Abstract
Fall armyworm (FAW), Spodoptera frugiperda, has recently invaded Africa where it is seriously threatening food security. Current management methods rely heavily on synthetic insecticides which are harmful to humans, the environment, and non-target beneficial insects. Cotesia icipe was recently identified as a major FAW-associated indigenous parasitoid causing a high parasitism rate on the pest in Kenya. Previous studies have demonstrated the efficacy of Metarhizium anisopliae ICIPE 7, ICIPE 41, ICIPE 78, and Beauveria bassiana ICIPE 621 against FAW. However, limited information is available on the interactions between these potent isolates and C. icipe. This study therefore assessed direct and indirect infection effects of these fungal isolates on C. icipe, induced 2nd instar FAW mortality, and parasitism rates of the infected C. icipe. Results showed that when C. icipe were directly exposed to dry conidia of the fungal isolates, ICIPE 7 and ICIPE 41 caused the highest (100%) C. icipe adult mortality seven days post-exposure. Both isolates also induced the highest FAW larval mortality of 55% and 53%, respectively. ICIPE 78 recorded the highest parasitism rates after direct infection. In the indirect exposure (fungal-infected FAW larvae exposed to the parasitoid), 1 × 109 conidia mL−1 recorded high C. icipe adult and FAW 2nd instar mortalities for all fungal isolates. This study provides an important baseline for effective fungal-based biopesticides development that could also be used in augmentative biological control. However, further studies are warranted to assess the performance of C. icipe in combination with these potent biopesticides in the field.
1. Introduction
Many agricultural systems are cereal-based in tropical Africa. These cereals include maize, sorghum, millet, wheat, and rice, which constitute major staple food crops for the majority of the populations living in the area [1]. However, currently, their production and productivity are threatened by several abiotic and biotic factors. For instance, damage by lepidopteran pests such as stemborers inflict 15–90% losses in cereals [2]. These losses have been aggravated by the recent invasion of fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), which constitutes a high threat to food security in Africa. The pest was first reported in Africa in early 2016 in the rainforest zones of Nigeria [3]. In Kenya, the pest was first reported in Trans Nzoia County by farmers in March 2017 and immediately confirmed by the Kenya Plant Health Inspectorate Service [4]. FAW is native to tropical and sub-tropical America and is known as one of the most damaging crop pests. It has been reported to feed on over 80 host plants and devastate most cereals (with a special preference for maize and sorghum causing yield losses of up to 70%) and has spread across the African and Asian continents [5].
Due to the presence of several host plants, favorable climatic conditions and probably the absence of its effective native natural enemies in Sub-Saharan Africa, this pest has spread very fast [2,3,4]. It is capable of persisting over long periods causing serious damage to crops, therefore, affecting the livelihoods of resource-poor farmers [5]. The absence of adequate management strategies in the newly invaded zones has led to smallholder farmers relying on the use of synthetic pesticides, which in most cases are ineffective and unsustainable in the long term, with negative impacts on humans, non-target organisms, and the environment [6]. However, entomopathogenic fungi (EPF) have been found to be effective against several species of insect pests including lepidopterans in Africa [7], and could also be explored to tackle this notorious invasive fall armyworm.
In the pursuit of developing sustainable and environmentally friendly approaches, some key potent entomopathogenic fungal-based biopesticides were identified to be effective against eggs and neonates of FAW [8]. The authors reported that Metarhizium anisopliae isolates ICIPE 78, ICIPE 40, and ICIPE 20 outperformed all the other tested isolates by reducing eggs’ hatchability by 87%, 83%, and 79.5%, respectively. In addition to the eggs’ mortality, the fungal isolates also induced mortality to the newly emerged larvae (neonates) from the fungus-treated eggs 7 days post-emergence, where M. anisopliae isolates ICIPE 41 and ICIPE 7 outperformed all the others by causing 96.49% and 93.66% larval mortality, respectively [8]. Recently, Beauveria bassiana ICIPE 621 and M. anisopliae ICIPE 7 were also reported to be highly effective against adult FAW by causing 100% mortality of the moths with the lowest LT50 values of 3.6 ± 0.1 and 3.9 ± 0.0 days, respectively [9]. Both isolates were also found to be compatible with FALLTRACT lure, as the lure had no effect on the conidial germination in the laboratory [9].
Furthermore, some parasitoids such as Cotesia icipe, Chelonus curvimaculatus, Charops ater, Telenomus remus, Trichogramma chilonis, Palexorista zonata, and Coccygidium luteum were also locally found to be associated with and efficient against FAW egg and larval stages [10]. Among them, C. icipe has been identified as a major FAW larval parasitoid causing a more than 60% parasitism rate [11]. Cotesia icipe is a solitary larval endoparasitoid and it was observed to parasitize Spodoptera littoralis and Spodoptera exigua larvae (Lepidoptera: Noctuidae) under laboratory conditions at icipe in Kenya [12]. Therefore, for effective deployment of the potent fungal-based biopesticides in the same existing parasitoids environment, there is an urgent need to evaluate the interactions between these effective biopesticides and native parasitoids to boost the control of FAW under an integrated pest management (IPM) umbrella in Africa. The aim of this study was to assess the non-target effects of the key identified effective commercialized (M. anisopliae ICIPE 7, ICIPE 78) and non-commercialized (M. anisopliae ICIPE 41 and B. bassiana ICIPE 621) fungal-based biopesticides on C. icipe for sustainable management of FAW.
