Abstract
The corrosion behaviour of J55 steel in typical high-water-cut oil wells and the inhibition effects of different types of corrosion inhibitors were investigated. Using electrochemical experiments, the effects of temperature, Cl−, HCO3−, Ca2+, Mg2+ and pH on the corrosion of J55 steel were studied. Under experimental conditions, the corrosion rate slowed with increasing pH value when the temperature increased from 35 °C to 70 °C. The corrosion rate also increased first and then decreased with increasing Cl−, Ca2+ and Mg2+ ion concentrations, which notably affected the distribution of corrosion pits on the surfaces of the steel. The protection performances of various corrosion inhibitors and corrosion products were evaluated through electrochemical measurements. The results showed that the CT-2, UT2-2 and YC-2 inhibitors had obvious effects on the corrosion prevention of the J55 steel. In particular, the corrosion inhibition efficiencies of the three kinds of carbon steel were remarkable and exceeded 84% when the UT2-2 concentration reached 0.18 g/L.
1. Introduction
The evaluation of carbon steel corrosion in the produced water of crude oil is of great interest to the petrochemical industry due to costly economic and human losses [1]. Carbon steel is considered to be the most economical and common material used in the oil and gas industry [2,3]. However, the corrosion of carbon steel occurs frequently in harsh environments [4], which causes not only huge economic losses but also serious safety concerns. Specifically, most carbon steel pipes or tubes are buried underground [5,6]. The corrosion behaviours of carbon steel are generally affected by various factors [7], such as the pressure, temperature, ionic concentration, flow state or flow pattern in pipes [8,9]. A corrosion-resistant steel casing is the key to ensuring the safety and integrity of downhole tools in oil and gas wells [10]. It is essential to strengthen and protect the casing and other downhole tools in oil-water wells to avoid oil and gas leakage during production [11,12] and to ensure normal operation during the entire life of a production well [13,14,15].
Produced water is generally associated with crude oil from the oil reservoir going into the ground [16] and contains highly corrosive components, such as chlorides, acidic media and dissolved solids [17,18]. Strong acids are often pumped into wells to simulate production by increasing formation permeability in the near-wellbore region [19]. At present, to meet the increasing demand for global oil and gas resources, some oil and gas fields with severely corrosive environments have been continually exploited [20]. However, water cuts in oil and gas wells continue to increase during the production period [21,22]. The increasing severity of downhole tool damage in oil and gas fields has made their repair more challenging [23], and the number of accidents continues to rise every year [24], which can easily lead to the abandonment or closure of oil and gas wells [25]; disrupt the integrity of production and water injection well patterns [26]; and, finally, reduce the later exploitation efficiency of oil and gas fields, causing substantial losses of material and financial resources [27,28,29].
Since the corrosion failure of oil wells is a slow and long-term process under field conditions, it is considerably difficult to simulate actual production conditions via laboratory-scale tests in relatively short test durations [2,30]. Many scholars developed studies on the effects of HCO3− and Cl− on the corrosion characteristics of carbon steel, but most of the research is limited to neutral and acidic corrosive media that only contain a small amount of ions, and corrosive media are generally not prepared based on the actual ionic composition of the produced water [31,32,33]. Therefore, it is essential to investigate the corrosive behaviors of carbon steel tools under actual production conditions (water production wells).
The Xingzichuan area of Yanchang Oilfield is a typical “three low” oil field with low pressure, low permeability and low production. J55 low-carbon steel is widely used as a casing material. Its damage has always been a common problem in major oil regions. There are many reasons for the damage, and corrosion is one of the most important factors. The corrosion of downhole tools, including tubing, casing and sucker rods, is particularly serious and gravely threatens the normal production and safe operation of oilfields. Since 2012, the oil wells have been operated 11.4 times per year on average, and some wells have been operated as many as 21 times per year, with casing damage found in 60.2% of the total wells. Many scholars and field experts use the method of adding a corrosion inhibitor to prevent corrosion. In recent years, inorganic inhibitors were extensively investigated, and their mechanism of action can be attributed to the strong oxidative ability of the inhibitors [34,35].
In this study, the produced waters in Yanchang Oilfield and commonly used J55 steel were taken as the research objects. Through electrochemical experiments, the factors and rules that affect the corrosion of J55 steel were determined, and an efficient corrosion inhibitor was also screened out. The main innovation points were as follows: (1) The influential factors of J55 casing corrosion in the oil plant, including temperature and ionic composition of produced water, were clarified, and the influence rules of different factors were analyzed. (2) Based on the corrosion law and mechanism of the corrosion inhibitor, the suitable corrosion inhibitor and injection concentration were determined. It is expected that the results of the study provide important guidance for the development of anti-corrosion measures for downhole tools in the Yanchang and other similar oilfields.
2. Materials and Methods
2.1. Materials
Five types of produced waters were sampled from wells H132, W214-5, X124, H128 and X275 in Yanchang Oilfield. CO2 and H2S gases existed in the formation. The water cuts of the five oil wells were greater than 80%. The salinities and ion concentrations shown in Table 1 were measured using the petroleum and natural gas industry standard of China (SY/T 5523-2006). The ionic concentrations of HCO3−, Cl−, Na+ and K+ were much higher than those of CO32− and SO42− in the water samples. In particular, the Cl− concentration of the water from the H128 well was greater than 30 g/L, which may lead to severe corrosion of downhole tools [36]. All the salinities were greater than 20 g/L, and even the salinity of the water from the H128 well was greater than 50 g/L, which may also cause scale corrosion [37]. J55 casing, N80 tubing and grade D sucker rods are commonly used as downhole tool materials in Yanchang Oilfield, and the elemental compositions of the steel materials are shown in Table 2. In addition to Fe, the Mn contents of N80 tubing and grade D sucker rods were higher than those of other elements.

Table 1.
Quality of the produced water in typical oil wells, including the pH, ion concentration and salinity.

Table 2.
The chemical composition of the J55 casing, N80 tubing and grade D sucker rods.
2.2. Methods
2.2.1. Test Method Regarding Corrosion Characteristics
- (1)
- Material preparation: Steel samples of J55 and N80 were used as the materials of the oil casing and tubing pipes, respectively, and were polished using 400#, 800#, 1200# and 2000# sandpaper successively. The polished test pieces were put in a beaker filled with acetone and then wiped with absorbent cotton to remove impurities. The test pieces were immersed in absolute ethanol for 5 min and put on filter paper. Finally, the test pieces were kept in a drying oven for 24 h and weighed by an electronic balance with an accuracy of 1/10,000 g. The surfaces of the J55 steel were flat before corrosion took place, as shown in Figure 1.
 Figure 1. Surface morphology of a J55 casing test piece before corrosion took place.
Figure 1. Surface morphology of a J55 casing test piece before corrosion took place.
- (2)
- The corrosion products of a J55 casing in the Xingzichuan oil production plant were analyzed. The method of combining Quanta 450 environmental scanning electron microscope and X-ray energy spectrum analyzer (MX2) was used to analyze the surface morphology of test pieces before and after the indoor weight loss test and the composition of the corrosion products. The main components of the corrosion products were determined according to the experimental results to determine the cause of the J55 casing corrosion in the Xingzichuan oil production plant. A J55 casing taken from the Wangjiawan block of the Xingzichuan oil production plant due to corrosion failure was processed into test pieces, which were put into separate water samples of H132, W214-5, X124 and H128 blocks, with a temperature of 60 °C and static corrosion of 72 h.
- (3)
- Weight loss method: First, the test pieces were hung in wide-mouth bottles filled with corrosive media and kept in a constant-temperature water bath. Second, the test pieces were removed after the corrosion test, and the surfaces of the pieces were recorded using an HD camera. Third, the surfaces of the pieces were washed and wiped with filter paper, and then the test pieces were put in acetone and absolute ethanol to remove oil and water successively. Finally, the cleaning liquid (3.36% HCl and 0.5% HMTA) was used to clean the test pieces for 3–5 min and immersed in absolute ethanol for 5 min to remove the water. The test pieces were put on filter papers for air drying and weighing after 12 h.
- (4)
- Data processing: the average corrosion rate was calculated using Equation (1) [38]:where Vcorr is the average corrosion rate of a test piece, mm/a; m0 and m1 are the masses of a test piece before and after the test, respectively, g; S is the area of a test piece, cm2; ρ is the density of a test piece, g/cm3; t is the test time, h; and 8.76 × 104 is the coefficient that converts corrosion quality into corrosion depth.
2.2.2. Corrosion Inhibitor Evaluation
Based on the corrosion data from the Yanchang Oilfield, ten kinds of corrosion inhibitors were screened, as shown in Table 3.

