Groundwater Risk Assessment Based on DRASTIC and Special Vulnerability of Solidified/Stabilized Heavy-Metal-Contaminated Sites
Abstract
:1. Introduction
2. Methods
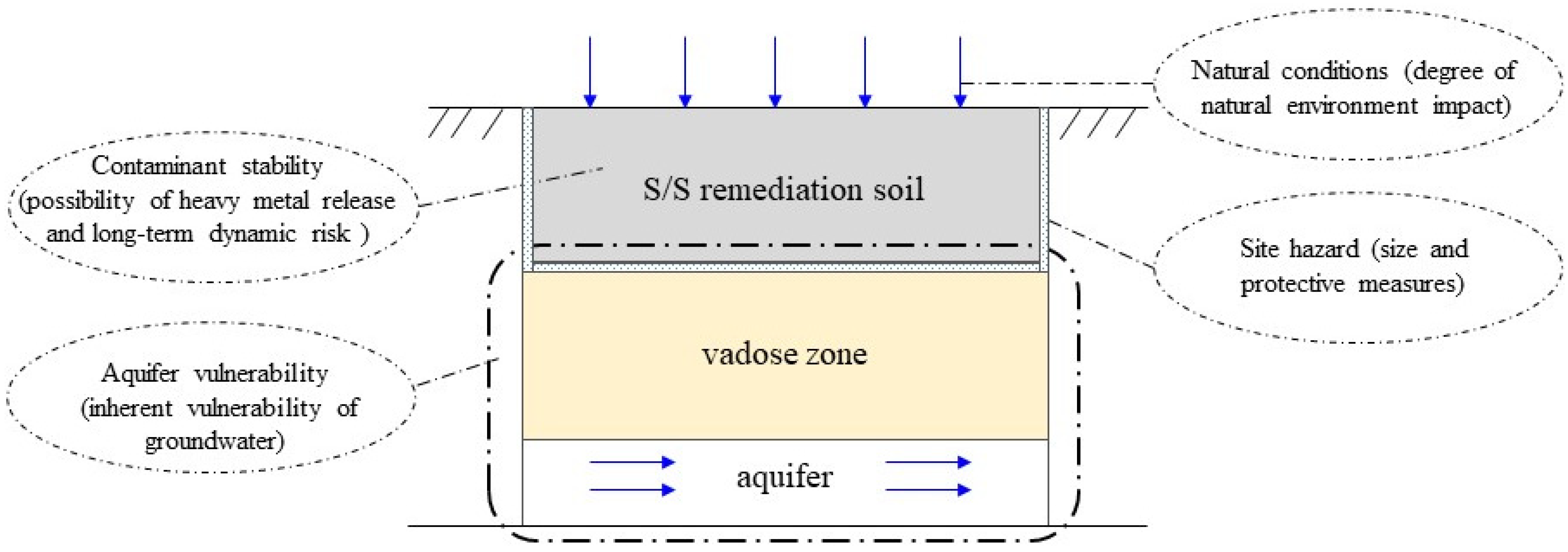
2.1. Conceptual Model
2.2. Index System Construction
2.2.1. Site Hazards
2.2.2. Contaminant Stability
2.2.3. Aquifer Vulnerability
2.2.4. Natural Conditions
2.2.5. Index System
2.3. Grading and Scoring Methods of Evaluation Indexes
2.4. Determination of Evaluation index Weights
2.5. Comprehensive Index Risk Evaluation Model
3. Results and discussion
3.1. Index Classification and Scoring
3.1.1. Site Hazard Index Classification and Scoring
3.1.2. Contaminant Stability Index Grading and Scoring
3.1.3. Aquifer Vulnerability Index Classification and Scoring
3.1.4. Grading and Scoring of Natural Condition Indexes
3.2. Calculation of Weights
3.3. Evaluation Level Classification
3.4. Case Study
3.4.1. Study Site
3.4.2. Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Environmental Protection; Ministry of Land and Resources. National Soil Pollution Status Survey Bulletin; Ministry of Environmental Protection: Beijing, China, 2014. [Google Scholar]
- US EPA. Innovative Treatment Technologies for Site Cleanup, 12th ed.; Annual Status Report, EPA 542-R-07-012; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- Moreno, A.M.; Quintana, J.R.; Pérez, L.; Parra, J.G. Factors influencing lead sorption–desorption at variable added metal concentrations in Rhodoxeralfs. Chemosphere 2006, 64, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Miao, C.; Yao, S.; Ding, H.; Zhang, K. Soil stabilization/solidification (S/S) agent—Water-soluble thiourea formaldehyde (WTF) resin: Mechanism and performance with cadmium (Ⅱ). Environ. Pollut. 2021, 272, 116025. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.A.; Solanki, C.H.; Kumar, S.; Reddy, K.R.; Du, Y.-J. Comparison of limestone calcined clay cement and ordinary Portland cement for stabilization/solidification of Pb-Zn smelter residue. Environ. Sci. Pollut. Res. 2021, 29, 11393–11404. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Hou, D.; Xu, W.; Zhang, J.; Jin, F.; Zhao, B.; Pan, S.; Peng, T.; Alessi, D.S. Assessing long-term stability of cadmium and lead in a soil washing residue amended with MgO-based binders using quantitative accelerated ageing. Sci. Total Environ. 2018, 643, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yoneda, M.; Feng, L. Risk dynamic evolution index based on fraction transformation and its application to site risk assessment. J. Hazard. Mater. 2021, 412, 125210. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, P.; Li, J.-S.; Zhang, T.-T.; Wang, S.