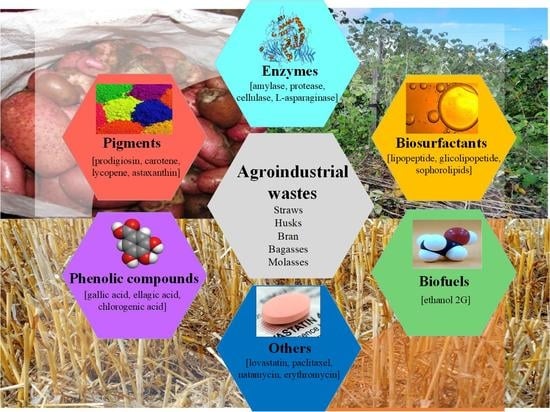
Advances in Agroindustrial Waste as a Substrate for Obtaining Eco-Friendly Microbial Products
Abstract
1. Introduction
2. Agroindustrial Waste: A Valuable Substrate for Fermentation Processes
| Waste | MC | Cel | Hem | Lig | CH | Pro | Phe | RS | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Banana leaf | - | 55 a | 20 a | 25 a | - | - | - | - | [22] |
| Bean Husk | - | - | - | - | - | 262 c | - | 327 c | [23] |
| Brewer’s spent grain | 64 b | 210 b | 243 b | 144 b | - | - | 0.55 b | 47.4 b | [20] |
| Coffee husk | 91 a | - | - | - | 14a | 14 a | 1 d | 14 a | [24] |
| Coffee pulp | 12 a | 33 a | 29 a | 26 a | - | 11 a | - | 97 c | [25] |
| Grape marc | - | 14 a | 10 a | 67 a | - | 14 a | 0.22 a | 0.4 a | [26] |
| Grape stalk | 570 b | 288 b | 133 b | 435 b | - | - | 4.4 b | 57 b | [20] |
| Mango seed | 40 a | 3 a | 14 a | 2 a | 82 a | 7 a | 14 c | [27] | |
| Olive pomace | - | 13a | 30a | 55a | - | 6 a | 0.7 a | 3 a | [26] |
| Pea pods | - | - | - | - | - | 414 c | - | 144 c | [23] |
| Peanut cake | 9 a | - | - | - | - | 44 a | - | [9] | |
| Potato skin | - | - | - | - | - | 165 c | - | 845 c | [23] |
| Sorghum waste | 7 a | 2 a | 82 a | 12 a | - | 13 a | - | 56 c | [25] |
3. Current Studies on High-Value-Added Microbial Compounds
3.1. Enzymes
| Microorganism | Enzyme | Fermentation | Agroindustrial Waste | Reference |
|---|---|---|---|---|
| Bacteria | ||||
| Anoxybacillus rupiensis | Amylase Protease | SmF | Potato peel powder | [33] |
| Bacillus sp. | α-amylase | SmF | Potato peels, mango peels and lemon peels | [38] |
| Bacillus subtilis | Milk clotting enzyme | SmF | Orange peel and rice straw | [39] |
| Bacillus aryabhattai | L-asparaginase | SmF | Olive mill wastewater | [36] |
| Bacillus tequilensis | α-amylase | SmF | Rice bran | [40] |
| Pseudomonas Aeruginosa | Lipase | SmF | Palm oil mill effluent | [34] |
| Bacillus amyloliquefaciens | α-amylase | SSF | Wheat bran with potato peel | [41] |
| Fungi | ||||
| Aspergillus heteromorphus | Cellulase Exoglucanase Xylanase | SmF | Anaerobically treated distillery spent wash and rice straw | [42] |
| Aspergillus flavipes | Proteases | SSF | Wheat bran | [10] |
