Comparative Investigation of the Effect of EggshellPowder and Calcium Carbonate as Additivesin Eco-Friendly Polymer Drilling Fluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Additives
2.2.1. Potato Starch
2.2.2. EggshellPowder
2.2.3. Preparation of Na-Bentonite
2.3. Characterization
2.4. Experimental Procedure
3. Results and Discussions
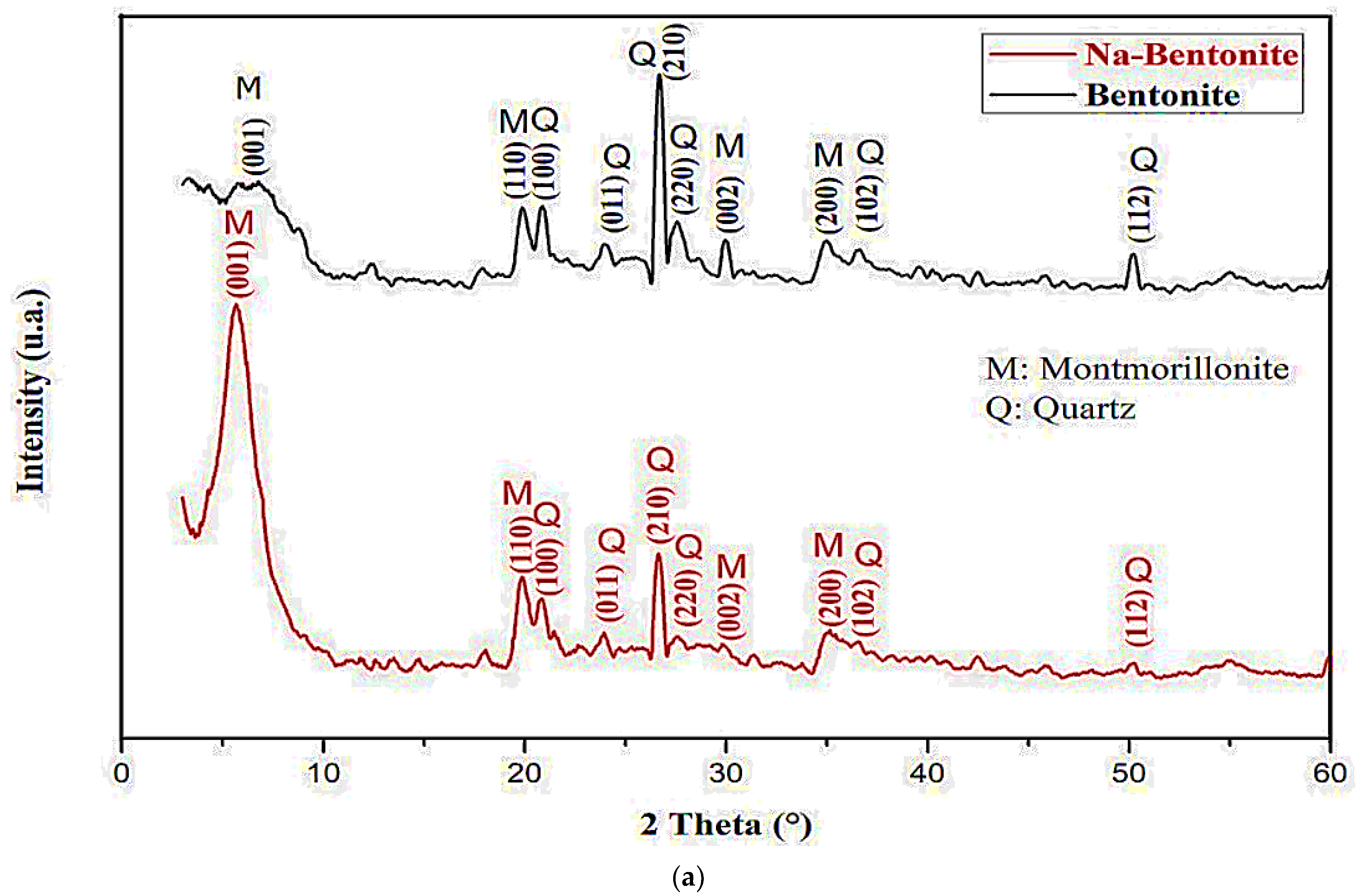
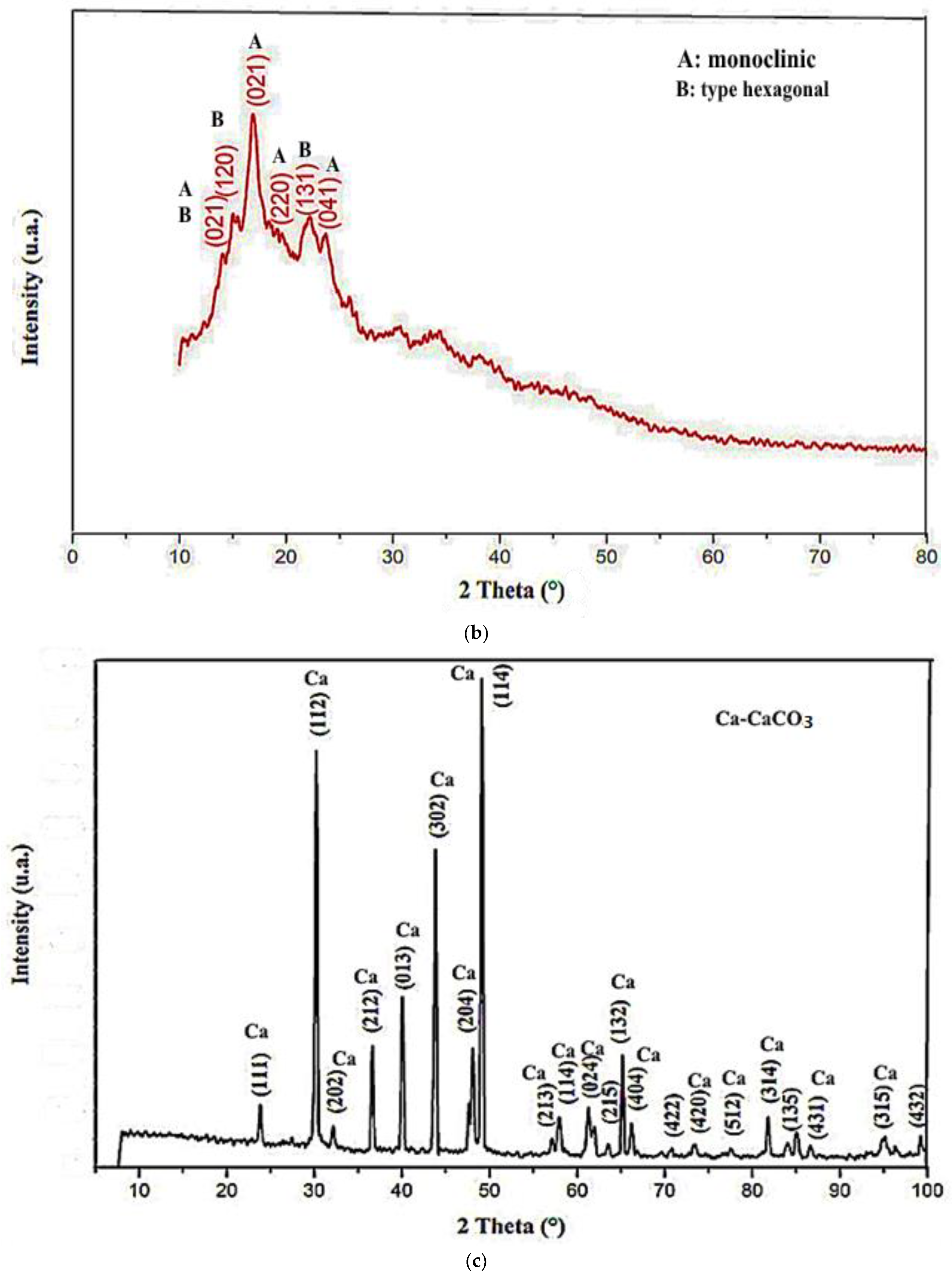
3.1. XRD Analysis
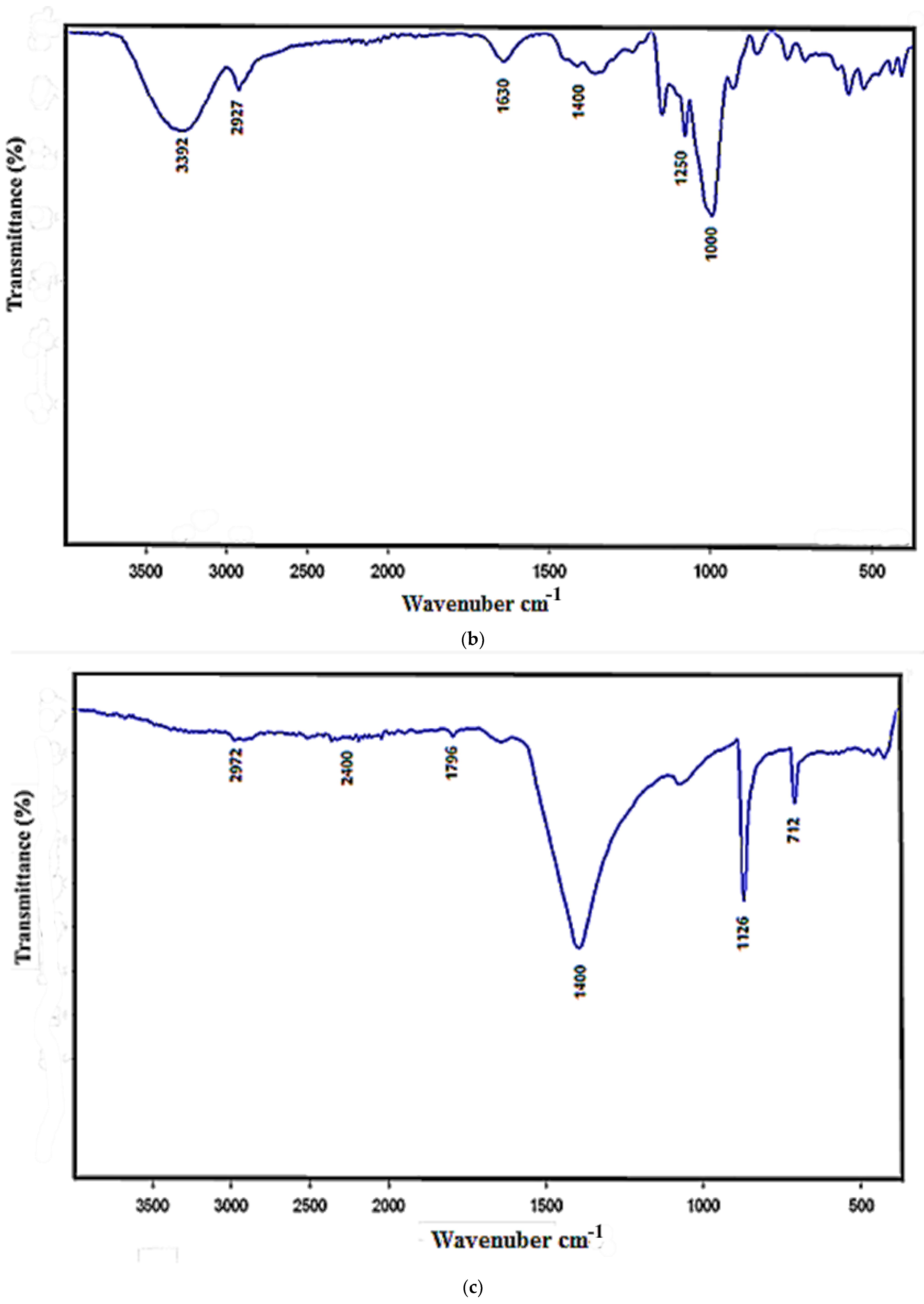
3.2. FTIR Analysis
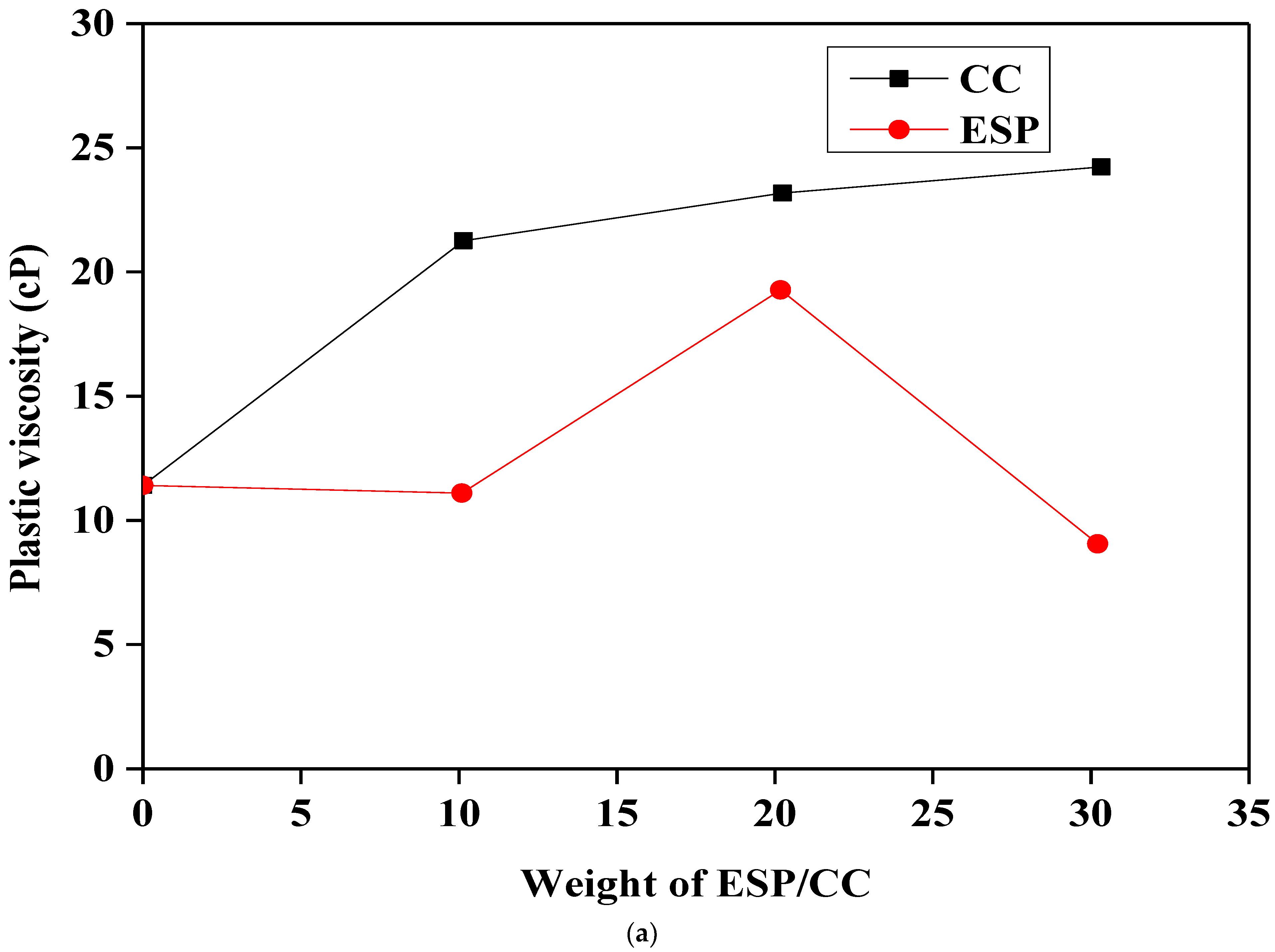