-Y. Investigation of the leaching behavior of lead in stabilized/solidified waste using a two-year semi-dynamic leaching test. Chemosphere 2017, 166, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, J.; Hu, B.; Wei, W. Adsorption and desorption for dynamics transport of hexavalent chromium (Cr(VI)) in soil column. Environ. Sci. Pollut. Res. 2017, 25, 459–468. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakamura, A.; Niinae, M.; Nakata, H.; Fujii, H.; Tasaka, Y. Lead immobilization in artificially contaminated kaolinite using magnesium oxide-based materials: Immobilization mechanisms and long-term evaluation. Chem. Eng. J. 2013, 232, 380–387. [Google Scholar] [CrossRef]
- Zha, F.; Liu, C.; Kang, B.; Yang, X.; Zhou, Y.; Yang, C. Acid rain leaching behavior of Zn-contaminated soils solidified/stabilized using cement–soda residue. Chemosphere 2021, 281, 130916. [Google Scholar] [CrossRef]
- Li, Z.; Gong, Y.; Zhao, D.; Deng, H.; Dang, Z.; Lin, Z. Field assessment of carboxymethyl cellulose bridged chlorapatite microparticles for immobilization of lead in soil: Effectiveness, long-term stability, and mechanism. Sci. Total Environ. 2021, 781, 146757. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Al-Tabbaa, A. Leachability and heavy metal speciation of 17-year old stabilised/solidified contaminated site soils. J. Hazard. Mater. 2014, 278, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, J.; Chen, H. Assessment of groundwater contamination risk using hazard quantification, a modified DRASTIC model and groundwater value, Beijing Plain, China. Sci. Total Environ. 2012, 432, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Chen, Z.; Zhang, W. Groundwater contamination risk assessment based on intrinsic vulnerability, pollution source assessment, and groundwater function zoning. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1907–1923. [Google Scholar] [CrossRef]
- Aller, L.; Bennett, T.; Lehr, J.; Petty, R.J.; Hackett, G. DRASTIC: A Standardized System for Evaluating Groundwater Pollution Potential Using Hydrogeologics; US Environmental Protection Agency: Washington, DC, USA, 1987. [Google Scholar]
- Huan, H.; Zhang, B.-T.; Kong, H.; Li, M.; Wang, W.; Xi, B.; Wang, G. Comprehensive assessment of groundwater pollution risk based on HVF model: A case study in Jilin City of northeast China. Sci. Total Environ. 2018, 628–629, 1518–1530. [Google Scholar] [CrossRef]
- Zhang, B.; Li, G.; Cheng, P.; Yeh, T.-C.J.; Hong, M. Landfill Risk Assessment on Groundwater Based on Vulnerability and Pollution Index. Water Resour. Manag. 2016, 30, 1465–1480. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Xu, H.; Ding, Z.; Shi, Y.; Lu, Z.; Cheng, Z. Groundwater pollution risk assessment based on groundwater vulnerability and pollution load on an isolated island. Chemosphere 2021, 289, 133134. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, P.; Lyu, Q.; Ren, X.; He, S. Groundwater contamination risk assessment using a modified DRATICL model and pollution loading: A case study in the Guanzhong Basin of China. Chemosphere 2021, 291, 132695. [Google Scholar] [CrossRef]
