Abstract
An acidophilic metal-resistant bacterial strain, Acidomyces acidothermus (A. acidothermus), was isolated and identified by morphology, physiology, biochemistry, and 16S rDNA. A. acidothermus culture conditions were optimized by response surface methodology (RSM). Results showed that with a temperature of 40 °C, a pH of 3 in a 9k medium, and a rotation speed of 140 r/min, the copper leaching rate reached the highest value of 39.8%. Furthermore, SEM images and a heatmap of differential metabolites indicated that A. acidothermus adsorbed on the surface of WPCBs and extracellular polymeric substances (EPS), mainly D-glucuronic acid, were secreted, suggesting the highly efficient mechanism of copper recovery from WPCBs.
1. Introduction
With the rapid development of information technology, large amounts of waste electrical and electronic equipment (WEEE) are being produced. According to the 2020 Global Electronic Waste Monitoring report released by the United Nations, the global electronic waste output reached 53.6 million tons in 2019, of which China was the largest producer at 10.129 million tons. Printed circuit boards have been found in almost all WEEE. Waste printed circuit boards (WPCBs) composed of polymers, inert oxides, and metals, which have a high resource value, are the most difficult type of electronic waste to dispose of. The metal content is up to 40% (20–30% copper, 5–6% zinc, 3–4% lead, 1–2% nickel, 0.5–0.9% silver, gold 0.04–0.07%, etc.) [1,2]. Obviously, improper treatment of WPCBs would cause serious harm to the environment.
At present, the commonly used methods for extracting metals from WPCBs include mechanical and physical methods [3], fire methods [4], and hydrometallurgy [5]. Mechanical and physical methods have a high evaluation of the effects of renewable resources, environmental friendliness, and industrial application rate, but the equipment used has a high investment and high energy consumption, and it is difficult to separate various metals in the products, so the economic feasibility is relatively poor [6,7]. The fire method has the advantages of simplicity, convenience, and a high recovery rate, but it also has many disadvantages: it produces a large amount of harmful gases (such as dioxins, etc.), the recovery rate of lead, tin, and other nonferrous metals is low, and a large number of nonmetallic components are lost in the incineration process [8,9]. The wet process has the advantages of less exhaust gas emissions and a simple process but requires a strong acid to dissolve, so the leaching solution and residue are highly toxic [10,11].
The biological treatment of recycled WPCB metals has the advantages of low energy consumption and less environmental pollution, but it is still in the experimental stage. Biological treatment of WPCBs began in the 1980s, and metals could be recovered by oxidation, reduction, dissolution, adsorption, and other methods through the interaction of microorganisms or their metabolites with metals [12]. At present, the microorganisms used to treat WPCBs include Acidithiobacillus ferrooxidans (A. ferrooxidans), Acidithiobacillus thiooxidans (A. thiooxidans), Aspergillus niger, Penicillium, cyanogen bacteria, etc. [13]. A. ferrooxidans and A. thiooxidans have been widely used in bioleaching technology [14]. However, as A. ferrooxidans and A. thiooxidans competed with each other for nutrient substrates, strict conditions (low pH and high reduction potential) needed to be maintained to promote balanced growth of the bacterium, thus enhancing metal leaching by WPCBs [15]. Most fungal strains, such as Aspergillus niger [16] or Penicillium chrysogenum [17], with the ability to yield high amounts of organic acids, were also applied in the bioleaching of WPCBs. Faraji et al. revealed that Aspergillus niger was capable of the simultaneous leaching of 100% of Zn, 80.39% of Ni, and 85.88% of Cu in 30 days [16]. Nevertheless, long reaction times and unclear leaching mechanisms limit the commercial application of bioleaching. To improve the metal leaching efficiency, the screening and separation of acidophilic metal-resistant bacteria is the key to metal recovery by biological methods.
Accordingly, the objective of this study was to screen for acidophilic metal-resistant bacteria and optimize culture conditions, to explore the leaching of copper from WPCBs under optimal growth conditions. The exploration of the bioleaching mechanism would provide a theoretical basis for improving the efficiency of metal recovery.
2. Material and methods
2.1. Experimental Materials
The main reagent used in the experiment, such as LB medium, (NH4)2SO4, K2HPO4, MgSO4·7H2O, KCl, FeSO4·7H2O, Ca(NO3)2, sulfur, H2SO4, NaOH, Agar powder, were purchased from Sinopharm Chemical Reagent Co., LTD. (Shanghai China)
WPCBs, without any physical or mechanical separation, were collected from an electronic waste recycling company in Changzhou. For experimental use, WPCBs were further ground by a grinder (YXD-100, Qingdao Yaoxin Intelligent Technology Co., Ltd., Qingdao, China) and sieved to obtain a mesh size of 100–200 μm.
The activated sludge (~3 g/L) was obtained from a municipal sewage treatment plant in Changzhou.
2.2. Microbial Screening and Isolation
Screening and acclimation of bacterial strains: A total of 110 mL of sludge and 1 g of waste circuit board were placed into 90 mL of LB liquid medium (pH = 3) and incubated at 30 °C at 140 r/min in an oscillatory incubator (ZHLY-300S, Shanghai Zhichu Instrument Co., Ltd., Shanghai, China). After five days, the inoculum was inoculated into fresh liquid medium with a volume ratio of 10%, and the culture was repeated four times. Enrichment of acidophilic metal-resistant bacteria liquid was obtained.
