Genetics of Resistance to Leaf Rust in Wheat: An Overview in a Genome-Wide Level
Abstract
1. Introduction
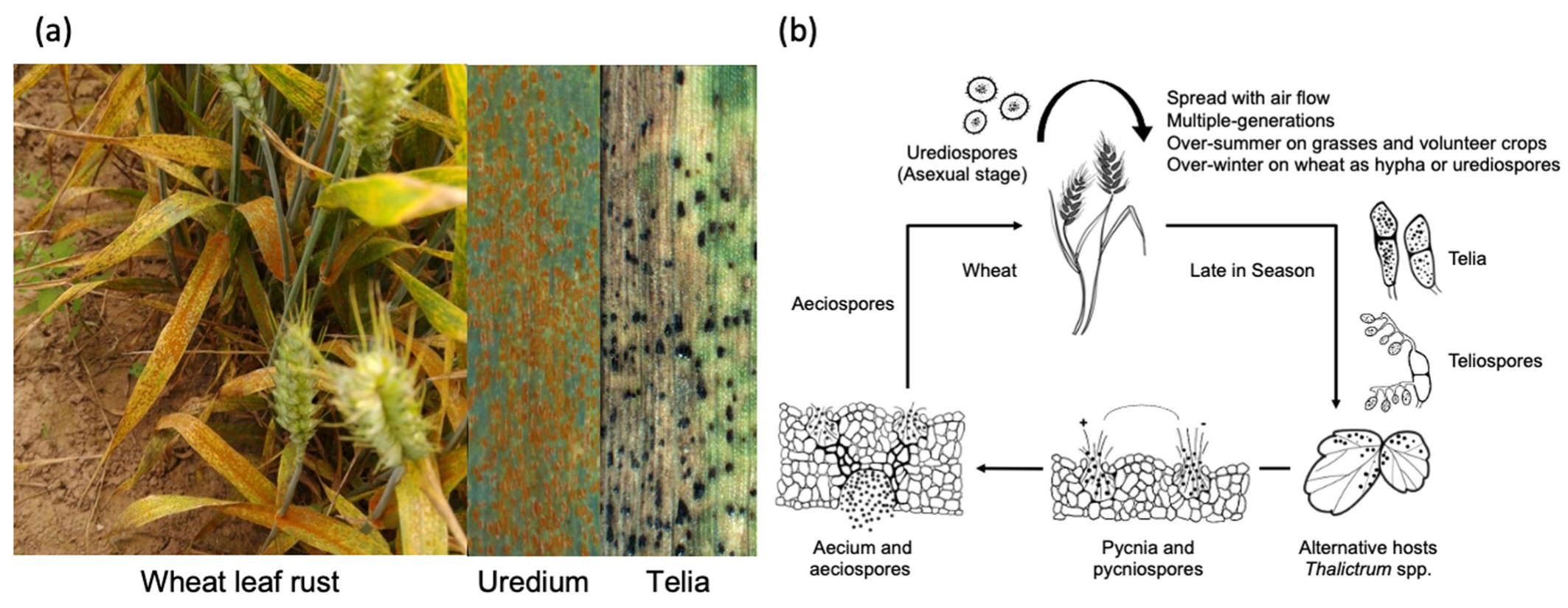
2. Pathogenic Profile
3. Types of Wheat Resistance to Leaf Rust
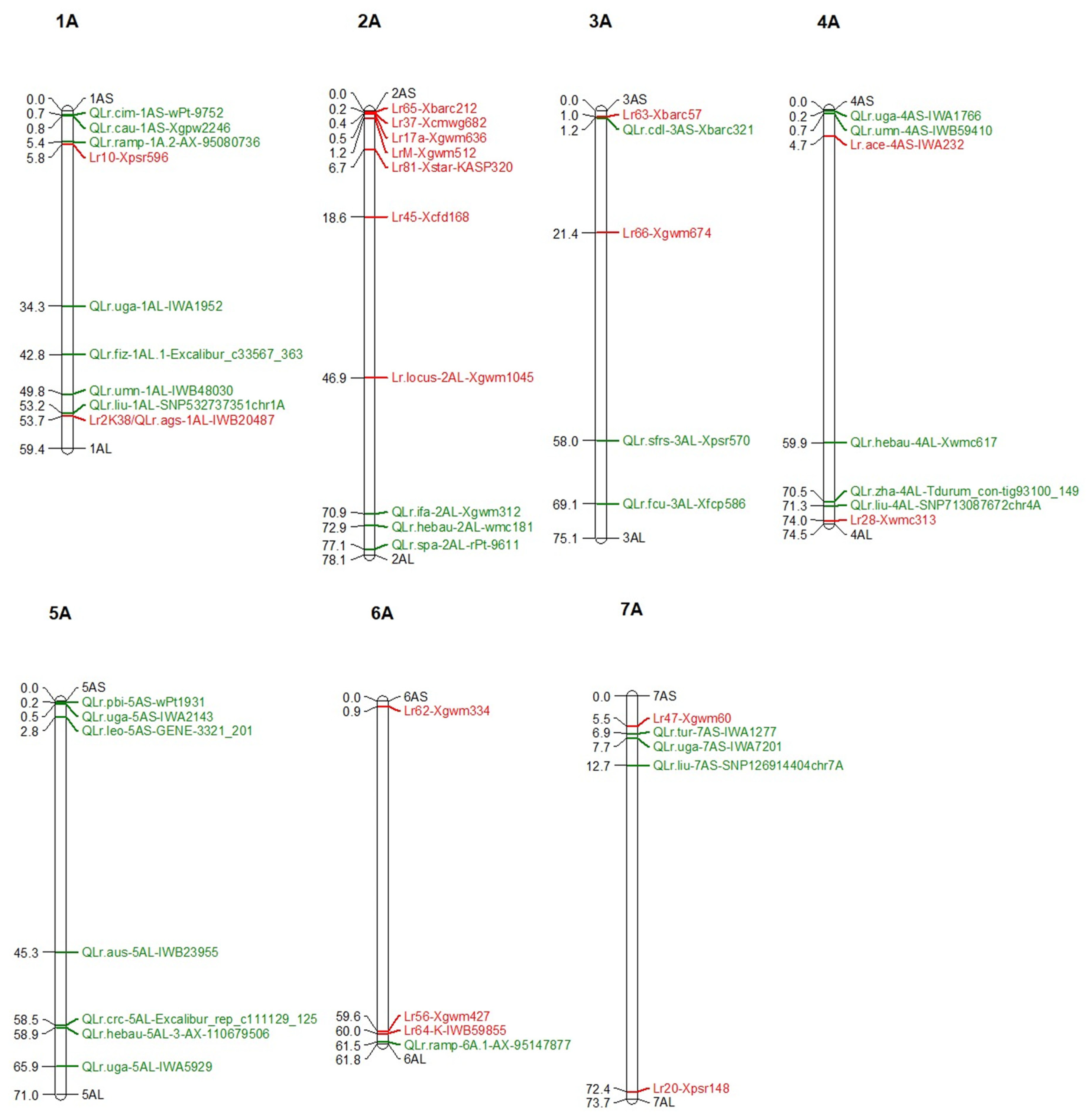
4. Genetic Loci Carrying Lr/QLr in A Sub-Genome
4.1. Lr/QLr on Chromosome 1A
4.2. Lr/QLr on Chromosome 2A
4.3. Lr/QLr on Chromosome 3A
4.4. Lr/QLr on Chromosome 4A
4.5. Lr/QLr on Chromosome 5A
4.6. Lr/QLr on Chromosome 6A
4.7. Lr/QLr on Chromosome 7A
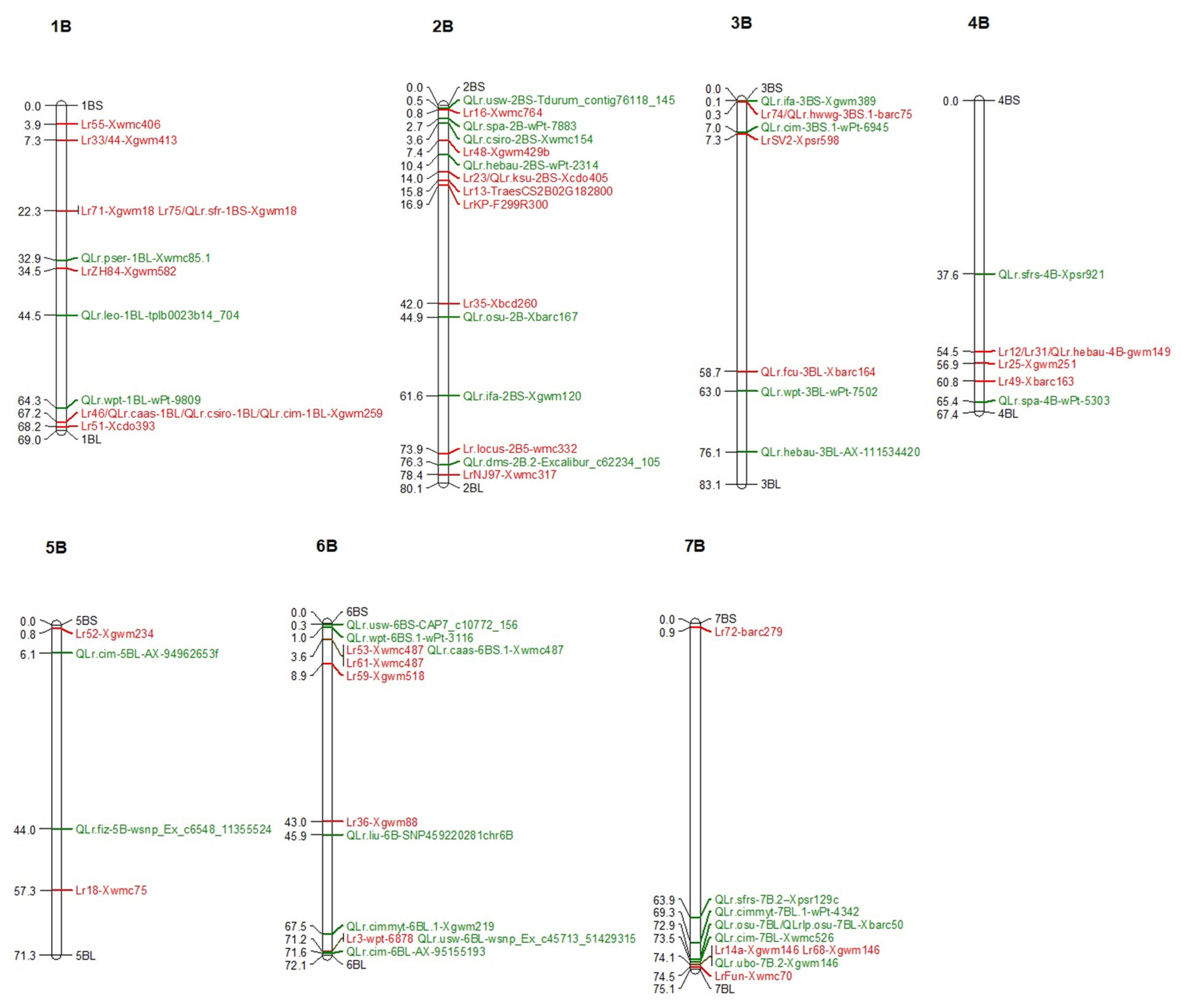
5. Genetic Loci Carrying Lr/QLr in B Sub-Genome
5.1. Lr/QLr on Chromosome 1B
5.2. Lr/QLr on Chromosome 2B
5.3. Lr/QLr on Chromosome 3B
5.4. Lr/QLr on Chromosome 4B
5.5. Lr/QLr on Chromosome 5B
5.6. Lr/QLr on Chromosome 6B
5.7. Lr/QLr on Chromosome 7B
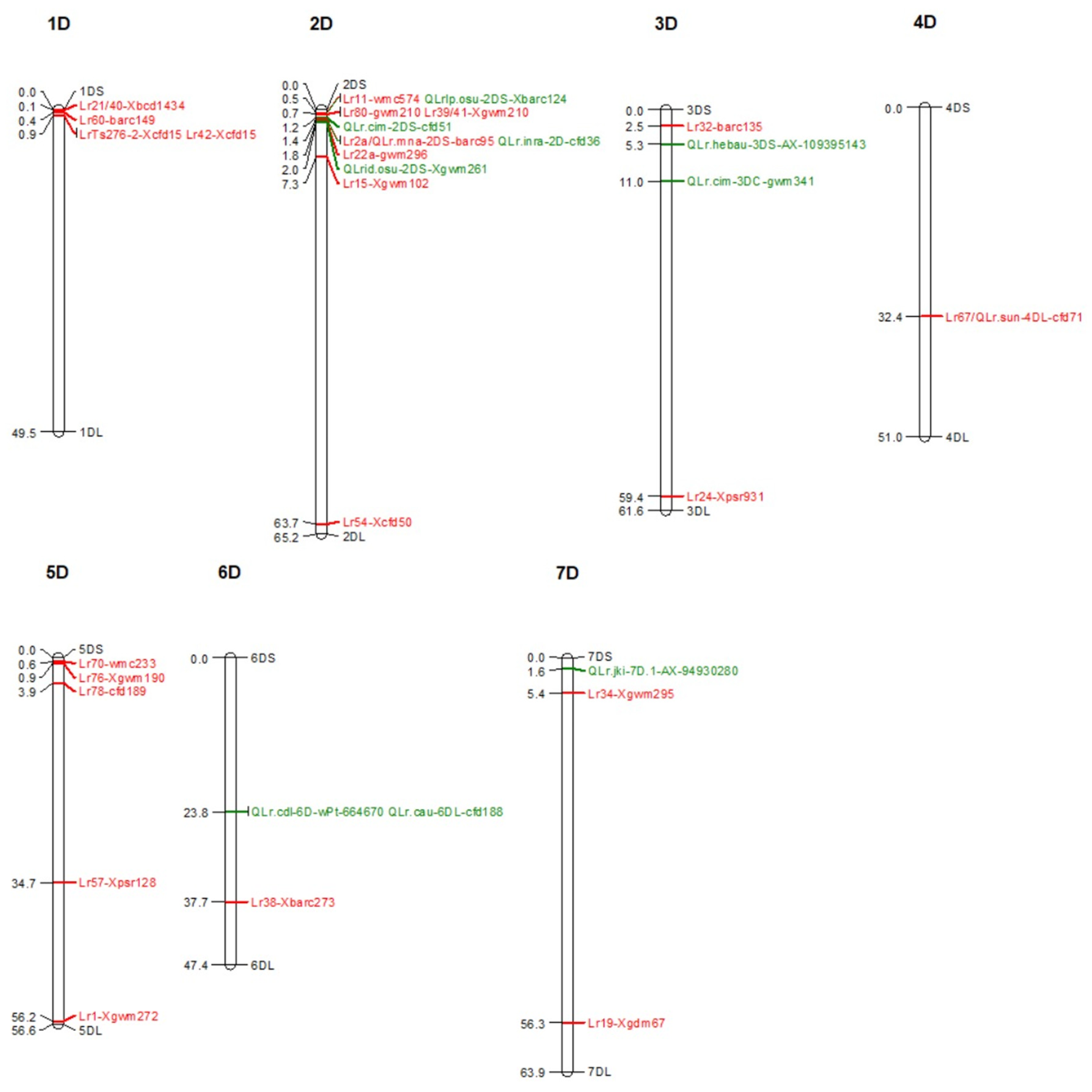
6. Genetic Loci Carrying Lr/QLr in D Sub-Genome
6.1. Lr/QLr on Chromosome 1D
6.2. Lr/QLr on Chromosome 2D
6.3. Lr/QLr on Chromosome 3D
6.4. Lr/QLr on Chromosome 4D
6.5. Lr/QLr on Chromosome 5D
6.6. Lr/QLr on Chromosome 6D
6.7. Lr/QLr on Chromosome 7D
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolton, M.D.; Kolmer, J.A.; Garvin, D.F. Wheat leaf rust caused by Puccinia triticina. Mol. Plant Pathol. 2008, 9, 563–575. [Google Scholar] [CrossRef]
- Eversmeyer, M.; Kramer, C. Epidemiology of wheat leaf and stem rust in the central great plains of the USA. Annu. Rev. Phytopathol. 2000, 38, 491. [Google Scholar] [CrossRef]
- Helfer, S. Rust fungi and global change. New Phytol. 2014, 201, 770–780. [Google Scholar] [CrossRef]
- Cloutier, S.; McCallum, B.D.; Loutre, C.; Banks, T.W.; Wicker, T.; Feuillet, C.; Keller, B.; Jordan, M.C. Leaf rust resistance gene Lr1, isolated from bread wheat (Triticum aestivum L.) is a member of the large psr567 gene family. Plant Mol. Biol. 2007, 65, 93–106. [Google Scholar] [CrossRef]
- Feuillet, C.; Travella, S.; Stein, N.; Albar, L.; Nublat, A.; Keller, B. Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploid wheat (Triticum aestivum L.) genome. Proc. Natl. Acad. Sci. USA 2003, 100, 15253–15258. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Zhang, P.; Yin, G.; Zhang, H.; Gebrewahid, T.W.; Zhang, J.; Dong, L.; Liu, D.; Liu, Z. High-temperature wheat leaf rust resistance gene Lr13 exhibits pleiotropic effects on hybrid necrosis. Mol. Plant 2021, 14, 1029–1032. [Google Scholar] [CrossRef]
- Huang, L.; Brooks, S.A.; Li, W.; Fellers, J.P.; Trick, H.N.; Gill, B.S. Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploid genome of bread wheat. Genetics 2003, 164, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Thind, A.K.; Wicker, T.; Šimková, H.; Fossati, D.; Moullet, O.; Brabant, C.; Vrána, J.; Doležel, J.; Krattinger, S.G. Rapid cloning of genes in hexaploid wheat using cultivar-specific long-range chromosome assembly. Nat. Biotech. 2017, 35, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Chen, H.; Tian, B.; Sehgal, S.K.; Singh, L.; Xie, J.; Rawat, N.; Juliana, P.; Singh, N.; Shrestha, S. Cloning of the broadly effective wheat leaf rust resistance gene Lr42 transferred from Aegilops tauschii. Nat. Commun. 2022, 13, 3044. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.-W.; Zhou, J.-M.; Chai, J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 2019, 364, eaav5870. [Google Scholar] [CrossRef]
- Zhao, Y.-B.; Liu, M.-X.; Chen, T.-T.; Ma, X.; Li, Z.-K.; Zheng, Z.; Zheng, S.-R.; Chen, L.; Li, Y.-Z.; Tang, L.-R.; et al. Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism. Sci. Adv. 2022, 8, eabq5108. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, M.C.; Singla, J.; Sánchez-Martín, J.; Zbinden, H.; Šimková, H.; Karafiátová, M.; Doležel, J.; Gronnier, J.; Poretti, M.; Glauser, G. A membrane-bound ankyrin repeat protein confers race-specific leaf rust disease resistance in wheat. Nat. Commun. 2021, 12, 956. [Google Scholar] [CrossRef]
- Wang, Y.; Abrouk, M.; Gourdoupis, S.; Koo, D.-H.; Karafiátová, M.; Molnár, I.; Doležel, J.; Athiyannan, N.; Cavalet-Giorsa, E.; Jaremko, Ł.; et al. An unusual tandem kinase fusion protein confers leaf rust resistance in wheat. Res. Sq. 2022. preprint. [Google Scholar]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.A.M.; Steuernagel, B.; Wulff, B.B. Rapid gene cloning in wheat. In Applications of Genetic and Genomic Research in Cereals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–95. [Google Scholar]
- Lan, C.; Zhang, Y.; Herrera-Foessel, S.A.; Basnet, B.R.; Huerta-Espino, J.; Lagudah, E.S.; Singh, R.P. Identification and characterization of pleiotropic and co-located resistance loci to leaf rust and stripe rust in bread wheat cultivar Sujata. Theor. Appl. Genet. 2015, 128, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Che, M.; Li, G.; Chen, J.; Quan, W.; Guo, Y.; Wang, Z.; Ren, J.; Zhang, H.; Zhang, Z. A QTL with major effect on reducing leaf rust severity on the short arm of chromosome 1A of wheat detected across different genetic backgrounds and diverse environments. Theor. Appl. Genet. 2015, 128, 1579–1594. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, A.; Chhokar, V.; Gangwar, O.P.; Bhardwaj, S.C.; Sivasamy, M.; Prasad, S.S.; Prakasha, T.; Khan, H.; Singh, R. Genome-wide association studies in diverse spring wheat panel for stripe, stem, and leaf rust resistance. Front. Plant Sci. 2020, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Loutre, C.; Wicker, T.; Travella, S.; Galli, P.; Scofield, S.; Fahima, T.; Feuillet, C.; Keller, B. Two different CC-NBS-LRR genes are required for Lr10-mediated leaf rust resistance in tetraploid and hexaploid wheat. Plant J. 2009, 60, 1043–1054. [Google Scholar] [CrossRef]
- Sapkota, S.; Hao, Y.; Johnson, J.; Buck, J.; Aoun, M.; Mergoum, M. Genome-wide association study of a worldwide collection of wheat genotypes reveals novel quantitative trait loci for leaf rust resistance. Plant Genome 2019, 12, 190033. [Google Scholar] [CrossRef]
- Fatima, F.; McCallum, B.D.; Pozniak, C.J.; Hiebert, C.W.; McCartney, C.A.; Fedak, G.; You, F.M.; Cloutier, S. Identification of new leaf rust resistance loci in wheat and wild relatives by array-based SNP genotyping and association genetics. Front. Plant Sci. 2020, 11, 583738. [Google Scholar] [CrossRef]
- Gao, L.; Turner, M.K.; Chao, S.; Kolmer, J.; Anderson, J.A. Genome wide association study of seedling and adult plant leaf rust resistance in elite spring wheat breeding lines. PLoS ONE 2016, 11, e0148671. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, Y.; Zhao, Y.; Schulthess, A.W.; Reif, J.C. Haplotype-based genome-wide association increases the predictability of leaf rust (Puccinia triticina) resistance in wheat. J. Exp. Bot. 2020, 71, 6958–6968. [Google Scholar] [CrossRef]