| Aspergillus flavus | Cellulase Xylanase | SSF | Rice straw | [28] |
| Aspergillus ibericus | Cellulase Xylanase β-Glucosidase | SSF | Brewer’s spent grain | [20] |
| Aspergillus niger | Lipase | SSF | Rice bran with Jathropa seed cake | [35] |
| Aspergillus oryzae | Proteases | SSF | Wheat bran | [10] |
| Rhyzopus oryzae | n-Demethylases | SSF | Coffee pulp and sorghum | [25] |
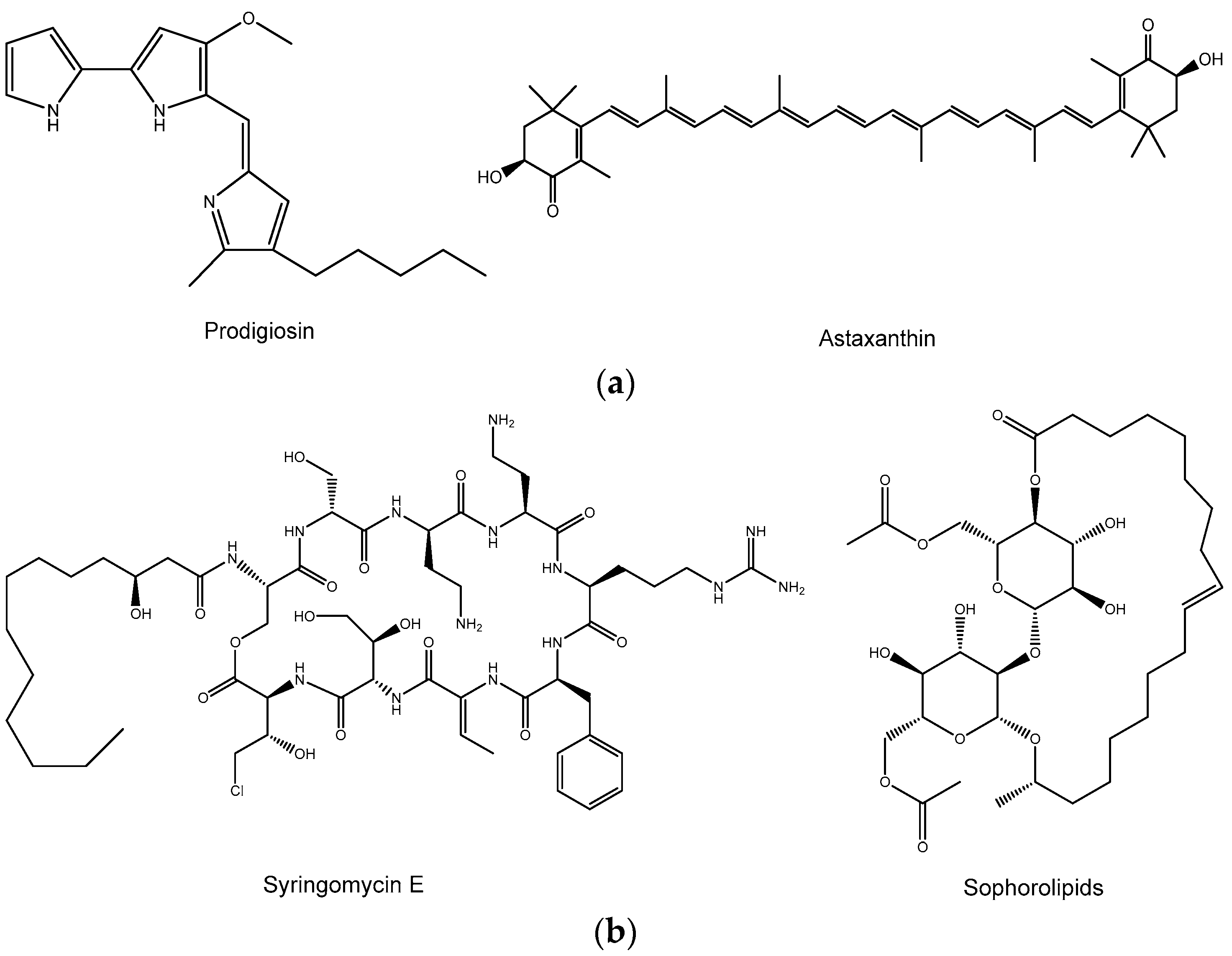
3.2. Pigments
| Microorganism | Pigment | Fermentation | Agroindustrial Waste | Reference |
|---|---|---|---|---|
| Bacteria | ||||
| Streptomyces sp. | Reddish-purple pigment | SmF | Discarded potato | [30] |
| Serratia nematodiphilia | Prodigiosin | SSF | Wheat bran | [49] |
| Rhodopseudomonas faecalis | Lycopene | SmF | Soybean meal | [50] |
| Fungus | ||||
| Monascus purpureus | Red pigments Yellow pigments | SSF | Potato pomace | [8] |
| Monascus purpureus | Red pigments | SmF | Soybean meal | [45] |
| Monascus sanguineus | Red pigments | SSF | Broken rice | [51] |
| Rhodotorula mucilaginosa | Carotenoids | SmF | Onion peels and mung bean husk | [23] |
| Sporidiobolus pararoseus | β-cryptoxanthin | SmF | Parboiled rice water and sugar cane molasses | [52] |
| β-carotene | ||||
| Xanthophyllomyces dendrorhous | Astaxanthin | SmF | Pineapple waste, orange waste and pomegranate waste | [53] |
| Xanthophyllomyces dendrorhou | Carotenoids | SmF | Mesquite pods | [54] |
3.3. Biosurfactans
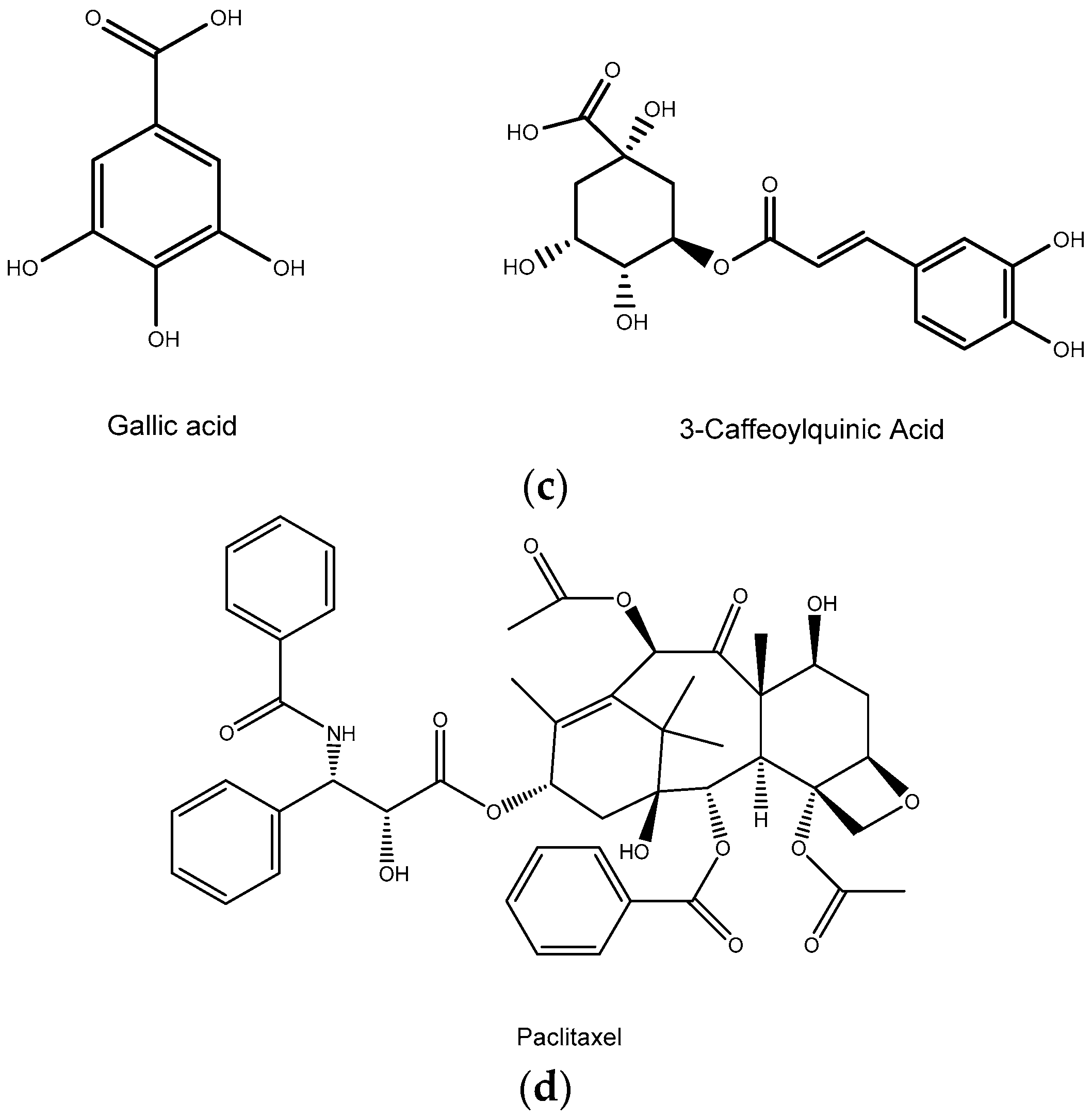
3.4. Phenolic Compounds
3.5. Others Bioactive Compounds
4. Improving Fermentation Processes and Sustainability
5. Advances for Scaling up Fermentation Processes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous yeasts as cell factories for the sustainable production of microbial lipids by the valorization of agri-food wastes. Fermentation 2021, 7, 50. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention. 2011. Available online: https://www.fao.org/fileadmin/user_upload/suistainability/pdf/Global_Food_Losses_and_Food_Waste.pdf (accessed on 13 December 2022).
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J Biotechnol 2018, 282, 46–66. [Google Scholar] [CrossRef]
