Experimental and Theoretical Studies of the Corrosion Inhibition Performance of a Quaternary Phosphonium-Based Ionic Liquid for Mild Steel in HCl Medium
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Electrochemical Measurements
2.3. Surface Analysis
2.4. UV-Visible Analysis of the Solution
2.5. Computational Methodology
2.5.1. DFT Calculations
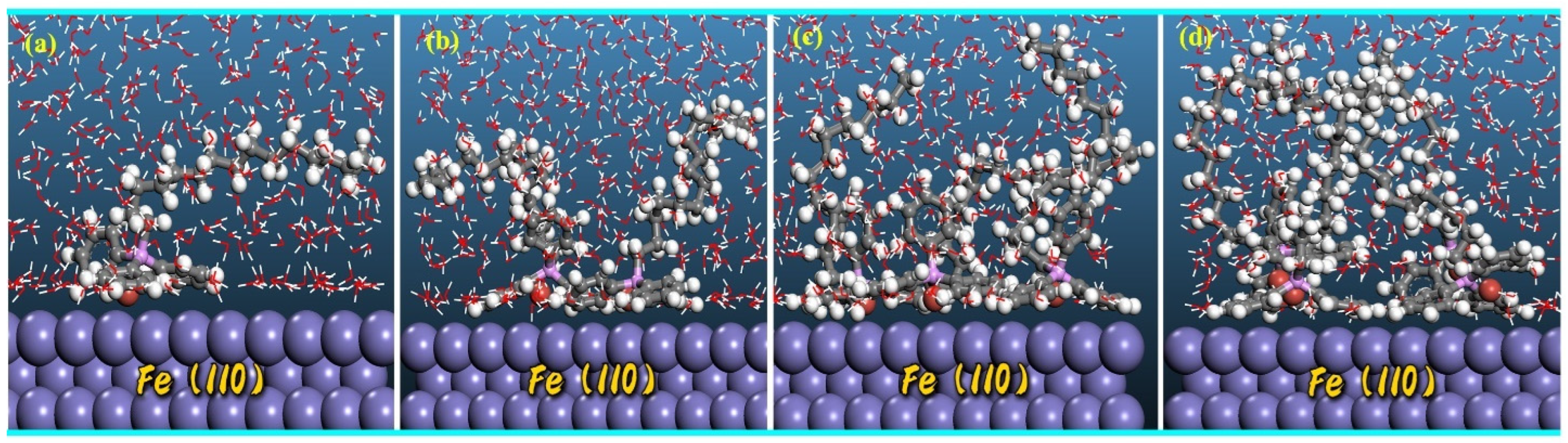
2.5.2. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Open Circuit Potential and PDP Analysis
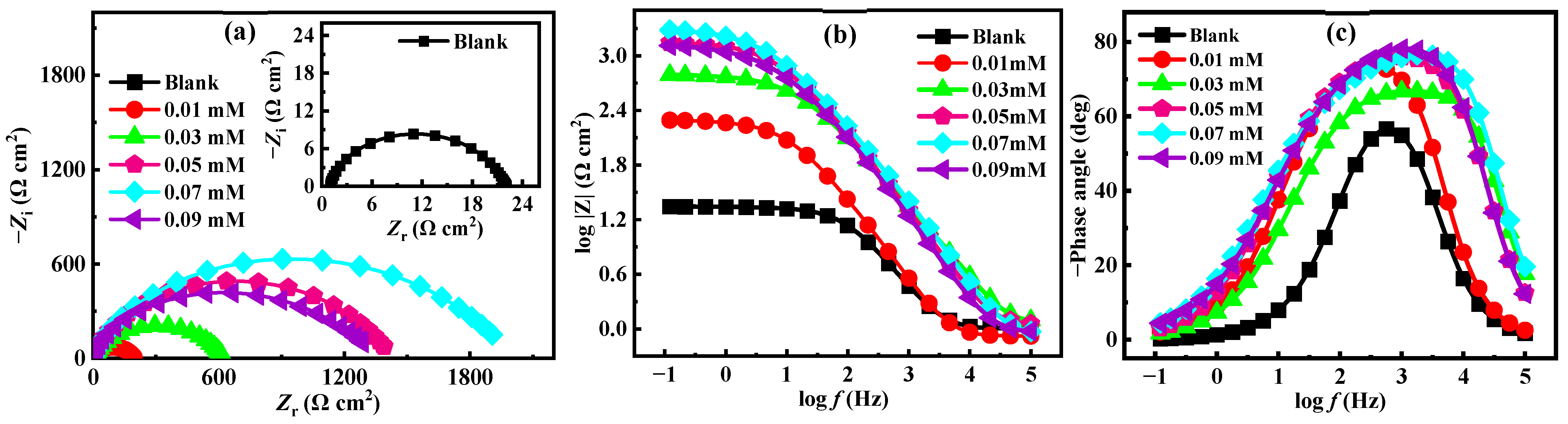
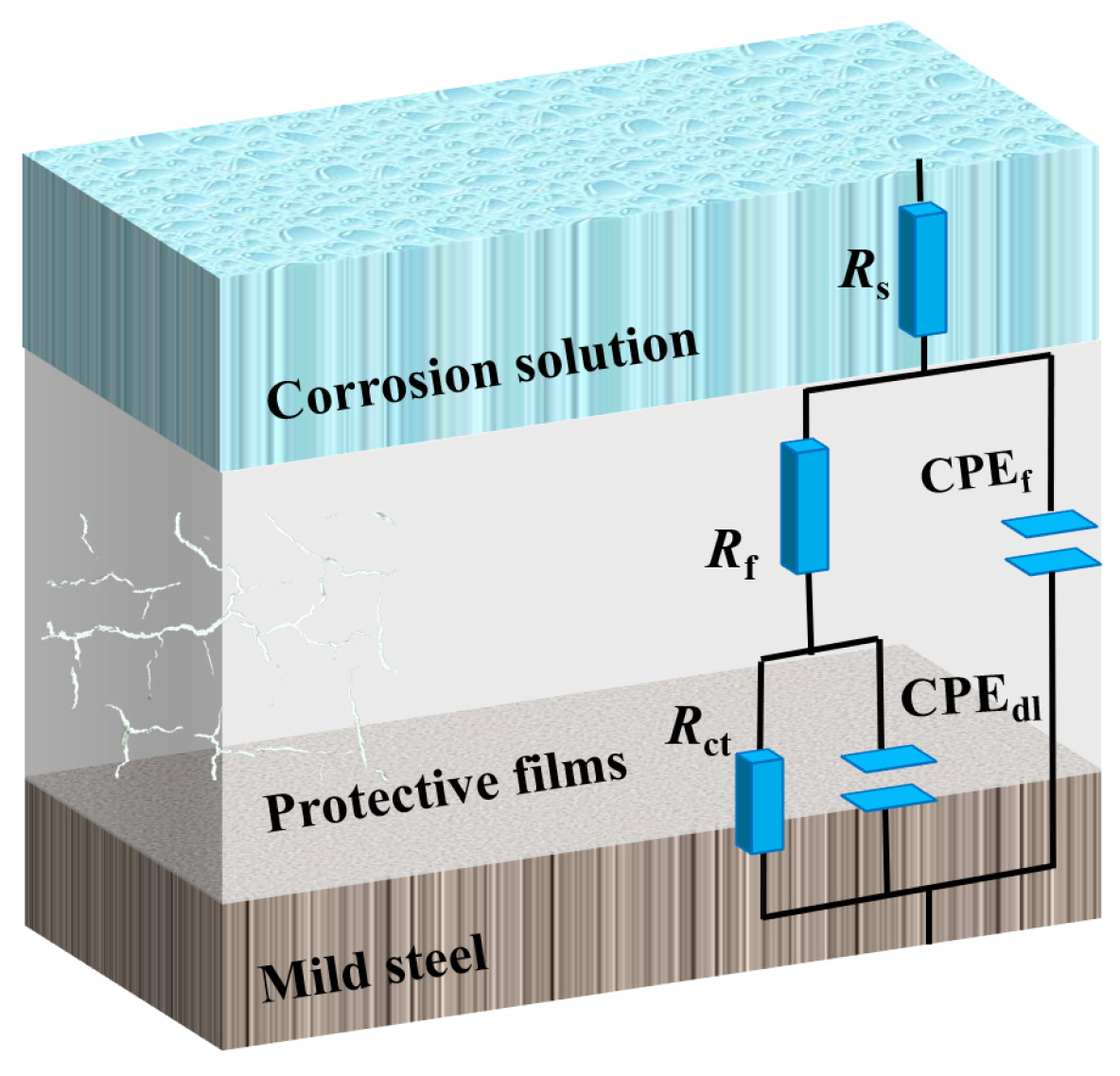
3.2. EIS Measurement
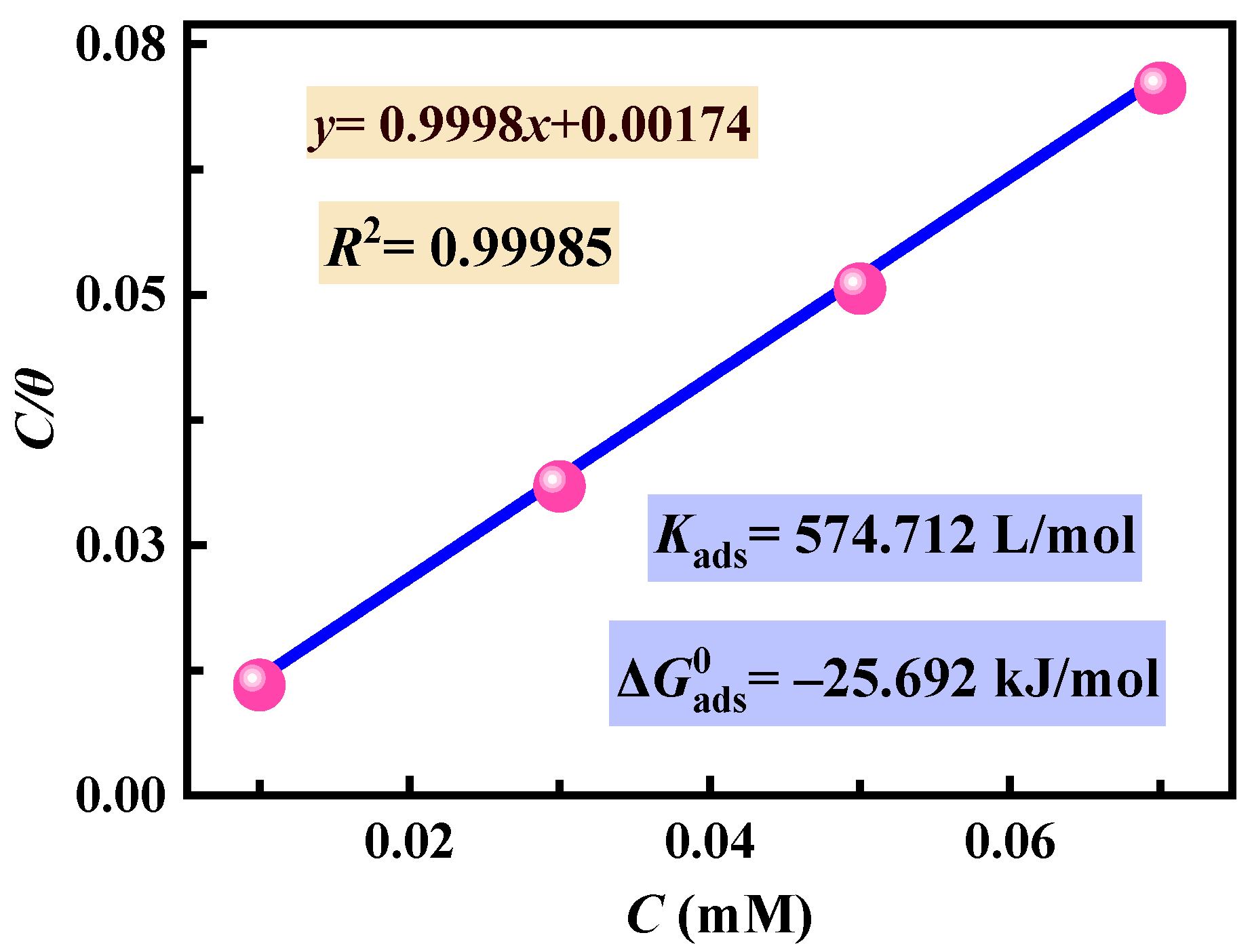
3.3. Adsorption Isotherm
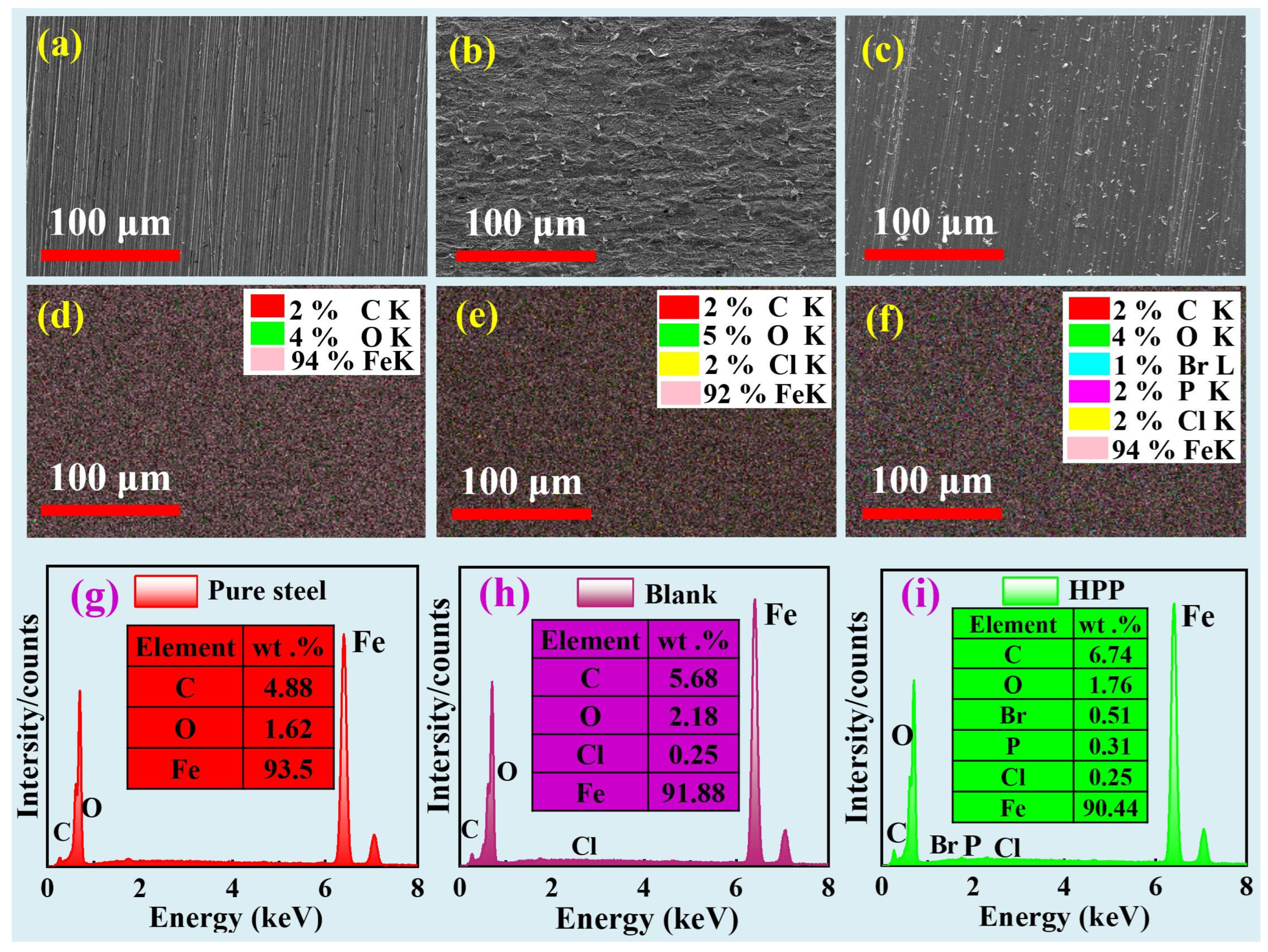
3.4. SEM-EDS Analysis
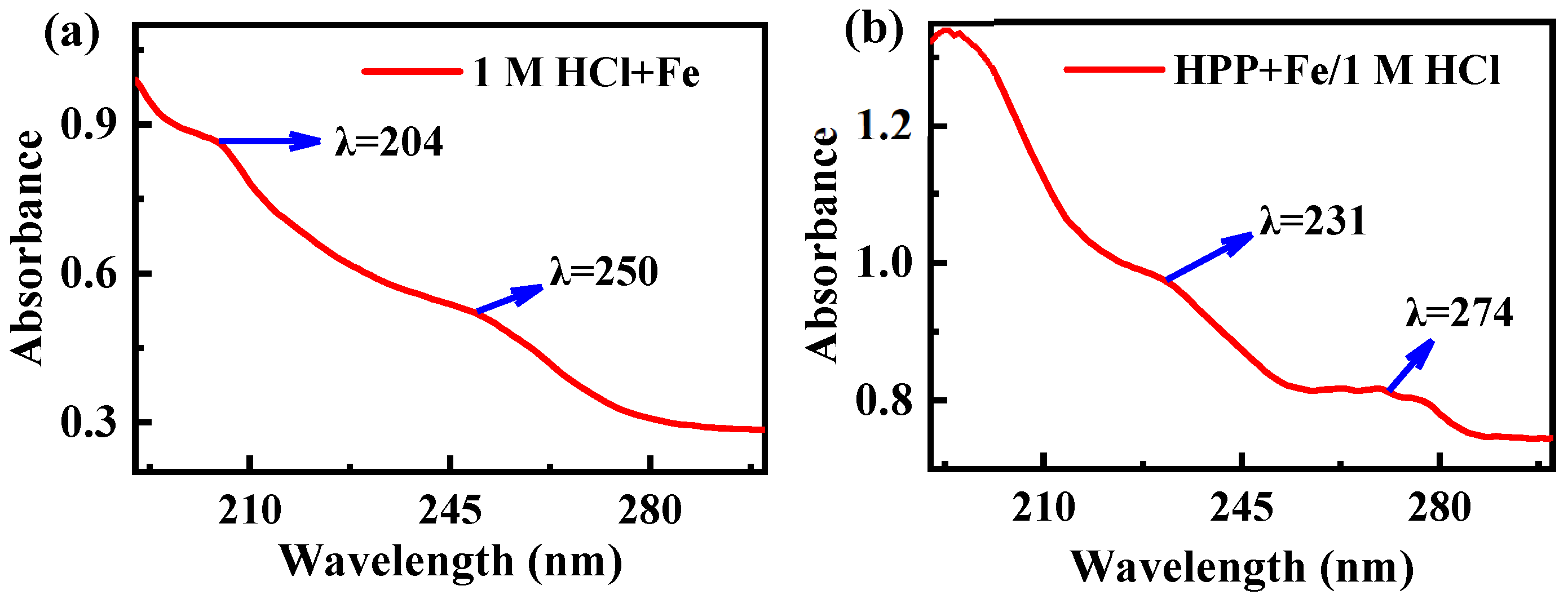
3.5. UV-Visible Spectroscopy Study
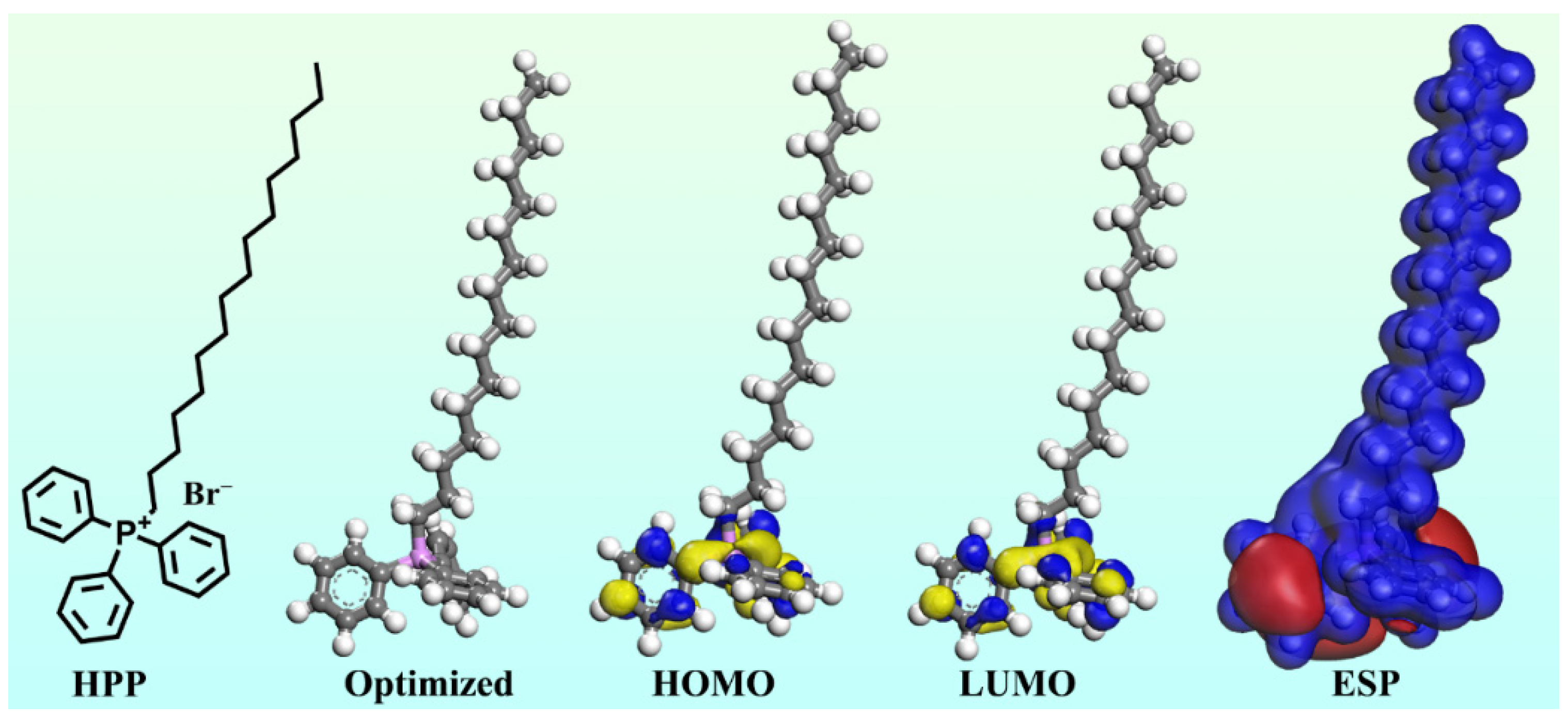
3.6. Computational Perspectives
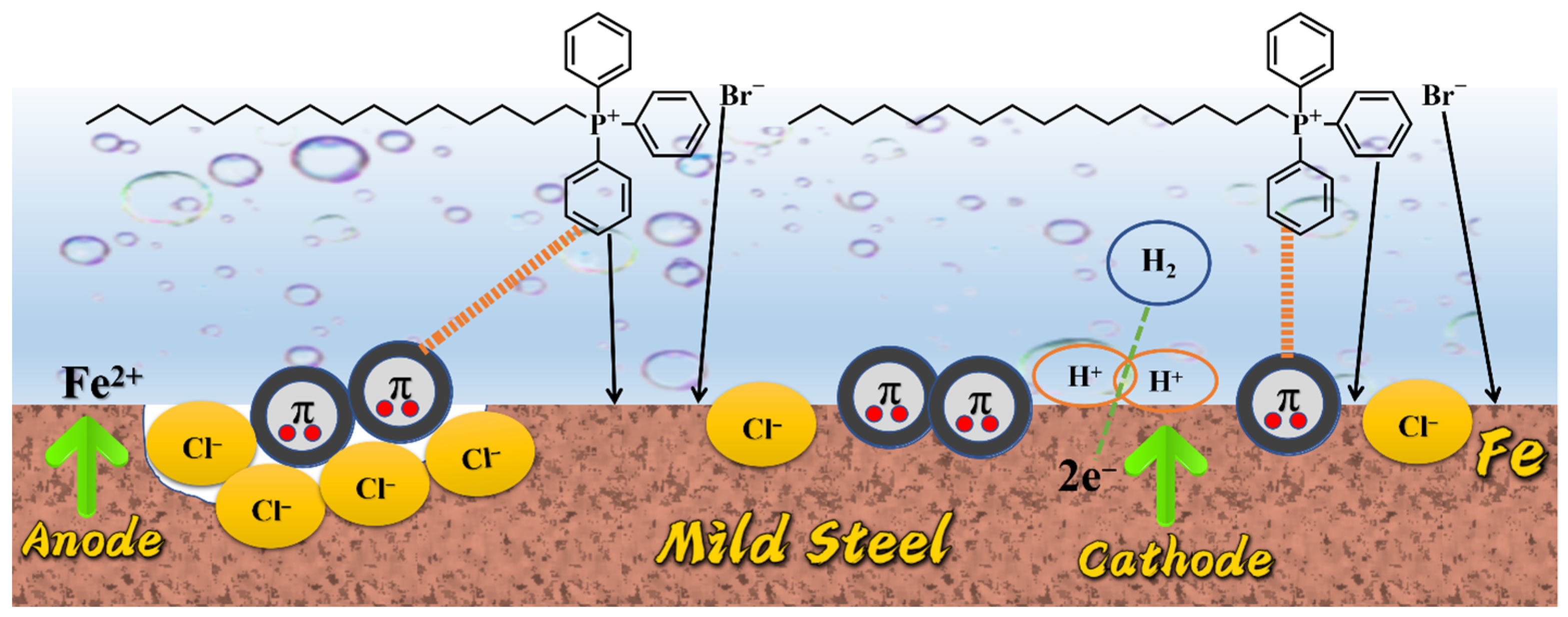
3.7. Analysis of Anticorrosive Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abd El-Lateef, H.M. Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions. Corros. Sci. 2015, 92, 104–117. [Google Scholar] [CrossRef]
- Slepski, P.; Gerengi, H.; Jazdzewska, A.; Orlikowski, J.; Darowicki, K. Simultaneous impedance and volumetric studies and additionally potentiodynamic polarization measurements of molasses as a carbon steel corrosion inhibitor in 1M hydrochloric acid solution. Constr. Build. Mater. 2014, 52, 482–487. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef]
- Cook, A.; Frankel, G.; Davenport, A.; Hughes, T.; Gibbon, S.; Williams, D.; Bluhm, H.; Maurice, V.; Lyth, S.; Marcus, P.; et al. Corrosion control: General discussion. Faraday Discuss. 2015, 180, 543–576. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A. Recent advances in metallic corrosion inhibition: A review. J. Mol. Liq. 2021, 322, 114862. [Google Scholar] [CrossRef]