- Sapkota, S.; Mergoum, M.; Kumar, A.; Fiedler, J.D.; Johnson, J.; Bland, D.; Lopez, B.; Sutton, S.; Ghimire, B.; Buck, J. A novel adult plant leaf rust resistance gene Lr2K38 mapped on wheat chromosome 1AL. Plant Genome 2020, 13, e20061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wei, W.; Zuansun, X.; Zhang, S.; Wang, C.; Liu, N.; Qiu, L.; Wang, W.; Guo, W.; Ma, J. Fine Mapping of the Leaf Rust Resistance Gene Lr65 in Spelt Wheat ‘Altgold’. Front. Plant Sci. 2021, 12, 666921. [Google Scholar] [CrossRef]
- Helguera, M.; Khan, I.; Kolmer, J.; Lijavetzky, D.; Zhong-Qi, L.; Dubcovsky, J. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci. 2003, 43, 1839–1847. [Google Scholar] [CrossRef]
- Bremenkamp-Barrett, B.; Faris, J.D.; Fellers, J.P. Molecular mapping of the leaf rust resistance gene Lr17a in wheat. Crop Sci. 2008, 48, 1124–1128. [Google Scholar] [CrossRef]
- Xu, X.; Kolmer, J.; Li, G.; Tan, C.; Carver, B.F.; Bian, R.; Bernardo, A.; Bai, G. Identification and characterization of the novel leaf rust resistance gene Lr81 in wheat. Theor. Appl. Genet. 2022, 135, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Raghu, B.; Jha, S.; Agarwal, P.; Mallick, N.; Niranjana, M.; Sharma, J.; Singh, A.; Sharma, N.; Rajkumar, S. A novel leaf rust resistance gene introgressed from Aegilops markgrafii maps on chromosome arm 2AS of wheat. Theor. Appl. Genet. 2020, 133, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.K.; Sharma, J.; Sivasamy, M.; Prabhu, K.; Tomar, R.; Tomar, S. Molecular mapping and validation of the microsatellite markers linked to the Secale cereale-derived leaf rust resistance gene Lr45 in wheat. Mol. Breed. 2015, 35, 61. [Google Scholar] [CrossRef]
- Aoun, M.; Breiland, M.; Kathryn Turner, M.; Loladze, A.; Chao, S.; Xu, S.S.; Ammar, K.; Anderson, J.A.; Kolmer, J.A.; Acevedo, M. Genome-wide association mapping of leaf rust response in a durum wheat worldwide germplasm collection. Plant Genome 2016, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, M.; Matiasch, L.; Mascher, F.; Vida, G.; Ittu, M.; Robert, O.; Holdgate, S.; Flath, K.; Neumayer, A.; Buerstmayr, H. Mapping of quantitative adult plant field resistance to leaf rust and stripe rust in two European winter wheat populations reveals co-location of three QTL conferring resistance to both rust pathogens. Theor. Appl. Genet. 2014, 127, 2011–2028. [Google Scholar] [CrossRef]
- Zhang, P.; Yin, G.; Zhou, Y.; Qi, A.; Gao, F.; Xia, X.; He, Z.; Li, Z.; Liu, D. QTL mapping of adult-plant resistance to leaf rust in the wheat cross Zhou 8425B/Chinese Spring using high-density SNP markers. Front. Plant Sci. 2017, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Knox, R.; DePauw, R.; Singh, A.; Cuthbert, R.; Campbell, H.; Shorter, S.; Bhavani, S. Stripe rust and leaf rust resistance QTL mapping, epistatic interactions, and co-localization with stem rust resistance loci in spring wheat evaluated over three continents. Theor. Appl. Genet. 2014, 127, 2465–2477. [Google Scholar] [CrossRef]
- Kolmer, J.; Anderson, J.; Flor, J. Chromosome location, linkage with simple sequence repeat markers, and leaf rust resistance conditioned by gene Lr63 in wheat. Crop Sci. 2010, 50, 2392–2395. [Google Scholar] [CrossRef]
- Kolmer, J.; Garvin, D.; Hayden, M.; Spielmeyer, W. Adult plant leaf rust resistance derived from the wheat landrace cultivar Americano 44d is conditioned by interaction of three QTL. Euphytica 2018, 214, 59. [Google Scholar] [CrossRef]
- Marais, G.; Bekker, T.; Eksteen, A.; McCallum, B.; Fetch, T.; Marais, A. Attempts to remove gametocidal genes co-transferred to common wheat with rust resistance from Aegilops speltoides. Euphytica 2010, 171, 71–85. [Google Scholar] [CrossRef]
- Messmer, M.; Seyfarth, R.; Keller, M.; Schachermayr, G.; Winzeler, M.; Zanetti, S.; Feuillet, C.; Keller, B. Genetic analysis of durable leaf rust resistance in winter wheat. Theor. Appl. Genet. 2000, 100, 419–431. [Google Scholar] [CrossRef]
- Chu, C.-G.; Friesen, T.; Xu, S.; Faris, J.; Kolmer, J. Identification of novel QTLs for seedling and adult plant leaf rust resistance in a wheat doubled haploid population. Theor. Appl. Genet. 2009, 119, 263–269. [Google Scholar] [CrossRef]
- Aoun, M.; Kolmer, J.A.; Rouse, M.N.; Elias, E.M.; Breiland, M.; Bulbula, W.D.; Chao, S.; Acevedo, M. Mapping of novel leaf rust and stem rust resistance genes in the Portuguese durum wheat landrace PI 192051. G3-Genes Genom. Genet. 2019, 9, 2535–2547. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, X.; Gebrewahid, T.-W.; Zhou, Y.; Yang, E.; Xia, X.; He, Z.; Li, Z.; Liu, D. Genome-wide association mapping of leaf rust and stripe rust resistance in wheat accessions using the 90K SNP array. Theor. Appl. Genet. 2021, 134, 1233–1251. [Google Scholar] [CrossRef]
- Bipinraj, A.; Honrao, B.; Prashar, M.; Bhardwaj, S.; Rao, S.; Tamhankar, S. Validation and identification of molecular markers linked to the leaf rust resistance gene Lr28 in wheat. J. Appl. Genet. 2011, 52, 171–175. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, D.; Satapathy, L.; Pathak, J.; Chandra, S.; Riaz, A.; Bhaganagre, G.; Dhariwal, R.; Kumar, M.; Prabhu, K.V.; et al. Insights of Lr28 mediated wheat leaf rust resistance: Transcriptomic approach. Gene 2017, 637, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Sinha, N.; Krishna, H.; Singh, P.K.; Gautam, T.; Prasad, P.; Balyan, H.S.; Gupta, P.K. A study of miRNAs and lncRNAs during Lr28-mediated resistance against leaf rust in wheat (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2020, 112, 101552. [Google Scholar] [CrossRef]
- Singh, D.; Simmonds, J.; Park, R.F.; Bariana, H.S.; Snape, J.W. Inheritance and QTL mapping of leaf rust resistance in the European winter wheat cultivar ‘Beaver’. Euphytica 2009, 169, 253–261. [Google Scholar] [CrossRef]
- Leonova, I.N.; Skolotneva, E.S.; Salina, E.A. Genome-wide association study of leaf rust resistance in Russian spring wheat varieties. BMC Plant Biol. 2020, 20, 135. [Google Scholar] [CrossRef]
- Pasam, R.K.; Bansal, U.; Daetwyler, H.D.; Forrest, K.L.; Wong, D.; Petkowski, J.; Willey, N.; Randhawa, M.; Chhetri, M.; Miah, H. Detection and validation of genomic regions associated with resistance to rust diseases in a worldwide hexaploid wheat landrace collection using BayesR and mixed linear model approaches. Theor. Appl. Genet. 2017, 130, 777–793. [Google Scholar] [CrossRef]
- Rosa, S.B.; Zanella, C.M.; Hiebert, C.W.; Brûlé-Babel, A.L.; Randhawa, H.S.; Shorter, S.; Boyd, L.A.; McCallum, B.D. Genetic characterization of leaf and stripe rust resistance in the Brazilian wheat cultivar Toropi. Phytopathology 2019, 109, 1760–1768. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Gebrewahid, T.-W.; Liu, H.; Xia, X.; He, Z.; Li, Z.; Liu, D. QTL mapping of adult-plant resistance to leaf and stripe rust in wheat cross SW 8588/Thatcher using the wheat 55K SNP array. Plant Dis. 2019, 103, 3041–3049. [Google Scholar] [CrossRef]
- Marais, F.; Marais, A.; McCallum, B.; Pretorius, Z. Transfer of leaf rust and stripe rust resistance genes Lr62 and Yr42 from Aegilops neglecta Req. ex Bertol. to common wheat. Crop Sci. 2009, 49, 871–879. [Google Scholar] [CrossRef]
