increasing the reaction efficiency of chlorine dioxide. The results are shown in Figure 4a. When the pulp consistency was 5–8%, the chlorine dioxide efficiency increased with the decrease of pulp consistency. When the pulp consistency was lower than 5%, the chlorine dioxide reaction efficiency did not change significantly. Hence, the higher the pulp consistency, the higher the chlorine dioxide concentration was in the reaction system. Figure 4b shows that the decomposition rates of chlorine dioxide gradually increased with the increase of the pulp consistency. When the pulp consistency was 2%, the decomposition rates were the lowest, 9.12%. When the pulp consistency was increased to 8%, the decomposition rates of chlorine dioxide finally reached 44.12%. This may be due to the solubility of a high proportion of chlorine dioxide solution that was affected by temperature, where the chlorine dioxide molecules decompose and escape at high temperature. On the assumption that there would be considerable water consumption during the reduction of the pulp consistency, a pulp consistency of 5% has the best and guaranteed effect of chlorine dioxide bleaching efficiency.
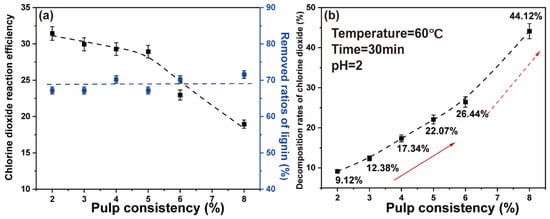
Figure 4.
(a) Effect of pulp consistency on the chlorine dioxide reaction efficiency and the ratios of lignin removed; (b) effect of pulp consistency on the chlorine dioxide decomposition rate (The two arrows represent the gradual improvement phase and the rapid improvement phase).
3.3. Effect of pH on a high Proportion Chlorine Dioxide Bleaching System
In the traditional chlorine dioxide bleaching research, pH has a great influence. Under alkaline conditions, chlorine dioxide reacts with OH− to generate chlorate ions and chlorite ions, which weaken the bleaching effect of chlorine dioxide. Generally, the optimal range of pH at the end of bleaching is 3.5–4.0 [40]. In this study, the pH in the system was changed by changing the amount of chlorine dioxide and pulp consistency in the system. Therefore, this experiment explored the influence of pH on the high proportion of chlorine dioxide bleaching system. The results are shown in Figure 5. The final data are the average of three parallel experiments.
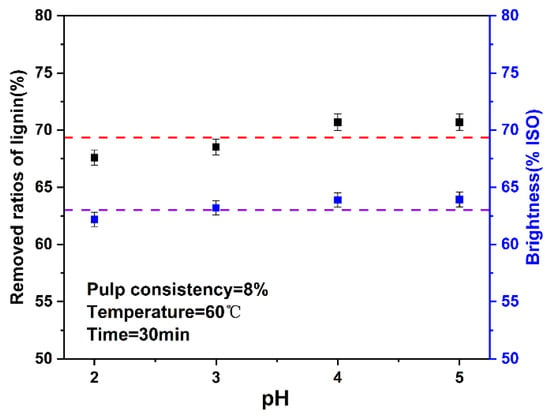
Figure 5.
Effect of pH on the ratios of lignin removed and brightness (The dotted lines are horizontal line that fitted with the data).
In the high proportion of chlorine dioxide system, when the pH was between 2–5, the change of ratios of lignin removed and brightness was not obvious, which was different from traditional chlorine dioxide bleaching. The high proportion of chlorine dioxide system was insensitive to the change of pH, which may be because the high proportion of chlorine dioxide mainly depends on the oxidation of chlorine dioxide, rather than the role of H+.
3.4. Effect of Temperature on a High Proportion of Chlorine Dioxide Bleaching System
Reaction temperature plays an important role in the process of a chemical reaction. High temperature will cause the ineffective decomposition of chlorine dioxide molecules, thereby forming chlorine, hypochlorous acid, and other substances through side reactions [16,41]. The effect of temperature on the decomposition rates of chlorine dioxide in a simulated bleaching system was experimentally investigated. The same volume of chlorine dioxide solution was added in the thermostatic water bath, and the temperatures were 40 °C, 60 °C, and 80 °C. The reaction time was between 0 and 45 min.