- Fierascu, R.; Fierascu, I.; Avramescu, S.; Sieniawska, E. Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef]
- Tavares, H. Raízen Raising the Roof on 2 New Cellulosic Ethanol Plants with $395M Investment. The Digest 2022. Available online: https://www.biofuelsdigest.com/bdigest/2022/05/15/raizen-raising-the-roof-on-2-new-cellulosic-ethanol-plants-with-395m-investment/ (accessed on 13 December 2022).
- Lane, J. The Big Launch: PM Modi Inaugurates India’s First 2G Ethanol Project, Using Praj Technology. The Digest 2022. Available online: https://www.biofuelsdigest.com/bdigest/2022/08/11/the-big-launch-pm-modi-inaugurates-indias-first-2g-ethanol-project-using-praj-technology/ (accessed on 13 December 2022).
- Ng, H.; Kee, P.; Yim, H.; Chen, P.; Wei, Y.; Chi-Wei Lan, J. Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef]
- Chen, X.; Yan, J.; Chen, J.; Gui, R.; Wu, Y.; Li, N. Potato pomace: An efficient resource for Monascus pigments production through solid-state fermentation. J. Biosci. Bioeng. 2021, 132, 167–173. [Google Scholar] [CrossRef]
- Duhan, J.; Chawla, P.; Kumar, S.; Bains, A.; Sadh, P. Proximate composition, polyphenols, and antioxidant activity of solid state fermented peanut press cake. Prep. Biochem. Biotech. 2021, 51, 340–349. [Google Scholar] [CrossRef]
- Zanutto-Elgui, M.; Vieira, J.; Prado, D.; Buzalaf, M.; Padilha, P.; Elgui de Oliveira, D.; Fleuri, L. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2019, 278, 823–831. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Acevedo-Benítez, J.A.; Hernández-Santiago, C. Distribution and potential of bioenergy resources from agricultural activities in Mexico. Renew Sust. Energ. Rev. 2010, 14, 2147–2153. [Google Scholar] [CrossRef]
- Ferreira, S.; Buller, L.; Maciel-Silva, F.; Sganzerla, W.; Berni, M.; Forster-Carneiro, T. Waste management and bioenergy recovery from açaí processing in the Brazilian Amazonian region: A perspective for a circular economy. Biofuel. Bioprod. Bior. 2020, 15, 37–46. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M. Losses, inefficiencies and waste in the global food system. Agr. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; Available online: http://www.fao.org/3/i6583e/i6583e.pdf (accessed on 30 June 2021).