- Marais, G.; Badenhorst, P.; Eksteen, A.; Pretorius, Z. Reduction of Aegilops sharonensis chromatin associated with resistance genes Lr56 and Yr38 in wheat. Euphytica 2010, 171, 15–22. [Google Scholar] [CrossRef]
- Kolmer, J.; Bernardo, A.; Bai, G.; Hayden, M.; Anderson, J. Thatcher wheat line RL6149 carries Lr64 and a second leaf rust resistance gene on chromosome 1DS. Theor. Appl. Genet. 2019, 132, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Helguera, M.; Khan, I.; Dubcovsky, J. Development of PCR markers for the wheat leaf rust resistance gene Lr47. Theor. Appl. Genet. 2000, 100, 1137–1143. [Google Scholar] [CrossRef]
- Brevis, J.C.; Chicaiza, O.; Khan, I.A.; Jackson, L.; Morris, C.F.; Dubcovsky, J. Agronomic and quality evaluation of common wheat near-isogenic lines carrying the leaf rust resistance gene Lr47. Crop Sci. 2008, 48, 1441–1451. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Bi, W.; Zhao, J.; Yu, X.; Li, Z.; Liu, D.; Liu, B.; Wang, X. Genome-wide expression profiling of genes associated with the Lr47-mediated wheat resistance to leaf rust (Puccinia triticina). Int. J. Mol. Sci. 2019, 20, 4498. [Google Scholar] [CrossRef]
- Xu, X.; Li, G.; Bai, G.; Bernardo, A.; Carver, B.F.; St. Amand, P.; Bian, R. Characterization of an incomplete leaf rust resistance gene on chromosome 1RS and development of KASP markers for Lr47 in wheat. Phytopathology 2021, 111, 649–658. [Google Scholar] [CrossRef]
- Turner, M.K.; Kolmer, J.A.; Pumphrey, M.O.; Bulli, P.; Chao, S.; Anderson, J.A. Association mapping of leaf rust resistance loci in a spring wheat core collection. Theor. Appl. Genet. 2017, 130, 345–361. [Google Scholar] [CrossRef]
- Neu, C.; Stein, N.; Keller, B. Genetic mapping of the Lr20 Pm1 resistance locus reveals suppressed recombination on chromosome arm 7AL in hexaploid wheat. Genome 2002, 45, 737–744. [Google Scholar] [CrossRef]
- Pietrusińska, A.; Tyrka, M. Linkage of Lr55 wheat leaf rust resistance gene with microsatellite and DArT-based markers. Physiol. Mol. Plant Pathol. 2021, 115, 101674. [Google Scholar] [CrossRef]
- Che, M.; Hiebert, C.W.; McCartney, C.A.; Zhang, Z.; McCallum, B.D. Mapping and DNA marker development for Lr33 from the leaf rust resistant line KU168-2. Euphytica 2019, 215, 29. [Google Scholar] [CrossRef]
- Dyck, P.; Sykes, E. Genetics of leaf-rust resistance in three spelt wheats. Can. J. Plant Sci. 1994, 74, 231–233. [Google Scholar] [CrossRef]
- Singh, D.; Mohler, V.; Park, R. Discovery, characterisation and mapping of wheat leaf rust resistance gene Lr71. Euphytica 2013, 190, 131–136. [Google Scholar] [CrossRef]
- Schnurbusch, T.; Paillard, S.; Schori, A.; Messmer, M.; Schachermayr, G.; Winzeler, M.; Keller, B. Dissection of quantitative and durable leaf rust resistance in Swiss winter wheat reveals a major resistance QTL in the Lr34 chromosomal region. Theor. Appl. Genet. 2004, 108, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Singla, J.; Lüthi, L.; Wicker, T.; Bansal, U.; Krattinger, S.G.; Keller, B. Characterization of Lr75: A partial, broad-spectrum leaf rust resistance gene in wheat. Theor. Appl. Genet. 2017, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bai, G. Lesion mimic associates with adult plant resistance to leaf rust infection in wheat. Theor. Appl. Genet. 2009, 119, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, T.; Xia, X.; He, Z.; Liu, D.; Yang, W.; Yin, G.; Li, Z. Molecular mapping of leaf rust resistance gene LrZH84 in Chinese wheat line Zhou 8425B. Theor. Appl. Genet. 2008, 117, 1069–1075. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, X.; He, Z.; Li, X.; Li, Z.; Liu, D. Fine mapping of leaf rust resistance gene LrZH84 using expressed sequence tag and sequence-tagged site markers, and allelism with other genes on wheat chromosome 1B. Phytopathology 2013, 103, 169–174. [Google Scholar] [CrossRef]
- William, M.; Singh, R.; Huerta-Espino, J.; Islas, S.O.; Hoisington, D. Molecular marker mapping of leaf rust resistance gene Lr46 and its association with stripe rust resistance gene Yr29 in wheat. Phytopathology 2003, 93, 153–159. [Google Scholar] [CrossRef]
- Mateos-Hernandez, M.; Singh, R.P.; Hulbert, S.H.; Bowden, R.L.; Huerta-Espino, J.; Gill, B.S.; Brown-Guedira, G. Targeted mapping of ESTs linked to the adult plant resistance gene Lr46 in wheat using synteny with rice. Funct. Integr. Genomic. 2006, 6, 122–131. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Z.; He, Z.; Wu, L.; Bai, B.; Lan, C.; Wang, C.; Zhou, G.; Zhu, H.; Xia, X. QTL mapping of adult-plant resistances to stripe rust and leaf rust in Chinese wheat cultivar Bainong 64. Theor. Appl. Genet. 2012, 125, 1253–1262. [Google Scholar] [CrossRef]
- Rosewarne, G.; Singh, R.; Huerta-Espino, J.; Rebetzke, G. Quantitative trait loci for slow-rusting resistance in wheat to leaf rust and stripe rust identified with multi-environment analysis. Theor. Appl. Genet. 2008, 116, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Helguera, M.; Vanzetti, L.; Soria, M.; Khan, I.; Kolmer, J.; Dubcovsky, J. PCR markers for Triticum speltoides leaf rust resistance gene Lr51 and their use to develop isogenic hard red spring wheat lines. Crop Sci. 2005, 45, 728–734. [Google Scholar] [CrossRef]
- De Froidmont, D. A co-dominant marker for the 1BL/1RS wheat-rye translocation via multiplex PCR. J. Cereal Sci. 1998, 27, 229–232. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Yang, J.; He, H.; Jin, H.; Li, X.; Ren, T.; Ren, Z.; Li, F.; Han, X. A high-quality genome assembly highlights rye genomic characteristics and agronomically important genes. Nat. Genet. 2021, 53, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Kthiri, D.; Loladze, A.; N’Diaye, A.; Nilsen, K.T.; Walkowiak, S.; Dreisigacker, S.; Ammar, K.; Pozniak, C.J. Mapping of genetic loci conferring resistance to leaf rust from three globally resistant durum wheat sources. Front. Plant Sci. 2019, 10, 1247. [Google Scholar] [CrossRef]
- McCartney, C.; Somers, D.; McCallum, B.; Thomas, J.; Humphreys, D.; Menzies, J.; Brown, P. Microsatellite tagging of the leaf rust resistance gene Lr16 on wheat chromosome 2BSc. Mol. Breed. 2005, 15, 329–337. [Google Scholar] [CrossRef]
- Kassa, M.T.; You, F.M.; Hiebert, C.W.; Pozniak, C.J.; Fobert, P.R.; Sharpe, A.G.; Menzies, J.G.; Humphreys, D.G.; Rezac Harrison, N.; Fellers, J.P. Highly predictive SNP markers for efficient selection of the wheat leaf rust resistance gene Lr16. BMC Plant Biol. 2017, 17, 45. [Google Scholar] [CrossRef]
- Bansal, U.K.; Hayden, M.J.; Venkata, B.; Khanna, R.; Saini, R.; Bariana, H.S. Genetic mapping of adult plant leaf rust resistance genes Lr48 and Lr49 in common wheat. Theor. Appl. Genet. 2008, 117, 307–312. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, Y.; Lillemo, M.; Yao, Z.; Zhang, P.; Xia, X.; He, Z.; Li, Z.; Liu, D. QTL mapping of adult-plant resistance to leaf rust in a RIL population derived from a cross of wheat cultivars Shanghai 3/Catbird and Naxos. Theor. Appl. Genet. 2014, 127, 1873–1883. [Google Scholar] [CrossRef]