2. Materials and Methods
2.1. Fall Armyworm Rearing
Spodoptera frugiperda originally collected from maize plants at Siaya and Homa Bay counties (−0.61401° Latitude, 34.09095° Longitude; 1215 m.a.s.l.), Kenya, from April–July 2017 were reared in small cages (30 × 30 × 30 cm) under laboratory-controlled ambient conditions of 25 ± 2 °C temperature, 70 ± 10% relative humidity, and 12L:12D photoperiod. The field-collected larvae were reared on a semi-synthetic diet until pupation [9]. The pupae were held in sleeved Perspex cages (40 × 40 × 45 cm) until adult emergence. Moths were fed on 10% honey solution soaked in cotton wool balls. Potted maize plants were placed in the cages and female moths were released for oviposition. Upon oviposition, the plants were removed after 24 h post-exposure (to ensure eggs and larvae of homogenous age for the experiments) and transferred to a separate ventilated cage (50 × 50 × 60 cm) to facilitate egg hatchability. Leaves with larvae were removed from these plants three days after the larvae hatched and placed into clean cages (50 × 50 × 60 cm) with a fine netting lid for ventilation. The cages were lined with paper towel to absorb excess moisture. The larvae were supplied daily with fresh maize leaves as food until they pupated. The pupae were collected from the cages using a fine camel hair brush and placed inside sleeved Perspex cages (40 × 40 × 45 cm) for adult emergence.
2.2. Cotesia icipe Parasitoid Rearing
The colony of C. icipe was initiated from parasitized Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) larvae collected from Amaranth plants from Yatta (01.23044° S; 37.45789° E) and Mwea (0.6309° S; 37.35117° E). A parasitoid colony was established in 2018 using S. frugiperda and maintained in the laboratory for several generations before any experiments were carried out. Cotesia icipe adults were reared in Perspex cages (30 × 30 × 30 cm) at room temperature of 25 °C, a 12L:12D photoperiod, and 60–70 percent relative humility (RH). Drops of 10% honey solution were supplied to the adult parasitoids, together with moist cotton wool balls placed in a Petri dish (9 cm). The wasps in the rearing cage were fed on (1st and 2nd instar larvae) of S. frugiperda on fresh maize leaves for colony maintenance. Upon 24 h, the exposed larvae were transferred into rectangular plastic jars measuring (20.5 × 14.5 × 8 cm) bearing fresh maize leaves as food. The larvae were kept until cocoons formed or FAW pupation occurred. The cocoons were regularly removed as they formed and kept in clean Perspex cages (40 × 40 × 45 cm) for parasitoid emergence.
2.3. Fungal Isolates Culture and Conidia Viability Assessment
The fungal isolates used in this study were obtained from icipe’s Arthropod Germplasm Centre. The source and origin of the fungal isolates (three Metarhizium and one Beauveria) are summarized in Table 1 below. Metarhizium anisopliae ICIPE 7, ICIPE 78, and ICIPE 41 isolates were cultured on Sabouraud dextrose agar (SDA), while B. bassiana ICIPE 621 was cultured on Potato dextrose agar (PDA) in Petri dishes (9 cm diameter). The inoculated plates were incubated at 25 ± 2 °C in complete darkness. Conidia were harvested after three weeks from surface cultures and suspended in 10 ml distilled water with 0.05% Triton X-100 in universal bottles (30 mL) containing glass beads (4 beads per bottle). The suspension was vortexed for 5 min at about 700 rpm to break the conidial clumps and ensure a homogeneous suspension. Conidial concentrations were quantified using a hemocytometer under a light microscope. Prior to bioassays, the conidial suspensions were adjusted to a concentration of 1 × 109 conidia mL−1 and other concentrations were achieved through serial dilution [13]. Conidia viability was determined by spread plating 0.1 mL of 3 × 106 conidia mL−1 onto 9 cm Petri dishes containing SDA or PDA medium. A sterile microscope cover slip (2 × 2 cm) was placed on top of the agar in each plate. Plates were sealed with parafilm and incubated in complete darkness at 25 ± 2 °C and examined after 18–20 h. The percentage of germination of conidia was determined from 100 random conidia on the surface area covered by each coverslip under the light microscope (400×) [14]. Conidia was considered to have germinated when the length of the germ tube was at least twice the diameter of the conidium [14]. Each plate represented a replicate and each isolate had four replicates.

Table 1.
Identity of fungal isolates screened against Cotesia icipe for pathogenicity under laboratory conditions.