- Hu, G.; Liu, H.; Chen, C.; Hou, H.; Li, J.; Hewage, K.; Sadiq, R. Low-temperature thermal desorption and secure landfill for oil-based drill cuttings management: Pollution control, human health risk, and probabilistic cost assessment. J. Hazard. Mater. 2021, 410, 124570. [Google Scholar] [CrossRef]
- Fleri, M.A.; Whetstone, G.T. In situ stabilisation/solidification: Project lifecycle. J. Hazard. Mater. 2007, 141, 441–456. [Google Scholar] [CrossRef]
- Al-Tabbaa, A.; Boes, N. Pilot in situ auger mixing treatment of a contaminated site. Part 4. Performance at five years. Proc. Inst. Civ. Eng. -Geotech. Eng. 2002, 155, 187–202. [Google Scholar] [CrossRef]
- Lu, S.F.; Wu, Y.L.; Chen, Z.; Li, T.; Shen, C.; Xuan, L.K.; Xu, L. Remediation of contaminated soil and groundwater using chemical reduction and solidification/stabilization method: A case study. Environ. Sci. Pollut. Res. 2021, 28, 12766–12779. [Google Scholar] [CrossRef]
- Khadhar, S.; Sdiri, A.; Chekirben, A.; Azouzi, R.; Charef, A. Integration of sequential extraction, chemical analysis and statistical tools for the availability risk assessment of heavy metals in sludge amended soils. Environ. Pollut. 2020, 263, 114543. [Google Scholar] [CrossRef]
- Setia, R.; Dhaliwal, S.S.; Singh, R.; Kumar, V.; Taneja, S.; Kukal, S.S.; Pateriya, B. Phytoavailability and human risk assessment of heavy metals in soils and food crops around Sutlej river, India. Chemosphere 2020, 263, 128321. [Google Scholar] [CrossRef] [PubMed]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Li, H.L.; Yin, C.Q.; Shan, B. Fraction distribution and risk assessment of heavy metals in sediments of Moshui Lake. J. Environ. Sci. 2008, 20, 390–397. [Google Scholar] [CrossRef]
- Lee, S.Z.; Allen, H.E.; Huang, C.P.; Sparks, D.L.; Sanders, P.F.; Peijnenburg, W.J. Predicting soil− water partition coefficients for cadmium. Environ. Sci. Technol. 1996, 30, 3418–3424. [Google Scholar] [CrossRef]
- Jing, Y.; He, Z.; Yang, X. Effects of pH, organic acids, and competitive cations on mercury desorption in soils. Chemosphere 2007, 69, 1662–1669. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Hansen, H.K.; Jensen, P.E. Relation between pH and desorption of Cu, Cr, Zn, and Pb from industrially polluted soils. Water Air Soil Pollut. 2009, 201, 295–304. [Google Scholar] [CrossRef]
- Strawn, D.G.; Sparks, D.L. Effects of Soil Organic Matter on the Kinetics and Mechanisms of Pb(II) Sorption and Desorption in Soil. Soil Sci. Soc. Am. J. 2000, 64, 144–156. [Google Scholar] [CrossRef]
- Yin, Y.; Allen, H.E.; Huang, C.P.; Sparks, D.L.; Sanders, P.F. Kinetics of Mercury(II) Adsorption and Desorption on Soil. Environ. Sci. Technol. 1997, 31, 496–503. [Google Scholar] [CrossRef]