Table 3.
Ten kinds of corrosion inhibitors used in the experiments and the main component of each one.
The weight loss method was used for the corrosion test of J55 steel. The produced water from the W214-5 well was taken as the corrosive medium. The corrosion inhibitors in Table 3 were added with a concentration of 50 g/L, and all the tests were conducted at 60 °C for 72 h. The performance of a corrosion inhibitor was expressed as , which was calculated using Equation (2) or Equation (3):
where η is the inhibition efficiency of corrosion, %; ∆m0 and ∆m1 are the masses of a test piece before and after adding an inhibitor, respectively, g; and ∆V0 and ∆V1 are the corrosion rates of a test piece before and after adding an inhibitor, respectively, mm/a.
2.3. Electrochemical Measurements
Electrochemical tests were conducted to evaluate the corrosion characteristics and corrosion inhibitor effect using a CS310 electrochemical workstation at 25 °C. Steel samples covered with thin or thick corrosion scales were obtained from the produced water environment of the oil field, and bare carbon steel was chosen for comparative experiments. The steel samples were cut into 10 mm × 10 mm × 10 mm cubes for the preparation of the electrode. Then, the cubes were welded with wire, and the edges of the specimens were sealed with epoxy for electrochemical measurements. The entire electrode was sealed with epoxy resin, and only a 1.0 cm2 working area was exposed. The bare steel electrode was polished and smoothed with 400#, 800# and 1200# metallographic papers successively; rinsed with deionized water; degreased with petroleum ether, alcohol and acetone; and air-dried. The electrochemical measurements were performed in a three-electrode cell system that included a reference electrode (mercury oxide electrode), an auxiliary electrode (platinum electrode) and a working electrode (J55 steel electrode). The scanning rate of the potentiodynamic potential was 0.5 mV/s, the frequency range of electrochemical impedance (EIS) measurement was 5–100 kHz and the measurement signal was a sine wave with an amplitude of 5 mV.
3. Results and Discussion
3.1. Factors That Affected the Corrosion
The surfaces of the J55 steel were covered with reddish-brown corrosion products, and the structure was loose, as shown in Figure 2.

Figure 2.
Macro-appearance of J55 steel specimens after static corrosion: (a) H132; (b) W214-5; (c) X124; (d) H128.
The local morphology analysis results are shown in Figure 3. The corrosion products on the surface were granular and loose in structure. After removing the corrosion products on the surface, there was obvious local corrosion on the surface of the test piece and the sizes were different.
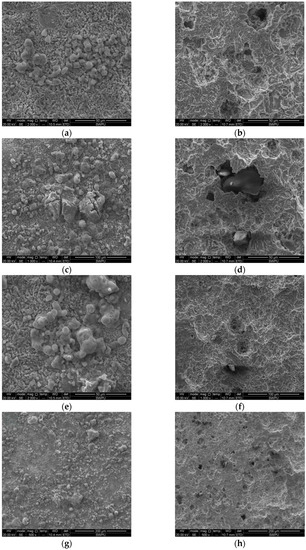
Figure 3.
Local corrosion morphologies of J55 steel before and after the removal of corrosion products: (a,b) H132 before and after removing the corrosion products; (c,d) W214-5 before and after removing the corrosion products; (e,f) X124 before and after removing the corrosion products; (g,h) H128 before and after removing the corrosion products.
Table 4 shows the corresponding energy spectrum analysis results. The contents of carbon, oxygen, sulfur and chlorine were relatively high, and thus, it can be judged that the corrosion products on the J55 steel were mainly iron carbonate and sulfide. According to the characteristics of oil well water quality and corrosion products, the J55 steel corrosion was local corrosion caused by CO2 and H2S. CO2 and H2S can bring great changes to the pH of a solution. In addition, HCO3−, Cl−, Ca2+ and Mg2+ also have an important impact on corrosion.

Table 4.
Element composition analysis of the corrosion products of the J55 steel.
3.2. Effects of Different Factors on the Corrosion Rate
Taking carbon J55 steel as the study object, the weight loss test and electrochemical test were conducted to investigate the influences of the temperature, Cl−, HCO3−, Ca2+, Mg2+ and pH on the corrosion characteristics of the steel sample.
3.2.1. Effect of the Temperature
The formation temperature in Yanchang Oilfield generally varies between 40 °C and 65 °C. Thus, the indoor test temperatures were set between 35 and 70 °C. The produced waters from the H132, W214-5, X124 and H128 wells were taken as the corrosive media. The experimental period was 72 h.
The effect of temperature on the corrosion rate of the J55 steel is shown in Figure 4. The corrosion rates of the J55 steel increased with increasing temperature in the range of 35–70 °C. The reason for this may be that with the increase in temperature, the activation dissolution rate of the metal increased and the corrosion loss accelerated [39]. In addition, the decomposition of bicarbonate was accelerated to produce more CO32− [40].
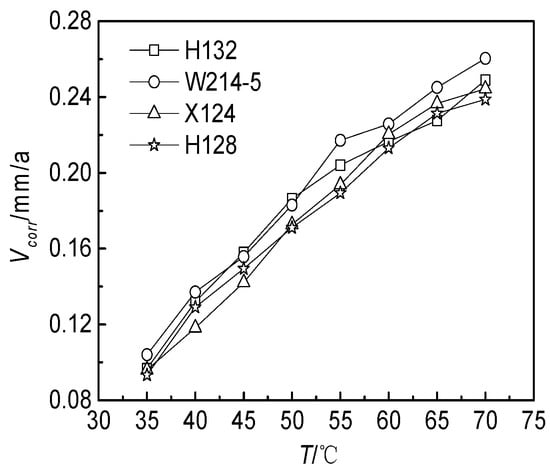
Figure 4.
Effect of temperature on the corrosion rates of the J55 steel in 4 kinds of produced waters.
Because the corrosion of the samples exposed to water from the W214-5 well in the Wangjiawan block was more serious than that of the other samples, the produced water from W214-5 well was used as a representative corrosive medium. The electrochemical test results of the J55 steel in the produced water at different temperatures are shown in Figure 5. Figure 5a shows that the corrosion potential of J55 steel in the water shifted negatively first and then became more positive, while the corrosion current increased first and then decreased as the temperature increased from 30 to 90 °C. Both the corrosion potential and current reached the most negative values at 80 °C. Below this temperature, the corrosion rate of the J55 steel increased as the temperature increased, but when the temperature was above 80 °C, the corrosion rate slowed with a continuous increase in temperature [41]. Figure 5b shows that the Nyquist plot of the J55 steel at various temperatures was a time-constant single-capacitance arc, whose diameter decreased as the temperature increased to 80 °C. Figure 5c shows that the Bode curves were upturned in the low-frequency region above 60 °C, which implied the generation of Warburg impedance, and the active polarization-controlled corrosion changed to active material diffusion-controlled corrosion [42].
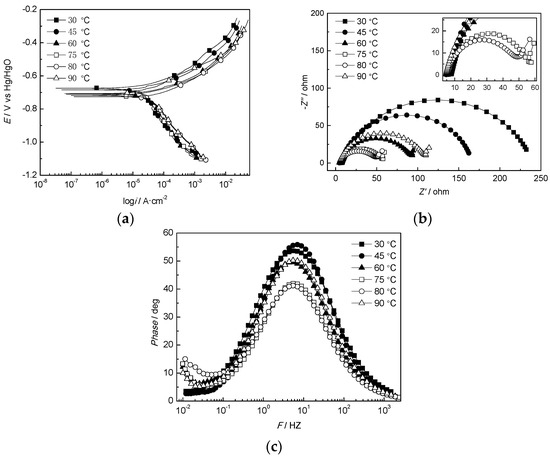
Figure 5.
Electrochemical behaviour of the J55 steel in the produced water obtained from the W214-5 well at various temperatures: (a) polarization curves of the J55 steel at various temperatures; (b) Nyquist plots of the J55 steel at various temperatures; (c) Bode curves of the J55 steel at various temperatures.