Strain isolation: after repeated domestication and culture, the bacterial liquids was diluted and inoculated on the acidic LB solid medium plate (pH = 3) at 30 °C in a constant temperature incubator (DHP, Shanghai a constant scientific instrument co., LTD, Shanghai, China). After 48 h of training, single colonies of different shapes and colors were selected and incubated on fresh acidic LB solid medium plates. After seven days of culture at 30 °C in a 100 r/min shaking incubator, 5 mL of culture medium was collected and cultured in fresh LB solid medium. The above separation operation was repeated six times.
For strain purification, took the above culture solution and dilute it in a gradient of 10−1 to 10−8, coating on acid LB solid medium (pH = 3), and inverted culture for 1–7 days. The growth of colonies was observed every day, and pure single colonies were obtained. The purified strain was stored in a refrigerator at −81°C for later use.
2.3. Optimization of Culture Conditions
Firstly, a single-factor test was conducted. The pH (0, 0.5, 1.5, 2.0, 3.0, and 5.0), temperature (30, 35, and 40 °C), rotational speed (0, 100, 140, and 180 r/min), and medium (9K medium, LB medium) were changed, respectively, to investigate the growth of the purified strain and determine the corresponding optimum culture conditions.
On the basis of a single-factor test, a response surface test was designed according to Box-Benhnken. By establishing a multiple quadratic regression model equation, the relationship between each factor and index in this experiment was approximated by a polynomial equation, and the optimal culture conditions were obtained by response surface graph analysis of the interaction of each factor.
2.4. Bioleaching of WPCBs
The purified strain was inoculated into an acid-liquid 9k medium and cultured until the OD600 value reached 0.8. 1 g of WPCBs was added to different volumes of the cultured bacterial solution, stirred at a speed of 140 r/min. The sample liquid was centrifuged. The supernatant, after high-speed and low-temperature centrifugation, was filtered to obtain the required clear and transparent leaching solution. The concentration of copper ions in waste circuit board wastewater when different amounts of bacteria were added was measured by an inductively coupled plasma mass spectrometer (ICP TQ, Thermo Fisher Scientific Co, Bremen, Germany) after 250 times dilution of the leaching solution.
2.5. Analytical Methods
The cell morphology of the purified strain was observed by optical microscope (BX51, Shanghai Xinu Optical Technology Co., LTD, Shanghai, China). The purified strain was sent to Shanghai Shenggong Bioengineering Co., Ltd. (Shanghai, China), for 16S rDNA sequencing to determine the species [18]. The growth of bacteria was expressed as a value of OD600. A microplate reader (Infinite 200PRO, Beijing Longyue Biotechnology Development Co., Ltd., Beijing, China) was used to measure the OD600 value. Scanning electron microscopy (SEM, SU1510, Hitachi, Tokyo, Japan) was used to observe the surface morphology in the residue of WPCBs. The metal content in the residue was observed by X-ray diffraction (XRD, X’PERT POWDER, Panaco, Amsterdam, The Netherlands). The metabolomics were used to analyze the metabolites in the bioleaching process [19].
3. Results and Discussion
3.1. Biological Characteristics and Identification Results of the Strain
The colonies of the obtained strain were grey-black, round, raised in the middle, hard and difficult to pick, and presented filamentous colonies under the microscope (Figure 1).
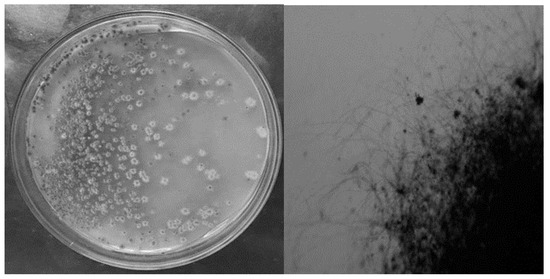
Figure 1.
Morphology and cell morphology of the purified strain.
The 16Sr DNA gene sequences of the strain were found to have the highest sequence similarity to Acidomyces acidothermus (A. acidothermus) at 100% in the ribosome database http://rdp.cme.msu.edu/index.jsp (accessed 10 May 2021). The A. acidothermus has been deposited in China General Microbiological Culture Collection Center with the deposit number of CGMCC No. 22431.
3.2. Optimization of Culture Conditions
Rapid cultivation of massive, highly active microorganisms under optimal conditions is important for efficient bioleaching of metals. The growth and reproduction of microorganisms are significantly sensitive to changes in the environment (e.g., nutrients, pH, temperature, dissolved oxygen, etc.).
The medium is the place where microorganisms are supplied with energy and multiply. Different mediums can affect the growth and multiplication of microorganisms due to differences in nutrients or nutrient ratios. The A. acidothermus was inoculated into 100 mL of 9k liquid medium and LB liquid medium, respectively, at a volume ratio of 5% and incubated for 48 h at 30 °C and 100 r/min with shaking. As shown in Figure 2A, from 0 h to 12 h, the OD600 values of the bacterial solution in 9k medium kept increasing, and after 12 h, the OD600 values tended to be flat. From 0 h to 24 h, the OD600 values of the bacterial solution in LB medium kept increasing, and after 12h, the OD600 values tended to be flat. Significantly, the OD600 value of bacterial solution at 9k was always higher than that in LB. Therefore, the suitable medium is 9k medium.