- Yusuf, M. Agro-industrial waste materials and their recycled value-added applications: Review. Handb. Ecomater. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Panesar, R.; Kaur, S.; Panesar, P. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin Food Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- Barros, M.; Salvador, R.; de Francisco, A.; Piekarski, C. Mapping of research lines on circular economy practices in agriculture: From waste to energy. Renew Sustain. Energy Rev. 2020, 131, 109958. [Google Scholar] [CrossRef]
- de la Rosa, O.; Flores-Gallegos, A.; Muñíz-Marquez, D.; Nobre, C.; Contreras-Esquivel, J.; Aguilar, C. Fructooligosaccharides production from agro-wastes as alternative low-cost source. Trends Food Sci. Tech. 2019, 91, 139–146. [Google Scholar] [CrossRef]
- Sadh, P.; Kumar, S.; Chawla, P.; Duhan, J. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Zerva, A.; Tsafantakis, N.; Topakas, E. Evaluation of Basidiomycetes wild strains grown in agro-industrial residues for their anti-tyrosinase and antioxidant potential and for the production of biocatalysts. Ferment 2021, 7, 19. [Google Scholar] [CrossRef]
- Tarrés, Q.; Espinosa, E.; Domínguez-Robles, J.; Rodríguez, A.; Mutjé, P.; Delgado-Aguilar, M. The suitability of banana leaf residue as raw material for the production of high lignin content micro/nano fibers: From residue to value-added products. Ind. Crop Prod. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Moreira, M.; Melo, M.; Coimbra, J.; Reis, K.; Schwan, R.; Silva, C. Solid coffee waste as alternative to produce carotenoids with antioxidant and antimicrobial activities. Waste Manag. 2018, 82, 93–99. [Google Scholar] [CrossRef]
- Peña-Lucio, E.; Londoño-Hernández, L.; Ascacio-Valdes, J.; Chavéz-González, M.; Bankole, O.; Aguilar, C. Use of coffee pulp and sorghum mixtures in the production of n-demethylases by solid-state fermentation. Bioresour. Technol. 2020, 305, 123112. [Google Scholar] [CrossRef]
- Filipe, D.; Fernandes, H.; Castro, C.; Peres, H.; Oliva-Teles, A.; Belo, I.; Salgado, J. Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Biofuels Bioprod. Biorefining 2019, 14, 78–91. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.; Contreras-Esquivel, J.; Aguilar, C. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Singh, A.; Bajar, S.; Devi, A.; Bishnoi, N. Adding value to agro-industrial waste for cellulase and xylanase production via solid-state bioconversion. Biomass Convers. Biorefin. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gea, T.; Sánchez, A.; Font, X. Agro-wastes and inert materials as supports for the production of biosurfactants by solid-state fermentation. Waste Biomass Valoriz. 2021, 12, 1963–1976. [Google Scholar] [CrossRef]
- Schalchli, H.; Hormazábal, E.; Astudillo, Á.; Briceño, G.; Rubilar, O.; Diez, M. Bioconversion of potato solid waste into antifungals and biopigments using Streptomyces spp. PLoS ONE 2021, 16, e0252113. [Google Scholar] [CrossRef]
- Pham, J.; Yilma, M.; Feliz, A.; Majid, M.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A review of the microbial production of bioactive natural products and biologics. Front Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef]
- Souza, P.; Werneck, G.; Aliakbarian, B.; Siqueira, F.; Ferreira Filho, E.; Perego, P.; Converti, A.; Magalhaes, P.O.; Junior, A.P. Production, purification and characterization of an aspartic protease from Aspergillus foetidus. Food Chem. Toxicol. 2017, 109, 1103–1110. [Google Scholar] [CrossRef]
- Tuysuz, E.; Gonul-Baltaci, N.; Omeroglu, M.; Adiguzel, A.; Taskin, M.; Ozkan, H. Co-production of amylase and protease by locally isolated thermophilic bacterium Anoxybacillus rupiensis T2 in sterile and non-sterile media using waste potato peels as substrate. Waste Biomass Valoriz. 2020, 11, 6793–6802. [Google Scholar] [CrossRef]
- Hermansyah, H.; Maresya, A.; Putri, D.; Sahlan, M.; Meyer, M. Production of dry extract lipase from Pseudomonas aeruginosa by the submerged fermentation method in palm oil mill effluent. Int. J. Technol. 2018, 9, 325. [Google Scholar] [CrossRef]
- Putri, D.; Khootama, A.; Perdani, M.; Utami, T.; Hermansyah, H. Optimization of Aspergillus niger lipase production by solid state fermentation of agro-industrial waste. Energy Rep. 2020, 6, 331–335. [Google Scholar] [CrossRef]
- Paz, A.; Nikolaivits, E.; Topakas, E. Valorization of olive mill wastewater towards the production of L-asparaginases. Biomass Convers. Biorefin. 2021, 11, 539–546. [Google Scholar] [CrossRef]