The decomposition rates of chlorine dioxide increase gradually with an increase in temperature. When the reaction time reaches 5 min, the decomposition rate of chlorine dioxide at 40 °C, 60 °C, and 80 °C were 7.13%, 12.29%, and 16.99%, respectively. The results are shown in Figure 6a. The final data are the average of three parallel experiments.
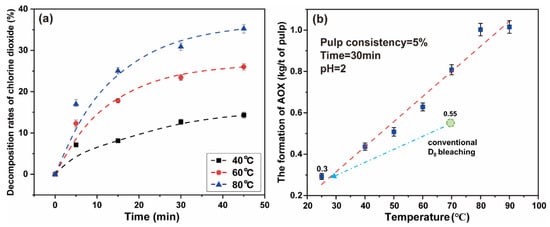
Figure 6.
(a) The effect of temperature on the decomposition of chlorine dioxide under simulated bleaching conditions; (b) the effect of high chlorine dioxide bleaching temperature on the formation of AOX.
With increasing time, the decomposition rates of chlorine dioxide gradually slowed down. When the time was 45 min, the decomposition rates of chlorine dioxide gradually reached 14.33%, 26.00%, and 35.25% at the respective temperature. As time continued to increase, the decomposition rates of chlorine dioxide still had an upward trend. Because the chlorine dioxide was gaseous, and as the temperature of the solution increases the solubility changes, the decomposition and loss of chlorine dioxide were carried out at the same time. To avoid the ineffective decomposition and loss of chlorine dioxide, a high proportion of chlorine dioxide bleaching system should adopt the short-term and low-temperature reaction strategy. This can improve the reaction efficiency of chlorine dioxide to a considerable extent.
Additionally, the reaction temperature has an impact on the amount of AOX generated in the bleaching liquid. When the temperature rises, the formation of chlorine and hypochlorite is promoted, resulting in an increase in the efficiency of the chlorination substitution reaction [10]. An experiments was undertaken to explore the effect of temperature on the high proportion of chlorine dioxide bleaching system. The chlorine dioxide/lignin mass ratio in the system was 4 and the pulp consistency was 5%. The concentration of chlorine dioxide in the system was 2.29 g/L. The polythene sealed bag containing the paper pulp was placed in a thermostatic water bath for 30 min at 25–90 °C.
It can be observed from Figure 6b that the formation of AOX increases with the increase in reaction temperature. When the temperature was 25 °C, the formation of AOX was 0.29 kg/t of pulp. If the temperature continues to increase, the formation of AOX rapidly increased. When the temperature reached 80 °C, the formation of generated AOX reached the highest value, which was 1.00 kg/t of pulp. Under low temperature conditions, the high proportion of chlorine dioxide bleaching system inhibits the formation of AOX (below 0.30 kg/t of pulp), which may originate from the accelerated oxidation of chlorine dioxide and the inhibition of the chlorinated substitution reaction [12]. The new system can reduce AOX to about 0.3 kg/t of pulp, which is 45% lower than conventional D0 bleaching. The low temperature greatly reduced the side reactions in the bleaching process, thus the amount of AOX was much less. It is completely feasible to adopt a low temperature strategy for the high proportion of chlorine dioxide bleaching system.
3.5. Preliminary Exploration of DPI-Ded Bleaching Process
To reduce the consumption of chlorine dioxide and the formation of AOX, this study experimented on lignin removal of a high proportion of chlorine dioxide bleaching system under 25 °C and low pulp consistency. Chlorine dioxide was added into the polythene sealed bag containing the pulp to ensure the chlorine dioxide/lignin mass ratio in the system was 4. The pulp consistency was adjusted to 5%, and the bag was placed in a thermostatic water bath at 25 °C. The reaction time was in a range of 0–60 min. The results are shown in Figure 7. The final data are the average of three parallel experiments.