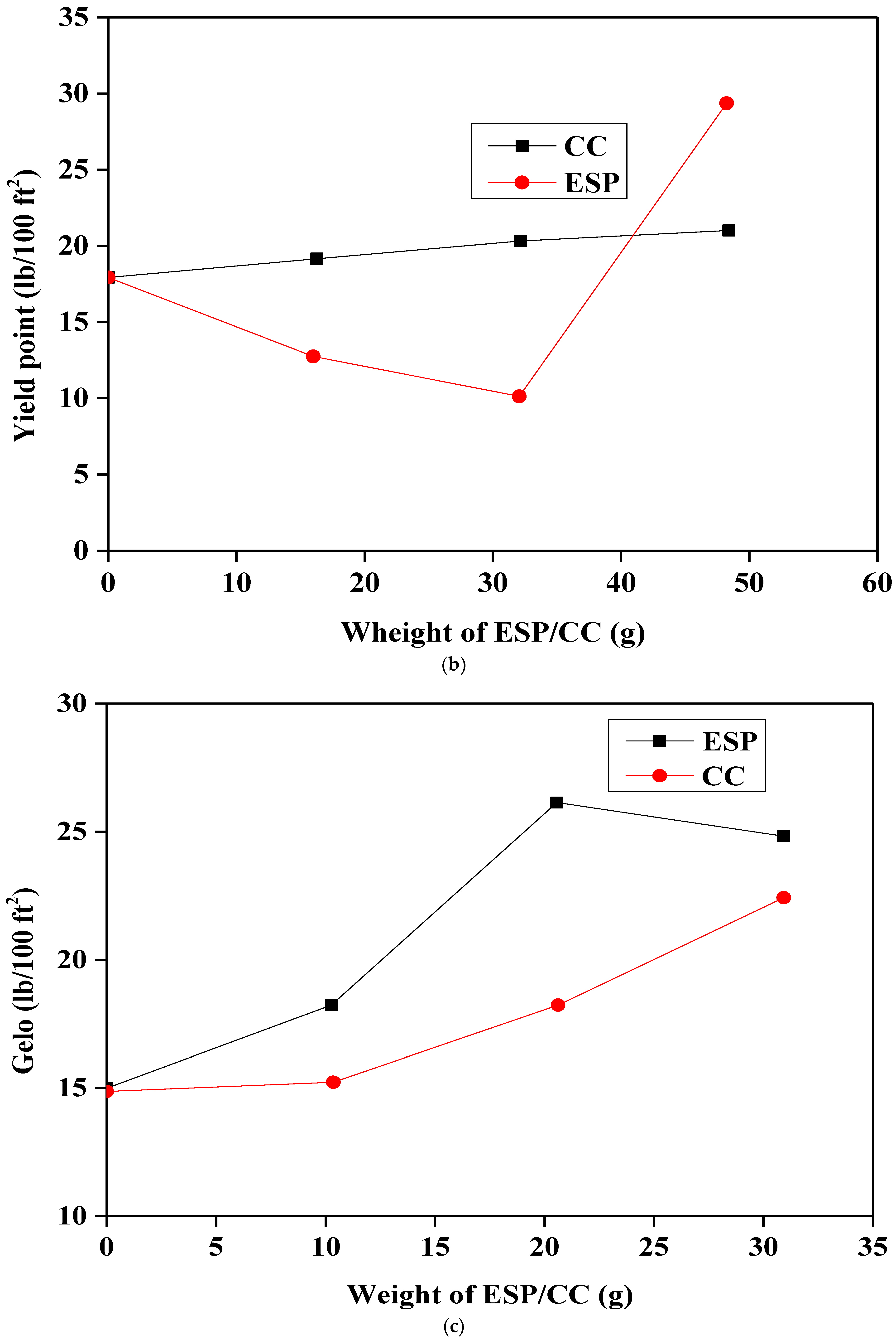
3.3. Rheological Properties of Different Samples
- Pseudoplastic—decrease in viscosity.
- Thixotropic—time-dependent decrease in viscosity.
- Dilatant—increase in viscosity.
- Rheopectic—time-dependent increase in viscosity.