2.4. Direct Infection Effects of Metarhizium anisopliae and Beauveria bassiana Isolates on Cotesia icipe
Four entomopathogenic fungal isolates M. anisopliae ICIPE 7, 78, 41, and B. bassiana ICIPE 621 were screened for their pathogenicity/virulence against C. icipe adult parasitoids. For each fungal isolate (treatment), 20 1-day-old adult C. icipe parasitoids (at a ratio of 1:2/male:female) were contaminated with EPF using velvet-coated plastic jars (150 × 80 mm) following the procedure described by Migiro et al. [15] and Opisa et al. [16]. The devices were contaminated with 1 g of dry conidia. The 20 adult parasitoids were introduced into the contamination device for 3 min to pick up spores. Control treatments were exposed to fungus-free velvet plastic jars also for 3 min. After 3 min of exposure, contaminated insects were transferred into Perspex cages as described above and provided with honey as food. Adult mortality was recorded daily for seven days by counting all dead parasitoids. A mycosis test was conducted to confirm mortality due to infection by the fungus treated, where the dead insects were surface sterilized with 70% alcohol and then rinsed thrice in distilled water. The surface-sterilized cadavers were kept separately in Petri dishes lined with sterile moistened filter paper to record fungal outgrowth and verify if mortality could be attributed to the respective fungal isolates they were treated with. Mortality due to fungal infection was confirmed by the presence of hyphae and conidia on the surface of the cadaver [9,16]. All the treatments were arranged in a completely randomized design with each treatment replicated four times as well as the controls.
In addition, after infection, the 20 infected parasitoids (ratio 1:2 male to female) were exposed to 50 2nd instar larvae for 24 h. The larvae were then removed and kept in separate clean lunch boxes (19 × 13 × 8 cm) and fed with fresh maize leaves until adults emerged. Adult parasitoids were kept in the cages and fed with honey when assessing their mortality until all parasitoids died. In addition to adult mortality, FAW larval mortality and parasitism rates were also recorded.
2.5. Indirect Infection Effects of Metarhizium anisopliae and Beauveria bassiana Isolates on Cotesia icipe
The 50 2nd instar of S. frugiperda larvae were sprayed directly with 10 ml fungal suspension at different concentrations (1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia mL−1) of M. anisopliae isolates (ICIPE 7, 78, and 41) and B. bassiana ICIPE 621, using the Burgerjon’s spray tower [17], before transferring them onto potted maize plants. Control treatments were sprayed with sterile distilled water containing 0.05% Triton X-100. The sprayed larvae were air-dried before being transferred into clean Perspex cages containing the potted maize plants. A total of 20 1-day-old adult C. icipe parasitoids at a ratio of 1:2/male:female were then introduced into each of the cages containing sprayed 2nd instar FAW larvae for 24 h for parasitism assessment. After 24 h, sprayed and exposed larvae were transferred into clean cages containing fresh maize leaves, and C. icipe were maintained with honey as the food source. The bioassays were maintained at 25–27 °C, 50–70% RH, and a 12L:12D photoperiod. Mortality rates were recorded daily until all parasitoids died. Dead parasitoids were surface sterilized and placed in Petri dishes lined with damp sterilized filter paper to allow fungal growth on the surface of the cadaver, and mycosis rate was determined. Mortality of the FAW larvae and parasitism rates were also assessed. The experiment was replicated four times and arranged in a complete randomized design.
2.6. Effect of Entomopathogenic fungi on Cotesia icipe Emergence and Sex Ratio
The cocoons that formed from the parasitized larvae both in the direct and indirect infection experiments were counted and kept in petri dishes inside the Perspex cages until emergence of adult parasitoids. The total number of parasitoids that emerged in each treatment was counted and their sex ratios were determined.
2.7. Data Analysis
FAW larvae and adult C. icipe mortality data were corrected for natural mortality in controls using Abbott’s formula [18] and their assumption of homogeneity of variance was tested using Bartlett’s test before subjecting the data to one-way analysis of variance (ANOVA). The percentage mortalities were analyzed using a binomial generalized linear model (GLM) with logit link [19]. Whenever treatments were found to be significantly different (p < 0.05), means were separated using the Student–Newman–Keuls (SNK) test. The lethal time for 50% mortality (LT50) was analyzed by GLM using the function ‘dose.p’ from the MASS library to estimate the lethal time to 50% mortality (LT50). Parasitism rate was calculated as the percentage of the number of parasitoid adults divided by the sum of larvae of the host and subjected to ANOVA. The sex ratios were analyzed using the chi-squared test (χ2 test), while mycosis data were analyzed using one-way ANOVA. All data analyses were performed using R (version 3.2.5) statistical software packages [20].
3. Results
3.1. Direct Effects of Entomopathogenic fungal Isolates on Cotesia icipe and FAW Larvae
There were significant differences in mortality of C. icipe adults between the treatments (F = 1.72; df = 3, 12; p = 0.02) 7 days after treatment (Table 2). However, the mortality rates of M. anisopliae ICIPE 7 and ICIPE 41 did not differ significantly among these isolates and similar trends were also observed between M. anisopliae ICIPE 78 and B. bassiana ICIPE 621. The lowest adult mortality rate was recorded in the M. anisopliae ICIPE 78 treatment, whereas the highest mortality was recorded in ICIPE 7 and ICIPE 41 (Table 2). The most virulent isolates with LT50 of less than 3 days were M. anisopliae isolates ICIPE 7 and ICIPE 41, whereas ICIPE 78 and B. bassiana ICIPE 621 had LT50 values of 5.2 and 5.0 days, respectively (Table 2).

Table 2.
Percentage of mortality of Cotesia icipe adults; LT50 and FAW larvae induced mortality by the different fungal isolates.
In addition, significant differences in mortality of FAW larvae exposed to the infected parasitoid were observed between M. anisopliae ICIPE 7 and ICIPE 41 versus M. anisopliae ICIPE 78 and B. bassiana ICIPE 621 treatments (F = 7.27; df = 3, 12; p < 0.001), 7 days post-treatment (Table 2). Metarhizium anisopliae ICIPE 7 caused the highest FAW larval mortality, while ICIPE 78 recorded the lowest among the isolates (Table 2).