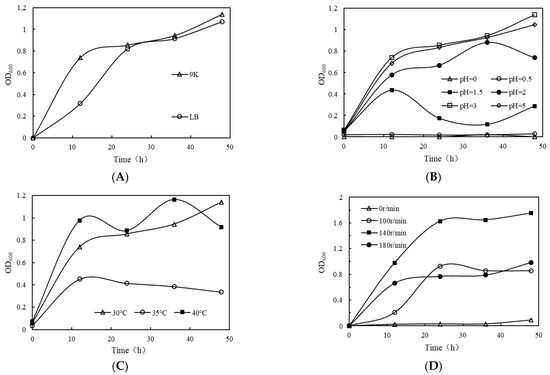
Figure 2.
Growth of A. acidothermus under different conditions. (A) Growth of strain in different media. (B) Growth of strain in different pH. (C) Growth of strain in different temperature. (D) Growth of strain in different rotation speed.
Changes in pH affect not only the activity of the microbial metabolic enzymes but also the absorption of nutrients from the environment. The A. acidothermus was inoculated into 100 mL of 9k liquid medium at a 5% volume ratio, with initial pH values of 0, 0.5, 1.5, 2.0, 3.0, and 5.0 and incubated at 30 °C and 100 r/min for 48 h in shock culture. Figure 2B showed that after 12 h of culture, the OD600 values of the A. acidothermus bacterial solution with a pH of 1.5–5 increased rapidly. Moreover, during the whole culture cycle, the OD600 values of the A. acidothermus bacterial solution with a pH of 3–5 always increased, while the OD600 values of the bacterial solution with a pH of 0–0.5 were always lower than 0.03. It indicated that the A. acidothermus was not suitable to grow at a pH less than 0.5. In comparison, the OD600 value of A. acidothermus at a pH of 3 was always higher than that at other pH values, so the optimal initial pH was 3.
Changes in temperature affect the biochemical reactions in microorganisms, thus affecting their metabolic activity. The A. acidothermus was inoculated into 100 mL of 9k liquid medium (pH = 3) at a 5% volume ratio, and the initial temperatures were 30 °C, 35 °C, and 40 °C, respectively. The bacterial solution was incubated for 48 h at 100 r/min with shaking. As shown in Figure 2C, from 0 h to 12 h, the OD600 value of A. acidothermus at temperatures ranging from 30 °C to 40 °C continued to rise. At 24 h, the OD600 value of A. acidothermus at 30 °C increased compared with that at 12 h, and the OD600 value of A. acidothermus at 35 °C to 40 °C decreased compared with that at 12 h. However, the OD600 value of the strain at 40 °C was still higher than that at other temperatures, indicating that the strain grew well under this condition. In comparison, A. acidothermus entered the rapid growth phase earlier at 40 °C, so the optimal initial temperature was 40 °C.
Changes in the dissolved oxygen affect the respiration rate of microorganisms and therefore the efficiency of the reaction. The speed of the shaker controls the dissolved oxygen content in the culture medium to a certain extent. The A. acidothermus was inoculated into 100 mL of 9k liquid medium (pH = 3) at a 5% volume ratio, and the initial rotation speeds were 0, 100 r/min, 140 r/min, and 180 r/min, respectively. The bacterial solution was incubated for 48 h at 40 °C with shaking. As shown in Figure 2D, the OD600 value of A. acidothermus at a rotation speed of 100–180 r/min increased from 0 h to 12 h. At 100 r/min and 180 r/min, the OD600 values of A. acidothermus were approximately 0.2 and 0.6, respectively, while at 140 r/min, the OD600 value of A. acidothermus reached approximately 0.9. At 24 h, the OD600 value of A. acidothermus increased at all speeds, and the OD600 value of bacteria increased steadily at 140 r/min. At 36 h and 48 h, the OD600 value of A. acidothermus at 140 r/min was always higher than that at other speeds, indicating that the strain grew well under this condition. In comparison, A. acidothermus entered the rapid growth phase earlier under the condition of 140 r/min, so the optimal speed was 140 r/min.
Design-Expert 8.0.6 software was used to optimize the experimental design and analysis of the culture conditions of A. acidothermus. The response surface analysis and variance analysis were shown in Table 1, Table 2 and Table 3, respectively. As shown in Table 1, OD600 was the response value, and pH (A), temperature (B), and rotational speed (C) were used as independent variables to optimize the culture conditions of the purified strain.

Table 1.
Response surface test factor levels.

Table 2.
Sample results of response surface analysis.

Table 3.
The variance analysis.
As seen from Table 3, the Model p < 0.0001 indicates that the model is highly significant, and the misfit term p = 0.3045 > 0.05 indicates that the difference is not significant. The calculation of R2R was 0.9812, which indicates that the model is well correlated. From the analysis results of the F value, it can be seen that the order of influence of each factor on the OD600 value is temperature, rotational speed, and pH. The interaction between the optimal conditions and parameters can be clearly seen from the response surface analysis diagram. The interaction between the elliptic shape is the best, and the circular shape is the worst [20]. Figure 3 showed that the interaction between pH and rotational speed was the strongest, while the interaction between pH and temperature was the weakest.