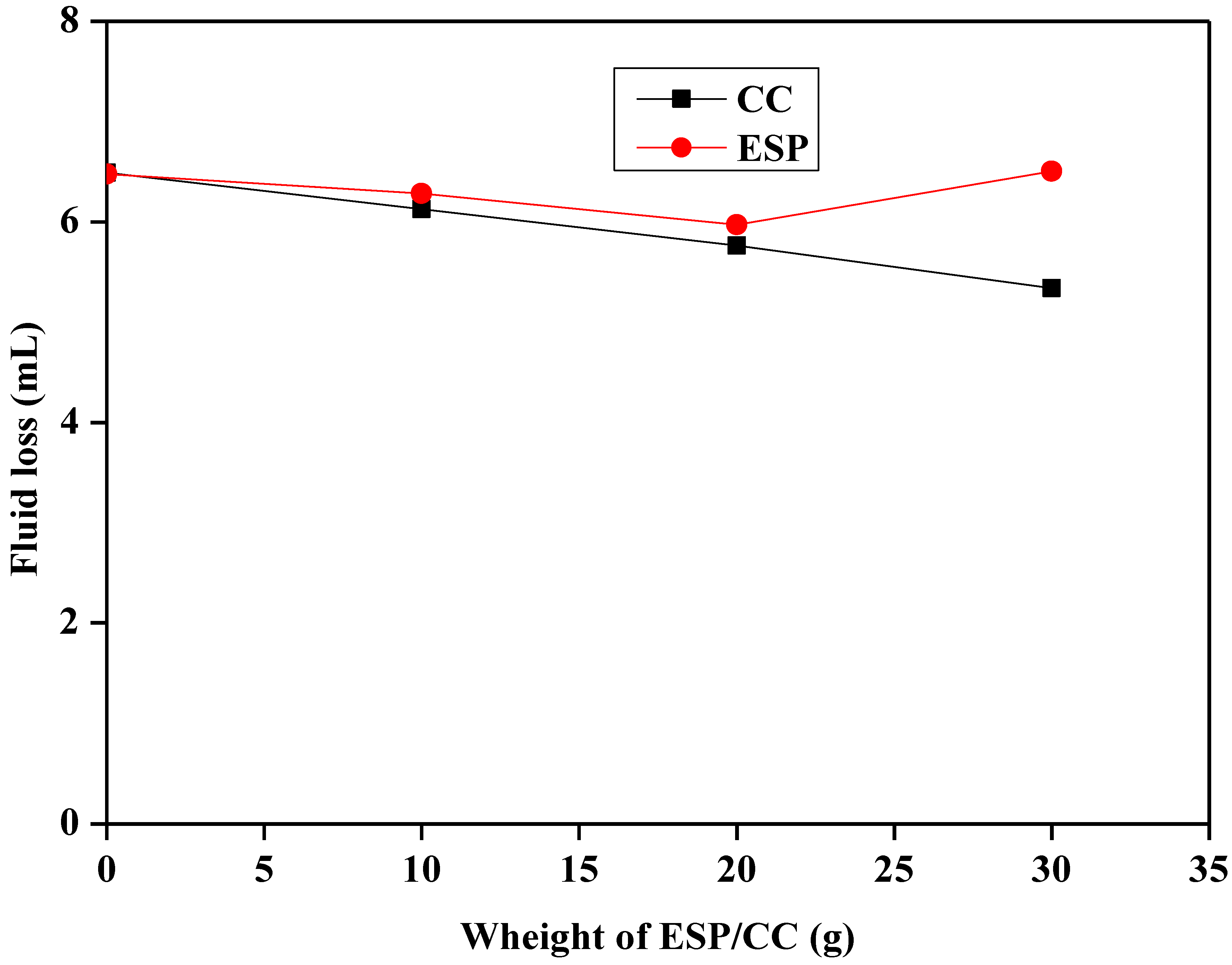
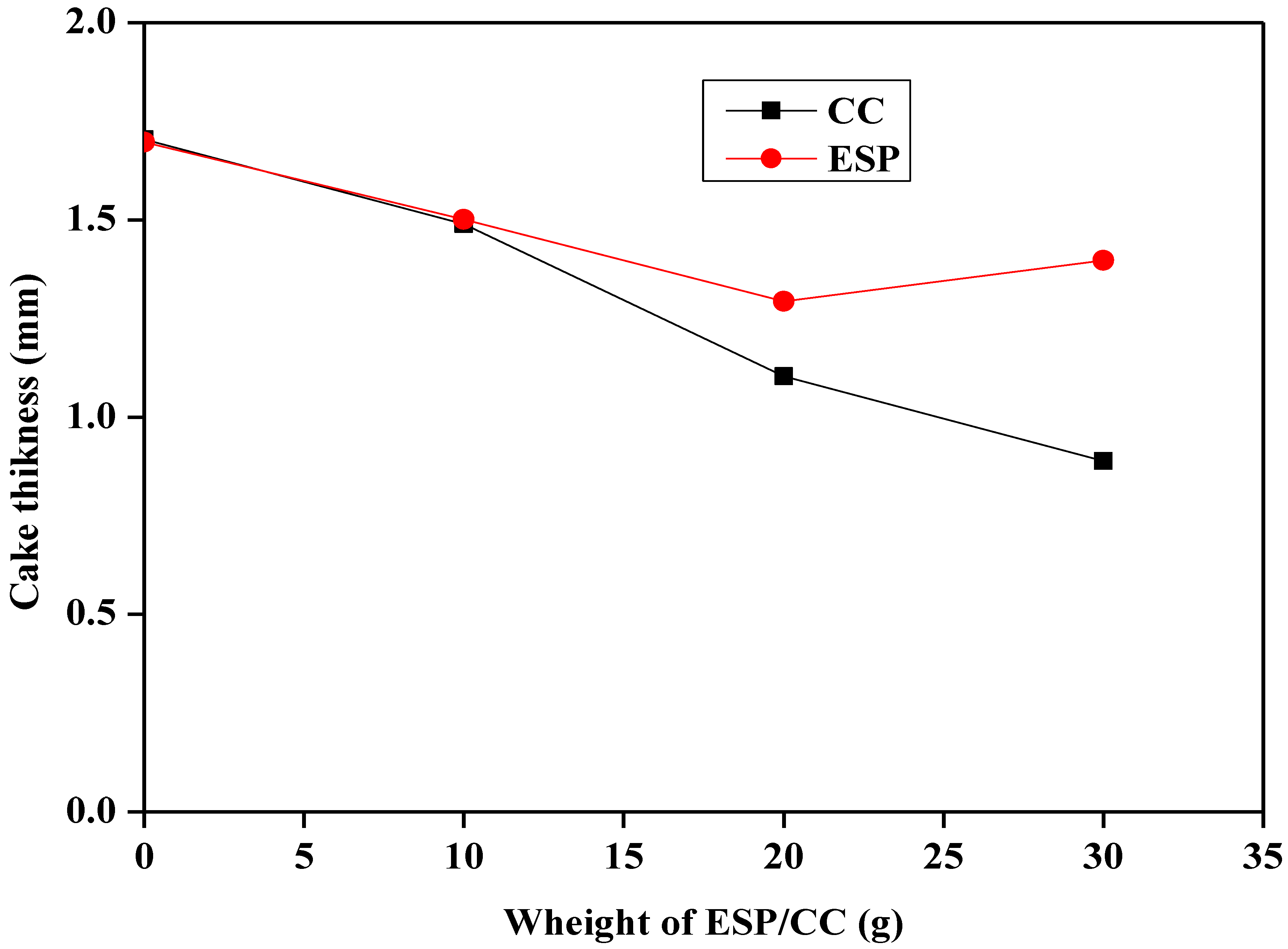
3.3.1. Filtration Properties of Different Samples
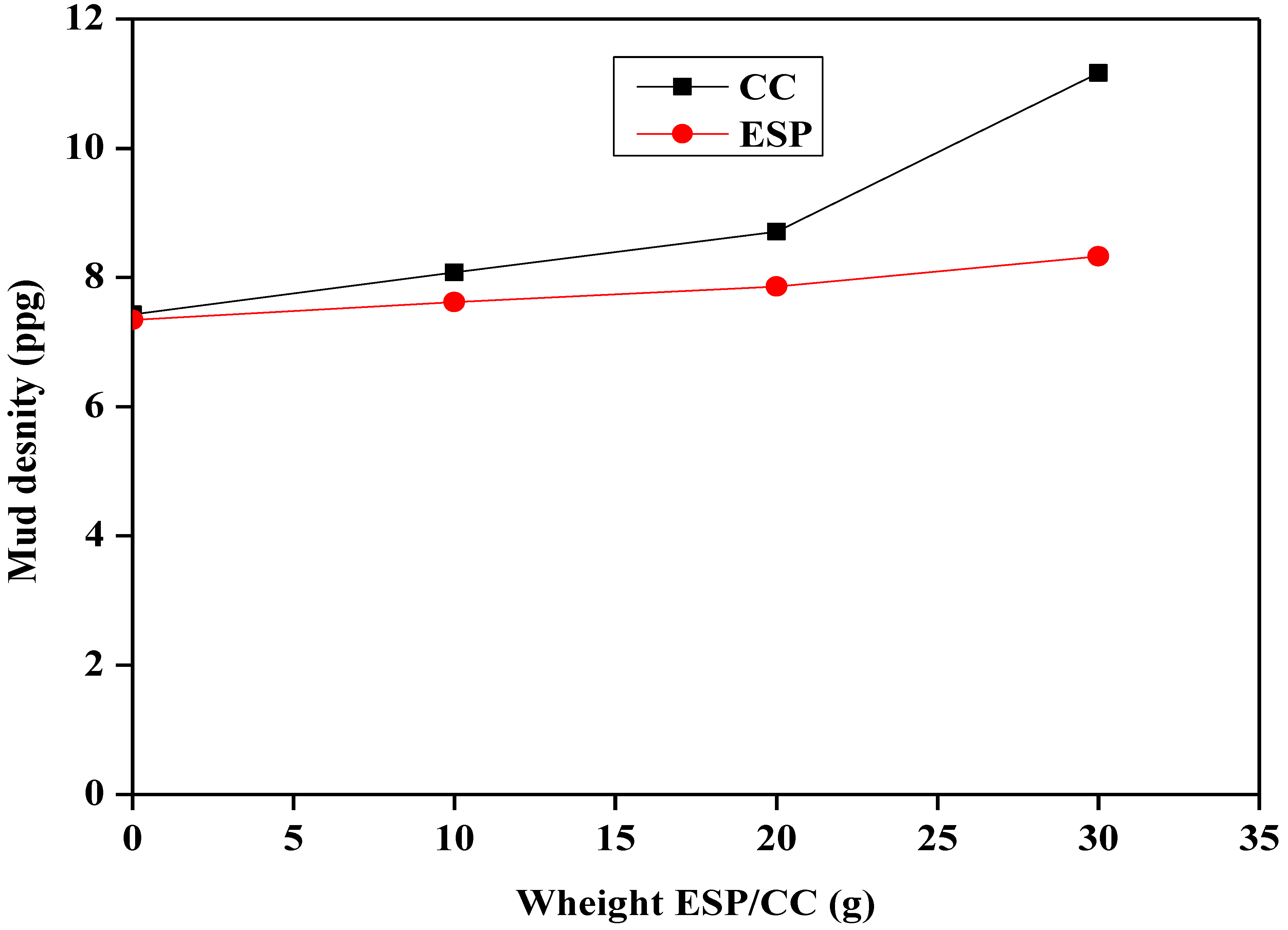
3.3.2. Effect of Additives on the Mud Density and the pH
4. Conclusions
- The addition of ESP and CC significantly improves the rheological properties of Na-bentonite/starch-based drilling fluids; this observation was confirmed by the increase in the plastic viscosity, the yield point, and the gel strength.
- The addition of ESP and CC has a positive impact on the filtration properties of both Na-bentonite and starch-based drilling fluids and their effect was observed in the increase in the fluid loss and cake thickness.
- A 30 g ratio of eggshell powder produces unfavorable outcomes.
- The pH levels are slightly influenced by the eggshell powder.
- The mud density is improved by the presence of both ESP and CC.
- The rheological and filtration properties were proportionally adjusted by the addition of both ESP and CC, and they are changed by increasing the concentration.
- The major goal behind this study is to develop drilling fluid additives that are environmentally eco-friendly and locally accessible.