Furthermore, significant variations in parasitism rates were also observed among the tested fungal isolates (F = 8.61; df = 3, 12; p < 0.001) 7 days post-treatment (Table 2). The highest and lowest parasitism rates were recorded in the ICIPE 78 (62.0%) and ICIPE 7 (35.7%) treatments, respectively (Table 2). However, there were no significant differences between ICIPE 78 and ICIPE 621, as well as between ICIPE 7 and ICIPE 41.
There were also no significant differences in the sex ratios among the different fungal isolates (p > 0.05, χ2 test), but the F1 parasitoid generations were female biased.
3.2. Indirect Effects of Entomopathogenic fungal Isolates on Cotesia icipe Adults
3.2.1. Mortality Rates of Cotesia icipe Adults and 2nd Instar Larvae of FAW after Indirect Exposure to FAW Larvae infected with Different Fungal Isolate Concentrations
The results showed significant differences in mortality of C. icipe adults in all the concentrations of M. anisopliae isolates: ICIPE 41 (F = 60.63; df = 4, 15; p < 0.0001); ICIPE 7 (F = 273.8; df = 4, 15; p < 0.001); ICIPE 78 (F = 6.03; df = 4, 15; p < 0.001); and B. bassiana ICIPE 621 (F = 152.3; df = 4, 15; p < 0.001) 7 days after treatment. For all the tested fungal isolates, the highest concentration always recorded the highest C. icipe adult mortality with cumulative mortalities of 94.1%, 81.25%, 67.00%, and 88.75% for M. anisopliae ICIPE 41, ICIPE 7, ICIPE 78, and B. bassiana 621, respectively (Figure 1).
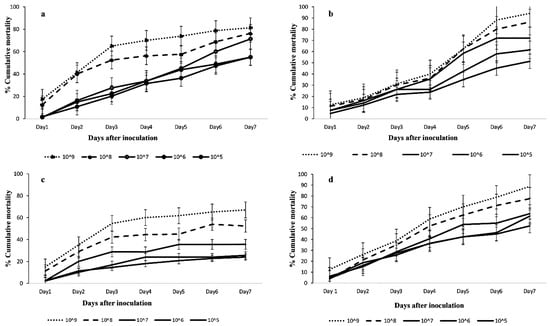
Figure 1.
Cumulative mortalities of Cotesia icipe indirectly exposed to FAW larvae infected with Metarhizium anisopliae: (a) ICIPE 7, (b) ICIPE 41, (c) ICIPE 78, and (d) Beauveria bassiana ICIPE 621 at different concentrations. Line graph points denote means ± standard errors.
Moreover, significant differences in mortality of FAW larvae were observed among all the concentrations of M. anisopliae: ICIPE 41 (F = 17.13; df = 4, 15; p < 0.001); ICIPE 7 (F = 38.88; df = 4, 15; p < 0.001); ICIPE 78 (F = 24.85; df = 4, 15; p < 0.001); and B. bassiana 621 (F = 10.86; df = 4, 15; p < 0.001) 7 days post-treatment. For all the tested fungal isolates, the highest concentration recorded the highest FAW larval mortality with cumulative larval mortalities of 25.75%, 31.58%, 29.50%, and 19.00% for M. anisopliae ICIPE 41, ICIPE 7, ICIPE 78, and B. bassiana ICIPE 621, respectively (Figure 2).
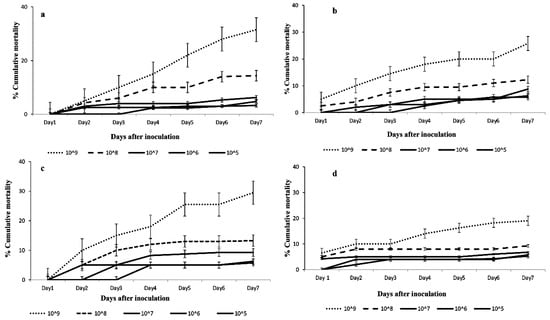
Figure 2.
Cumulative mortalities of 2nd instar larvae of FAW infected with Metarhizium anisopliae: (a) ICIPE 7, (b) ICIPE 41, (c) ICIPE 78 and Beauveria bassiana, and (d) ICIPE 621 at different concentrations. Line graph points denote means ± standard errors.
3.2.2. Effect of Entomopathogenic fungal Isolate Concentrations on Cotesia icipe Emergence
Parasitism rates of C. icipe varied among the fungal isolates after exposing the infected FAW larvae to the parasitoid. Significant differences in emergence of C. icipe were observed among all the concentrations of M. anisopliae: ICIPE 41 (F = 4.70; df = 5, 18; p = 0.006); ICIPE 7 (F = 3.291; df = 5, 18; p = 0.0027); and ICIPE 78 (F = 10.83; df = 5, 18; p < 0.0001). However, no significant difference was observed for B. bassiana ICIPE 621 (F = 2.10; df = 5, 18; p = 0.1). Control treatments had the highest parasitoid emergence. For all the tested fungal isolates, the least concentration always recorded the highest parasitoid emergence, while the high concentrations yielded moderate parasitism rates (Table 3).