Benamor et al. proposed the idea that an increase in temperature could enhance the activities of ions in produced water from oil wells, leading to an increased corrosion rate of the steel [27]. The fitting results of equivalent circuit elements of the J55 steel from W214-5 water are shown in Figure 6 and Table 5, wherein Rs is the solution resistance, Rct is the charge transfer resistance, CPEdl is the constant phase element, Y is a constant, n is a deviation coefficient and Zw is the Warburg impedance. It can be found that Rs decreased with increasing temperature, indicating that increasing temperature could enhance the conductivity of the solution. The trend of Rct with temperature was similar to that of the capacitive reactance arc diameter. Warburg impedance began to occur as the temperature increased to 60 °C, which showed that the corrosion product film formed on the surface of the electrode specimen started to affect the electrochemical reaction. As the temperature increased from 60 to 80 °C, the capacitive reactance arc diameter continued to decrease to the minimum value, which indicated that the corrosion product film formed in this temperature range did not protect the J55 steel. However, the arc diameter began to increase, and the corrosion product film could protect the J55 steel above 80 °C. In addition, the Warburg resistance did not change obviously with temperature. This implied that the temperature had little effect on the Warburg resistance [43].
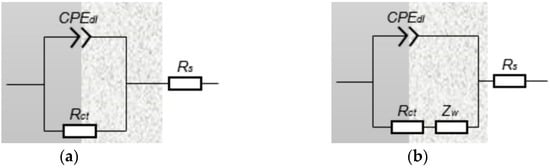
Figure 6.
Equivalent circuit diagram of the electrochemical impedance spectrum of the J55 steel at different temperatures. (a) 30 °C ≤ T < 75 °C; (b) 75 °C ≤ T ≤ 90 °C.

Table 5.
Fitting results of equivalent circuit elements of the system at various temperatures.
3.2.2. Effect of the Cl− Concentration
Based on the ion concentration of the produced water from the W214-5 well, the corrosion media were prepared with distilled water and analytical reagents. The concentrations of CO32−, HCO3−, SO42−, Ca2+ and Mg2+ were maintained at 0.12, 0.8, 0.02, 0.7 and 0.2 g/L, respectively, and the Cl− concentration was set at 2, 5, 10, 15, 20 and 25 g/L. The effect of the Cl− concentration on the corrosion rate of the J55 steel was studied at 60 °C for 72 h. As shown in Figure 7, the corrosion rate of J55 steel gradually increased and reached a maximum at a Cl− concentration of 10 g/L after increasing from 2 to 10 g/L. Once the Cl− concentration was higher than 10 g/L, the corrosion rate began to decrease.
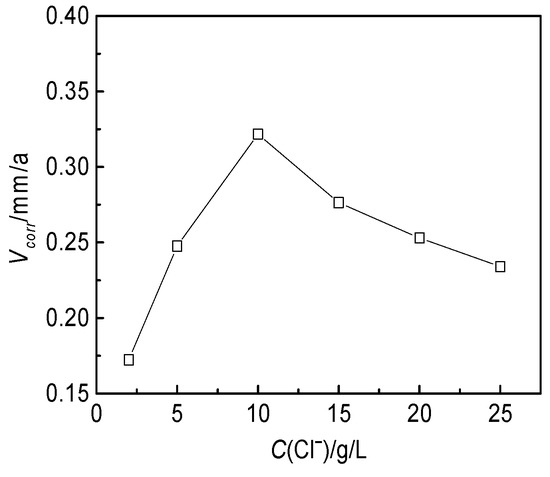
Figure 7.
Effect of the Cl− concentration on the corrosion rate of the J55 steel in the W214-5 well water.
The results of equivalent circuit elements of J55 steel in the produced water from the W214-5 well at various Cl− concentrations are shown in Figure 8 and the fitting circuit at different Cl− concentrations is shown in Figure 6a. The quantity of Rct reached the minimum at 10 g/L, which was consistent with the measured result of the polarization curves.
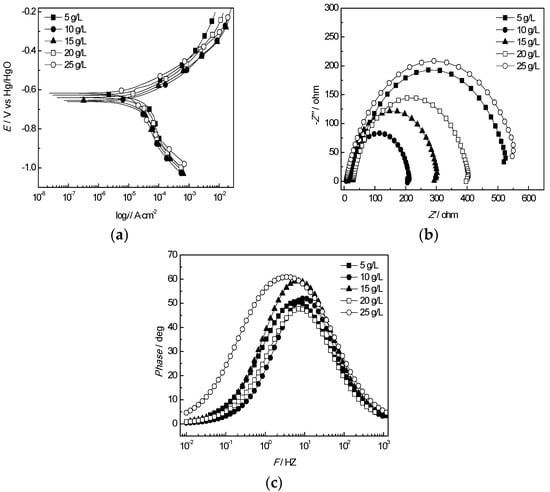
Figure 8.
Electrochemical behaviour of the J55 steel in the produced water from the W214-5 well at various Cl− concentrations: (a) polarization curves of the J55 steel at various Cl− concentrations; (b) Nyquist plots of the J55 steel at various Cl− concentrations; (c) Bode curves of the J55 steel at various Cl− concentrations.
With the corrosion process, the corrosion products on the surface of J55 steel would gradually thicken and play a protective role [44]. The conductivity of the solution increased with increasing Cl− concentration, and the radius of Cl− with strong penetrability is relatively small when its concentration is less than 10 g/L. Under the action of an electric field, Cl− could immigrate in the corrosion products, adsorb on the steel surface and combine with Fe2+ to generate FeClOH, which could promote an anodic reaction [45]. Finally, the corrosion product film was damaged [46]. The strong adsorption capacity of Cl− could cause it to occupy additional cathode active sites when the Cl− concentration exceeded 10 g/L, resulting in a decrease in the concentration of the cathode depolarizer HCO3−. The addition of a large amount of NaCl could increase the ion strength of the solution and inhibit the ionization of H2CO3, leading to a decrease in the corrosion rate, which is consistent with the phenomenon found in [47].
3.2.3. Effect of the HCO3− Concentration
The effect of the HCO3− concentration on the J55 steel was also investigated at 60 °C for 72 h. The concentrations of CO32−, Cl−, SO42−, Ca2+ and Mg2+ were set at constant values of 0.12, 17.0, 0.02, 0.7 and 0.2 g/L, respectively, and the HCO3− concentration varied from 0.2 to 1.5 g/L. The corrosive media were prepared with distilled water and analytical reagents according to the ion concentrations of the produced water from the W214-5 well. A group of parallel experiments was set for each test. The effect of HCO3− concentration on the corrosion of the J55 steel is shown in Figure 9. The corrosion rate of the J55 steel increased rapidly as the HCO3− concentration increased from 0.2 to 0.8 g/L and plateaued as the concentration reached 1.5 g/L.
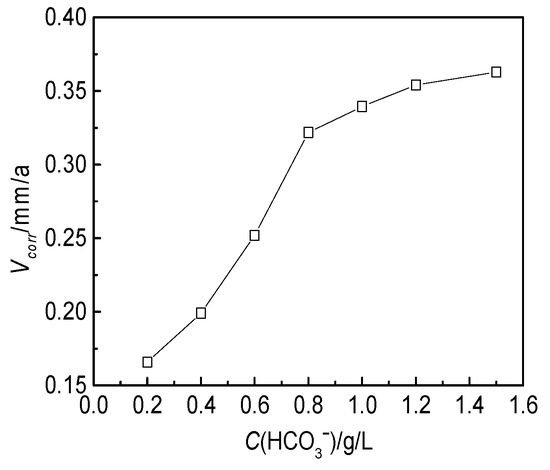
Figure 9.
Effect of the HCO3− concentration on the corrosion rate of the J55 steel in the W214-5 well water.
The polarization curves of the J55 steel are shown in Figure 10a. As the HCO3− concentration increased from 0.5 to 7 g/L, both the cathode and anode reactions were accelerated, the corrosion potential gradually shifted negatively and the corrosion current continued to increase. When the HCO3− concentration increased from 7 to 10 g/L, the anode polarization curves became passivated, the corrosion potential began to shift positively and the corrosion current decreased, which indicated that the corrosion of J55 steel can be promoted when the HCO3− concentration is less than 7 g/L. Otherwise, the corrosion could be suppressed. The results of equivalent circuit elements of the J55 steel in the produced water from the W214-5 well at different concentrations of HCO3− are presented in Figure 10b,c. In addition, the fitting circuit at different HCO3−concentrations is as the same as that at different temperatures (Figure 6). Warburg impedance appeared at 5 g/L, which showed that the corrosion product film formed on the surface of the J55 steel electrode specimen began to affect the electrochemical reaction. Rct reached the minimum value at 7 g/L and began to increase as the concentration surpassed 7 g/L, which verified a certain protection effect of the corrosion product film. This may have been because a low concentration of HCO3− could be used as a cathode depolarizer, similar to O2 and H+ ions, to accelerate the cathode reaction. However, the corrosion rate increase of the J55 steel slowed because a high concentration of HCO3− could react with iron on the steel surface and form a passivation film to keep the steel in a passivated state [48]. Wright et al. also found a decrease in corrosion growth magnitude, which was correlated with the increasing concentration of HCO3− as reactive ions [49].