- The ESP concentrations ranging from 10 to 20 g aremore effective to improve both rheological and filtration properties. It was demonstrated that CaCO3 has greater potential and an alternative uses to improve water-based drilling fluid mud density in comparison to eggshell powder. Finally, the addition of CC or ESP had a slight influence on the pH of the drilling fluids. This means that ESP is feasible to generate waste and study local products that represent an alternative to the use of chemicals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bleier, R.; Leuterman, A.J.; Stark, C. Drilling Fluids: Making Peace with the Environment; OnePetro: Richardson, TX, USA, 1992. [Google Scholar]
- Luo, Z.; Pei, J.; Wang, L.; Yu, P.; Chen, Z. Influence of an Ionic Liquid on Rheological and Filtration Properties of Water-Based Drilling Fluids at High Temperatures. Appl. Clay Sci. 2017, 136, 96–102. [Google Scholar] [CrossRef]
- Barrett, M.L. Drilling Mud: A 20th Century History; AAPG: Tulsa, OK, USA, 2011. [Google Scholar]
- Gürgen, S.; Sofuoğlu, A. Advancements in Conventional Machining: A Case of Vibration and Heat-Assisted Machining of Aerospace Alloys. InAdvanced Machining and Finishing; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-817452-4. [Google Scholar]
- Sofuoğlu, A.; Çakir, F.; Gürgen, S.; Orak, S.; Kushan, M. Numerical Investigation of Hot Ultrasonic Assisted Turning of Aviation Alloys. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 122. [Google Scholar] [CrossRef]
- Al-Hameedi, A.T.; Alkinani, H.; Dunn-Norman, S.; Salem, E.; Knickerbocker, M.; Alashwak, N.; Mutar, R.; Al-Bazzaz, W. Laboratory Study of Environmentally Friendly Drilling Fluid Additives Banana Peel Powder for Modifying the Drilling Fluid Characteristics in Water-Based Muds; OnePetro: Richardson, TX, USA, 2020. [Google Scholar]
- Ikram, R.; Mohamed Jan, B.; Sidek, A.; Kenanakis, G. Utilization of Eco-Friendly Waste Generated Nanomaterials in Water-Based Drilling Fluids; State of the Art Review. Materials 2021, 14, 4171. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.-C.; Bouzaza, A.; Amrane, A.; Tahraoui, H.; Mouni, L. Aleppo Pine Seeds (Pinus Halepensis Mill.) as a Promising Novel Green Coagulant for the Removal of Congo Red Dye: Optimization via Machine Learning Algorithm. J. Environ. Manag. 2023, 331, 117286. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Hadadi, A.; Hamri, N.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Manseri, A.; Mouni, L. Adsorption Performance of Zeolite for the Removal of Congo Red Dye: Factorial Design Experiments, Kinetic, and Equilibrium Studies. Separations 2023, 10, 57. [Google Scholar] [CrossRef]
- De Nino, A.; Tallarida, M.; Algieri, V.; Olivito, F.; Costanzo, P.; Filpo, G.; Maiuolo, L. Sulfonated Cellulose-Based Magnetic Composite as Useful Media for Water Remediation from Amine Pollutants. Appl. Sci. 2020, 10, 8155. [Google Scholar] [CrossRef]
- Dintcheva, N.; Infurna, G.; Baiamonte, M.; D’Anna, F. Natural Compounds as Sustainable Additives for Biopolymers. Polymers 2020, 12, 732. [Google Scholar] [CrossRef]
- Gioia, M.L. Synthesis and Preliminary Evaluation of the Anti-Cancer Activity on A549 Lung Cancer Cells of a Series of Unsaturated Disulfides. MedChemComm 2019, 10, 116–119. [Google Scholar]
- Tan, J.; Liu, L.; Li, F.; Chen, Z.; Chen, G.; Fang, F.; Guo, J.-S.; He, M.; Zhou, X. Screening of Endocrine Disrupting Potential of Surface Waters via an Affinity-Based Biosensor in a Rural Community in the Yellow River Basin, China. Environ. Sci. Technol. 2022, 56, 14350–14360. [Google Scholar] [CrossRef]
- Al-Hameedi, A.T.; Alkinani, H.; Dun-Norman, S.; Al-Alwani, M.; Alshammari, A.; Albazzaz, H.; Alkhamis, M.; Alashwak, N.; Mutar, R. Insights into the Application of New Eco-Friendly Drilling Fluid Additive to Improve the Fluid Properties in Water-Based Drilling Fluid Systems. J. Pet. Sci. Eng. 2019, 183, 106424. [Google Scholar] [CrossRef]
- Al-Hameedi, A.T.; Alkinani, H.; Dunn-Norman, S.; Albazzaz, H.; Alkhamis, M. Insights into Eco-Friendly and Conventional Drilling Additives: Applications, Cost Analysis, Health, Safety, and Environmental Considerations; OnePetro: Richardson, TX, USA, 2019. [Google Scholar]
- Aftab, A.; Ali, M.; Sahito, M.; Mohanty, U.; Jha, N.K.; Akhondzadeh, H.; Azhar, M.R.; Ismail, A.; Keshavarz, A.; Iglauer, S. Environmental Friendliness and High Performance of Multifunctional Tween 80/ZnO-Nanoparticles-Added Water-Based Drilling Fluid: An Experimental Approach. ACS Sustain. Chem. Eng. 2020, 8, 11224–11243. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, Z.; Zhong, H.; Zhao, X.; Kong, X. Study of Environmentally Friendly Wild Jujube Pit Powder as a Water-Based Drilling Fluid Additive. ACS Omega 2021, 6, 1436–1444. [Google Scholar] [CrossRef]
- Al-Hameedi, A.T.; Alkinani, H.; Dunn-Norman, S.; Al-Alwani, M.; Al-Bazzaz, W.; Alshammari, A.; Albazzaz, H.; Mutar, R. Experimental Investigation of Bio-Enhancer Drilling Fluid Additive: Can Palm Tree Leaves Be Utilized as a Supportive Eco-Friendly Additive in Water-Based Drilling Fluid System? J. Pet. Explor. Prod. Technol. 2019, 10, 595–603. [Google Scholar] [CrossRef]
- Zha, X.; Liao, X.; Zhao, X.; Liu, F.; He, A.Q.; Xiong, W.X. Turning Waste Drilling Fluids into a New, Sustainable Soil Resources for Landscaping. Ecol. Eng. 2017, 121, 130–136. [Google Scholar] [CrossRef]