Table 3.
Effects of the various fungal isolate concentrations on the number of emerged Cotesia icipe (parasitism rates) in the laboratory (Mean ± SE) and their sex ratios (F:M).
In addition, the sex ratios of the F1 C. icipe generations were females biased for all the tested isolates and at all concentrations, except for ICIPE 41 and ICIPE 78 where the sex ratios were balanced at the concentrations of 1 × 109, and 1 × 109 and 1 × 108, respectively (Table 3).
4. Discussion
A simultaneous use of entomopathogenic fungi (EPF) and parasitoids could be harmful to the parasitoids when host searching occurs at sites that are contaminated with the EPF conidia. The potential of an entomopathogenic fungus to attach, germinate, penetrate, and colonize the body of the host insect determines its efficacy [21]. The results of this study showed that direct infection of parasitoids with dry conidia has significant effects on the parasitoid. Cotesia icipe adults were susceptible to infection by the tested fungal isolates. It was observed that M. anisopliae isolates have a more detrimental effect on C. icipe adults leading to their high mortality rates. Metarhizium anisopliae ICIPE 7 and ICIPE 41 recorded 100% adult parasitoid mortality, whereas B. bassiana ICIPE 621 showed less effect on direct infection of the conidia. The differences in the pathogenicity and virulence of the different isolates was also reported for fungal pathogens tested in different insect species [15,22]. Differences in components that induce virulence, including proteinases, chitinases, and lipases, which are essential for cuticle destruction and entry into the insect, could explain the variation in pathogenicity among isolates. Given that various fungal species or isolates produce these proteins differently, this could explain the discrepancies in pathogenicity and “speed of kill” identified among the isolates in the study [23]. These results are also similar to studies conducted by Ansari et al. [24] that showed that dry conidia considerably outperformed wet-formulated conidia. The authors found that the dry conidia of M. anisopliae were shown to be effective in infecting mosquitoes. They further reported that dry conidia were more virulent to the mosquitoes compared with the oil-formulated ones, and there was also transfer of M. anisopliae conidia between Anopheles gambiae mosquitoes (Diptera: Culicidae) [24]. However, it is important to note that the virulence of dry conidia applied in the field may decrease faster due to the environmental conditions, thus posing an advantage to the parasitoids. Furthermore, since dry conidia are always applied in combination with the pest attractants to target the adult of the pests, there would be less interactions of the fungus with the natural enemies that might not negatively affect the parasitoids performance under field conditions.
Alves et al. [25] confirmed horizontal transfer of M. anisopliae isolate from honeybees to the pollen beetle Meligethes aeneus (Coleoptera: Nitidulidae). Other studies have also reported high mortality of adult parasitoids caused by EPF applications. For example, Abbas et al. [26] reported 40% mortality from M. anisopliae to Cephalonomia stephanoderis Betrem (Hymenoptera: Bethylidae) adult, the parasitoid of coffee berry borer (Hypothenemus hampei). Sisay et al. [27] reported that parasitoids Bracon hebetor (Hymenoptera: Braconidae) and Anagyrus lopezi (Hymenoptera: Encyrtidae) were highly susceptible to infection by 11 strains of M. anisopliae. Uma Devi et al. [28] showed a low risk for the parasitoid wasp Trybliographa rapae after they parasitized larvae of the cabbage root fly Delia radicum previously infected with a strain of M. brunneum; therefore, they recommended the combined use of both agents to control D. radicum. The isolates also showed different killing speeds on the parasitoid with the most virulent isolates with a LT50 of less than 3 days being those of M. anisopliae.
The results also showed that there was subsequent transmission of conidia between C. icipe-infected adults and FAW larvae during parasitization, which was evident in the FAW larval mortality that was recorded. Horizontal pathogen transmission among insects is a key measure of how well a pathogen can establish and propagate in the environment, resulting in increased epizootics in target host populations. The results of this study also showed that direct infection of M. anisopliae ICIPE 7 and ICIPE 41 isolates had the potential to affect C. icipe emergence. Metarhizium anisopliae ICIPE 7 recorded the lowest parasitism rates as compared with the control treatments.
Studies have shown that different fungal isolates have varying degrees of specificity. Such interaction is complementary and not pathogenic. For instance, Encarsia formosa, a parasitoid of greenhouse whitefly, was able to detect virus-infected hosts and, therefore, only oviposits in hosts not infected with Aschersonia aleyrodis [29]. Furthermore, Caballero et al. [30] reported that the survival of Serangium parcesetosum Sicard (Coleoptera: Coccinellidae), a whitefly predator, was lower when they were sprayed with B. bassiana as opposed to sprays from Isaria fumosorosea, and that survivorship was not affected by the concentration of each pathogen. The ability of parasitoids to detect and avoid hosts infected by entomopathogenic fungi seems to be crucial for the combined use of both biocontrol agents [26]. Further studies are therefore warranted to assess this behavioral aspect of C. icipe after infecting FAW larvae. Dimbi et al. [31] found that Eretmocerus sp. avoided oviposition on Bemisia argentifolii Bellows infected with B. bassiana. Production of antifungal compounds by both host and parasitoid has also been reported as a response to the presence of B. bassiana.