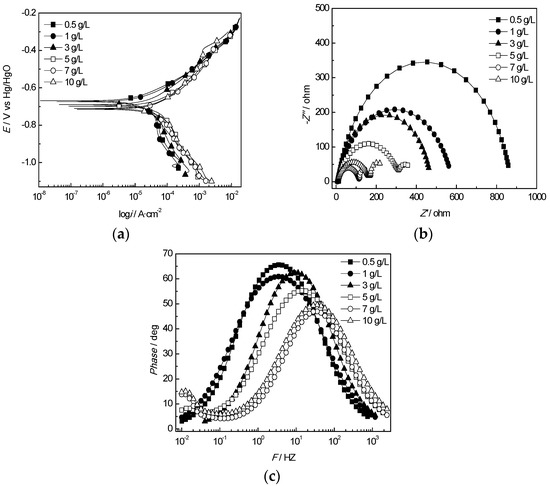
Figure 10.
Electrochemical behavior of the J55 steel in the produced water from the W214-5 well at various HCO3− concentrations: (a) polarization curves of the J55 steel at various HCO3− concentrations; (b) Nyquist plots of the J55 steel at various HCO3− concentrations; (c) Bode curves of the J55 steel at various HCO3− concentrations.
3.2.4. Effects of the Ca2+ and Mg2+ Concentrations
Cations such as calcium ions (Ca2+) and magnesium ions (Mg2+) also have a potential effect on the corrosion of downhole tools. The concentrations of CO32−, HCO3−, Cl− and SO42− were maintained at 0.12, 0.8, 17.0 and 0.02 g/L, respectively. Six types of corrosive media were prepared with a constant concentration of Mg2+ (0.2 g/L) and concentrations of Ca2+ varying from 0.3 to 2.0 g/L, and another six types were prepared with a fixed concentration of Ca2+ (0.7 g/L) and concentrations of Mg2+ in the range of 0.1–0.6 g/L. The corrosive media were also prepared with distilled water and analytical reagents, and the test temperature and time were kept the same as those in the above tests.
As shown in Figure 11, the corrosion rate of the J55 steel first increased and then decreased with the increases in the Ca2+ and Mg2+ concentrations. The maximum value was obtained when the Ca2+ concentration was 1.2 g/L and the Mg2+ concentration was 0.3 g/L due to the increase in the hardness and ionic strength of the aqueous solution [50].
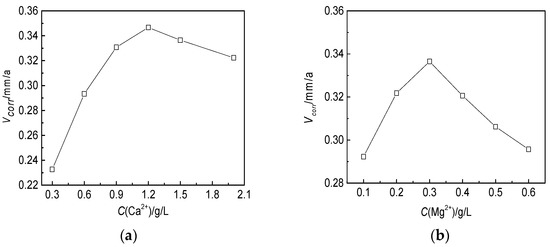
Figure 11.
Effects of the Ca2+ and Mg2+ concentrations on the corrosion rate of the J55 steel in W214-5 well water: (a) corrosion rate of the J55 steel at different Ca2+ concentrations; (b) corrosion rate of the J55 steel at different Mg2+ concentrations.
The results of equivalent circuit elements of the J55 steel in the produced water from the W214-5 well at different concentrations of Ca2+ and Mg2+ are shown in Figure 12 and Figure 13. As shown in Figure 12a and Figure 13a, consistent with the weight loss test results, the corrosion current of the J55 steel increased first and then decreased with the increases in the Ca2+ and Mg2+ concentrations. The fitting circuit at different Ca2+ and Mg2+ concentrations is shown in Figure 14. Rct decreased first and then increased with increasing Ca2+ or Mg2+ concentrations, and the corrosion rate reached the minimum value at a Ca2+ concentration of 1.2 g/L and Mg2+ concentration of 0.3 g/L. Therefore, the variation in the concentrations of Ca2+ and Mg2+ in the corrosive medium had an obvious effect on Rct.
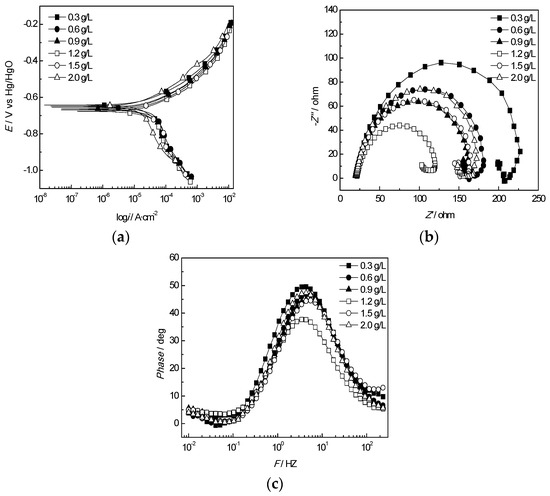
Figure 12.
Electrochemical behavior of the J55 steel in the produced water from the W214-5 well at various Ca2+ concentrations: (a) polarization curves of the J55 steel at various Ca2+ concentrations; (b) Nyquist plots of the J55 steel at various Ca2+ concentrations; (c) Bode curves of the J55 steel at various Ca2+ concentrations.
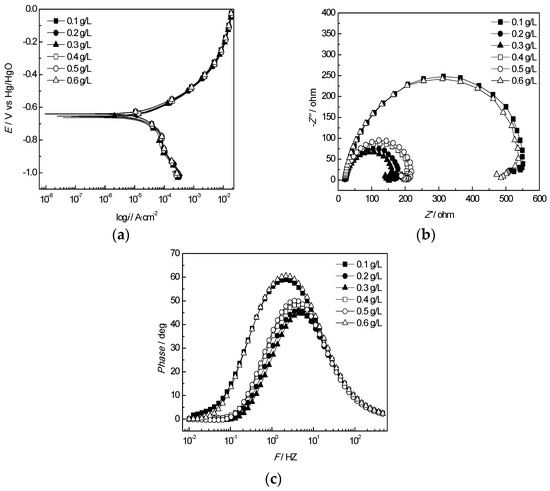
Figure 13.
Electrochemical behavior of the J55 steel in the produced water from the W214-5 well at various Mg2+ concentrations: (a) polarization curves of the J55 steel at various Mg2+ concentrations; (b) Nyquist plots of the J55 steel at various Mg2+ concentrations; (c) Bode curves of the J55 steel at various Mg2+ concentrations.
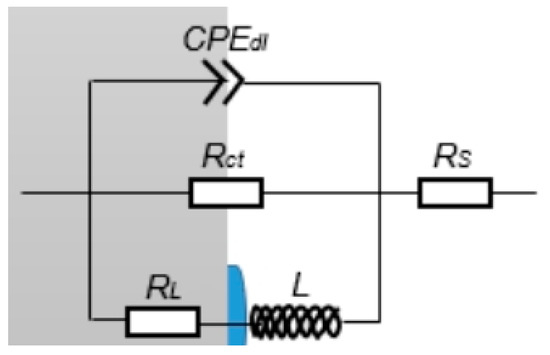
Figure 14.
Equivalent circuit diagram of the electrochemical impedance spectrum of the J55 steel in different concentrations of Ca2+ and Mg2+ solutions.
The increases in the Ca2+ and Mg2+ concentrations could intensify the scaling tendency of the solution and cause corrosion under the scale [51]. However, galvanic corrosion may form between the product film and the exposed metallic substrate, which could accelerate metallic substrate corrosion. Nevertheless, as the concentrations of Ca2+ and Mg2+ continued to increase, a complete and dense protective film gradually formed on the metallic surface, which could reduce the corrosion rate [52].
3.2.5. Effect of the pH Value
The pH value of the produced water from the W214-5 well was adjusted with NaOH and acetic acid. The corrosion rate of the J55 steel in the pH range of 5–10 was measured at 60 °C. A group of parallel experiments was designed for each test. The macromorpholgies of the test pieces after corrosion at different pH values and the effects of pH on the corrosion rate are shown in Figure 15. The corrosion rate of the J55 steel decreased as the pH value of the corrosion medium increased. A rapid decrease appeared when the pH value increased from 5 to 7, but the declining rate was moderated as the pH value exceeded 7 due to the effective cathode depolarization of hydrogen ions. The lower the pH value, the higher the corrosion rate.