- Nelson, J.C. QGENE: Software for marker-based genomic analysis and breeding. Mol. Breed. 1997, 3, 239–245. [Google Scholar] [CrossRef]
- Faris, J.; Li, W.; Liu, D.; Chen, P.; Gill, B. Candidate gene analysis of quantitative disease resistance in wheat. Theor. Appl. Genet. 1999, 98, 219–225. [Google Scholar] [CrossRef]
- Hewitt, T.; Zhang, J.; Huang, L.; Upadhyaya, N.; Li, J.; Park, R.; Hoxha, S.; McIntosh, R.; Lagudah, E.; Zhang, P. Wheat leaf rust resistance gene Lr13 is a specific Ne2 allele for hybrid necrosis. Mol. Plant 2021, 14, 1025–1028. [Google Scholar] [CrossRef]
- Bai, S.; Pang, S.; Li, H.; Yang, J.; Yu, H.; Chen, S.; Wang, X. Broad-Spectrum Resistance to Leaf Rust in the Argentinean Wheat Cultivar “Klein Proteo” Is Controlled by LrKP Located on Chromosome 2BS. Agriculture 2022, 12, 1836. [Google Scholar] [CrossRef]
- Seyfarth, R.; Feuillet, C.; Schachermayr, G.; Winzeler, M.; Keller, B. Development of a molecular marker for the adult plant leaf rust resistance gene Lr35 in wheat. Theor. Appl. Genet. 1999, 99, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, F.; Liang, F.; Zhang, Y.; Ma, L.; Wang, H.; Liu, D. Functional analysis of a pathogenesis-related thaumatin-like protein gene TaLr35PR5 from wheat induced by leaf rust fungus. BMC Plant Biol. 2018, 18, 76. [Google Scholar] [CrossRef]
- Liang, F.; Du, X.; Zhang, J.; Li, X.; Wang, F.; Wang, H.; Liu, D. Wheat TaLr35PR2 gene is required for Lr35-mediated adult plant resistance against leaf rust fungus. Funct. Plant Biol. 2019, 47, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Zhang, W.; Zhang, J.; Wang, H.; Liu, D. Expression profiles of pathogenesis-related gene, TaLr35PR1, as it relate to Lr35-mediated adult plant leaf rust resistance. Plant Mol. Biol. Rep. 2016, 34, 1127–1135. [Google Scholar] [CrossRef]
- Xu, X.; Bai, G.; Carver, B.F.; Shaner, G.E.; Hunger, R.M. Molecular characterization of slow leaf-rusting resistance in wheat. Crop Sci. 2005, 45, 758–765. [Google Scholar] [CrossRef]
- Iqbal, M.; Semagn, K.; Jarquin, D.; Randhawa, H.; McCallum, B.D.; Howard, R.; Aboukhaddour, R.; Ciechanowska, I.; Strenzke, K.; Crossa, J. Identification of Disease Resistance Parents and Genome-Wide Association Mapping of Resistance in Spring Wheat. Plants 2022, 11, 2905. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, X.; He, Z.; Li, X.; Wang, C.; Li, Z.; Liu, D. Molecular mapping of leaf rust resistance gene LrNJ97 in Chinese wheat line Neijiang 977671. Theor. Appl. Genet. 2013, 126, 2141–2147. [Google Scholar] [CrossRef]
- Brown-Guedira, G.; Singh, S.; Fritz, A. Performance and mapping of leaf rust resistance transferred to wheat from Triticum timopheevii subsp. armeniacum. Phytopathology 2003, 93, 784–789. [Google Scholar] [CrossRef]
- Kolmer, J.; Chao, S.; Brown-Guedira, G.; Bansal, U.; Bariana, H. Adult plant leaf rust resistance derived from the soft red winter wheat cultivar ‘Caldwell’maps to chromosome 3BS. Crop Sci. 2018, 58, 152–158. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Li, C.; Bowden, R.; Bai, G.; Li, C.; Li, C.; Su, Z.; Carver, B.F. Mapping of quantitative trait loci for leaf rust resistance in the wheat population Ning7840× Clark. Plant Dis. 2017, 101, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Rosewarne, G.M.; Singh, R.P.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Basnet, B.R.; Zhang, Y.; Yang, E. QTL characterization of resistance to leaf rust and stripe rust in the spring wheat line Francolin# 1. Mol. Breed. 2014, 34, 789–803. [Google Scholar]
- Dieguez, M.J.; Pergolesi, M.F.; Velásquez, S.M.; Ingala, L.; López, M.; Darino, M.; Paux, E.; Feuillet, C.; Sacco, F. Fine mapping of LrSV2, a race-specific adult plant leaf rust resistance gene on wheat chromosome 3BS. Theor. Appl. Genet. 2014, 127, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Gerard, G.S.; Kobiljski, B.; Lohwasser, U.; Börner, A.; Simon, M.R. Genetic architecture of adult plant resistance to leaf rust in a wheat association mapping panel. Plant Pathol. 2018, 67, 584–594. [Google Scholar] [CrossRef]
- Qureshi, N.; Bariana, H.; Kumran, V.V.; Muruga, S.; Forrest, K.L.; Hayden, M.J.; Bansal, U. A new leaf rust resistance gene Lr79 mapped in chromosome 3BL from the durum wheat landrace Aus26582. Theor. Appl. Genet. 2018, 131, 1091–1098. [Google Scholar] [CrossRef]
- Singh, S.; Bowden, R.L. Molecular mapping of adult-plant race-specific leaf rust resistance gene Lr12 in bread wheat. Mol. Breed. 2011, 28, 137–142. [Google Scholar] [CrossRef]
- Singh, A.; Pallavi, J.; Gupta, P.; Prabhu, K. Identification of microsatellite markers linked to leaf rust resistance gene Lr25 in wheat. J. Appl. Genet. 2012, 53, 19–25. [Google Scholar] [CrossRef]
- Hiebert, C.; Thomas, J.; McCallum, B. Locating the broad-spectrum wheat leaf rust resistance gene Lr52 (LrW) to chromosome 5B by a new cytogenetic method. Theor. Appl. Genet. 2005, 110, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, C.; Herrera-Foessel, S.A.; Randhawa, M.S.; Huerta-Espino, J.; Liu, D.; Dreisigacker, S.; Singh, R.P.; Lan, C. Four consistent loci confer adult plant resistance to leaf rust in the durum Wheat Lines Heller#1 and Dunkler. Phytopathology 2020, 110, 892–899. [Google Scholar]
- Sadeghabad, A.A.; Dadkhodaie, A.; Heidari, B.; Razi, H.; Mostowfizadeh-Ghalamfarsa, R. Microsatellite markers for the Triticum timopheevi-derived leaf rust resistance gene Lr18 on wheat 5BL chromosome. Breed. Sci. 2017, 67, 129–134. [Google Scholar] [CrossRef]
- Dadkhodaie, N.; Karaoglou, H.; Wellings, C.; Park, R. Mapping genes Lr53 and Yr35 on the short arm of chromosome 6B of common wheat with microsatellite markers and studies of their association with Lr36. Theor. Appl. Genet. 2011, 122, 479–487. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.; Singh, R.; Huerta-Espino, J.; William, H.; Djurle, A.; Yuen, J. Molecular mapping of a leaf rust resistance gene on the short arm of chromosome 6B of durum wheat. Plant Dis. 2008, 92, 1650–1654. [Google Scholar] [CrossRef]
- Pirseyedi, S.-M.; Somo, M.; Poudel, R.S.; Cai, X.; McCallum, B.; Saville, B.; Fetch, T.; Chao, S.; Marais, F. Characterization of recombinants of the Aegilops peregrina-derived Lr59 translocation of common wheat. Theor. Appl. Genet. 2015, 128, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Pourkhorshid, Z.; Dadkhodaie, A.; Niazi, A. Molecular mapping of the Aegilops speltoides-derived leaf rust resistance gene Lr36 in common wheat (Triticum aestivum). Euphytica 2022, 218, 26. [Google Scholar] [CrossRef]
- Kuraparthy, V.; Sood, S.; Chhuneja, P.; Dhaliwal, H.S.; Kaur, S.; Bowden, R.L.; Gill, B.S. A cryptic wheat–Aegilops triuncialis translocation with leaf rust resistance gene Lr58. Crop Sci. 2007, 47, 1995–2003. [Google Scholar] [CrossRef]