- Aslam, R.; Serdaroglu, G.; Zehra, S.; Kumar Verma, D.; Aslam, J.; Guo, L.; Verma, C.; Ebenso, E.E.; Quraishi, M.A. Corrosion inhibition of steel using different families of organic compounds: Past and present progress. J. Mol. Liq. 2022, 348, 118373. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors: Design, performance and industrial scale applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Sorour, A.A.; Verma, C. A review on corrosion inhibitors for high-pressure supercritical CO2 environment: Challenges and opportunities. J. Pet. Sci. Eng. 2022, 215, 110695. [Google Scholar] [CrossRef]
- Zhuang, W.; Wang, X.; Zhu, W.; Zhang, Y.; Sun, D.; Zhang, R.; Wu, C. Imidazoline gemini surfactants as corrosion inhibitors for carbon steel X70 in NaCl solution. ACS Omega 2021, 6, 5653–5660. [Google Scholar] [CrossRef]
- Amendola, R.; Acharjee, A. Microbiologically influenced corrosion of copper and its alloys in anaerobic aqueous environments: A review. Front. Microbiol. 2022, 13, 806688. [Google Scholar] [CrossRef]
- Han, P.; Chen, C.; Li, W.; Yu, H.; Xu, Y.; Ma, L.; Zheng, Y. Synergistic effect of mixing cationic and nonionic surfactants on corrosion inhibition of mild steel in HCl: Experimental and theoretical investigations. J. Colloid Interface Sci. 2018, 516, 398–406. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Fatyeyeva, K. Ionic liquids as green and sustainable steel corrosion inhibitors: Recent developments. Chem. Eng. J. 2021, 425, 131480. [Google Scholar] [CrossRef]
- Ardakani, E.K.; Kowsari, E.; Ehsani, A.; Ramakrishna, S. Performance of all ionic liquids as the eco-friendly and sustainable compounds in inhibiting corrosion in various media: A comprehensive review. Microchem. J. 2021, 165, 106049. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial applications of ionic liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- El Hajjaji, F.; Ech-chihbi, E.; Salim, R.; Titi, A.; Messali, M.; El Ibrahimi, B.; Kaya, S.; Taleb, M. A detailed electronic-scale DFT modeling/MD simulation, electrochemical and surface morphological explorations of imidazolium-based ionic liquids as sustainable and non-toxic corrosion inhibitors for mild steel in 1 M HCl. Mater. Sci. Eng. B 2023, 289, 116232. [Google Scholar] [CrossRef]
- Liu, P.; Dai, S.; Lan, J.; Lu, H.; Wang, B.; Zhu, Y. Corrosion inhibition mechanism of imidazole ionic liquids with high temperature in 20% HCl solution. J. Mol. Model. 2022, 29, 29. [Google Scholar] [CrossRef]
- Zheng, X.W.; Zhang, S.T.; Li, W.P.; Yin, L.L.; He, J.H.; Wu, J.F. Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodide as inhibitor for mild steel in sulfuric acid solution. Corros. Sci. 2014, 80, 383–392. [Google Scholar] [CrossRef]
- Meunier, M.; Robertson, S. Materials studio 20th anniversary. Mol. Simul. 2021, 47, 537–539. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.-M.; Wang, S.-S.; Wang, Y.-H.; Li, H.-J.; Wu, Y.-C. Environmentally benign cinchonain IIa from Uncaria laevigata for corrosion inhibition of Q235 steel in HCl corrosive medium: Experimental and theoretical investigation. Environ. Res. 2022, 215, 114376. [Google Scholar] [CrossRef]
- Li, E.; Li, Y.; Liu, S.; Yao, P. Choline amino acid ionic liquids as green corrosion inhibitors of mild steel in acidic medium. Colloid. Surface A 2023, 657, 130541. [Google Scholar] [CrossRef]
- Ahmad, Z.U.; Chao, B.; Konggidinata, M.I.; Lian, Q.; Zappi, M.E.; Gang, D.D. Molecular simulation and experimental validation of resorcinol adsorption on ordered mesoporous carbon (OMC). J. Hazard. Mater. 2018, 354, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qi, C.; Zheng, X.; Zhang, R.; Shen, X.; Kaya, S. Toward understanding the adsorption mechanism of large size organic corrosion inhibitors on an Fe(110) surface using the DFTB method. RSC Adv. 2017, 7, 29042–29050. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Abu-Rayyan, A.; Al Jahdaly, B.A.; AlSalem, H.S.; Alhadhrami, N.A.; Hajri, A.K.; Bukhari, A.A.H.; Waly, M.M.; Salem, A.M. A study of the synthesis and characterization of new acrylamide derivatives for use as corrosion inhibitors in nitric acid solutions of copper. Nanomaterials 2022, 12, 3685. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Nwankwo, H.U.; Ateba, C.N.; Olasunkanmi, L.O.; Adekunle, A.S.; Isabirye, D.A.; Onwudiwe, D.C.; Ebenso, E.E. Synthesis, characterization, antimicrobial studies and corrosion inhibition potential of 1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane: Experimental and quantum chemical studies. Materials 2016, 9, 107. [Google Scholar] [CrossRef]
- Betti, N.; Al-Amiery, A.A.; Al-Azzawi, W.K. Experimental and quantum chemical investigations on the anticorrosion efficiency of a nicotinehydrazide derivative for mild steel in HCl. Molecules 2022, 27, 6254. [Google Scholar] [CrossRef]
- Singh, P.; Quraishi, M.A. Corrosion inhibition of mild steel using Novel Bis Schiff’s Bases as corrosion inhibitors: Electrochemical and surface measurement. Measurement 2016, 86, 114–124. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Li, B.; Guo, H.; Dou, X.; Lu, K.; Feng, Y. High-performance corrosion resistance of chemically-reinforced chitosan as ecofriendly inhibitor for mild steel. Bioelectrochemistry 2023, 150, 108330. [Google Scholar] [CrossRef]
- Laadam, G.; Benhiba, F.; El Faydy, M.; Titi, A.; Al-Gorair, A.S.; Alshareef, M.; Hawsawi, H.; Touzani, R.; Warad, I.; Bellaouchou, A.; et al. Anti-corrosion performance of novel pyrazole derivative for carbon steel corrosion in 1 M HCl: Computational and experimental studies. Inorg. Chem. Commun. 2022, 145, 109963. [Google Scholar] [CrossRef]