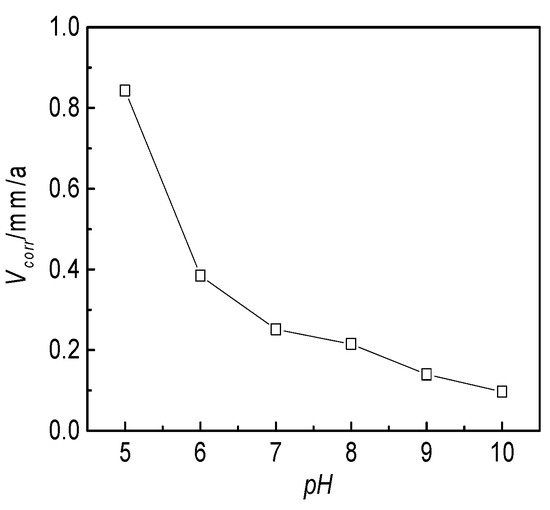
Figure 15.
Effect of the pH value on the corrosion rate of the J55 steel pieces in the W214-5 well water.
Figure 16 shows the electrochemical test results of the J55 steel in corrosive media with different pH values. In Figure 16a, it is shown that both the corrosion currents of the anode and cathode decreased and the corrosion potential shifted positively as the pH value increased. The minimum and maximum corrosion currents were 5.01 and 24.38 µA, corresponding to pH values of 10 and 5, respectively. In the experimental corrosive environment, the greater the pH value was, the lower the concentration of the cathode depolarizer H+. Thus, the corrosion rate of the J55 steel decreased. In addition, increasing the pH value was good for the passivation of the anode. The Nyquist plot of the J55 steel and the Bode phase diagram in the corrosive media with different pH values are shown in Figure 16b,c, respectively. The Nyquist plot exhibits a single-capacitance arc resistance characteristic with a time constant as the pH value varied from 5 to 9 and a dual-capacitance arc resistance characteristic with a double time constant at a pH value of 10. The capacitive arc was caused by the double electron layer in the high-frequency region and the corrosion product film in the low-frequency region. The equivalent circuit diagram of the electrochemical impedance spectrum of the J55 steel in pH range of 5-9 is as the same as that at temperatures ranging from 30 to 75 °C (Figure 6a). It can be found that Rct increased with the pH value, and the corrosion rate increased when the pH value exceeded 9. As shown in Figure 17, maximum CPEf and Rp values appeared at a pH value of 10, which indicated that a loose corrosion product film easily formed on the surface of the substrate.
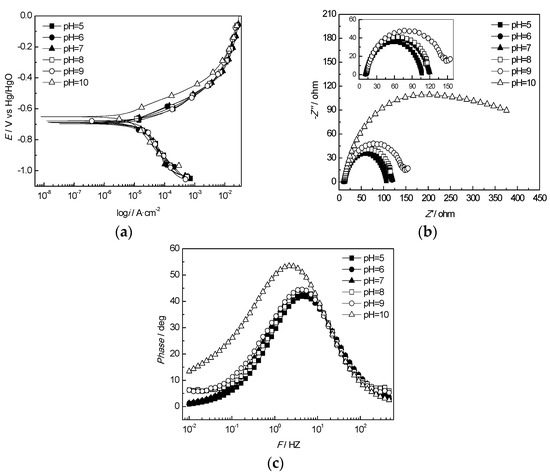
Figure 16.
Electrochemical test of the J55 steel in different pH solutions in the W214-5 well-produced water: (a) polarization curves of the J55 steel at different pH values; (b) Nyquist plots of the J55 steel at different pH values; (c) Bode curves of the J55 steel at different pH values.
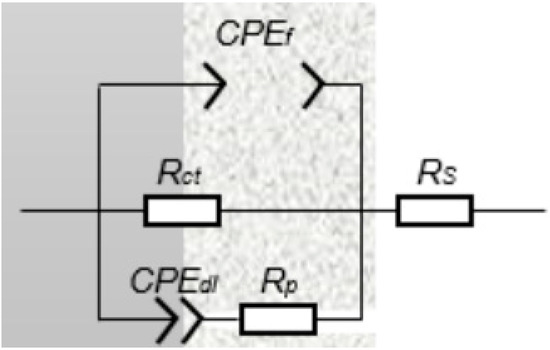
Figure 17.
Equivalent circuit diagram of the electrochemical impedance spectrum of the J55 steel at pH = 10.
3.3. Evaluation of the Corrosion Inhibitor Effects
The corrosion inhibition effect of inhibitors can be evaluated using the corrosion inhibition efficiency. The corrosion inhibition efficiencies of ten corrosion inhibitors at the same concentration are shown in Table 6. At a concentration of 50 mg/L, three corrosion inhibitors, namely, CT-2, UT2-2 and YC-2, showed the highest corrosion inhibition efficiency (>60%) for the J55 steel, followed by KHS-1 and CRS2-4, and the corrosion inhibition effects of the remaining inhibitors were relatively low. Therefore, the corrosion inhibitors CT-2, UT2-2 and YC-2 were taken as preselected corrosion inhibitors.

Table 6.
Inhibition effects of ten types of inhibitors at 0.05 g/L on the J55 steel in the X275 produced water.
3.3.1. Polarization Curves
Three corrosion inhibitors, namely, CT-2, UT2-2 and YC-2, were combined, and the produced water from the X275 well was selected as the corrosive medium. Two combinations were investigated: one combination was that of inhibitors CT-2 and UT2-2 in ratios of 2:1, 1:1 and 1:2, and the other combination was a mixture of CT-2 and YC-2 in the same ratios listed above. The total concentrations of the inhibitors were fixed at 0.3 g/L.
After the addition of the CT-2 and UT2-2 inhibitors, the polarization curves of the J55 steel in X275 well-produced water were obtained and are shown in Figure 18. It was found that the inhibitors had a remarkable effect on the corrosion potential. When 0.3 g/L CT-2 or 0.3 g/L UT2-2 was added, almost no change in the corrosion potential occurred. However, the polarization curves moved significantly in an upward left direction, and the current densities of the cathode and anode decreased after combining the inhibitors, indicating that the corrosion inhibition effect could be significantly improved when the compound inhibitors were combined.
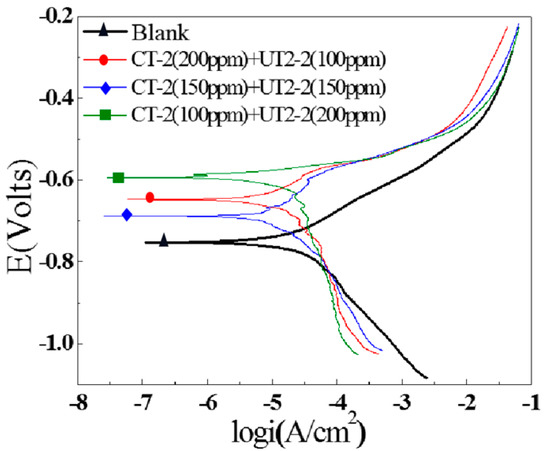
Figure 18.
Polarization curves of the J55 steel with different ratios of CT-2 and UT2-2.
When the inhibitors CT-2 or UT2-2 were added separately, the surface coverage effect played a leading role in the corrosion inhibition. However, the anode corrosion process was mainly suppressed, and the “negative catalytic effect” was dominant when the two inhibitors were added together. Moreover, it can be observed from Figure 19 that a “platform” appeared on the anode polarization curve when CT-2 and UT2-2 were added together, which implied that anode desorption occurred. Among the three compounding ratios, the anode adsorption potential range was the largest, and the metal anode became more stable when an inhibitor ratio of 1:1 for CT-2 and UT2-2 was implemented. When the inhibitor ratio was 1:2, the smallest slope of the anode polarization curve was obtained; the anode corrosion current increased sharply; and the anode was unstable, as the potential change was small. After strong polarization, the anodic polarization curves with added corrosion inhibitor nearly coincided with the polarization curves of the blank solution. The cathodic reaction slowed down obviously, especially under CT-2 (200 ppm) + YC-2 (100 ppm). A corrosion inhibitor mainly blocks the material transfer of a cathodic reaction to achieve a corrosion inhibition effect [53].
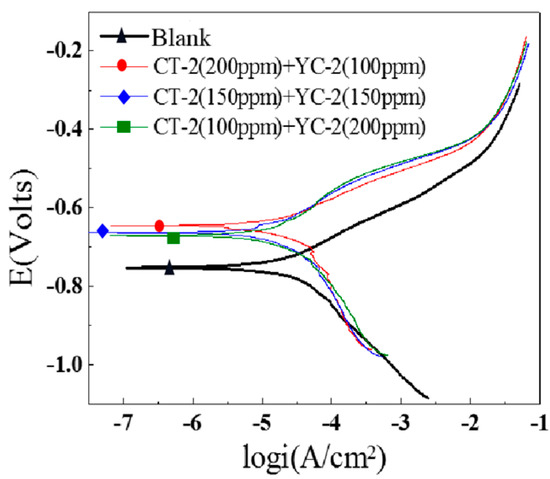
Figure 19.