- Rosewarne, G.; Singh, R.; Huerta-Espino, J.; Herrera-Foessel, S.; Forrest, K.; Hayden, M.; Rebetzke, G. Analysis of leaf and stripe rust severities reveals pathotype changes and multiple minor QTLs associated with resistance in an Avocet× Pastor wheat population. Theor. Appl. Genet. 2012, 124, 1283–1294. [Google Scholar] [CrossRef]
- Kolmer, J. Leaf rust resistance in wheat line RL6062 is an allele at the Lr3 locus. Crop Sci. 2015, 55, 2186–2190. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.; Huerta-Espino, J.; Calvo-Salazar, V.; Lan, C.; Singh, R. Lr72 confers resistance to leaf rust in durum wheat cultivar Atil C2000. Plant Dis. 2014, 98, 631–635. [Google Scholar] [CrossRef] [PubMed]
- William, H.; Hoisington, D.; Singh, R.; Gonzalez-de-Leon, D. Detection of quantitative trait loci associated with leaf rust resistance in bread wheat. Genome 1997, 40, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Bai, G.-H.; Carver, B.; Shaner, G.; Hunger, R. Mapping of QTLs prolonging the latent period of Puccinia triticina infection in wheat. Theor. Appl. Genet. 2005, 110, 244–251. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Rosewarne, G.M.; Periyannan, S.K.; Viccars, L.; Calvo-Salazar, V.; Lan, C.; Lagudah, E.S. Lr68: A new gene conferring slow rusting resistance to leaf rust in wheat. Theor. Appl. Genet. 2012, 124, 1475–1486. [Google Scholar] [CrossRef]
- Maccaferri, M.; Mantovani, P.; Tuberosa, R.; DeAmbrogio, E.; Giuliani, S.; Demontis, A.; Massi, A.; Sanguineti, M. A major QTL for durable leaf rust resistance widely exploited in durum wheat breeding programs maps on the distal region of chromosome arm 7BL. Theor. Appl. Genet. 2008, 117, 1225–1240. [Google Scholar] [CrossRef]
- Xing, L.; Wang, C.; Xia, X.; He, Z.; Chen, W.; Liu, T.; Li, Z.; Liu, D. Molecular mapping of leaf rust resistance gene LrFun in Romanian wheat line Fundulea 900. Mol. Breed. 2014, 33, 931–937. [Google Scholar] [CrossRef]
- Tar, M.; Purnhauser, L.; Csősz, M. Identification and localization of molecular markers linked to the Lr52 leaf rust resistance gene of wheat. Cereal Res. Commun. 2008, 36, 409–415. [Google Scholar] [CrossRef]
- Huang, L.; Gill, B. An RGA–like marker detects all known Lr21 leaf rust resistance gene family members in Aegilops tauschii and wheat. Theor. Appl. Genet. 2001, 103, 1007–1013. [Google Scholar] [CrossRef]
- Scofield, S.R.; Huang, L.; Brandt, A.S.; Gill, B.S. Development of a Virus-Induced Gene-Silencing System for Hexaploid Wheat and Its Use in Functional Analysis of the Lr21-Mediated Leaf Rust Resistance Pathway. Plant Physiol. 2005, 138, 2165–2173. [Google Scholar] [CrossRef]
- Hiebert, C.W.; Thomas, J.B.; McCallum, B.D.; Somers, D.J. Genetic mapping of the wheat leaf rust resistance gene Lr60 (LrW2). Crop Sci. 2008, 48, 1020–1026. [Google Scholar] [CrossRef]
- Dinkar, V.; Jha, S.; Mallick, N.; Niranjana, M.; Agarwal, P.; Sharma, J. Molecular mapping of a new recessive wheat leaf rust resistance gene originating from Triticum spelta. Sci. Rep. 2020, 10, 22113. [Google Scholar] [CrossRef]
- Darino, M.; Dieguez, M.; Singh, D.; Ingala, L.; Pergolesi, M.; Park, R.; McIntosh, R.; Sacco, F. Detection and location of Lr11 and other leaf rust resistance genes in the durably resistant wheat cultivar Buck Poncho. Euphytica 2015, 206, 135–147. [Google Scholar] [CrossRef]
- Kumar, S.; Bhardwaj, S.C.; Gangwar, O.P.; Sharma, A.; Qureshi, N.; Kumaran, V.V.; Khan, H.; Prasad, P.; Miah, H.; Singh, G.P. Lr80: A new and widely effective source of leaf rust resistance of wheat for enhancing diversity of resistance among modern cultivars. Theor. Appl. Genet. 2021, 134, 849–858. [Google Scholar] [CrossRef]
- Singh, S.; Franks, C.; Huang, L.; Brown-Guedira, G.; Marshall, D.; Gill, B.; Fritz, A. Lr41, Lr39, and a leaf rust resistance gene from Aegilops cylindrica may be allelic and are located on wheat chromosome 2DS. Theor. Appl. Genet. 2004, 108, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bai, G.; Carver, B.F. Molecular markers for wheat leaf rust resistance gene Lr41. Mol. Breed. 2009, 23, 311–321. [Google Scholar] [CrossRef]
- Lan, C.; Hale, I.L.; Herrera-Foessel, S.A.; Basnet, B.R.; Randhawa, M.S.; Huerta-Espino, J.; Dubcovsky, J.; Singh, R.P. Characterization and mapping of leaf rust and stripe rust resistance loci in hexaploid wheat lines UC1110 and PI610750 under Mexican environments. Front. Plant Sci. 2017, 8, 1450. [Google Scholar] [CrossRef] [PubMed]
- Azzimonti, G.; Marcel, T.; Robert, O.; Paillard, S.; Lannou, C.; Goyeau, H. Diversity, specificity and impacts on field epidemics of QTLs involved in components of quantitative resistance in the wheat leaf rust pathosystem. Mol. Breed. 2014, 34, 549–567. [Google Scholar] [CrossRef]
- Dholakia, B.; Rajwade, A.; Hosmani, P.; Khan, R.; Chavan, S.; Reddy, D.; Lagu, M.; Bansal, U.; Saini, R.; Gupta, V. Molecular mapping of leaf rust resistance gene Lr15 in hexaploid wheat. Mol. Breed. 2013, 31, 743–747. [Google Scholar] [CrossRef]
- Lu, Y.; Bowden, R.L.; Zhang, G.; Xu, X.; Fritz, A.K.; Bai, G. Quantitative trait loci for slow-rusting resistance to leaf rust in doubled-haploid wheat population CI13227× Lakin. Phytopathology 2017, 107, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Heyns, I.; Pretorius, Z.; Marais, F. Derivation and characterization of recombinants of the Lr54/Yr37 translocation in common wheat. Open Plant Sci. J. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Thomas, J.; Nilmalgoda, S.; Hiebert, C.; McCallum, B.; Humphreys, G.; DePauw, R. Genetic markers and leaf rust resistance of the wheat gene Lr32. Crop Sci. 2010, 50, 2310–2317. [Google Scholar] [CrossRef]
- Zhang, P.; Lan, C.; Asad, M.A.; Gebrewahid, T.W.; Xia, X.; He, Z.; Li, Z.; Liu, D. QTL mapping of adult-plant resistance to leaf rust in the Chinese landraces Pingyuan 50/Mingxian 169 using the wheat 55K SNP array. Mol. Breed. 2019, 39, 98. [Google Scholar] [CrossRef]
- Schachermayr, G.; Messmer, M.; Feuillet, C.; Winzeler, H.; Winzeler, M.; Keller, B. Identification of molecular markers linked to the Agropyron elongatum-derived leaf rust resistance gene Lr24 in wheat. Theor. Appl. Genet. 1995, 90, 982–990. [Google Scholar] [CrossRef]
- Gupta, S.; Charpe, A.; Koul, S.; Haque, Q.; Prabhu, K. Development and validation of SCAR markers co-segregating with an Agropyron elongatum derived leaf rust resistance gene Lr24 in wheat. Euphytica 2006, 150, 233–240. [Google Scholar] [CrossRef]
- Hiebert, C.W.; McCallum, B.D.; Thomas, J.B. Lr70, a new gene for leaf rust resistance mapped in common wheat accession KU3198. Theor. Appl. Genet. 2014, 127, 2005–2009. [Google Scholar] [CrossRef]
- Bansal, M.; Kaur, S.; Dhaliwal, H.; Bains, N.; Bariana, H.; Chhuneja, P.; Bansal, U. Mapping of Aegilops umbellulata-derived leaf rust and stripe rust resistance loci in wheat. Plant Pathol. 2017, 66, 38–44. [Google Scholar] [CrossRef]