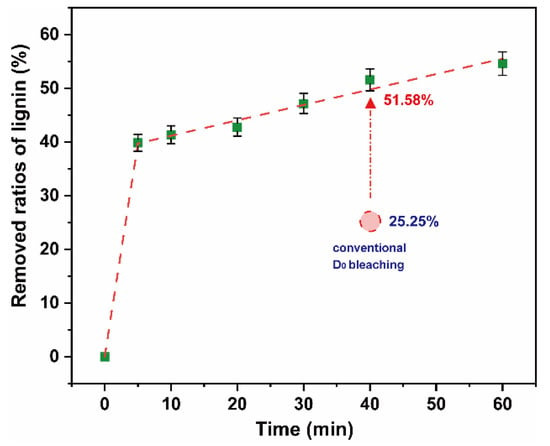
Figure 7.
Removed ratios of lignin in high proportion of chlorine dioxide bleaching system.
There was a rapid removal of ~40% lignin within 5 min. On extension of the reaction time, the ratios of lignin removed gradually increased and stabilized. When the reaction time reached 60 min, the ratios of lignin removed increased to ~55%. This was 26.33% higher than that of the D0 section of the conventional bleaching system in the same time frame. The decomposition of chlorine dioxide was the main reason for the low delignification efficiency of chlorine dioxide in a high proportion of chlorine dioxide bleaching system. The utilization efficiency of chlorine dioxide can be significantly improved by reducing the temperature. It can be observed that a high proportion of chlorine dioxide bleaching system was feasible in a pulp, at normal temperature and low pulp consistency.
To explore the feasibility of maintaining a normal temperature in a high proportion of chlorine dioxide bleaching system for pulp bleaching, a reference to the conventional ECF bleaching method is mentioned in this paper [29,31]. At normal temperature, a high proportion of chlorine dioxide was added as pretreatment before conventional DED bleaching (DPI-D0E0D1). The advantages of the DPI-D0E0D1 method for the bleached pulp were analyzed in terms of kappa number, brightness, and overall chlorine dioxide reaction efficiency. The bleaching results are shown in Figure 8a,b. The final data are the average of three parallel experiments.
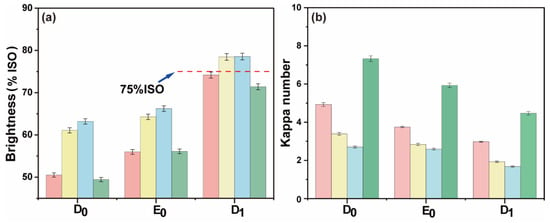
Figure 8.
(a) Brightness comparison between DPI-D0E0D1 and conventional bleaching; (b) the kappa number comparison between DPI-D0E0D1 and conventional bleaching (red: D0 chlorine dioxide consumption 0.00%, yellow: D0 chlorine dioxide consumption 0.50%, blue: D0 chlorine dioxide consumption 0.75%, green: conventional chlorine dioxide bleaching).
The amount of chlorine dioxide in the D0 stage had an effect on the final kappa number and brightness of pulp. The final bleaching effect is shown in Figure 9. The final data are the average of three parallel experiments. The final kappa number was reduced to ~2.0, and the brightness was ~75% ISO. Without adding chlorine dioxide in the D0 section, the brightness of the pulp after DPI-D0E0D1 bleaching reached the same amount as that of the pulp after conventional chlorine dioxide bleaching. The formation of AOX was lower than that of conventional bleaching. However, its chlorine dioxide efficiency was also calculated to be slightly lower than that of conventional bleaching. The bleaching results are shown in Figure 9. To summarize, it was feasible to combine the DPI pulp with the D0 stage of direct heat treatment and the conventional chlorine dioxide bleaching E0 and D1 stages.
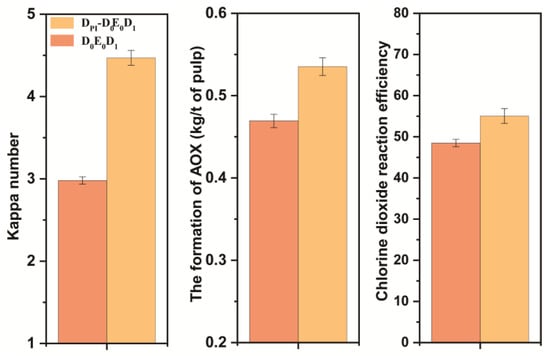
Figure 9.