- Mahfoud, H.; Rouag, N.; Boudiba, S.; Benahmed, M.; Morakchi, K.; Akkal, S. Mathematical and electrochemical investigation of Lamium flexuosum extract as effective corrosion inhibitor for CS in acidic solution using multidimensional minimization program system. Arab. J. Sci. Eng. 2022, 47, 6605–6616. [Google Scholar] [CrossRef]
- Brug, G.J.; Vandeneeden, A.L.G.; Sluytersrehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- El-Haitout, B.; Hejjaj, C.; Lgaz, H.; Al-Hadeethi, M.R.; Ali, O.M.; Lee, H.S.; Ali, I.H.; Salghi, R. Superior long-term corrosion inhibition of N80 steel by new eco-friendly hydrazone-based compounds in a simulated oil well acidizing environment: Establishing the mechanism at the molecular level. Langmuir 2022, 38, 15937–15949. [Google Scholar] [CrossRef]
- El Aatiaoui, A.; Daoudi, W.; El Boutaybi, A.; Guo, L.; Benchat, N.-E.; Aouinti, A.; Oussaid, A.; Loutou, M. Synthesis and anticorrosive activity of two new imidazo[1, 2-a]pyridine Schiff bases. J. Mol. Liq. 2022, 350, 118458. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, M.; He, Z.; Zhang, R.; Kaya, S.; Lin, Y.; Saji, V.S. One-pot hydrothermal synthesized nitrogen and sulfur codoped carbon dots for acid corrosion inhibition of Q235 steel. Langmuir 2022, 38, 3984–3992. [Google Scholar] [CrossRef]
- Hu, K.; Zhuang, J.; Ding, J.; Ma, Z.; Wang, F.; Zeng, X. Influence of biomacromolecule DNA corrosion inhibitor on carbon steel. Corros. Sci. 2017, 125, 68–76. [Google Scholar] [CrossRef]
- Huang, L.; Wang, S.S.; Li, H.J.; Wang, J.Y.; Li, Z.G.; Wu, Y.C. Highly effective Q235 steel corrosion inhibition in 1 M HCl solution by novel green strictosamide from Uncaria laevigata: Experimental and theoretical approaches. J. Environ. Chem. Eng. 2022, 10, 107581. [Google Scholar] [CrossRef]