- Kolmer, J.; Bernardo, A.; Bai, G.; Hayden, M.; Chao, S. Adult plant leaf rust resistance derived from Toropi wheat is conditioned by Lr78 and three minor QTL. Phytopathology 2018, 108, 246–253. [Google Scholar] [CrossRef]
- Kuraparthy, V.; Chhuneja, P.; Dhaliwal, H.S.; Kaur, S.; Bowden, R.L.; Gill, B.S. Characterization and mapping of cryptic alien introgression from Aegilops geniculata with new leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theor. Appl. Genet. 2007, 114, 1379–1389. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Zhang, Y.; Du, Z.; Feng, J.; Quan, W.; Ren, J.; Che, M.; Zhang, Z. Identification and Validation of a Major Quantitative Trait Locus for Adult Plant Resistance Against Leaf Rust From the Chinese Wheat Landrace Bai Qimai. Front. Plant Sci. 2022, 13, 812002. [Google Scholar] [CrossRef]
- Mebrate, S.; Oerke, E.; Dehne, H.; Pillen, K. Mapping of the leaf rust resistance gene Lr38 on wheat chromosome arm 6DL using SSR markers. Euphytica 2008, 162, 457–466. [Google Scholar] [CrossRef]
- Rollar, S.; Serfling, A.; Geyer, M.; Hartl, L.; Mohler, V.; Ordon, F. QTL mapping of adult plant and seedling resistance to leaf rust (Puccinia triticina Eriks.) in a multiparent advanced generation intercross (MAGIC) wheat population. Theor. Appl. Genet. 2021, 134, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Kang, J.; Bräunlich, S.; Boni, R.; Chauhan, H.; Selter, L.L.; Robinson, M.D.; Schmid, M.W.; Wiederhold, E.; Hensel, G. Abscisic acid is a substrate of the ABC transporter encoded by the durable wheat disease resistance gene Lr34. New Phytol. 2019, 223, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Sucher, J.; Boni, R.; Yang, P.; Rogowsky, P.; Büchner, H.; Kastner, C.; Kumlehn, J.; Krattinger, S.G.; Keller, B. The durable wheat disease resistance gene Lr34 confers common rust and northern corn leaf blight resistance in maize. Plant Biotech. J. 2017, 15, 489–496. [Google Scholar] [CrossRef]
- Schnippenkoetter, W.; Lo, C.; Liu, G.; Dibley, K.; Chan, W.L.; White, J.; Milne, R.; Zwart, A.; Kwong, E.; Keller, B. The wheat Lr34 multipathogen resistance gene confers resistance to anthracnose and rust in sorghum. Plant Biotech. J. 2017, 15, 1387–1396. [Google Scholar] [CrossRef]
- Krattinger, S.G.; Sucher, J.; Selter, L.L.; Chauhan, H.; Zhou, B.; Tang, M.; Upadhyaya, N.M.; Mieulet, D.; Guiderdoni, E.; Weidenbach, D. The wheat durable, multipathogen resistance gene Lr34 confers partial blast resistance in rice. Plant Biotech. J. 2016, 14, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tar, M.; Purnhauser, L.s.; Csôsz, L.s.n.; Mesterházy, A.k.; Gyulai, G.b. Identification of molecular markers for an efficient leaf rust resistance gene (Lr29) in wheat. Acta Biol. Szeged. 2002, 46, 133–134. [Google Scholar]
- Gupta, S.K.; Charpe, A.; Prabhu, K.V.; Haque, Q.M.R. Identification and validation of molecular markers linked to the leaf rust resistance gene Lr19 in wheat. Theor. Appl. Genet. 2006, 113, 1027–1036. [Google Scholar] [CrossRef]
- Prins, R.; Groenewald, J.; Marais, G.; Snape, J.; Koebner, R. AFLP and STS tagging of Lr19, a gene conferring resistance to leaf rust in wheat. Theor. Appl. Genet. 2001, 103, 618–624. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Tsilo, T.J.; Kolmer, J.A.; Anderson, J.A. Molecular mapping and improvement of leaf rust resistance in wheat breeding lines. Phytopathology 2014, 104, 865–870. [Google Scholar] [CrossRef]
- Wulff, B.B.; Krattinger, S.G. The long road to engineering durable disease resistance in wheat. Curr. Opin. Biotech. 2022, 73, 270–275. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G.; Lagudah, E.S.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 2014, 5, 641. [Google Scholar] [CrossRef]
- Luo, M.; Xie, L.; Chakraborty, S.; Wang, A.; Matny, O.; Jugovich, M.; Kolmer, J.A.; Richardson, T.; Bhatt, D.; Hoque, M. A five-transgene cassette confers broad-spectrum resistance to a fungal rust pathogen in wheat. Nat. Biotech. 2021, 39, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Z.; Chang, C. Susceptibility Is New Resistance: Wheat Susceptibility Genes and Exploitation in Resistance Breeding. Agriculture 2022, 12, 1419. [Google Scholar] [CrossRef]
| Type of Resistance | Resistance Stage | Resistant Features | Cloned Resistance Genes |
|---|---|---|---|
| Race-specific resistance | Seedling stage | Seedling resistance. Immune or hypersensitive response (cell death/necrosis) observed on the leaf surface. May be lost at adult plant stage against multiple Pt pathotypes in the field. | Lr1, Lr10, Lr13, Lr21, Lr22a, Lr42 (NBS-LRR) [4,5,6,7,8,9] Lr14a (Ankyrin repeats and Ca2+ channels) [12] Lr9/Lr58 (Tandem kinase) [13] |
| Adult plant stage | Hypersensitive adult plant resistance (APR)/All-stage (AS) race-specific resistance. Immune or hypersensitive response (cell death/necrosis) observed on the leaf surface. | ||
| Slow rusting | Adult plant stage | Non-race-specific resistance. A lower level but more durable resistance. Rust infection and sporulation can be accomplished in a much delayed and reduced manner. | Lr34 (ATP-binding cassette transporter) [14] Lr67 (Hexose transporter) [15] |
| Chromosome | Lr Gene/Major QTL | Resistance Type | Donor | Associated Markers or SNPs a | Reference |
|---|---|---|---|---|---|
| 1AS | QLr.cim-1AS | APR | T. aestivum: Sujata | wPt-9752, Xgdm33, Xcfd15 | [17] |
| 1AS | QLr.cau-1AS | APR | T. aestivum: Luke | Xgpw2246 | [18] |
| 1AS | QLr.ramp-1A.2 | APR | T. aestivum: spring wheat collection (GWAS) | AX-95080736 | [19] |
| 1AS | Lr10 | AS | T. aestivum: TcLr10 | Lrk10D1, Xgwm136, Xpsr596 | [5] |
| 1AL | QLr.uga-1AL | AS | T. aestivum: wheat genotypes collection (GWAS) | IWA1952 | [21] |
| 1AL | QLr.fiz-1AL | APR | Triticum and Aegilops species collection (GWAS) | Excalibur_c33567_363 | [22] |
| 1AL | QLr.umn-1AL | APR | T. aestivum: spring wheat breeding lines (GWAS) | IWB48030 | [23] |
| 1AL | QLr.liu-1AL | APR | T. aestivum: wheat collections and their hybrids (GWAS) | SNP532737351chr1A | [24] |
| 1AL | Lr2K38/QLr.ags-1AL | APR | T. aestivum: AGS 2038 | IWB20487, IWA4022 | [25] |
| 2AS | Lr65 | AS | T. spelta: Altgold Rotkorn | Xbarc124, Xbarc212, Xgwm614 | [26] |
| 2AS | Lr37 | AS | Ae. ventricosa: Madsen | Xcmwg682, Xbcd348, Xpsr933 | [27] |
| 2AS | Lr17a | AS | T. aestivum: Klein Lucero (CI 14047) T. aestivum: Maria Escobar (PI 150604) | Xgwm614, Xgwm614, Xwmc407 | [28] |
| 2AS | Lr81 | AS | T. aestivum: PI 470121 | Xstar-KASP320, Xstar-KASP323 | [29] |
| 2AS | LrM | AS | Ae. markgrafii: ER9-700 | Xgwm512, Xcfd36 | [30] |
| 2A | Lr45 | AS | S. cereale: TcLr45 | Xcfd168, Xgwm372 | [31] |
| 2AL | Lr.locus-2AL | AS | T. turgidum: durum wheat collection (GWAS) | Xgwm1045 | [32] |
| 2AL | QLr.ifa-2AL | APR | T. aestivum: Capo | Xgwm312 | [33] |
| 2AL | QLr.hebau-2AL | APR | T. aestivum: Zhou 8425B | wmc181, BS00057060_51 | [34] |
| 2AL | QLr.spa-2AL | APR | T. aestivum: AC Cadillac | rPt-9611 | [35] |