- Naser, S.; Saber, W.; El-Metwally, M.; Moustafa, M.; El-Kott, A. Fungal assembly of L-asparaginase using solid-state fermentation: A review. BIOCELL 2020, 44, 147–155. [Google Scholar] [CrossRef]
- Saleh, F.; Hussain, A.; Younis, T.; Ali, S.; Rashid, M.; Ali, A.; Mustafa, G.; Jabeen, F.; Al-Surhanee, A.A.; Alnoman, M.M.; et al. Comparative growth potential of thermophilic amylolytic Bacillus sp. on unconventional media food wastes and its industrial application. Saudi J. Biol. Sci. 2020, 27, 3499–3504. [Google Scholar] [CrossRef]
- Wehaidy, H.; Abdel Wahab, W.; Kholif, A.; Elaaser, M.; Bahgaat, W.; Abdel-Naby, M. Statistical optimization of B. subtilis MK775302 milk clotting enzyme production using agro-industrial residues, enzyme characterization and application in cheese manufacture. Biocatal. Agric. Biotechnol. 2020, 25, 101589. [Google Scholar] [CrossRef]
- Paul, J.; Beliya, E.; Tiwari, S.; Patel, K.; Gupta, N.; Jadhav, S. Production of biocatalyst α-amylase from agro-waste ‘rice bran’ by using Bacillus tequilensis TB5 and standardizing its production process. Biocatal. Agric. Biotechnol. 2020, 26, 101648. [Google Scholar] [CrossRef]
- Mojumdar, A.; Deka, J. Recycling agro-industrial waste to produce amylase and characterizing amylase–gold nanoparticle composite. Int. J. Recycl. Org. Waste Agric. 2019, 8, 263–269. [Google Scholar] [CrossRef]
- Bajar, S.; Singh, A.; Bishnoi, N. Exploration of low-cost agro-industrial waste substrate for cellulase and xylanase production using Aspergillus heteromorphus. Appl. Water Sci. 2020, 10, 153. [Google Scholar] [CrossRef]
- Narsing Rao, M.; Xiao, M.; Li, W. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Venil, C.; Dufossé, L.; Renuka Devi, P. Bacterial pigments: Sustainable compounds with market potential for pharma and food industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Keivani, H.; Jahadi, M.; Ghasemisepero, N. Optimizing submerged cultivation for the production of red pigments by Monascus purpureus on soybean meals using response surface methodology. Appl. Food Biotechnol. 2020, 7, 143–151. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, G.; Arora, A.; Paul, D. Carotenoid production by red yeast isolates grown in agricultural and “mandi” waste. Waste Biomass Valoriz. 2021, 12, 3939–3949. [Google Scholar] [CrossRef]
- Venil, C.; Malathi, M.; Velmurugan, P.; Renuka Devi, P. Green synthesis of silver nanoparticles using canthaxanthin from Dietzia maris AURCCBT01 and their cytotoxic properties against human keratinocyte cell line. J. Appl. Microbiol. 2021, 130, 1730–1744. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.; Ligabue-Braun, R. Agro-industrial residues: Eco-friendly and inexpensive substrates for microbial pigments production. Front. Sustain. Food Syst. 2021, 5, 589414. [Google Scholar] [CrossRef]
- Maurya, K.; Tripathi, A.; Kumar, D.; Srivastava, S. Production, purification and characterization of prodigiosin by Serratia nematodiphilia (NCIM 5606) using solid-state fermentation with various substrate. Ann. Phytomed. 2020, 9, 302–306. [Google Scholar] [CrossRef]
- Patthawaro, S.; Lomthaisong, K.; Saejung, C. Bioconversion of agro-industrial waste to value-added product lycopene by photosynthetic bacterium Rhodopseudomonas faecalis and its carotenoid composition. Waste Biomass Valoriz. 2020, 11, 2375–2386. [Google Scholar] [CrossRef]
- Kumar, A.; Dave, N.; Murugesan, G.; Pai, S.; Pugazhendhi, A.; Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Production and extraction of red pigment by solid-state fermentation of broken rice using Monascus sanguineus NFCCI 2453. Biocat. Agric Biotechnol. 2021, 33, 101964. [Google Scholar] [CrossRef]
- Otero, D.; Bulsing, B.; Huerta, K.; Rosa, C.; Zambiazi, R.; Burkert, C.; Burkert, J. Carotenoid-producing yeasts in the brazilian biodiversity: Isolation, identification and cultivation in agroindustrial waste. Braz. J. Chem. Eng. 2019, 36, 117–129. [Google Scholar] [CrossRef]
- Korumilli, T.; Mishra, S.; Korukonda, J.R. Production of astaxanthin by Xanthophyllomyces dendrorhous on fruit waste extract and optimization of key parameters using Taguchi method. J. Biochem. Technol. 2020, 11, 25–31. [Google Scholar]
- Villegas-Méndez, M.; Aguilar-Machado, D.; Balagurusamy, N.; Montañez, J.; Morales-Oyervides, L. Agro-industrial wastes for the synthesis of carotenoids by Xanthophyllomyces dendrorhous: Mesquite pods-based medium design and optimization. Biochem. Eng. J. 2019, 150, 107260. [Google Scholar] [CrossRef]