- Daniel, J.; Penn, C.; Antonangelo, J.A.; Zhang, H. Land Application of Urban Horizontal Directional Drilling Residuals to Established Grass and Bare Soils. Sustainability 2020, 12, 10264. [Google Scholar] [CrossRef]
- Al-saba, M.; Amadi, K.; Al-Hadramy, K.; Al Dushaishi, M.; Al-Hameedi, A.T.; Alkinani, H. Experimental Investigation of Bio-Degradable Environmental Friendly Drilling Fluid Additives Generated from Waste; OnePetro: Richardson, TX, USA, 2018. [Google Scholar]
- Al-Hameedi, A.T.; Alkinani, H.; Dunn-Norman, S.; Al-Alwani, M.; Alshammari, A.; Alkhamis, M.; Mutar, R.; Al-Bazzaz, W. Experimental Investigation of Environmentally Friendly Drilling Fluid Additives (Mandarin Peels Powder) to Substitute the Conventional Chemicals Used in Water-Based Drilling Fluid. J. Pet. Explor. Prod. Technol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Olamigoke, O.; Aghante, T.; Iyalla, F. Petroleum and Coal Article Open Access An Experimental Study of Effects of Egg Shell and Snail Shell Powder on Rheological and Filtration Properties of Potassium Chloride Polymer Drilling Fluids. Pet. Coal 2020, 62, 1138–1143. [Google Scholar]
- Hossain, M.E.; Wajheeuddin, M. The Use of Grass as an Environmentally Friendly Additive in Water-Based Drilling Fluids. Pet. Sci. 2016, 13, 292–303. [Google Scholar] [CrossRef]
- Nik Ab Lah, N.K.I.; Ngah, K.; Sauki, A. Study on the Viability of Egg Shell as a Lost Circulation Material in Synthetic Based Drilling Fluid. J. Phys. Conf. Ser. 2019, 1349, 012135. [Google Scholar] [CrossRef]
- Iqbal, R.; Zubair, M.; Pirzada, F.; Abro, F.; Ali, M. An Experimental Study on the Performance of Calcium Carbonate Extracted from Eggshells as Weighting Agent in Drilling Fluid. Eng. Technol. Appl. Sci. Res. 2019, 9, 3859–3862. [Google Scholar] [CrossRef]
- Al-Hameedi, A.; Alkinani, H.; Dunn-Norman, S. Green Fluid Technology: How Food Wastes Can Revolutionize the Oil and Gas Industry; OnePetro: Richardson, TX, USA, 2021. [Google Scholar]
- Al-Hameedi, A.; Alkinani, H.H.; Dunn-Norman, S.; Hamoud, Z. Investigation Study of the Effectiveness of Eggshells Powder as a Multifunctional Eco-Friendly Additive in Water-Based Fluid; OnePetro: Richardson, TX, USA, 2020. [Google Scholar]
- Sid, A.N.E.H.; Kouini, B.; Abdelkrim, H.; Rabah, D.; Gherraf, N.; Bououdina, M. The Synergistic Effect of Algerian Na-Bentonite/Potato Starch/Grass Powder on the Enhancement of Aged Water-Based Drilling Fluids. Arab. J. Sci. Eng. 2022, 47, 11721–11732. [Google Scholar] [CrossRef]
- Sid, A.N.E.H.; Kouini, B.; Abdelkrim, H.; Gherraf, N.; Benmounnah, A.; Bououdina, M. Eco-Friendly Potato/Corn Starch Mediated Algerian Na-Bentonite as a Potential Water-Based Drilling Fluid. Pet. Sci. Technol. 2023, 1–16. [Google Scholar] [CrossRef]
- Cherifi, B.I.; Belbachir, M.; Rahmouni, A. Green Anionic Polymerization of Vinyl Acetate Using Maghnite-Na+ (Algerian MMT): Synthesis Characterization and Reactional Mechanism. Discov. Chem. Eng. 2021, 1, 5. [Google Scholar] [CrossRef]
- Li, Q.; Geng, H.; Shui, Z.; Huang, Y. Effect of Metakaolin Addition and Seawater Mixing on the Properties and Hydration of Concrete. Appl. Clay Sci. 2015, 115, 51–60. [Google Scholar] [CrossRef]
- Pinto, C.; Campelo, P.; Souza, S. Rietveld-Based Quantitative Phase Analysis of B-Type Starch Crystals Subjected to Ultrasound and Hydrolysis Processes. J. Appl. Polym. Sci. 2020, 137, 49529. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Bezzekhami, M.; Amine, H.; Belalia, M.; Mostefai, A.; Belkhir, N.; Bououdina, M. Green Synthesis of Starch Nanoparticles (SNPs) by Esterification with Rosin Acid Catalyzed by Maghnite-H+ (Algerian Montmorillonite) with Enhanced Antioxidant Activity. Arab. J. Sci. Eng. 2022, 48, 311–326. [Google Scholar] [CrossRef]
- Bezzekhami, M.; Belalia, M.; Hamed, D.; Bououdina, M.; Badredine, B.; Amine, H. Nanoarchitectonics of Starch Nanoparticles Rosin Catalyzed by Algerian Natural Montmorillonite (Maghnite-H) for Enhanced Antimicrobial Activity. J. Inorg. Organomet. Polym. Mater. 2022, 33, 1–14. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the ‘Debye-Scherrer Equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Lechtanski, V. Calcium Carbonate Content of Egg Shells; Oxford: New York, NY, USA, 2000; pp. 159–165. [Google Scholar]
- Chraibi, S.; Moussout, H.; Boukhlifi, F.; Ahlafi, H.; Alami, M. Utilization of Calcined Eggshell Waste as an Adsorbent for the Removal of Phenol from Aqueous Solution. J. Encapsulation Adsorpt. Sci. 2016, 6, 132–146. [Google Scholar] [CrossRef]
- Fertl Chilingar, G.V.; Robertson, J.O. Chapter 6 Drilling Parameters. InDevelopments in Petroleum Science; Chilingar, G.V., Serebryakov, V.A., Robertson, J.O., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 151–167. [Google Scholar]
- Zaman, T.; Mostari, M.; Al Mahmood, A.; Rahman, M.S. Evolution and Characterization of Eggshell as a Potential Candidate of Raw Material. Ceramica 2018, 64, 236–241. [Google Scholar] [CrossRef]
- Aroun, I. Mise En Forme Du Biopolymère Amidon, Corrélation Structure, Propriété et Application Dans l’élimination Du Cadmium Présent Dans l’eau. 2012. Available online: https://di.univ-blida.dz/jspui/handle/123456789/5047 (accessed on 4 January 2023).