| 3AS | Lr63 | AS | T. monococcum: RL6137 | Xbarc321, Xbarc57 | [36] |
| 3AS | QLr.cdl-3AS | APR | T. aestivum: Americano 44d | Xbarc321 | [37] |
| 3AS | Lr66 | AS | Ae. speltoides | Xgwm674, Xbarc57 | [38] |
| 3AL | QLr.sfrs-3AL | APR | T. aestivum: Forno | Xpsr570, Xpsr543 | [39] |
| 3AL | QLr.fcu-3AL | APR | Synthetic hexaploid wheat: TA4152-60 | Xcfa2183, Xgwm666, Xfcp586 | [40] |
| 4AS | QLr.uga-4AS | AS | T. aestivum: wheat genotypes collection (GWAS) | IWA1766 | [21] |
| 4AS | QLr.umn-4AS | APR | T. aestivum: spring wheat breeding lines (GWAS) | IWB59410 | [23] |
| 4AS | Lr.ace-4A | AS | T. turgidum: PI 192051 | IWA232, IWA603, IWA4657 | [41] |
| 4AL | QLr.hebau-4AL | APR | T. aestivum: Zhou 8425B | Xwmc617, BobWhite_c15697_675 | [34] |
| 4AL | QLr.zha-4AL | APR | T. aestivum: wheat lines collection (GWAS) | Tdurum_con-tig93100_149 | [42] |
| 4AL | QLr.liu-4AL | APR | T. aestivum: wheat collections and their hybrids (GWAS) | SNP713087672chr4A | [24] |
| 4AL | Lr28 | AS | Ae. speltoides | Xwmc313, SCS421 | [43] |
| 5AS | QLr.pbi-5AS | APR | T. aestivum: Beaver | wPt-1931, wPt-8756 | [46] |
| 5AS | QLr.uga-5AS | APR | T. aestivum: wheat genotypes collection (GWAS) | IWA2143, Xwmc47, Xbarc122 | [21] |
| 5AS | QLr.leo-5AS | APR | T. aestivum: Spring wheat collection (GWAS) | GENE-3321_201 | [47] |
| 5AL | QLr.aus-5AL | APR | T. aestivum: wheat landrace collection (GWAS) | IWB23955, IWB34703 | [48] |
| 5AL | QLr.crc-5AL | APR | T. aestivum: Toropi-6.4 | Excalibur_rep_c111129_125 | [49] |
| 5AL | QLr.hebau-5AL | APR | T. aestivum: SW 8588 | AX-110679506, AX-110996595 | [50] |
| 5AL | QLr.uga-5AL | AS | T. aestivum: wheat genotypes collection (GWAS) | IWA5929, Xgpw2273 | [21] |
| 6AS | Lr62 | AS | Ae. neglecta: 03M119-71A | Xgwm334, Xcfd190, Xcfa2173 | [51] |
| 6AL | Lr56 | AS | Ae. sharonensis | Xgwm427, Xwmc59 | [52] |
| 6AL | Lr64 | AS | T. dicoccoides: RL6149 | K-IWB59855 | [53] |
| 6AL | QLr.ramp-6A.1 | APR | T. aestivum: spring wheat collection (GWAS) | AX-94653398 | [19] |
| 7AS | Lr47 | AS | Ae. speltoides: Pavon | Xgwm60, PS10 | [54] |
| 7AS | QLr.tur-7AS | AS/APR | T. aestivum: spring wheat collection (GWAS) | IWA1277 | [58] |
| 7AS | QLr.uga-7AS | AS | T. aestivum: wheat genotypes collection (GWAS) | IWA7201 | [21] |
| 7AS | QLr.liu-7AS | APR | T. aestivum: wheat collections and their hybrids (GWAS) | SNP126914404chr7A | [24] |
| 7AL | Lr20 | AS | T. aestivum: Thew | Xpsr148, Xcdo347, STS638 | [59] |
| Chromosome | Gene/QTL | Resistance Type | Donor | Associated Markers or SNPs a | Reference |
|---|---|---|---|---|---|
| 1BS | Lr55 | AS/APR | E. trachycaulis: KS04WGRC45 | Xgwm374, Xwmc406 | [60] |
| 1BL | Lr33 | AS/APR | T. aestivum: PI 58548, KU168-2 | Xgwm413 | [61] |
| 1BL | Lr44 | AS | T. spelta: Accession 7831 | Linked with Lr33 | [62] |
| 1B | Lr71 | AS | T. spelta: Altgold Rotkorn | Xgwm18, Xbarc187 | [63] |
| 1BS | Lr75/QLr.sfr-1BS | APR | T. aestivum: Forno | Xgwm18,Xpsr949, Xgwm604 | [64,65] |
| 1BL | QLr.pser-1BL | AS/APR | T. aestivum: Ning7840 | Xscm9, Xwmc85.1 | [66] |
| 1BL | LrZH84 | AS | T. aestivum: Zhou 8425B | Xgwm582, barc8, BF474863, BE497107 | [67,68] |
| 1BL | QLr.leo-1BL | APR | T. aestivum: spring wheat collection (GWAS) | tplb0023b14_704, wsnp_Ra_c8484_14372815, BobWhite_c1456_615 | [47] |
| 1BL | QLr.wpt-1BL | APR | T. aestivum | wPt-9809 | [97] |
| 1BL | Lr46/QLr.caas-1BL/QLr.csiro-1BL/QLr.cim-1BL | Slow rusting | T. aestivum: Pavon 76, Bainong 64, Attila, Toropi-6.4, Francolin#1 | Xwmc719, Xgwm140, Xwms259 Xgwm153.2, Xwmc44 Excalibur_c35888_208, csLV46, wPt-9028, wPt-1770 | [49,69,70,71,72,95] |
| 1BL | Lr51 | AS | Ae. speltoides: F-7-3 | Xcdo393 | [73] |
| 1BL | Lr26 | AS | S. cereale: Petkus | 1BL/1RS translocation | [74] |
| 1BL | QLr.pbi-1B | APR | T. aestivum: Beaver | 1BL/1RS translocation | [46] |
| 1B | QLr.ramp-1B.3 | APR | T. aestivum: spring wheat collection | AX-94517050 | [19] |
| 2BS | QLr.usw-2BS | APR | T. turgidum: Saragolla | Tdurum_contig76118_145, wsnp_Ex_c18354_27181086 | [76] |
| 2BS | Lr16 | AS | T. aestivum: BW278, AC Majestic, AC Domain, Kenyon | Xwmc764, Xgwm210, Xwmc661, kwm677, kwm744 | [77,78] |
| 2B | QLr.spa-2B | APR | T. aestivum: Carberry | wPt-732018, wPt-7883 | [35] |
| 2BS | QLr.csiro-2BS | APR | T. aestivum: Attila | Xwmc154, Xgwm682, XP32/M62 | [72] |
| 2BS | Lr48 | APR | T. aestivum: CSP44 | Xgwm429b, Xbarc07 | [79] |
| 2BS | QLr.hebau-2BS | APR | T. aestivum: Shanghai 3/Catbird | wPt-8548, wPt-2314 | [80] |
| 2BS | Lr23/QLr.ksu-2BS | AS/APR | Synthetic hexaploid wheat: W-7984 | Xtam72, Per2, Xcdo405 | [81,82] |
| 2BS | Lr13 | HTAS/APR | T. aestivum: TcLr13 | TraesCS2B02G182800 | [6,83] |
| 2BS | LrKP | AS | T. aestivum: Klein Proteo | Lrkp2B114, LrkpF299R300 | [84] |
| 2BS | Lr35 | APR | T. aestivum: TcLr35 | Xwg996, Xpsr540, Xbcd260 | [85] |
| 2BL | QLr.osu-2B | Slow rusting | T. aestivum: CI 13227 | Xbarc167, Xagc.tgc135, Xcatg.atgc60 | [89] |
| 2BS | QLr.ifa-2BS | APR | T. aestivum: Capo | Xgwm120 | [33] |
| 2BL | Lr.locus-2B5 | AS | T. turgidum: durum wheat collection (GWAS) | IWA1765, wmc332 | [32] |
| 2B | QLr.dms-2B.2 | APR | T. aestivum: spring wheat collection (GWAS) | Excalibur_c62234_105 | [90] |
| 2BL | LrNJ97 | AS | T. aestivum: Neijiang 977671 | Xwmc317, Xbarc159 | [91] |
| 2BL | Lr50 | AS | T. timopheevii: TA 870, TA 874, KS96WGRC36 | Xgwm382, Xgdm87 | [92] |
| 3BS | QLr.ifa-3BS | APR | T. aestivum: Capo | Xgwm389 | [33] |
| 3BS | Lr74/QLr.hwwg-3BS.1 | APR | T. aestivum: Caldwell, Clark | gwm533, cfb5006, barc75, IWA4654, IWA1702, Xgwm389 | [93,94] |
| 3BS | QLr.cim-3BS.1 | APR | T. aestivum: Francolin#1 | wPt-6945, wPt-664393, wPt-5390 | [95] |
| 3BS | LrSV2 | APR | T. aestivum: Sinvalocho MA | Xpsr598, swm13, gwm533 | [96] |
| 3BL | QLr.fcu-3BL | AS | Synthetic hexaploid wheat: TA4152–60 | Xbarc164, Xfcp544 | [40] |
| 3BL | QLr.wpt-3BL | APR | T. aestivum: wheat cultivar collection (GWAS) | wPt-7502 | [97] |
| 3BL | QLr.hebau-3BL | APR | T. aestivum: SW 8588 | AX-111014259, AX-111534420 | [50] |
| 3BL | Lr79 | APR | T. turgidum: Aus26582 | sun770, sun786 | [98] |
| 4B | QLr.sfrs-4B | APR | T. aestivum: Forno | Xpsr921, Xpsr953b | [39] |
| 4BL | Lr12/Lr31/QLr.hebau-4B | APR | T. aestivum: Chinese Spring | gwm149, BS00109813_51 | [34,99] |
| 4BL | Lr25 | AS | S. cereale: TcLr25 | Xgwm251, Xgwm538, Xgwm6 | [100] |
| 4BL | Lr49 | APR | T. aestivum: VL404 | Xbarc163, Xwmc349 | [79] |
| 4BL | QLr.spa-4B | APR | T. aestivum: Carberry | wPt-5303, wPt-1849 | [35] |
| 5BS | Lr52 | AS | T. aestivum: RL6107 | Xwmc149, Xtxw200, Xgwm234 | [101,117] |
| 5BL | QLr.cim-5BL | APR | T. aestivum: Heller#1, Dunkler | AX-94480675f, AX-94394039f, AX-94962653 | [102] |
| 5BL | QLr.fiz-5B | APR | Triticum and Aegilops species collection (GWAS) | wsnp_Ex_c6548_11355524 | [22] |
| 5BL | Lr18 | AS | T. timopheevii | Xwmc75, Xgpw7425 | [103] |
| 6BS | QLr.usw-6BS | APR | T. turgidum ssp. durum: Gaza | CAP7_c10772_156 | [76] |