- Curtin, D.; Trolove, S. Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics. Soil Res. 2013, 51, 494–502. [Google Scholar] [CrossRef]
- Shaheen, S.M. Sorption and lability of cadmium and lead in different soils from Egypt and Greece. Geoderma 2009, 153, 61–68. [Google Scholar] [CrossRef]
- Gwenzi, W.; Gora, D.; Chaukura, N.; Tauro, T. Potential for leaching of heavy metals in open-burning bottom ash and soil from a non-engineered solid waste landfill. Chemosphere 2016, 147, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Li, P.; Howard, K.W.F.; Yang, C.; Zhang, X. Assessment of groundwater vulnerability in the Yinchuan Plain, Northwest China using OREADIC. Environ. Monit. Assess. 2011, 184, 3613–3628. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.L.; Du, Y.J.; Reddy, K.R.; Wu, H.L. Effects of freeze-thaw on characteristics of new KMP binder stabilized Zn-and Pb-contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 19473–19484. [Google Scholar] [CrossRef]
- McIntyre, A.M.; Guéguen, C. Binding interactions of algal-derived dissolved organic matter with metal ions. Chemosphere 2013, 90, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Şener, Ş.; Şener, E.; Nas, B.; Karagüzel, R. Combining AHP with GIS for landfill site selection: A case study in the Lake Beyşehir catchment area (Konya, Turkey). Waste Manag. 2010, 30, 2037–2046. [Google Scholar] [CrossRef]
- Liu, Z.C.; He, L.S.; Dong, J.; Meng, R.; Song, B.Y.; Xi, B.D. Risk assessment of groundwater pollution for simple waste landfill. Res. Environ. Sci. 2012, 25, 833–839. [Google Scholar]
- Ma, Y.; Dong, B.; Bai, Y.; Zhang, M.; Xie, Y.; Shi, Y.; Du, X. Remediation status and practices for contaminated sites in China: Survey-based analysis. Environ. Sci. Pollut. Res. 2018, 25, 33216–33224. [Google Scholar] [CrossRef]
- Ibe, K.M.; Nwankwor, G.I.; Onyekuru, S.O. Assessment of ground water vulnerability and its application to the development of protection strategy for the water supply aquifer in Owerri, Southeastern Nigeria. Environ. Monit. Assess. 2001, 67, 323–360. [Google Scholar] [CrossRef]
- Reddy, V.A.; Solanki, C.H.; Kumar, S.; Reddy, K.R.; Du, Y.-J. Stabilization/Solidification of Zinc- and Lead-Contaminated Soil Using Limestone Calcined Clay Cement (LC3): An Environmentally Friendly Alternative. Sustainability 2020, 12, 3725. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Feng, Y.-S.; Jin, F.; Zhang, L.-M.; Du, Y.-J. Stabilization and solidification of a heavy metal contaminated site soil using a hydroxyapatite based binder. Constr. Build. Mater. 2017, 156, 199–207. [Google Scholar] [CrossRef]