Polarization curves of the J55 steel with different ratios of CT−2 and YC−2.
The polarization curves of the J55 steel in the X275 well-produced water with different ratios of CT−2 and YC−2 are shown in Figure 16. The three polarization curves tended to coincide when the inhibitors CT−2 and YC−2 were combined at all three ratios, which indicated that the ratio of CT−2 and YC−2 had little effect on mitigating corrosion. However, compared with the two single inhibitors, the combination showed a synergistic effect.
The corrosion potentials of the J55 steel at different combined concentrations are represented in Table 7. The corrosion potentials with different combined inhibitor concentrations were higher than those of the blank group. The minimum corrosion potential difference was 64 mV when CT-2 and UT2-2 were combined at a ratio of 1:1.

Table 7.
Corrosion potential of the J55 steel with different compounds.
3.3.2. EIS Measurement
CT-2 and UT2-2 were added into the produced water of the X275 well; the associated EIS curves of the J55 steel are shown in Figure 20. As seen in Figure 20a, the capacitance resistance arcs of the waters with the combined inhibitors were obviously smaller than those with CT-2 alone at the concentration of 0.3 g/L, which showed that the inhibition effect of the combined inhibitors was not as good as that of the single inhibitor CT-2. In the high-frequency region, the shape of the impedance arc curve was similar, but the size was different, indicating that the addition of the corrosion inhibitor increased the reaction resistance. In the low-frequency region, except for the blank group and CT-2 (300 ppm), the second arc was formed. This showed that the addition of the corrosion inhibitor and material transfer needed to be carried out through the pores in the inhibitor film [53,54]. When CT-2 (300 ppm) was added, the curve shape was consistent with that of the blank group, and only the arc radius was enlarged, that is, the mass spectrum corrosion mechanism did not change when two compounds were added, only the reaction resistance was increased [55,56]. From the EIS curves in Figure 20b, it can be seen that the system with concentration ratios of 1:2 and 1:1 for CT-2 to UT2-2 had three time constants; meanwhile, with a ratio of 2:1, it only had two time constants.
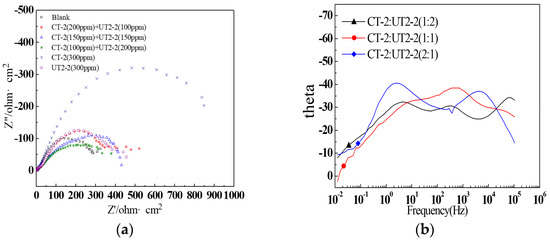
Figure 20.
EIS curves of the J55 steel in the X275 produced water with different concentrations of CT-2 and UT2-2: (a) impedance Nyquist curves; (b) impedance phase curves.
As shown in Figure 21, two different equivalent circuits were fitted to the systems with three and two time constants. The inhibition efficiencies with three concentration ratios shown in Table 8 were calculated based on the charge transfer resistance Rct. The highest inhibition efficiency was only 38.4%, corresponding to the system with a ratio of 2:1 for CT-2 to UT2-2. In addition, the inhibition efficiency increased with the increasing compound proportion of the inhibitor CT-2, which showed a dominating effect. The three-time constants represented the electrochemical elements related to the corrosion of surface films, inhibitor coatings and metallic substrates/solution interfaces [57].
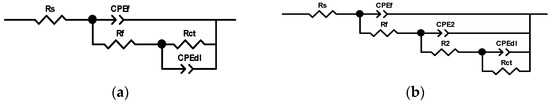
Figure 21.
Equivalent circuits for the systems with concentration ratios of CT-2 and UT2-2: (a) three time constants for the 1:2 and 1:1 ratios; (b) two time constants for the 2:1 ratio.

Table 8.
EIS fitting parameters of the systems with concentration ratios of CT-2 and UT2-2.
The impedance spectra of the systems with different concentrations of CT-2 and YC-2 were obtained via EIS testing and are shown in Figure 22. The Nyquist diagrams seemed to be composed of a small arc and a long diagonal line. However, the oblique line could not reach the condition of the diffusion process (45° oblique line), which only represented a circular arc with a relatively large diameter. Therefore, the Nyquist diagrams were still composed of capacitive reactance arcs. From the EIS phase diagram (Figure 22b), we can see that the curves of the systems with three concentration ratios had two-time constants, but the curve of the system with the ratio of 1:1 for CT-2 to YC-2 was not obvious.
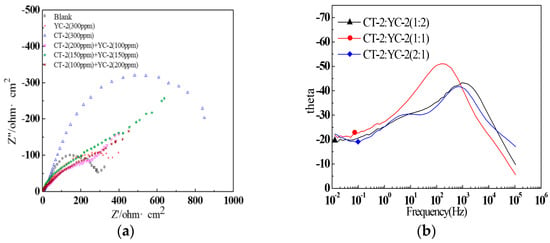
Figure 22.
EIS of the J55 steel in the X275 produced water with different concentrations of CT-2 and YC-2: (a) impedance Nyquist curves; (b) impedance phase curves.
The equivalent circuit with two time constants is as the same as Figure 21a, which was fitted based on the impedance spectra of the systems with concentration ratios of CT-2 and YC-2. The EIS parameters are listed in Table 9. The inhibition efficiency of the systems with a concentration ratio of 1:1 reached the highest value of 59.8% and was higher than that of the systems with CT-2 or YC-2 alone at the same concentration of 0.3 g/L. This also verified the synergistic effect of CT-2 and YC-2.

Table 9.
EIS fitted parameters of the systems with concentration ratios of CT-2 and YC-2.
3.3.3. Comparison of the Inhibition Effect of UT2-2 for J55, N80 and Grade D
For the system of the J55 steel in the water produced from the X275 well, the inhibitor UT2-2 showed an optimal corrosion inhibition effect among the studied inhibitors. Therefore, UT2-2 could be regarded as a preselected reagent in subsequent studies.
The corrosion results of the three kinds of carbon steel pieces in water with UT2-2 are shown in Table 10. The corrosion rates decreased gradually with increasing UT2-2 concentration, and the inhibition efficiencies also showed an increasing trend. In particular, the brightness of the three kinds of steel pieces significantly improved, and the rust spots on the surfaces of the pieces were obviously reduced. The corrosion inhibition efficiency exceeded 69% when the concentration of UT2-2 reached 0.1 g/L. Furthermore, the corrosion inhibition efficiencies of the three kinds of carbon steel were remarkable and exceeded 84% when the UT2-2 concentration reached 0.18 g/L.

Table 10.
Inhibition effect of UT2-2 on the three kinds of carbon steel (J55, N80 and grade D).
4. Conclusions
In this study, the main factors affecting the J55 steel corrosion in Yanchang Oilfield were identified through corrosion experiments. The influences of different factors on corrosion were studied, and the efficient corrosion inhibitor was optimized.
- (1)
- The morphology and element analysis of corrosion products of the J55 steel were carried out using a scanning electron microscope. The corrosion product film was loose and there were elements such as C, O and Ca2+. After combining the characteristics of the produced water, the main corrosion factors that affected the J55 steel were pH, HCO3−, Cl−, Ca2+ and Mg2+.
- (2)
- Based on the electrochemical experiment, the effects of temperature, Clˉ, HCO3−, Ca2+, Mg2+ and pH on the corrosion of the J55 steel were studied. Under experimental conditions, the corrosion rate slowed with increasing pH value when the temperature increased from 35 °C to 70 °C. The corrosion rate also increased first and then decreased with increasing Cl−, Ca2+ and Mg2+ ion concentrations, which notably affected the distribution of corrosion pits on the surfaces of the steel.
- (3)
- The inhibition effects of different inhibitors on the J55 steel were evaluated through corrosion experiments, and the inhibitor UT2-2 with the optimal comprehensive performance was screened. The distributions of corrosion pits on the surfaces of the downhole carbon steel were notably affected by the ion concentrations in the produced water. Three types of inhibitors, namely, CT-2, UT2-2 and YC-2, were evaluated. Furthermore, a concentration ratio of 1:1 for CT-2 and UT2-2 showed a prominent film-forming performance. In particular, the corrosion inhibition efficiencies of the three kinds of carbon steel were remarkable and exceeded 84% when the UT2-2 concentration reached 0.18 g/L.