- Luft, L.; Confortin, T.; Todero, I.; Zabot, G.; Mazutti, M. An overview of fungal biopolymers: Bioemulsifiers and biosurfactants compounds production. Crit. Rev. Biotechnol. 2020, 40, 1059–1080. [Google Scholar] [CrossRef]
- Rane, A.; Baikar, V.; Ravi Kumar, V.; Deopurkar, R. Agro-industrial wastes for production of biosurfactant by Bacillus subtilis ANR 88 and its application in synthesis of silver and gold nanoparticles. Front. Microbiol. 2017, 8, 492. [Google Scholar] [CrossRef]
- Vera, E.; de Azevedo, P.; Domínguez, J.; Oliveira, R. Optimization of biosurfactant and bacteriocin-like inhibitory substance (BLIS) production by Lactococcus lactis CECT-4434 from agroindustrial waste. Biochem. Eng. J. 2018, 133, 168–178. [Google Scholar] [CrossRef]
- Anaukwu, C.; Ogbukagu, C.; Ekwealor, I. Optimized biosurfactant production by Pseudomonas aeruginosa strain CGA1 using agro-industrial waste as sole carbon source. Adv. Microbiol. 2020, 10, 543–562. [Google Scholar] [CrossRef]
- Rastogi, S.; Tiwari, S.; Ratna, S.; Kumar, R. Utilization of agro-industrial waste for biosurfactant production under submerged fermentation and its synergistic application in biosorption of Pb2+. Bioresour. Technol. Rep. 2021, 15, 100706. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.; Huerta-Ochoa, S.; Aguilar, C.; Prado-Barragán, L. Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Dulf, F.; Vodnar, D.; Dulf, E.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef]
- Nisa, K.; Rosyida, V.; Nurhayati, S.; Indrianingsih, A.; Darsih, C.; Apriyana, W. Total phenolic contents and antioxidant activity of rice bran fermented with lactic acid bacteria. IOP Conf. Ser. Earth Environ. Sc. 2019, 251, 012020. [Google Scholar] [CrossRef]
- Sadh, P.; Chawla, P.; Duhan, J. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Biosci. 2018, 22, 113–120. [Google Scholar] [CrossRef]
- Yepes-Betancur, D.; Márquez-Cardozo, C.; Cadena-Chamorro, E.; Martinez-Saldarriaga, J.; Torres-León, C.; Ascacio-Valdes, A.; Aguilar, C. Solid-state fermentation—Assisted extraction of bioactive compounds from hass avocado seeds. Food Bioprod. Process. 2021, 126, 155–163. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Laredo-Alcalá, E.; Buenrostro-Figueroa, J.; Ascacio-Valdés, J.; Genisheva, Z.; Aguilar, C.; Teixeira, J. Ellagic acid production using polyphenols from orange peel waste by submerged fermentation. Electron. J. Biotechnol. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Zeng, X.; Miao, W.; Zeng, H.; Zhao, K.; Zhou, Y.; Zhang, J.; Zhao, Q.; Tursun, D.; Xu, D.; Li, F. Production of natamycin by Streptomyces gilvosporeus Z28 through solid-state fermentation using agro-industrial residues. Bioresour. Technol. 2019, 273, 377–385. [Google Scholar] [CrossRef]
- El-Sayed, E.; Ahmed, A.; Al-Hagar, O. Agro-industrial wastes for production of paclitaxel by irradiated Aspergillus fumigatus under solid-state fermentation. J. Appl. Microbiol. 2020, 128, 1427–1439. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef]
- Shata, H. Statistical optimization of erythromycin production by Saccharopolyspora erythraea under solid state fermentation of agro-industrial materials using response surface methodology. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 692–697. [Google Scholar] [CrossRef]
- El-Housseiny, G.; Ibrahim, A.; Yassien, M.; Aboshanab, K. Production and statistical optimization of paromomycin by Streptomyces rimosus NRRL 2455 in solid state fermentation. BMC Microbiol. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- El-Bondkly, A.; El-Gendy, M.; El-Bondkly, A. Construction of efficient recombinant strain through genome shuffling in marine endophytic Fusarium sp. ALAA-20 for improvement lovastatin production using agro-industrial wastes. Arab. J. Sci. Eng. 2020, 46, 175–190. [Google Scholar] [CrossRef]
- Guneser, O.; Demirkol, A.; Yuceer, Y.K.; Togay, S.O.; Hosoglu, M.I.; Elibol, M. Production of flavor compounds from olive mill waste by Rhizopus oryzae and Candida tropicalis. Braz. J. Microbiol. 2017, 48, 275–285. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-phenylethanol (rose aroma) production potential of an isolated Pichia kudriavzevii through solid-state fermentation. Process Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, P.; Singh, J.; Singh, S.; Nain, L. Prospecting the potential of agroresidues as substrate for microbial flavor production. Front. Sustain. Food Syst. 2020, 4, 18. [Google Scholar] [CrossRef]