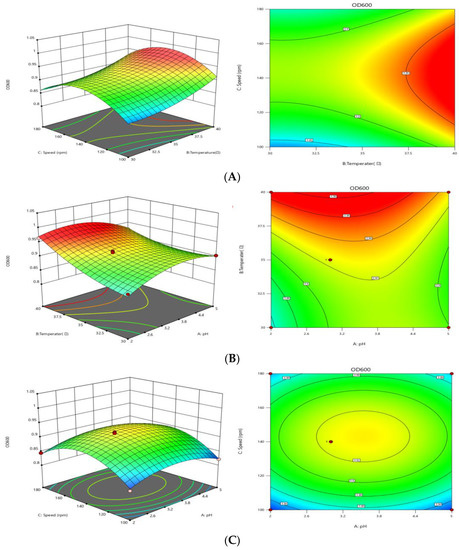
Figure 3.
Response surface curves. (A) Response surface and contour of temperature and rotation speed. (B) Response surface and contour of temperature and pH. (C) Response surface and contour of pH and rotation speed.
After fitting the data, the multiple quadratic regression equation was obtained:
OD600= 0.9285 + 0.0014 + 0.0358 + 0.0099 C to 0.0210 AB + B 0.0006 AC − 0.0029 – 0.0335 − A BC2+ 0.0196 B2 − 0.0630 − C2.
According to the model prediction, when the pH is 3.33374, the temperature is 39.8628 °C, the rotation speed is 147.857 r/min, and the OD600 value reaches the maximum value of 0.9824. The optimized conditions of the model are similar to those of the single-factor experiment. Considering the practical reasons, a pH of 3, a temperature of 40 °C, and a rotation speed of 140 r/min were selected to verify the predicted value. The results of the predicted value and the actual value were essentially the same. This shows that the regression equation can compare the real reaction of the influence of various factors on OD600 [21].
3.3. Bioleaching of WPCBs
Under optimized culture conditions, 1g of WPCBs was put into different volumes of A. acidothermus solution (10, 20, 30, 40, 50, and 100 mL), respectively. The leaching effect of the volume of the A. acidothermus solution on copper in WPCBs is shown in Table 4.

Table 4.
Copper leaching rate.
Table 4 exhibited that the initial bacterial liquid product for 10, 20, 30, 40, 50, and 100 mL, under the condition of acid and heavy metal, the copper leaching rate of A. acidothermus over time increased first, then decreased, and at the age of 4 h, the leaching rate was highest. This may be that the microbes in the logarithmic growth phase into environment containing waste circuit board, when the strains thrive in the first 4 h. The strain grew vigorously during the first 4 h, and the leaching rate was high. After 4 h, the leaching rate decreased. This might be because secondary precipitation occurs during the decomposition of WPCBs, and the released elements might be immobilised by the precipitation and enter the solution in lower amounts. [22]. Moreover, Rogers et al. pointed out that the WPCBs might contain nutrients needed for bacterial metabolism [23]. It could be inferred that part of the copper ions leached from WPCBs were absorbed by A. acidothermus for growth and reproduction. As the concentration of the A. acidothermus bacterial solution increased, the leaching rate increased. The leaching rate of 100 mL of bacterial solution was the highest, up to 39.8 ± 0.1%. Due to the high concentration of bacterial solution, the number of microorganisms and secreted metabolites increased, and the adsorption of microorganisms on the surface of WPCBs was promoted to improve the leaching rate [24].
After 4 h of leaching of A. acidothermus WPCBs, the residue in the bacterial solution was removed, baked in an oven at 70 °C for 24 h, and ground into a powder. The XRD was scanned continuously from 10° to 80° with a step distance of 0.00657° to obtain the XRD pattern (Figure 4).
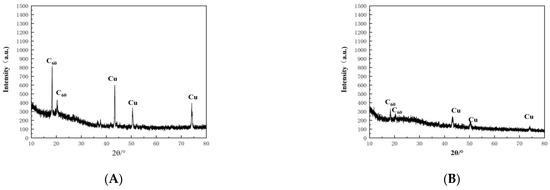
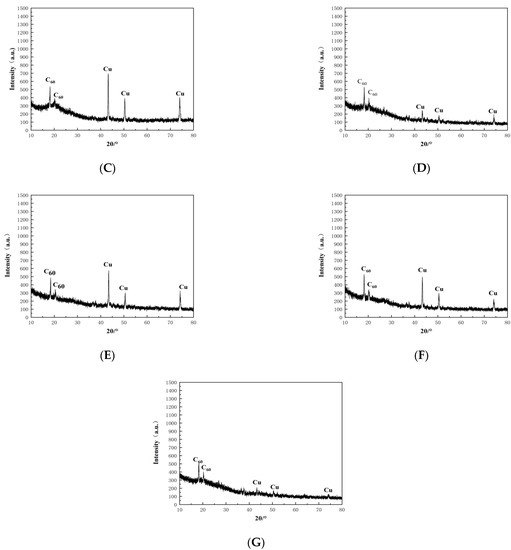
Figure 4.