Author Contributions
All authors made substantial contributions to the manuscript. The manuscript to be submitted was approved by all the authors. The authors did the following work: methodology, investigation, data curation and original draft preparation, W.L.; investigation, methodology and supervision, J.J.; investigation, validation, and review and editing, J.S.; formal analysis and resources, S.W.; review and editing, F.Z.; formal analysis and validation, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. U19B2012).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deyab, M.A.; Abd El-Rehim, S.S. Effect of succinic acid on carbon steel corrosion in produced water of crude oil. J. Taiwan Inst. Chem. Eng. 2014, 45, 1065–1072. [Google Scholar] [CrossRef]
- Deyab, M.A.; Eddahaoui, K.; Essehli, R.; Rhadfi, T.; Benmokhtar, S.; Mele, G. Experimental evaluation of new inorganic phosphites as corrosion inhibitors for carbon steel in saline water from oil source wells. Desalination 2016, 383, 38–45. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Shuang, S.; Roostaei, M.; Fattahpour, V.; Mahmoudi, M.; Zeng, H.; Luo, J. Investigation on the flow-induced corrosion and degradation behavior of underground J55 pipe in a water production well in the Athabasca oil sands reservoir. J. Petrol. Sci. Eng. 2019, 182, 106325. [Google Scholar] [CrossRef]
- Zhu, S.D.; Wei, J.F.; Cai, R.; Bai, Z.Q.; Zhou, G.S. Failure analysis of P110 tubing string in the ultra-deep oil well. Eng. Fail. Anal. 2011, 18, 950–962. [Google Scholar] [CrossRef]
- Cirimello, P.G.; Otegui, J.L.; Carfi, G.; Morris, W. Failure and integrity analysis of casings used for oil well drilling. Eng. Fail. Anal. 2017, 75, 1–14. [Google Scholar] [CrossRef]
- El-Askalany, A.H.; Mostafa, S.I.; Shalabi, K.; Eid, A.M.; Shaaban, S. Novel tetrazole-based symmetrical diselenides as corrosion inhibitors for N80 carbon steel in 1 M HCl solutions: Experimental and theoretical studies. J. Mol. Liq. 2016, 223, 497–508. [Google Scholar] [CrossRef]
- Ituen, E.B.; James, A.O.; Akaranta, O. Fluvoxamine-based corrosion inhibitors for J55 steel in aggressive oil and gas well treatment fluids. Egy. J. Pet. 2017, 26, 745–756. [Google Scholar] [CrossRef]
- Hegazy, M.A.; El-Etre, A.Y.; El-Shafaie, M.; Berry, K.M. Novel cationic surfactants for corrosion inhibition of carbon steel pipelines in oil and gas wells applications. J. Mol. Liq. 2016, 214, 347–356. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Umoren, S.A.; Quraishi, M.A.; Sorour, A.A.; Chen, T.; Aljeaban, N.; Wang, Q. High temperature sweet corrosion and inhibition in the oil and gas industry: Progress, challenges and future perspectives. J. Petrol. Sci. Eng. 2020, 185, 106469. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; Mohamed, M.A.A. Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J. Mol. Struct. 2017, 1130, 522–542. [Google Scholar] [CrossRef]
- Ammar, S.; Iling, A.W.M.; Ramesh, K.; Ramesh, S. Development of fully organic coating system modified with epoxidized soybean oil with superior corrosion protection performance. Prog. Org. Coat. 2020, 140, 105523. [Google Scholar] [CrossRef]
- Shi, Z.; Ouyang, Y.; Qiu, R.; Hu, S.; Zhang, Y.; Chen, M.; Wang, P. Bioinspired superhydrophobic and oil-infused nanostructured surface for Cu corrosion inhibition: A comparison study. Prog. Org. Coat. 2019, 131, 49–59. [Google Scholar] [CrossRef]
- Wongpanya, P.; Saramas, Y.; Chumkratoke, C.; Wannakomol, A. Erosion–corrosion behaviors of 1045 and J55 steels in crude oil. J. Petrol. Sci. Eng. 2020, 189, 106965. [Google Scholar] [CrossRef]
- Zhang, H.; Lan, H.; Lin, N. A numerical simulation of water distribution associated with internal corrosion induced by water wetting in upward inclined oil pipes. J. Petrol. Sci. Eng. 2019, 173, 351–361. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Ghaffari, S.; Hajizadeh, A. Film former corrosion inhibitors for oil and gas pipelines-A technical review. J Nat. Gas Sci. Eng. 2018, 58, 92–114. [Google Scholar] [CrossRef]
- Farelas, F.; Galicia, M.; Brown, B.; Nesic, S.; Castaneda, H. Evolution of dissolution processes at the interface of carbon steel corroding in a CO2 environment studied by EIS. Corros. Sci. 2010, 52, 509–517. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.H.; Li, J.; Lu, J.L.; Wang, F.S. Long-term anticorrosion behaviour of polyaniline on mild steel. Corros. Sci. 2007, 49, 3052–3063. [Google Scholar] [CrossRef]
- Lukács, Z.; Molnár, F.; Kovács, I.; Horváth, I.W.; Hancsók, J.; Kristóf, T. Corrosion testing and evaluation of gas oil desulfurization reactor structure material in the presence of fatty acids. Eng. Fail. Anal. 2020, 107, 104221. [Google Scholar] [CrossRef]
- Feng, Q.; Yan, B.; Chen, P.; Shirazi, S.A. Failure analysis and simulation model of pinhole corrosion of the refined oil pipeline. Eng. Fail. Anal. 2019, 106, 104177. [Google Scholar] [CrossRef]
- Fadhil, A.A.; Khadom, A.A.; Fu, C.; Liu, H.; Mahood, H.B.; Mahmoudd, A.K.; . Khalafe, M.Z.; Karimb, A.M.A. Ceramics coating materials for corrosion control of crude oil distillation column: Experimental and theoretical studies. Corros. Sci. 2020, 162, 108220. [Google Scholar] [CrossRef]
- Smith, P.; Roy, S.; Swailes, D.; Maxwell, S.; Page, D.; Lawson, J. A model for the corrosion of steel subjected to synthetic produced water containing sulfate, chloride and hydrogen sulfide. Chem. Eng. Sci. 2011, 66, 5775–5790. [Google Scholar] [CrossRef]
- Hou, B.S.; Zhang, Q.H.; Lia, Y.Y.; Zhu, G.Y.; Liu, H.F.; Zhang, G.A. A pyrimidine derivative as a high efficiency inhibitor for the corrosion of carbon steel in oilfield produced water under supercritical CO2 conditions. Corros. Sci. 2020, 164, 108334. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Lv, Y.; Asif, M.; Xiong, F.; Zhang, G.; Liu, H. Corrosion inhibition and anti-bacterial efficacy of benzalkonium chloride in artificial CO2-saturated oilfield produced water. Corros. Sci. 2017, 117, 24–34. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Zhang, G.; Liu, H.; Cheng, Y.F. Corrosion of X80 pipeline steel under sulfate-reducing bacterium biofilms in simulated CO2-saturated oilfield produced water with carbon source starvation. Corros. Sci. 2018, 136, 47–59. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, Y.F.; Sharma, M.; Voordouw, G. Effect of fluid flow on biofilm formation and microbiologically influenced corrosion of pipelines in oilfield produced water. J. Pet. Sci. Eng. 2017, 156, 451–459. [Google Scholar] [CrossRef]
- McMahon, P.B.; Kulongoski, J.T.; Vengosh, A.; Cozzarelli, I.M.; Landon, M.K.; Kharaka, Y.K.; Gillespie, J.M.; Davis, T.A. Regional patterns in the geochemistry of oil-field water, southern San Joaquin Valley, California, USA. Appl. Geochem. 2018, 98, 127–140. [Google Scholar] [CrossRef]