- Conway, B.E.; Bockris, J.O.M.; Ammar, I.A. The dielectric constant of the solution in the diffuse and Helmholtz double layers at a charged interface in aqueous solution. Trans. Faraday Soc. 1951, 47, 756–766. [Google Scholar] [CrossRef]
- Zhu, M.; He, Z.; Guo, L.; Zhang, R.; Anadebe, V.C.; Obot, I.B.; Zheng, X. Corrosion inhibition of eco-friendly nitrogen-doped carbon dots for carbon steel in acidic media: Performance and mechanism investigation. J. Mol. Liq. 2021, 342, 117583. [Google Scholar] [CrossRef]
- Jalab, R.; Saad, M.A.; Sliem, M.H.; Abdullah, A.M.; Hussein, I.A. An eco-friendly quaternary ammonium salt as a corrosion inhibitor for carbon steel in 5 M HCl solution: Theoretical and experimental investigation. Molecules 2022, 27, 6414. [Google Scholar] [CrossRef]
- Mittal, A.; Kurup, L.; Mittal, J. Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J. Hazard. Mater. 2007, 146, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Martin, U.; Bastidas, D.M. Adsorption and surface analysis of sodium phosphate corrosion inhibitor on carbon steel in simulated concrete pore solution. Materials 2022, 15, 7429. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Rajeev, R.; Varghese, A.; Rajendran, N. Surface adsorption and anticorrosive behavior of benzimidazolium inhibitor in acid medium for carbon steel corrosion. J. Appl. Electrochem. 2022, 52, 1659–1674. [Google Scholar] [CrossRef]
- Costa, M.; Carvalho, A.; Uryga, A.; Paiva, A. Solvent extraction of iron(III) from hydrochloric acid solutions using N,N′-Dimethyl-N,N′-diphenylmalonamide and N,N′-Dimethyl-N,N′-diphenyltetradecylmalonamide. Solvent Extr. Ion Exch. 2003, 21, 653–686. [Google Scholar] [CrossRef]
- Rbaa, M.; Benhiba, F.; Obot, I.B.; Oudda, H.; Warad, I.; Lakhrissi, B.; Zarrouk, A. Two new 8-hydroxyquinoline derivatives as an efficient corrosion inhibitors for mild steel in hydrochloric acid: Synthesis, electrochemical, surface morphological, UV–visible and theoretical studies. J. Mol. Liq. 2019, 276, 120–133. [Google Scholar] [CrossRef]