Comparison of DPI-D0E0D1 and D0E0D1 results.
4. Conclusions
The high proportion of chlorine dioxide bleaching system is a new, clean, efficient and energy-saving system for removing lignin from pulp. The ratios of lignin removed can reach 50% under the following conditions: chlorine dioxide/lignin mass ratio in the system was 4, the reaction temperature and time were 25 °C and 30 min, and the pulp consistency was 5%. This method can quickly remove lignin from pulp and greatly reduce AOX production. We connected the normal temperature bleaching system of low pulp consistency and high proportion of chlorine dioxide with the D0E0D1 stages of the conventional chlorine dioxide bleaching, as DPI-D0E0D1. The kappa number, brightness, and overall chlorine dioxide reaction efficiency of bleached pulp was studied. The results show that DPI- D0E0D1 is feasible as a new bleaching method.
Author Contributions
Conceptualization, J.H.; validation, C.C., G.H. and P.H.; formal analysis, Y.H. and T.L.; investigation, B.L. and S.Y.; resources, S.W., C.Q. and C.X.; writing—original draft, B.L. and S.Y.; writing—review & editing, J.H.; supervision, C.L.; project administration, C.L.; funding acquisition, S.W., C.Q. and C.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [22168007], and the Natural Science Foundation of Guangxi [20220GXNSFAA035466].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Tran, A. Decreasing effluent loads through bleaching modification. Water Res. 2006, 40, 487–494. [Google Scholar] [CrossRef]
- Tabassum, S.; Sulaiman, O.; Ibrahim, M.; Hashim, R.; Altamash, T. Removal of chemically hazardous p-hydroxybenzoic acid during total chlorine free bleaching process of Hevea Brasiliensis. J. Clean. Prod. 2012, 25, 68–72. [Google Scholar] [CrossRef]
- Abrantes, S.; Amaral, E.; Costa, A.P.; Shatalov, A.A.; Duarte, A.P. Hydrogen peroxide bleaching of arundo donax l. Kraft-anthraquinone pulp - effect of a chelating stage. Ind. Crop. Prod. 2007, 25, 288–293. [Google Scholar]
- Cadena, E.M.; Vidal, T.; Torres, A.L. Influence of the hexenuronic acid content on refining and ageing in eucalyptus TCF pulp. Bioresour. Technol. 2010, 101, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, Z.; Liu, J.; Yang, J.; Yin, Y.; Zhan, L. A Method to Reduce Steam Consumption of ECF Bleaching Based on Operation Optimizing. Processes 2021, 9, 928. [Google Scholar] [CrossRef]
- Kaur, D.; Bhardwaj, N.K.; Lohchab, R.K. Reduction in chlorophenolic compounds during bleaching of rice straw pulp by replacing elemental chlorine with chlorine dioxide. Int. J. Environ. Sci. Technol. 2017, 15, 1113–1122. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, S.; Ma, Z.; Zhao, J.R.H.; Kerekes, R.J.; McDonald, J.D.; Man, Y. Optimizing Bleaching Operating Conditions Based on Mathematical Programming to Reduce AOX Emissions. ACS Omega 2022, 7, 5421–5428. [Google Scholar] [CrossRef]
- Brogdon, B.N.; Mancosky, D.G.; Lucia, L.A. New Insights into Lignin Modification During Chlorine Dioxide Bleaching Sequences (I): Chlorine Dioxide Delignification. J. Wood Chem. Technol. 2005, 24, 201–219. [Google Scholar] [CrossRef]
- Lachenal, D.; Hamzeh, Y.; Chirat, C.; Mortha, G. Getting the best from chlorine dioxide bleaching. J. Pulp Pap. Sci. 2008, 34, 9–12. [Google Scholar]