- Reddy, V.A.; Solanki, C.H.; Kumar, S.; Reddy, K.R.; Du, Y.-J. New ternary blend limestone calcined clay cement for solidification/stabilization of zinc contaminated soil. Chemosphere 2019, 235, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Kogbara, R.B.; Al-Tabbaa, A.; Yi, Y.; Stegemann, J.A. pH-dependent leaching behaviour and other performance properties of cement-treated mixed contaminated soil. J. Environ. Sci. 2012, 24, 1630–1638. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Voglar, G.E.; Leštan, D. Solidification/stabilisation of metals contaminated industrial soil from former Zn smelter in Celje, Slovenia, using cement as a hydraulic binder. J. Hazard. Mater. 2010, 178, 926–933. [Google Scholar] [CrossRef]
- Redman, A.D.; Macalady, D.L.; Ahmann, D. Natural Organic Matter Affects Arsenic Speciation and Sorption onto Hematite. Environ. Sci. Technol. 2002, 36, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Henriques, B.; da Silva, E.F.; Pereira, M.; Duarte, A.; Groenenberg, J.; Römkens, P. Evaluation of an approach for the characterization of reactive and available pools of 20 potentially toxic elements in soils: Part II–Solid-solution partition relationships and ion activity in soil solutions. Chemosphere 2010, 81, 1560–1570. [Google Scholar] [CrossRef]
- Yu, Y.; Qu, G.; Wu, B.; Lu, Q.; Ning, P. Characterization of the Stabilization of Arsenic in Mine Tailings. Anal. Lett. 2017, 50, 1862–1875. [Google Scholar] [CrossRef]
- Rodrfguez-Piñero, M.; Pereira, C.F.; de Elvira Francoy, C.R.; Vale Parapar, J.F. Stabilization of a chromium-containing solid waste: Immobilization of hexavalent chromium. J. Air Waste Manag. Assoc. 2011, 48, 1093–1099. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Saiers, J.E.; Ryan, J.N. Colloid-facilitated mobilization of metals by freeze–thaw cycles. Environ. Sci. Technol. 2014, 48, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Zhongping, Y.; Yao, W.; Xuyong, L.; Shupei, R.; Hui, X.; Jiazhuo, C. The effect of long-term freeze-thaw cycles on the stabilization of lead in compound solidified/stabilized lead-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 37413–37423. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Taki, G.; Nguyen, X.P.; Jo, Y.-T.; Kim, J.; Park, J.-H. Heavy metal stabilization in contaminated soil by treatment with calcined cockle shell. Environ. Sci. Pollut. Res. 2017, 24, 7177–7183. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, Z.; Song, Y.; Li, J.; You, Y.; Li, J. Evaluation of ferrihydrite-humic acid coprecipitate as amendment to remediate a Cd-and Pb-contaminated soil. Geoderma 2020, 361, 114131. [Google Scholar] [CrossRef]


| Heavy Metal Type | Pb | Cu | Zn | Cd | Ni | Cr(VI) | As | Hg |
|---|---|---|---|---|---|---|---|---|
| Screening value (mg/kg) | 400 | 2000 | - | 20 | 150 | 3 | 20 | 8 |
| Leaching concentration limit (mg/L) | 5 | 100 | 100 | 1 | 5 | 5 | 5 | 0.1 |