- Mathew, S.; Abraham, T. Physico-Chemical Characterization of Starch Ferulates of Different Degrees of Substitution. Food Chem. 2007, 105, 579–589. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.; Tomkinson, J.; Hill, C. The Preparation and Characterisation of a Series of Chemically Modified Potato Starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Shujun, W.; Wenyuan, G.; Jia, W.; Xiao, P.-G. Crystallography, Morphology and Thermal Properties of Starches from Four Different Medicinal Plants of Fritillaria Species. Food Chem. 2006, 96, 591–596. [Google Scholar] [CrossRef]
- Borhade, A.; Kale, S. Calcined Eggshell as a Cost Effective Material for Removal of Dyes from Aqueous Solution. Appl. Water Sci. 2017, 7, 4255–4268. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Kunanuruksapong, R.; Sirivat, A. Preparation and Properties of Calcium Oxide from Eggshells via Calcination. Mater. Sci.-Pol. 2012, 30, 313–322. [Google Scholar] [CrossRef]
- Mohadi, R.; Anggraini, K.; Riyanti, F.; Lesbani, A. Preparation Calcium Oxide From Chicken Eggshells. Sriwij. J. Environ. 2016, 1, 32–35. [Google Scholar] [CrossRef]
- Jaiswal, K.; Dutta, S.; Pohrmen, C.; Verma, R.; Kumar, A.; Ramaswamy, A.P. Bio-Waste Chicken Eggshell-Derived Calcium Oxide for Photocatalytic Application in Methylene Blue Dye Degradation under Natural Sunlight Irradiation. Inorg. Nano-Met. Chem. 2020, 51, 1–10. [Google Scholar] [CrossRef]
- Lala, S.; Deb, P.; Barua, E.; Deoghare, A.B.; Chatterjee, S. Characterization of Hydroxyapatite Derived from Eggshells for Medical Implants. Mater. Today Proc. 2019, 15, 323–327. [Google Scholar] [CrossRef]
- Onolemhemhen, R.; Olamigoke, O.; Kaka, A.-Q.O. The Suitability of Egg Shell and Snail Shell Waste for PH and Mud Weight Enhancement of Water Based Drilling Mud. Pet. Coal 2019, 61, 371–376. [Google Scholar]



| Component | Amount | Function |
|---|---|---|
| Water | 330 mL | Base fluid |
| Na-Bentonite | 22.4 g | Viscosifier |
| NaOH | 1 g | Alkalinity control |
| KCl | 1 g | Inhibitor |
| CMC | 1 g | Viscosifier |
| Potato starch | 6.6 g | Filtration control |
| CC | (10, 20, and 30 g) | Weighting agent |
| ESP | (10, 20, and 30 g) | Weighting agent |
| Property | Plastic Viscosity (cP) | Yield Point (cP) | Gel0 (lb/100 ft2) | Gel10 (lb/100 ft2) | Fluid Loss (mL) | Filter Cake (mm) | Mud Density (ppg) | pH |
|---|---|---|---|---|---|---|---|---|
| Value | 11.3 | 28.1 | 15 | 21 | 6.5 | 1.7 | 7.7 | 9.16 |
| Sample | 2θ (°) | (hkl) | d hkl (Å) | D (nm) |
|---|---|---|---|---|
| Bentonite | 6.77 19.85 20.81 24.02 26.70 27.59 30.00 34.91 36.60 50.25 | (001) (110) (100) (011) (210) (220) (002) (200) (102) (112) | 13.07 4.45 4.33 3.78 3.43 3.32 3.08 2.69 2.58 2.00 | 4.30 13.93 4.28 5.14 11.36 14.05 7.15 6.07 14.36 10.79 = 9.14 |
| Na-Bentonite | 5.64 19.85 20.81 24.02 26.70 27.59 30.00 34.91 36.60 50.25 | (001) (110) (100) (011) (210) (220) (002) (200) (102) (112) | 15.68 4.45 4.33 3.78 3.43 3.32 3.08 2.69 2.58 2.00 | 5.23 10.59 4.28 5.14 11.34 12.35 8.75 5.10 14.26 11.39 = 8.84 |
| PV (cp) | YP (lb/100 ft2) | Gel0 (lb/100 ft2) | Gel10 (lb/100 ft2) | Fluid Loss (mL) | Mud Density (ppg) | Cake Thickness (mm) | pH | |
|---|---|---|---|---|---|---|---|---|
| References | 11.3 | 28.1 | 15 | 21 | 6.5 | 7.7 | 1.7 | 9.16 |
| CC | 21 | 30 | 15.4 | 21.3 | 6.1 | 8.4 | 1.5 | 9.17 |
| 23 | 32 | 18.3 | 24.6 | 5.8 | 9.1 | 1.1 | 9.18 | |
| 24 | 33 | 22.6 | 26.6 | 5.4 | 11.6 | 0.9 | 9.21 | |
| ESP | 11 | 20 | 18.2 | 22.3 | 6.3 | 7.9 | 1.5 | 9.16 |
| 19 | 16 | 26.4 | 28.4 | 6 | 8.2 | 1.3 | 9.17 | |
| 9 | 46 | 24.8 | 26.2 | 6.5 | 8.7 | 1.4 | 9.2 |
| Eco-Friendly Additive | PV (cp) | YP (cp) | Gels (lb/100 ft2) | Range of Weight (g) | Mud Density (ppg) | pH |
|---|---|---|---|---|---|---|
| ESP (Rita U et al.) [51] | - | - | - | 0–30 | Increase with the increase of the weight of ESP | Increase with the increase of the weight of ESP |
| ESP (Olamigoke et al.) [23] | Effective at low concentration (2 g) | Effective at low concentration (2 g) | Effective at low concentration (2 g) | 0–20 | - | - |
| ESP (Alhameedi et al.) [28] | Increase with both 0.75% and 1.5% of ESP | Increase with both 0.75% and 1.5% of ESP | Increase with both 0.75% and 1.5% of ESP | 0–1.5% | Increase with both 0.75% and 1.5% of ESP | Slight decrease |
| ESP (Iqbal et al.) [26] | Increase | increase | Increase | 275 g–410 g | Increase | Same values of CaCO3 |
| ESP (current work) | Effective for 10 g and 20 g | Effective for 10 g and 20 g | Effective for 10 g and 20 g | 10 g–30 g | Increase | Slight increase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sid, A.N.E.H.; Tahraoui, H.; Kebir, M.; Bezzekhami, M.A.; Kouini, B.; Hassein-Bey, A.H.; Selma, T.; Amrane, A.; Imessaoudene, A.; Mouni, L. Comparative Investigation of the Effect of EggshellPowder and Calcium Carbonate as Additivesin Eco-Friendly Polymer Drilling Fluids. Sustainability 2023, 15, 3375. https://doi.org/10.3390/su15043375
Sid ANEH, Tahraoui H, Kebir M, Bezzekhami MA, Kouini B, Hassein-Bey AH, Selma T, Amrane A, Imessaoudene A, Mouni L. Comparative Investigation of the Effect of EggshellPowder and Calcium Carbonate as Additivesin Eco-Friendly Polymer Drilling Fluids. Sustainability. 2023; 15(4):3375. https://doi.org/10.3390/su15043375
Chicago/Turabian StyleSid, Asma Nour El Houda, Hichem Tahraoui, Mohammed Kebir, Mohammed Amin Bezzekhami, Benalia Kouini, Amel Hind Hassein-Bey, Toumi Selma, Abdeltif Amrane, Ali Imessaoudene, and Lotfi Mouni. 2023. "Comparative Investigation of the Effect of EggshellPowder and Calcium Carbonate as Additivesin Eco-Friendly Polymer Drilling Fluids" Sustainability 15, no. 4: 3375. https://doi.org/10.3390/su15043375
APA StyleSid, A. N. E. H., Tahraoui, H., Kebir, M., Bezzekhami, M. A., Kouini, B., Hassein-Bey, A. H., Selma, T., Amrane, A., Imessaoudene, A., & Mouni, L. (2023). Comparative Investigation of the Effect of EggshellPowder and Calcium Carbonate as Additivesin Eco-Friendly Polymer Drilling Fluids. Sustainability, 15(4), 3375. https://doi.org/10.3390/su15043375