- Svenson, D.R.; Chang, H.-M.; Jameel, H.; Kadla, J.F. The role of non-phenolic lignin in chlorate-forming reactions during chlorine dioxide bleaching of softwood kraft pulp. Holzforschung 2005, 59, 110–115. [Google Scholar] [CrossRef]
- Xu, R.Y.; Xie, Y.W.; Tian, J.P.; Chen, L.J. Adsorbable organic halogens in contaminated water environment: A review of sources and removal technologies. J. Clean Prod. 2021, 283, 17. [Google Scholar] [CrossRef]
- Svenson, D.R.; Jameel, H.; Chang, H.; Kadla, J.F. Inorganic Reactions in Chlorine Dioxide Bleaching of Softwood Kraft Pulp. J. Wood Chem. Technol. 2006, 26, 201–213. [Google Scholar] [CrossRef]
- Tarkpea, M.; Eklund, B.; Linde, M.; Bengtsson, B.E. Toxicity of conventional, elemental chlorine-free, and totally chlorine-free kraft-pulp bleaching effluents assessed by short-term lethal and sublethal bioassays. Environ. Toxicol. Chem. 1999, 18, 2487–2496. [Google Scholar] [CrossRef]
- Ragnar, M.; Törngren, A. Ways to reduce the amount of organically bound chlorine in bleached pulp and the AOX discharges from ECF bleaching. Nord. Pulp Pap. Res. J. 2002, 17, 234–239. [Google Scholar] [CrossRef]
- Parthasarathy, V.R.; Rudie, G.F. Modified chlorine dioxide delignification of softwood pulps for high brightness and ultra-low aox. Tappi J. 1996, 79, 171–179. [Google Scholar]
- Nie, S.; Wang, S.; Qin, C.; Yao, S.; Ebonka, J.F.; Song, X.; Li, K. Removal of hexenuronic acid by xylanase to reduce adsorbable organic halides formation in chlorine dioxide bleaching of bagasse pulp. Bioresour. Technol. 2015, 196, 413–417. [Google Scholar] [CrossRef]
- Törngren, A.; Ragnar, M. Hexenuronic acid reactions in chlorine dioxide bleaching – aspects on in situ formation of molecular chlorine. Nord. Pulp Pap. Res. J. 2002, 17, 179–182. [Google Scholar] [CrossRef]
- Nong, G.-Z.; Xing, D.-Y.; Li, Y.-J.; Zhu, T.; Wu, J.-L.; Gan, W.-X.; Wang, S.-F.; Li, X.-R. Recycle cooking wood chips with the residue liquid removed out of lignin by calcification for increasing pulp yield and reducing waste water discharge. J. Clean. Prod. 2020, 277, 124028. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Effects of sodium hydroxide pretreatment on structural components of biomass. Trans. Asabe 2014, 57, 1187–1198. [Google Scholar]
- Al-Dajani, W.W.; Gellerstedt, G. On the isolation and structure of softwood residual lignins. Nord. Pulp Pap. Res. J. 2002, 17, 193–198. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Zhu, H.; Wang, S.; Song, X.; Qin, C. Extraction of hemicellulose by hot water to reduce adsorbable organic halogen formation in chlorine dioxide bleaching of bagasse pulp. Ind. Crop. Prod. 2017, 96, 178–185. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.-M.; Jameel, H. Phenolation to Improve Lignin Reactivity toward Thermosets Application. ACS Sustain. Chem. Eng. 2018, 6, 5504–5512. [Google Scholar] [CrossRef]
- Kihlman, M.; Aldaeus, F.; Chedid, F.; Germgård, U. Effect of various pulp properties on the solubility of cellulose in sodium hydroxide solutions. Holzforschung 2012, 66, 601–606. [Google Scholar] [CrossRef]
- Costa, M.M.; Colodette, J.L. The impact of kappa number composition on eucalyptus kraft pulp bleachability. Braz. J. Chem. Eng. 2007, 24, 61–71. [Google Scholar] [CrossRef]