- Saeed, S.; Raza, S.Q.; Zafar, S.S.; Mujahid, H.; Irfan, M.; Mehmood, T. Microbial conversion of pomegranate peels to biovanillin using submerged fermentation and process optimization through statistical design. Biomass Convers. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Saeed, S.; Baig, U.U.R.; Tayyab, M.; Altaf, I.; Irfan, M.; Raza, S.Q.; Nadeem, F.; Mehmood, T. Valorization of banana peels waste into biovanillin and optimization of process parameters using submerged fermentation. Biocatal. Agric. Biotechnol. 2021, 36, 102154. [Google Scholar] [CrossRef]
- Ndao, A.; Sellamuthu, B.; Kumar, L.R.; Tyagi, R.D.; Valéro, J.R. Biopesticide production using Bacillus thuringiensis kurstaki by valorization of starch industry wastewater and effluent from aerobic, anaerobic digestion. Syst. Microbiol. Biomanuf. 2021, 1, 494–504. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Kumar, P.; Samrat, K.; Moovendhan, M.; Kavisri, M.; Sivakumar, L.; Bharani, R.S.A.; Shyamsundar, D. Development of high organic-rich low-cost medium derived from microbial consortium decomposed vegetable wastes for the viable inocula production of potential fungal biopesticide Metarhizium anisopliae. Biomass Convers. Biorefin. 2022, 1–17. [Google Scholar] [CrossRef]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Hanson, J. Chemistry of Fungi, 1st ed.; Royal Society of Chemistry: London, UK, 2008; p. 240. [Google Scholar]
- Ali, H.K.Q.; Zulkali, M.M.D. Design aspects of bioreactors for solid-state fermentation: A review. Chem. Biochem. Eng. Q. 2011, 25, 255–266. [Google Scholar]
- Zhang, L.; Li, Z.; Dai, B.; Zhang, W.; Yuan, Y. Effect of submerged and solid-state fermentation on pigment and citrinin production by Monascus purpureus. Acta Biol. Hung. 2013, 64, 385–394. [Google Scholar] [CrossRef]
- Asghari, M.; Jahadi, M.; Hesam, F.; Ghasemi-Sepro, N. Optimization of Monascus pigment production on date waste substrates using solid state fermentation. Appl. Food Biotechnol. 2020, 8, 247–254. [Google Scholar]
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-state fermentation as a novel paradigm for organic waste valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Singhania, R.; Sukumaran, R.; Patel, A.; Larroche, C.; Pandey, A. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb. Technol. 2010, 46, 541–549. [Google Scholar] [CrossRef]
- Terán Hilares, R.; de Souza, R.; Marcelino, P.; da Silva, S.; Dragone, G.; Mussatto, S.; Santos, J. Sugarcane bagasse hydrolysate as a potential feedstock for red pigment production by Monascus ruber. Food Chem. 2018, 245, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery. Bioresour. Technol. 2022, 362, 127790. [Google Scholar] [CrossRef]
- Soccol, C.; Costa, E.; Letti, L.; Karp, S.; Woiciechowski, A.; Vandenberghe, L. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Sadh, P.; Duhan, S.; Duhan, J. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Xu, M.; Yang, M.; Sun, H.; Gao, M.; Wang, Q.; Wu, C. Bioconversion of biowaste into renewable energy and resources: A sustainable strategy. Environ. Res. 2022, 214, 113929. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Coelho, E.; Aguiar, T.Q.; Domingues, L. Microbial biosynthesis of lactones: Gaps and opportunities towards sustainable production. Appl. Sci. 2021, 11, 8500. [Google Scholar] [CrossRef]
- Crater, J.; Lievense, J. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 2018, 365, fny138. [Google Scholar] [CrossRef]
- Deljou, A.; Arezi, I.; Khanahmad, M. Scale-up thermostable α-amylase production in lab-scale fermenter using rice husk as an elicitor by Bacillus licheniformis-AZ2 isolated from Qinarje Hot Spring (Ardebil Prov. of Iran). Period Biol. 2018, 120, 11–21. [Google Scholar] [CrossRef]
- Yegin, S.; Buyukkileci, A.; Sargin, S.; Goksungur, Y. Exploitation of agricultural wastes and by-products for production of Aureobasidium pullulans Y-2311-1 xylanase: Screening, bioprocess optimization and scale up. Waste Biomass Valoriz. 2016, 8, 999–1010. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.-L.; Doan, M.D.; Nguyen, T.H.; Tran, T.H.T.; Tran, T.N.; Doan, C.T.; Ngo, V.A.; Ho, N.D.; Do, V.C.; et al. Utilization of by-product of groundnut oil processing for production of prodigiosin by microbial fermentation and its novel potent anti-nematodes effect. Agronomy 2021, 12, 41. [Google Scholar] [CrossRef]