The results of C. icipe adults after their indirect exposure to FAW larvae infected with different fungal isolate concentrations also showed that the fungal inoculum was transmitted from infected larvae to the adult parasitoids. Metarhizium anisopliae ICIPE 41 caused the highest C. icipe cumulated adult mortality rate of 94.1%, whereas B. bassiana ICIPE 621 recorded adult mortality of 88.75%. Moreover, the highest concentrations of 1 × 109 conidia mL−1 in M. anisopliae ICIPE 78 and ICIPE 41 recorded low parasitoid emergence. Similar results were reported by Migiro et al. [15] where M. anisopliae has the potential to infect the ectoparasitoid of Liriomyza leafminer Diglyphus isaea after indirect infection and thus could not be used indiscriminately.
Förster et al. [32] also evaluated the effect of five entomopathogenic fungi, M. anisopliae, Isaria fumosorosea, Nomuraea rileyi, and two strains of B. bassiana against the predatory lady beetle, Hippodamia convergens (Colepotera: Coccinellidae) in the laboratory and reported that the fungal isolates caused mortality rates ranging from 56 to 95%, and the mortality was dose-dependent except for Nomuraea rileyi. In addition, the four fungal isolates tested in the current study on the FAW larvae showed low larval mortality rates at all the concentrations, where only M. anisopliae ICIPE 7 recorded the highest moderate larval mortality of 31.58%. Ramanujam et al. [33] reported that strains of M. anisopliae ICAR-NBAIR Ma-35 and B. bassiana ICAR-NBAIR Bb-45 were effective against 2nd instar larvae of FAW with mortalities of 67.8% and 64.3%, respectively. Reports by Akutse et al. [8] also revealed that, M. anisopliae isolates caused 92% and 97% mortality to FAW eggs and 1st instar larvae, respectively, but these isolates were less pathogenic to the 2nd instar larvae of FAW, where only 30% larval mortality was recorded in B. bassiana ICIPE 676. Akutse et al. [9] further reported that M. anisopliae ICIPE 7 and B. bassiana ICIPE 621 caused 100% mortality to adult FAW but less pathogenic to its 2nd instar larvae. Morales-Reyes et al. [34] reported that M. anisopliae and B. bassiana at the concentrations of 1 × 106 and 1 × 107 caused FAW 2nd instar larval mortality ranging between 45% to 65%. García-Munguía et al. [35] reported 96.6% and 78.6% mortality on 2nd instar larvae of FAW with B. bassiana and M. anisopliae strains, respectively, at a concentration of 1 × 109 conidia mL−1. Mumo et al. [36] also documented the important role played by entomopathogenic fungal enzymes in the virulence of insect pest species, with variations in different strains, which could also explain the differential responses of the FAW and its associated larval parasitoid C. icipe to the tested fungal isolates.
When compared with the controls, the fungal isolates had no effect on the sex ratios of the emerging C. icipe parasitoids in both direct and indirect applications. Furthermore, at all concentrations, the F1 progenies revealed dominant female bias populations, which is critical for parasitoid reproduction in the system. More research is needed to determine the lifetime and survival of F1 progenies from the different infection approaches. Similarly, Polanczyk et al. [37] found that M. anisopliae did not influence the sex ratios of Trichogramma atopovirilia (Hymenoptera: Trichogrammatidae) by contaminating the diet and immersing eggs in conidial solutions.
These results show that the adult parasitoids have the potential to pick dry conidia of M. anisopliae when they come into contact, but autodissemination application of the dry spores of these potent isolates in combination with an attractant [9] will limit this effect. The indirect exposure, however, indicates that the lowest concentrations had less or no effect on the parasitoids, whereas the highest concentrations had significant effects on the parasitoids; therefore, lower concentrations could be recommended for use in association with the parasitoid to suppress the pest populations because these lower concentrations do not affect C. icipe performance.
5. Conclusions
This laboratory experiment showed that direct application of the fungi may cause detrimental effects to the parasitoids. Therefore, the use of autodissemination devices that combine pheromones/attractants with these potent isolates (especially for ICIPE 7 and ICIPE 621 which are very virulent against adult FAW) should also be evaluated under field conditions, as this application strategy may limit parasitoids’ contact with the fungal inoculum. Direct infection of both M. anisopliae ICIPE 7 and ICIPE 41 were more pathogenic to C. icipe, while the indirect exposure of the parasitoids to infected FAW eggs and larvae showed negative effects on the parasitoids with significant mortalities at high concentrations compared with the lowest concentrations. The highest concentrations of 1 × 109 conidia mL−1 of M. anisopliae ICIPE 78 and ICIPE 41 recorded low emergence of the parasitoid compared with B. bassiana 621. It is worth noting that, the lower concentrations (1 × 105–1 × 107 conidia mL−1) of all the tested isolates did not show any significant detrimental effects to the parasitoids, especially with B. bassiana ICIPE 621. Low concentrations of these potent fungal isolates could therefore be applied together with the parasitoids for sustainable management of the FAW management without affecting the C. icipe performance. Metarhizium anisopliae ICIPE 7 showed a high potential in the management of the FAW larvae, as it recorded a higher mortality rate in both direct and indirect applications. However, applications of high concentrations (1 × 109 and 1 × 108 conidia mL−1) together with the parasitoid should be timed in order to achieve more effective management of FAW larvae under different cropping systems. More field evaluations are thus required to identify the degree of impact due to different environmental conditions which could also interfere with fungus transmission.