XRD patterns of WPCBs residues. (A) original WPCBs. (B) WPCBs residues after 10 mL bacterial solution leaching. (C) WPCBs residues after 20 mL bacterial solution leaching. (D) WPCBs residues after 30 mL bacterial solution leaching. (E) WPCBs residues after 40 mL bacterial solution leaching. (F) WPCBs residues after 50 mL bacterial solution leaching. (G) WPCBs residues after 100 mL bacterial solution leaching.
XRD patterns showed that the characteristic peaks of elemental copper and C60 retrieved from PDF0178–2076 by computer were consistent. Using HighScore for semiquantitative analysis of XRD data, the results are shown in Figure 5. As seen in Figure 5G, the copper accounted for 37% and the C60 accounted for 63% of the original WPCBs, while the copper accounted for 9% and the C60 accounted for 91% of the WPCB residues. Therefore, it could be concluded that the leaching rate of copper in the 100 mL bacterial solution was the highest.
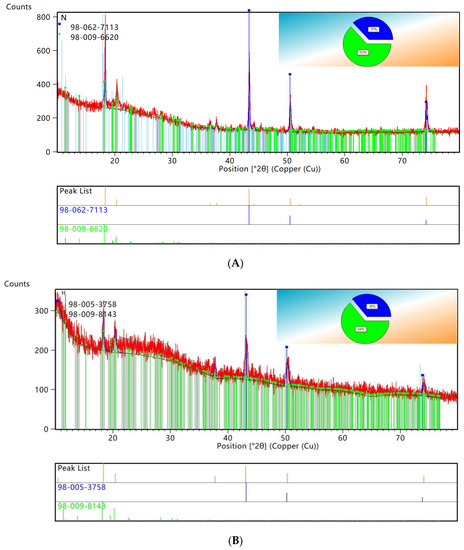
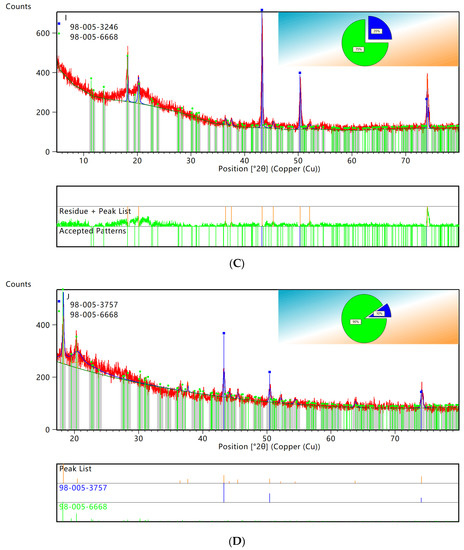
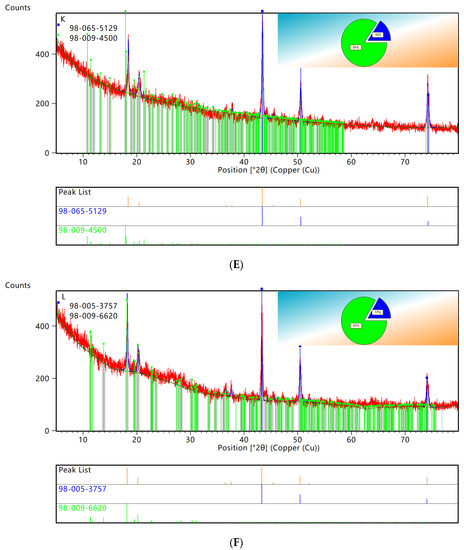
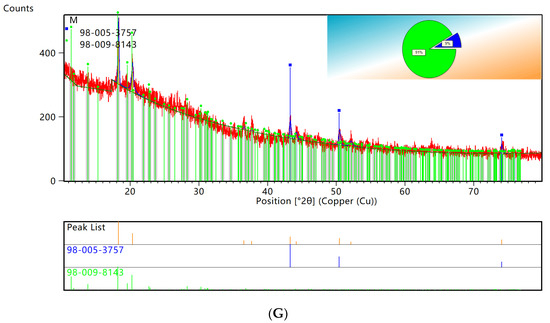
Figure 5.
XRD patterns semiquantitative analysis. (A) original WPCBs. (B) WPCBs residues after 10 mL bacterial solution leaching. (C) WPCBs residues after 20 mL bacterial solution leaching. (D) WPCBs residues after 30 mL bacterial solution leaching. (E) WPCBs residues after 40 mL bacterial solution leaching. (F) WPCBs residues after 50 mL bacterial solution leaching. (G) WPCBs residues after 100 mL bacterial solution leaching.
3.4. Bioleaching Mechanisms
In general, bioleaching mechanisms included direct action mechanisms, indirect action mechanisms, and combined action mechanisms. WPCBs were amorphous, non-crystalline substances that were better suited to acid attacks [25]. 9k medium, with a large amount of Fe2+, was highly acidic. Moreover, A. acidothermus, which secrete acidic enzymes, contributed significantly to the leaching of metals [26].
To illustrate the direct effect of bacterial leaching, the adsorption of bacteria on the WPCB’s surface should be demonstrated first. After leaching out the WPCBs, the residue in the bacterial solution was removed, baked in an oven at 70 °C for 24 h, and ground into a powder. SEM was used to observe the surface morphology of the residue (Figure 6).