- Liu, B.; Qin, C.; Zhang, F.; Wang, S.; Liang, C.; Nie, S.; Wang, S.; Yao, S. Reaction Mechanism of Phenolic Lignin and High Concentration Chlorine Dioxide and Its Application. ACS Omega 2020, 5, 22475–22481. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, B.J.; Liang, J.R.; Li, J.; Liu, B.J.; Wang, F.; Qin, C.R.; Yao, S.Q. Effects of the preferential oxidation of phenolic lignin using chlorine dioxide on pulp bleaching efficiency. Int. J. Mol. Sci. 2022, 23, 13301. [Google Scholar] [CrossRef]
- Yao, S.Q.; Liu, B.J.; Nie, S.X.; Wang, S.W.; Qin, C.R.; Wang, S.F. Pretreatment of chlorine dioxide solution for pulp bleaching. J. Biobased Mater. Bioenergy 2019, 13, 523–531. [Google Scholar] [CrossRef]
- Parkås, J.; Brunow, G.; Lundquist, K. Quantitative lignin analysis based on permanganate oxidation. Bioresources 2007, 2. [Google Scholar] [CrossRef]
- Ragnar, M.; Dahllof, H. Ecf bleaching of eucalypt kraft pulp - bleaching chemical needs and yellowing characteristics of different sequences. Nord. Pulp Paper Res. J. 2002, 17, 228–233. [Google Scholar] [CrossRef]
- Ferdous, T.; Quaiyyum, M.A.; Jahan, M.S. Chlorine dioxide bleaching of nineteen non-wood plant pulps. Nord. Pulp Paper Res. J. 2020, 35, 569–576. [Google Scholar] [CrossRef]
- Bikova, T.; Klevinska, V.; Eremeeva, T.; Treimanis, A. Study on Alkali-Accessible Chromophores from Unbleached Kraft Pulp. Holzforschung 2001, 55, 554–558. [Google Scholar] [CrossRef]
- Li, J.; Gellerstedt, G. Kinetics and mechanism of kappa number determination. Nord. Pulp Pap. Res. J. 1998, 13, 147–152. [Google Scholar] [CrossRef]
- Popson, S.J.; Malthouse, D.D.; Robertson, P.C. Applying brightness, whiteness, and color measurements to color removal. Tappi J. 1997, 80, 137–147. [Google Scholar]
- Sullivan, J.; Douek, M. Method and sample-related problems in the determination of aox in effluents. Tappi J. 1996, 79, 145–154. [Google Scholar]
- Tessier, P.; Savoie, M. Chlorine dioxide delignification kinetics and eop extraction of softwood kraft pulp. Can. J. Chem. Eng. 1997, 75, 23–30. [Google Scholar] [CrossRef]
- Yoon, B.H.; Wang, L.J.; Lee, M.K. Empirical modeling of chlorine dioxide delignification of oxygen-delignified hardwood kraft pulp. J. Wood Sci. 2004, 50, 524–529. [Google Scholar] [CrossRef]
- Nie, S.; Liu, X.; Wu, Z.; Zhan, L.; Yin, G.; Yao, S.; Song, H.; Wang, S. Kinetics study of oxidation of the lignin model compounds by chlorine dioxide. Chem. Eng. J. 2014, 241, 410–417. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Mortha, G.; Lachenal, D.; Hostachy, J.C.; Calais, C. Comparative Studies of Chlorine Dioxide Reactions with Muconic Acid Derivatives and Lignin Model Compounds. J. Wood Chem. Technol. 2006, 26, 153–164. [Google Scholar] [CrossRef]
- Ma, Q.; Hirth, K.; Agarwal, U.P.; Zhu, J. Oxidative delignification: The roles of lignin reactivity and accessibility. J. Clean. Prod. 2022, 363. [Google Scholar] [CrossRef]
- Chandranupap, P.; Nguyen, K.L. Effect of ph on kinetics and bleaching efficiency of chlorine dioxide delignification. Appita J. 2000, 53, 108. [Google Scholar]
- Ferro, E.I.; Ruuttunen, K.; Perrin, J.; Vuorinen, T. Sustainable bleaching of eucalyptus sp. Kraft pulp with hypochlorous acid, ozone and hydrogen peroxide. Ind. Crop. Prod. 2021, 172, 114004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).