Author Contributions
Conceptualization, J.C., K.O.F. and K.S.A.; formal analysis, J.C., F.C.A., K.O.F., J.M. and K.S.A.; funding acquisition, K.S.A.; investigation, J.C., K.O.F., J.M. and K.S.A.; writing—original draft, J.C., K.O.F., F.C.A. and K.S.A.; writing—review and editing, J.C., K.O.F., F.C.A. and K.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the UK’s Foreign, Commonwealth and Development Office (FCDO) (B2329A-FCDO- FAW and B2291A-FCDO -BIOPESTICIDE) and the European Union (EU) project “Integrated pest management strategy to counter the threat of invasive fall armyworm to food security and Eastern Africa (FAW-IPM)” (FOOD/2018402-634) through the International Centre of Insect Physiology and Ecology (icipe).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data related to this work are available upon request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the icipe core funding provided by the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Australian Centre for International Agricultural Research (ACIAR); the Federal Democratic Republic of Ethiopia; and the Government of the Republic of Kenya. The first author was supported through the Dissertation and Research Internship Program (DRIP) of icipe and received a scholarship through a MPhil fellowship provided by the German Academic Exchange Service/Deutcher Akademischer Austausch Dienst (DAAD) through the African Regional Postgraduate Programme in Insect Science (ARPPIS) at the University of Ghana. We are also thankful to Sospeter Wafula, Jane Kimemia, and Levi Ombura for their technical assistance. The views expressed herein do not necessarily reflect the official opinion of the donors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pérez-Escamilla, R. Food security and the 2015–2030 sustainable development goals: From human to planetary health: Perspectives and opinions. Curr. Dev. Nutr. 2017, 1, e000513. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Goergen, G.; Lava, K.P.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae): A New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Tindo, M.; Tigui, A.; Kengni, F.; Atanga, J.; Bila, S.; Doumtsop, A.; Abega, R. First report of the fall army worm, Spodoptera frugiperda (Lepidoptera, Noctuidae) in Cameroon. Cameroon J. Biol. Biochem. Sci. 2017, 25, 30–32. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Sustainable Management of the Fall Armyworm in Africa; FAO Programme for Action; FAO: Rome, Italy, 2017; Available online: http://www.fao.org/3/a-bt417e.pdf (accessed on 19 July 2019).
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Akutse, K.S.; Subramanian, S.; Maniania, K.N.; Dubois, T.; Ekesi, S. Biopesticide Research and Product Development in Africa for Sustainable Agriculture and Food Security—Experiences from the International Centre of Insect Physiology and Ecology (icipe). Front. Sustain. Food Syst. 2020, 4, 563016. [Google Scholar] [CrossRef]
- Akutse, K.S.; Kimemia, J.W.; Ekesi, S.; Khamis, F.M.; Ombura, O.L.; Subramanian, S. Ovicidal effects of entomopathogenic fungal isolates on the invasive Fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Appl. Entomol. 2019, 143, 626–634. [Google Scholar] [CrossRef]
- Akutse, K.S.; Khamis, F.M.; Ambele, F.C.; Kimemia, J.W.; Ekesi, S.; Subramanian, S. Combining insect pathogenic fungi and a pheromone trap for sustainable management of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 2020, 177, 107477. [Google Scholar] [CrossRef]
- Sisay, B.; Simiyu, J.; Malusi, P.; Likhayo, P.; Mendesil, E.; Elibariki, N.; Wakgari, M.; Ayalew, G.; Tefera, T. First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J. Appl. Entomol. 2018, 142, 800–804. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Wamalwa, M.; Obala, F.; Tonnang, H.E.Z.; Tefera, T.; Calatayud, P.A.; Subramanian, S.; Ekesi, S. A deadly encounter: Alien invasive Spodoptera frugiperda in Africa and indigenous natural enemy, Cotesia icipe (Hymenoptera, Braconidae). PLoS ONE 2021, 16, e0253122. [Google Scholar] [CrossRef]
- Fiaboe, K.R.; Agboka, K.; Agboyi, L.K.; Koffi, D.; Ofoe, R.; Kpadonou, G.E.; Agnamba, A.O.; Assogba, K.; Adjevi, M.K.A.; Zanou, K.T.; et al. First report and distribution of the South American tomato pinworm, Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae) in Togo. Phytoparasitica 2021, 49, 167–177. [Google Scholar] [CrossRef]
- Akutse, K.S.; Maniania, N.K.; Fiaboe, K.K.M.; Van den Berg, J.; Ekesi, S. Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life-history parameters of Liriomyza huidobrensis (Diptera: Agromyzidae). Fungal Ecol. 2013, 6, 293–301. [Google Scholar] [CrossRef]
- Goettel, M.S.; Inglis, G.D. Fungi: Hyphomycetes. In Manual of Techniques in Insect Pathology; Academic Press: Cambridge, MA, USA, 1997; pp. 213–249. [Google Scholar]
- Migiro, L.N.; Maniania, N.K.; Chabi-Olaye, A.; Vandenberg, J. Pathogenicity of entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana (Hypocreales: Clavicipitaceae) isolates to the adult pea leafminer (Diptera: Agromyzidae) and prospects of an autoinoculation device for infection in the field. Environ. Entomol. 2010, 39, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Opisa, S.; du Plessis, H.; Akutse, K.S.; Fiaboe, K.K.M.; Ekesi, S. Horizontal transmission of Metarhizium anisopliae between Spoladea recurvalis (Lepidoptera: Crambidae) adults and compatibility of the fungus with the attractant phenylacetaldehyde. Microb. Pathog. 2019, 131, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Burgerjon, A. An improved laboratory apparatus for applying direct sprays and surface films, with data on the electrostatic charge on atomized fluids. Ann. Appl. Biol. 1956, 39, 1–28. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J. Generalized Linear Models, 2nd ed.; Chapman and Hall: London, UK, 1989. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 23 June 2021).