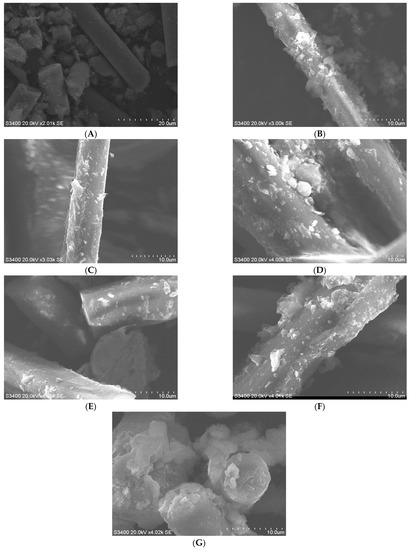
Figure 6.
SEM images of WPCBs residues. (A) original WPCBs. (B) WPCBs residues after 10 mL bacterial solution leaching. (C) WPCBs residues after 20 mL bacterial solution leaching. (D) WPCBs residues after 30 mL bacterial solution leaching. WPCBs residues after 40 mL bacterial solution leaching. (F) WPCBs residues after 50 mL bacterial solution leaching. (G) WPCBs residues after 100 mL bacterial solution leaching.
Figure 6A displays the morphology of the original WPCBs. It can be seen from the figure that the WPCBs are composed of rod-shaped and globular polymers, and some rod-shaped surfaces are smooth. Figure 6B was the SEM image of the leaching residue of 10 mL of A. acidothermus bacterial solution, the surface of which was no longer smooth, and there were flocculent materials and lumps attached to the WPCBs. Figure 6C was the SEM image of the leaching residue of 20 mL of A. acidothermus bacterial solution, and the WPCBs had tiny protrusions and clumps attached to them. Figure 6D was the SEM image of the leaching residue of 30 mL of A. acidothermus bacterial solution, and the WPCBs were attached to large and small lumps and flocculents, whose surfaces were unevenly eroded by microorganisms. Figure 6E was the SEM image of the leaching residue of 40 mL of A. acidothermus bacterial solution, and there were small lumps on the surface of the WPCBs. Figure 6F was the SEM image of the leaching residue of 50 mL of A. acidothermus bacterial solution. The surface of the WPCBs, eroded by microorganisms, was uneven, with lumps and tiny protrusions. Figure 6G was the SEM image of the leaching residue of 100 mL of A. acidothermus bacterial solution. The end of the powder rod of the WPCBs was no longer smooth and became bumpy after being eroded by microorganisms, and a large number of lumps were attached to it.
Figure 6 showed bacteria tended to adhere to the WPCB’s surface, which is similar to the research results of Roberts et al. [24,27]. With the extension of reaction times, microorganisms grew on the surface of WPCBs and secreted extracellular polymers to form biofilms. It had been illustrated that the microorganisms adsorbed on the surface of WPCBs and secreted extracellular polymeric substances (EPS) [28]. The heatmap of differential metabolites (Figure 7) revealed that in the process of microbial leaching, metabolites such as ketoleucine, 2-Funancarboxaldichyde, thymine, etc. constituted EPS. The EPS matrix also contained D-glucuronic acid, which during bioleaching was able to form stable complexes with iron ions, thus giving the cells a positive net charge and thus allowing them to adsorb onto the negatively charged WPCB’s surface [29]. On the one hand, iron-D-glucuronic complex could strengthen the EPS matrix of biofilm. On the other hand, the complexes were able to enhance bioleaching. EPS forms a biofilm leaching area, which created conditions for H+ to go deep into the interior of WPCBs for acid dissolution. EPS matrix enabled microbial biofilm to capture nutrients from the aqueous phase or WPCB’s surface, and EPS matrix acted as a common external digestive system. In addition, EPS could ensure the activity of enzymes and promote the bioleaching reaction. In conclusion, biofilm could make microorganisms attach more firmly to the mineral surface, and it was also the micro-environment with the most active material exchange and chemical reaction between biological and WPCBs, becoming the external force for the continuous decomposition of WPCBs [30].
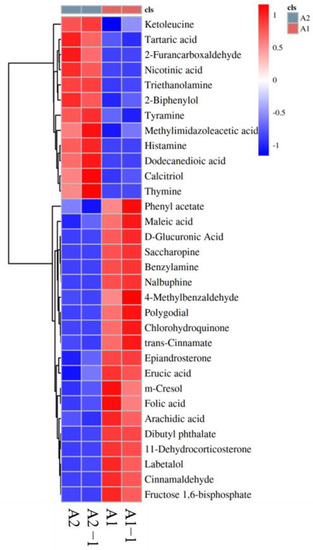
Figure 7.
The heatmap of differential metabolites ((A1): Before bioleaching reaction, (A2): After bioleaching reaction).
Although bacteria can directly decompose WPCBs, Fe3+, the most important oxidising agent, produced by bacterial oxidation is also the key to copper bioleaching [31]. Fe2+ could be oxidized to Fe3+ by floating A. acidothermus with O2 in existence [32], and then Fe3+ oxidized copper into copper ions while Fe2+ was reduced to Fe3+. Afterwards, the next cycle of reactions would begin [33].