- Benamora, A.; Talkhana, A.G.; Nasser, M.; Hussein, I.; Okonkwo, P.C. Effect of temperature and fluid speed on the corrosion behavior of carbon steel pipeline in Qatari oilfield produced water. J. Electroanal. Chem. 2018, 808, 218–227. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Xu, N.; Xiong, W.; Liu, H.F.; Zhang, G.A. Effective inhibition on the corrosion of X65 carbon steel in the oilfield produced water by two Schiff bases. J. Mol. Liq. 2019, 285, 223–236. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Zhang, Y.; Ma, L.; Yang, P. Electrochemical polarization study on crude oil pipeline corrosion by the produced water with high salinity. Eng. Fail. Anal. 2016, 60, 307–315. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yuan, J. Influence of produced water with high salinity and corrosion inhibitors on the corrosion of water injection pipe in Tuha oil field. Eng. Fail. Anal. 2014, 45, 225–233. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Zhang, G.A. Inhibitive and adsorption behavior of thiadiazole derivatives on carbon steel corrosion in CO2-saturated oilfield produced water: Effect of substituent group on efficiency. J. Colloid. Interf. Sci. 2020, 572, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Tavares, S.S.M.; Pardal, J.M.; Mainier, F.B.; da Igreja, H.R.; Barbosa, E.S.; Rodrigues, C.R.; Barbosa, C.; Pardal, J.P. Investigation of the failure in a pipe of produced water from an oil separator due to internal localized corrosion. Eng. Fail. Anal. 2016, 61, 100–107. [Google Scholar] [CrossRef]
- Velázquez, J.C.; Cruz-Ramirez, J.C.; Valor, A.; Venegas, V.; Caleyo, F.; Hallen, J.M. Modeling localized corrosion of pipeline steels in oilfield produced water environments. Eng. Fail. Anal. 2017, 79, 216–231. [Google Scholar] [CrossRef]
- Deyab, M.A.; Bali, B.E.; Essehli, R.; Ouarsal, R.; Lachkar, M.; Fuess, H. NaNi(H2PO3)3·H2O as a novel corrosion inhibitor for X70-steel in saline produced water. J Mol. Liq. 2016, 216, 636–640. [Google Scholar] [CrossRef]
- Nezhad, A.H.N.; Davoodi, A.; Zahrani, E.M.; Arefinia, R. The effects of an inorganic corrosion inhibitor on the electrochemical behavior of superhydrophobic micro-nano structured Ni films in 3.5% NaCl solution. Surf. Coat. Tech. 2020, 395, 125946. [Google Scholar] [CrossRef]
- Liao, K.; Zhou, F.; Song, X.; Wang, Y.; Zhao, S.; Liang, J.; Chen, L.; He, G. Synergistic effect of O2 and H2S on the corrosion behavior of N80 steel in a simulated high-pressure flue gas injection system. J. Mater. Eng. Perform. 2020, 29, 155–166. [Google Scholar] [CrossRef]
- Tang, Z.; Hong, S.; Xiao, W.; Taylor, J. Characteristics of iron corrosion scales established under blending of ground, surface, and saline waters and their impacts on iron release in the pipe distribution system. Corros. Sci. 2006, 48, 322–342. [Google Scholar] [CrossRef]
- Qin, M.; Liao, K.; He, G.; Huang, Y.; Wang, M.; Zhang, S. Main control factors and prediction model of flow-accelerated CO2/H2S synergistic corrosion for X65 steel. Process Saf. Environ. Prot. 2022, 160, 749–762. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Wang, M.; Wang, X.; Liu, G.; Huang, Y. Understanding the influences of temperature and microstructure on localized corrosion of subsea pipeline weldment using an integrated multi-electrode array. Ocean Eng. 2019, 189, 106351. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, K.; Zhou, F.; Leng, J.; Huang, Q.; He, G. Effect of Temperature on the corrosion behavior of L245NS steel in a CO2/H2S/O2 multi-component thermal fluid collection and transportation system. Arabian J. Sci. Eng. 2022, 47, 11223–11237. [Google Scholar] [CrossRef]
- Stoulil, J.; Kaňok, J.; Kouřil, M.; Parschová, H.; Novák, P. Influence of temperature on corrosion rate and porosity of corrosion products of carbon steel in anoxic bentonite environment. J. Nucl. Mater. 2013, 443, 20–25. [Google Scholar] [CrossRef]
- Azghandi, M.V.; Davoodi, A.; Farzi, G.A.; Kosari, A. Water-base acrylic terpolymer as a corrosion inhibitor for SAE1018 in simulated sour petroleum solution in stagnant and hydrodynamic conditions. Corros. Sci. 2012, 64, 44–54. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Yin, B.; Zhao, S.; Liu, X. Impedance spectroscopic study of corrosion inhibition of copper by surfactants in the acidic solutions. Corros. Sci. 2003, 45, 867–882. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, D.; Xiao, G.; Shang, J.; Liu, Y.; Long, D.; He, Q.; Singh, A. Effect of Cl− accumulation on corrosion behavior of steels in H2S/CO2 methyldiethanolamine (MDEA) gas sweetening aqueous solution. J. Nat. Gas Sci. Eng. 2016, 30, 444–454. [Google Scholar] [CrossRef]
- Qin, M.; Liao, K.; He, G.; Zou, Q.; Zhao, S.; Zhang, S. Corrosion mechanism of X65 steel exposed to H2S/CO2 brine and H2S/CO2 vapor corrosion environments. J. Nat. Gas Sci. Eng. 2022, 106, 104774. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, Y.; Matsuda, K.; Zou, Z. Characterization of the effect of hydrogen sulfide on the corrosion of X80 pipeline steel in saline solution. Corros. Sci. 2016, 102, 455–468. [Google Scholar] [CrossRef]
- Deng, K.; Lin, Y.; Ning, H.; Liu, W.; Singh, A. Influences of temperature and pressure on CO2 solubility in saline solutions in simulated oil and gas well environments. Appl. Geochem. 2018, 99, 22–30. [Google Scholar] [CrossRef]
- Vatankhah, G.; Drogowska, M.; Menard, H.; Brossard, L. Electrodissolution of iron in sodium sulfate and sodium bicarbonate solutions at pH8. J. Appl. Electrochem. 1998, 28, 173–183. [Google Scholar] [CrossRef]
- Kytopoulos, V.N.; Altzoumailis, A.; Panagopoulos, C.; Riga, C. Investigation of certain mechanical and magnetic properties of a stressed low-carbon steel after corrosion in NaCl-water solution. Procedia Struct. Integr. 2020, 26, 113–119. [Google Scholar] [CrossRef]
- Wright, R.F.; Brand, E.R.; Ziomek-Moroz, M.; Tylczak, J.H.; Ohodnicki, P.R. Effect of HCO3− on electrochemical kinetics of carbon steel corrosion in CO2-saturated brines. Electrochim. Acta 2018, 290, 626–638. [Google Scholar] [CrossRef]
- Cheng, Q.; Tao, B.; Song, L.; Zhang, W.; Liu, X.; Li, W.; Hou, B.; Liu, Q. Corrosion behaviour of Q235B carbon steel in sediment water from crude oil. Corros. Sci. 2016, 111, 61–71. [Google Scholar] [CrossRef]
- Guo, W.; Xu, L.; Xu, B.; Yang, Y.; Sun, Z.; Liu, S. A modified composite film electrode of polyoxometalate/carbon nanotubes and its electrocatalytic reduction. J. Appl. Electrochem. 2009, 39, 647–652. [Google Scholar] [CrossRef]
- Dahmani, K.; Galai, M.; Elhasnaoui, A.; Temmar, B.; El Hessni, A.; Cherkaoui, M. Corrosion resistance of electrochemical copper coating realized in the presence of essential oils. Der PharmaChemica 2015, 7, 566–572. [Google Scholar]
- Dkhireche, N.; Galai, M.; El Kacimi, Y.; Rbaa, M.; Ouakki, M.; Lakhrissi, B.; Touhami, M.E. New quinoline derivatives as sulfuric acid inhibitor’s for mild steel. Anal. Bioanal. Electrochem. 2018, 10, 111–135. [Google Scholar]
- Ouakki, M.; Galai, M.; Benzekri, Z.; Aribou, Z.; Ech-Chihbi, E.; Guo, L.; Dahmani, K.; Nouneh, K.; Briche, S.; Boukhris, S.; et al. A detailed investigation on the corrosion inhibition effect of by newly synthesized pyran derivative on mild steel in 1.0 M HCl: Experimental, surface morphological (SEM-EDS, DRX& AFM) and computational analysis (DFT & MD simulation). J. Mol. Liq. 2021, 344, 117777. [Google Scholar]
- Ouass, A.; Galai, M.; Ouakki, M.; Ech-Chihbi, E.; Kadiri, L.; Hsissou, R.; Essaadaoui, Y.; Berisha, A.; Cherkaoui, M.; Lebkiri, A.; et al. Poly (sodium acrylate) and Poly (acrylic acid sodium) as an eco-friendly corrosion inhibitor of mild steel in normal hydrochloric acid: Experimental, spectroscopic and theoretical approach. J. Appl. Electrochem. 2021, 51, 1009–1032. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Lin, Y.; Quraishi, M.A.; Lgaz, H.; Chung, I.M. Corrosion inhibition performance of imidazolidine derivatives for J55 pipeline steel in acidic oilfield formation water: Electrochemical, surface and theoretical studies. J. Taiwan Inst. Chem. Eng. 2019, 95, 341–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).