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. Available online: https://www.entomoljournal.com/archives/?year=2018&vol=6&issue=1&ArticleId=2930 (accessed on 18 November 2021).
- Opisa, S.; du Plessis, H.; Akutse, K.S.; Fiaboe, K.K.M.; Ekesi, S. Effects of Entomopathogenic fungi and Bacillus thuringiensis -based biopesticides on Spoladea recurvalis (Lepidoptera: Crambidae). J. Appl. Entomol. 2018, 142, 617–626. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Ansari, M.A.; Pope, E.C.; Carpenter, S.; Scholte, E.J.; Butt, T.M. Entomopathogenic fungus as a biological control for an important vector of livestock disease: The culicoides biting midge. PLoS ONE 2011, 6, e16108. [Google Scholar] [CrossRef]
- Alves, R.T.; Bateman, R.P.; Gunn, J.; Prior, C.; Leather, S.R. Effects of different formulations on viability and medium-term storage of Metarhizium anisopliae conidia. Neotrop. Entomol. 2002, 31, 91–99. [Google Scholar] [CrossRef]
- Abbas, M.S.T. Interactions between Entomopathogenic Fungi and Entomophagous Insects. Adv. Entomol. 2020, 8, 130–146. [Google Scholar] [CrossRef]
- Sisay, B.; Simiyu, J.; Mendesil, E.; Likhayo, P.; Ayalew, G.; Mohamed, S.; Subramanian, S.; Tefera, T. Fall armyworm, Spodoptera frugiperda infestations in East Africa: Assessment of damage and parasitism. Insects 2019, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Uma Devi, K.; Padmavathi, J.; Uma Maheswara Rao, C.; Khan, A.A.P.; Mohan, M.C. A study of host specificity in the entomopathogenic fungus Beauveria bassiana (Hypocreales, Clavicipitaceae). Biocontrol Sci. Technol. 2008, 18, 975–989. [Google Scholar] [CrossRef]
- Montesinos, E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Caballero, R.; Cyman, S.; Schuster, D.J.; Portillo, H.E.; Slater, R. Baseline susceptibility of Bemisia tabaci (Genn.) biotype B in southern Florida to cyantraniliprole. Crop. Prot. 2013, 44, 104–108. [Google Scholar] [CrossRef]
- Dimbi, S.; Maniania, N.K.; Lux, S.A.; Ekesi, S.; Mueke, J.K. Pathogenicity of Metarhizium anisopliae (Metsch.) Sorokin and Beauveria bassiana (Balsamo) Vuillemin, to three adult fruit fly species: Ceratitis capitata (Weidemann), C. rosa var. fasciventris Karsch and C. cosyra (Walker) (Diptera: Tephritidae). Mycopathologia 2003, 156, 375–382. [Google Scholar] [CrossRef]
- Förster, H.; Kanetis, L.; Adaskaveg, J.E. Spiral gradient dilution, a rapid method for determining growth responses and 50% effective concentration values in fungus-fungicide interactions. Phytopathology 2004, 94, 163–170. [Google Scholar] [CrossRef]
- Ramanujam, B.; Poornesha, B.; Shylesha, A.N. Effect of entomopathogenic fungi against invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in maize. Egypt J. Biol. Pest Control 2020, 30, 1–5. [Google Scholar] [CrossRef]
- Morales-Reyes, C.; Mascarin, G.M.; Jackson, M.A.; Hall, D.; Sánchez-Peña, S.R.; Arthurs, S.P. Comparison of aerial conidia and blastospores from two entomopathogenic fungi against Diaphorina citri (Hemiptera: Liviidae) under laboratory and greenhouse conditions. Biocontrol. Sci. Technol. 2018, 28, 737–749. [Google Scholar] [CrossRef]
- García-Munguía, A.M.; Garza-Hernndez, J.A.; Rebollar-Tellez, E.A.; Rodríguez-Pérez, M.A.; Reyes-Villanueva, F. Transmission of Beauveria bassiana from male to female Aedes aegypti mosquitoes. Parasites Vectors 2011, 4, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Mumo, L.; Yu, J.; Fang, K. Assessing impacts of seasonal climate variability on maize yield in Kenya. Int. J. Plant Prod. 2018, 12, 297–307. [Google Scholar] [CrossRef]
- Polanczyk, R.A.; Pratissoli, D.; Dalvi, L.P.; Grecco, E.D.; Franco, C.R. Efeito de Beauveria bassiana (Bals.) Vuillemin e Metarhizium anisopliae (Metsch.) Sorokin nos parâmetros biológicos de Trichogramma atopovirilia Oatman & Platner, 1983 (Hymenoptera: Trichogrammatidade). Cienc. Agrotec. 2010, 34, 1412–1416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).