In addition, organic acids and inorganic acids secreted through bacterial metabolism can also dissolve WPCBs and release metal ions. Figure 7 also showed that in the process of bioleaching, metabolites included tartaric acid, nicotinic acid, methylimidazoleacetic acid, dodecanedioic acid, maleic acid, D-glucuronic acid, erueic acid, folic acid, arachidic acid, etc. It could be inferred that these organic acids were conducive to the leaching of metals.
4. Conclusions
In this study, one strain was screened and isolated from the sludge. According to the physiological, biochemical, and 16rDNA characteristics, the obtained strain was identified as Acidomyces acidothermus. The optimal culture conditions for A. acidothermus were as follows: temperature: 40 °C, pH: 3, rotational speed: 140 r/min by single-factor experiment and response surface experiment. The interaction between pH and rotational speed was the strongest, while the interaction between pH and temperature was the weakest. Under the optimal conditions, 1 g of WPCBs (mesh size of 100–200 μm) were added to 100 mL of A. acidothermus bacterial solution and shaken for 4 h, and the highest copper leaching rate of 39.8% was achieved. A. acidothermus adsorbed on the surface of WPCBs and secreted EPS and organic acids, which promoted copper leaching. The use of the bioleaching method to leach copper ions from WPCBs is a technical concept that follows the current trend of green and sustainable development.
Author Contributions
Conceptualization, Q.Z.; Investigation, X.S. and W.L.; Project administration, X.Y.; Software, X.S.; Writing—original draft, X.Y. and X.S.; Writing—review & editing, X.Y. and G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Natural Science Foundation of the Jiangsu Higher Education Institutions of China, grant number 20KJA610005, The Natural Science Foundation of the Jiangsu Higher Education Institutions of China, grant number 21KJB610010, Changzhou Longcheng Talent Program, grant number CQ20210092 and Changzhou Science and Technology Bureau, grant number CM20223017. The APC was funded by The Natural Science Foundation of the Jiangsu Higher Education Institutions of China, grant number 21KJB610010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Horațiu, V.; Ancuța-Elena, T.; Marius, P. Advanced recovery techniques for waste materials from IT and telecommunication equipment printed circuit boards. Sustainability 2019, 12, 74. [Google Scholar]
- Gu, W.; Bai, J.; Dong, B.; Zhuang, X.; Zhao, J.; Zhang, C.; Wang, J.; Shih, K. Enhanced bioleaching efficiency of copper from waste printed circuit board driven by nitrogen-doped carbon nanotubes modified electrode. Chem. Eng. J. 2017, 324, 122–129. [Google Scholar] [CrossRef]
- Ning, C.; Lin, C.S.K.; Hui, D.C.W.; McKay, G. Waste printed circuit board (PCB) recycling techniques. Top. Curr. Chem. 2017, 375, 43. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Liu, C.; Liu, L.; Xiong, M.; Chen, J.; Zhang, Z.; Zhong, S.; Xu, Z.; Huang, J. Pyrolysis behaviour and combustion kinetics of waste printed circuit boards. Int. J. Miner. Metall. Mater. 2022, 29, 1722–1732. [Google Scholar] [CrossRef]
- Sronsri, C.; Panitantum, N.; Sittipol, W.; U-Yen, K.; Kerdphol, P. Optimization of selective gold recovery from electronic wastes through hydrometallurgy and adsorption. Trans. Inst. Chem. Engineers. Process Saf. Environ. Prot. Part B 2022, 163, 659–668. [Google Scholar] [CrossRef]
- Kaya, M. Waste Printed Circuit Board (WPCB) Recycling: Conventional and Emerging Technology Approach. Encycl. Renew. Sustain. Mater. 2020, 4, 677–694. [Google Scholar]
- Kaya, M. Waste Printed Circuit Board (WPCB) Recovery Technology: Disassembly and Desoldering Approach. Encycl. Renew. Sustain. Mater. 2020, 4, 658–676. [Google Scholar]
- Jungho, H.; Jooho, P.; Joo, H. Effect of pyro-processing conditions on impurity removal and precious metal enrichment in waste printed circuit board (WPCB) recycling process. Resour. Conserv. Recycl. 2022, 179, 106068. [Google Scholar]
- Yang, J.; Feng, J.; Li, W.; Chen, X.; Liu, X.; Ruan, J.; Qiu, R.; Xiong, Y.; Tian, S. A resource-utilization way of the waste printed circuit boards to prepare silicon carbide nanoparticles and their photocatalytic application. J. Hazard. Mater. 2019, 373, 640–648. [Google Scholar] [CrossRef]
- Moosakazemi, F.; Ghassa, S.; Soltani, F.; Mohammadi, M.R.T. Regeneration of Sn-Pb solder from waste printed circuit boards: A hydrometallurgical approach to treating waste with waste. J. Hazard. Mater. 2020, 385, 121589. [Google Scholar] [CrossRef]
- Awasthi, A.; Zlamparet, G.; Zeng, X.; Li, J. Evaluating waste printed circuit boards recycling: Opportunities and challenges, a mini review. Waste Manag. Res. 2017, 3, 346–356. [Google Scholar] [CrossRef]