- Tran, L.T.; Techato, K.; Nguyen, V.B.; Wang, S.-L.; Nguyen, A.D.; Phan, T.Q.; Doan, C.T.; Phoungthong, K. Utilization of cassava wastewater for low-cost production of prodigiosin via Serratia marcescens TNU01 fermentation and its novel potent α-glucosidase inhibitory effect. Molecules 2021, 26, 6270. [Google Scholar] [CrossRef] [PubMed]
- Hölker, U.; Lenz, J. Solid-state fermentation—Are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Jeevarathinam, G.; Kumar, S.; Muniraj, I.; Uthandi, S. Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes. BMC Biotechnol. 2021, 21, 33. [Google Scholar] [CrossRef] [PubMed]
| Microorganism | Biosurfactant | Fermentation | Agroindustrial Waste | Reference |
|---|---|---|---|---|
| Bacteria | ||||
| Bacillus subtilis | Lipopeptide | SmF | Molasses | [56] |
| Lactococcus lactis | Glycolipopeptide | SmF | Vinasse | [57] |
| Pseudomonas aeruginosa | Octadecanoic acid Lipopeptide | SmF | Sugar cane molasses | [58] |
| Cyclododecanol Lipopeptide | ||||
| Bacillus haynesii | Lipopeptide | SmF | Orange peel | [59] |
| Fungus | ||||
| Starmerella bombicola ATCC 22214 | Sophorolipids | SSF | Wheat straw, rice husk and coconut fiber | [29] |
| Microorganism | Antioxidant Compound | Fermentation | Agroindustrial Waste | Reference |
|---|---|---|---|---|
| Bacteria | ||||
| Lactobacillus lactic Lactobacillus plantarum | Total phenols | SSF | Rice bran | [63] |
| Fungus | ||||
| Aspergillus awamori | Phenolic compounds | SSF | Peanut press cake | [64] |
| Aspergillus niger | Procyanidin B2 monomers | SSF | Hass avocado seeds | [65] |
| Aspergillus niger | Pentagalloylglucose | SSF | Mango seed waste | [27] |
| Ellagic acid | ||||
| Aspergillus oryzae | Gallic acid | SSF | Peanut press cake | [9] |
| Chlorogenic acid | ||||
| 4-hydroxy butyric acid | ||||
| p-Coumeric acid | ||||
| Rhizopus oligosporus | 3-caffeoylquinic acid | SSF | Apricot pomace | [62] |
| 5-caffeoylquinic acid | ||||
| Quercetin-3-rutino-side | ||||
| Quercetin-3(6″acetyl-glucoside) | ||||
| Rhizopus oryzae | Hydroxycinnamic acids | SSF | Olive mill waste | [20] |
| Aspergillus fumigatus | Ellagic acid | SmF | Orange peel waste | [66] |
| Microorganism | Product | Fermentation | Agroindustrial Waste | Reference |
|---|---|---|---|---|
| Bacteria | ||||
| Bacterial consortium | Volatile fatty acids | Anaerobic fermentation | Cucumber, tomato and lettuce waste | [69] |
| Saccharopolyspora erythraea | Erythromycin | SSF | Sugarcane bagasse, beet sugar root and oatmeal | [70] |
| Streptomyces rimosus | Paromomycin | SSF | Corn bran | [71] |
| Streptomyces gilvosporeus | Natamycin | SSF | Wheat bran, rapeseed cake, rice hull and crude glycerol | [67] |
| Fungi | ||||
| Aspergillus fumigatus | Paclitaxel | SSF | Sugarcane bagasse | [68] |
| Wheat bran | ||||
| Aspergillus niger Aspergillus ibericus | Ergosterol Lignocellulolytic enzymes | SSF | Olive mill waste with winery waste | [26] |
| Fusarium sp. (Recombinant) | Lovastatin | SSF | Groundnut oil and soybean oil cakes | [72] |
| Pleurotus citrinopileatus | Antityrosinase compounds | SmF | Olive-oil mill wastewater | [21] |
| Rhizopus oryzae | 2-pentanone d-limonene 2-phenylethanol | SmF | Olive mill waste | [73] |
| Candida tropicalis | d-limonene methyl butanoate | SmF | Olive mill waste | [73] |
| Pichia kudriavzevii | 2-phenylethanol | SSF | Sugarcane bagasse | [74] |
| SSF | SmF | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astudillo, Á.; Rubilar, O.; Briceño, G.; Diez, M.C.; Schalchli, H. Advances in Agroindustrial Waste as a Substrate for Obtaining Eco-Friendly Microbial Products. Sustainability 2023, 15, 3467. https://doi.org/10.3390/su15043467
Astudillo Á, Rubilar O, Briceño G, Diez MC, Schalchli H. Advances in Agroindustrial Waste as a Substrate for Obtaining Eco-Friendly Microbial Products. Sustainability. 2023; 15(4):3467. https://doi.org/10.3390/su15043467
Chicago/Turabian StyleAstudillo, Álvaro, Olga Rubilar, Gabriela Briceño, María Cristina Diez, and Heidi Schalchli. 2023. "Advances in Agroindustrial Waste as a Substrate for Obtaining Eco-Friendly Microbial Products" Sustainability 15, no. 4: 3467. https://doi.org/10.3390/su15043467
APA StyleAstudillo, Á., Rubilar, O., Briceño, G., Diez, M. C., & Schalchli, H. (2023). Advances in Agroindustrial Waste as a Substrate for Obtaining Eco-Friendly Microbial Products. Sustainability, 15(4), 3467. https://doi.org/10.3390/su15043467