| Target Layer A | System Layer B | Index Layer C | |||
|---|---|---|---|---|---|
| Influencing Factors | Weight | Evaluation Indexes | Original Weight | Combined Weight | |
| Risk assessment of groundwater contamination at S/S heavy metal sites | Site Hazard B1 | 0.1893 | Site Size C11 | 0.5132 | 0.0972 |
| Top protection C12 | 0.1478 | 0.0280 | |||
| Side protection C13 | 0.0594 | 0.0112 | |||
| Bottom protection C14 | 0.2800 | 0.0530 | |||
| Contaminant stability B2 | 0.3509 | Exceedance multiple of heavy metals C21 | 0.2218 | 0.0778 | |
| Stabilization efficiency C22 | 0.0459 | 0.0161 | |||
| Toxicity leaching factor C23 | 0.0459 | 0.0161 | |||
| Leachable form ratio C24 | 0.3831 | 0.1344 | |||
| Soil pH C25 | 0.2218 | 0.0778 | |||
| Organic matter and clay C26 | 0.0814 | 0.0286 | |||
| Aquifer Vulnerability B3 | 0.3509 | Depth to water table C31 | 0.2887 | 0.1013 | |
| Net recharge of aquifer C32 | 0.1724 | 0.0605 | |||
| Topography C33 | 0.0451 | 0.0158 | |||
| Impact of vadose zone C34 | 0.2887 | 0.1013 | |||
| Hydraulic conductivity C35 | 0.1024 | 0.0359 | |||
| Aquifer media C36 | 0.1024 | 0.0359 | |||
| Natural conditions B4 | 0.1094 | Rainfall pH C41 | 0.8559 | 0.0936 | |
| Freeze-thaw cycle C42 | 0.1427 | 0.0156 | |||
| Scale | Definition (Compare Impact Factors a and b) |
|---|---|
| 1 | Equal importance |
| 3 | Weak importance of one over another |
| 5 | Essential or strong importance |
| 7 | Demonstrated importance |
| 9 | Absolute importance |
| 2,4,6,8 | Intermediate values between the two adjacent judgments |
| Reciprocals | If activity a has one of the above nonzero numbers assigned to it when compared with activity b, then b has the reciprocal value when compared with a |
| Evaluation Indexes | Classification of Indexes | ||||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | |
| Site size/m3 | <0.25 × 103 | 0.25 × 103~1.5 × 104 | 1.5 × 104~5 × 104 | 5 × 104~20 × 104 | ≥20 × 104 |
| Rating | 1 | 2 | 4 | 7 | 10 |
| Top protection | Concrete | Compacted clay | Soil | None | |
| Rating | 1 | 3 | 5 | 10 | |
| Side protection | Double-layer composite impermeable | Single layer protection | Concrete | None | |
| Rating | 1 | 3 | 4 | 10 | |
| Bottom protection | Double-layer composite impermeable | Single layer protection | Concrete | Natural powdered clay | Natural gravel |
| Rating | 1 | 3 | 4 | 5 | 10 |
| Evaluation Indexes | Classification of Indexes | ||||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | |
| Exceedance multiple of heavy metals/times | <1 | [1, 5) | [5, 10) | [10, 20) | ≥20 |
| Rating | 1 | 3 | 5 | 7 | 10 |
| Stabilization efficiency/% | ≥95 | [90, 95) | [80, 90) | [70, 80) | <70 |
| Rating | 1 | 2 | 4 | 7 | 10 |
| Toxicity leaching factor | <0.1 | [0.1, 0.3) | [0.3, 0.6) | [0.6, 0.9) | ≥0.9 |