- Pathak, A.; Morrison, L.; Healy, M.G. Catalytic potential of selected metal ions for bioleaching, and potential techno-economic and environmental issues: A critical review. Bioresour. Technol. 2017, 229, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, N.; Shen, W.; Zhang, T.; Wu, P. Bioleaching of copper from metal concentrates of waste printed circuit boards by a newly isolated Acidithiobacillus ferrooxidans strain Z1. J. Mater. Cycles Waste Manag. 2015, 19, 247–255. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Liu, C.; Dong, F.; Liu, B. Column bioleaching copper and its kinetics of waste printed circuit boards (WPCBs) by Acidithiobacillus ferrooxidans. Chemosphere 2015, 141, 162–168. [Google Scholar] [CrossRef]
- Norris, P.R.; Gould, O.J.; Ogden, T.J. Iron solubilization during anaerobic growth of acidophilic microorganisms with a polymetallic sulfide ore. Miner. Eng. 2015, 75, 77–84. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef]
- Xia, M.-C.; Bao, P.; Liu, A.-J.; Zhang, S.-S.; Peng, T.-J.; Shen, L.; Yu, R.-L.; Wu, X.-L.; Li, J.-K.; Liu, Y.-D.; et al. Isolation and identification of Penicillium chrysogenum strain Y5 and its copper extraction characterization from waste printed circuit boards. J. Biosci. Bioeng. 2018, 126, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Lu, X.; Li, N.; Zhang, M.; Qiao, X. Characterization of garlic endophytes isolated from the black garlic processing. MicrobiologyOpen 2018, 7, e00547. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, X.; Yang, Y.L.; Xing, Y.M.; Zhang, Q.; Li, J.M.; Ma, K.; Liu, H.W.; Guo, S.X. Genomic and transcriptomic analyses reveal differential regulation of diverse terpenoid and polyketides secondary metabolites in Hericium erinaceus. Sci. Rep. 2017, 7, 10151. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Liu, D.; Lv, M.; Liu, L. Response surface methodology for optimization of copper leaching from a low-grade flotation middling. Miner. Metall. Process. 2011, 28, 139–145. [Google Scholar] [CrossRef]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of response surface methodology(RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Min. Sci. Technol. 2018, 4, 621–629. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Guo, Y.; An, N.; Liang, C.; Ge, Z. Bioleaching of gold from waste printed circuit boards by alkali-tolerant Pseudomonas fluorescens. Hydrometallurgy 2020, 194, 105260. [Google Scholar] [CrossRef]
- Rogers, J.R.; Bennet, P.C. Mineral simulation of subsurface microorganisms: Release of limiting nutrients from silicates. Chem. Geol. 2004, 203, 91–108. [Google Scholar] [CrossRef]
- Roberts, J.A.; Fowle, D.A.; Hughes, B.T.; Kulczycki, E. Attachment behavior of Shewanella putrefaciens onto magnetite under aerobic and anaerobic conditions. Geomicrobiol. J. 2006, 23, 631–640. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Tian, X.; Che, L.; Wu, X.; Zhao, F. Leaching of indium from end-of-life LCD panels via catalysis by synergistic microbial communities. Sci. Total Environ. 2019, 655, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Rafiq, M.; Rehman, M.; Sajjad, W.; Hasan, F.; Abdullah, S. Fungi in acidic fire: A potential source of industrially important enzymes. Fungal Biol. Rev. 2019, 33, 58–71. [Google Scholar] [CrossRef]
- Nie, H.; Yang, C.; Zhu, N.; Wu, P.; Zhang, T.; Zhang, Y.; Xing, Y. Isolation of Acidithiobacillus ferrooxidans strain Z1 and its mechanism of bioleaching copper from waste printed circuit boards. J. Chem. Technol. Biotechnol. 2015, 90, 714–721. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr. There’s plenty of room at the bottom: Nanoscience in geochemistry. Geochim. Et Cosmochim. Acta 2002, 66, 735–743. [Google Scholar] [CrossRef]
- Fomchenko, N.; Muravyov, M. Sequential bioleaching of pyritic tailings and ferric leaching of nonferrous slags as a method for metal recovery from mining and metallurgical wastes. Minerals 2020, 10, 1097. [Google Scholar] [CrossRef]
- Ye, M.; Yan, P.; Sun, S.; Han, D.; Xiao, X.; Zheng, L.; Huang, S.; Chen, Y.; Zhuang, S. Bioleaching combined brine leaching of heavy metals from lead-zinc mine tailings: Transformations during the leaching process. Chemosphere 2016, 168, 1115. [Google Scholar] [CrossRef]
- Liang, G.; Mo, Y.; Zhou, Q. Novel strategies of bioleaching metals from printed circuit boards (PCBs) in mixed cultivation of two acidophiles. Enzym. Microb. Technol. 2010, 47, 322–326. [Google Scholar] [CrossRef]
- Nkulu, G.; Gaydardzhiev, S.; Mwema, E.; Compere, P. SEM and EDS observations of carrollite bioleaching with a mixed culture of acidophilic bacteria. Miner. Eng. 2015, 75, 70–76. [Google Scholar] [CrossRef]
- Li, Q.; Sand, W. Mechanical and chemical studies on EPS from Sulfobacillus thermosulfidooxidans: From planktonic to biofilm cells. Colloids Surf. B Biointerfaces 2017, 153, 34–40. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).