- Serin, S. DFT-based computations on some structurally related N-substituted piperazines. J. Indian Chem. Soc. 2022, 99, 100766. [Google Scholar] [CrossRef]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2021, 406, 126863. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Z.; Zhao, X.; Fan, B.; Liu, H.; Guo, M.; Hao, H. Efficient corrosion inhibition by sugarcane purple rind extract for carbon steel in HCl solution: Mechanism analyses by experimental and in silico insights. RSC Adv. 2021, 11, 31693–31711. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, S.; Zhang, S.; He, Q.; Li, W. Theoretical studies of three triazole derivatives as corrosion inhibitors for mild steel in acidic medium. Corros. Sci. 2014, 87, 366–375. [Google Scholar] [CrossRef]
- Shahsavari, M.; Imani, A.; Asselin, E. Pomegranate arils extract as a green corrosion inhibitor for mild steel: Effect of concentration and temperature in hydrochloric acid. Mater. Res. Express 2022, 9, 116517. [Google Scholar] [CrossRef]
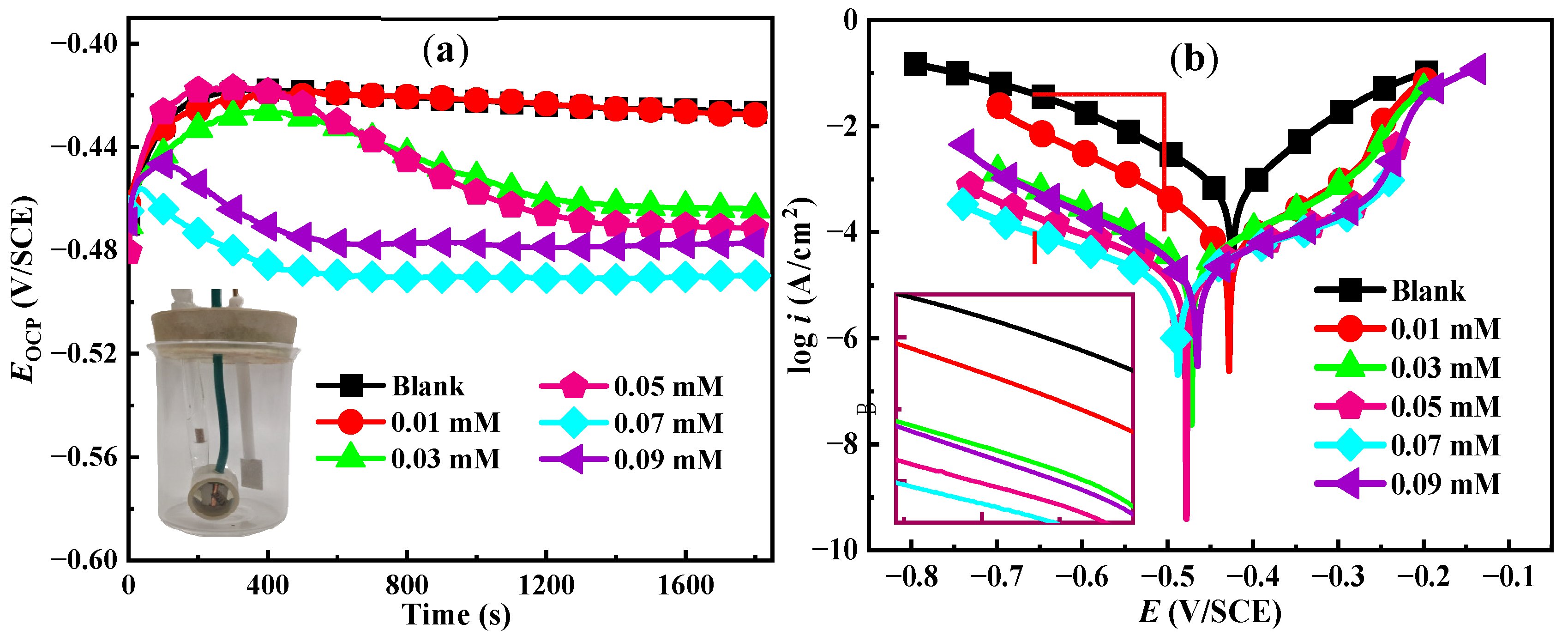
| C (mM) | Ecorr (V/SCE) | icorr (μA/cm2) | βc (mV/dec−1) | βa (mV/dec−1) | ηPDP (%) |
|---|---|---|---|---|---|
| Blank | −0.426 | 669.2 | −115 | 82 | / |
| 0.01 | −0.427 | 77.39 | −105 | 104 | 88.4 |
| 0.03 | −0.464 | 38.63 | −136 | 134 | 94.2 |
| 0.05 | −0.472 | 21.41 | −161 | 165 | 96.8 |
| 0.07 | −0.489 | 15.79 | −176 | 155 | 97.6 |
| 0.09 | −0.477 | 26.37 | −129 | 177 | 96.1 |
| C (mM) | Rs (Ω cm2) | CPEf | Cf (μF cm−2) | Rf (Ω cm2) | CPEdl | Cdl (μF cm−2) | Rct (Ω cm2) | χ2 (10−3) | θ | ηEIS (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y0 (10−5 S Sn cm−2) | n1 | Y0 (10−5 S Sn cm−2) | n2 | |||||||||