| Rating | 1 | 2 | 4 | 7 | 10 |
| Organic matter and clay/% | ≥65 | [40, 65) | [30, 40) | [10, 30) | <10 |
| Rating | 1 | 3 | 5 | 7 | 10 |
| Soil pH (cation) | ≥10 | [7.5, 10) | [6.5, 7.5) | [5, 6.5) | <5 |
| Rating | 3 | 1 | 4 | 7 | 10 |
| Soil pH (anionic) | ≥10 | [7.5, 10) | [6.5, 7.5) | [5, 6.5) | <5 |
| Rating | 7 | 4 | 1 | 3 | 10 |
| Leachable form ratio/% | <1 | [1, 10) | [10, 30) | [30, 50) | ≥50 |
| Rating | 1 | 3 | 5 | 7 | 10 |
| Evaluation Indexes | Classification of Indexes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Level 6 | Level 7 | Level 8 | Level 9 | Level 10 | |
| Depth to water table/m | ≥30.5 | [22.9, 30.5) | [15.2, 22.9) | [9.1, 15.2) | [4.6, 9.1) | [1.5, 4.6) | <1.5 | |||
| Rating | 1 | 2 | 3 | 5 | 7 | 9 | 10 | |||
| Net recharge of aquifer/mm | <51 | [51, 102) | [102, 178) | [178, 254) | ≥254 | |||||
| Rating | 1 | 3 | 6 | 8 | 10 | |||||
| Topography/% | ≥18 | [12, 18) | [6, 12) | [2, 6) | <2 | |||||
| Rating | 1 | 3 | 5 | 9 | 10 | |||||
| Impact of vadose zone | Clay | Loam | Sandy loam | Silt | Fine Sand | Sand | Medium sand | Coarse sand | Sand and gravel | Gravel |
| Rating | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Aquifer media | Clay | Loam | Sandy loam | Silt | Fine Sand | Sand | Medium sand | Coarse sand | Sand and gravel | Gravel |
| Rating | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Hydraulic conductivity (m/day) | <4 | [4, 12) | [12, 28) | [28, 40) | [40, 80) | ≥80 | ||||
| Rating | 1 | 2 | 4 | 6 | 8 | 10 | ||||
| Evaluation Indexes | Classification of Indexes | ||||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | |
| Rainfall pH | ≥5.6 | [5, 5.6) | [4, 5.5) | <4.5 | |
| Rating | 1 | 5 | 7 | 10 | |
| Freeze–thaw cycle/time | <5 | [5, 15) | [15, 30) | [30, 50) | ≥50 |
| Rating | 1 | 3 | 5 | 7 | 10 |
| Contaminant Stability B2 | Exceedance Multiple of Heavy Metals C21 | Stabilization Efficiency C22 | Toxicity Leaching Factor C23 | Leachable Form Ratio C24 | Soil pH C25 | Organic Matter and Clay C26 |
|---|---|---|---|---|---|---|
| Exceedance multiple of heavy metals C21 | 1 | 5 | 5 | 1/2 | 1 | 3 |
| Stabilization efficiency C22 | 1/5 | 1 | 1 | 1/7 | 1/5 | 1/2 |
| Toxicity leaching factor C23 | 1/5 | 1 | 1 | 1/7 | 1/5 | 1/2 |
| Leachable form ratio C24 | 2 | 7 | 7 | 1 | 2 | 5 |
| Soil pH C25 | 1 | 5 | 5 | 1/2 | 1 | 3 |
| Organic matter and clay C26 | 1/3 | 2 | 2 | 1/5 | 1/3 | 1 |
| Grading | Very Low Risk | Low Risk | Medium Risk | High Risk | Very High Risk |
|---|---|---|---|---|---|
| Rating value (R) | [0, 2) | [2, 4) | [4, 6) | [6, 8) | [8, 10] |
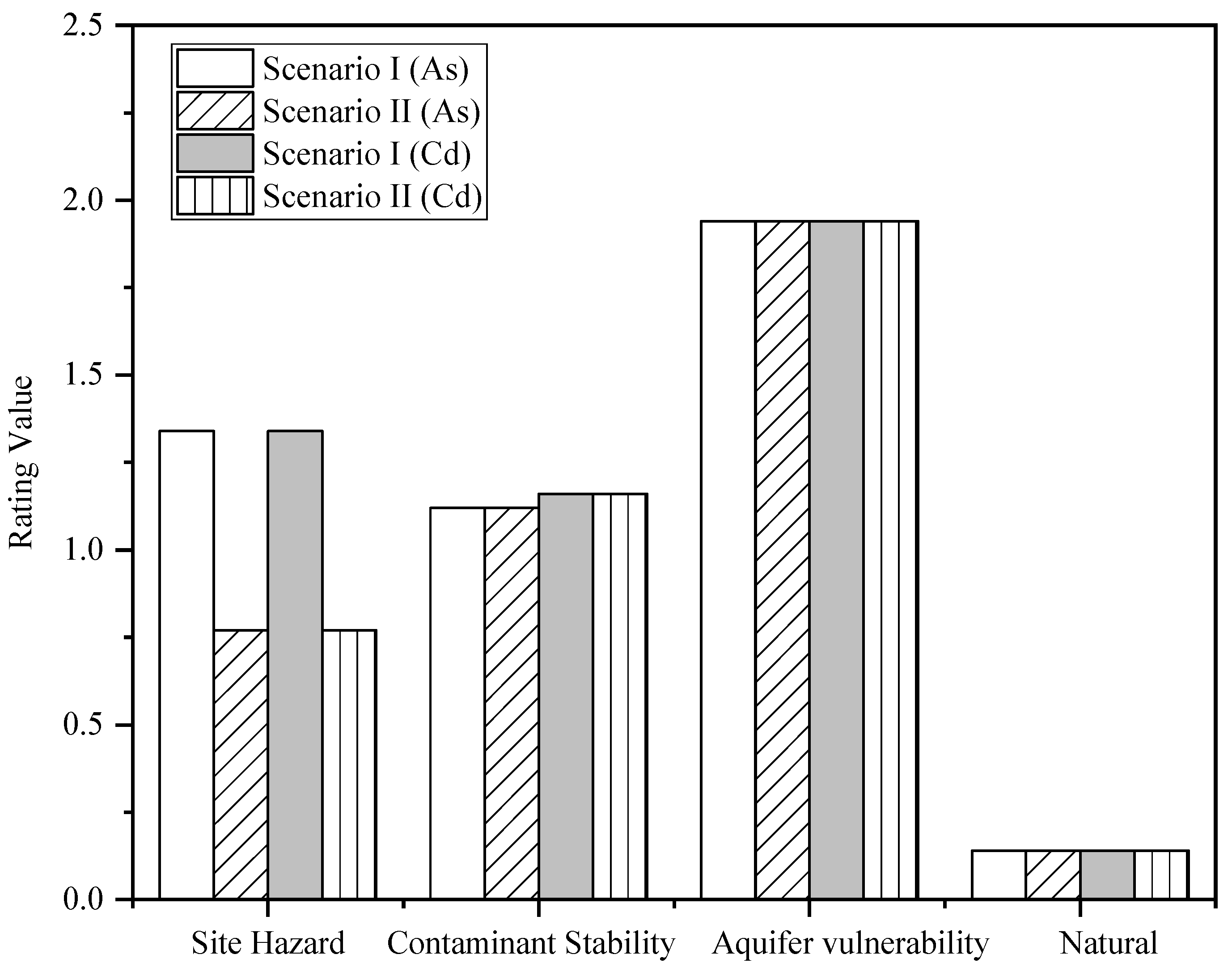
| Influencing Factors | Evaluation Indexes | Indicator Values | Rating | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Scenario 1 | Scenario 2 | Scenario 1 | Scenario 2 | ||||||
| As | Cd | As | Cd | As | Cd | As | Cd | ||