| 6BS | QLr.wpt-6BS.1 | APR | T. aestivum: wheat cultivar collection (GWAS) | wPt-3116 | [97] |
| 6BS | Lr53 | AS | T. dicoccoides: 98M71 | Xwmc487, Xcfd1, Xgwm508 | [104] |
| 6BS | QLr.caas-6BS.1 | APR | T. aestivum: Bainong 64 | Xwmc487, Xcfd13 | [71] |
| 6BS | Lr61 | AS | T. turgidum ssp. durum: Guayacan INIA | Xwmc487, Xwmc104, Xwmc398 | [105] |
| 6BS | Lr59 | AS | Ae. peregrina | Xgwm518, Xdupw217 | [106] |
| 6BS | Lr36 | AS | Ae. speltoides: ER84018 | Xgwm88, Xcfd13 | [107] |
| 6B | QLr.liu-6B | APR | T. aestivum: wheat collections and their hybrids (GWAS) | SNP459220281chr6B | [24] |
| 6BL | Lr9/Lr58-2BL | AS | Ae. umbellulate Ae. triuncialis: TA10438 | Xpsr546 | [13,108] |
| 6BL | QLr.cimmyt-6BL.1 | APR | T. aestivum: Pastor | wPT6329, wPt-5176, Xgwm219 | [109] |
| 6BL | Lr3 | AS | T. aestivum: RL6062 | wPt-6878 | [110] |
| 6BL | QLr.usw-6BL | APR | T. turgidum: Gaza | wsnp_Ex_c45713_51429315, GENE-3689_293 | [76] |
| 6BL | QLr.cim-6BL | APR | T. aestivum: Heller#1, Dunkler | AX-95155193, AX-94469158 | [102] |
| 7BS | Lr72 | AS | T. durum: Atil C2000 | barc279, wmc606 | [111] |
| 7BL | QLr.sfrs-7B.2 | APR | T. aestivum: Forno | Xpsr593c, Xpsr129c | [39] |
| 7BL | QLr.cimmyt-7BL | APR | T. aestivum: Pastor | wPt-4342, wPt-8921 | [109] |
| 7BL | QLr.osu-7BL/QLrlp.osu-7BL | Slow rusting | T. aestivum: CI 13227 | Xaca.cacg126, Xbarc50, Xbarc182, Xcatg.atgc125 | [89,113] |
| 7BL | QLr.cim-7BL | AS | T. aestivum: Sujata | Xcfa2040, Xwmc526 | [17] |
| 7BL | Lr14a | AS | T. aestivum: Selkirk T. aestivum: ArinaLrFor | Xgwm146, gwm344 | [12] |
| 7BL | Lr68 | Slow rusting | T. aestivum: Parula | Xgwm146, csGS, cs7BLNLRR, Psy1-1 | [114] |
| 7BL | QLr.ubo-7B.2 | APR | T. durum: Colosseo | Xbarc340.2, Xgwm146, Xgwm344.2 | [115] |
| 7BL | LrFun | AS | T. aestivum: Fundulea 900 | Xgwm344, Xwmc70 | [116] |
| 7BL | QLr.ksu-7BL | APR | T. aestivum: Opata 85 | Cht1b, Tha1, Cat, Xfbb189 | [82] |
| 7BL | QLr.cimmyt-7BL.1 | Slow rusting | T. aestivum: Parula | Xcmtg05-500, Xcmti16-1500 | [112] |
| 7BL | QLr.usw-7BL | APR | T. turgidum: Arnacoris | Tdurum_contig30545_715, Bobwhite_c42202_158 | [76] |
| Chromosome | Gene/QTL | Resistance Type | Donor | Associated Markers or SNPs a | Reference |
|---|---|---|---|---|---|
| 1DS | Lr21/40 | AS | Ae. tauschii: TA1649 Ae. tauschii: KS89WGRC7 | Xksud14, Xksu936, Xksu937, Xksu027, Gli-D1, Xbcd1434 | [7,118] |
| 1DS | Lr60 | AS | T. aestivum: RL6172 | barc149, WMC432, CFD61 | [120] |
| 1DS | LrTs276-2 | AS | T. Spelta: TSD276-2 | Xcfd15 | [121] |
| 1DS | Lr42 | AS | Ae. tauschii: TA2450 | Xwmc432, Xgdm33, Xcfd15 | [9] |
| 1DS | QLr.wpt-1DS | APR | T. aestivum: wheat cultivar collection (GWAS) | wPt-0413 | [97] |
| 1D | QLr.zha-1D | APR | T. aestivum: wheat lines collection (GWAS) | BS00014671_51 | [42] |
| 2DS | Lr11 | AS | T. aestivum: Buck Poncho | Xscar32/35, wmc574, wms1099 | [122] |
| 2DS | QLrlp.osu-2DS | Slow rusting | T. aestivum: CI 13227 | Xactg.gtg185, Xbarc124 | [113] |
| 2DS | Lr80 | AS | T. aestivum: Hango-2 | Cau96, gwm210, barc124 | [123] |
| 2DS | Lr39/41 | AS | Ae. tauschii: TA4186 Ae. tauschii: TA2460 | Xgwm210
, Xgwm296, Xgwm455 , Xbarc124, Xgdm35, Xcfd36 | [124,125] |
| 2DS | QLr.cim-2DS | APR | T. aestivum: UC1110 | cfd51, cfd36 | [126] |
| 2DS | Lr2a/QLr.mna-2DS | AS/APR | T. aestivum: MN98550-5/MN99394-1 | wmc453, wPt-0330, barc95 | [150] |
| 2DS | QLr.inra-2D | APR | T. aestivum: Balance | gpw3320, cfd36 | [127] |
| 2DS | Lr22a | AS | Ae. tauschii: RL6044 | gwm455, gwm296, gwm261 | [8] |
| 2DS | QLrid.osu-2DS | Slow rusting | T. aestivum: CI 13227 | Xgwm261 | [89] |
| 2DS | Lr15 | AS | T. aestivum: TcLr15 | Xgwm4562, Xgwm102 | [128] |
| 2DL | Lr54 | AS | Ae. kotschyi: CS-Lr54/Yr37 | Xcfd50, Xgdm6 | [130] |
| 2DS | QLr.hwwg-2DS | APR | T. aestivum: CI13227 | IWB8545 | [129] |
| 2DL | QLr.tur-2DL | AS/APR | T. aestivum: spring wheat collection (GWAS) | IWA5637 | [58] |
| 2D | QLr.fiz-2D | APR | Triticum and Aegilops species collection (GWAS) | Kukri_c59403_339 | [22] |
| 2D | QLr.dms-2D | APR | T. aestivum: spring wheat collection (GWAS) | RAC875_c52856_250 | [90] |
| 3DS | Lr32 | AS | Ae. tauschii: RL5497-1 | barc135 | [131] |
| 3DS | QLr.hebau-3DS | APR | T. aestivum: Pingyuan 50 | AX-109395143 | [132] |
| 3DC | QLr.cim-3DC | APR | T. aestivum: UC1110 | gwm341, barc1119 | [126] |
| 3DL | Lr24 | AS | A. elongatum: Agent | Xpsr904, Xpsr931, Xpsr1205 | [133,134] |
| 3D | QLr.liu-3D | APR | T. aestivum: wheat collections and their hybrids (GWAS) | SNP597179288chr3D | [24] |
| 3D | QLr.cdl-3D | APR | T. aestivum: Americano 44d | wPt-741949 | [37] |
| 4DL | Lr67 | Slow rusting | T. aestivum: RL6077 | csSNP856, gwm4670, cfd71 | [15] |
| 4DL | QLr.sfrs-4DL | APR | T. aestivum: Forno | Xglk302b, Xpsr1101a | [39] |
| 5DS | Lr70 | AS | T. aestivum: KU3198 | wmc233, gwm190 | [135] |
| 5DS | Lr76 | AS | Ae. umbellulate: IL 393-4 | Xcfd18, Xwmc233, Xgwm190 | [136] |
| 5DS | Lr78 | Slow rusting | T. aestivum: Toropi (PI 344200) | IWA6289, cfd189, wmc233 | [137] |
| 5D | Lr57 | AS/APR | Ae. geniculata: TA6675 | Xbcd1087, Xabg705, Xpsr128 | [138] |
| 5DL | Lr1 | AS | T. aestivum: 87E03-S2B1 | Xgwm272, Xgwm65 | [4] |
| 6D | QLr.cdl-6D | APR | T. aestivum: Americano 44d | wPt-664670 | [37] |
| 6DL | QLr.cau-6DL | APR | T. aestivum: Bai Qimai | Cfd188 | [139] |
| 6DL | Lr38 | AS/APR | A. intermedium: RL6079 | Xwmc773, Xbarc273 | [140] |
| 7DS | QLr.jki-7D.1 | APR | T. aestivum: MAGIC population | AX-94930280 | [141] |
| 7DS | Lr34 | Slow rusting | T. aestivum: SAAR | Xgwm37, XcsLV34, Xgwm295 | [14] |
| 7DS | Lr29 | AS | A. elongatum: RL6080 | OPY10950, UBC2191000 | [146] |
| 7DS | QLr.wpt-7DS | APR | T. aestivum: wheat cultivar collection (GWAS) | wPt-2565 | [97] |
| 7DL | Lr19 | AS | A. elongatum: TcLr19 | Xwmc221, Xgdm46, Xgdm67 | [147,148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Wang, C.; Ren, Z.; Wang, J.; Zhang, P.; Zhao, S.; Li, M.; Yuan, M.; Yu, X.; Li, Z.; et al. Genetics of Resistance to Leaf Rust in Wheat: An Overview in a Genome-Wide Level. Sustainability 2023, 15, 3247. https://doi.org/10.3390/su15043247
Ren X, Wang C, Ren Z, Wang J, Zhang P, Zhao S, Li M, Yuan M, Yu X, Li Z, et al. Genetics of Resistance to Leaf Rust in Wheat: An Overview in a Genome-Wide Level. Sustainability. 2023; 15(4):3247. https://doi.org/10.3390/su15043247
Chicago/Turabian StyleRen, Xiaopeng, Chuyuan Wang, Zhuang Ren, Jing Wang, Peipei Zhang, Shuqing Zhao, Mengyu Li, Meng Yuan, Xiumei Yu, Zaifeng Li, and et al. 2023. "Genetics of Resistance to Leaf Rust in Wheat: An Overview in a Genome-Wide Level" Sustainability 15, no. 4: 3247. https://doi.org/10.3390/su15043247
APA StyleRen, X., Wang, C., Ren, Z., Wang, J., Zhang, P., Zhao, S., Li, M., Yuan, M., Yu, X., Li, Z., Chen, S., & Wang, X. (2023). Genetics of Resistance to Leaf Rust in Wheat: An Overview in a Genome-Wide Level. Sustainability, 15(4), 3247. https://doi.org/10.3390/su15043247