| Blank | 1.003 | 5.177 | 1.000 | 51.7 | 3.92 | 415.60 | 0.686 | 1241.0 | 17.1 | 8.22 | / | / |
| 0.01 | 0.832 | 4.336 | 1.000 | 43.3 | 13.90 | 24.74 | 0.644 | 45.0 | 185.2 | 2.17 | 0.907 | 90.7 |
| 0.03 | 1.171 | 42.20 | 1.000 | 422.0 | 12.07 | 6.39 | 0.689 | 14.8 | 612.6 | 2.88 | 0.972 | 97.2 |
| 0.05 | 1.106 | 64.20 | 0.999 | 639.2 | 19.50 | 4.82 | 0.664 | 12.4 | 1412.0 | 5.43 | 0.988 | 98.8 |
| 0.07 | 0.877 | 50.59 | 1.000 | 505.9 | 25.56 | 5.44 | 0.633 | 15.0 | 1986.0 | 8.10 | 0.991 | 99.1 |
| 0.09 | 0.919 | 78.92 | 1.000 | 789.2 | 17.24 | 8.19 | 0.582 | 16.6 | 1351.0 | 9.61 | 0.987 | 98.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Huang, Y.; Wu, Y.; Shi, W.; Abbas, F.; Lin, Y.; Marzouki, R.; Zheng, X. Experimental and Theoretical Studies of the Corrosion Inhibition Performance of a Quaternary Phosphonium-Based Ionic Liquid for Mild Steel in HCl Medium. Sustainability 2023, 15, 3103. https://doi.org/10.3390/su15043103
Guo L, Huang Y, Wu Y, Shi W, Abbas F, Lin Y, Marzouki R, Zheng X. Experimental and Theoretical Studies of the Corrosion Inhibition Performance of a Quaternary Phosphonium-Based Ionic Liquid for Mild Steel in HCl Medium. Sustainability. 2023; 15(4):3103. https://doi.org/10.3390/su15043103
Chicago/Turabian StyleGuo, Lei, Yue Huang, Yundong Wu, Wei Shi, Faheem Abbas, Yuanhua Lin, Riadh Marzouki, and Xingwen Zheng. 2023. "Experimental and Theoretical Studies of the Corrosion Inhibition Performance of a Quaternary Phosphonium-Based Ionic Liquid for Mild Steel in HCl Medium" Sustainability 15, no. 4: 3103. https://doi.org/10.3390/su15043103
APA StyleGuo, L., Huang, Y., Wu, Y., Shi, W., Abbas, F., Lin, Y., Marzouki, R., & Zheng, X. (2023). Experimental and Theoretical Studies of the Corrosion Inhibition Performance of a Quaternary Phosphonium-Based Ionic Liquid for Mild Steel in HCl Medium. Sustainability, 15(4), 3103. https://doi.org/10.3390/su15043103