| Site Hazard | Site size/m3 | 118,439 | 118,439 | 118,439 | 118,439 | 0.68 | 0.68 | 0.68 | 0.68 |
| Top protection | None | None | Concrete | Concrete | 0.28 | 0.28 | 0.03 | 0.03 | |
| Side protection | None | None | Double-layer composite impermeable | Double-layer composite impermeable | 0.11 | 0.11 | 0.01 | 0.01 | |
| Bottom protection | Natural silty clay | Natural silty clay | Double-layer composite impermeable | Double-layer composite impermeable | 0.27 | 0.27 | 0.05 | 0.05 | |
| Contaminant Stability | exceedance multiple of heavy metals/times | 3.25 | 3.55 | 3.25 | 3.55 | 0.23 | 0.23 | 0.23 | 0.23 |
| Stabilization efficiency/% | 96.5% | 95.1% | 96.5% | 95.1% | 0.02 | 0.02 | 0.02 | 0.02 | |
| Toxicity leaching factor/% | 0.55 | 0.02 | 0.55 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Leachable form ratio/% | 3.02 | 12.5 | 3.02 | 12.5 | 0.40 | 0.67 | 0.40 | 0.67 | |
| Soil pH | 9.05 | 9.05 | 9.05 | 9.05 | 0.31 | 0.08 | 0.31 | 0.08 | |
| Organic matter and clay/% | 36.5 | 36.5 | 36.5 | 36.5 | 0.14 | 0.14 | 0.14 | 0.14 | |
| Aquifer Vulnerability | Depth to water table /m | 2.4 | 2.4 | 2.4 | 2.4 | 0.91 | 0.91 | 0.91 | 0.91 |
| Net recharge of aquifer /mm | 93.09 | 93.09 | 93.09 | 93.09 | 0.18 | 0.18 | 0.18 | 0.18 | |
| Topography e/% | 1.2 | 1.2 | 1.2 | 1.2 | 0.16 | 0.16 | 0.16 | 0.16 | |
| Impact of vadose zone | Clay/Gravel | Clay/Gravel | Clay/Gravel | Clay/Gravel | 0.41 | 0.41 | 0.41 | 0.41 | |
| Hydraulic conductivity (m/d) | 18.5 | 18.5 | 18.5 | 18.5 | 0.14 | 0.14 | 0.14 | 0.14 | |
| Aquifer media | Clay/Gravel | Clay/Gravel | Clay/Gravel | Clay/Gravel | 0.14 | 0.14 | 0.14 | 0.14 | |
| Natural Conditions | Rainfall pH | 5.6 | 5.6 | 5.6 | 5.6 | 0.09 | 0.09 | 0.09 | 0.09 |
| Freeze–thaw cycle/times | 12 | 12 | 12 | 12 | 0.05 | 0.05 | 0.05 | 0.05 | |
| Composite Index (R) | 4.55 | 4.58 | 3.98 | 4.02 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Chi, Z. Groundwater Risk Assessment Based on DRASTIC and Special Vulnerability of Solidified/Stabilized Heavy-Metal-Contaminated Sites. Sustainability 2023, 15, 2997. https://doi.org/10.3390/su15042997
Wei Z, Chi Z. Groundwater Risk Assessment Based on DRASTIC and Special Vulnerability of Solidified/Stabilized Heavy-Metal-Contaminated Sites. Sustainability. 2023; 15(4):2997. https://doi.org/10.3390/su15042997
Chicago/Turabian StyleWei, Zhiyong, and Zifang Chi. 2023. "Groundwater Risk Assessment Based on DRASTIC and Special Vulnerability of Solidified/Stabilized Heavy-Metal-Contaminated Sites" Sustainability 15, no. 4: 2997. https://doi.org/10.3390/su15042997
APA StyleWei, Z., & Chi, Z. (2023). Groundwater Risk Assessment Based on DRASTIC and Special Vulnerability of Solidified/Stabilized Heavy-Metal-Contaminated Sites. Sustainability, 15(4), 2997. https://doi.org/10.3390/su15042997







